Dual Protective Effects of Postbiotics and Cichorium intybus L. Mixture in the Sarcopenic and Inflammatory Models
Abstract
1. Introduction
2. Materials and Methods
2.1. DuoX Formulation
2.2. Establishment of the Cellular Sarcopenia and Inflammation Models
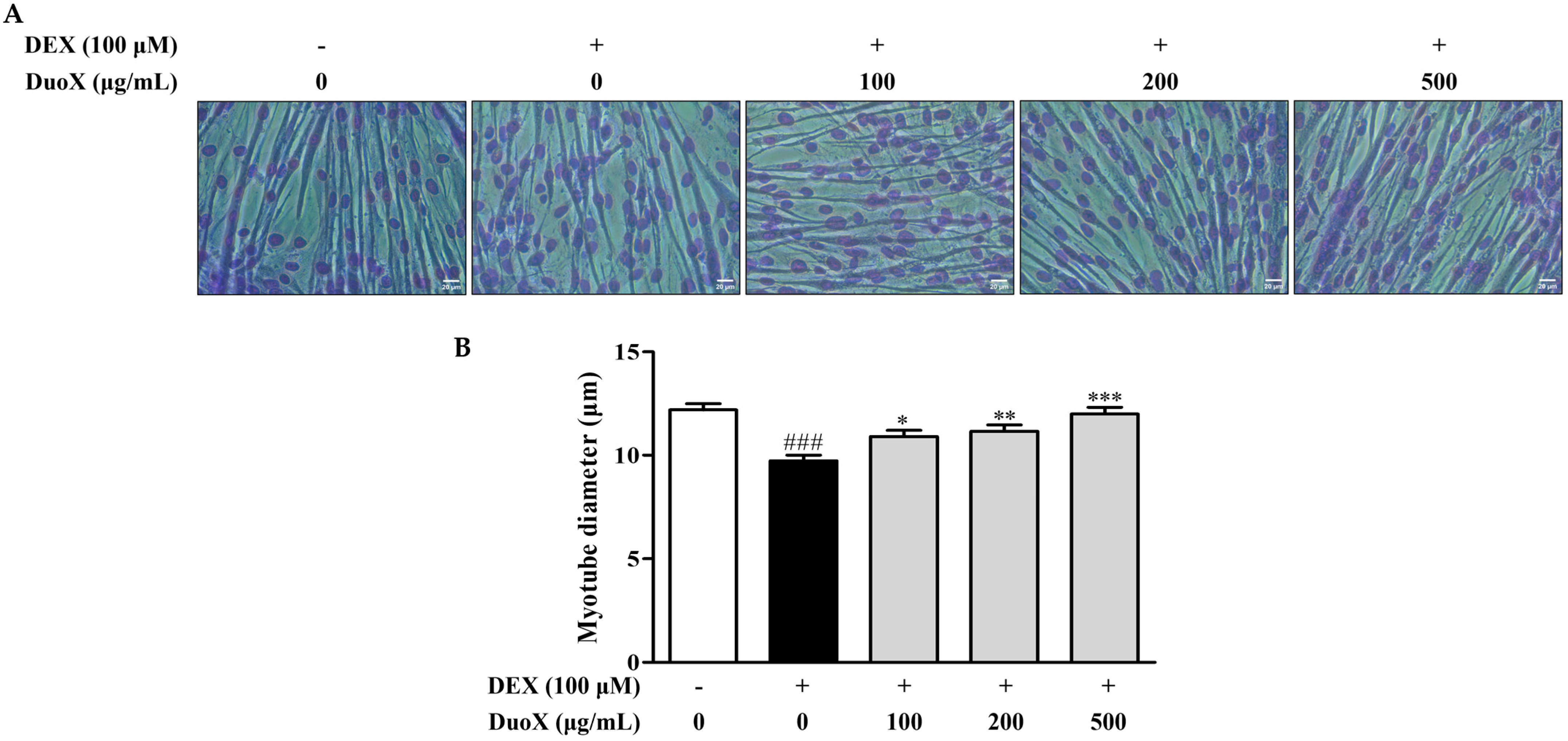
2.3. Measurements of Myotube Diameters
2.4. Animal Experiments
2.5. Grip Strength Test
2.6. Nitric Oxide (NO) Assay
2.7. Protein Extraction and Western Blotting
2.8. Statistical Analysis
3. Results
3.1. DuoX Increases the Dexamethasone-Induced Reduction in Myotube Diameter in C2C12 Cells
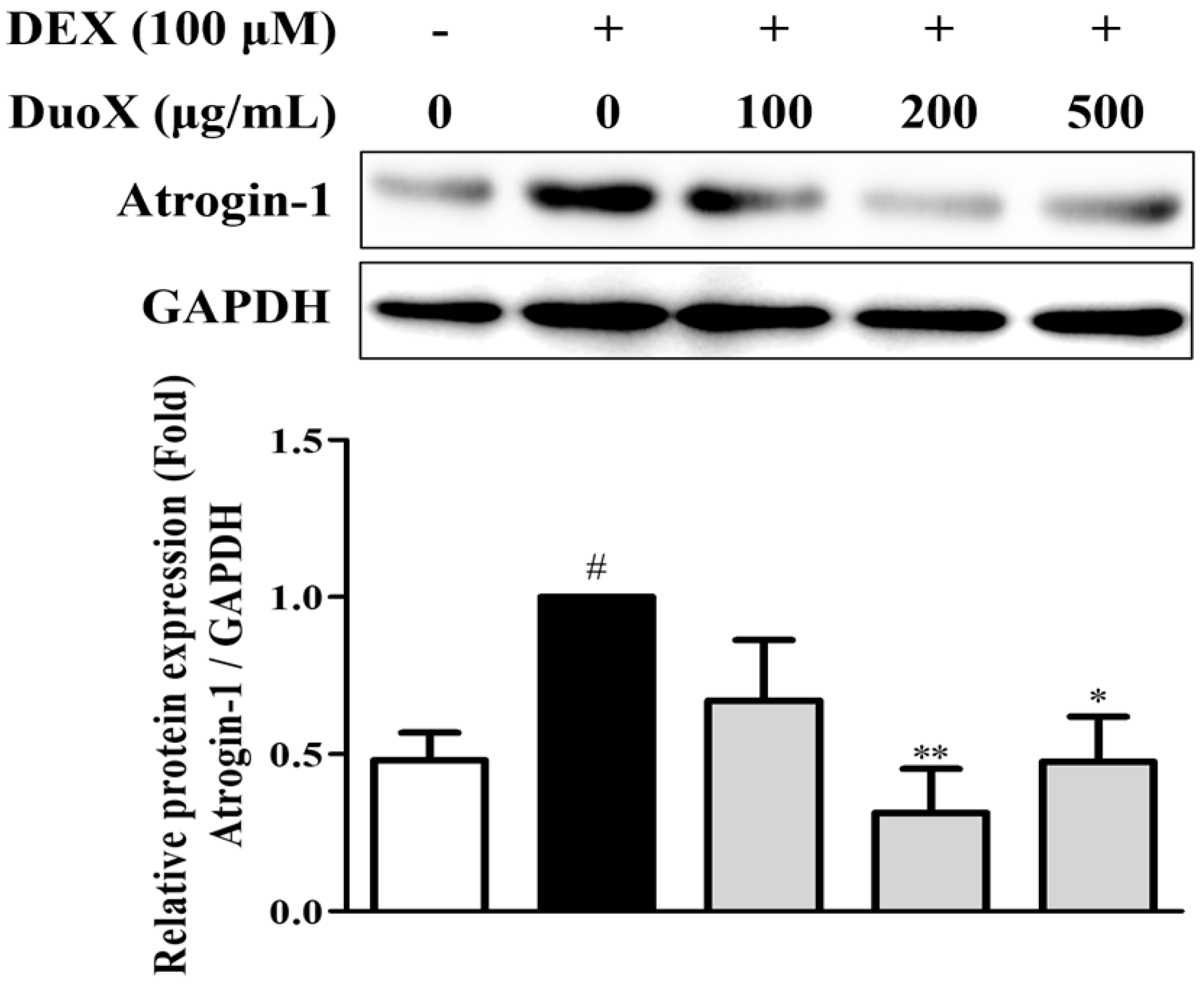
3.2. DuoX Decreases Atrogin-1 Protein Expression in Dexamethasone-Induced C2C12 Cells
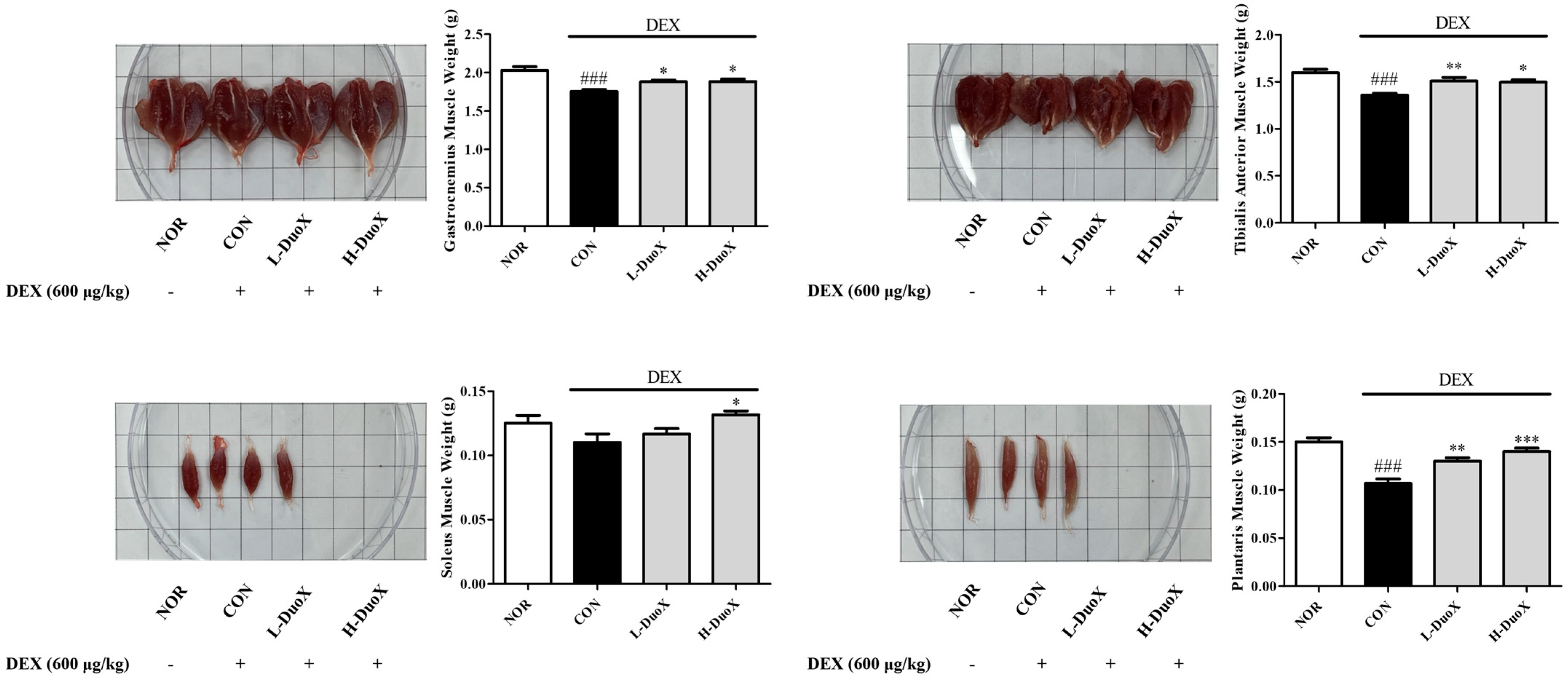
3.3. DuoX Prevents Muscle Weight Loss in a Rat Model of Dexamethasone-Induced Sarcopenia
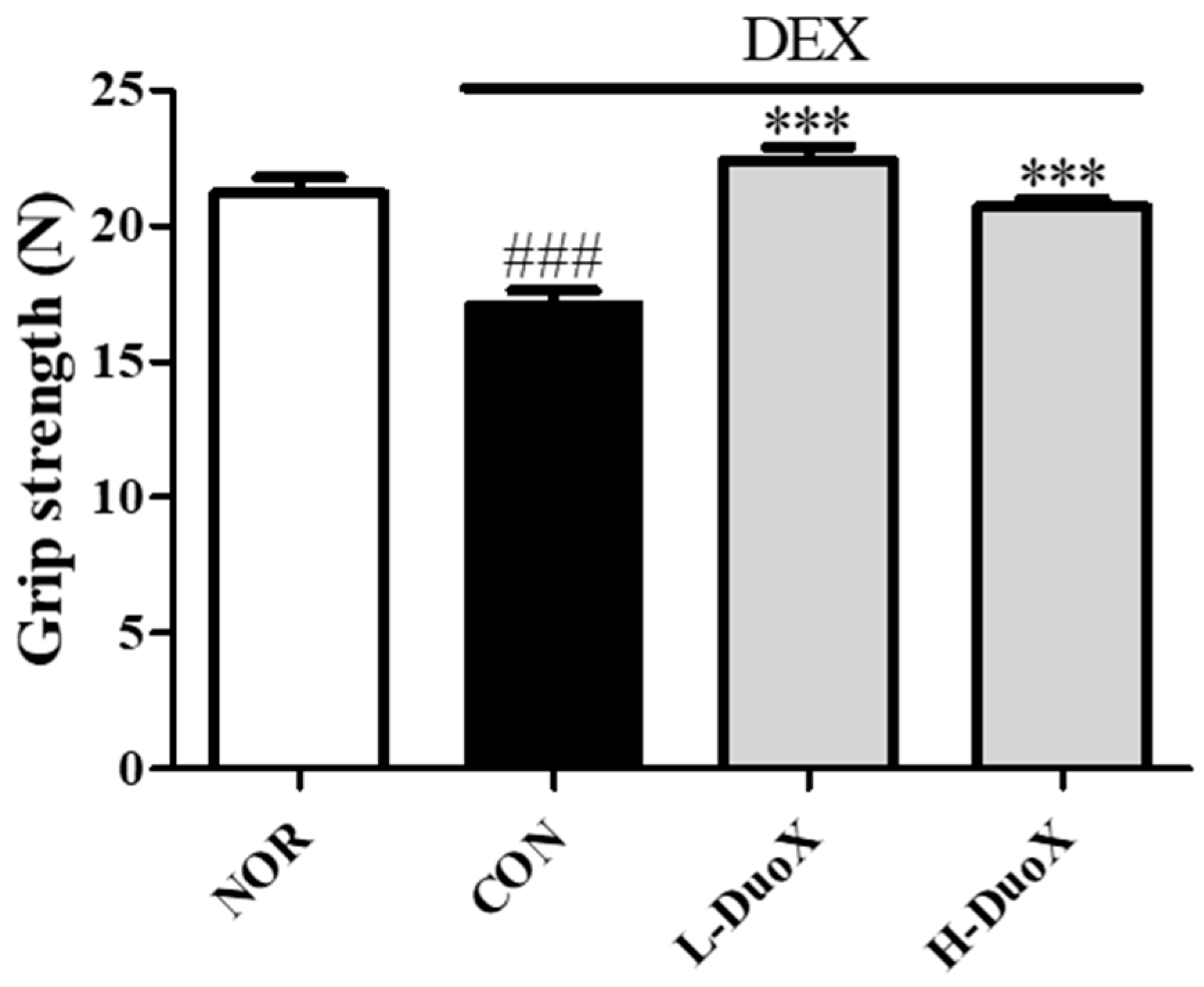
3.4. DuoX Improves Grip Strength in the Rat Model of Dexamethasone-Induced Sarcopenia
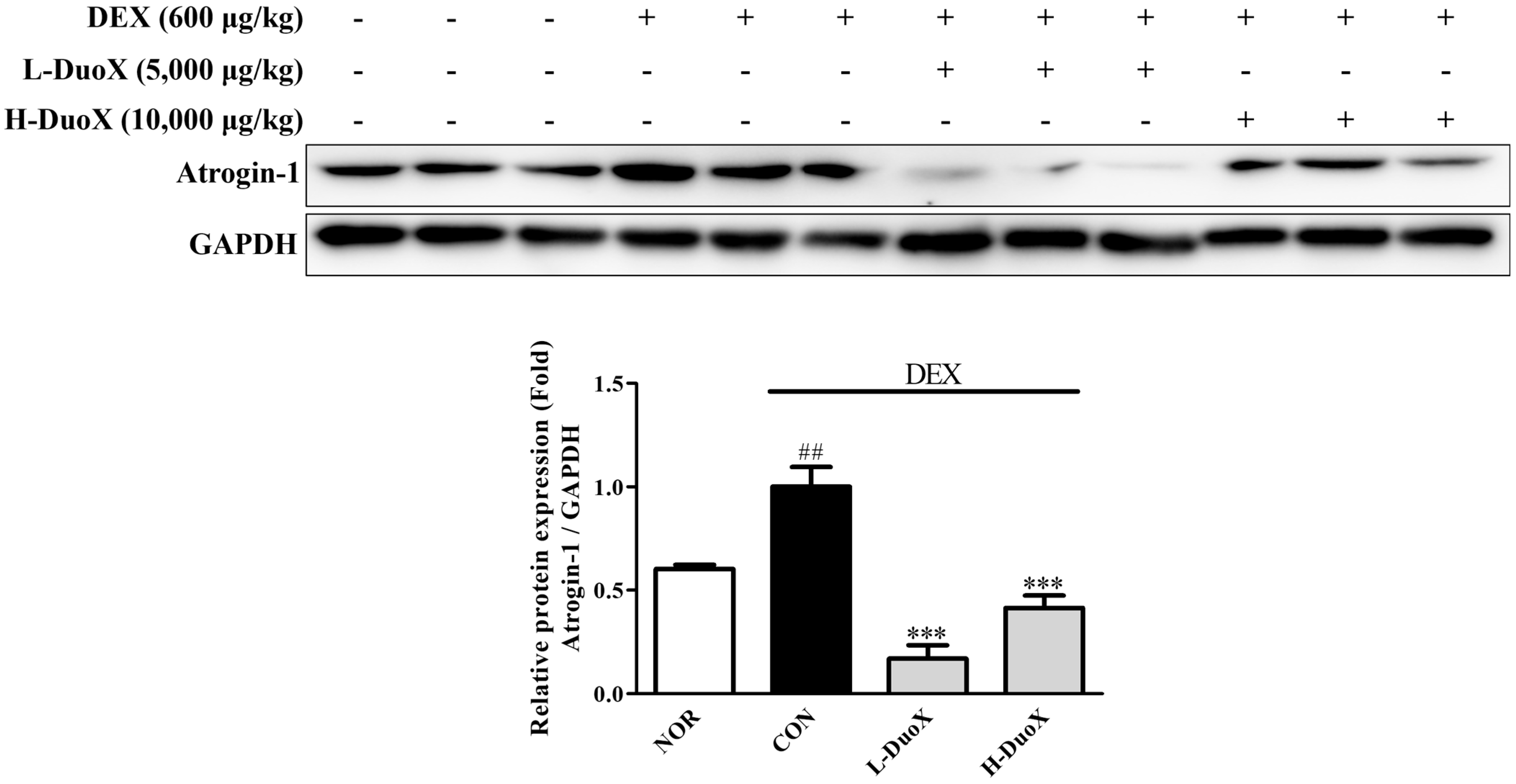
3.5. DuoX Decreases Atrogin-1 Protein Expression in the Rat Model of Dexamethasone-Induced Sarcopenia
3.6. DuoX Decreases NO Production in LPS-Induced RAW 264.7 Cells
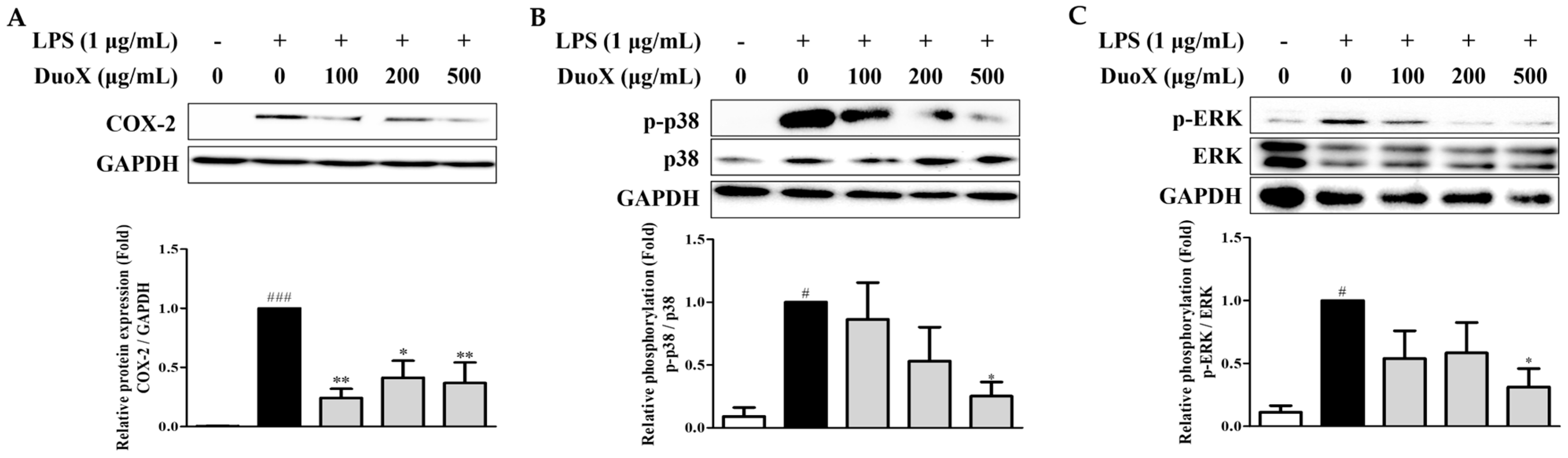
3.7. DuoX Decreases COX-2 Protein Expression and MAPK Pathway Phosphorylation in LPS-Induced RAW 264.7 Cells
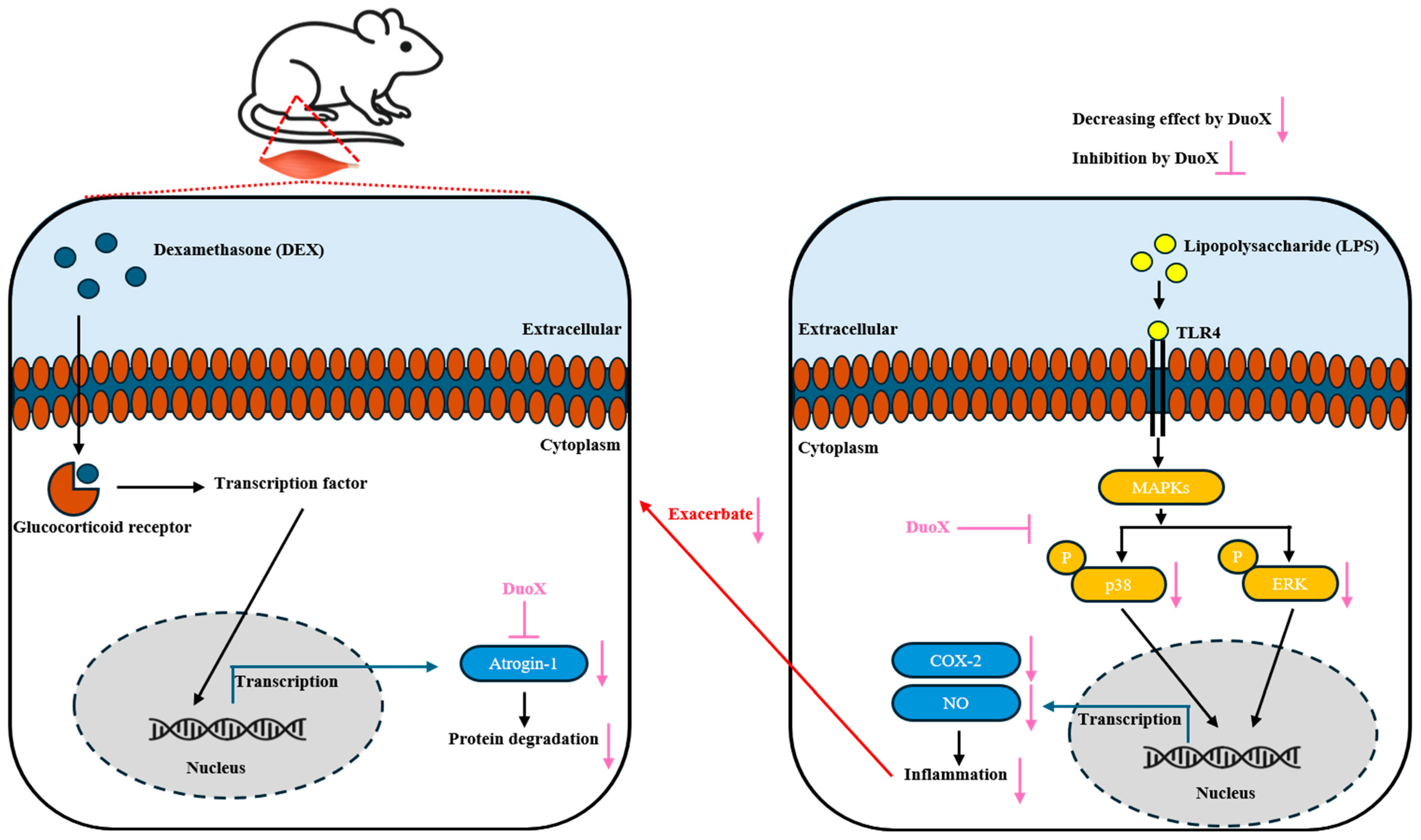
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| COX-2 | Cyclooxygenase-2 |
| DEX | Dexamethasone |
| DMEM | Dulbecco’s modified Eagle’s medium |
| DW | Distilled water |
| ERK | Extracellular signal-regulated kinase |
| FBS | Fetal bovine serum |
| FDA | Food and Drug Administration |
| GA | Gastrocnemius |
| LPS | Lipopolysaccharide |
| MAPK | Mitogen-activated protein kinase |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NO | Nitric oxide |
| PBS | Phosphate-buffered saline |
| PLA | Plantaris |
| SOL | Soleus |
| TA | Tibialis anterior |
| UPS | Ubiquitin-proteasome system |
References
- Crimmins, E.M. Lifespan and healthspan: Past, present, and promise. Gerontologist 2015, 55, 901–911. [Google Scholar] [CrossRef]
- Martinez, R.; Morsch, P.; Soliz, P.; Hommes, C.; Ordunez, P.; Vega, E. Life expectancy, healthy life expectancy, and burden of disease in older people in the Americas, 1990–2019: A population-based study. Rev. Panam. Salud Pública 2021, 45, e114. [Google Scholar] [CrossRef]
- Najm, A.; Niculescu, A.G.; Grumezescu, A.M.; Beuran, M. Emerging therapeutic strategies in sarcopenia: An updated review on pathogenesis and treatment advances. Int. J. Mol. Sci. 2024, 25, 4300. [Google Scholar] [CrossRef]
- Santilli, V.; Bernetti, A.; Mangone, M.; Paoloni, M. Clinical definition of sarcopenia. Clin. Cases Miner. Bone Metab. 2014, 11, 177. [Google Scholar] [CrossRef] [PubMed]
- Dodds, R.M.; Syddall, H.E.; Cooper, R.; Benzeval, M.; Deary, I.J.; Dennison, E.M.; Der, G.; Gale, C.R.; Inskip, H.M.; Jagger, C. Grip strength across the life course: Normative data from twelve British studies. PLoS ONE 2014, 9, e113637. [Google Scholar] [CrossRef]
- Mitchell, W.K.; Williams, J.; Atherton, P.; Larvin, M.; Lund, J.; Narici, M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front. Physiol. 2012, 3, 260. [Google Scholar] [CrossRef]
- González-Blanco, L.; Bermúdez, M.; Bermejo-Millo, J.C.; Gutiérrez-Rodríguez, J.; Solano, J.J.; Antuña, E.; Menéndez-Valle, I.; Caballero, B.; Vega-Naredo, I.; Potes, I.; et al. Cell interactome in sarcopenia during aging. J. Cachexia Sarcopenia Muscle 2022, 13, 919–931. [Google Scholar] [CrossRef] [PubMed]
- Clavel, S.; Coldefy, A.S.; Kurkdjian, E.; Salles, J.; Margaritis, I.; Derijard, B. Atrophy-related ubiquitin ligases, atrogin-1 and MuRF1 are up-regulated in aged rat Tibialis Anterior muscle. Mech. Ageing Dev. 2006, 127, 794–801. [Google Scholar] [CrossRef]
- Gumucio, J.P.; Mendias, C.L. Atrogin-1, MuRF-1, and sarcopenia. Endocrine 2013, 43, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Garagnani, P.; Vitale, G.; Capri, M.; Salvioli, S. Inflammaging and ‘Garb-aging’. Trends Endocrinol. Metab. 2017, 28, 199–212. [Google Scholar] [CrossRef]
- Pugin, J. How tissue injury alarms the immune system and causes a systemic inflammatory response syndrome. Ann. Intensive Care 2012, 2, 27. [Google Scholar] [CrossRef]
- Byars, S.G.; Voskarides, K. Antagonistic pleiotropy in human disease. J. Mol. Evol. 2020, 88, 12–25. [Google Scholar] [CrossRef]
- Moens, U.; Kostenko, S.; Sveinbjørnsson, B. The role of mitogen-activated protein kinase-activated protein kinases (MAPKAPKs) in inflammation. Genes 2013, 4, 101–133. [Google Scholar] [CrossRef]
- Ci, X.; Li, H.; Yu, Q.; Zhang, X.; Yu, L.; Chen, N.; Song, Y.; Deng, X. Avermectin exerts anti-inflammatory effect by down-regulating the nuclear transcription factor kappa-B and mitogen-activated protein kinase activation pathway. Fundam. Clin. Pharmacol. 2009, 23, 449–455. [Google Scholar] [CrossRef]
- Binder, E.; Bermúdez-Silva, F.J.; André, C.; Elie, M.; Romero-Zerbo, S.Y.; Leste-Lasserre, T.; Belluomo l Duchampt, A.; Clark, S.; Aubert, A. Leucine supplementation protects from insulin resistance by regulating adiposity levels. PLoS ONE 2013, 8, e74705. [Google Scholar] [CrossRef]
- Schakman, O.; Dehoux, M.; Bouchuari, S.; Delaere, S.; Lause, P.; Decroly, N.; Shoelson, S.E.; Thissen, J.-P. Role of IGF-I and the TNFα/NF-κB pathway in the induction of muscle atrogenes by acute inflammation. Am. J. Physiol.-Endocrinol. Metab. 2012, 303, E729–E739. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, A.E.; Chapman, K.E. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol. Cell. Endocrinol. 2011, 335, 2–13. [Google Scholar] [CrossRef]
- Schakman, O.; Kalista, S.; Barbé, C.; Loumaye, A.; Thissen, J.P. Glucocorticoid-induced skeletal muscle atrophy. Int. J. Biochem. Cell Biol. 2013, 45, 2163–2172. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.Y.H.; Hsiao, A.W.T.; Wong, N.; Chen, Y.F.; Lee, C.W.; Lee, W.Y.W. Is dexamethasone-induced muscle atrophy an alternative model for naturally aged sarcopenia model? J. Orthop. Transl. 2023, 39, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Tucureanu, M.M.; Rebleanu, D.; Constantinescu, C.A.; Deleanu, M.; Voicu, G.; Butoi, E.; Calin, M.; Manduteanu, I. Lipopolysaccharide-induced inflammation in monocytes/macrophages is blocked by liposomal delivery of Gi-protein in-hibitor. Int. J. Nanomed. 2018, 13, 63–76. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef]
- Hankittichai, P.; Buacheen, P.; Pitchakarn, P.; Na Takuathung, M.; Wikan, N.; Smith, D.R.; Potikanond, S.; Nimlamool, W. Artocarpus lakoocha extract inhibits LPS-induced inflammatory response in RAW 264.7 macrophage cells. Int. J. Mol. Sci. 2020, 21, 1355. [Google Scholar] [CrossRef]
- Yang, G.; Lee, K.; Lee, M.; Ham, I.; Choi, H.-Y. Inhibition of lipopolysaccharide-induced nitric oxide and prosta-glandin E 2 production by chloroform fraction of Cudrania tricuspidata in RAW 264.7 macrophages. BMC Complement. Altern. Med. 2012, 12, 250. [Google Scholar] [CrossRef]
- Maldonado, R.F.; Sá-Correia, I.; Valvano, M.A. Lipopolysaccharide modification in Gram-negative bacteria during chronic infection. FEMS Microbiol. Rev. 2016, 40, 480–493. [Google Scholar] [CrossRef] [PubMed]
- Park, B.S.; Lee, J.O. Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp. Mol. Med. 2013, 45, e66. [Google Scholar] [CrossRef]
- Scott, E.; De Paepe, K.; Van de Wiele, T. Postbiotics and their health modulatory biomolecules. Biomolecules 2022, 12, 1640. [Google Scholar] [CrossRef] [PubMed]
- Proestos, C. The benefits of plant extracts for human health. Foods 2020, 9, 1653. [Google Scholar] [CrossRef]
- Vinderola, G.; Sanders, M.E.; Salminen, S. The Concept of Postbiotics. Foods 2022, 11, 1077. [Google Scholar] [CrossRef] [PubMed]
- Żółkiewicz, J.; Marzec, A.; Ruszczyński, M.; Feleszko, W. Postbiotics—A Step Beyond Pre- and Probiotics. Nutrients 2020, 12, 2189. [Google Scholar] [CrossRef]
- Jeong, Y.-J.; Kim, J.-H.; Jung, Y.-J.; Kwak, M.-S.; Sung, M.-H.; Imm, J.-Y. KL-Biome (Postbiotic Formulation of Lactiplantibacillus plantarum KM2) Improves dexamethasone-induced muscle atrophy in mice. Int. J. Mol. Sci. 2024, 25, 7499. [Google Scholar] [CrossRef]
- Han, S.; Seo, K.-H.; Lee, H.G.; Kim, H. Effect of Cucumis melo L. peel extract supplemented postbiotics on reprograming gut microbiota and sarcopenia in hindlimb-immobilized mice. Food Res. Int. 2023, 173, 113476. [Google Scholar] [CrossRef]
- Bueno, E.B.T.; Silva, K.d.O.; Mendes, M.E.F.; de Oliveira, L.B.; Menezes, F.P.d.; Imperador, A.C.; Correia, L.F.; Winkelstroter, L.K. Postbiotics Derived from Lactic Acid Bacteria Fermentation: Therapeutic Potential in the Treatment of Muscular Complications in Inflammatory Bowel Disease. Fermentation 2025, 11, 362. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, X.; Liu, X.; Chen, D.; Wang, M.; Jiang, X.; Xiong, Z. The roles of the gut microbiota and chronic low-grade inflammation in older adults with frailty. Front. Cell. Infect. Microbiol. 2021, 11, 675414. [Google Scholar] [CrossRef]
- Li, K.; Zhang, L.; Xue, J.; Yang, X.; Dong, X.; Sha, L.; Lei, H.; Zhang, X.; Zhu, L.; Wang, Z. Dietary inulin alleviates diverse stages of type 2 diabetes mellitus via anti-inflammation and modulating gut microbiota in db/db mice. Food Funct. 2019, 10, 1915–1927. [Google Scholar] [CrossRef]
- Zou, Y.-F.; Li, C.-Y.; Fu, Y.-P.; Feng, X.; Peng, X.; Feng, B.; Li, L.-X.; Jia, R.-Y.; Huang, C.; Song, X. Restorative effects of inulin from codonopsis pilosula on intestinal mucosal immunity, anti-inflammatory activity and gut microbiota of immunosuppressed mice. Front. Pharmacol. 2022, 13, 786141. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Xiao, P.; Zhang, X.; Yang, Y.; Yang, M.; Wang, T.; Lu, H.; Tian, H.; Wang, H.; Liu, J. Inulin ameliorates schizophrenia via modulation of the gut microbiota and anti-inflammation in mice. Food Funct. 2021, 12, 1156–1175. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Jeong, E.; Park, H.; Song, H.-Y.; Moon, J.; Kim, M.-A.; Koo, B.S.; Lee, J.-H.; Hong, J.K.; Han, K.-I.; et al. Heat-Killed Lactobacillus plantarum beLP1 Attenuates Dexamethasone-Induced Sarcopenia in Rats by Increasing AKT Phosphorylation. Biomedicines 2025, 13, 1668. [Google Scholar] [CrossRef] [PubMed]
- Farabegoli, F.; Santaclara, F.J.; Costas, D.; Alonso, M.; Abril, A.G.; Espiñeira, M.; Ortea, I.; Costas, C. Exploring the anti-inflammatory effect of inulin by integrating transcriptomic and proteomic analyses in a murine macrophage cell model. Nutrients 2023, 15, 859. [Google Scholar] [CrossRef]
- Bodine, S.C.; Latres, E.; Baumhueter, S.; Lai, V.K.-M.; Nunez, L.; Clarke, B.A.; Poueymirou, W.T.; Panaro, F.J.; Na, E.; Dharmarajan, K. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 2001, 294, 1704–1708. [Google Scholar] [CrossRef]
- Saha, R.N.; Jana, M.; Pahan, K. MAPK p38 regulates transcriptional activity of NF-κB in primary human astrocytes via acetylation of p65. J. Immunol. 2007, 179, 7101–7109. [Google Scholar] [CrossRef]
- Sawada, D.; Sugawara, T.; Ishida, Y.; Aihara, K.; Aoki, Y.; Takehara, I.; Takano, K.; Fujiwara, S. Effect of continuous ingestion of a beverage prepared with Lactobacillus gasseri CP2305 inactivated by heat treatment on the regulation of intestinal function. Food Res. Int. 2016, 79, 33–39. [Google Scholar] [CrossRef]
- Maehata, H.; Arai, S.; Iwabuchi, N.; Abe, F. Immuno-modulation by heat-killed Lacticaseibacillus paracasei MCC1849 and its application to food products. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211008291. [Google Scholar] [CrossRef]
- Birkeland, E.; Gharagozlian, S.; Birkeland, K.I.; Valeur, J.; Måge, I.; Rud, I.; Aas, A.-M. Prebiotic effect of inulin-type fructans on faecal microbiota and short-chain fatty acids in type 2 diabetes: A randomised controlled trial. Eur. J. Nutr. 2020, 59, 3325–3338. [Google Scholar] [CrossRef] [PubMed]
- Hiel, S.; Gianfrancesco, M.A.; Rodriguez, J.; Portheault, D.; Leyrolle, Q.; Bindels, L.B.; da Silveira Cauduro, C.G.; Mulders, M.D.; Zamariola, G.; Azzi, A.-S. Link between gut microbiota and health outcomes in inulin-treated obese patients: Lessons from the Food4Gut multicenter randomized placebo-controlled trial. Clin. Nutr. 2020, 39, 3618–3628. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.; Choi, J.; Jeong, E.; Song, H.-Y.; Moon, J.; Kim, M.-a.; Lee, C.; Park, J.; Hong, J.K.; Kim, T.-J. Dual Protective Effects of Postbiotics and Cichorium intybus L. Mixture in the Sarcopenic and Inflammatory Models. Biomedicines 2025, 13, 2046. https://doi.org/10.3390/biomedicines13092046
Park H, Choi J, Jeong E, Song H-Y, Moon J, Kim M-a, Lee C, Park J, Hong JK, Kim T-J. Dual Protective Effects of Postbiotics and Cichorium intybus L. Mixture in the Sarcopenic and Inflammatory Models. Biomedicines. 2025; 13(9):2046. https://doi.org/10.3390/biomedicines13092046
Chicago/Turabian StylePark, Harang, Jinsu Choi, Eunwoo Jeong, Hye-Yeong Song, Juyeong Moon, Min-ah Kim, Chunghyeon Lee, Junsoo Park, Jong Kwang Hong, and Tack-Joong Kim. 2025. "Dual Protective Effects of Postbiotics and Cichorium intybus L. Mixture in the Sarcopenic and Inflammatory Models" Biomedicines 13, no. 9: 2046. https://doi.org/10.3390/biomedicines13092046
APA StylePark, H., Choi, J., Jeong, E., Song, H.-Y., Moon, J., Kim, M.-a., Lee, C., Park, J., Hong, J. K., & Kim, T.-J. (2025). Dual Protective Effects of Postbiotics and Cichorium intybus L. Mixture in the Sarcopenic and Inflammatory Models. Biomedicines, 13(9), 2046. https://doi.org/10.3390/biomedicines13092046






