Exploring the Role of Polyunsaturated Fatty Acids in Children’s Sleep
Abstract
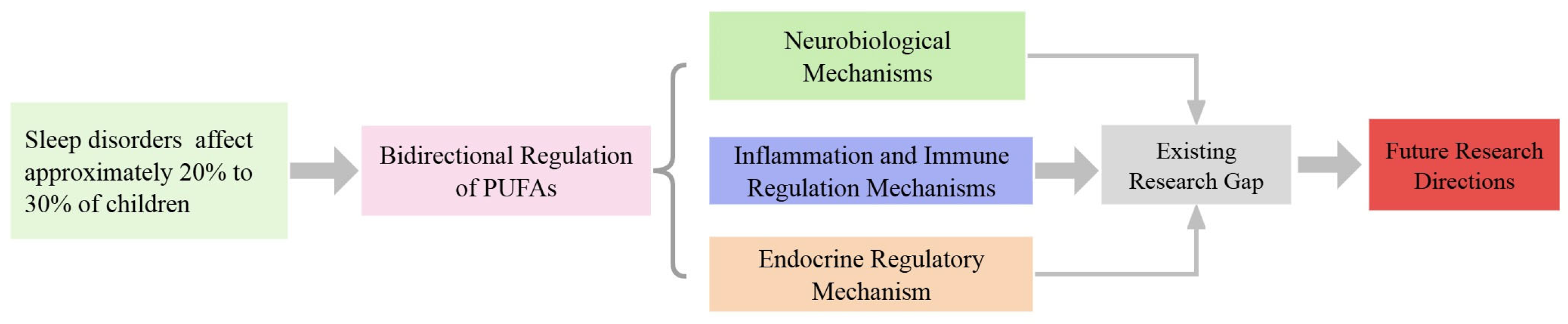
1. Introduction
2. Concise Overview of Polyunsaturated Fatty Acids
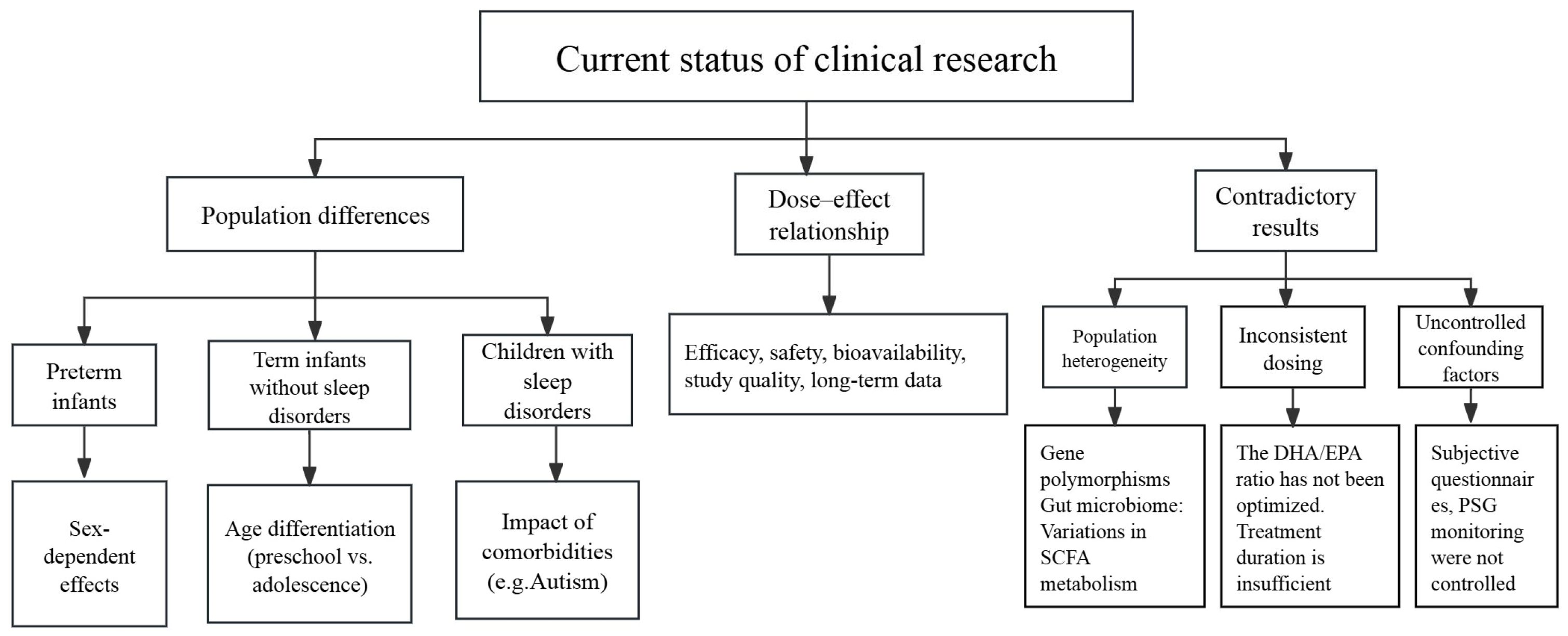
3. Current Research Status on Polyunsaturated Fatty Acids and Sleep
4. Mechanisms by Which Polyunsaturated Fatty Acids Improve Sleep
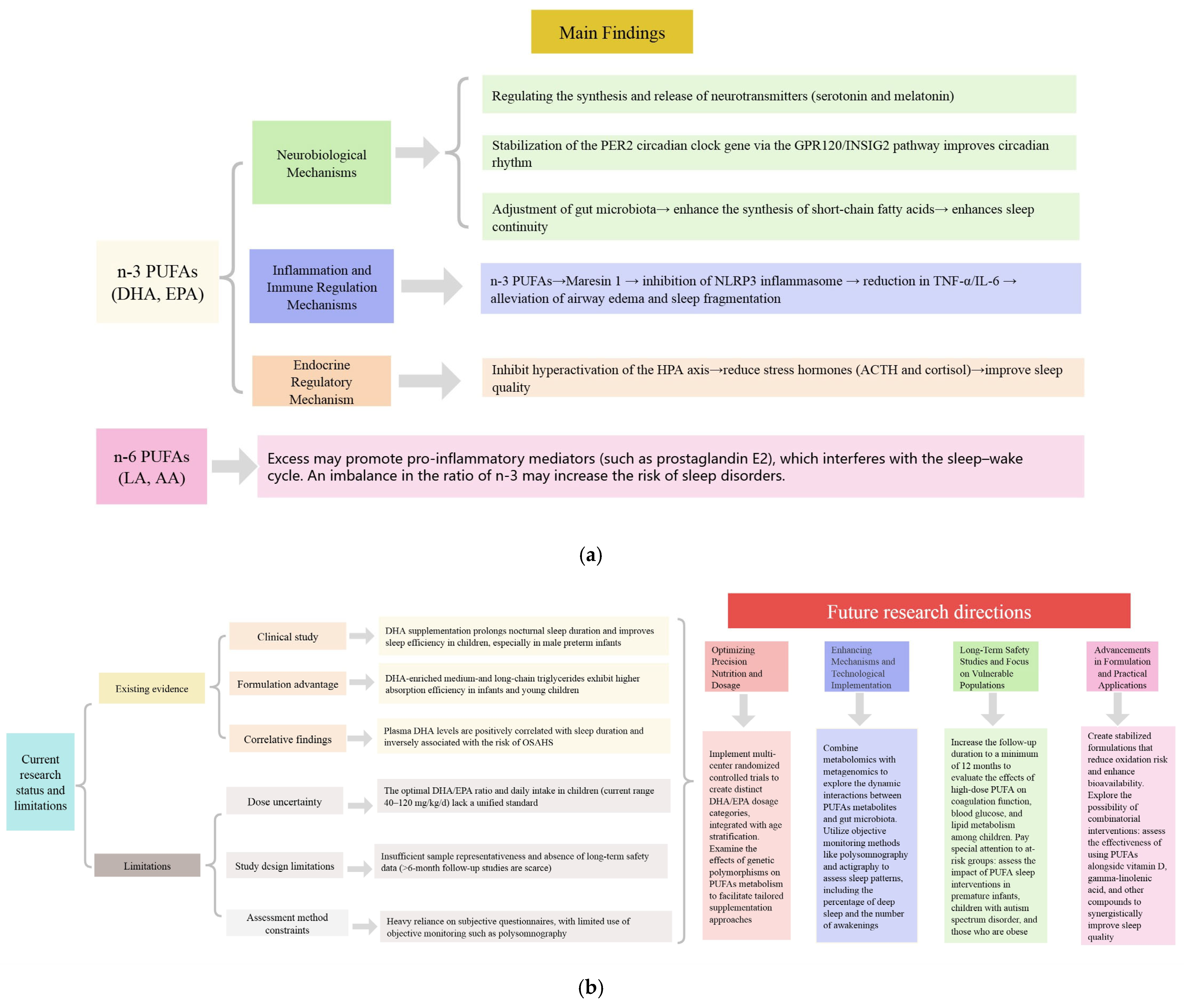
4.1. Neurobiological Mechanisms
4.2. Inflammation and Immune Regulation Mechanisms
4.3. Endocrine Regulatory Mechanism
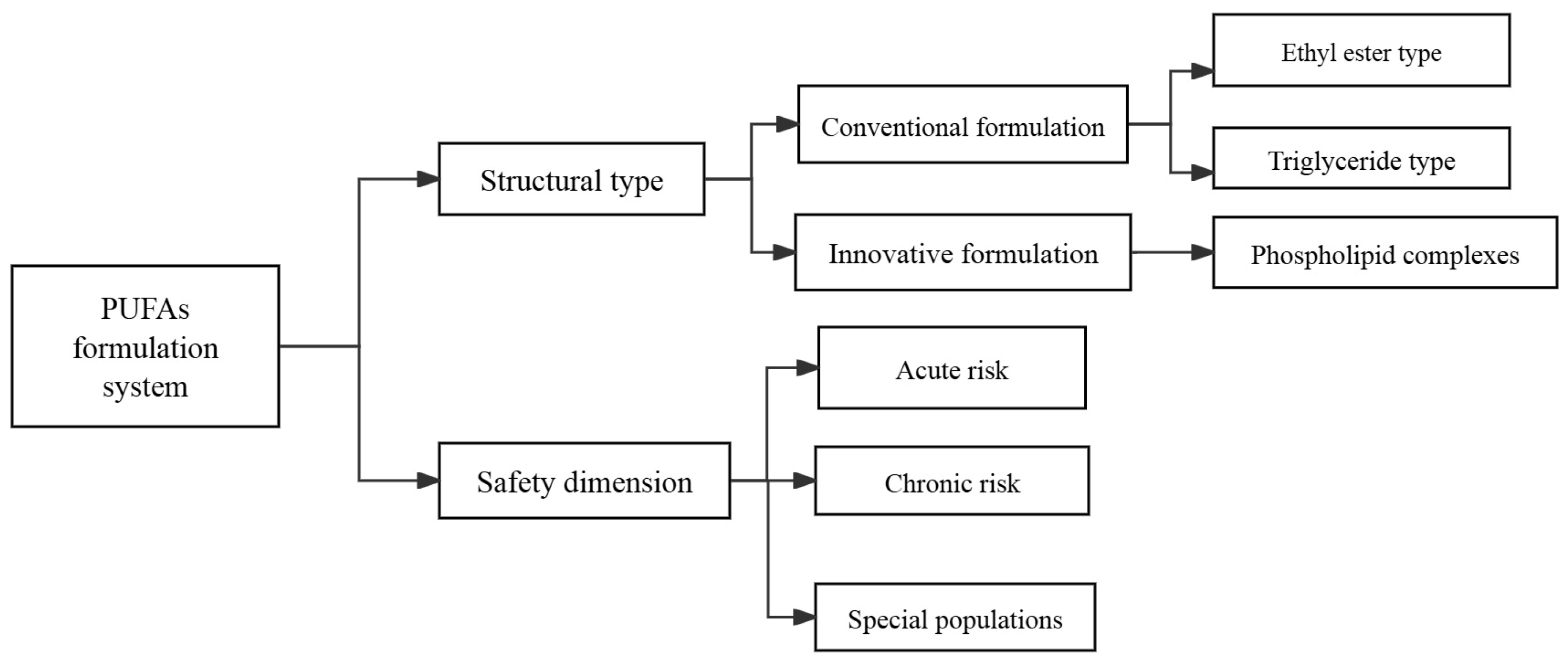
5. Types of Polyunsaturated Fatty Acid Preparations and Safety in Clinical Studies
6. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Abbreviations’ | Full Terms |
| PUFAs | Polyunsaturated fatty acids |
| DHA | Docosahexaenoic acid |
| OSAHS | Obstructive sleep apnea hypopnea syndrome |
| EPA | Eicosapentaenoic acid |
| α-LA | Alpha-linolenic acid |
| LA | Linoleic acid |
| AA | Arachidonic acid |
| GABA | Gamma-aminobutyric acid |
| INSIG2 | Insulin-induced gene 2 |
| RORα | Retinoic acid-related orphan receptor alpha |
| IL-6 | Interleukin-6 |
| TNF-α | Tumor necrosis factor-alpha |
| AEA | Anandamide |
| AMPK | AMP-activated protein kinase |
| HPA | Hypothalamic-pituitary-adrenal |
| CRF | Corticotropin-releasing factor |
| ACTH | Adrenocorticotropic hormone |
| RxOME3FAs | Prescription omega-3 polyunsaturated fatty acids |
| PSG | Polysomnography |
References
- Deng, Y.; Zhang, Z.; Gui, Y.; Li, W.; Rong, T.; Jiang, Y.; Zhu, Q.; Zhao, J.; Zhang, Y.; Wang, G.; et al. Sleep disturbances and emotional and behavioral difficulties among preschool-aged children. JAMA Netw. Open 2023, 6, e2347623. [Google Scholar] [CrossRef]
- Gemke, R.J.B.J.; Burger, P.; Steur, L.M.H. Sleep disorders in children: Classification, evaluation, and management. A review. Eur. J. Pediatr. 2024, 184, 39. [Google Scholar] [CrossRef]
- Cortese, S.; Fusetto Veronesi, G.; Gabellone, A.; Margari, A.; Marzulli, L.; Matera, E.; Petruzelli, M.G.; Piarulli, F.M.; Tar-antino, F.; Bellato, A.; et al. The management of sleep disturbances in children with attention-deficit/hyperactivity disorder (ADHD): An update of the literature. Expert Rev. Neurother. 2024, 24, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.A.; Bower, J.L.; Cho, K.W.; Clementi, M.A.; Lau, S.; Oosterhoff, B.; Alfano, C.A. Sleep loss and emotion: A systematic review and meta-analysis of over 50 years of experimental research. Psychol. Bull. 2024, 150, 440–463. [Google Scholar] [CrossRef]
- Simola, P.; Liukkonen, K.; Pitkäranta, A.; Pirinen, T.; Aronen, E.T. Psychosocial and somatic outcomes of sleep problems in children: A 4-year follow-up study. Child Care Health Dev. 2014, 40, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Uren, J.; Richdale, A.L.; Cotton, S.M.; Whitehouse, A.J.O. Sleep problems and anxiety from 2 to 8 years and the influence of autistic traits: A longitudinal study. Eur. Child Adolesc. Psychiatry 2019, 28, 1117–1127. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, C.; Luo, J.; Dibaba, D.T.; Fly, A.D.; Haas, D.M.; Shikany, J.M.; Kahe, K. Long-chain omega-3 fatty acids, selenium, and mercury in relation to sleep duration and sleep quality: Findings from the CARDIA study. Eur. J. Nutr. 2022, 1, 753–762. [Google Scholar] [CrossRef]
- Heath, R.J.; Klevebro, S.; Wood, T.R. Maternal and Neonatal Polyunsaturated Fatty Acid Intake and Risk of Neurodevelopmental Impairment in Premature Infants. Int. J. Mol. Sci. 2022, 23, 700. [Google Scholar] [CrossRef]
- Jansen, E.C.; Conroy, D.A.; Burgess, H.J.; O’Brien, L.M.; Cantoral, A.; Téllez-Rojo, M.M.; Peterson, K.E.; Baylin, A. Plasma DHA is related to sleep timing and duration in a cohort of Mexican adolescents. J. Nutr. 2020, 150, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.A.; Tintle, N.; Harris, W.S.; Darvishian, M.; Marklund, M.; Virtanen, J.K.; Hantunen, S.; de Mello, V.D.; Tuomilehto, J.; Lindström, J.; et al. PUFA omega-3 and omega-6 biomarkers and sleep: A pooled analysis of cohort studies on behalf of the Fatty Acids and Outcomes Research Consortium (FORCE). Am. J. Clin. Nutr. 2022, 115, 864–876. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Ge, H.; Sun, J.; Hao, K.; Yao, W.; Zhang, D. Associations of dietary omega-3 and omega-6 fatty acids consumption with sleep disorders and sleep duration among adults. Nutrients 2021, 13, 1475. [Google Scholar] [CrossRef]
- Murphy, R.A.; Devarshi, P.P.; Mun, J.G.; Marshall, K.; Mitmesser, S.H. Association of omega-3 levels and sleep in US adults: National Health and Nutrition Examination Survey, 2011–2012. Sleep Health 2022, 8, 294–297. [Google Scholar] [CrossRef]
- Boone, K.M.; Klebanoff, M.A.; Rogers, L.K.; Rausch, J.; Coury, D.L.; Keim, S.A. Effects of omega-3-6-9 fatty acid supplementation on behavior and sleep in preterm toddlers with autism symptomatology: Secondary analysis of a randomized clinical trial. Early Hum. Dev. 2022, 169, 105588. [Google Scholar] [CrossRef]
- Scoditti, E.; Tumolo, M.R.; Garbarino, S. Mediterranean diet on sleep: A health alliance. Nutrients 2022, 14, 2998. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhao, A.; Szeto, I.M.; Wang, Y.; Meng, L.; Li, T.; Zhang, J.; Wang, M.; Tian, Z.; Zhang, Y. Diet quality, consumption of seafood and eggs are associated with sleep quality among Chinese urban adults: A cross-sectional study in eight cities of China. Food Sci. Nutr. 2019, 7, 2091–2102. [Google Scholar] [CrossRef]
- Patan, M.J.; Kennedy, D.O.; Husberg, C.; Hustvedt, S.O.; Calder, P.C.; Middleton, B.; Khan, J.; Forster, J.; Jackson, P.A. Differential effects of DHA- and EPA-rich oils on sleep in healthy young adults: A randomized controlled trial. Nutrients 2021, 13, 248. [Google Scholar] [CrossRef] [PubMed]
- Hysing, M.; Kvestad, I.; Kjellevold, M.; Kolden Midtbø, L.; Graff, I.E.; Lie, Ø.; Hurum, H.; Stormark, K.M.; Øyen, J. Fatty fish intake and the effect on mental health and sleep in preschool children in FINS-KIDS, a randomized controlled trial. Nutrients 2018, 10, 1478. [Google Scholar] [CrossRef]
- Dai, Y.; Liu, J.H. Omega-3 long-chain polyunsaturated fatty acid and sleep: A systematic review and meta-analysis of randomized controlled trials and longitudinal studies. Nutr. Rev. 2021, 79, 847–868. [Google Scholar] [CrossRef]
- Zailani, H.; Satyanarayanan, S.K.; Liao, W.C.; Liao, H.F.; Huang, S.Y.; Gałecki, P.; Su, K.P.; Chang, J.P. Omega-3 polyunsaturated fatty acids in managing comorbid mood disorders in chronic obstructive pulmonary disease (COPD): A review. J. Clin. Med. 2023, 12, 2653. [Google Scholar] [CrossRef]
- Bercea, C.I.; Cottrell, G.S.; Tamagnini, F.; McNeish, A.J. Omega-3 polyunsaturated fatty acids and hypertension: A review of vasodilatory mechanisms of docosahexaenoic acid and eicosapentaenoic acid. Br. J. Pharmacol. 2021, 178, 860–877. [Google Scholar] [CrossRef] [PubMed]
- Alagawany, M.; Elnesr, S.S.; Farag, M.R.; El-Sabrout, K.; Alqaisi, O.; Dawood, M.A.O.; Soomro, H.; Abdelnour, S.A. Nutritional significance and health benefits of omega-3, -6 and -9 fatty acids in animals. Anim. Biotechnol. 2022, 33, 1678–1690. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, W.; Cheng, X.; Yang, H.; She, Z.G.; Cai, J.; Li, H.; Zhang, X.J. Emerging Roles and Therapeutic Applications of Arachidonic Acid Pathways in Cardiometabolic Diseases. Circ. Res. 2024, 135, 222–260. [Google Scholar] [CrossRef]
- Decoeur, F.; Benmamar-Badel, A.; Leyrolle, Q.; Persillet, M.; Layé, S.; Nadjar, A. Dietary N-3 PUFA deficiency affects sleep-wake activity in basal condition and in response to an inflammatory challenge in mice. Brain Behav. Immun. 2020, 85, 162–169. [Google Scholar] [CrossRef]
- Yang, R.; Ding, H.; Shan, J.; Li, X.; Zhang, J.; Liu, G.; Zheng, H.; Su, Y.; Yao, H.; Qi, K.; et al. Association of fish oil containing lipid emulsions with retinopathy of prematurity: A retrospective observational study. BMC Pediatr. 2022, 22, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef]
- Madore, C.; Leyrolle, Q.; Morel, L.; Rossitto, M.; Greenhalgh, A.D.; Delpech, J.C.; Martinat, M.; Bosch-Bouju, C.; Bourel, J.; Rani, B.; et al. Essential omega-3 fatty acids tune microglial phagocytosis of synaptic elements in the mouse developing brain. Nat. Commun. 2020, 11, 6133. [Google Scholar] [CrossRef]
- Zhao, M.; Tuo, H.; Wang, S.; Zhao, L. The Effects of Dietary Nutrition on Sleep and Sleep Disorders. Mediat. Inflamm. 2020, 2020, 3142874. [Google Scholar] [CrossRef]
- Checa-Ros, A.; D’Marco, L. Role of Omega-3 Fatty Acids as Non-Photic Zeitgebers and Circadian Clock Synchronizers. Int. J. Mol. Sci. 2022, 23, 12162. [Google Scholar] [CrossRef]
- Judge, M.P.; Cong, X.; Harel, O.; Courville, A.B.; Lammi-Keefe, C.J. Maternal consumption of a DHA-containing functional food benefits infant sleep patterning: An early neurodevelopmental measure. Early Hum. Dev. 2012, 88, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, P.; Li, H.; Jia, W.; Shou, Q.; Zhu, Y.; Mao, L.; Wang, W.; Wu, F.; Chen, X.; Wan, X.; et al. Eicosapentaenoic and docosahexaenoic acids attenuate hyperglycemia through the microbiome-gut-organs axis in db/db mice. Microbiome 2021, 9, 185. [Google Scholar] [CrossRef]
- Yang, S.C. A New Perspective on Fish Oil: The Prevention of Alcoholic Liver Disease. J. Oleo Sci. 2021, 70, 1531–1538. [Google Scholar] [CrossRef]
- Shrestha, N.; Sleep, S.L.; Cuffe, J.S.M.; Holland, O.J.; Perkins, A.V.; Yau, S.Y.; McAinch, A.J.; Hryciw, D.H. Role of omega-6 and omega-3 fatty acids in fetal programming. Clin. Exp. Pharmacol. Physiol. 2020, 47, 907–915. [Google Scholar] [CrossRef]
- Mani, I.; Dwarkanath, P.; Thomas, T.; Thomas, A.; Kurpad, A.V. Maternal fat and fatty acid intake and birth outcomes in a South Indian population. Int. J. Epidemiol. 2016, 45, 523–531. [Google Scholar] [CrossRef]
- Shrestha, N.; Cuffe, J.S.M.; Holland, O.J.; Bulmer, A.C.; Hill, M.; Perkins, A.V.; Muhlhausler, B.S.; McAinch, A.J.; Hryciw, D.H. Elevated maternal linoleic acid reduces circulating leptin concentrations, cholesterol levels and male fetal survival in a rat model. J. Physiol. 2019, 597, 3349–3361. [Google Scholar] [CrossRef]
- Rosenfeld, C.S.; Roberts, R.M. Maternal diet and other factors affecting offspring sex ratio: A review. Biol. Reprod. 2004, 71, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Niseteo, T.; Hojsak, I.; Ožanić Bulić, S.; Pustišek, N. Effect of Omega-3 Polyunsaturated Fatty Acid Supplementation on Clinical Outcome of Atopic Dermatitis in Children. Nutrients 2024, 16, 2829. [Google Scholar] [CrossRef]
- Garaulet, M.; Lee, Y.C.; Shen, J.; Parnell, L.D.; Arnett, D.K.; Tsai, M.Y.; Lai, C.Q.; Ordovas, J.M. CLOCK genetic variation and metabolic syndrome risk: Modulation by monounsaturated fatty acids. Am. J. Clin. Nutr. 2009, 90, 1466–1475. [Google Scholar] [CrossRef]
- Lavialle, M.; Champeil-Potokar, G.; Alessandri, J.M.; Balasse, L.; Guesnet, P.; Papillon, C.; Pévet, P.; Vancassel, S.; Vivien-Roels, B.; Denis, I.; et al. An (n-3) polyunsaturated fatty acid–deficient diet disturbs daily locomotor activity, melatonin rhythm, and striatal dopamine in Syrian hamsters. J. Nutr. 2008, 138, 1719–1724. [Google Scholar] [CrossRef]
- Almaspour, M.B.; Nasehi, M.; Khalifeh, S.; Zarrindast, M.R. The effect of fish oil on social interaction memory in total sleep-deprived rats with respect to the hippocampal level of stathmin, TFEB, synaptophysin, and LAMP-1 proteins. Prostaglandins Leukot. Essent. Fat. Acids 2020, 157, 102097. [Google Scholar] [CrossRef] [PubMed]
- Boone, K.M.; Rausch, J.; Pelak, G.; Li, R.; Turner, A.N.; Klebanoff, M.A.; Keim, S.A. Docosahexaenoic acid and arachidonic acid supplementation and sleep in toddlers born preterm: Secondary analysis of a randomized clinical trial. J. Clin. Sleep. Med. 2019, 15, 1197–1208. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, P.; Burton, J.R.; Sewell, R.P.; Spreckelsen, T.F.; Richardson, A.J. Fatty acids and sleep in UK children: Subjective and pilot objective sleep results from the DOLAB study—A randomized controlled trial. J. Sleep Res. 2014, 23, 364–388. [Google Scholar] [CrossRef]
- Yehuda, S.; Rabinovitz-Shenkar, S.; Carasso, R.L. Effects of essential fatty acids in iron-deficient and sleep-disturbed attention deficit hyperactivity disorder (ADHD) children. Eur. J. Clin. Nutr. 2011, 65, 1167–1169. [Google Scholar] [CrossRef]
- Barion, G.R.; Marghetti, P.G.; Cagliari, P.Z.; Mastroeni, M.F. Docosahexaenoic acid and sleep quality in very and extreme preterm infants. Int. J. Environ. Res. Public. Health 2024, 21, 1362. [Google Scholar] [CrossRef]
- Uauy, R.; Hoffman, D.R.; Peirano, P.; Birch, D.G.; Birch, E.E. Essential fatty acids in visual and brain development. Lipids 2001, 36, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Yan, Y.; Zheng, J.S.; Mi, J.; Li, D. Association between Erythrocyte Membrane Phospholipid Fatty Acids and Sleep Disturbance in Chinese Children and Adolescents. Nutrients 2018, 10, 344. [Google Scholar] [CrossRef] [PubMed]
- Cespuglio, R. Serotonin: Its place today in sleep preparation, triggering or maintenance. Sleep. Med. 2018, 49, 31–39. [Google Scholar] [CrossRef]
- Oikonomou, G.; Altermatt, M.; Zhang, R.W.; Coughlin, G.M.; Montz, C.; Gradinaru, V.; Prober, D.A. The serotonergic raphe promote sleep in zebrafish and mice. Neuron 2019, 103, 686–701. [Google Scholar] [CrossRef]
- Patrick, R.P.; Ames, B.N. Vitamin D and the omega-3 fatty acids control serotonin synthesis and action, part 2: Relevance for ADHD, bipolar disorder, schizophrenia, and impulsive behavior. FASEB J. 2015, 29, 2207–2222. [Google Scholar] [CrossRef] [PubMed]
- Petra, A.I.; Panagiotidou, S.; Hatziagelaki, E.; Stewart, J.M.; Conti, P.; Theoharides, T.C. Gut-Microbiota-Brain Axis and Its Effect on Neuropsychiatric Disorders with Suspected Immune Dysregulation. Clin. Ther. 2015, 37, 984–995. [Google Scholar] [CrossRef]
- Barrett, E.; Ross, R.P.; O’Toole, P.W.; Fitzgerald, G.F.; Stanton, C. γ-Aminobutyric Acid Production by Culturable Bacteria from the Human Intestine. J. Appl. Microbiol. 2012, 113, 411–417. [Google Scholar] [CrossRef]
- Xie, Z.; Chen, F.; Li, W.A.; Geng, X.; Li, C.; Meng, X.; Feng, Y.; Liu, W.; Yu, F. A review of sleep disorders and melatonin. Neurol. Res. 2017, 39, 559–565. [Google Scholar] [CrossRef]
- Padilla, S.L.; Perez, J.G.; Ben-Hamo, M.; Johnson, C.W.; Sanchez, R.E.A.; Bussi, I.L.; Palmiter, R.D.; de la Iglesia, H.O. Kisspeptin Neurons in the Arcuate Nucleus of the Hypothalamus Orchestrate Circadian Rhythms and Metabolism. Curr. Biol. 2019, 29, 592–604. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Yang, Y.; Selby, C.P.; Liu, Z.; Sancar, A. Molecular mechanism of the repressive phase of the mammalian circadian clock. Proc. Natl. Acad. Sci. USA 2021, 118, e2021174118. [Google Scholar] [CrossRef]
- Wefers, J.; van Moorsel, D.; Hansen, J.; Connell, N.J.; Havekes, B.; Hoeks, J.; van Marken Lichtenbelt, W.D.; Duez, H.; Phielix, E.; Kalsbeek, A.; et al. Circadian misalignment induces fatty acid metabolism gene profiles and compromises insulin sensitivity in human skeletal muscle. Proc. Natl. Acad. Sci. USA 2018, 115, 7789–7794. [Google Scholar] [CrossRef] [PubMed]
- Furutani, A.; Ikeda, Y.; Itokawa, M.; Nagahama, H.; Ohtsu, T.; Furutani, N.; Kamagata, M.; Yang, Z.H.; Hirasawa, A.; Tahara, Y.; et al. Fish oil accelerates diet-induced entrainment of the mouse peripheral clock via GPR120. PLoS ONE 2015, 10, e0132472. [Google Scholar] [CrossRef]
- Chen, R.; Zuo, Z.; Li, Q.; Wang, H.; Li, N.; Zhang, H.; Yu, X.; Liu, Z. DHA substitution overcomes high-fat diet-induced disturbance in the circadian rhythm of lipid metabolism. Food Funct. 2020, 11, 3621–3631. [Google Scholar] [CrossRef] [PubMed]
- Challet, E. The Circadian Regulation of Food Intake. Nat. Rev. Endocrinol. 2019, 15, 393–405. [Google Scholar] [CrossRef]
- Sejbuk, M.; Siebieszuk, A.; Witkowska, A.M. The Role of Gut Microbiome in Sleep Quality and Health: Dietary Strategies for Microbiota Support. Nutrients 2024, 16, 2259. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, Y.; Gao, H.; Li, D.; Jiang, R.; Ge, L.; Tong, C.; Xu, K. Associations among Dietary Omega-3 Polyunsaturated Fatty Acids, the Gut Microbiota, and Intestinal Immunity. Mediat. Inflamm. 2021, 2021, 8879227. [Google Scholar] [CrossRef]
- Lai, W.D.; Tung, T.H.; Teng, C.Y.; Chang, C.H.; Chen, Y.C.; Huang, H.Y.; Lee, H.C.; Huang, S.Y. Fish oil ameliorates neuropsychiatric behaviors and gut dysbiosis by elevating selected microbiota-derived metabolites and tissue tight junctions in rats under chronic sleep deprivation. Food Funct. 2022, 13, 2662–2680. [Google Scholar] [CrossRef]
- Cao, W.; Wang, C.; Chin, Y.; Chen, X.; Gao, Y.; Yuan, S.; Xue, C.; Wang, Y.; Tang, Q. DHA-Phospholipids (DHA--PL) and EPA-Phospholipids (EPA-PL) Prevent Intestinal Dysfunction Induced by Chronic Stress. Food Funct. 2019, 10, 277–288. [Google Scholar] [CrossRef]
- Warner, D.R.; Warner, J.B.; Hardesty, J.E.; Song, Y.L.; King, T.N.; Kang, J.X.; Chen, C.Y.; Xie, S.; Yuan, F.; Prodhan, M.A.I.; et al. Decreased ω-6:ω-3 PUFA Ratio Attenuates Ethanol-Induced Alterations in Intestinal Homeostasis, Microbiota, and Liver Injury. J. Lipid Res. 2019, 60, 2034–2049. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Q.; Wang, H.; Zhao, X.; Li, N.; Zhang, H.; Chen, G.; Liu, Z. Fish oil alleviates circadian bile composition dysregulation in male mice with NAFLD. J. Nutr. Biochem. 2019, 69, 53–62. [Google Scholar] [CrossRef]
- Stahl, S.T.; Smagula, S.F.; Rodakowski, J.; Dew, M.A.; Karp, J.F.; Albert, S.M.; Butters, M.; Gildengers, A.; Reynolds, C.F., 3rd. Subjective Sleep Quality and Trajectories of Interleukin-6 in Older Adults. Am. J. Geriatr. Psychiatry 2021, 29, 204–208. [Google Scholar] [CrossRef]
- Waitzberg, D.L.; Torrinhas, R.S. Fish Oil Lipid Emulsions and Immune Response: What Clinicians Need to Know. Nutr. Clin. Pract. 2009, 24, 487–499. [Google Scholar] [CrossRef]
- Lee, E.; Ramsey, M.; Malhotra, A.; Ancoli-Israel, S.; Kaufmann, C.N.; Soontornniyomkij, B.; Graham, S.A.; Depp, C.; Eyler, L.T. Links between objective sleep and sleep variability measures and inflammatory markers in adults with bipolar disorder. J. Psychiatr. Res. 2021, 134, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.; Mayeli, M.; Saghazadeh, A.; Rezaei, N. Cytokines in narcolepsy: A systematic review and meta-analysis. Cytokine 2020, 131, 155103. [Google Scholar] [CrossRef] [PubMed]
- Zhan, S.; Che, P.; Zhao, X.K.; Li, N.; Ding, Y.; Liu, J.; Li, S.; Ding, K.; Han, L.; Huang, Z.; et al. Molecular mechanism of tumour necrosis factor alpha regulates hypocretin (orexin) expression, sleep and behaviour. J. Cell Mol. Med. 2019, 23, 6822–6834. [Google Scholar] [CrossRef]
- da Silveira Cruz-Machado, S.; Guissoni Campos, L.M.; Fadini, C.C.; Anderson, G.; Markus, R.P.; Pinato, L. Disrupted nocturnal melatonin in autism: Association with tumor necrosis factor and sleep disturbances. J. Pineal Res. 2021, 70, e12715. [Google Scholar] [CrossRef]
- Dolsen, M.R.; Harvey, A.G. IL-6, sTNF-R2, and CRP in the context of sleep, circadian preference, and health in adolescents with eveningness chronotype: Cross-sectional and longitudinal treatment effects. Psychoneuroendocrinology 2021, 129, 105241. [Google Scholar] [CrossRef] [PubMed]
- Imani, M.M.; Sadeghi, M.; Khazaie, H.; Emami, M.; Sadeghi Bahmani, D.; Brand, S. Evaluation of Serum and Plasma Interleukin-6 Levels in Obstructive Sleep Apnea Syndrome: A Meta-Analysis and Meta-Regression. Front. Immunol. 2020, 11, 1343. [Google Scholar] [CrossRef]
- Han, Y.H.; Shin, K.O.; Kim, J.Y.; Khadka, D.B.; Kim, H.J.; Lee, Y.M.; Cho, W.J.; Cha, J.Y.; Lee, B.J.; Lee, M.O.; et al. A Maresin 1/RORα12-Lipoxygenase Autoregulatory Circuit Prevents Inflammation and Progression of Nonalcoholic Steatohepatitis. J. Clin. Investig. 2019, 129, 1684–1698. [Google Scholar] [CrossRef]
- Im, D.S. Maresin-1 Resolution with RORα and LGR6. Prog. Lipid Res. 2020, 78, 101034. [Google Scholar] [CrossRef]
- Szentirmai, É.; Millican, N.S.; Massie, A.R.; Kapás, L. Butyrate, a Metabolite of Intestinal Bacteria, Enhances Sleep. Sci. Rep. 2019, 9, 7035. [Google Scholar] [CrossRef]
- Patterson, E.; Wall, R.; Fitzgerald, G.F.; Ross, R.P.; Stanton, C. Health Implications of High Dietary Omega-6 Polyunsaturated Fatty Acids. J. Nutr. Metab. 2012, 2012, 539426. [Google Scholar] [CrossRef]
- Bagga, D.; Wang, L.; Farias-Eisner, R.; Glaspy, J.A.; Reddy, S.T. Differential Effects of Prostaglandin Derived from Omega-6 and Omega-3 Polyunsaturated Fatty Acids on COX-2 Expression and IL-6 Secretion. Proc. Natl. Acad. Sci. USA 2003, 100, 1751–1756. [Google Scholar] [CrossRef]
- Pfitzer, G. New Insights into the Mechanisms of Anandamide-Induced Airway Dilation Placing Its Degradation Enzyme, FAAH Center Stage. Pflügers Arch. 2023, 475, 557–559. [Google Scholar] [CrossRef]
- Wang, X.; Yu, Q.; Yue, H.; Zhang, J.; Zeng, S.; Cui, F. Circulating Endocannabinoids and Insulin Resistance in Patients with Obstructive Sleep Apnea. Biomed Res. Int. 2016, 2016, 9782031. [Google Scholar] [CrossRef] [PubMed]
- Currenti, W.; Godos, J.; Castellano, S.; Mogavero, M.P.; Ferri, R.; Caraci, F.; Grosso, G.; Galvano, F. Time Restricted Feeding and Mental Health: A Review of Possible Mechanisms on Affective and Cognitive Disorders. Int. J. Food Sci. Nutr. 2021, 72, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Hennebelle, M.; Morgan, R.K.; Sethi, S.; Zhang, Z.; Chen, H.; Grodzki, A.C.; Lein, P.J.; Taha, A.Y. Linoleic Acid-Derived Metabolites Constitute the Majority of Oxylipins in the Rat Pup Brain and Stimulate Axonal Growth in Primary Rat Cortical Neuron-Glia Co-Cultures in a Sex-Dependent Manner. J. Neurochem. 2020, 152, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Zhou, Y.; Wu, M.; Li, X.; Mai, K.; Ai, Q. ω-6 Polyunsaturated Fatty Acids (Linoleic Acid) Activate Both Autophagy and Antioxidation in a Synergistic Feedback Loop via TOR-Dependent and TOR-Independent Signaling Pathways. Cell Death Dis. 2020, 11, 607. [Google Scholar] [CrossRef]
- Wang, R.; Kern, J.T.; Goodfriend, T.L.; Ball, D.L.; Luesch, H. Activation of the Antioxidant Response Element by Specific Oxidized Metabolites of Linoleic Acid. J. Prostaglandins Leukot. Essent. Fat. Acids 2009, 81, 53–59. [Google Scholar] [CrossRef]
- Parati, G.; Ochoa, J.E.; Bilo, G.; Mattaliano, P.; Salvi, P.; Kario, K.; Lombardi, C. Obstructive Sleep Apnea Syndrome as a Cause of Resistant Hypertension. Hypertens. Res. 2014, 37, 601–613. [Google Scholar] [CrossRef]
- Peres, B.U.; Allen, A.J.H.; Shah, A.; Fox, N.; Laher, I.; Almeida, F.; Jen, R.; Ayas, N. Obstructive Sleep Apnea and Circulating Biomarkers of Oxidative Stress: A Cross-Sectional Study. J. Antioxid. 2020, 9, 476. [Google Scholar] [CrossRef]
- Patchen, B.K.; Balte, P.; Bartz, T.M.; Barr, R.G.; Fornage, M.; Graff, M.; Jacobs, D.R., Jr.; Kalhan, R.; Lemaitre, R.N.; O’Connor, G.; et al. Investigating Associations of Omega-3 Fatty Acids, Lung Function Decline, and Airway Obstruction. Am. J. Respir. Crit. Care Med. 2023, 208, 846–857. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.S.; Ottallah, H.; Maciel, C.B.; Strickland, M.; Doré, S. Role of the L-PGDS-PGD2-DP1 Receptor Axis in Sleep Regulation and Neurologic Outcomes. Sleep 2019, 42, zsz073. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.Y.; Choi, J.E.; Kim, M.; Hong, J.; Park, Y. N-3 PUFA Have Antidepressant-Like Effects Via Improvement of the HPA-Axis and Neurotransmission in Rats Exposed to Combined Stress. Mol. Neurobiol. 2020, 57, 3860–3874. [Google Scholar] [CrossRef] [PubMed]
- Šunderić, M.; Robajac, D.; Gligorijević, N.; Miljuš, G.; Nedić, O.; Smilkov, K.; Ackova, D.G.; Rudić-Grujić, V.; Penezić, A. There Something Fishy About Fish Oil? Curr. Pharm. Des. 2019, 25, 1747–1759. [Google Scholar] [CrossRef]
- Zou, X.; Ye, L.; He, X.; Wu, S.; Zhang, H.; Jin, Q. Preparation of DHA-Rich Medium-and Long-Chain Triacylglycerols by Lipase-Catalyzed Acidolysis of Microbial Oil from Schizo-chytrium sp. with Medium-Chain Fatty Acids. Appl. Biochem. Biotechnol. 2020, 191, 1294–1314. [Google Scholar] [CrossRef]
- Xie, D.; Chen, Y.; Yu, J.; Yang, Z.; Wang, X.; Wang, X. Progress in Enrichment of N-3 Polyunsaturated Fatty Acid: A Review. Crit. Rev. Food Sci. Nutr. 2023, 63, 11310–11326. [Google Scholar] [CrossRef]
- Kazuo, K. Prevention of Fish Oil Oxidation. J. Oleo Sci. 2019, 68, 1–11. [Google Scholar] [CrossRef]
- Yang, M.; Li, L.; Zhu, X.; Liang, L.; Chen, J.; Cao, W.; Liu, W.; Duan, X.; Ren, G.; Liu, Z.; et al. Microencapsulation of Fish Oil by Spray Drying, Spray Freeze-Drying, Freeze-Drying, and Microwave Freeze-Drying: Microcapsule Characterization and Storage Stability. J. Food Sci. 2024, 89, 3276–3289. [Google Scholar] [CrossRef] [PubMed]
- Racey, M.; MacFarlane, A.; Carlson, S.E.; Stark, K.D.; Plourde, M.; Field, C.J.; Yates, A.A.; Wells, G.; Grantham, A.; Ba-zinet, R.P.; et al. Dietary Reference Intakes Based on Chronic Disease Endpoints: Outcomes from a Case Study Workshop for Omega 3’s EPA and DHA. Appl. Physiol. Nutr. Metab. 2021, 46, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Frost, B.L.; Patel, A.L.; Robinson, D.T.; Berseth, C.L.; Cooper, T.; Caplan, M. Randomized Controlled Trial of Early Docosahexaenoic Acid and Arachidonic Acid Enteral Supplementation in Very Low Birth Weight Infants. J. Pediatr. 2021, 232, 23–30.e21. [Google Scholar] [CrossRef] [PubMed]
- Hellström, A.; Nilsson, A.K.; Wackernagel, D.; Pivodic, A.; Vanpee, M.; Sjöbom, U.; Hellgren, G.; Hallberg, B.; Domellöf, M.; Klevebro, S.; et al. Effect of Enteral Lipid Supplement on Severe Retinopathy of Prematurity: A Randomized Clinical Trial. JAMA Pediatr. 2021, 175, 359–367. [Google Scholar] [CrossRef]
- Brunton, S.; Collins, N. Differentiating Prescription Omega-3-Acid Ethyl Esters (P-OM3) from Dietary-Supplement Omega-3 Fatty Acids. Curr. Med. Res. Opin. 2007, 23, 1139–1145. [Google Scholar] [CrossRef]
- Chang, J.P.; Tseng, P.T.; Zeng, B.S.; Chang, C.H.; Su, H.; Chou, P.H.; Su, K.P. Safety of Supplementation of Omega-3 Polyunsaturated Fatty Acids: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2023, 14, 1326–1336. [Google Scholar] [CrossRef]
| Type | Main Components | Sources | Functions | Relationship with Health |
|---|---|---|---|---|
| n-3 | DHA, EPA | Marine fish, such as mackerel, salmon, herring, and sardines | Involved in processes such as synaptogenesis, gene expression, neuroplasticity, cell membrane fluidity, neurotransmitter transmission, and myelination, which are crucial for the functioning of the nervous system | Helps to reduce the risk of chronic inflammation-related diseases in the brain and enhances cognitive function |
| n-6 | LA AA | Soybean oil, corn oil, sunflower oil, peanut oil, meat, eggs, and dairy | Plays an important role in regulating inflammatory responses, with its metabolites involved in various physiological processes | Under pathological conditions such as oxidative stress, an excess of AA is linked to the development of cardiovascular and metabolic diseases |
| Type | Mechanism Classification | Specific Pathways | Effects on Children’s Sleep |
|---|---|---|---|
| Omega-3 series | Neurobiological Mechanisms—Regulation of Neurotransmitters |
| Contributes to the preparation, initiation, and maintenance of sleep; a deficiency may negatively impact the oscillatory activity of cortical neurons during sleep and the sleep–wake cycle |
| Neurobiological Mechanisms—Effects on Circadian Rhythm |
| Reduced levels of DHA can lead to poor sleep quality and a shorter total sleep duration | |
| Neurobiological Mechanisms—Gut–Brain Axis |
| Regulating circadian rhythms, improving lipid metabolism, and supporting gut health, thereby promoting sleep | |
| Inflammation and Immune Regulatory Mechanisms—Anti-Inflammatory Effects |
| Reducing inflammation’s interference with sleep may positively impact sleep duration | |
| Inflammation and Immune Regulatory Mechanisms—Interaction Between Sleep and Inflammation |
| Affecting circadian rhythms and reducing total sleep duration | |
| Endocrine Regulatory Mechanisms |
| Inhibiting the excessive activation of the HPA axis alleviates stress-induced dysregulation of HPA axis function, thereby regulating sleep | |
| Omega-6 series | Inflammation and Immune Regulatory Mechanisms |
| Lead to sleep disturbances, the intrinsic mechanisms by which dietary n-6 fatty acids contribute to sleep disorders are still unclear |
| Affect the continuity and quality of sleep |
| PUFAs | Molecular Formula | Simplified Structure | Key Sleep–Cognition Benefits | References |
|---|---|---|---|---|
| DHA | C22H32O2 | CH3-CH2-CH=CH-CH2-CH=CH-CH2-CH=CH-CH2-CH=CH-CH2-CH=CH-CH2-CH=CH-CH2-CH2-COOH | DHA is associated with increased levels of serotonin and melatonin, reduced risk of OSAHS, and improved sleep efficiency. | [9,10,11,41,42] |
| EPA | C20H30O2 | CH3-CH2-CH=CH-CH2--CH=CH-CH2-CH=CH-CH2-CH=CH-CH2-CH=CH-CH2-CH2-COOH | EPA is associated with decreased levels of TNF-α and IL-6, stabilization of the circadian rhythm, and improved sleep duration. | [7,10,11] |
| α-LA | C18H30O2 | CH3-(CH2-CH=CH)3--(CH2),-COOH | Precursor to EPA and DHA, limited direct sleep evidence. | [19,21] |
| LA | C18H32O2 | CH3-(CH2)4-CH=CH-CH2-CH=CH-(CH2),-COOH | A balanced intake of LA is necessary, as excessive consumption may promote inflammation and disrupt sleep. | [22,26] |
| AA | C20H32O2 | CH3-(CH2)4-CH=CH-CH2-CH=CH-CH2-CH=CH-CH2-CH=CH-(CH2)3-COOH | AA serves as a precursor to pro-inflammatory eicosanoids, and elevated levels of AA are associated with poor sleep. | [22,77,78] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, L.; Zhu, B.; Yao, D. Exploring the Role of Polyunsaturated Fatty Acids in Children’s Sleep. Biomedicines 2025, 13, 2045. https://doi.org/10.3390/biomedicines13092045
Zhu L, Zhu B, Yao D. Exploring the Role of Polyunsaturated Fatty Acids in Children’s Sleep. Biomedicines. 2025; 13(9):2045. https://doi.org/10.3390/biomedicines13092045
Chicago/Turabian StyleZhu, Liuyan, Bingquan Zhu, and Dan Yao. 2025. "Exploring the Role of Polyunsaturated Fatty Acids in Children’s Sleep" Biomedicines 13, no. 9: 2045. https://doi.org/10.3390/biomedicines13092045
APA StyleZhu, L., Zhu, B., & Yao, D. (2025). Exploring the Role of Polyunsaturated Fatty Acids in Children’s Sleep. Biomedicines, 13(9), 2045. https://doi.org/10.3390/biomedicines13092045






