Clinical Impact of CT-Based FFR in Everyday Cardiology: Bridging Computation and Decision-Making
Abstract
1. Introduction
2. Clinical Indications for CCTA: Guidelines-Based Recommendations
3. An Overview of FFRCT Techniques, Including Their Diagnostic Accuracy and Findings from Major Validation Studies
3.1. FFRCT Based on the Heartflow Software
3.2. FFRCT Based on the Siemens Healthcare Software
3.3. DeepVessel FFR Based on the Keya Medical Software
3.4. uFFRCT Based on the United Imaging Healthcare Software
3.5. esFFR Based on the CAscope Software
4. FFRCT in Clinical Applications
4.1. Stable CAD
4.2. Acute Chest Pain
4.3. Non-ST-Segment Elevation Myocardial Infarction (NSTEMI)
4.4. Clinical Decision Making
4.5. Transcatheter Aortic Valve Implantation (TAVI)
4.6. In-Stent Restenosis (ISR)
5. Cost-Utility and Validation Evidence: A Critical Synthesis of FFRCT
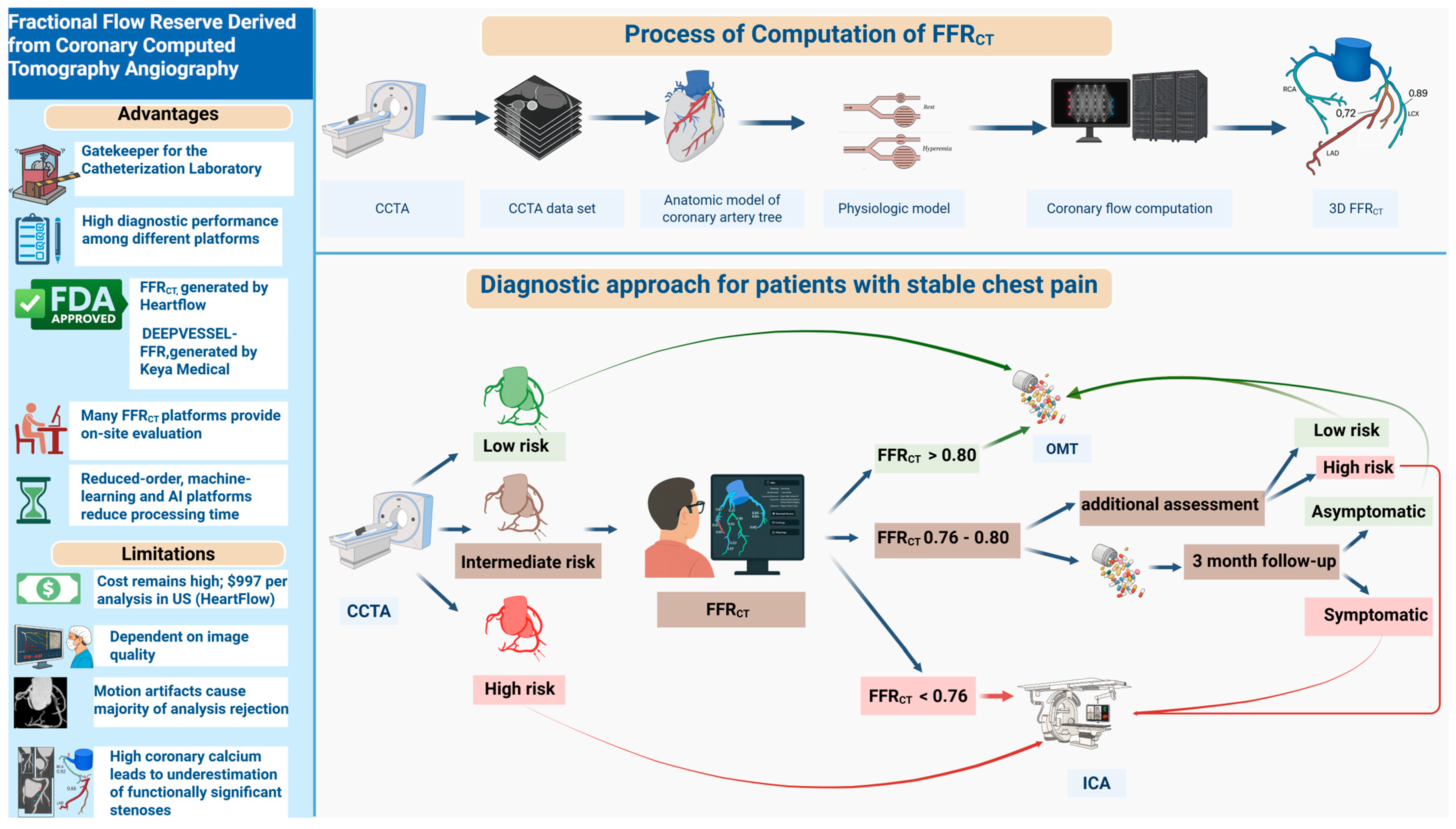
6. Proposed Algorithm for FFRCT Integration in Clinical Practice
- (A)
- Advantages and Limitations: The left panel summarizes the principal advantages of FFRCT, including its role as a non-invasive “gatekeeper” to invasive angiography, high diagnostic performance across platforms, U.S. Food and Drug Administration (FDA) approval, on-site evaluation availability, and the potential for rapid processing via reduced-order, machine learning-based, or AI-based approaches. Key limitations include high per-analysis cost, dependency on image quality, frequent analysis rejection due to motion artifacts, and reduced accuracy in the setting of high coronary calcium.
- (B)
- Process of Computation: The upper panel depicts the FFRCT computational pipeline: acquisition of coronary computed tomography angiography (CCTA) data, 3D reconstruction of the coronary artery tree, development of a physiologic model, and computational simulation of coronary flow to yield vessel- and lesion-specific FFRCT values.
- (C)
- Diagnostic Approach: The lower panel demonstrates a contemporary diagnostic algorithm for patients presenting with stable chest pain. Following CCTA-based anatomical risk stratification (low, intermediate, high), patients at intermediate risk undergo FFRCT analysis. Management is then tailored according to FFRCT values, as follows:
- FFRCT > 0.80: Low risk, optimal medical therapy (OMT) recommended.
- FFRCT: 0.76–0.80: Further clinical assessment and short-term follow-up
- FFRCT < 0.76: Referral for invasive coronary angiography (ICA).
7. Limitations
8. Future Directions
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Benz, D.C.; Giannopoulos, A.A. Fractional flow reserve as the standard of reference: All that glistens is not gold. J. Nucl. Cardiol. 2020, 27, 1314–1316. [Google Scholar] [CrossRef]
- Nallamothu, B.K.; Spertus, J.A.; Lansky, A.J.; Cohen, D.J.; Jones, P.G.; Kureshi, F.; Dehmer, G.J.; Drozda, J.P., Jr.; Walsh, M.N.; Brush, J.E., Jr.; et al. Comparison of clinical interpretation with visual assessment and quantitative coronary angiography in patients undergoing percutaneous coronary intervention in contemporary practice: The Assessing Angiography (A2) project. Circulation 2013, 127, 1793–1800. [Google Scholar] [CrossRef] [PubMed]
- Leape, L.L.; Park, R.E.; Bashore, T.M.; Harrison, J.K.; Davidson, C.J.; Brook, R.H. Effect of variability in the interpretation of coronary angiograms on the appropriateness of use of coronary revascularization procedures. Am. Heart J. 2000, 139 Pt 1, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Detre, K.M.; Wright, E.; Murphy, M.L.; Takaro, T. Observer agreement in evaluating coronary angiograms. Circulation 1975, 52, 979–986. [Google Scholar] [CrossRef]
- Tonino, P.A.; De Bruyne, B.; Pijls, N.H.; Siebert, U.; Ikeno, F.; van’t Veer, M.; Klauss, V.; Manoharan, G.; Engstrøm, T.; Oldroyd, K.G.; et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N. Engl. J. Med. 2009, 360, 213–224. [Google Scholar] [CrossRef]
- Boden, W.E.; O’Rourke, R.A.; Teo, K.K.; Hartigan, P.M.; Maron, D.J.; Kostuk, W.J.; Knudtson, M.; Dada, M.; Casperson, P.; Harris, C.L.; et al. Optimal medical therapy with or without PCI for stable coronary disease. N. Engl. J. Med. 2007, 356, 1503–1516. [Google Scholar] [CrossRef]
- Pijls, N.H.; van Schaardenburgh, P.; Manoharan, G.; Boersma, E.; Bech, J.W.; van’t Veer, M.; Bär, F.; Hoorntje, J.; Koolen, J.; Wijns, W.; et al. Percutaneous coronary intervention of functionally nonsignificant stenosis: 5-year follow-up of the DEFER Study. J. Am. Coll. Cardiol. 2007, 49, 2105–2111. [Google Scholar] [CrossRef]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Shiomi, H.; Kuribayashi, S.; Isshiki, T.; Kanazawa, S.; Ito, H.; Ikeda, S.; Forrest, B.; Zarins, C.K.; Hlatky, M.A.; et al. Cost analysis of non-invasive fractional flow reserve derived from coronary computed tomographic angiography in Japan. Cardiovasc. Interv. Ther. 2015, 30, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, U.; Ferencik, M.; Udelson, J.E.; Picard, M.H.; Truong, Q.A.; Patel, M.R.; Huang, M.; Pencina, M.; Mark, D.B.; Heitner, J.F.; et al. Prognostic Value of Noninvasive Cardiovascular Testing in Patients With Stable Chest Pain: Insights From the PROMISE Trial (Prospective Multicenter Imaging Study for Evaluation of Chest Pain). Circulation 2017, 135, 2320–2332. [Google Scholar] [CrossRef]
- Mordi, I.R.; Badar, A.A.; Irving, R.J.; Weir-McCall, J.R.; Houston, J.G.; Lang, C.C. Efficacy of noninvasive cardiac imaging tests in diagnosis and management of stable coronary artery disease. Vasc. Health Risk Manag. 2017, 13, 427–437. [Google Scholar] [CrossRef]
- Nagel, E.; Greenwood, J.P.; McCann, G.P.; Bettencourt, N.; Shah, A.M.; Hussain, S.T.; Perera, D.; Plein, S.; Bucciarelli-Ducci, C.; Paul, M.; et al. Magnetic Resonance Perfusion or Fractional Flow Reserve in Coronary Disease. N. Engl. J. Med. 2019, 380, 2418–2428. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, K.M.; Chen, M.Y.; Dey, A.K.; Virmani, R.; Finn, A.V.; Khamis, R.Y.; Choi, A.D.; Min, J.K.; Williams, M.C.; Buckler, A.J.; et al. Coronary Computed Tomography Angiography From Clinical Uses to Emerging Technologies: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 76, 1226–1243. [Google Scholar] [CrossRef] [PubMed]
- Manolis, A.J.; Collins, P.; Kallistratos, M.S.; Rosano, G. Key messages and critical approach of the 2024 guidelines of the European Society of Cardiology on chronic coronary syndromes. Hell. J. Cardiol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Tsigkas, G.; Apostolos, A.; Synetos, A.; Latsios, G.; Toutouzas, K.; Xenogiannis, I.; Hamilos, M.; Sianos, G.; Ziakas, A.; Tsiafoutis, I.; et al. Computed tomoGRaphy guidEd invasivE Coronary angiography in patiEnts with a previous coronary artery bypass graft surgery trial (GREECE trial): Rationale and design of a multicenter, randomized control trial. Hell. J. Cardiol. 2021, 62, 470–472. [Google Scholar] [CrossRef]
- Tsigkas, G.; Toulgaridis, F.; Apostolos, A.; Kalogeropoulos, A.; Karamasis, G.V.; Vasilagkos, G.; Pappas, L.; Toutouzas, K.; Tsioufis, K.; Korkonikitas, P.; et al. CCTA-Guided Invasive Coronary Angiography in Patients With CABG: A Multicenter, Randomized Study. Circ. Cardiovasc. Interv. 2024, 17, e014045. [Google Scholar] [CrossRef]
- Jones, D.A.; Beirne, A.M.; Kelham, M.; Rathod, K.S.; Andiapen, M.; Wynne, L.; Godec, T.; Forooghi, N.; Ramaseshan, R.; Moon, J.C.; et al. Computed Tomography Cardiac Angiography Before Invasive Coronary Angiography in Patients With Previous Bypass Surgery: The BYPASS-CTCA Trial. Circulation 2023, 148, 1371–1380. [Google Scholar] [CrossRef]
- Min, J.K.; Leipsic, J.; Pencina, M.J.; Berman, D.S.; Koo, B.-K.; van Mieghem, C.; Erglis, A.; Lin, F.Y.; Dunning, A.M.; Apruzzese, P.; et al. Diagnostic Accuracy of Fractional Flow Reserve From Anatomic CT Angiography. JAMA 2012, 308, 1237–1245. [Google Scholar] [CrossRef]
- Taylor, C.A.; Fonte, T.A.; Min, J.K. Computational fluid dynamics applied to cardiac computed tomography for noninvasive quantification of fractional flow reserve: Scientific basis. J. Am. Coll. Cardiol. 2013, 61, 2233–2241. [Google Scholar] [CrossRef]
- Baumann, S.; Wang, R.; Schoepf, U.J.; Steinberg, D.H.; Spearman, J.V.; Bayer, R.R., 2nd; Hamm, C.W.; Renker, M. Coronary CT angiography-derived fractional flow reserve correlated with invasive fractional flow reserve measurements--initial experience with a novel physician-driven algorithm. Eur. Radiol. 2015, 25, 1201–1207. [Google Scholar] [CrossRef]
- Coenen, A.; Lubbers, M.M.; Kurata, A.; Kono, A.; Dedic, A.; Chelu, R.G.; Dijkshoorn, M.L.; Gijsen, F.J.; Ouhlous, M.; van Geuns, R.J.; et al. Fractional flow reserve computed from noninvasive CT angiography data: Diagnostic performance of an on-site clinician-operated computational fluid dynamics algorithm. Radiology 2015, 274, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Itu, L.; Rapaka, S.; Passerini, T.; Georgescu, B.; Schwemmer, C.; Schoebinger, M.; Flohr, T.; Sharma, P.; Comaniciu, D. A machine-learning approach for computation of fractional flow reserve from coronary computed tomography. J. Appl. Physiol. 2016, 121, 42–52. [Google Scholar] [CrossRef]
- Koo, B.-K.; Erglis, A.; Doh, J.-H.; Daniels, D.V.; Jegere, S.; Kim, H.-S.; Dunning, A.; DeFrance, T.; Lansky, A.; Leipsic, J.; et al. Diagnosis of Ischemia-Causing Coronary Stenoses by Noninvasive Fractional Flow Reserve Computed from Coronary Computed Tomographic Angiograms. J. Am. Coll. Cardiol. 2011, 58, 1989–1997. [Google Scholar] [CrossRef]
- Luo, Y.; Mao, M.; Xiang, R.; Han, B.; Chang, J.; Zuo, Z.; Wu, F.; Ma, K. Diagnostic performance of computed tomography-based fraction flow reserve in identifying myocardial ischemia caused by coronary artery stenosis: A meta-analysis. Hell. J. Cardiol. 2022, 63, 1–7. [Google Scholar] [CrossRef]
- Gulati, M.; Levy, P.D.; Mukherjee, D.; Amsterdam, E.; Bhatt, D.L.; Birtcher, K.K.; Blankstein, R.; Boyd, J.; Bullock-Palmer, R.P.; Conejo, T.; et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 144, e368–e454. [Google Scholar] [CrossRef] [PubMed]
- Narula, J.; Chandrashekhar, Y.; Ahmadi, A.; Abbara, S.; Berman, D.S.; Blankstein, R.; Leipsic, J.; Newby, D.; Nicol, E.D.; Nieman, K.; et al. SCCT 2021 Expert Consensus Document on Coronary Computed Tomographic Angiography: A Report of the Society of Cardiovascular Computed Tomography. J. Cardiovasc. Comput. Tomogr. 2021, 15, 192–217. [Google Scholar] [CrossRef]
- Sandoval, Y.; Leipsic, J.; Collet, C.; Ali, Z.A.; Azzalini, L.; Barbato, E.; Cavalcante, J.L.; Costa, R.A.; Garcia-Garcia, H.M.; Jones, D.A.; et al. Coronary computed tomography angiography to guide percutaneous coronary intervention: Expert opinion from a SCAI/SCCT roundtable. J. Cardiovasc. Comput. Tomogr. 2025, 19, 277–290. [Google Scholar] [CrossRef]
- Nakazato, R.; Park, H.-B.; Berman, D.S.; Gransar, H.; Koo, B.-K.; Erglis, A.; Lin, F.Y.; Dunning, A.M.; Budoff, M.J.; Malpeso, J.; et al. Noninvasive Fractional Flow Reserve Derived From Computed Tomography Angiography for Coronary Lesions of Intermediate Stenosis Severity. Circ. Cardiovasc. Imaging 2013, 6, 881–889. [Google Scholar] [CrossRef]
- Nørgaard, B.L.; Leipsic, J.; Gaur, S.; Seneviratne, S.; Ko, B.S.; Ito, H.; Jensen, J.M.; Mauri, L.; De Bruyne, B.; Bezerra, H.; et al. Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: The NXT trial (Analysis of Coronary Blood Flow Using CT Angiography: Next Steps). J. Am. Coll. Cardiol. 2014, 63, 1145–1155. [Google Scholar] [CrossRef]
- Yang, D.H.; Kim, Y.-H.; Roh, J.H.; Kang, J.-W.; Ahn, J.-M.; Kweon, J.; Lee, J.B.; Choi, S.H.; Shin, E.-S.; Park, D.-W.; et al. Diagnostic performance of on-site CT-derived fractional flow reserve versus CT perfusion. Eur. Heart J. Cardiovasc. Imaging 2016, 18, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Driessen, R.S.; Danad, I.; Stuijfzand, W.J.; Raijmakers, P.G.; Schumacher, S.P.; Diemen, P.A.v.; Leipsic, J.A.; Knuuti, J.; Underwood, S.R.; Ven, P.M.v.d.; et al. Comparison of Coronary Computed Tomography Angiography, Fractional Flow Reserve, and Perfusion Imaging for Ischemia Diagnosis. J. Am. Coll. Cardiol. 2019, 73, 161–173. [Google Scholar] [CrossRef]
- Renker, M.; Schoepf, U.J.; Wang, R.; Meinel, F.G.; Rier, J.D.; Bayer, R.R.; Möllmann, H.; Hamm, C.W.; Steinberg, D.H.; Baumann, S. Comparison of Diagnostic Value of a Novel Noninvasive Coronary Computed Tomography Angiography Method Versus Standard Coronary Angiography for Assessing Fractional Flow Reserve. Am. J. Cardiol. 2014, 114, 1303–1308. [Google Scholar] [CrossRef]
- De Geer, J.; Sandstedt, M.; Björkholm, A.; Alfredsson, J.; Janzon, M.; Engvall, J.; Persson, A. Software-based on-site estimation of fractional flow reserve using standard coronary CT angiography data. Acta Radiol. 2016, 57, 1186–1192. [Google Scholar] [CrossRef] [PubMed]
- Ko, B.S.; Cameron, J.D.; Munnur, R.K.; Wong, D.T.L.; Fujisawa, Y.; Sakaguchi, T.; Hirohata, K.; Hislop-Jambrich, J.; Fujimoto, S.; Takamura, K.; et al. Noninvasive CT-Derived FFR Based on Structural and Fluid Analysis: A Comparison With Invasive FFR for Detection of Functionally Significant Stenosis. JACC Cardiovasc. Imaging 2017, 10, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Q.; Zhou, Y.J.; Zhao, Y.X.; Shi, D.M.; Liu, Y.Y.; Liu, W.; Liu, X.L.; Li, Y.P. Diagnostic accuracy of a deep learning approach to calculate FFR from coronary CT angiography. J. Geriatr. Cardiol. JGC 2019, 16, 42–48. [Google Scholar] [CrossRef] [PubMed]
- The ADAPT Study: Assessment of the DiAgnostic Performance of DeepVessel FFR in SuspecTed Coronary Artery Disease. 2021. Available online: https://clinicaltrials.gov/study/NCT04828590 (accessed on 9 July 2025).
- Keya Medical. DeepVessel FFR. 2024. Available online: https://www.keyamedical.com/ (accessed on 9 July 2025).
- Tang, C.X.; Liu, C.Y.; Lu, M.J.; Schoepf, U.J.; Tesche, C.; Bayer, R.R., 2nd; Hudson, H.T., Jr.; Zhang, X.L.; Li, J.H.; Wang, Y.N.; et al. CT FFR for Ischemia-Specific CAD With a New Computational Fluid Dynamics Algorithm: A Chinese Multicenter Study. JACC Cardiovasc. Imaging 2020, 13, 980–990. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Li, Q.; Chen, Q.; Tang, Y.; Zhang, H.; He, Y.; Fu, G.; Yang, Q.; Shou, X.; Ye, Y.; et al. Diagnostic performance of a novel automated CT-derived FFR technology in detecting hemodynamically significant coronary artery stenoses: A multicenter trial in China. Am. Heart J. 2023, 265, 180–190. [Google Scholar] [CrossRef]
- Cook, C.M.; Petraco, R.; Shun-Shin, M.J.; Ahmad, Y.; Nijjer, S.; Al-Lamee, R.; Kikuta, Y.; Shiono, Y.; Mayet, J.; Francis, D.P.; et al. Diagnostic Accuracy of Computed Tomography-Derived Fractional Flow Reserve: A Systematic Review. JAMA Cardiol. 2017, 2, 803–810. [Google Scholar] [CrossRef]
- Coenen, A.; Kim, Y.H.; Kruk, M.; Tesche, C.; De Geer, J.; Kurata, A.; Lubbers, M.L.; Daemen, J.; Itu, L.; Rapaka, S.; et al. Diagnostic Accuracy of a Machine-Learning Approach to Coronary Computed Tomographic Angiography-Based Fractional Flow Reserve: Result From the MACHINE Consortium. Circ. Cardiovasc. Imaging 2018, 11, e007217. [Google Scholar] [CrossRef] [PubMed]
- von Knebel Doeberitz, P.L.; De Cecco, C.N.; Schoepf, U.J.; Duguay, T.M.; Albrecht, M.H.; van Assen, M.; Bauer, M.J.; Savage, R.H.; Pannell, J.T.; De Santis, D.; et al. Coronary CT angiography-derived plaque quantification with artificial intelligence CT fractional flow reserve for the identification of lesion-specific ischemia. Eur. Radiol. 2019, 29, 2378–2387. [Google Scholar] [CrossRef]
- Douglas, P.S.; Pontone, G.; Hlatky, M.A.; Patel, M.R.; Norgaard, B.L.; Byrne, R.A.; Curzen, N.; Purcell, I.; Gutberlet, M.; Rioufol, G.; et al. Clinical outcomes of fractional flow reserve by computed tomographic angiography-guided diagnostic strategies vs. usual care in patients with suspected coronary artery disease: The prospective longitudinal trial of FFRCT: Outcome and resource impacts study. Eur. Heart J. 2015, 36, 3359–3367. [Google Scholar] [CrossRef]
- Douglas, P.S.; Bruyne, B.D.; Pontone, G.; Patel, M.R.; Norgaard, B.L.; Byrne, R.A.; Curzen, N.; Purcell, I.; Gutberlet, M.; Rioufol, G.; et al. 1-Year Outcomes of FFRCT-Guided Care in Patients with Suspected Coronary Disease: The PLATFORM study. J. Am. Coll. Cardiol. 2016, 68, 435–445. [Google Scholar] [CrossRef]
- Curzen, N.; Nicholas, Z.; Stuart, B.; Wilding, S.; Hill, K.; Shambrook, J.; Eminton, Z.; Ball, D.; Barrett, C.; Johnson, L.; et al. Fractional flow reserve derived from computed tomography coronary angiography in the assessment and management of stable chest pain: The FORECAST randomized trial. Eur. Heart J. 2021, 42, 3844–3852. [Google Scholar] [CrossRef]
- Rabbat, M.; Leipsic, J.; Bax, J.; Kauh, B.; Verma, R.; Doukas, D.; Allen, S.; Pontone, G.; Wilber, D.; Mathew, V.; et al. Fractional Flow Reserve Derived from Coronary Computed Tomography Angiography Safely Defers Invasive Coronary Angiography in Patients with Stable Coronary Artery Disease. J. Clin. Med. 2020, 9, 604. [Google Scholar] [CrossRef]
- Becker, L.M.; Peper, J.; Verhappen, B.; Swart, L.A.; Dedic, A.; van Dockum, W.G.; van der Ent, M.; Royaards, K.J.; Niezen, A.; Hensen, J.J.; et al. Real world impact of added FFR-CT to coronary CT angiography on clinical decision-making and patient prognosis - IMPACT FFR study. Eur. Radiol. 2023, 33, 5465–5475. [Google Scholar] [CrossRef]
- Qiao, H.Y.; Tang, C.X.; Schoepf, U.J.; Bayer, R.R., 2nd; Tesche, C.; Di Jiang, M.; Yin, C.Q.; Zhou, C.S.; Zhou, F.; Lu, M.J.; et al. One-year outcomes of CCTA alone versus machine learning-based FFR(CT) for coronary artery disease: A single-center, prospective study. Eur. Radiol. 2022, 32, 5179–5188. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Shan, D.; Wang, X.; Sun, X.; Shao, M.; Wang, K.; Pan, Y.; Wang, Z.; Schoepf, U.J.; Savage, R.H.; et al. On-Site Computed Tomography-Derived Fractional Flow Reserve to Guide Management of Patients With Stable Coronary Artery Disease: The TARGET Randomized Trial. Circulation 2023, 147, 1369–1381. [Google Scholar] [CrossRef] [PubMed]
- Giannopoulos, A.A.; Keller, L.; Sepulcri, D.; Boehm, R.; Garefa, C.; Venugopal, P.; Mitra, J.; Ghose, S.; Deak, P.; Pack, J.D.; et al. High-Speed On-Site Deep Learning-Based FFR-CT Algorithm: Evaluation Using Invasive Angiography as the Reference Standard. AJR Am. J. Roentgenol. 2023, 221, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhu, T.; Wang, Y.; Deng, Y.; Guan, H. Impact of coronary computed tomography angiography-derived fractional flow reserve based on deep learning on clinical management. Front. Cardiovasc. Med. 2023, 10, 1036682. [Google Scholar] [CrossRef]
- Di Pietro, G.; Improta, R.; De Filippo, O.; Bruno, F.; Birtolo, L.I.; Bruno, E.; Galea, N.; Francone, M.; Dewey, M.; D’Ascenzo, F.; et al. Clinical impact of CCT-FFR as first-strategy in patients with symptomatic stable coronary artery disease: A systematic review and meta-analysis. J. Cardiovasc. Comput. Tomogr. 2025, 19, 174–182. [Google Scholar] [CrossRef]
- Lee, J.M.; Choi, G.; Koo, B.K.; Hwang, D.; Park, J.; Zhang, J.; Kim, K.J.; Tong, Y.; Kim, H.J.; Grady, L.; et al. Identification of High-Risk Plaques Destined to Cause Acute Coronary Syndrome Using Coronary Computed Tomographic Angiography and Computational Fluid Dynamics. JACC Cardiovasc. Imaging 2019, 12, 1032–1043. [Google Scholar] [CrossRef]
- Koo, B.-K.; Yang, S.; Jung, J.W.; Zhang, J.; Lee, K.; Hwang, D.; Lee, K.-S.; Doh, J.-H.; Nam, C.-W.; Kim, T.H.; et al. Artificial Intelligence–Enabled Quantitative Coronary Plaque and Hemodynamic Analysis for Predicting Acute Coronary Syndrome. JACC Cardiovasc. Imaging 2024, 17, 1062–1076. [Google Scholar] [CrossRef] [PubMed]
- Ferencik, M.; Lu, M.T.; Mayrhofer, T.; Puchner, S.B.; Liu, T.; Maurovich-Horvat, P.; Ghemigian, K.; Ivanov, A.; Adami, E.; Nagurney, J.T.; et al. Non-invasive fractional flow reserve derived from coronary computed tomography angiography in patients with acute chest pain: Subgroup analysis of the ROMICAT II trial. J. Cardiovasc. Comput. Tomogr. 2019, 13, 196–202. [Google Scholar] [CrossRef]
- Fischer, A.M.; van Assen, M.; Schoepf, U.J.; Matuskowitz, A.J.; Varga-Szemes, A.; Golden, J.W.; Giovagnoli, D.A.; Tesche, C.; Bayer, R.R., 2nd. Non-invasive fractional flow reserve (FFR(CT)) in the evaluation of acute chest pain—Concepts and first experiences. Eur. J. Radiol. 2021, 138, 109633. [Google Scholar] [CrossRef]
- Chinnaiyan, K.M.; Safian, R.D.; Gallagher, M.L.; George, J.; Dixon, S.R.; Bilolikar, A.N.; Abbas, A.E.; Shoukfeh, M.; Brodsky, M.; Stewart, J.; et al. Clinical Use of CT-Derived Fractional Flow Reserve in the Emergency Department. JACC Cardiovasc. Imaging 2020, 13 Pt 1, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Gaur, S.; Taylor, C.A.; Jensen, J.M.; Bøtker, H.E.; Christiansen, E.H.; Kaltoft, A.K.; Holm, N.R.; Leipsic, J.; Zarins, C.K.; Achenbach, S.; et al. FFR Derived From Coronary CT Angiography in Nonculprit Lesions of Patients With Recent STEMI. JACC Cardiovasc. Imaging 2017, 10, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Hoshino, M.; Kanaji, Y.; Sugiyama, T.; Misawa, T.; Hada, M.; Nagamine, T.; Nogami, K.; Ueno, H.; Sayama, K.; et al. Predictors and prognostic value of coronary computed tomography angiography for unrecognized myocardial infarction in patients with chronic coronary syndrome. Hell. J. Cardiol. 2024. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Group, E.S.D. Fourth universal definition of myocardial infarction (2018). Eur. Heart J. 2018, 40, 237–269. [Google Scholar] [CrossRef]
- Fujimoto, S.; Nozaki, Y.O.; Sakamoto, T.; Nakanishi, R.; Asano, T.; Kadota, K.; Komiyama, K.; Taguchi, E.; Okubo, R.; Saito, A.; et al. Clinical impacts of CT-derived fractional flow reserve under insurance reimbursement: Results from multicenter, prospective registry. J. Cardiol. 2024, 84, 126–132. [Google Scholar] [CrossRef]
- Nozaki, Y.O.; Fujimoto, S.; Takahashi, D.; Kudo, A.; Kawaguchi, Y.O.; Sato, H.; Kudo, H.; Takamura, K.; Hiki, M.; Dohi, T.; et al. Additional prognostic impact of plaque characterization with on-site CT-derived fractional flow reserve in coronary CT angiography. J. Cardiol. 2024, 84, 336–341. [Google Scholar] [CrossRef]
- Takagi, H.; Ihdayhid, A.R.; Leipsic, J.A. Integration of fractional flow reserve derived from CT into clinical practice. J. Cardiol. 2023, 81, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Meier, D.; Andreini, D.; Cosyns, B.; Skalidis, I.; Storozhenko, T.; Mahendiran, T.; Assanelli, E.; Sonck, J.; Roosens, B.; Rotzinger, D.C.; et al. Usefulness of FFR-CT to exclude haemodynamically significant lesions in high-risk NSTE-ACS. EuroIntervention 2025, 21, 73–81. [Google Scholar] [CrossRef]
- Zimmerli, A.; Meier, D.; Salihu, A.; Liabot, Q.; Weerts, V.; Skalidis, I.; Andreini, D.; Cosyns, B.; Storozhenko, T.; Mahendiran, T.; et al. Impact of FFR-CT before coronary angiography on the management of non-culprit lesions among high-risk NSTE-ACS patients. J. Cardiol. 2025, 86, 211–213. [Google Scholar] [CrossRef] [PubMed]
- Tassetti, L.; Sfriso, E.; Torlone, F.; Baggiano, A.; Mushtaq, S.; Cannata, F.; Del Torto, A.; Fazzari, F.; Fusini, L.; Junod, D.; et al. The Role of Multimodality Imaging (CT & MR) as a Guide to the Management of Chronic Coronary Syndromes. J. Clin. Med. 2024, 13, 3450. [Google Scholar] [CrossRef]
- Nørgaard, B.L.; Hjort, J.; Gaur, S.; Hansson, N.; Bøtker, H.E.; Leipsic, J.; Mathiassen, O.N.; Grove, E.L.; Pedersen, K.; Christiansen, E.H.; et al. Clinical Use of Coronary CTA–Derived FFR for Decision-Making in Stable CAD. JACC Cardiovasc. Imaging 2017, 10, 541–550. [Google Scholar] [CrossRef]
- Patel, M.R.; Nørgaard, B.L.; Fairbairn, T.A.; Nieman, K.; Akasaka, T.; Berman, D.S.; Raff, G.L.; Hurwitz Koweek, L.M.; Pontone, G.; Kawasaki, T.; et al. 1-Year Impact on Medical Practice and Clinical Outcomes of FFR(CT): The ADVANCE Registry. JACC Cardiovasc. Imaging 2020, 13 Pt 1, 97–105. [Google Scholar] [CrossRef]
- Andreini, D.; Modolo, R.; Katagiri, Y.; Mushtaq, S.; Sonck, J.; Collet, C.; De Martini, S.; Roberto, M.; Tanaka, K.; Miyazaki, Y.; et al. Impact of Fractional Flow Reserve Derived From Coronary Computed Tomography Angiography on Heart Team Treatment Decision-Making in Patients With Multivessel Coronary Artery Disease: Insights From the SYNTAX III REVOLUTION Trial. Circ. Cardiovasc. Interv. 2019, 12, e007607. [Google Scholar] [CrossRef]
- Sonck, J.; Nagumo, S.; Norgaard, B.L.; Otake, H.; Ko, B.; Zhang, J.; Mizukami, T.; Maeng, M.; Andreini, D.; Takahashi, Y.; et al. Clinical Validation of a Virtual Planner for Coronary Interventions Based on Coronary CT Angiography. JACC Cardiovasc. Imaging 2022, 15, 1242–1255. [Google Scholar] [CrossRef]
- Van Belle, E.; Raposo, L.; Bravo Baptista, S.; Vincent, F.; Porouchani, S.; Cosenza, A.; Rogers, C.; Leipsic, J. Impact of an Interactive CT/FFR(CT) Interventional Planner on Coronary Artery Disease Management Decision Making. JACC Cardiovasc. Imaging 2021, 14, 1068–1070. [Google Scholar] [CrossRef]
- Madsen, K.T.; Nørgaard, B.L.; Øvrehus, K.A.; Jensen, J.M.; Parner, E.; Grove, E.L.; Fairbairn, T.A.; Nieman, K.; Patel, M.R.; Rogers, C.; et al. Prognostic Value of Coronary CT Angiography-derived Fractional Flow Reserve on 3-year Outcomes in Patients with Stable Angina. Radiology 2023, 308, e230524. [Google Scholar] [CrossRef] [PubMed]
- Karampinos, K.; Ktenopoulos, N.; Apostolos, A.; Koliastasis, L.; Kachrimanidis, I.; Vlachakis, P.; Katsaros, O.; Tsalamandris, S.; Karanasos, A.; Drakopoulou, M.; et al. Navigating the silence: Reconsidering treatment paradigms in asymptomatic severe aortic stenosis. Hell. J. Cardiol. 2025. [Google Scholar] [CrossRef]
- Custódio, P.; Madeira, S.; Teles, R.; Almeida, M. Coronary artery disease and its management in TAVI. Hell. J. Cardiol. 2024, 78, 36–41. [Google Scholar] [CrossRef]
- Ktenopoulos, N.; Karanasos, A.; Katsaros, O.; Apostolos, A.; Latsios, G.; Moulias, A.; Papafaklis, M.I.; Tsigkas, G.; Tsioufis, C.; Toutouzas, K.; et al. Coronary Artery Disease and Severe Aortic Stenosis: Contemporary Treatment Options for Patients Undergoing Transcatheter Aortic Valve Implantation. J. Clin. Med. 2024, 13, 7625. [Google Scholar] [CrossRef]
- Peper, J.; Becker, L.M.; Berg, H.v.d.; Bor, W.L.; Brouwer, J.; Nijenhuis, V.J.; Ginkel, D.-J.v.; Rensing, B.J.M.W.; Berg, J.M.t.; Timmers, L.; et al. Diagnostic Performance of CCTA and CT-FFR for the Detection of CAD in TAVR Work-Up. Cardiovasc. Interv. 2022, 15, 1140–1149. [Google Scholar] [CrossRef]
- Brandt, V.; Schoepf, U.J.; Aquino, G.J.; Bekeredjian, R.; Varga-Szemes, A.; Emrich, T.; Bayer, R.R., 2nd; Schwarz, F.; Kroencke, T.J.; Tesche, C.; et al. Impact of machine-learning-based coronary computed tomography angiography-derived fractional flow reserve on decision-making in patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. Eur. Radiol. 2022, 32, 6008–6016. [Google Scholar] [CrossRef]
- Michail, M.; Ihdayhid, A.R.; Comella, A.; Thakur, U.; Cameron, J.D.; McCormick, L.M.; Gooley, R.P.; Nicholls, S.J.; Mathur, A.; Hughes, A.D.; et al. Feasibility and Validity of Computed Tomography-Derived Fractional Flow Reserve in Patients With Severe Aortic Stenosis: The CAST-FFR Study. Circ. Cardiovasc. Interv. 2021, 14, e009586. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Pan, D.; Sun, X.; Yang, G.; Yao, J.; Shen, X.; Xiao, W. Two birds with one stone: Pre-TAVI coronary CT angiography combined with FFR helps screen for coronary stenosis. BMC Med. Imaging 2025, 25, 192. [Google Scholar] [CrossRef] [PubMed]
- Gohmann, R.F.; Pawelka, K.; Seitz, P.; Majunke, N.; Heiser, L.; Renatus, K.; Desch, S.; Lauten, P.; Holzhey, D.; Noack, T.; et al. Combined cCTA and TAVR Planning for Ruling Out Significant CAD: Added Value of ML-Based CT-FFR. JACC Cardiovasc. Imaging 2022, 15, 476–486. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiong, T.Y.; Li, Y.M.; Huang, F.Y.; Peng, Y.; Li, Q.; Wei, J.F.; He, S.; Cao, K.L.; Feng, Y.; et al. Variation of computed tomographic angiography-based fractional flow reserve after transcatheter aortic valve implantation. Eur. Radiol. 2021, 31, 6220–6229. [Google Scholar] [CrossRef]
- Andreini, D.; Mushtaq, S.; Pontone, G.; Rogers, C.; Pepi, M.; Bartorelli, A.L. Severe in-stent restenosis missed by coronary CT angiography and accurately detected with FFR(CT). Int. J. Cardiovasc. Imaging 2017, 33, 119–120. [Google Scholar] [CrossRef] [PubMed]
- Mueller, A.; Konje, S.K.; Barman, N.B.; Argulian, E.A.; Maslov, P.M.; Namdarizandi, V.M.; Leipsic, J.L.; Tomey, M.T.; Narula, J.N.; Ahmadi, A.A. Symptoms and ASCVD score fail to identify majority of the patients at risk of first myocardial infarction. Eur. Heart J. 2024, 45 (Suppl. S1), ehae666-1657. [Google Scholar] [CrossRef]
- Douglas, P.S.; Nanna, M.G.; Kelsey, M.D.; Yow, E.; Mark, D.B.; Patel, M.R.; Rogers, C.; Udelson, J.E.; Fordyce, C.B.; Curzen, N.; et al. Comparison of an Initial Risk-Based Testing Strategy vs Usual Testing in Stable Symptomatic Patients With Suspected Coronary Artery Disease: The PRECISE Randomized Clinical Trial. JAMA Cardiol. 2023, 8, 904–914. [Google Scholar] [CrossRef]
- Rasoul, H.; Fyyaz, S.; Noakes, D.; Shakespeare, C.; David, S.; Khawaja, Z.M.; Papamichail, N.; Alfakih, K. NHS England-funded CT fractional flow reserve in the era of the ISCHEMIA trial. Clin. Med. 2021, 21, 90–95. [Google Scholar] [CrossRef]
- Graby, J.; Metters, R.; Kandan, S.R.; McKenzie, D.; Lowe, R.; Carson, K.; Hudson, B.J.; Rodrigues, J.C.L. Real-world clinical and cost analysis of CT coronary angiography and CT coronary angiography-derived fractional flow reserve (FFR(CT))-guided care in the National Health Service. Clin. Radiol. 2021, 76, 862.e19–862.e28. [Google Scholar] [CrossRef]
- Karády, J.; Mayrhofer, T.; Ivanov, A.; Foldyna, B.; Lu, M.T.; Ferencik, M.; Pursnani, A.; Salerno, M.; Udelson, J.E.; Mark, D.B.; et al. Cost-effectiveness Analysis of Anatomic vs Functional Index Testing in Patients With Low-Risk Stable Chest Pain. JAMA Netw. Open 2020, 3, e2028312. [Google Scholar] [CrossRef]
- Mittal, T.K.; Hothi, S.S.; Venugopal, V.; Taleyratne, J.; O’Brien, D.; Adnan, K.; Sehmi, J.; Daskalopoulos, G.; Deshpande, A.; Elfawal, S.; et al. The Use and Efficacy of FFR-CT: Real-World Multicenter Audit of Clinical Data With Cost Analysis. JACC Cardiovasc. Imaging 2023, 16, 1056–1065. [Google Scholar] [CrossRef]
- Hlatky, M.A.; De Bruyne, B.; Pontone, G.; Patel, M.R.; Norgaard, B.L.; Byrne, R.A.; Curzen, N.; Purcell, I.; Gutberlet, M.; Rioufol, G.; et al. Quality-of-Life and Economic Outcomes of Assessing Fractional Flow Reserve With Computed Tomography Angiography: PLATFORM. J. Am. Coll. Cardiol. 2015, 66, 2315–2323. [Google Scholar] [CrossRef]
- Wu, J.; Yang, D.; Zhang, Y.; Xian, H.; Weng, Z.; Ji, L.; Yang, F. Non-invasive imaging innovation: FFR-CT combined with plaque characterization, safeguarding your cardiac health. J. Cardiovasc. Comput. Tomogr. 2025, 19, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.; Wang, M.; Kong, L.; Li, L.; Chi Chan, L.W. Clinical Applications of Fractional Flow Reserve Derived from Computed Tomography in Coronary Artery Disease. Mayo Clin. Proc. Digit. Health 2025, 3, 100187. [Google Scholar] [CrossRef]
- Javid, R.N.; Hosseini, S.K. CT-derived Fractional Flow Reserve: How, When, and Where to use this Novel Cardiac Imaging Tool. Curr. Cardiol. Rev. 2024, 20, e040624230662. [Google Scholar] [CrossRef] [PubMed]
- Tesche, C.; Otani, K.; De Cecco, C.N.; Coenen, A.; De Geer, J.; Kruk, M.; Kim, Y.H.; Albrecht, M.H.; Baumann, S.; Renker, M.; et al. Influence of Coronary Calcium on Diagnostic Performance of Machine Learning CT-FFR: Results from MACHINE Registry. JACC Cardiovasc. Imaging 2020, 13, 760–770. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Tu, C.; Zhang, B.; Zhang, D.; Song, X.; Zhang, H. A meta-analysis comparing the diagnostic performance of computed tomography-derived fractional flow reserve and coronary computed tomography angiography at different levels of coronary artery calcium score. Eur. Radiol. 2024, 34, 5621–5632. [Google Scholar] [CrossRef] [PubMed]
- Katsaros, O.; Sagris, M.; Karakasis, P.; Ktenopoulos, N.; Soulaidopoulos, S.; Theofilis, P.; Apostolos, A.; Tzoumas, A.; Patsourakos, N.; Toutouzas, K.; et al. The Role of Calcified Nodules in Acute Coronary Syndrome: Diagnosis and Management. Int. J. Mol. Sci. 2025, 26, 2581. [Google Scholar] [CrossRef] [PubMed]
- Chlorogiannis, D.D.; Pargaonkar, S.; Apostolos, A.; Vythoulkas-Biotis, N.; Kokkinidis, D.G.; Nagraj, S. The Predictive Value of Aortic Calcification on Computed Tomography for Major Cardiovascular Events. J. Clin. Med. 2024, 13, 4019. [Google Scholar] [CrossRef]
- Liu, L.; Pan, Y.; Ma, Z.; Tian, J.; Xing, H.; Zhang, M.; Zhang, M.; Xu, F.; Ren, Y.; Zhang, L.; et al. Improved evaluation of coronary artery diseases from patients with coronary calcification utilizing FFR(CT): A comparative study against CCTA. Hell. J. Cardiol. 2025. [Google Scholar] [CrossRef]
- Pontone, G.; Weir-McCall, J.R.; Baggiano, A.; Del Torto, A.; Fusini, L.; Guglielmo, M.; Muscogiuri, G.; Guaricci, A.I.; Andreini, D.; Patel, M.; et al. Determinants of Rejection Rate for Coronary CT Angiography Fractional Flow Reserve Analysis. Radiology 2019, 292, 597–605. [Google Scholar] [CrossRef]
- van Assen, M.; Muscogiuri, G.; Caruso, D.; Lee, S.J.; Laghi, A.; De Cecco, C.N. Artificial intelligence in cardiac radiology. Radiol. Med. 2020, 125, 1186–1199. [Google Scholar] [CrossRef]
- Chlorogiannis, D.D.; Apostolos, A.; Chlorogiannis, A.; Palaiodimos, L.; Giannakoulas, G.; Pargaonkar, S.; Xesfingi, S.; Kokkinidis, D.G. The Role of ChatGPT in the Advancement of Diagnosis, Management, and Prognosis of Cardiovascular and Cerebrovascular Disease. Healthcare 2023, 11, 2906. [Google Scholar] [CrossRef]
| Clinical Scenario | SCCT 2021 Expert Consensus | 2021 AHA/ACC/ASE/Chest/SAEM/SCCT/SCMR Multisociety Guidelines |
|---|---|---|
| Stable chest pain (no known CAD) | First-line test in low- to intermediate-risk patients | Class I (Level of Evidence A): Recommended when intermediate stenosis present; improves lesion-specific assessment |
| Acute chest pain (low- to intermediate-risk) | Supports ECG—gated CCTA in emergency setting for early rule-out, including the “triple rule-out” approach (CAD, pulmonary embolism, aortic dissection) in appropriately selected patients (men > 45, women > 55). | Acute chest pain (low- to intermediate-risk) |
| Inconclusive CCTA | Supports use of additional non-invasive imaging (FFRCT, CTP, or alternative modalities such as CMR, nuclear perfusion imaging, or stress echocardiography); selection among these non-invasive functional tests is limited. | Class IIa (Level of Evidence B): Recommends downstream non-invasive testing: FFRCT or myocardial perfusion imaging, and notes that the choice should be individualized based on test availability, patient-specific contraindications (e.g., MRI compatibility); suggesting a shared decision-making approach. |
| Intermediate stenosis (30–90% diameter stenosis) | Recommends functional assessment with FFRCT or CTP to clarify ischemic significance | Class IIa (Level of Evidence B): Supports downstream testing (FFRCT or MPI); acknowledges improved diagnostic accuracy when paired with CCTA |
| Asymptomatic high-risk individuals (diabetes, smoking, inflammatory conditions, familial hypercholesterolemia, HIV, or a strong family history) | Conditionally appropriate for detecting subclinical CAD and initiating preventive therapies | Not routinely recommended, but may be considered in selected high-risk patients (e.g., diabetes, strong family history, chronic inflammatory conditions) |
| Asymptomatic low- or intermediate-risk individuals | Rarely appropriate due to low yield and risk of downstream harm | Not recommended due to low diagnostic yield |
| Prior CABG | Recommended for graft patency evaluation; native vessel assessment limited by artifacts | Class IIb (Level of Evidence B): Recommended for evaluating graft patency |
| Stents ≥ 3 mm | Appropriate for ISR evaluation | N/A |
| Congenital coronary anomalies | Recommended as first-line due its ability to accurately depict complex three-dimensional anatomical relationships, vessel courses, and potential high-risk features such as interarterial or intramural courses. | N/A |
| New cardiomyopathy | Supports use of CCTA to identify ischemic etiology in unexplained cardiomyopathy; Appropriate to exclude obstructive coronary artery disease | N/A |
| Heart transplant (coronary allograft vasculopathy surveillance) | Recognized as non-invasive alternative to invasive angiography in transplant recipients, when renal function allows contrast use | N/A |
| Viability/scar assessment (when MRI contraindicated) | Not guideline-emphasized; considered investigational or conditional in MRI-limited patients Delayed-enhancement CCTA is an acceptable alternative in specific revascularization or ablation planning settings | N/A |
| 3D Software Provider | Study | Parameter | Patients (n) | Vessels (n) | Design | AUC | Accuracy (%) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Correlation Coefficient |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Heartflow | DISCOVER FLOW 2011 [23] | FFRCT | 103 | 159 | Prospective, multicenter | 0.90 | 84.3 | 87.9 | 82.2 | 73.9 | 92.2 | 0.678 |
| Heartflow | DeFACTO 2013 [28] | FFRCT | 252 | 407 | Prospective, multicenter | 0.79 | 69 | 74 | 67 | 41 | 90 | 0.50 |
| Heartflow | HTNXT 2014 [29] | FFRCT | 254 | 484 | Prospective, multicenter | 0.93 | 86 | 84 | 86 | 61 | 95 | 0.82 |
| Heartflow | Yang et al. 2016 [30] | FFRCT | 72 | 138 | Retrospective | 0.919 | 81 | 87 | 77 | 71 | 90 | 0.671 |
| Heartflow | Driesen et al. 2019 [31] | FFRCT | 157 | 505 | Post-hoc analysis of PACIFIC trial | 0.94 | 87 | 90 | 86 | 65 | 96 | 0.80 |
| Siemens (1st generation) | Renker et al. 2014 [32] | cFFR | 53 | 67 | Retrospective | 0.92 | N/A | 85 | 85 | 71 | 93 | 0.66 |
| Siemens (1st generation) | Coenen et al. 2015 [21] | cFFR | 106 | 189 | Retrospective | 0.83 | 74.6 | 87.5 | 65.1 | 64.8 | 87.7 | 0.59 |
| Siemens (1st generation) | De Geer et al. 2016 [33] | cFFR | 21 | 23 | Retrospective | N/A | 78 | 83 | 76 | 56 | 93 | 0.77 |
| Siemens (2nd generation) | Itu et al. 2016 [22] | cFFRML | 87 | 125 | Prospective, single center | N/A | 83.2 | 81.6 | 83.9 | 68.9 | 91.2 | 0.729 |
| Toshiba | Ko et al. 2017 [34] | FFRCT | 30 | 56 | Prospective, single center | 0.88 | 83.9 | 77.8 | 86.8 | 73.7 | 89.2 | 0.57 |
| Keya Medical Technology | Wang et al. 2019 [35] | DEEPVESSEL-FFR | 63 | 71 | Prospective, single center | 0.933 | 88.73 | 97.56 | 76.67 | 85.11 | 95.83 | 0.683 |
| Keya Medical Technology | ADAPT 2021 [36] | DEEPVESSEL-FFR | 302 | N/A | Retrospective, multicenter | N/A | 86.8 | 86.9 | 86.7 | 79.4 | 91.9 | N/A |
| United-Imaging Healthcare | Tang 2020 [38] | uCT-FFR | 338 | 422 | Retrospective, multicenter | 0.92 | 91 | 89 | 91 | 86 | 94 | 0.69 |
| CAscope | Ding 2023 [39] | esFFR | 329 | 350 | Prospective, multicenter | 0.97 | 93 | 95 | 92 | 90 | 96 | 0.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bozika, M.; Apostolos, A.; Nastouli, K.-M.; Papafaklis, M.I.; Skalidis, I.; Terentes-Printzios, D.; Karanasos, A.; Koutsogiannis-Korkontzelos, C.; Boliaris, G.; Floropoulos, S.; et al. Clinical Impact of CT-Based FFR in Everyday Cardiology: Bridging Computation and Decision-Making. Biomedicines 2025, 13, 1969. https://doi.org/10.3390/biomedicines13081969
Bozika M, Apostolos A, Nastouli K-M, Papafaklis MI, Skalidis I, Terentes-Printzios D, Karanasos A, Koutsogiannis-Korkontzelos C, Boliaris G, Floropoulos S, et al. Clinical Impact of CT-Based FFR in Everyday Cardiology: Bridging Computation and Decision-Making. Biomedicines. 2025; 13(8):1969. https://doi.org/10.3390/biomedicines13081969
Chicago/Turabian StyleBozika, Maria, Anastasios Apostolos, Kassiani-Maria Nastouli, Michail I. Papafaklis, Ioannis Skalidis, Dimitrios Terentes-Printzios, Antonios Karanasos, Christos Koutsogiannis-Korkontzelos, Georgios Boliaris, Spyridon Floropoulos, and et al. 2025. "Clinical Impact of CT-Based FFR in Everyday Cardiology: Bridging Computation and Decision-Making" Biomedicines 13, no. 8: 1969. https://doi.org/10.3390/biomedicines13081969
APA StyleBozika, M., Apostolos, A., Nastouli, K.-M., Papafaklis, M. I., Skalidis, I., Terentes-Printzios, D., Karanasos, A., Koutsogiannis-Korkontzelos, C., Boliaris, G., Floropoulos, S., Mavromati, A., Katsanos, K., Davlouros, P., & Tsigkas, G. (2025). Clinical Impact of CT-Based FFR in Everyday Cardiology: Bridging Computation and Decision-Making. Biomedicines, 13(8), 1969. https://doi.org/10.3390/biomedicines13081969










