Sex-Related Differences in On-Treatment Platelet Reactivity in Patients with Acute Coronary Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Blood Sampling
2.3. Light-Transmission Aggregometry (LTA)
2.4. Determination of Platelet Surface P-Selectin
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Platelet Aggregation and Platelet Surface P-Selectin Expression According to Sex
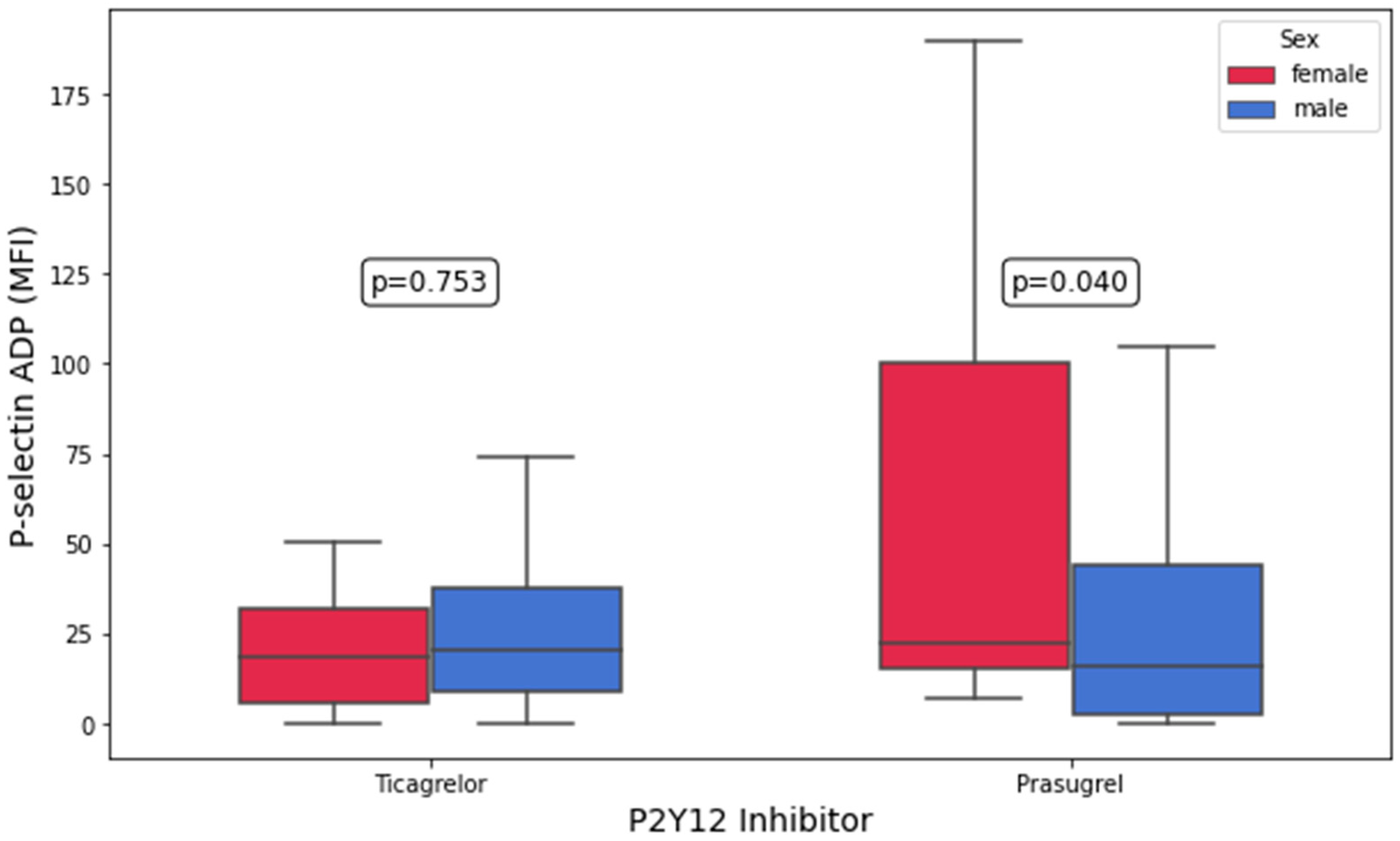
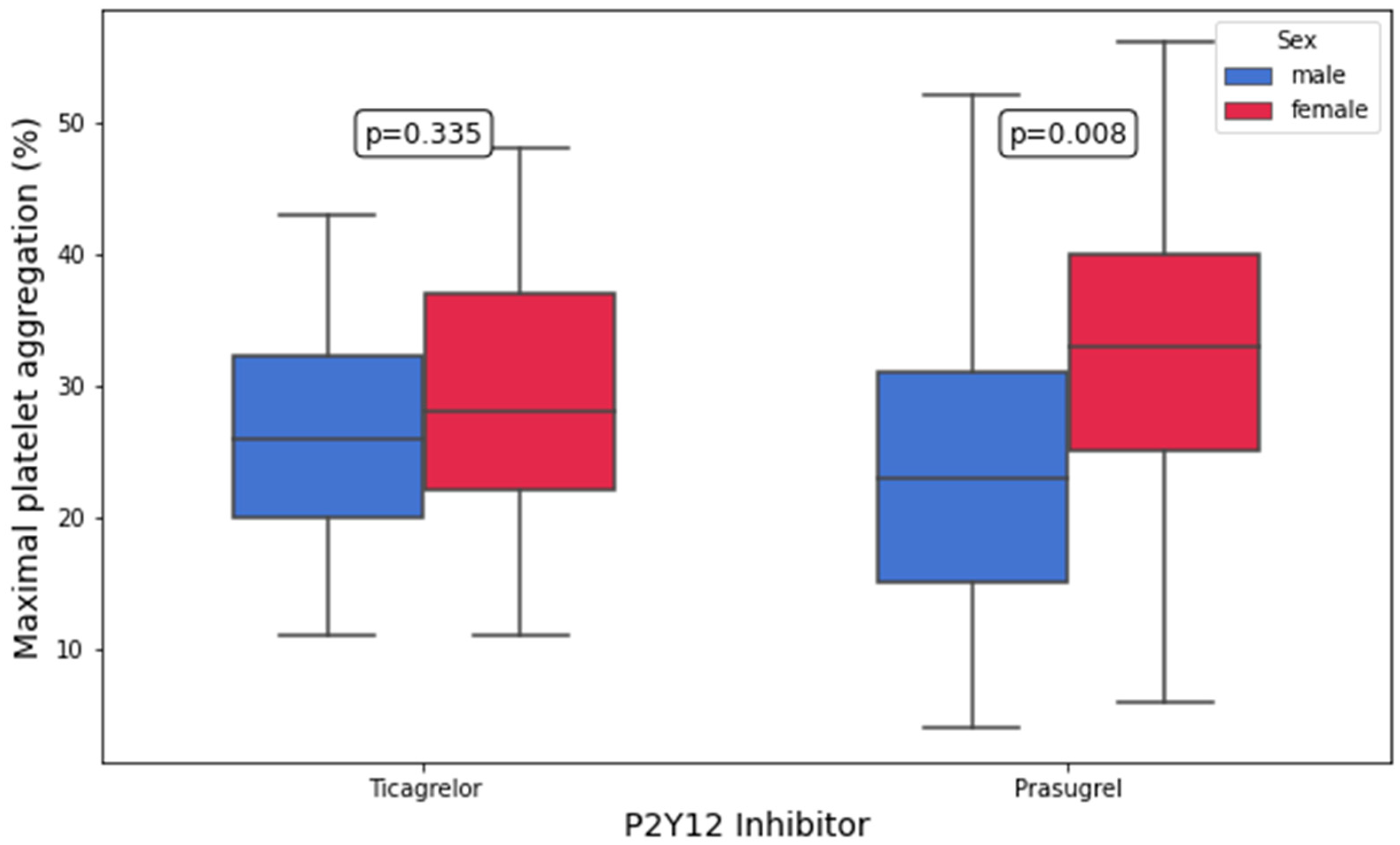
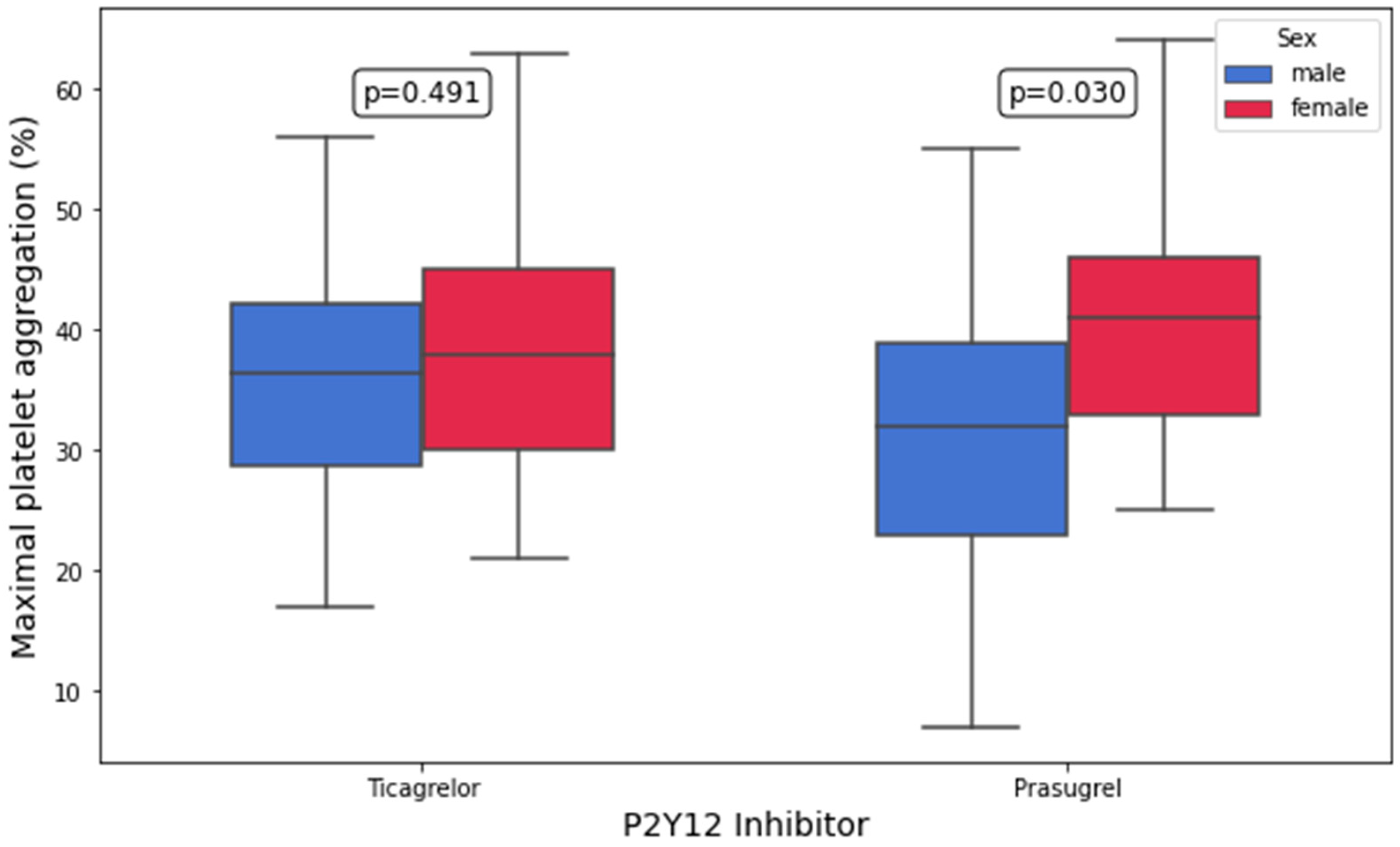
3.3. Platelet Aggregation and Platelet Surface P-Selectin Expression According to P2Y12 Inhibitor
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bergmark, B.A.; Mathenge, N.; Merlini, P.A.; Lawrence-Wright, M.B.; Giugliano, R.P. Acute coronary syndromes. Lancet 2022, 399, 1347–1358. [Google Scholar] [CrossRef]
- Gremmel, T.; Michelson, A.D.; Frelinger, A.L., III; Bhatt, D.L. Novel aspects of antiplatelet therapy in cardiovascular disease. Res. Pract. Thromb. Haemost. 2018, 2, 439–449. [Google Scholar] [CrossRef]
- Tersalvi, G.; Biasco, L.; Cioffi, G.M.; Pedrazzini, G. Acute Coronary Syndrome, Antiplatelet Therapy, and Bleeding: A Clinical Perspective. J. Clin. Med. 2020, 9, 2064. [Google Scholar] [CrossRef]
- Tscharre, M.; Michelson, A.D.; Gremmel, T. Novel Antiplatelet Agents in Cardiovascular Disease. J. Cardiovasc. Pharmacol. Ther. 2020, 25, 191–200. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Braunwald, E.; McCabe, C.H.; Montalescot, G.; Ruzyllo, W.; Gottlieb, S.; Neumann, F.J.; Ardissino, D.; De Servi, S.; Murphy, S.A.; et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 2007, 357, 2001–2015. [Google Scholar] [CrossRef]
- Wallentin, L.; Becker, R.C.; Budaj, A.; Cannon, C.P.; Emanuelsson, H.; Held, C.; Horrow, J.; Husted, S.; James, S.; Katus, H.; et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 2009, 361, 1045–1057. [Google Scholar] [CrossRef] [PubMed]
- Gremmel, T.; Eslam, R.B.; Koppensteiner, R.; Lang, I.M.; Panzer, S. Prasugrel reduces agonists’ inducible platelet activation and leukocyte-platelet interaction more efficiently than clopidogrel. Cardiovasc. Ther. 2013, 31, e40–e45. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]
- Levine, G.N.; Bates, E.R.; Bittl, J.A.; Brindis, R.G.; Fihn, S.D.; Fleisher, L.A.; Granger, C.B.; Lange, R.A.; Mack, M.J.; Mauri, L.; et al. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2016, 68, 1082–1115. [Google Scholar] [CrossRef]
- Dorsam, R.T.; Kunapuli, S.P. Central role of the P2Y12 receptor in platelet activation. J. Clin. Investig. 2004, 113, 340–345. [Google Scholar] [CrossRef]
- Gremmel, T.; Kopp, C.W.; Eichelberger, B.; Koppensteiner, R.; Panzer, S. Sex differences of leukocyte-platelet interactions and on-treatment platelet reactivity in patients with atherosclerosis. Atherosclerosis 2014, 237, 692–695. [Google Scholar] [CrossRef] [PubMed]
- Haider, A.; Bengs, S.; Luu, J.; Osto, E.; Siller-Matula, J.M.; Muka, T.; Gebhard, C. Sex and gender in cardiovascular medicine: Presentation and outcomes of acute coronary syndrome. Eur. Heart J. 2020, 41, 1328–1336. [Google Scholar] [CrossRef]
- Idris, H.; French, J.K.; Shugman, I.M.; Hopkins, A.P.; Juergens, C.P.; Thomas, L. Influence of Age and Gender on Clinical Outcomes Following Percutaneous Coronary Intervention for Acute Coronary Syndromes. Heart Lung Circ. 2017, 26, 554–565. [Google Scholar] [CrossRef]
- Mennuni, M.G.; Dangas, G.D.; Mehran, R.; Ben-Gal, Y.; Xu, K.; Genereux, P.; Brener, S.J.; Feit, F.; Lincoff, A.M.; Ohman, E.M.; et al. Coronary Artery Bypass Surgery Compared With Percutaneous Coronary Intervention for Proximal Left Anterior Descending Artery Treatment in Patients With Acute Coronary Syndrome: Analysis From the ACUITY Trial. J. Invasive Cardiol. 2015, 27, 468–473. [Google Scholar]
- Milcent, C.; Dormont, B.; Durand-Zaleski, I.; Steg, P.G. Gender differences in hospital mortality and use of percutaneous coronary intervention in acute myocardial infarction: Microsimulation analysis of the 1999 nationwide French hospitals database. Circulation 2007, 115, 833–839. [Google Scholar] [CrossRef]
- Hanratty, B.; Lawlor, D.A.; Robinson, M.B.; Sapsford, R.J.; Greenwood, D.; Hall, A. Sex differences in risk factors, treatment and mortality after acute myocardial infarction: An observational study. J. Epidemiol. Community Health 2000, 54, 912–916. [Google Scholar] [CrossRef]
- Mutschlechner, D.; Tscharre, M.; Wittmann, F.; Kitzmantl, D.; Schloglhofer, T.; Wadowski, P.P.; Laufer, G.; Eichelberger, B.; Lee, S.; Wiedemann, D.; et al. Platelet reactivity is associated with pump thrombosis in patients with left ventricular assist devices. Res. Pract. Thromb. Haemost. 2024, 8, 102564. [Google Scholar] [CrossRef]
- Sibbing, D.; Braun, S.; Morath, T.; Mehilli, J.; Vogt, W.; Schomig, A.; Kastrati, A.; von Beckerath, N. Platelet reactivity after clopidogrel treatment assessed with point-of-care analysis and early drug-eluting stent thrombosis. J. Am. Coll. Cardiol. 2009, 53, 849–856. [Google Scholar] [CrossRef]
- Sibbing, D.; Schulz, S.; Braun, S.; Morath, T.; Stegherr, J.; Mehilli, J.; Schomig, A.; von Beckerath, N.; Kastrati, A. Antiplatelet effects of clopidogrel and bleeding in patients undergoing coronary stent placement. J. Thromb. Haemost. 2010, 8, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Grifoni, E.; Gori, A.M.; Giusti, B.; Valenti, R.; Migliorini, A.; Basili, S.; Paniccia, R.; Elmahdy, M.F.; Pulli, R.; Pratesi, C.; et al. On-Treatment Platelet Reactivity is a Predictor of Adverse Events in Peripheral Artery Disease Patients Undergoing Percutaneous Angioplasty. Eur. J. Vasc. Endovasc. Surg. 2018, 56, 545–552. [Google Scholar] [CrossRef]
- Gremmel, T.; Michelson, A.D.; Wadowski, P.P.; Pultar, J.; Weikert, C.; Tscharre, M.; Lee, S.; Panzer, S.; Frelinger, A.L., III. Sex-specific platelet activation through protease-activated receptor-1 in patients undergoing cardiac catheterization. Atherosclerosis 2021, 339, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Basili, S.; Raparelli, V.; Proietti, M.; Tanzilli, G.; Franconi, F. Impact of sex and gender on the efficacy of antiplatelet therapy: The female perspective. J. Atheroscler. Thromb. 2015, 22, 109–125. [Google Scholar] [CrossRef] [PubMed]
- Wadowski, P.P.; Weikert, C.; Pultar, J.; Lee, S.; Eichelberger, B.; Koppensteiner, R.; Lang, I.M.; Panzer, S.; Gremmel, T. Ticagrelor Inhibits Toll-Like and Protease-Activated Receptor Mediated Platelet Activation in Acute Coronary Syndromes. Cardiovasc. Drugs Ther. 2020, 34, 53–63. [Google Scholar] [CrossRef]
- Badr Eslam, R.; Lang, I.M.; Koppensteiner, R.; Calatzis, A.; Panzer, S.; Gremmel, T. Residual platelet activation through protease-activated receptors (PAR)-1 and -4 in patients on P2Y12 inhibitors. Int. J. Cardiol. 2013, 168, 403–406. [Google Scholar] [CrossRef]
- Gremmel, T.; Kopp, C.W.; Seidinger, D.; Giurgea, G.A.; Koppensteiner, R.; Steiner, S.; Panzer, S. The formation of monocyte-platelet aggregates is independent of on-treatment residual agonists’-inducible platelet reactivity. Atherosclerosis 2009, 207, 608–613. [Google Scholar] [CrossRef]
- Tscharre, M.; Wittmann, F.; Kitzmantl, D.; Lee, S.; Eichelberger, B.; Wadowski, P.P.; Laufer, G.; Wiedemann, D.; Forstner-Bergauer, B.; Ay, C.; et al. Platelet activation and aggregation in different centrifugal-flow left ventricular assist devices. Platelets 2022, 33, 249–256. [Google Scholar] [CrossRef]
- Tscharre, M.; Wittmann, F.; Kitzmantl, D.; Schloglhofer, T.; Cichra, P.; Lee, S.; Eichelberger, B.; Wadowski, P.P.; Laufer, G.; Wiedemann, D.; et al. Impact of ABO Blood Group on Thromboembolic and Bleeding Complications in Patients with Left Ventricular Assist Devices. Thromb. Haemost. 2023, 123, 336–346. [Google Scholar] [CrossRef]
- Gum, P.A.; Kottke-Marchant, K.; Welsh, P.A.; White, J.; Topol, E.J. A prospective, blinded determination of the natural history of aspirin resistance among stable patients with cardiovascular disease. J. Am. Coll. Cardiol. 2003, 41, 961–965. [Google Scholar] [CrossRef]
- Bonello, L.; Tantry, U.S.; Marcucci, R.; Blindt, R.; Angiolillo, D.J.; Becker, R.; Bhatt, D.L.; Cattaneo, M.; Collet, J.P.; Cuisset, T.; et al. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J. Am. Coll. Cardiol. 2010, 56, 919–933. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Dong, Z.; Ma, J.; Teng, J.; Wang, T.; Zhang, X.; Gu, Q.; Ye, Z.; Ullah, I.; et al. An optimal window of platelet reactivity by LTA assay for patients undergoing percutaneous coronary intervention. Thromb. J. 2021, 19, 73. [Google Scholar] [CrossRef]
- Gurbel, P.A.; Bliden, K.P.; DiChiara, J.; Newcomer, J.; Weng, W.; Neerchal, N.K.; Gesheff, T.; Chaganti, S.K.; Etherington, A.; Tantry, U.S. Evaluation of dose-related effects of aspirin on platelet function: Results from the Aspirin-Induced Platelet Effect (ASPECT) study. Circulation 2007, 115, 3156–3164. [Google Scholar] [CrossRef]
- Sibbing, D.; Braun, S.; Jawansky, S.; Vogt, W.; Mehilli, J.; Schomig, A.; Kastrati, A.; von Beckerath, N. Assessment of ADP-induced platelet aggregation with light transmission aggregometry and multiple electrode platelet aggregometry before and after clopidogrel treatment. Thromb. Haemost. 2008, 99, 121–126. [Google Scholar] [CrossRef]
- Gremmel, T.; Steiner, S.; Seidinger, D.; Koppensteiner, R.; Panzer, S.; Kopp, C.W. Comparison of methods to evaluate clopidogrel-mediated platelet inhibition after percutaneous intervention with stent implantation. Thromb. Haemost. 2009, 101, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Sibbing, D.; Aradi, D.; Alexopoulos, D.; Ten Berg, J.; Bhatt, D.L.; Bonello, L.; Collet, J.P.; Cuisset, T.; Franchi, F.; Gross, L.; et al. Updated Expert Consensus Statement on Platelet Function and Genetic Testing for Guiding P2Y(12) Receptor Inhibitor Treatment in Percutaneous Coronary Intervention. JACC Cardiovasc. Interv. 2019, 12, 1521–1537. [Google Scholar] [CrossRef] [PubMed]
- Romo, G.M.; Dong, J.F.; Schade, A.J.; Gardiner, E.E.; Kansas, G.S.; Li, C.Q.; McIntire, L.V.; Berndt, M.C.; Lopez, J.A. The glycoprotein Ib-IX-V complex is a platelet counterreceptor for P-selectin. J. Exp. Med. 1999, 190, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Gremmel, T.; Koppensteiner, R.; Kaider, A.; Eichelberger, B.; Mannhalter, C.; Panzer, S. Impact of variables of the P-selectin—P-selectin glycoprotein ligand-1 axis on leukocyte-platelet interactions in cardiovascular disease. Thromb. Haemost. 2015, 113, 806–812. [Google Scholar]
- Somodi, L.; Beke Debreceni, I.; Kis, G.; Cozzolino, M.; Kappelmayer, J.; Antal, M.; Panyi, G.; Bardos, H.; Mutch, N.J.; Muszbek, L. Activation mechanism dependent surface exposure of cellular factor XIII on activated platelets and platelet microparticles. J. Thromb. Haemost. 2022, 20, 1223–1235. [Google Scholar] [CrossRef]
- Veninga, A.; De Simone, I.; Heemskerk, J.W.M.; Cate, H.T.; van der Meijden, P.E.J. Clonal hematopoietic mutations linked to platelet traits and the risk of thrombosis or bleeding. Haematologica 2020, 105, 2020–2031. [Google Scholar] [CrossRef]
- Michelson, A.D.; Frelinger, A.L., III; Furman, M.I. Current options in platelet function testing. Am. J. Cardiol. 2006, 98, 4N–10N. [Google Scholar] [CrossRef]
- Breet, N.J.; Sluman, M.A.; van Berkel, M.A.; van Werkum, J.W.; Bouman, H.J.; Harmsze, A.M.; Kelder, J.C.; Zijlstra, F.; Hackeng, C.M.; Ten Berg, J.M. Effect of gender difference on platelet reactivity. Neth. Heart J. 2011, 19, 451–457. [Google Scholar] [CrossRef]
- Capodanno, D.; Angiolillo, D.J. Impact of race and gender on antithrombotic therapy. Thromb. Haemost. 2010, 104, 471–484. [Google Scholar] [CrossRef]
- Gewalt, S.; Lahu, S.; Ndrepepa, G.; Pellegrini, C.; Bernlochner, I.; Neumann, F.J.; Menichelli, M.; Morath, T.; Witzenbichler, B.; Wohrle, J.; et al. Efficacy and Safety of Ticagrelor Versus Prasugrel in Women and Men with Acute Coronary Syndrome: A Pre-specified, Sex-Specific Analysis of the ISAR-REACT 5 Trial. J. Atheroscler. Thromb. 2022, 29, 747–761. [Google Scholar] [CrossRef]
- Angiolillo, D.J.; Galli, M.; Alexopoulos, D.; Aradi, D.; Bhatt, D.L.; Bonello, L.; Capodanno, D.; Cavallari, L.H.; Collet, J.P.; Cuisset, T.; et al. International Consensus Statement on Platelet Function and Genetic Testing in Percutaneous Coronary Intervention: 2024 Update. JACC Cardiovasc. Interv. 2024, 17, 2639–2663. [Google Scholar] [CrossRef]
| Characteristic | Women (n = 32) | Men (n = 125) | p |
|---|---|---|---|
| Demographics | |||
| Age, years | 62.8 ± 12.2 | 57.5 ± 10.8 | 0.02 |
| Caucasian, white No. (%) | 32 (100) | 125 (100) | 1.0 |
| Body mass index, kg/m2 | 29.2 ± 5.4 | 28.4 ± 4.6 | 0.5 |
| Medical history | |||
| Prior myocardial infarction, No. (%) | 5 (16) | 22 (18) | 0.9 |
| Prior stroke or TIA, No. (%) | 0 (0) | 5 (4) | 0.5 |
| Arterial hypertension, No. (%) | 21 (66) | 86 (71) | 0.7 |
| Hyperlipoproteinemia, No. (%) | 25 (81) | 91 (76) | 0.7 |
| Peripheral artery disease, No. (%) | 2 (6) | 12 (10) | 0.7 |
| Diabetes mellitus type II, No. (%) | 10 (32) | 30 (25) | 0.5 |
| Smoking, No. (%) | 17 (52) | 92 (74) | 0.3 |
| HAS-BLED score ≥ 3, No. (%) | 21 (65.6) | 86 (68.8) | 0.7 |
| Laboratory data | |||
| Serum creatinine, mg/dL | 0.8 (0.7–0.9) | 1.0 (0.9–1.1) | <0.001 |
| Platelet count, G/L | 229 (203–279) | 226 (187–251) | 0.2 |
| Hemoglobin, g/dL | 12.5 (11.6–13.1) | 14.1 (13.3–14.9) | <0.001 |
| Hematocrit, % | 36.6 (34.2–38.3) | 41.6 (39.1–44.0) | <0.001 |
| Highly sensitive CRP, mg/dL | 1.4 (0.8–3.9) | 1.2 (0.6–3.4) | 0.7 |
| Medication | |||
| Aspirin, No. (%) | 32 (100) | 125 (100) | 1.0 |
| Prasugrel, No (%) | 15 (46.9) | 65 (52) | 0.6 |
| Ticagrelor, No. (%) | 17 (53.1) | 60 (48) | 0.8 |
| Statin, No. (%) | 32 (100) | 121 (98) | 1.0 |
| Beta-blocker, No. (%) | 32 (100) | 118 (95.9) | 0.6 |
| ACE inhibitor or ARB, No. (%) | 28 (90.3) | 121 (98.4) | 0.1 |
| Calcium-channel blocker, No. (%) | 6 (19) | 9 (7) | 0.1 |
| SGLT2 inhibitor, No. (%) | 2 (6.3) | 3 (2.4) | 0.3 |
| Parameter | Women (n = 32) | Men (n = 125) | p |
|---|---|---|---|
| HRPR AA (LTA ≥ 20% to AA) | 2 (6) | 2 (1.6) | 1 |
| HRPR ADP (LTA ≥ 70% to 10 μM of ADP) | 2 (6) | 5 (4) | 0.9 |
| LRPR AA (LTA ≤ 20% to AA) | 31 (96.9) | 122 (97.6) | 0.8 |
| LRPR ADP (LTA < 25.5% to 5 μM of ADP) | 10 (31.3) | 70 (56.0) | 0.01 |
| P-selectin, ADP (MFI) | 22.0 (11.5–50.5) | 17.7 (5.0–38.8) | 0.3 |
| P-selectin, ADP (%) | 11.3 (9.6–18.8) | 11.7 (7.6–17.0) | 0.4 |
| P-selectin, AA (MFI) | 0.6 (0.0–17.1) | 0.2 (0.0–13.0) | 0.8 |
| P-selectin, AA (%) | 5.1 (4.0–8.2) | 5.4 (3.4–9.1) | 0.6 |
| P-selectin, TRAP (MFI) | 2630.7 (1775.2–3228.2) | 2993.2 (1907.4–3915.2) | 0.3 |
| P-selectin, TRAP (%) | 84.7 (79.5–88.9) | 87.5 (80.2–92.5) | 0.2 |
| P-selectin, AYPGKF (MFI) | 188.3 (90.7–554.9) | 208.6 (92.7–741.7) | 0.3 |
| P-selectin, AYPGKF (%) | 39.7 (23.7–60.2) | 43.1 (31.4–64.8) | 0.2 |
| ADP-inducible platelet aggregation, 10 μM of ADP (%) | 38.5 (31.0–45.75) | 34.0 (25.0 −41.0) | 0.03 |
| ADP-inducible platelet aggregation, 5 μM of ADP (%) | 30.5 (24.0–37.75) | 24.0 (19.0–31.0) | <0.001 |
| AA-inducible platelet aggregation (%) | 3 (2–6.25) | 2.0 (1.0–4.0) | 0.3 |
| COL-inducible platelet aggregation (%) | 75.5 (37.5–93.75) | 70 (44–82) | 0.5 |
| TRAP-inducible platelet aggregation (%) | 80 (63.75–95.5) | 72.0 (61.0–86.0) | 0.1 |
| AYPGKF-inducible platelet aggregation (%) | 70 (58.0–90.0) | 71.5 (56.0–86.0) | 0.5 |
| Ticagrelor (n = 77) | Prasugrel (n = 80) | |||||
|---|---|---|---|---|---|---|
| Parameter | Women (n = 16) | Men (n = 61) | p | Women (n = 16) | Men (n = 64) | p |
| P-selectin, ADP (MFI) | 18.4 (5.8–31.9) | 20.6 (8.7–37.4) | 0.8 | 22.3 (15.1–100.1) | 15.8 (2.6–44.0) | 0.04 |
| P-selectin, ADP (%) | 10.2 (8.5–15.6) | 11.8 (8.7–16.5) | 0.6 | 11.8 (10.1–30.7) | 11.7 (7.1–18.0) | 0.09 |
| P-selectin, AA (MFI) | 0.0 (0.0–3.0) | 1.4 (0.0–11.7) | 0.3 | 10.4 (0.0–20.4) | 0.0 (0.0–13.3) | 0.1 |
| P-selectin, AA (%) | 4.7 (3.2–5.0) | 5.4 (3.1–8.1) | 0.3 | 7.8 (5.5–13.8) | 5.4 (3.5–9.9) | 0.05 |
| P-selectin, TRAP (MFI) | 2380.2 (1835.7–2718.2) | 2719.2 (1629.8–3625.5) | 0.4 | 3151.3 (1829.6–3810.4) | 3367.5 (2272.0–4107.3) | 0.7 |
| P-selectin, TRAP (%) | 84.5 (79.8–86.9) | 87.0 (78.9–90.6) | 0.3 | 86.8 (73.3–94.1) | 90.1 (82.5–93.8) | 0.6 |
| P-selectin, AYPGKF (MFI) | 168.1 (74.3–426.7) | 187.4 (88.8–607.2) | 0.7 | 248.5 (90.7–636.8) | 291.7 (104.9–889.2) | 0.3 |
| P-selectin, AYPGKF (%) | 40.2 (23.8–52.9) | 41.7 (29.6–62.0) | 0.6 | 37.1 (23.1–61.1) | 47.5 (32.6–68.2) | 0.3 |
| ADP-inducible platelet aggregation, 10 μM of ADP (%) | 38.0 (30.0–45.0) | 36.5 (28.8–42.2) | 0.5 | 41.0 (33.0–46.0) | 32.0 (23.0–39.0) | 0.03 |
| ADP-inducible platelet aggregation, 5 μM of ADP (%) | 28.0 (22.0–37.0) | 26.0 (20.0–32.2) | 0.3 | 33.0 (25.0–40.0) | 23.0 (15.0–31.0) | 0.008 |
| AA-inducible platelet aggregation (%) | 2.0 (2.0–5.0) | 3.0 (2.0–5.2) | 0.8 | 4.0 (2.0–6.5) | 2.0 (1.0–4.0) | 0.1 |
| COL-inducible platelet aggregation (%) | 66.0 (38.0–83.0) | 74.0 (56.0–83.0) | 0.5 | 79.0 (35.0–97.0) | 55.0 (27.0–81.0) | 0.2 |
| TRAP-inducible platelet aggregation (%) | 80.0 (64.0–94.0) | 73.0 (60.8–85.0) | 0.2 | 79.0 (63.0–95.0) | 72.0 (61.0–87.0) | 0.4 |
| AYPGKF-inducible platelet aggregation (%) | 65.0 (58.5–78.0) | 72.5 (61.8–86.0) | 0.6 | 85.0 (58.0–96.0) | 71.5 (55.0–86.5) | 0.1 |
| Parameter | Variable | B (Unstandardized Coefficient) | 95% Confidence Interval | p |
|---|---|---|---|---|
| P-selectin, ADP (MFI) | Female vs. male | 27.380 | −0.378–55.138 | 0.053 |
| P-selectin, AA (MFI) | Female vs. male | 5.843 | −2.736–14.423 | 0.179 |
| ADP-inducible platelet aggregation, 5 μM of ADP (%) | Female vs. male | 20.368 | 5.325–35.410 | 0.009 |
| ADP-inducible platelet aggregation, 5 μM of ADP (%) | Age | 0.339 | −0.105–0.783 | 0.131 |
| ADP-inducible platelet aggregation, 5 μM of ADP (%) | Hemoglobin | 0.780 | −11.359–12.919 | 0.898 |
| ADP-inducible platelet aggregation, 5 μM of ADP (%) | Hematocrit | −0.265 | −4.526–3.995 | 0.901 |
| ADP-inducible platelet aggregation, 5 μM of ADP (%) | Creatinine | −5.183 | −39.948–20.582 | 0.687 |
| ADP-inducible platelet aggregation, 5 μM of ADP (%) | Highly sensitive CRP | −0.300 | −1.742–1.141 | 0.677 |
| ADP-inducible platelet aggregation, 5 μM of ADP (%) | SGLT2 inhibitor | −1.041 | −32.413–30.331 | 0.947 |
| ADP-inducible platelet aggregation, 10 μM of ADP (%) | Female vs. male | 10.712 | −2.536–23.960 | 0.111 |
| ADP-inducible platelet aggregation, 10 μM of ADP (%) | Age | 0.166 | −0.234–0.567 | 0.410 |
| ADP-inducible platelet aggregation, 10 μM of ADP (%) | Hemoglobin | 5.984 | −5.545–17.514 | 0.303 |
| ADP-inducible platelet aggregation, 10 μM of ADP (%) | Hematocrit | −2.439 | −6.410–1.531 | 0.224 |
| ADP-inducible platelet aggregation, 10 μM of ADP (%) | SGLT2 inhibitor | 9.122 | −23.670–41.913 | 0.580 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mutschlechner, D.; Tsarouchas, A.; Tscharre, M.; Wadowski, P.P.; Lee, S.; Pultar, J.; Weikert, C.; Panzer, S.; Gremmel, T. Sex-Related Differences in On-Treatment Platelet Reactivity in Patients with Acute Coronary Syndrome. Biomedicines 2025, 13, 2068. https://doi.org/10.3390/biomedicines13092068
Mutschlechner D, Tsarouchas A, Tscharre M, Wadowski PP, Lee S, Pultar J, Weikert C, Panzer S, Gremmel T. Sex-Related Differences in On-Treatment Platelet Reactivity in Patients with Acute Coronary Syndrome. Biomedicines. 2025; 13(9):2068. https://doi.org/10.3390/biomedicines13092068
Chicago/Turabian StyleMutschlechner, David, Anastasios Tsarouchas, Maximilian Tscharre, Patricia Pia Wadowski, Silvia Lee, Joseph Pultar, Constantin Weikert, Simon Panzer, and Thomas Gremmel. 2025. "Sex-Related Differences in On-Treatment Platelet Reactivity in Patients with Acute Coronary Syndrome" Biomedicines 13, no. 9: 2068. https://doi.org/10.3390/biomedicines13092068
APA StyleMutschlechner, D., Tsarouchas, A., Tscharre, M., Wadowski, P. P., Lee, S., Pultar, J., Weikert, C., Panzer, S., & Gremmel, T. (2025). Sex-Related Differences in On-Treatment Platelet Reactivity in Patients with Acute Coronary Syndrome. Biomedicines, 13(9), 2068. https://doi.org/10.3390/biomedicines13092068







