Machine Learning Prediction of Short Cervix in Mid-Pregnancy Based on Multimodal Data from the First-Trimester Screening Period: An Observational Study in a High-Risk Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants and Cervical Length Assessment
2.2. Data Processing and Cohort Division
2.3. Multimodal Data for Predicting Short Cervix in Mid-Pregnancy
2.4. Predictive Variables
2.5. Derivation and Validation Data
2.6. Model Development and Validation
2.7. Model Interpretation
2.8. Propensity Score Matching
2.9. Statistical Analysis
3. Results
3.1. Study Population Characteristics
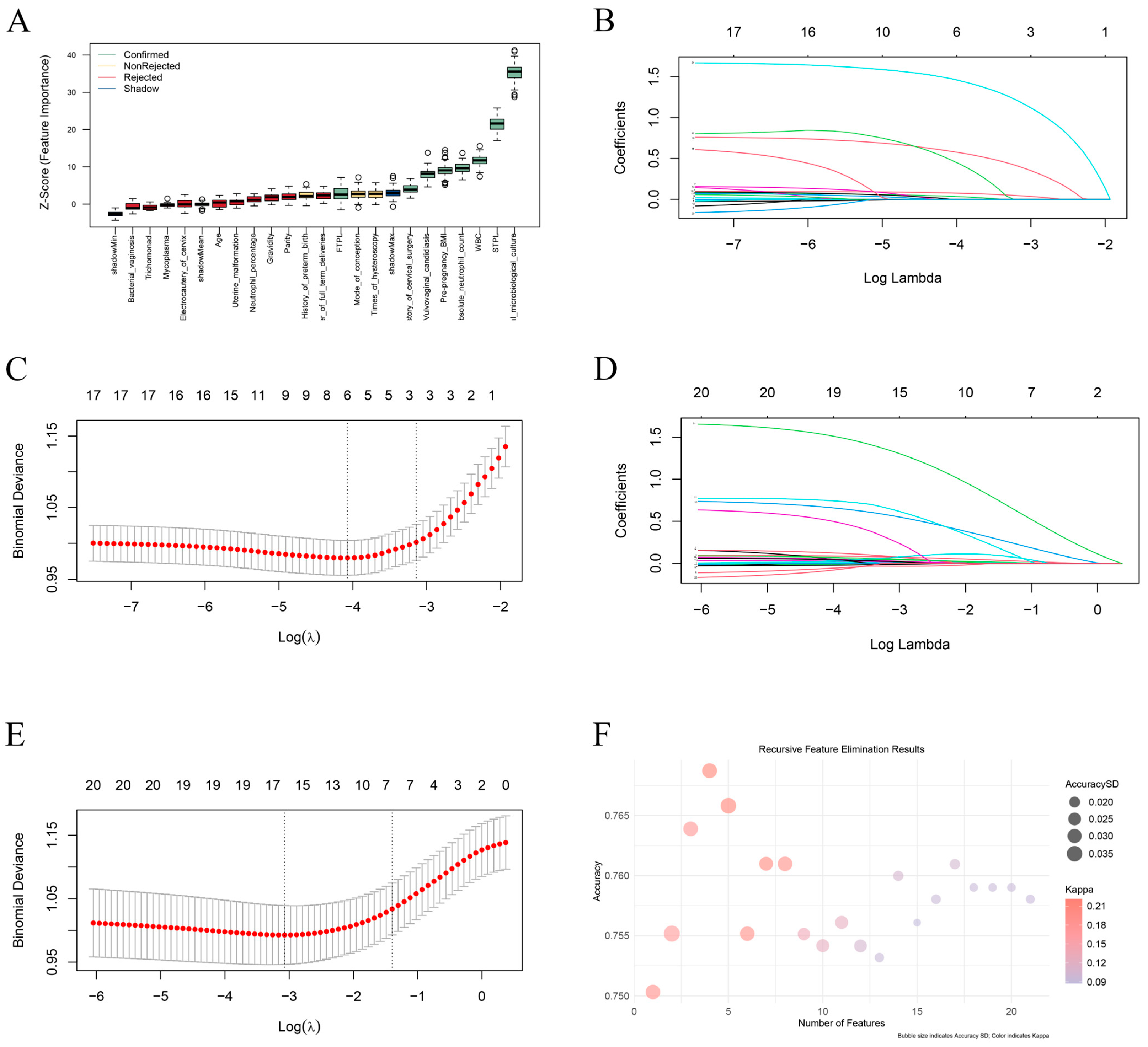
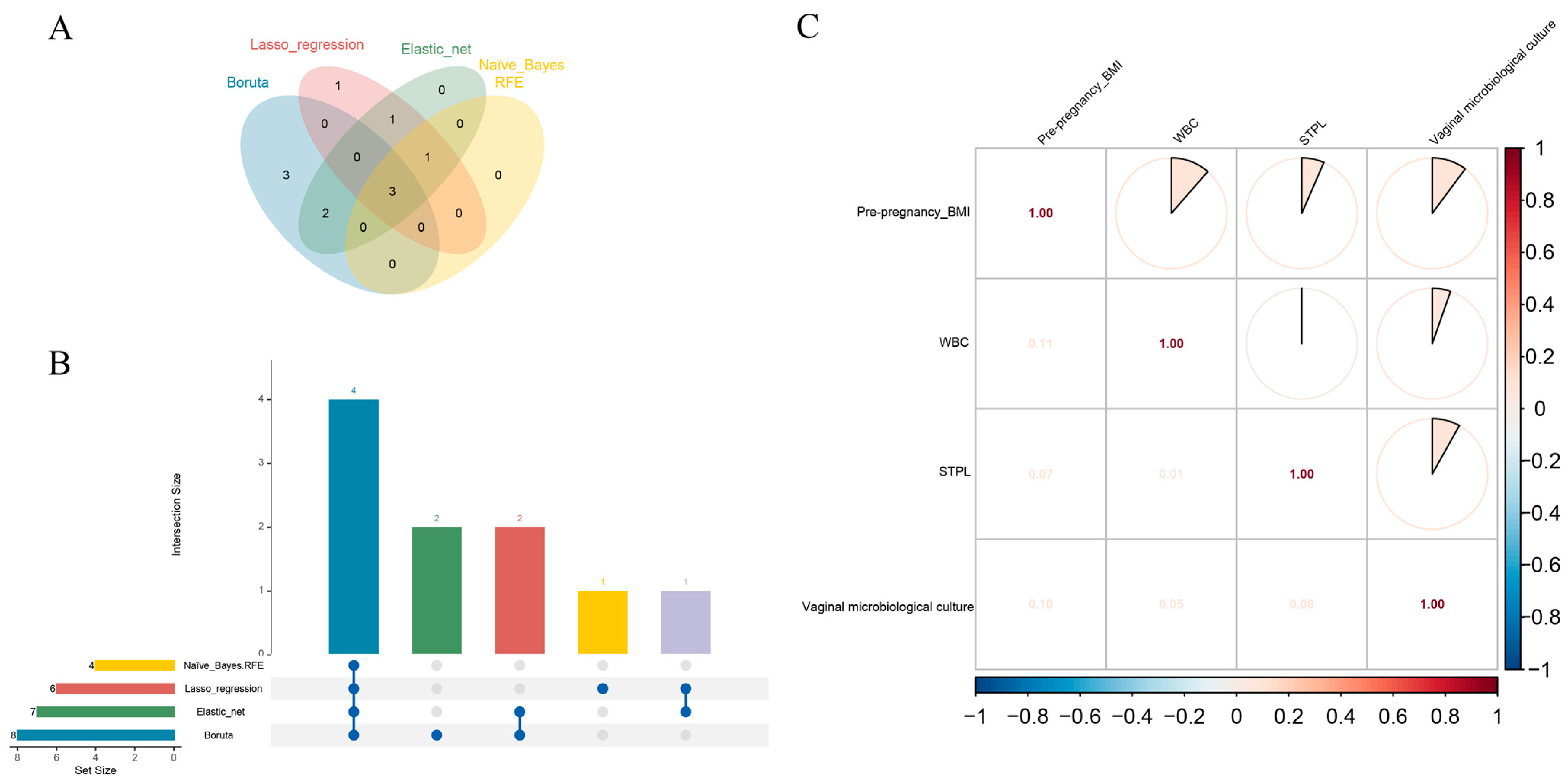
3.2. Variable Screening
3.3. Data Preprocessing
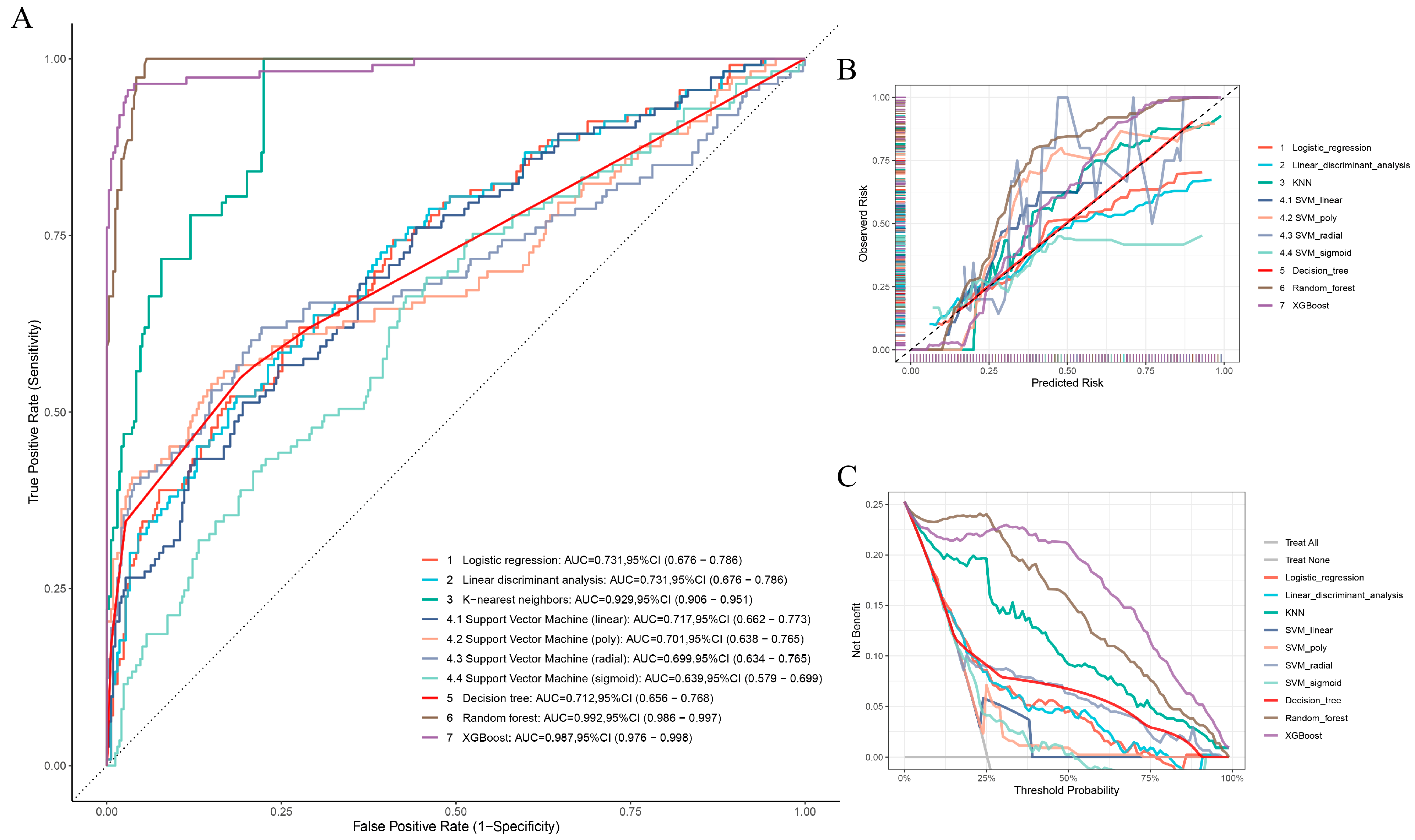
3.4. Construction and Assessment of ML Models
3.5. Verification of the ML Models
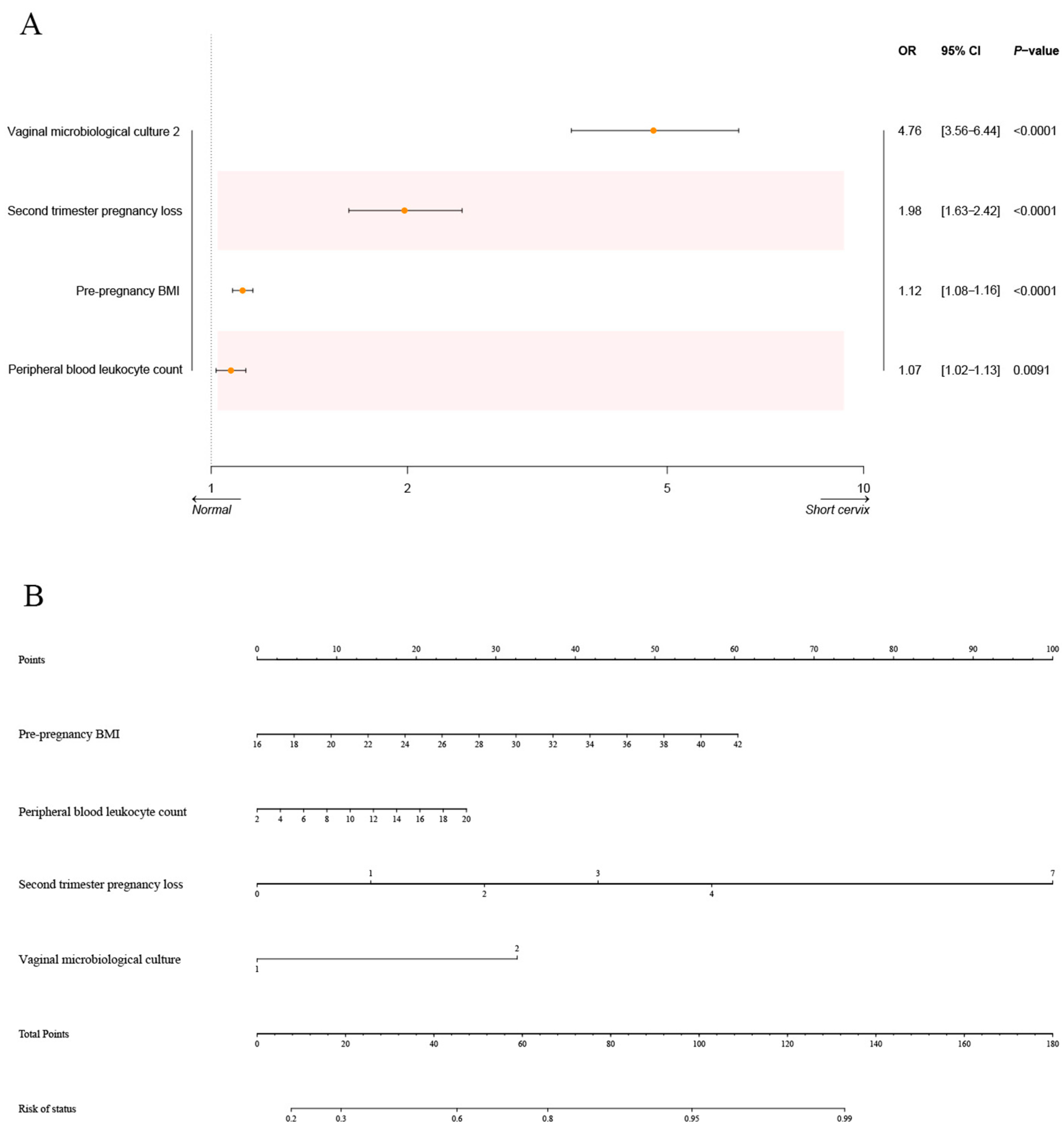
3.6. Interpretability Analysis for the Optimal Model
3.7. sPTB Rate in the Normal and Short Cervix Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

| Predictors | Types | Values |
|---|---|---|
| Age | Discrete | / |
| Pre-pregnancy BMI | Continuous | BMI = weight/height2 (kg/m2) |
| Gravidity | Discrete | Number of pregnancies (including the current pregnancy) |
| Parity | Discrete | Number of births (excluding the current pregnancy) |
| Number of full-term deliveries | Discrete | Times of delivery without pregnancy loss or preterm birth (excluding the current pregnancy) |
| First-trimester pregnancy loss | Discrete | Times of miscarriages in the first trimester (excluding the current pregnancy) |
| Second-trimester pregnancy loss | Discrete | Times of miscarriages in the second trimester (excluding the current pregnancy) |
| History of preterm birth | Discrete | Times of preterm birth (excluding the current pregnancy) |
| Mode of conception | Categorical | Mode of conception for this pregnancy 1- Natural conception 2- Ovulation induction 3- IVF-ET 4- ICSI 5- PGD |
| Uterine malformation | Categorical | 1- Normal uterus 2- Bicornuate uterus 3- Septate uterus |
| History of cervical surgery | Categorical | 1- None 2- Cervical LEEP cone resection 3- Cervical conization |
| Times of hysteroscopy | Discrete | Number of hysteroscopic examinations |
| Electrocautery of cervix | Categorical | 1- No 2- Yes |
| Vulvovaginal candidiasis | Categorical | Vulvovaginal candidiasis detection (tested between 11+0 and 13+6 weeks of gestation) 1- No 2- Yes |
| Trichomonad | Categorical | Trichomonad detection in vaginal secretions (tested between 11+0 and 13+6 weeks of gestation) 1- No 2- Yes |
| Mycoplasma | Categorical | Mycoplasma detection in vaginal secretions (tested between 11+0 and 13+6 weeks of gestation) 1- No 2- Yes |
| Bacterial vaginosis | Categorical | Bacterial vaginosis detection (tested between 11+0 and 13+6 weeks of gestation) 1- No 2- Yes |
| Vaginal microbiological culture (≥105CFU/mL) | Categorical | Bacterial culture of vaginal secretions (tested between 11 + 0 and 13 + 6 weeks of gestation) 1- No 2- Yes |
| WBC | Continuous | Absolute peripheral blood leukocyte count (tested between 11 + 0 and 13 + 6 weeks of gestation) |
| Neutrophil percentage (%) | Continuous | Percentage of neutrophils in peripheral blood (tested between 11+0 and 13+6 weeks of gestation)) |
| Absolute neutrophil count | Continuous | Absolute peripheral blood neutrophil values (tested between 11+0 and 13+6 weeks of gestation) |
| Predictive Variables | Trainset (N = 1033) | Test Set (N = 447) | p-Values | |
|---|---|---|---|---|
| General information | Age | |||
| Mean (SD) | 33.9 (3.42) | 34.0 (3.38) | 0.431 a | |
| Pre-pregnancy BMI | ||||
| Mean (SD) | 22.4 (3.35) | 22.3 (3.28) | 0.797 a | |
| Gravidity | ||||
| Median [Min, Max] | 3.00 [2.00, 9.00] | 3.00 [2.00, 9.00] | 0.296 b | |
| Parity | ||||
| Median [Min, Max] | 0 [0, 3.00] | 0 [0, 3.00] | 0.128 b | |
| Number of full-term deliveries | ||||
| Median [Min, Max] | 0 [0, 3.00] | 0 [0, 3.00] | 0.014 b | |
| Medical history | First trimester pregnancy loss * | |||
| Median [Min, Max] | 1.00 [0, 8.00] | 1.00 [0, 8.00] | 0.025 b | |
| Second trimester pregnancy loss * | ||||
| Median [Min, Max] | 0 [0, 7.00] | 0 [0, 3.00] | 0.628 b | |
| History of preterm birth | ||||
| Median [Min, Max] | 0 [0, 2.00] | 0 [0, 2.00] | 0.379 b | |
| Mode of conception | ||||
| Natural conception | 660 (63.9%) | 302 (67.6%) | 0.408 c | |
| Ovulation induction | 39 (3.8%) | 14 (3.1%) | ||
| IVF-ET | 14 (1.4%) | 3 (0.7%) | ||
| ICSI | 221 (21.4%) | 81 (18.1%) | ||
| PGD | 99 (9.6%) | 47 (10.5%) | ||
| Uterine malformation | ||||
| Normal uterus | 1018 (98.5%) | 440 (98.4%) | 0.859 c | |
| Bicornuate uterus | 4 (0.4%) | 1 (0.2%) | ||
| Septate uterus | 11 (1.1%) | 6 (1.3%) | ||
| History of cervical surgery | ||||
| None | 904 (87.5%) | 393 (87.9%) | 0.934 c | |
| Cervical LEEP cone resection | 92 (8.9%) | 40 (8.9%) | ||
| Cervical conization | 37 (3.6%) | 14 (3.1%) | ||
| Times of hysteroscopy | ||||
| Median [Min, Max] | 0 [0, 9.00] | 0 [0, 9.00] | 0.137 b | |
| Electrocautery of cervix | ||||
| No | 1026 (99.3%) | 447 (100%) | 0.11 c | |
| Yes | 7 (0.7%) | 0 (0%) | ||
| Laboratory examination | Vulvovaginal candidiasis | |||
| No | 925 (89.5%) | 396 (88.6%) | 0.584 c | |
| Yes | 108 (10.5%) | 51 (11.4%) | ||
| Trichomonad | ||||
| No | 1031 (99.8%) | 447 (100%) | 1 c | |
| Yes | 2 (0.2%) | 0 (0%) | ||
| Mycoplasma | ||||
| No | 806 (78.0%) | 336 (75.2%) | 0.252 c | |
| Yes | 227 (22.0%) | 111 (24.8%) | ||
| Bacterial vaginosis | ||||
| No | 936 (90.6%) | 410 (91.7%) | 0.554 c | |
| Yes | 97 (9.4%) | 37 (8.3%) | ||
| Vaginal microbiological culture (≥105CFU/mL) | ||||
| No | 865 (83.7%) | 379 (84.8%) | 0.643 c | |
| Yes | 168 (16.3%) | 68 (15.2%) | ||
| WBC | ||||
| Mean (SD) | 8.73 (2.21) | 8.75 (2.27) | 0.855 a | |
| Neutrophil percentage | ||||
| Mean (SD) | 72.0 (6.58) | 71.7 (6.83) | 0.359 a | |
| Absolute neutrophil count | ||||
| Mean (SD) | 6.34 (1.88) | 6.34 (1.99) | 0.982 a | |
| Outcome | Short cervix | |||
| No | 770 (74.5%) | 334 (74.7%) | 1 c | |
| Yes | 263 (25.5%) | 113 (25.3%) |
| Models | Parameters/Hyperparameters | Optimum Value |
|---|---|---|
| LR | Coefficients | Pre-pregnancy BMI = 0.11290, peripheral blood leukocyte = 0.07022, second trimester pregnancy loss = 0.68365, vaginal microbiological culture 2 = 1.54878 |
| LDA | Coefficients of linear discriminants | Pre-pregnancy BMI = 0.13325144, Peripheral blood leukocyte = 0.07801393, second trimester pregnancy loss = 0.73637384, vaginal microbiological culture 2 = 1.79646255 |
| KNN | k, kernel | k = 9, kernel = “optimal” |
| Linear SVM | cost | cost = 0.1 |
| Polynomial SVM | cost, degree, coef.0 | cost = 1, degree = 5, coef.0 = 2 |
| RBF-SVM | cost | cost = 1 |
| Sigmoid SVM | cost, coef.0 | cost = 0.1, coef.0 = 0 |
| DT | cp | cp = 0.01038961 |
| RF | mtry, ntree | mtry = 2, ntree = 400 |
| XGBoost | nrounds, max_depth, eta = 0.3, gamma, colsample_bytree, min_child_weight, subsample | nrounds = 100, max depth = 3, eta = 0.4, gamma = 0, colsample bytree = 1, min child weight = 1, subsample = 1 |
| Model | Accuracy | Precision | F1 Score | Sensitivity (Recall) | Specificity | Brier |
|---|---|---|---|---|---|---|
| Logistic regression | 0.7919463 | 0.7173913 | 0.4150943 | 0.2920354 | 0.9610778 | 0.1587173 |
| LDA | 0.7964206 | 0.7115385 | 0.4484848 | 0.3274336 | 0.9550898 | 0.1593799 |
| KNN | 0.8389262 | 0.8153846 | 0.5955056 | 0.4690265 | 0.9640719 | 0.1025336 |
| Linear SVM | 0.7606264 | 0.875 | 0.1157025 | 0.0619469 | 0.997006 | 0.168591 |
| Polynomial SVM | 0.7941834 | 0.8387097 | 0.3611111 | 0.2300885 | 0.9850299 | 0.1620557 |
| RBF-SVM | 0.8098434 | 0.8333333 | 0.4516129 | 0.3097345 | 0.9790419 | 0.1500501 |
| Sigmoid SVM | 0.7472036 | 0.5 | 0.1102362 | 0.0619469 | 0.9790419 | 0.1836746 |
| DT | 0.8143177 | 0.8125 | 0.484472 | 0.3451327 | 0.9730539 | 0.1477355 |
| RF | 0.9038031 | 1 | 0.7650273 | 0.619469 | 1 | 0.06315483 |
| XGBoost | 0.9574944 | 0.9795918 | 0.9099526 | 0.8495575 | 0.994012 | 0.04911613 |
| Model | AUC | Accuracy | Precision | F1 Score | Sensitivity (Recall) | Specificity | Brier |
|---|---|---|---|---|---|---|---|
| Logistic regression | 0.752 (0.711–0.794) | 0.7901726 | 0.6585366 | 0.406015 | 0.2934783 | 0.9507909 | 0.1539881 |
| LDA | 0.751 (0.71–0.793) | 0.7848606 | 0.6078431 | 0.4335664 | 0.3369565 | 0.9297012 | 0.1550962 |
| KNN | 0.935 (0.919–0.951) | 0.8472776 | 0.785124 | 0.6229508 | 0.5163043 | 0.9543058 | 0.09834036 |
| Linear SVM | 0.74 (0.697–0.782) | 0.7715803 | 0.5555556 | 0.4109589 | 0.326087 | 0.9156415 | 0.1685743 |
| Polynomial SVM | 0.708 (0.658–0.758) | 0.7901726 | 0.7954545 | 0.3070175 | 0.1902174 | 0.9841828 | 0.1848635 |
| RBF-SVM | 0.732 (0.684–0.781) | 0.8061089 | 0.75 | 0.4384615 | 0.3097826 | 0.9666081 | 0.1505253 |
| Sigmoid SVM | 0.68 (0.635–0.725) | 0.752988 | 0.375 | 0.03125 | 0.01630435 | 0.9912127 | 0.1718315 |
| DT | 0.739 (0.697–0.78) | 0.8180611 | 0.742268 | 0.5124555 | 0.3913043 | 0.9560633 | 0.1416904 |
| RF | 0.98 (0.973–0.987) | 0.8937583 | 0.9814815 | 0.7260274 | 0.576087 | 0.9964851 | 0.07299181 |
| XGBoost | 0.971 (0.96–0.983) | 0.9216467 | 0.9432624 | 0.8184615 | 0.7228261 | 0.9859402 | 0.06609167 |
| Model | AUC | Accuracy | Precision | F1 Score | Sensitivity (Recall) | Specificity | Brier |
|---|---|---|---|---|---|---|---|
| Logistic regression | 0.757 (0.716–0.798) | 0.7757909 | 0.6380952 | 0.4511785 | 0.3489583 | 0.928972 | 0.1592825 |
| LDA | 0.757 (0.716–0.798) | 0.7675378 | 0.592 | 0.466877 | 0.3854167 | 0.9046729 | 0.160957 |
| KNN | 0.928 (0.91–0.945) | 0.8363136 | 0.792 | 0.6246057 | 0.515625 | 0.9514019 | 0.1050932 |
| Linear SVM | 0.756 (0.715–0.797) | 0.7634113 | 0.578125 | 0.4625 | 0.3854167 | 0.8990654 | 0.1692026 |
| Polynomial SVM | 0.706 (0.657–0.755) | 0.7647868 | 0.8888889 | 0.2191781 | 0.125 | 0.9943925 | 0.1751815 |
| RBF-SVM | 0.735 (0.687–0.782) | 0.786795 | 0.7466667 | 0.4194757 | 0.2916667 | 0.964486 | 0.1577546 |
| Sigmoid SVM | 0.702 (0.658–0.747) | 0.7551582 | 0.5777778 | 0.3687943 | 0.2708333 | 0.928972 | 0.1740828 |
| DT | 0.75 (0.708–0.792) | 0.8115543 | 0.8115543 | 0.8115543 | 0.8115543 | 0.8115543 | 0.145428 |
| RF | 0.981 (0.974–0.988) | 0.9037139 | 1 | 0.7770701 | 0.6354167 | 1 | 0.07283245 |
| XGBoost | 0.972 (0.961–0.982) | 0.9202201 | 0.9294872 | 0.8333333 | 0.7552083 | 0.9794393 | 0.07035735 |
| Predictive Variables | Short Cervix (N = 24) | Normal Cervix (N = 117) | p-Values | |
|---|---|---|---|---|
| General information | Age | |||
| Mean (SD) | 36.6(3.05) | 38.6 (2.00) | 0.018 a | |
| Pre-pregnancy BMI | ||||
| Mean (SD) | 22.4 (2.89) | 21.98 (2.89) | <0.001 a | |
| Gravidity | ||||
| Median [Min, Max] | 2.00 [1.00, 9.00] | 2.00 [1.00, 9.00] | <0.001 b | |
| Parity | ||||
| Median [Min, Max] | 0 [0, 2.00] | 0 [0, 3.00] | 0.002 b | |
| Medical history | Second trimester pregnancy loss * | |||
| Median [Min, Max] | 0.00 [0, 1.00] | 0.00 [0, 1.00] | <0.001b | |
| Laboratory examination | Vaginal microbiological culture (≥105CFU/mL) | |||
| No | 5 (20.8%) | 112 (95.7%) | <0.001 c | |
| Yes | 19 (79.2%) | 5 (4.3%) | ||
| WBC | ||||
| Mean (SD) | 10.17(2.56) | 8.08 (1.45) | 0.002 a |
| Predictive Variables | Short Cervix (N = 308) | Normal Cervix (N = 308) | p-Values | |
|---|---|---|---|---|
| General information | Age | |||
| Mean (SD) | 33.9 (3.40) | 33.8 (3.26) | 0.762 a | |
| Pre-pregnancy BMI | ||||
| Mean (SD) | 23.1 (3.38) | 23.2 (3.56) | 0.575 a | |
| Gravidity | ||||
| Median [Min, Max] | 3.00 [2.00, 9.00] | 3.00 [2.00, 8.00] | 0.602 b | |
| Parity | ||||
| Median [Min, Max] | 0 [0, 3.00] | 0 [0, 3.00] | 0.884 b | |
| Number of full-term deliveries | ||||
| Median [Min, Max] | 0 [0, 3.00] | 0 [0, 3.00] | 0.842 b | |
| Medical history | First trimester pregnancy loss * | |||
| Median [Min, Max] | 1.00 [0, 8.00] | 1.00 [0, 6.00] | 0.821 b | |
| Second trimester pregnancy loss * | ||||
| Median [Min, Max] | 0 [0, 3.00] | 0 [0, 4.00] | 0.642 b | |
| History of preterm birth | ||||
| Median [Min, Max] | 0 [0, 2.00] | 0 [0, 1.00] | 0.883 b | |
| Mode of conception | ||||
| Natural conception | 191 (62.0%) | 197 (64.0%) | 0.718 c | |
| Ovulation induction | 18 (5.8%) | 17 (5.5%) | ||
| IVF-ET | 4 (1.3%) | 6 (1.9%) | ||
| ICSI | 71 (23.1%) | 59 (19.2%) | ||
| PGD | 24 (7.8%) | 29 (9.4%) | ||
| Uterine malformation | ||||
| Normal uterus | 303 (98.4%) | 303 (98.4%) | 1 c | |
| Bicornuate uterus | 0 (0%) | 0 (0%) | ||
| Septate uterus | 5 (1.6%) | 5 (1.6%) | ||
| History of cervical surgery | ||||
| None | 275 (89.3%) | 281 (91.2%) | 0.729 c | |
| Cervical LEEP cone resection | 20 (6.5%) | 17 (5.5%) | ||
| Cervical conization | 13 (4.2%) | 10 (3.2%) | ||
| Times of hysteroscopy | ||||
| Median [Min, Max] | 0 [0, 9.00] | 0 [0, 9.00] | 0.735 b | |
| Electrocautery of cervix | ||||
| No | 307 (99.7%) | 308 (100%) | 1 c | |
| Yes | 1 (0.3%) | 0 (0%) | ||
| Laboratory examination | Vulvovaginal candidiasis | |||
| No | 262 (85.1%) | 259 (84.1%) | 0.824 c | |
| Yes | 46 (14.9%) | 49 (15.9%) | ||
| Trichomonad | ||||
| No | 307 (99.7%) | 308 (100%) | 1 c | |
| Yes | 1 (0.3%) | 0 (0%) | ||
| Mycoplasma | ||||
| No | 233 (75.6%) | 233 (75.6%) | 1 c | |
| Yes | 75 (24.4%) | 75 (24.4%) | ||
| Bacterial vaginosis | ||||
| No | 279 (90.6%) | 272 (88.3%) | 0.432 c | |
| Yes | 29 (9.4%) | 36 (11.7%) | ||
| Vaginal microbiological culture (≥105CFU/mL) | ||||
| No | 224 (72.7%) | 229 (74.4%) | 0.715 c | |
| Yes | 84 (27.3%) | 79 (25.6%) | ||
| WBC | ||||
| Mean (SD) | 8.98 (2.33) | 8.94 (2.38) | 0.836 a | |
| Neutrophil percentage | ||||
| Mean (SD) | 72.1 (6.64) | 72.1 (7.01) | 0.981 a | |
| Absolute neutrophil count | ||||
| Mean (SD) | 6.54 (2.03) | 6.51 (2.05) | 0.884 a | |
| Secondary outcome | sPTB (<34 weeks) | |||
| No | 221 (71.8%) | 295 (95.8%) | <0.001c | |
| Yes | 87 (28.2%) | 13(4.2%) | ||
| sPTB (<37 weeks) | ||||
| No | 191 (62.0%) | 273 (88.6%) | <0.001c | |
| Yes | 117 (38.0%) | 35 (11.4%) |
References
- Mitha, A.; Chen, R.; Razaz, N.; Johansson, S.; Stephansson, O.; Altman, M.; Bolk, J. Neurological development in children born moderately or late preterm: National cohort study. BMJ 2024, 384, e075630. [Google Scholar] [CrossRef]
- Hoffman, M.K.; Clifton, R.G.; Biggio, J.R.; Saade, G.R.; Ugwu, L.G.; Longo, M.; Bousleiman, S.Z.; Clark, K.; Grobman, W.A.; Frey, H.A.; et al. Cervical Pessary for Prevention of Preterm Birth in Individuals With a Short Cervix: The TOPS Randomized Clinical Trial. JAMA 2023, 330, 340–348. [Google Scholar] [CrossRef]
- Iams, J.D.; Goldenberg, R.L.; Meis, P.J.; Mercer, B.M.; Moawad, A.; Das, A.; Thom, E.; McNellis, D.; Copper, R.L.; Johnson, F.; et al. The length of the cervix and the risk of spontaneous premature delivery. N. Engl. J. Med. 1996, 334, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Kuusela, P.; Jacobsson, B.; Hagberg, H.; Fadl, H.; Lindgren, P.; Wesström, J.; Wennerholm, U.B.; Valentin, L. Second-trimester transvaginal ultrasound measurement of cervical length for prediction of preterm birth: A blinded prospective multicentre diagnostic accuracy study. BJOG Int. J. Obstet. Gynaecol. 2021, 128, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Moroz, L.A.; Simhan, H.N. Rate of sonographic cervical shortening and biologic pathways of spontaneous preterm birth. Am. J. Obstet. Gynecol. 2014, 210, 555.e1–555.e5. [Google Scholar] [CrossRef]
- Ward, C.L.; Crouser, S.; Buhimschi, C.S.; Thung, S.F.; Samuels, P.; Lynch, C.D.; Landon, M.B.; Frey, H.A. Evaluation of ‘opt-in’ approach to cervical-length screening for reducing preterm birth. Ultrasound Obstet. Gynecol 2022, 59, 269–270. [Google Scholar] [CrossRef]
- Orzechowski, K.M.; Boelig, R.C.; Baxter, J.K.; Berghella, V. A universal transvaginal cervical length screening program for preterm birth prevention. Obstet. Gynecol. 2014, 124, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Esplin, M.S.; Elovitz, M.A.; Iams, J.D.; Parker, C.B.; Wapner, R.J.; Grobman, W.A.; Simhan, H.N.; Wing, D.A.; Haas, D.M.; Silver, R.M.; et al. Predictive Accuracy of Serial Transvaginal Cervical Lengths and Quantitative Vaginal Fetal Fibronectin Levels for Spontaneous Preterm Birth Among Nulliparous Women. JAMA 2017, 317, 1047–1056. [Google Scholar] [CrossRef]
- Miller, E.S.; Tita, A.T.; Grobman, W.A. Second-Trimester Cervical Length Screening Among Asymptomatic Women: An Evaluation of Risk-Based Strategies. Obstet. Gynecol. 2015, 126, 61–66. [Google Scholar] [CrossRef]
- Boelig, R.C.; Kripalu, V.; Chen, S.L.; Cruz, Y.; Roman, A.; Berghella, V. Utility of follow-up cervical length screening in low-risk women with a cervical length of 26 to 29 mm. Am. J. Obstet. Gynecol. 2021, 225, 179.e1–179.e6. [Google Scholar] [CrossRef]
- Temming, L.A.; Durst, J.K.; Tuuli, M.G.; Stout, M.J.; Dicke, J.M.; Macones, G.A.; Cahill, A.G. Universal cervical length screening: Implementation and outcomes. Am. J. Obstet. Gynecol. 2016, 214, 523.e1–523.e8. [Google Scholar] [CrossRef]
- Moon, I.; LoPiccolo, J.; Baca, S.C.; Sholl, L.M.; Kehl, K.L.; Hassett, M.J.; Liu, D.; Schrag, D.; Gusev, A. Machine learning for genetics-based classification and treatment response prediction in cancer of unknown primary. Nat. Med. 2023, 29, 2057–2067. [Google Scholar] [CrossRef]
- Obermeyer, Z.; Emanuel, E.J. Predicting the Future—Big Data, Machine Learning, and Clinical Medicine. N. Engl. J. Med. 2016, 375, 1216–1219. [Google Scholar] [CrossRef]
- Chernbumroong, S.; Johnson, J.; Gupta, N.; Miller, S.; McCormack, F.X.; Garibaldi, J.M.; Johnson, S.R. Machine learning can predict disease manifestations and outcomes in lymphangioleiomyomatosis. Eur. Respir. J. 2021, 57, 2003036. [Google Scholar] [CrossRef] [PubMed]
- Kindschuh, W.F.; Baldini, F.; Liu, M.C.; Liao, J.; Meydan, Y.; Lee, H.H.; Heinken, A.; Thiele, I.; Thaiss, C.A.; Levy, M.; et al. Preterm birth is associated with xenobiotics and predicted by the vaginal metabolome. Nat. Microbiol. 2023, 8, 246–259. [Google Scholar] [CrossRef] [PubMed]
- Souka, A.P.; Papamihail, M.; Pilalis, A. Very short cervix in low-risk asymptomatic singleton pregnancies: Outcome according to treatment and cervical length at diagnosis. Acta Obstet. Gynecol. Scand. 2020, 99, 1469–1475. [Google Scholar] [CrossRef]
- Fetal Medicine Foundation. Cervical Assessment. Available online: https://fetalmedicine.org/education/cervical-assessment (accessed on 6 August 2025).
- Gudicha, D.W.; Romero, R.; Kabiri, D.; Hernandez-Andrade, E.; Pacora, P.; Erez, O.; Kusanovic, J.P.; Jung, E.; Paredes, C.; Berry, S.M.; et al. Personalized assessment of cervical length improves prediction of spontaneous preterm birth: A standard and a percentile calculator. Am. J. Obstet. Gynecol. 2021, 224, 288.e1–288.e17. [Google Scholar] [CrossRef]
- Park, S.; Moon, J.; Kang, N.; Kim, Y.H.; You, Y.A.; Kwon, E.; Ansari, A.; Hur, Y.M.; Park, T.; Kim, Y.J. Predicting preterm birth through vaginal microbiota, cervical length, and WBC using a machine learning model. Front. Microbiol. 2022, 13, 912853. [Google Scholar] [CrossRef] [PubMed]
- Mitrogiannis, I.; Evangelou, E.; Efthymiou, A.; Kanavos, T.; Birbas, E.; Makrydimas, G.; Papatheodorou, S. Risk factors for preterm birth: An umbrella review of meta-analyses of observational studies. BMC Med. 2023, 21, 494. [Google Scholar] [CrossRef]
- Passos, I.; Britto, R.L. Diagnosis and treatment of müllerian malformations. Taiwan J. Obstet. Gynecol. 2020, 59, 183–188. [Google Scholar] [CrossRef]
- Souka, A.P.; Maritsa, V.; Antsaklis, P.; Pilalis, A.; Daskalakis, G. Cervical length evolution in pregnancy and prediction of preterm delivery. Arch. Gynecol. Obstet. 2024, 310, 2477–2485. [Google Scholar] [CrossRef]
- Basri, N.I.; Dasrilsyah, R.A.; Jamil, A.A.M.; Leong, C.S.Y. Cervical length screening among low-risk women; relationship of body mass index on cervical length and risk of preterm birth. BMC Pregnancy Childbirth 2024, 24, 363. [Google Scholar] [CrossRef]
- Humphries, R.; Bobenchik, A.M.; Hindler, J.A.; Schuetz, A.N. Overview of Changes to the Clinical and Laboratory Standards Institute Performance Standards for Antimicrobial Susceptibility Testing, M100, 31st Edition. J. Clin. Microbiol. 2021, 59, e0021321. [Google Scholar] [CrossRef]
- Bergman, J.J.; Berg, A.O.; Schneeweiss, R.; Heidrich, F.E. Clinical comparison of microscopic and culture techniques in the diagnosis of Candida vaginitis. J. Fam. Pract. 1984, 18, 549–552. [Google Scholar] [PubMed]
- Radonjic, I.V.; Dzamic, A.M.; Mitrovic, S.M.; Arsic Arsenijevic, V.S.; Popadic, D.M.; Kranjcic Zec, I.F. Diagnosis of Trichomonas vaginalis infection: The sensitivities and specificities of microscopy, culture and PCR assay. Eur. J. Obstet. Gynecol. Reprod. Biol. 2006, 126, 116–120. [Google Scholar] [CrossRef]
- Wroblewski, J.K.; Manhart, L.E.; Dickey, K.A.; Hudspeth, M.K.; Totten, P.A. Comparison of transcription-mediated amplification and PCR assay results for various genital specimen types for detection of Mycoplasma genitalium. J. Clin. Microbiol. 2006, 44, 3306–3312. [Google Scholar] [CrossRef] [PubMed]
- Priestley, C.J.; Kinghorn, G.R. Bacterial vaginosis. Br. J. Clin. Pract. 1996, 50, 331–334. [Google Scholar] [CrossRef]
- Zhang, Y.; Qian, Y.; Liu, C.; Fan, X.; Li, X.; Song, Y.; Fan, Y.; Hu, Z.; Yang, J. Association between white blood cell count and adverse pregnancy outcomes: A retrospective cohort study from a tertiary hospital in China. BMJ Open 2023, 13, e072633. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, K.K.; Cantonwine, D.E.; Ferguson, K.; Arjona, M.; Meeker, J.D.; McElrath, T.F. Inflammatory and oxidative stress markers associated with decreased cervical length in pregnancy. Am. J. Reprod. Immunol. 2016, 76, 376–382. [Google Scholar] [CrossRef]
- Pudjihartono, N.; Fadason, T.; Kempa-Liehr, A.W.; O’Sullivan, J.M. A Review of Feature Selection Methods for Machine Learning-Based Disease Risk Prediction. Front. Bioinform. 2022, 2, 927312. [Google Scholar] [CrossRef]
- Kohavi, R.; John, G.H. Wrappers for feature subset selection. Artif. Intell. 1997, 97, 273–324. [Google Scholar] [CrossRef]
- Chawla, N.V.; Bowyer, K.W.; Hall, L.O.; Kegelmeyer, W.P. SMOTE: Synthetic minority over-sampling technique. J. Artif. Intell. Res. 2002, 16, 321–357. [Google Scholar] [CrossRef]
- Lundberg, S.M.; Lee, S.I. A Unified Approach to Interpreting Model Predictions. Adv. Neural Inf. Process. Syst. 2017, 30. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, K.; Zhang, J.; Zhang, Y.; Xia, Z.; Wang, Y.; Fan, X.; Mu, X.; Xu, L.; Xiong, C.; et al. Association of gestational weight gain patterns with preterm birth subtypes in a population based cohort study from China. Sci. Rep. 2025, 15, 23324. [Google Scholar] [CrossRef]
- Sağlam, E.; Özler, M.R.; Yılmaz, E.B.S.; Yılmaz, S.; Bucak, M. Predictive value of inflammatory indices for disease severity and perinatal outcomes in intrahepatic cholestasis of pregnancy. BMC Pediatr. 2025, 25, 483. [Google Scholar] [CrossRef]
- Chun, R.P.C.; Chan, H.G.; Lim, G.Y.S.; Kanagalingam, D.; Partana, P.; Tan, K.H.; Teoh, T.G.; Tan, I. Preterm birth trends and risk factors in a multi-ethnic Asian population: A retrospective study from 2017 to 2023, can we screen and predict this? Ann. Acad. Med. Singap. 2025, 54, 296–304. [Google Scholar] [CrossRef]
- Marquart, K.G.F.; Silva, T.V.; Mol, B.W.; Cecatti, J.G.; Passini, R., Jr.; Pereira, C.M.; Guedes, T.B.; Fanton, T.F.; Pacagnella, R.C. Cervical length distribution among Brazilian pregnant population and risk factors for short cervix: A multicenter cross-sectional study. PLoS ONE 2022, 17, e0272128. [Google Scholar] [CrossRef]
- Panagiotopoulos, M.; Pergialiotis, V.; Trimmi, K.; Varthaliti, A.; Koutras, A.; Antsaklis, P.; Daskalakis, G. Differences in cervical length during the second trimester among normal weight, overweight and obese women: A systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. X 2024, 21, 100291. [Google Scholar] [CrossRef]
- Soto-Torres, E.E.; Hernandez-Andrade, E.; Huntley, E.S.; Blackwell, S.C. Maternal and obstetrical factors associated with short cervical length at midtrimester in women with no history of preterm delivery. J. Matern. Fetal Neonatal Med. 2023, 36, 2228448. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Li, S.; Gong, X.; Li, J.; Li, X.; Zhai, Y.; Huang, J.; Li, X.; Li, L.; Yang, J.; et al. Longitudinal Cervical Length Measurements and Spontaneous Preterm Birth in Singleton and Twin Pregnancies. JAMA Netw. Open 2024, 7, e244592. [Google Scholar] [CrossRef] [PubMed]
- Donders, G.G.; Van Calsteren, C.; Bellen, G.; Reybrouck, R.; Van den Bosch, T.; Riphagen, I.; Van Lierde, S. Association between abnormal vaginal flora and cervical length as risk factors for preterm birth. Ultrasound Obstet. Gynecol. 2010. [Google Scholar] [CrossRef] [PubMed]
- Care, A.; Nevitt, S.J.; Medley, N.; Donegan, S.; Good, L.; Hampson, L.; Tudur Smith, C.; Alfirevic, Z. Interventions to prevent spontaneous preterm birth in women with singleton pregnancy who are at high risk: Systematic review and network meta-analysis. BMJ 2022, 376, e064547. [Google Scholar] [CrossRef]
- Patel, K.; Pirie, D.; Heazell, A.E.P.; Morgan, B.; Woolner, A. Subsequent pregnancy outcomes after second trimester miscarriage or termination for medical/fetal reason: A systematic review and meta-analysis of observational studies. Acta Obstet. Gynecol. Scand. 2024, 103, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.; Motiwale, T.; Barney, O.; Dudbridge, F.; McParland, P.C.; Moss, E.L. Impact of past obstetric history and cervical excision on preterm birth rate. Acta Obstet. Gynecol. Scand. 2021, 100, 1995–2002. [Google Scholar] [CrossRef] [PubMed]


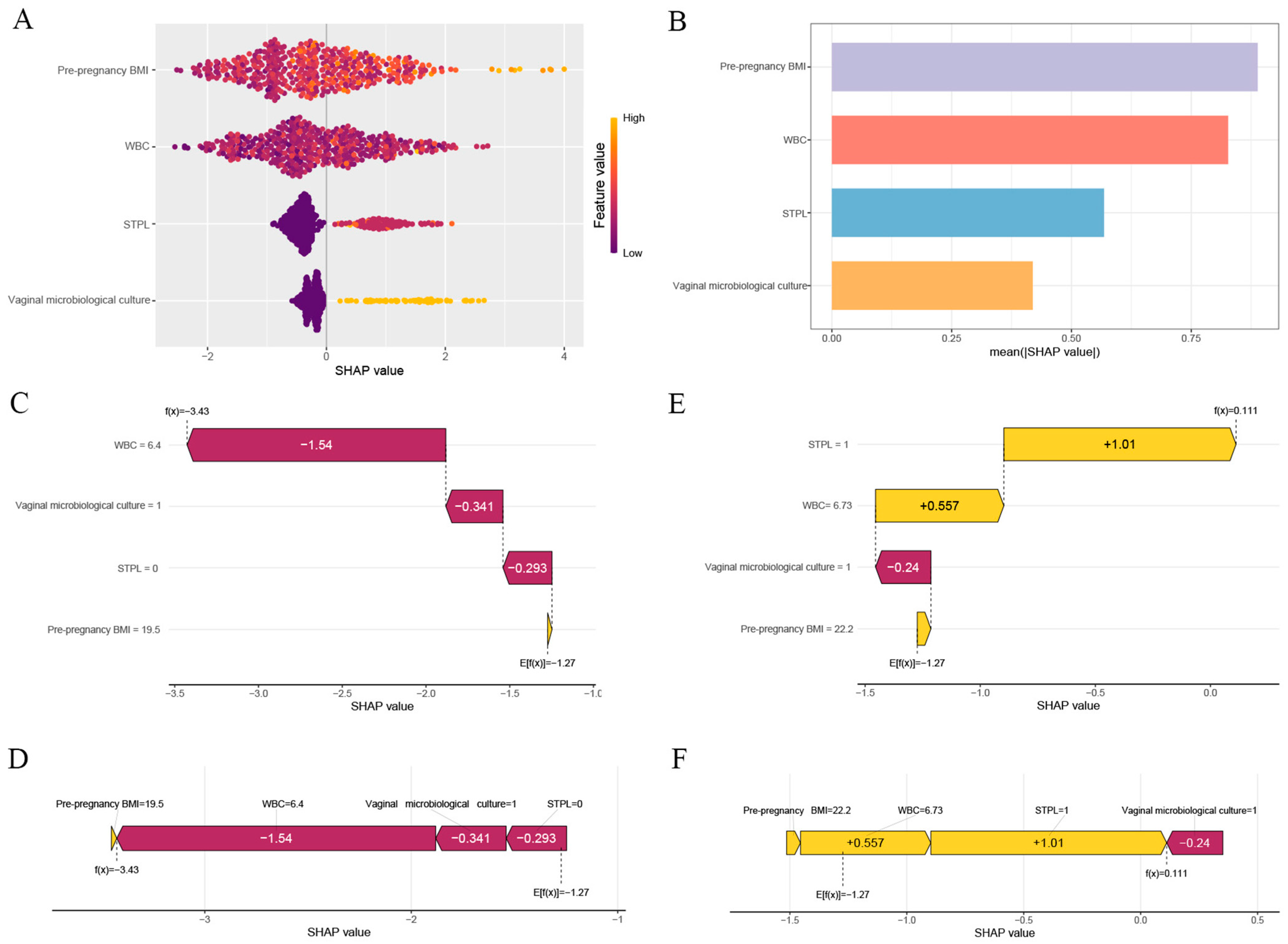
| Predictive Variables | Short Cervix (N = 376) | Normal Cervix (N = 1104) | p-Values | |
|---|---|---|---|---|
| General information | Age | |||
| Mean (SD) | 33.8 (3.37) | 33.9 (3.42) | 0.484 a | |
| Pre-pregnancy BMI | ||||
| Mean (SD) | 23.5 (3.61) | 22.0 (3.14) | <0.001 a | |
| Gravidity | ||||
| Median [Min, Max] | 3.00 [2.00, 9.00] | 3.00 [2.00, 9.00] | 0.021 b | |
| Parity | ||||
| Median [Min, Max] | 0 [0, 3.00] | 0 [0, 3.00] | 0.623 b | |
| Number of full-term deliveries | ||||
| Median [Min, Max] | 0 [0, 3.00] | 0 [0, 3.00] | 0.005 b | |
| Medical history | First trimester pregnancy loss (FTPL) * | |||
| Median [Min, Max] | 1.00 [0, 8.00] | 1.00 [0, 8.00] | 0.17 b | |
| Second trimester pregnancy loss (STPL) * | ||||
| Median [Min, Max] | 0 [0, 7.00] | 0 [0, 4.00] | <0.001 b | |
| History of preterm birth | ||||
| Median [Min, Max] | 0 [0, 2.00] | 0 [0, 1.00] | 0.015 b | |
| Mode of conception | ||||
| Natural conception | 230 (61.2%) | 732 (66.3%) | <0.001 c | |
| Ovulation induction | 26 (6.9%) | 27 (2.4%) | ||
| IVF-ET | 8 (2.1%) | 9 (0.8%) | ||
| ICSI | 84 (22.3%) | 218 (19.7%) | ||
| PGD | 28 (7.4%) | 118 (10.7%) | ||
| Uterine malformation | ||||
| Normal uterus | 369 (98.1%) | 1089 (98.6%) | 0.148 c | |
| Bicornuate uterus | 0 (0%) | 5 (0.5%) | ||
| Septate uterus | 7 (1.9%) | 10 (0.9%) | ||
| History of cervical surgery | ||||
| None | 963 (87.2%) | 334 (88.8%) | 0.015 c | |
| Cervical LEEP cone resection | 109 (9.9%) | 23 (6.1%) | ||
| Cervical conization | 32 (2.9%) | 19 (5.1%) | ||
| Times of hysteroscopy | ||||
| Mean (SD) | 0.601 (1.22) | 0.544 (0.988) | 0.415 b | |
| Median [Min, Max] | 0 [0, 9.00] | 0 [0, 9.00] | ||
| Electrocautery of cervix | ||||
| No | 1098 (99.5%) | 375 (99.7%) | 0.686 c | |
| Yes | 6 (0.5%) | 1 (0.3%) | ||
| Laboratory examination | Vulvovaginal candidiasis | |||
| No | 305 (81.1%) | 1016 (92.0%) | <0.001 c | |
| Yes | 71 (18.9%) | 88 (8.0%) | ||
| Trichomonad | ||||
| No | 375 (99.7%) | 1103 (99.9%) | 0.444 c | |
| Yes | 1 (0.3%) | 1 (0.1%) | ||
| Mycoplasma | ||||
| No | 276 (73.4%) | 866 (78.4%) | 0.047 c | |
| Yes | 100 (26.6%) | 238 (21.6%) | ||
| Bacterial vaginosis | ||||
| No | 336 (89.4%) | 1010 (91.5%) | 0.213 c | |
| Yes | 40 (10.6%) | 94 (8.5%) | ||
| Vaginal microbiological culture (≥105CFU/mL) | ||||
| No | 242 (64.4%) | 1002 (90.8%) | <0.001 c | |
| Yes | 134 (35.6%) | 102 (9.2%) | ||
| WBC | ||||
| Mean (SD) | 9.15 (2.38) | 8.60 (2.15) | <0.001 a | |
| Neutrophil percentage | ||||
| Mean (SD) | 72.3 (6.60) | 71.8 (6.67) | 0.258 a | |
| Absolute neutrophil count | ||||
| Mean (SD) | 6.67 (2.07) | 6.23 (1.85) | <0.001 a | |
| Secondary outcome | sPTB (<34 weeks) | |||
| No | 257 (68.4%) | 1072 (97.1%) | <0.001 c | |
| Yes | 119 (31.6%) | 32 (2.9%) | ||
| sPTB (<37 weeks) | ||||
| No | 221 (58.8%) | 997 (90.3%) | <0.001 c | |
| Yes | 155 (41.2%) | 107 (9.7%) |
| Model | Accuracy | Precision | F1 Score | Sensitivity (Recall) | Specificity | Brier |
|---|---|---|---|---|---|---|
| Logistic regression | 0.6831169 | 0.7288961 | 0.6479076 | 0.5831169 | 0.7831169 | 0.2086745 |
| LDA | 0.6818182 | 0.708559 | 0.6391753 | 0.5636364 | 0.8 | 0.2090479 |
| KNN | 0.8272727 | 0.8173804 | 0.8299233 | 0.8428571 | 0.8116883 | 0.1185919 |
| Linear SVM | 0.6831169 | 0.7085799 | 0.6625173 | 0.6220779 | 0.7441558 | 0.2184744 |
| Polynomial SVM | 0.6798701 | 0.6520307 | 0.7067222 | 0.7714286 | 0.5883117 | 0.2038918 |
| RBF-SVM | 0.696104 | 0.732308 | 0.670423 | 0.618182 | 0.774026 | 0.2037133 |
| Sigmoid SVM | 0.672727 | 0.685237 | 0.66129 | 0.638961 | 0.706494 | 0.215311 |
| DT | 0.701299 | 0.760943 | 0.662757 | 0.587013 | 0.815584 | 0.2057295 |
| RF | 0.792208 | 0.901786 | 0.759399 | 0.655844 | 0.928571 | 0.1248432 |
| XGBoost | 0.849351 | 0.857713 | 0.847569 | 0.837662 | 0.861039 | 0.1153128 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Dong, J.; Shi, J.; Qu, X.; Bao, Y.; Mao, X.; Lv, M.; Chen, X.; Ying, H. Machine Learning Prediction of Short Cervix in Mid-Pregnancy Based on Multimodal Data from the First-Trimester Screening Period: An Observational Study in a High-Risk Population. Biomedicines 2025, 13, 2057. https://doi.org/10.3390/biomedicines13092057
Wu S, Dong J, Shi J, Qu X, Bao Y, Mao X, Lv M, Chen X, Ying H. Machine Learning Prediction of Short Cervix in Mid-Pregnancy Based on Multimodal Data from the First-Trimester Screening Period: An Observational Study in a High-Risk Population. Biomedicines. 2025; 13(9):2057. https://doi.org/10.3390/biomedicines13092057
Chicago/Turabian StyleWu, Shengyu, Jiaqi Dong, Jifan Shi, Xiaoxian Qu, Yirong Bao, Xiaoyuan Mao, Mu Lv, Xuan Chen, and Hao Ying. 2025. "Machine Learning Prediction of Short Cervix in Mid-Pregnancy Based on Multimodal Data from the First-Trimester Screening Period: An Observational Study in a High-Risk Population" Biomedicines 13, no. 9: 2057. https://doi.org/10.3390/biomedicines13092057
APA StyleWu, S., Dong, J., Shi, J., Qu, X., Bao, Y., Mao, X., Lv, M., Chen, X., & Ying, H. (2025). Machine Learning Prediction of Short Cervix in Mid-Pregnancy Based on Multimodal Data from the First-Trimester Screening Period: An Observational Study in a High-Risk Population. Biomedicines, 13(9), 2057. https://doi.org/10.3390/biomedicines13092057







