The Transgenerational Impact of High-Fat Diet and Diabetic Pregnancy on Embryonic Transcriptomics and Mitochondrial Health
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Care and Ethics
2.2. Diet Characteristics
2.3. Timed Breeding
2.4. Late-Gestation Diabetes Induction
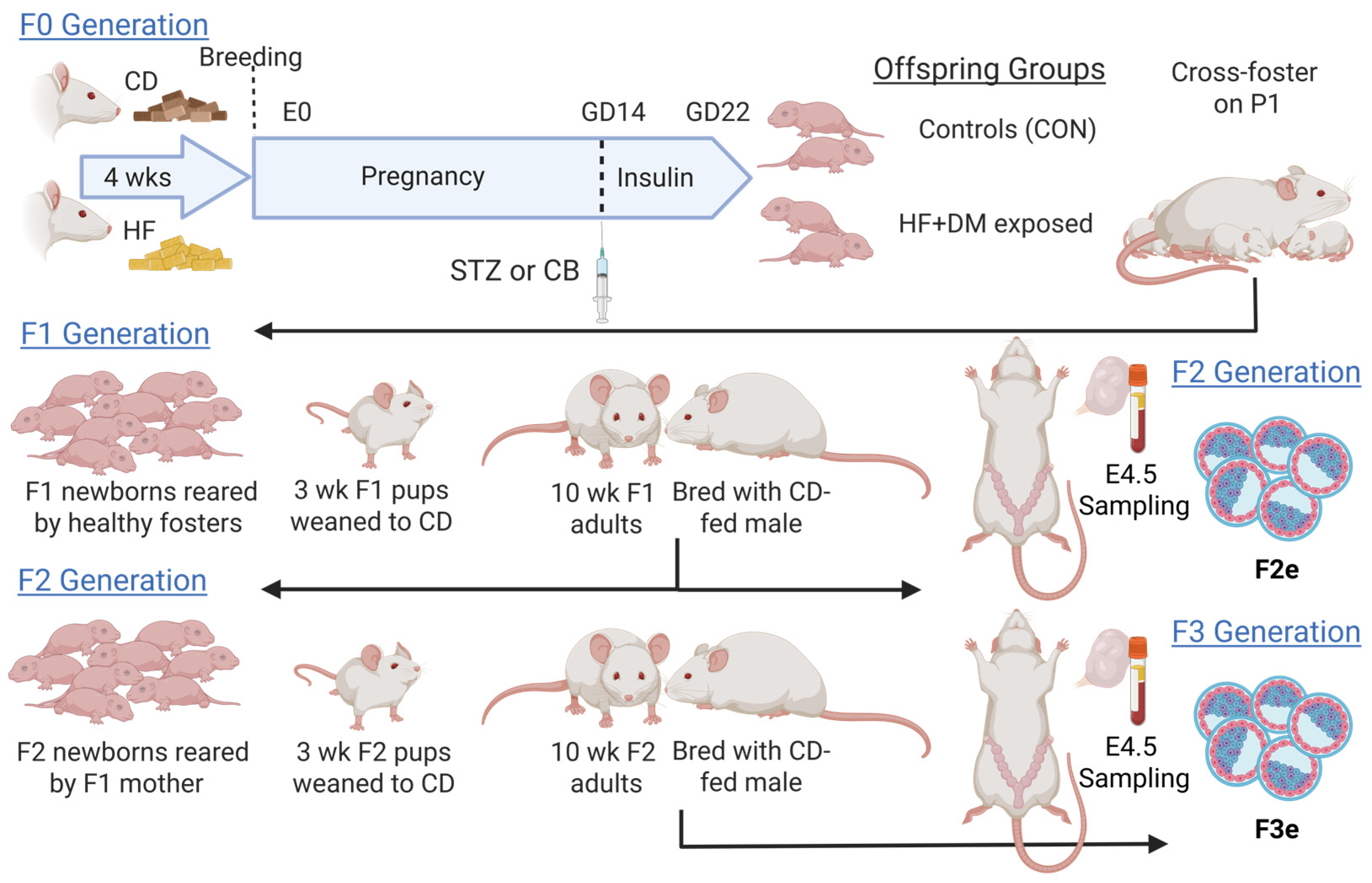
2.5. Generational Animal Model
2.6. Body Weights and Blood Sampling
2.7. Embryo Collection and Culture
2.8. Embryo Grade and Viability
2.9. Embryo Staining and Imaging
2.10. Embryo Dissociation
2.11. Single Cell-RNA Sequencing and Bioinformatics Processing
2.12. Statistical Analysis
3. Results
3.1. Phenotypes of F0 Dams
3.2. Phenotypes of F1 and F2 Offspring
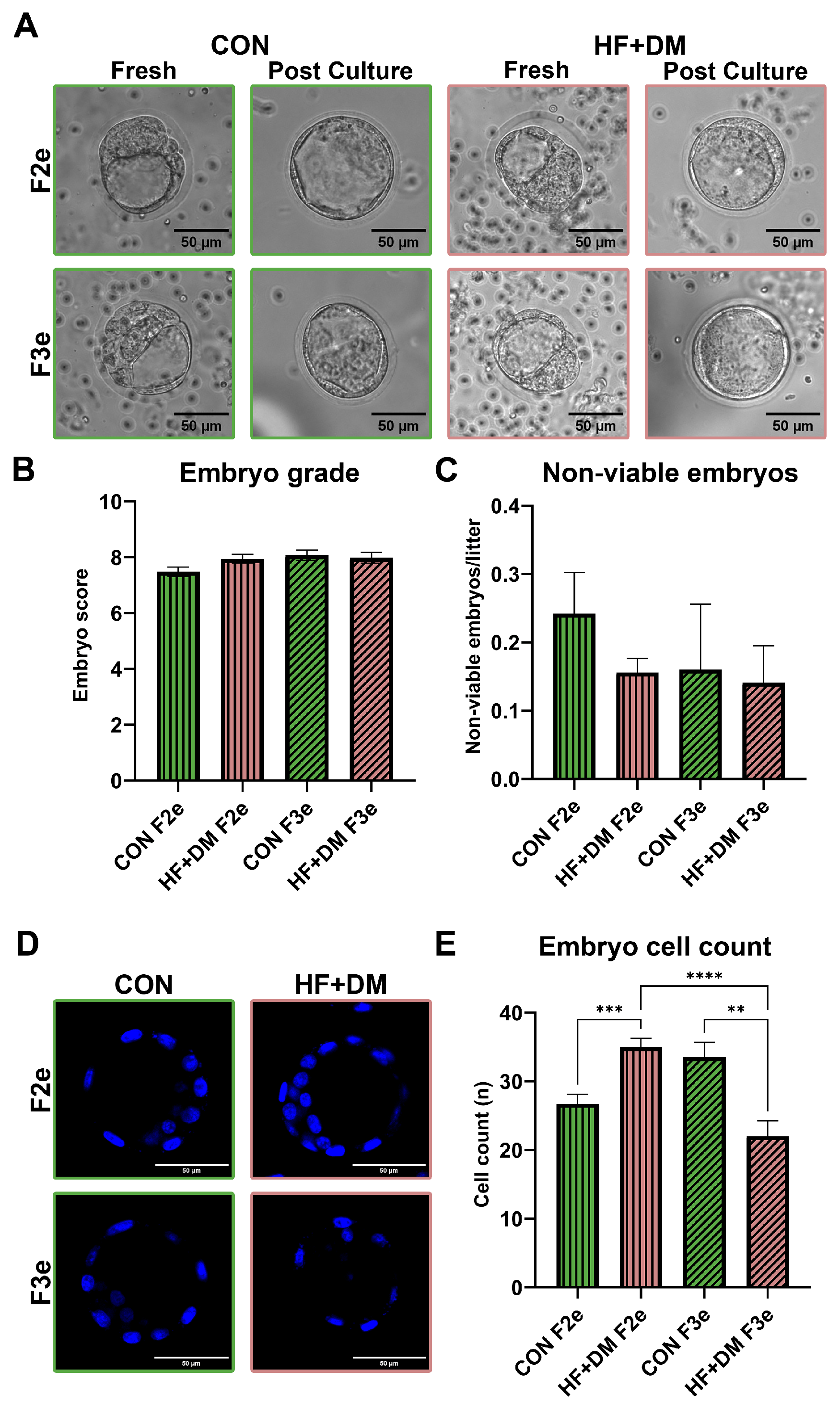
3.3. Multigenerational Effect of Diet and Diabetes on Embryo Morphology and Maturation
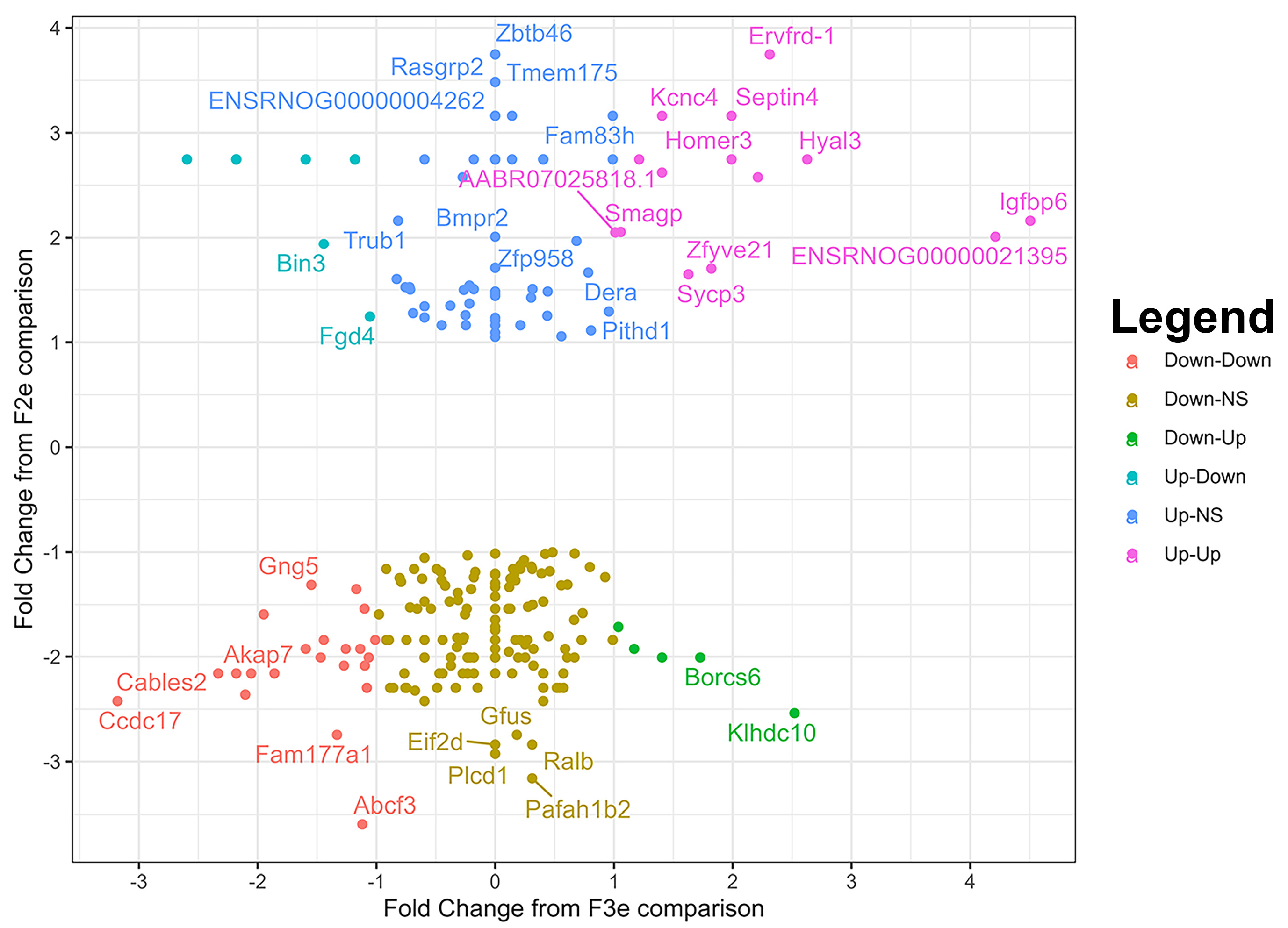
3.4. Exposure-Mediated Transcriptomic Analysis of 2nd and 3rd Generation Embryos
3.5. Exposure-Mediated Transcriptomic Analysis of 2nd and 3rd Generation Embryos in Trophectoderm and Inner Cell Mass Cells
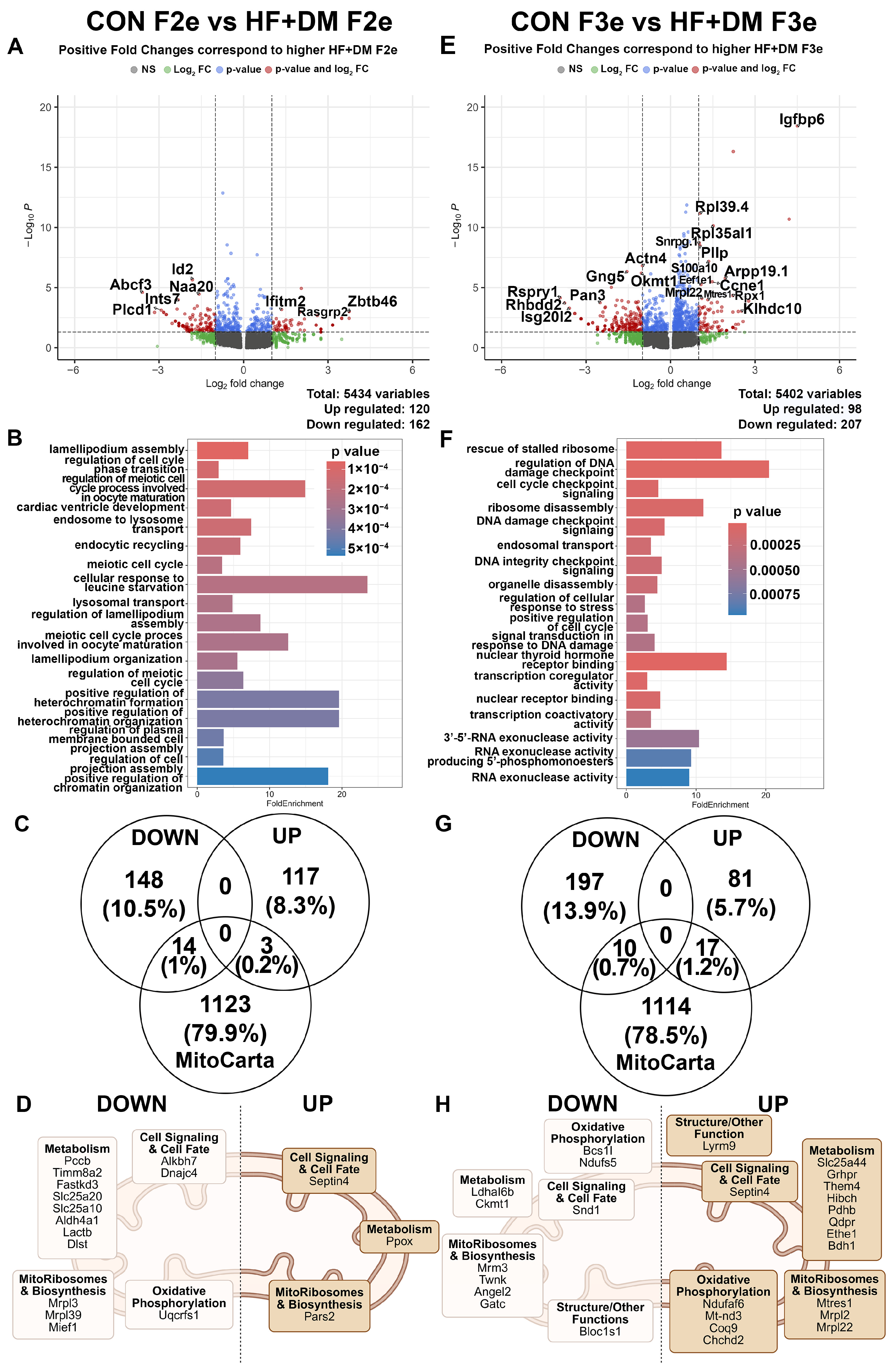

3.6. Generational Transcriptomic Analysis in Exposed Embryos

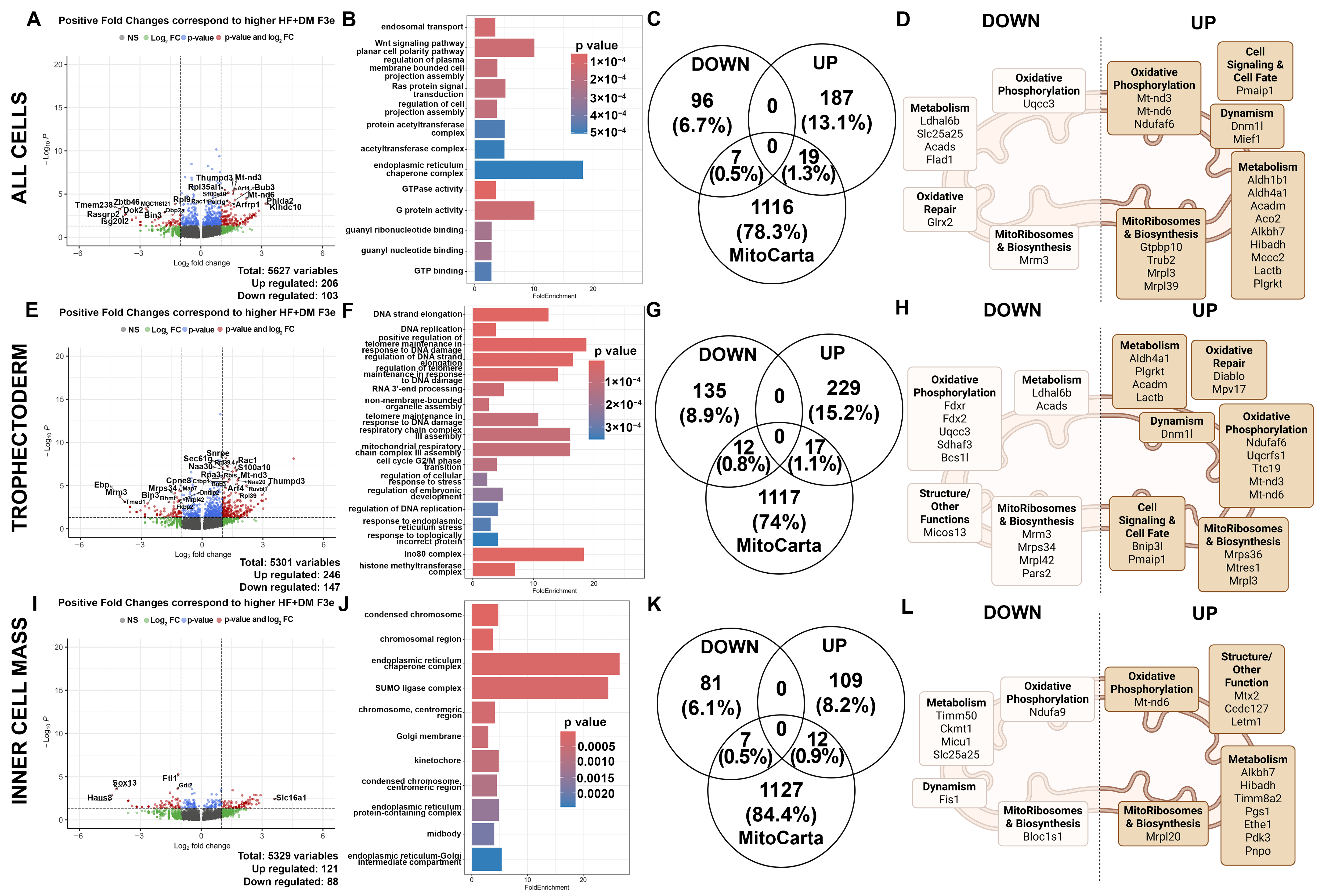
3.7. Exposure-Mediated and Generational Transcriptomic Analysis of 2nd and 3rd Generation Embryos in Female/Indeterminate Cells
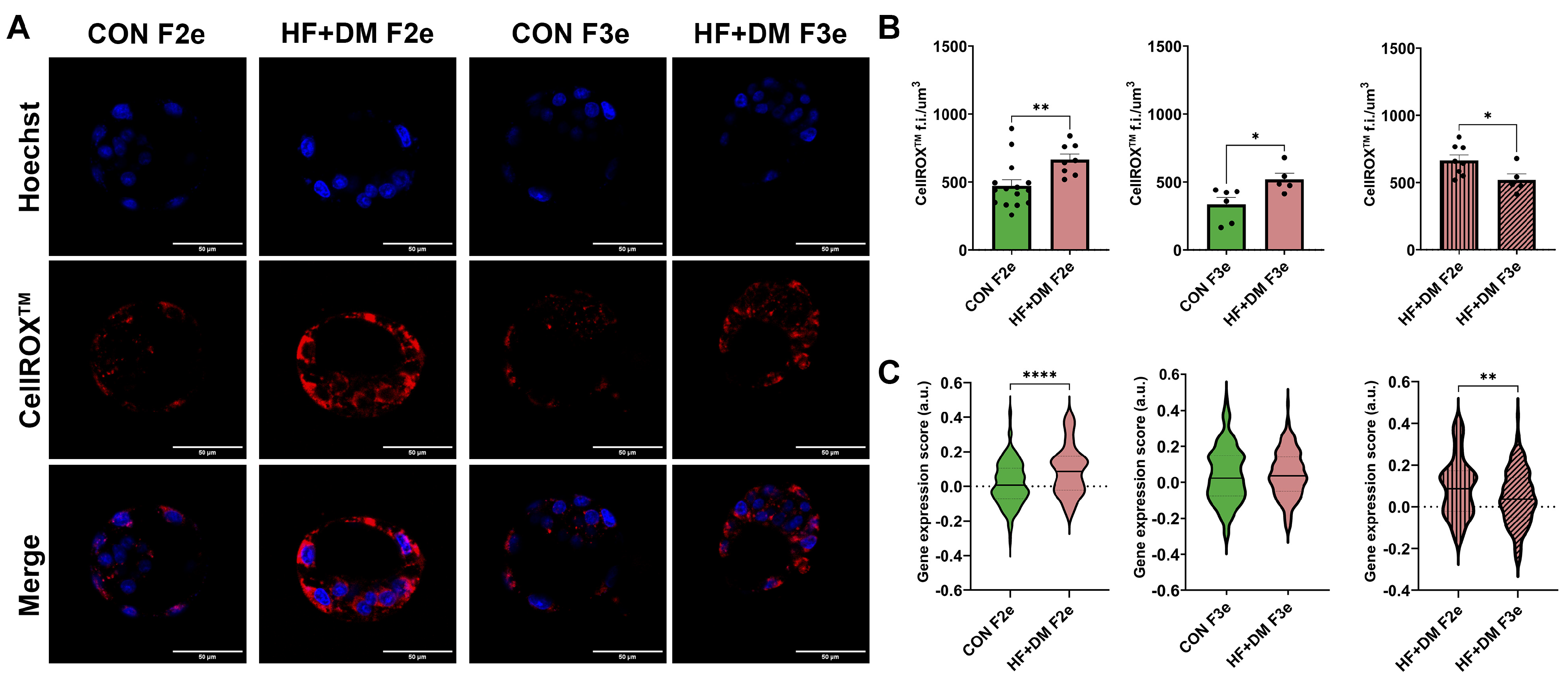
3.8. Mitochondria and Oxidative Stress in F2 and F3 Embryos
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CON | Control |
| HF+DM | High fat+diabetes |
| F2e | Second-generation embryos |
| F3e | Third-generation embryos |
| STZ | Streptozotocin |
| E | Embryonic day |
| GD | Gestational day |
| P | Postnatal day |
| ROS | Reactive oxygen species |
| ETC | Electron transport chain |
References
- Bryan Stierman, M.P.H.; Joseph Afful, M.S.; Margaret, D.; Carroll, M.S.P.H.; Chen, T.; Orlando Davy, M.P.H.; Steven Fink, M.A.; Cheryl, D.; Fryar, M.S.P.H.; Gu, Q.; et al. National Health and Nutrition Examination Survey 2017–March 2020 Prepandemic Data Files Development of Files and Prevalence Estimates for Selected Health Outcomes; National Health Statistics Reports; National Center for Health Statistics: Hyattsville, MD, USA, 2021.
- Vasudevan, C.; Renfrew, M.; McGuire, W. Fetal and perinatal consequences of maternal obesity. Arch. Dis. Child. Fetal Neonatal Ed. 2011, 96, F378–F382. [Google Scholar] [CrossRef]
- Hohos, N.M.; Skaznik-Wikiel, M.E. High-Fat Diet and Female Fertility. Endocrinology 2017, 158, 2407–2419. [Google Scholar] [CrossRef]
- Hou, Y.-J.; Zhu, C.-C.; Duan, X.; Liu, H.-L.; Wang, Q.; Sun, S.-C. Both diet and gene mutation induced obesity affect oocyte quality in mice. Sci. Rep. 2016, 6, 18858. [Google Scholar] [CrossRef]
- Reynolds, K.A.; Boudoures, A.L.; Chi, M.M.; Wang, Q.; Moley, K.H. Adverse effects of obesity and/or high-fat diet on oocyte quality and metabolism are not reversible with resumption of regular diet in mice. Reprod. Fertil. Dev. 2015, 27, 716–724. [Google Scholar] [CrossRef]
- Boudoures, A.L.; Saben, J.; Drury, A.; Scheaffer, S.; Modi, Z.; Zhang, W.; Moley, K.H. Obesity-exposed oocytes accumulate and transmit damaged mitochondria due to an inability to activate mitophagy. Dev. Biol. 2017, 426, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Kirillova, A.; Smitz, J.E.J.; Sukhikh, G.T.; Mazunin, I. The Role of Mitochondria in Oocyte Maturation. Cells 2021, 10, 2484. [Google Scholar] [CrossRef] [PubMed]
- Houghton, F.D. Energy metabolism of the inner cell mass and trophectoderm of the mouse blastocyst. Differentiation 2006, 74, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Siemers, K.M.; Baack, M.L. The importance of placental lipid metabolism across gestation in obese and non-obese pregnancies. Clin. Sci. 2023, 137, 31–34. [Google Scholar] [CrossRef]
- Louwen, F.; Kreis, N.N.; Ritter, A.; Yuan, J. Maternal obesity and placental function: Impaired maternal-fetal axis. Arch. Gynecol. Obstet. 2024, 309, 2279–2288. [Google Scholar] [CrossRef]
- Siemers, K.M.; Joss-Moore, L.A.; Baack, M.L. Gestational Diabetes-like Fuels Impair Mitochondrial Function and Long-Chain Fatty Acid Uptake in Human Trophoblasts. Int. J. Mol. Sci. 2024, 25, 11534. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Fernandez, J.; Ochoa, J.J.; Lopez-Frias, M.; Diaz-Castro, J. Impact of Early Nutrition, Physical Activity and Sleep on the Fetal Programming of Disease in the Pregnancy: A Narrative Review. Nutrients 2020, 12, 3900. [Google Scholar] [CrossRef]
- Marciniak, A.; Patro-Małysza, J.; Kimber-Trojnar, Ż.; Marciniak, B.; Oleszczuk, J.; Leszczyńska-Gorzelak, B. Fetal programming of the metabolic syndrome. Taiwan J. Obstet. Gynecol. 2017, 56, 133–138. [Google Scholar] [CrossRef]
- Hedermann, G.; Hedley, P.L.; Thagaard, I.N.; Krebs, L.; Ekelund, C.K.; Sørensen, T.I.A.; Christiansen, M. Maternal obesity and metabolic disorders associate with congenital heart defects in the offspring: A systematic review. PLoS ONE 2021, 16, e0252343. [Google Scholar] [CrossRef]
- Iessa, N.; Bérard, A. Update on Prepregnancy Maternal Obesity: Birth Defects and Childhood Outcomes. J. Pediatr. Genet. 2015, 4, 71–83. [Google Scholar] [CrossRef]
- Sutton, E.F.; Gilmore, L.A.; Dunger, D.B.; Heijmans, B.T.; Hivert, M.F.; Ling, C.; Martinez, J.A.; Ozanne, S.E.; Simmons, R.A.; Szyf, M.; et al. Developmental programming: State-of-the-science and future directions-Summary from a Pennington Biomedical symposium. Obesity 2016, 24, 1018–1026. [Google Scholar] [CrossRef] [PubMed]
- Yen, I.W.; Lee, C.N.; Lin, M.W.; Fan, K.C.; Wei, J.N.; Chen, K.Y.; Chen, S.C.; Tai, Y.Y.; Kuo, C.H.; Lin, C.H.; et al. Overweight and obesity are associated with clustering of metabolic risk factors in early pregnancy and the risk of GDM. PLoS ONE 2019, 14, e0225978. [Google Scholar] [CrossRef]
- Kapur, A.; McIntyre, H.D.; Hod, M. Type 2 Diabetes in Pregnancy. Endocrinol. Metab. Clin. N. Am. 2019, 48, 511–531. [Google Scholar] [CrossRef]
- Lu, M.; Sferruzzi-Perri, A.N. Placental mitochondrial function in response to gestational exposures. Placenta 2021, 104, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Napso, T.; Lean, S.C.; Lu, M.; Mort, E.J.; Desforges, M.; Moghimi, A.; Bartels, B.; El-Bacha, T.; Fowden, A.L.; Camm, E.J.; et al. Diet-induced maternal obesity impacts feto-placental growth and induces sex-specific alterations in placental morphology, mitochondrial bioenergetics, dynamics, lipid metabolism and oxidative stress in mice. Acta Physiol. 2022, 234, e13795. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S. Impact of Maternal Diet on the Epigenome during In Utero Life and the Developmental Programming of Diseases in Childhood and Adulthood. Nutrients 2015, 7, 9492–9507. [Google Scholar] [CrossRef]
- Youngson, N.A.; Morris, M.J. What obesity research tells us about epigenetic mechanisms. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20110337. [Google Scholar] [CrossRef]
- Shasa, D.R.; Odhiambo, J.F.; Long, N.M.; Tuersunjiang, N.; Nathanielsz, P.W.; Ford, S.P. Multigenerational impact of maternal overnutrition/obesity in the sheep on the neonatal leptin surge in granddaughters. Int. J. Obes. 2015, 39, 695–701. [Google Scholar] [CrossRef]
- King, S.E.; Skinner, M.K. Epigenetic Transgenerational Inheritance of Obesity Susceptibility. Trends Endocrinol. Metab. 2020, 31, 478–494. [Google Scholar] [CrossRef]
- Schoonejans, J.M.; Ozanne, S.E. Developmental programming by maternal obesity: Lessons from animal models. Diabet. Med. 2021, 38, e14694. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Ye, T.T.; Liu, C.X.; Wang, L.; Chen, Y.W.; Dong, Y. Maternal high-fat diet impairs glucose metabolism, β-cell function and proliferation in the second generation of offspring rats. Nutr. Metab. 2017, 14, 67. [Google Scholar] [CrossRef] [PubMed]
- Graus-Nunes, F.; Dalla Corte Frantz, E.; Lannes, W.R.; da Silva Menezes, M.C.; Mandarim-de-Lacerda, C.A.; Souza-Mello, V. Pregestational maternal obesity impairs endocrine pancreas in male F1 and F2 progeny. Nutrition 2015, 31, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Gregorio, B.M.; Souza-Mello, V.; Mandarim-de-Lacerda, C.A.; Aguila, M.B. Maternal high-fat diet is associated with altered pancreatic remodelling in mice offspring. Eur. J. Nutr. 2013, 52, 759–769. [Google Scholar] [CrossRef]
- Dunn, G.A.; Bale, T.L. Maternal high-fat diet promotes body length increases and insulin insensitivity in second-generation mice. Endocrinology 2009, 150, 4999–5009. [Google Scholar] [CrossRef]
- Mdaki, K.S.; Larsen, T.D.; Wachal, A.L.; Schimelpfenig, M.D.; Weaver, L.J.; Dooyema, S.D.; Louwagie, E.J.; Baack, M.L. Maternal high-fat diet impairs cardiac function in offspring of diabetic pregnancy through metabolic stress and mitochondrial dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H681–H692. [Google Scholar] [CrossRef]
- Mdaki, K.S.; Larsen, T.D.; Weaver, L.J.; Baack, M.L. Age Related Bioenergetics Profiles in Isolated Rat Cardiomyocytes Using Extracellular Flux Analyses. PLoS ONE 2016, 11, e0149002. [Google Scholar] [CrossRef]
- Larsen, T.D.; Sabey, K.H.; Knutson, A.J.; Gandy, T.C.T.; Louwagie, E.J.; Lauterboeck, L.; Mdaki, K.S.; Baack, M.L. Diabetic Pregnancy and Maternal High-Fat Diet Impair Mitochondrial Dynamism in the Developing Fetal Rat Heart by Sex-Specific Mechanisms. Int. J. Mol. Sci. 2019, 20, 3090. [Google Scholar] [CrossRef]
- Upadhyaya, B.; Larsen, T.; Barwari, S.; Louwagie, E.J.; Baack, M.L.; Dey, M. Prenatal Exposure to a Maternal High-Fat Diet Affects Histone Modification of Cardiometabolic Genes in Newborn Rats. Nutrients 2017, 9, 407. [Google Scholar] [CrossRef]
- Preston, C.C.; Larsen, T.D.; Eclov, J.A.; Louwagie, E.J.; Gandy, T.C.T.; Faustino, R.S.; Baack, M.L. Maternal High Fat Diet and Diabetes Disrupts Transcriptomic Pathways That Regulate Cardiac Metabolism and Cell Fate in Newborn Rat Hearts. Front. Endocrinol. 2020, 11, 570846. [Google Scholar] [CrossRef]
- Baack, M.L.; Forred, B.J.; Larsen, T.D.; Jensen, D.N.; Wachal, A.L.; Khan, M.A.; Vitiello, P.F. Consequences of a Maternal High-Fat Diet and Late Gestation Diabetes on the Developing Rat Lung. PLoS ONE 2016, 11, e0160818. [Google Scholar] [CrossRef] [PubMed]
- Louwagie, E.J.; Larsen, T.D.; Wachal, A.L.; Baack, M.L. Placental lipid processing in response to a maternal high-fat diet and diabetes in rats. Pediatr. Res. 2018, 83, 712–722. [Google Scholar] [CrossRef]
- Louwagie, E.J.; Larsen, T.D.; Wachal, A.L.; Gandy, T.C.T.; Eclov, J.A.; Rideout, T.C.; Kern, K.A.; Cain, J.T.; Anderson, R.H.; Mdaki, K.S.; et al. Age and Sex Influence Mitochondria and Cardiac Health in Offspring Exposed to Maternal Glucolipotoxicity. iScience 2020, 23, 101746. [Google Scholar] [CrossRef]
- Ayyappan, P.; Larsen, T.D.; Gandy, T.C.T.; Louwagie, E.J.; Baack, M.L. Impact of Prenatal Exposure to Maternal Diabetes and High-Fat Diet on Postnatal Myocardial Ketone Body Metabolism in Rats. Int. J. Mol. Sci. 2023, 24, 3684. [Google Scholar] [CrossRef] [PubMed]
- Gabory, A.; Attig, L.; Junien, C. Epigenetic mechanisms involved in developmental nutritional programming. World J. Diabetes 2011, 2, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Louwagie, E.J.; Larsen, T.D.; Wachal, A.L.; Gandy, T.C.T.; Baack, M.L. Mitochondrial Transfer Improves Cardiomyocyte Bioenergetics and Viability in Male Rats Exposed to Pregestational Diabetes. Int. J. Mol. Sci. 2021, 22, 2382. [Google Scholar] [CrossRef] [PubMed]
- Marei, W.F.A.; Smits, A.; Mohey-Elsaeed, O.; Pintelon, I.; Ginneberge, D.; Bols, P.E.J.; Moerloose, K.; Leroy, J. Differential effects of high fat diet-induced obesity on oocyte mitochondrial functions in inbred and outbred mice. Sci. Rep. 2020, 10, 9806. [Google Scholar] [CrossRef]
- Mack, L.R.; Tomich, P.G. Gestational Diabetes: Diagnosis, Classification, and Clinical Care. Obstet. Gynecol. Clin. N. Am. 2017, 44, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Klein, A. Generational Experimental Animal Model. 2025. Available online: https://BioRender.com/q3nxb80 (accessed on 24 June 2025).
- Gardner, D.K.; Lane, M.; Stevens, J.; Schlenker, T.; Schoolcraft, W.B. Blastocyst score affects implantation and pregnancy outcome: Towards a single blastocyst transfer. Fertil. Steril. 2000, 73, 1155–1158. [Google Scholar] [CrossRef]
- Capalbo, A.; Rienzi, L.; Cimadomo, D.; Maggiulli, R.; Elliott, T.; Wright, G.; Nagy, Z.P.; Ubaldi, F.M. Correlation between standard blastocyst morphology, euploidy and implantation: An observational study in two centers involving 956 screened blastocysts. Hum. Reprod. 2014, 29, 1173–1181. [Google Scholar] [CrossRef]
- Ai, J.; Jin, L.; Zheng, Y.; Yang, P.; Huang, B.; Dong, X. The Morphology of Inner Cell Mass Is the Strongest Predictor of Live Birth After a Frozen-Thawed Single Embryo Transfer. Front. Endocrinol. 2021, 12, 621221. [Google Scholar] [CrossRef]
- Hao, Y.; Stuart, T.; Kowalski, M.H.; Choudhary, S.; Hoffman, P.; Hartman, A.; Srivastava, A.; Molla, G.; Madad, S.; Fernandez-Granda, C.; et al. Dictionary learning for integrative, multimodal and scalable single-cell analysis. Nat. Biotechnol. 2024, 42, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Min, Z.; Alsolami, S.; Ma, Z.; Zhang, E.; Chen, W.; Zhong, K.; Pei, W.; Kang, X.; Zhang, P.; et al. Generation of human blastocyst-like structures from pluripotent stem cells. Cell Discov. 2021, 7, 81. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Chen, Y.; Ren, Y.; Yuan, P.; Wang, N.; Liu, Q.; Yang, C.; Yan, Z.; Yang, M.; Wang, J.; et al. Primary specification of blastocyst trophectoderm by scRNA-seq: New insights into embryo implantation. Sci. Adv. 2022, 8, eabj3725. [Google Scholar] [CrossRef]
- Proks, M.; Salehin, N.; Brickman, J.M. Deep learning-based models for preimplantation mouse and human embryos based on single-cell RNA sequencing. Nat. Methods 2025, 22, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Guo, G.; Yuan, P.; Ralston, A.; Sun, L.; Huss, M.; Mistri, T.; Pinello, L.; Ng, H.H.; Yuan, G.; et al. The role of Cdx2 as a lineage specific transcriptional repressor for pluripotent network during the first developmental cell lineage segregation. Sci. Rep. 2017, 7, 17156. [Google Scholar] [CrossRef]
- Wicklow, E.; Blij, S.; Frum, T.; Hirate, Y.; Lang, R.A.; Sasaki, H.; Ralston, A. HIPPO Pathway Members Restrict SOX2 to the Inner Cell Mass Where It Promotes ICM Fates in the Mouse Blastocyst. PLoS Genet. 2014, 10, e1004618. [Google Scholar] [CrossRef]
- Pan, G.; Thomson, J.A. Nanog and transcriptional networks in embryonic stem cell pluripotency. Cell Res. 2007, 17, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Frankenberg, S.; Renfree, M.B. On the origin of POU5F1. BMC Biol. 2013, 11, 56. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Schöler, H.R. Role of Oct4 in the early embryo development. Cell Regen. 2014, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Hu, E.; Cai, Y.; Xie, Z.; Luo, X.; Zhan, L.; Tang, W.; Wang, Q.; Liu, B.; Wang, R.; et al. Using clusterProfiler to characterize multiomics data. Nat. Protoc. 2024, 19, 3292–3320. [Google Scholar] [CrossRef]
- Rath, S.; Sharma, R.; Gupta, R.; Ast, T.; Chan, C.; Durham, T.J.; Goodman, R.P.; Grabarek, Z.; Haas, M.E.; Hung, W.H.W.; et al. MitoCarta3.0: An updated mitochondrial proteome now with sub-organelle localization and pathway annotations. Nucleic Acids Res. 2021, 49, D1541–D1547. [Google Scholar] [CrossRef] [PubMed]
- Wooldridge, A.L.; Kirschenman, R.; Spaans, F.; Pasha, M.; Davidge, S.T.; Cooke, C.M. Advanced maternal age alters cardiac functional and structural adaptations to pregnancy in rats. Am. J. Physiol. Heart Circ. Physiol. 2024, 326, H1131–H1137. [Google Scholar] [CrossRef] [PubMed]
- Napso, T.; Hung, Y.-P.; Davidge, S.T.; Care, A.S.; Sferruzzi-Perri, A.N. Advanced maternal age compromises fetal growth and induces sex-specific changes in placental phenotype in rats. Sci. Rep. 2019, 9, 16916. [Google Scholar] [CrossRef]
- Belletti, B.; Prisco, M.; Morrione, A.; Valentinis, B.; Navarro, M.; Baserga, R. Regulation of Id2 Gene Expression by the Insulin-like Growth Factor I Receptor Requires Signaling by Phosphatidylinositol 3-Kinase. J. Biol. Chem. 2001, 276, 13867–13874. [Google Scholar] [CrossRef]
- Zhou, J.X.; Fan, L.X.; Li, X.; Calvet, J.P.; Li, X. TNFα Signaling Regulates Cystic Epithelial Cell Proliferation through Akt/mTOR and ERK/MAPK/Cdk2 Mediated Id2 Signaling. PLoS ONE 2015, 10, e0131043. [Google Scholar] [CrossRef]
- Zhang, Y.; Turunen, M.; Appelkvist, E.L. Restricted uptake of dietary coenzyme Q is in contrast to the unrestricted uptake of alpha-tocopherol into rat organs and cells. J. Nutr. 1996, 126, 2089–2097. [Google Scholar] [CrossRef]
- McKenzie, M.; Tucker, E.J.; Compton, A.G.; Lazarou, M.; George, C.; Thorburn, D.R.; Ryan, M.T. Mutations in the gene encoding C8orf38 block complex I assembly by inhibiting production of the mitochondria-encoded subunit ND1. J. Mol. Biol. 2011, 414, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Parmar, G.; Fong-McMaster, C.; Pileggi, C.A.; Patten, D.A.; Cuillerier, A.; Myers, S.; Wang, Y.; Hekimi, S.; Cuperlovic-Culf, M.; Harper, M.-E. Accessory subunit NDUFB4 participates in mitochondrial complex I supercomplex formation. J. Biol. Chem. 2024, 300, 105626. [Google Scholar] [CrossRef]
- Formosa, L.E.; Dibley, M.G.; Stroud, D.A.; Ryan, M.T. Building a complex complex: Assembly of mitochondrial respiratory chain complex I. Semin. Cell Dev. Biol. 2018, 76, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Klein, A. Exposure-Mediated Transcriptomic Analysis of E4.5 F2 and F3 Embryos. 2025. Available online: https://BioRender.com/av3p88u (accessed on 10 June 2025).
- Klein, A. Exposure-Mediated Transcriptomic Analysis of E4.5 F2 and F3 Embryos in Trophectoderm Cells. 2025. Available online: https://BioRender.com/s0dyit1 (accessed on 10 June 2025).
- Liang, L.; Zhao, Z.; Jin, Q.; Zhang, S.; Zhao, Z.; Li, X. RPA3 promotes the proliferation, migration, and invasion of gliomas by activating the PI3K-AKT-mTOR pathway. Cell. Mol. Biol. 2023, 69, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Zhang, Y.; Sun, Y.; He, L. CCDC127 regulates lipid droplet homeostasis by enhancing mitochondria-ER contacts. Biochem. Biophys. Res. Commun. 2023, 683, 149116. [Google Scholar] [CrossRef]
- Klein, A. Exposure-Mediated Transcriptomic Analysis of E4.5 F2 and F3 Embryos in Inner Cell Mass Cells. 2025. Available online: https://BioRender.com/inj79ef (accessed on 10 June 2025).
- Klein, A. Generational Transcriptomic Analysis of HF+DM E4.5 Embryos. 2025. Available online: https://BioRender.com/4b93xep (accessed on 10 June 2025).
- Sekine, Y.; Hatanaka, R.; Watanabe, T.; Sono, N.; Iemura, S.; Natsume, T.; Kuranaga, E.; Miura, M.; Takeda, K.; Ichijo, H. The Kelch repeat protein KLHDC10 regulates oxidative stress-induced ASK1 activation by suppressing PP5. Mol. Cell 2012, 48, 692–704. [Google Scholar] [CrossRef]
- Bell, C.J.; Gupta, N.; Tremblay, K.D.; Mager, J. Borcs6 is required for endo-lysosomal degradation during early development. Mol. Reprod. Dev. 2022, 89, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhang, Y.; Zhao, S.; Sun, Y. Septin4 as a novel binding partner of PARP1 contributes to oxidative stress induced human umbilical vein endothelial cells injure. Biochem. Biophys. Res. Commun. 2018, 496, 621–627. [Google Scholar] [CrossRef]
- Wang, N.; Xu, F.; Lu, S.; Zhang, N.; Sun, Y. Septin4 as an autophagy modulator regulates Angiotensin-II mediated VSMCs proliferation and migration. Biochem. Biophys. Res. Commun. 2020, 525, 272–279. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, Y.; You, S.; Lu, S.; Zhang, N.; Sun, Y. Septin4 promotes cardiomyocytes apoptosis by enhancing the VHL-mediated degradation of HIF-1α. Cell Death Discov. 2021, 7, 172. [Google Scholar] [CrossRef]
- Bach, L.A. Recent insights into the actions of IGFBP-6. J. Cell Commun. Signal. 2015, 9, 189–200. [Google Scholar] [CrossRef]
- Blyth, A.J.; Kirk, N.S.; Forbes, B.E. Understanding IGF-II Action through Insights into Receptor Binding and Activation. Cells 2020, 9, 2276. [Google Scholar] [CrossRef]
- Soygur, B.; Sati, L. The role of syncytins in human reproduction and reproductive organ cancers. Reproduction 2016, 152, R167–R178. [Google Scholar] [CrossRef]
- Tug, E.; Yirmibes Karaoguz, M.; Nas, T. Expression of the syncytin-1 and syncytin-2 genes in the trophoblastic tissue of the early pregnancy losses with normal and abnormal karyotypes. Gene 2020, 741, 144533. [Google Scholar] [CrossRef]
- Vargas, A.; Moreau, J.; Landry, S.; LeBellego, F.; Toufaily, C.; Rassart, E.; Lafond, J.; Barbeau, B. Syncytin-2 plays an important role in the fusion of human trophoblast cells. J. Mol. Biol. 2009, 392, 301–318. [Google Scholar] [CrossRef]
- Dinh, T.T.H.; Iseki, H.; Mizuno, S.; Iijima-Mizuno, S.; Tanimoto, Y.; Daitoku, Y.; Kato, K.; Hamada, Y.; Hasan, A.S.H.; Suzuki, H.; et al. Disruption of entire Cables2 locus leads to embryonic lethality by diminished Rps21 gene expression and enhanced p53 pathway. eLife 2021, 10, e50346. [Google Scholar] [CrossRef] [PubMed]
- Mak, W.; Nesterova, T.B.; de Napoles, M.; Appanah, R.; Yamanaka, S.; Otte, A.P.; Brockdorff, N. Reactivation of the paternal X chromosome in early mouse embryos. Science 2004, 303, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Shiura, H.; Abe, K. Xist/Tsix expression dynamics during mouse peri-implantation development revealed by whole-mount 3D RNA-FISH. Sci. Rep. 2019, 9, 3637. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Isotani, A.; Mise, N.; Yamamoto, M.; Fujihara, Y.; Kaseda, K.; Nakanishi, T.; Ikawa, M.; Hamada, H.; Abe, K.; et al. Comparison of gene expression in male and female mouse blastocysts revealed imprinting of the X-linked gene, Rhox5/Pem, at preimplantation stages. Curr. Biol. 2006, 16, 166–172. [Google Scholar] [CrossRef]
- Petropoulos, S.; Edsgärd, D.; Reinius, B.; Deng, Q.; Panula, S.P.; Codeluppi, S.; Plaza Reyes, A.; Linnarsson, S.; Sandberg, R.; Lanner, F. Single-Cell RNA-Seq Reveals Lineage and X Chromosome Dynamics in Human Preimplantation Embryos. Cell 2016, 165, 1012–1026. [Google Scholar] [CrossRef] [PubMed]
- Matos, L.; Stevenson, D.; Gomes, F.; Silva-Carvalho, J.L.; Almeida, H. Superoxide dismutase expression in human cumulus oophorus cells. Mol. Hum. Reprod. 2009, 15, 411–419. [Google Scholar] [CrossRef]
- Asano, E.; Hasegawa, H.; Hyodo, T.; Ito, S.; Maeda, M.; Chen, D.; Takahashi, M.; Hamaguchi, M.; Senga, T. SHCBP1 is required for midbody organization and cytokinesis completion. Cell Cycle 2014, 13, 2744–2751. [Google Scholar] [CrossRef]
- He, X.; Lin, Z.; Ning, J.; Li, N.; Cui, X.; Zhao, B.; Hong, F.; Miao, J. Promoting TTC4 and HSP70 interaction and translocation of annexin A7 to lysosome inhibits apoptosis in vascular endothelial cells. FASEB J. 2020, 34, 12932–12945. [Google Scholar] [CrossRef]
- Crevel, G.; Bennett, D.; Cotterill, S. The human TPR protein TTC4 is a putative Hsp90 co-chaperone which interacts with CDC6 and shows alterations in transformed cells. PLoS ONE 2008, 3, e0001737. [Google Scholar] [CrossRef]
- De Pace, R.; Maroofian, R.; Paimboeuf, A.; Zamani, M.; Zaki, M.S.; Sadeghian, S.; Azizimalamiri, R.; Galehdari, H.; Zeighami, J.; Williamson, C.D.; et al. Biallelic BORCS8 variants cause an infantile-onset neurodegenerative disorder with altered lysosome dynamics. Brain 2024, 147, 1751–1767. [Google Scholar] [CrossRef]
- Anufrieva, K.S.; Shender, V.; Arapidi, G.P.; Pavlyukov, M.S.; Shakhparonov, M.I.; Shnaider, P.V.; Butenko, I.O.; Lagarkova, M.A.; Govorun, V.M. Therapy-induced stress response is associated with downregulation of pre-mRNA splicing in cancer cells. Genome Med. 2018, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Asada, S.; Goyama, S.; Inoue, D.; Shikata, S.; Takeda, R.; Fukushima, T.; Yonezawa, T.; Fujino, T.; Hayashi, Y.; Kawabata, K.C.; et al. Mutant ASXL1 cooperates with BAP1 to promote myeloid leukaemogenesis. Nat. Commun. 2018, 9, 2733. [Google Scholar] [CrossRef]
- Selesniemi, K.; Albers, R.E.; Brown, T.L. Id2 Mediates Differentiation of Labyrinthine Placental Progenitor Cell Line, SM10. Stem Cells Dev. 2016, 25, 959–974. [Google Scholar] [CrossRef] [PubMed]
- Tiane, A.; Schepers, M.; Riemens, R.; Rombaut, B.; Vandormael, P.; Somers, V.; Prickaerts, J.; Hellings, N.; van den Hove, D.; Vanmierlo, T. DNA methylation regulates the expression of the negative transcriptional regulators ID2 and ID4 during OPC differentiation. Cell. Mol. Life Sci. 2021, 78, 6631–6644. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Vogel, G.; Yu, Z.; Almazan, G.; Richard, S. Type II arginine methyltransferase PRMT5 regulates gene expression of inhibitors of differentiation/DNA binding Id2 and Id4 during glial cell differentiation. J. Biol. Chem. 2011, 286, 44424–44432. [Google Scholar] [CrossRef] [PubMed]
- Richard, E.; Marchuk, H.; Álvarez, M.; He, W.; Chen, X.; Desviat, L.R.; Zhang, G.F. Metabolic flux analysis in hiPSC-CMs reveals insights into cardiac dysfunction in propionic acidemia. Cell. Mol. Life Sci. 2025, 82, 137. [Google Scholar] [CrossRef]
- Hsu, C.C.; Wang, C.Y.; Manne, R.K.; Cai, Z.; Penugurti, V.; Kant, R.; Bai, L.; Pan, B.S.; Chen, T.; Chen, Y.R.; et al. ALDH4A1 functions as an active component of the MPC complex maintaining mitochondrial pyruvate import for TCA cycle entry and tumour suppression. Nat. Cell Biol. 2025, 27, 847–862. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Yu, M.; Qin, J.; Luo, Y.; Zhong, M. LACTB Regulates PIK3R3 to Promote Autophagy and Inhibit EMT and Proliferation Through the PI3K/AKT/mTOR Signaling Pathway in Colorectal Cancer. Cancer Manag. Res. 2020, 12, 5181–5200. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, N.R.; Nandan, D.; Nair, B.G.; Nair, V.A.; Venugopal, P.; Aradhya, R. Oxidative Stress and Redox Imbalance: Common Mechanisms in Cancer Stem Cells and Neurodegenerative Diseases. Cells 2025, 14, 511. [Google Scholar] [CrossRef] [PubMed]
- Pizzuti, V.; Paris, F.; Marrazzo, P.; Bonsi, L.; Alviano, F. Mitigating Oxidative Stress in Perinatal Cells: A Critical Step toward an Optimal Therapeutic Use in Regenerative Medicine. Biomolecules 2023, 13, 971. [Google Scholar] [CrossRef]
- Liang, J.; Wu, M.; Chen, C.; Mai, M.; Huang, J.; Zhu, P. Roles of Reactive Oxygen Species in Cardiac Differentiation, Reprogramming, and Regenerative Therapies. Oxidative Med. Cell. Longev. 2020, 2020, 2102841. [Google Scholar] [CrossRef]
- Weng, Z.; Wang, Y.; Ouchi, T.; Liu, H.; Qiao, X.; Wu, C.; Zhao, Z.; Li, L.; Li, B. Mesenchymal Stem/Stromal Cell Senescence: Hallmarks, Mechanisms, and Combating Strategies. Stem Cells Transl. Med. 2022, 11, 356–371. [Google Scholar] [CrossRef]
- Zhang, B.; Pan, C.; Feng, C.; Yan, C.; Yu, Y.; Chen, Z.; Guo, C.; Wang, X. Role of mitochondrial reactive oxygen species in homeostasis regulation. Redox Rep. 2022, 27, 45–52. [Google Scholar] [CrossRef]
- Maldonado, E.; Morales-Pison, S.; Urbina, F.; Solari, A. Aging Hallmarks and the Role of Oxidative Stress. Antioxidants 2023, 12, 651. [Google Scholar] [CrossRef]
- Morganti, C.; Ito, K. Mitochondrial Contributions to Hematopoietic Stem Cell Aging. Int. J. Mol. Sci. 2021, 22, 11117. [Google Scholar] [CrossRef]
- Arai, F.; Suda, T. Quiescent stem cells in the niche. In StemBook; Harvard Stem Cell Institute: Cambridge, MA, USA; Fumio Arai and Toshio Suda: Cambridge, MA, USA, 2008. [Google Scholar]
- Roato, I.; Visca, M.; Mussano, F. Suppressing the Aging Phenotype of Mesenchymal Stromal Cells: Are We Ready for Clinical Translation? Biomedicines 2024, 12, 2811. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Yu, X.; Zhang, C.; Wang, Y.; Sun, Y.; Sun, H.; Zhang, H.; Shi, Y.; He, X. Telomeres and Mitochondrial Metabolism: Implications for Cellular Senescence and Age-related Diseases. Stem Cell Rev. Rep. 2022, 18, 2315–2327. [Google Scholar] [CrossRef] [PubMed]
- Mongelli, A.; Mengozzi, A.; Geiger, M.; Gorica, E.; Mohammed, S.A.; Paneni, F.; Ruschitzka, F.; Costantino, S. Mitochondrial epigenetics in aging and cardiovascular diseases. Front. Cardiovasc. Med. 2023, 10, 1204483. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Z.; Li, C.; Song, Y.; Wang, Y.; Bo, H.; Zhang, Y. Impact of Exercise and Aging on Mitochondrial Homeostasis in Skeletal Muscle: Roles of ROS and Epigenetics. Cells 2022, 11, 2086. [Google Scholar] [CrossRef]
- Musson, R.; Gąsior, Ł.; Bisogno, S.; Ptak, G.E. DNA damage in preimplantation embryos and gametes: Specification, clinical relevance and repair strategies. Hum. Reprod. Update 2022, 28, 376–399. [Google Scholar] [CrossRef]
- Ye, Q.; Zeng, X.; Cai, S.; Qiao, S.; Zeng, X. Mechanisms of lipid metabolism in uterine receptivity and embryo development. Trends Endocrinol. Metab. 2021, 32, 1015–1030. [Google Scholar] [CrossRef]
- de Andrade Melo-Sterza, F.; Poehland, R. Lipid Metabolism in Bovine Oocytes and Early Embryos under In Vivo, In Vitro, and Stress Conditions. Int. J. Mol. Sci. 2021, 22, 3421. [Google Scholar] [CrossRef]
- Chevalley, T.; Dübi, M.; Fumeaux, L.; Merli, M.S.; Sarre, A.; Schaer, N.; Simeoni, U.; Yzydorczyk, C. Sexual Dimorphism in Cardiometabolic Diseases: From Development to Senescence and Therapeutic Approaches. Cells 2025, 14, 467. [Google Scholar] [CrossRef]
- Eriksson, J.G.; Kajantie, E.; Osmond, C.; Thornburg, K.; Barker, D.J. Boys live dangerously in the womb. Am. J. Hum. Biol. 2010, 22, 330–335. [Google Scholar] [CrossRef]
- Sandovici, I.; Fernandez-Twinn, D.S.; Hufnagel, A.; Constância, M.; Ozanne, S.E. Sex differences in the intergenerational inheritance of metabolic traits. Nat. Metab. 2022, 4, 507–523. [Google Scholar] [CrossRef] [PubMed]
- Tiberi, J.; Cesarini, V.; Stefanelli, R.; Canterini, S.; Fiorenza, M.T.; La Rosa, P. Sex differences in antioxidant defence and the regulation of redox homeostasis in physiology and pathology. Mech. Ageing Dev. 2023, 211, 111802. [Google Scholar] [CrossRef]
- Kander, M.C.; Cui, Y.; Liu, Z. Gender difference in oxidative stress: A new look at the mechanisms for cardiovascular diseases. J. Cell. Mol. Med. 2017, 21, 1024–1032. [Google Scholar] [CrossRef]
- Ramakrishna, N.B.; Murison, K.; Miska, E.A.; Leitch, H.G. Epigenetic Regulation during Primordial Germ Cell Development and Differentiation. Sex. Dev. 2021, 15, 411–431. [Google Scholar] [CrossRef]
- Gabory, A.; Attig, L.; Junien, C. Developmental programming and epigenetics. Am. J. Clin. Nutr. 2011, 94, S1943–S1952. [Google Scholar] [CrossRef]
- Inoue, A.; Jiang, L.; Lu, F.; Suzuki, T.; Zhang, Y. Maternal H3K27me3 controls DNA methylation-independent imprinting. Nature 2017, 547, 419–424. [Google Scholar] [CrossRef]
- Hanna, C.W.; Kelsey, G. Genomic imprinting beyond DNA methylation: A role for maternal histones. Genome Biol. 2017, 18, 177. [Google Scholar] [CrossRef]
- Shao, T.; Ji, J.F.; Zheng, J.Y.; Li, C.; Zhu, L.Y.; Fan, D.D.; Lin, A.F.; Xiang, L.X.; Shao, J.Z. Zbtb46 Controls Dendritic Cell Activation by Reprogramming Epigenetic Regulation of cd80/86 and cd40 Costimulatory Signals in a Zebrafish Model. J. Immunol. 2022, 208, 2686–2701. [Google Scholar] [CrossRef]
- Zeng, Q.; Jin, F.; Qian, H.; Chen, H.; Wang, Y.; Zhang, D.; Wei, Y.; Chen, T.; Guo, B.; Chai, C. The miR-345-3p/PPP2CA signaling axis promotes proliferation and invasion of breast cancer cells. Carcinogenesis 2022, 43, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.J.; Hashimoto, K.; Fujio, K.; Hayashi, K.; Paul, S.K.; Yuzuriha, A.; Qiu, W.-Y.; Nakamura, E.; Kanashiro, M.A.; Kabata, M.; et al. A let-7 microRNA-RALB axis links the immune properties of iPSC-derived megakaryocytes with platelet producibility. Nat. Commun. 2024, 15, 2588. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Li, X.; Bai, C.; Yang, Z.; Zhang, L.; Luo, J.; Zhang, W. lncRNA MIR503HG Targets miR-191-5p/PLCD1 Axis and Negatively Modulates Apoptosis, Extracellular Matrix Disruption, and Inflammation in Abdominal Aortic Aneurysm. Mediat. Inflamm. 2023, 2023, 4003618. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ouyang, Y.; Feng, Z.; Jiang, Z.; Ma, J.; Zhou, X.; Cai, C.; Han, Y.; Zeng, S.; Liu, S.; et al. RASGRP2 is a potential immune-related biomarker and regulates mitochondrial-dependent apoptosis in lung adenocarcinoma. Front. Immunol. 2023, 14, 1100231. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.D.; Tseng, Y.T.; Shrestha, S.; Lin, Y.L.; Khaleel, A.; Chou, C.H.; Chu, C.F.; Huang, H.Y.; Lin, C.M.; Ho, S.Y.; et al. miRTarBase update 2014: An information resource for experimentally validated miRNA-target interactions. Nucleic Acids Res. 2014, 42, D78–D85. [Google Scholar] [CrossRef]
- Garcia, D.M.; Baek, D.; Shin, C.; Bell, G.W.; Grimson, A.; Bartel, D.P. Weak seed-pairing stability and high target-site abundance decrease the proficiency of lsy-6 and other microRNAs. Nat. Struct. Mol. Biol. 2011, 18, 1139–1146. [Google Scholar] [CrossRef] [PubMed]
- Kurzawa, R.; Głabowski, W.; Baczkowski, T.; Brelik, P. Evaluation of mouse preimplantation embryos exposed to oxidative stress cultured with insulin-like growth factor I and II, epidermal growth factor, insulin, transferrin and selenium. Reprod. Biol. 2002, 2, 143–162. [Google Scholar] [PubMed]
- Gali Ramamoorthy, T.; Allen, T.-J.; Davies, A.; Harno, E.; Sefton, C.; Murgatroyd, C.; White, A. Maternal overnutrition programs epigenetic changes in the regulatory regions of hypothalamic Pomc in the offspring of rats. Int. J. Obes. 2018, 42, 1431–1444. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.H.; Zhu, C.C.; Ju, J.Q.; Xu, Y.; Luo, S.M.; Sun, S.C.; Ou, X.H. Single-cell transcriptome analysis reveals that maternal obesity affects DNA repair, histone methylation, and autophagy level in mouse embryos. J. Cell. Physiol. 2021, 236, 4944–4953. [Google Scholar] [CrossRef]
- de Barros Mucci, D.; Kusinski, L.C.; Wilsmore, P.; Loche, E.; Pantaleão, L.C.; Ashmore, T.J.; Blackmore, H.L.; Fernandez-Twinn, D.S.; Carmo, M.d.G.T.d.; Ozanne, S.E. Impact of maternal obesity on placental transcriptome and morphology associated with fetal growth restriction in mice. Int. J. Obes. 2020, 44, 1087–1096. [Google Scholar] [CrossRef]
- Stuart, T.J.; O’Neill, K.; Condon, D.; Sasson, I.; Sen, P.; Xia, Y.; Simmons, R.A. Diet-induced obesity alters the maternal metabolome and early placenta transcriptome and decreases placenta vascularity in the mouse. Biol. Reprod. 2018, 98, 795–809. [Google Scholar] [CrossRef]
- McCoski, S.R.; Poole, R.K.; Vailes, M.T.; Ealy, A.D. Maternal obesity alters the expression of embryonic regulatory transcripts in the preimplantation ovine conceptus. Reprod. Biol. 2018, 18, 198–201. [Google Scholar] [CrossRef]
- Ravisankar, S.; Murphy, M.J.; Redmayne-Titley, N.; Davis, B.; Luo, F.; Takahashi, D.; Hennebold, J.D.; Chavez, S.L. Long-term Hyperandrogenemia and/or Western-style Diet in Rhesus Macaque Females Impairs Preimplantation Embryogenesis. Endocrinology 2022, 163, bqac019. [Google Scholar] [CrossRef]
- Klein, A. Graphical Abstract. 2025. Available online: https://BioRender.com/vfdg46q (accessed on 30 June 2025).


| Poor | Good | Great | |
|---|---|---|---|
| Expansion Score (EXS) | 1: No discernable blastocoel cavity 2: Blastocoel present but is less than half of the embryo volume | 3: Blastocoel that fills more than half of the embryo, but not fully expanded; space between blastomeres and zona pellucida | 4: Fully expanded with a blastocoel that is flush with the zona pellucida |
| Trophectoderm Score (TS) | C: TE layer not cohesive or unable to be differentiated from other blastomeres | B: TE forms a cohesive layer but individual blastomeres can still be appreciated | A: TE forms a thin, cohesive layer |
| Inner Cell Mass Score (ICMS) | C: No discernable ICM or unable to be localized to one side of the embryo | B: ICM localized to one side but individual blastomeres can still be appreciated instead of being compact | A: ICM is localized to one side and completely compact |
| Parameter | CON n = 15 | HF+DM n = 10 | p-Value |
|---|---|---|---|
| Maternal age (weeks) | 14.48 ± 0.48 | 15.16 ± 0.27 | 0.0913 |
| Maternal age range (weeks) | 13.14–19.29 | 14.00–16.00 | 0.0913 |
| Weight at baseline (g) | 208.20 ± 2.25 | 173.30 ± 2.40 | <0.0001 |
| Weight at post diet (g) | 230.00 ± 2.80 | 252.10 ± 2.29 | <0.0001 |
| Weight at GD14 (g) | 278.10 ± 4.88 | 302.60 ± 2.83 | 0.0008 |
| Weight at P1 (g) | 248.10 ± 3.53 | 273.40 ± 5.28 | 0.0004 |
| Weight gain post diet (g) | 21.80 ± 1.46 | 78.80 ± 2.83 | <0.0001 |
| Weight gain post diet to GD14 (g) | 46.82 ± 3.35 | 51.00 ± 2.12 | 0.3299 |
| Weight gain GD14 to P1 (g) | −37.64 ± 9.96 | −29.11 ± 4.17 | 0.475 |
| Weight gain post diet to P1 (g) | 18.36 ± 3.73 | 21.89 ± 4.54 | 0.5563 |
| Glucose at baseline (mg/dL) | 103.10 ± 2.77 | 99.50 ± 2.69 | 0.387 |
| Glucose post diet (mg/dL) | 97.47 ± 3.31 | 89.60 ± 1.31 | 0.0749 |
| Glucose GD15-GD21 (mg/dL) | 87.50 ± 1.87 | 357.00 ± 18.62 | <0.0001 |
| Glucose at P1 (mg/dL) | 108.60 ± 4.05 | 516.10 ± 35.43 | <0.0001 |
| Triglycerides at baseline (mg/dL) | 74.16 ± 4.49 | 91.96 ± 6.16 | 0.0254 |
| Triglycerides post diet (mg/dL) | 92.40 ± 6.96 | 130.70 ± 12.63 | 0.0131 |
| Triglycerides at P1 (mg/dL) | 31.06 ± 4.52 | 122.20 ± 12.72 | <0.0001 |
| Total placentations at P1 | 13.42 ± 1.21 | 14.88 ± 0.72 | 0.5039 |
| Live offspring at P1 | 11.14 ± 0.97 | 12.50 ± 1.91 | 0.1211 |
| Parameter | CON n = 30 | HF+DM n = 24 | p-Value |
|---|---|---|---|
| Birthweight (g) | 5.95 ± 0.12 | 6.15 ± 0.48 | 0.218 |
| Adult weight (g) | 286.00 ± 3.27 | 293.60 ± 2.74 | 0.0943 |
| Maternal age (weeks) | 20.09 ± 0.34 | 24.60 ± 0.57 | <0.0001 |
| Maternal age range (weeks) | 17.00–23.43 | 21.43–30.71 | <0.0001 |
| Ovary weight (mg) | 52.02 ± 1.27 | 62.57 ± 1.45 | <0.0001 |
| Ovary–body weight (mg/g) | 0.18 ± 0.01 | 0.22 ± 0.01 | <0.0001 |
| Breeding days | 2.35 ± 0.33 | 1.94 ± 0.23 | 0.5521 |
| Embryos/litter | 8.00 ± 0.94 | 9.90 ± 0.83 | 0.158 |
| Glucose at E4.5 (mg/dL) | 92.52 ± 3.00 | 94.79 ± 3.26 | 0.6107 |
| Triglycerides at E4.5 (mg/dL) | 109.40 ± 5.19 | 120.00 ± 6.19 | 0.1942 |
| Parameter | CON n = 14 | HF+DM n = 16 | p-Value |
|---|---|---|---|
| Birthweight (g) | 6.77 ± 0.20 | 6.75 ± 0.07 | 0.9228 |
| Adult weight (g) | 289.20 ± 3.83 | 270.90 ± 4.72 | 0.0062 |
| Maternal age (weeks) | 16.64 ± 0.54 | 16.82 ± 0.89 | 0.4162 |
| Maternal age range (weeks) | 11.43–20.00 | 13.71–27.86 | 0.4162 |
| Ovary weight (mg) | 45.84 ± 1.23 | 52.06 ± 2.04 | 0.0146 |
| Ovary–body weight (mg) | 0.16 ± 0.00 | 0.19 ± 0.01 | 0.0005 |
| Breeding days | 2.67 ± 0.30 | 2.30 ± 0.30 | 0.4106 |
| Embryos/litter | 10.25 ± 0.91 | 8.60 ± 0.79 | 0.0459 |
| Glucose at E4.5 (mg/dL) | 88.67 ± 4.05 | 98.50 ± 2.92 | 0.0533 |
| Triglycerides at E4.5 (mg/dL) | 114.70 ± 7.61 | 91.67 ± 9.76 | 0.0747 |
| Gene ID | p-Value | Log2_FC | Adjusted p-Value |
|---|---|---|---|
| Id2 | 2.23 × 10−6 | −1.81609 | 0.027468 |
| AABR07025818.1 | 1.16 × 10−5 | 2.050856 | 0.142563 |
| Abcf3 | 2.5 × 10−5 | −3.593 | 0.306855 |
| Naa20 | 3.43 × 10−5 | −1.58134 | 0.42136 |
| Ints7 | 0.000103 | −2.32354 | 1 |
| Mrpl3 | 0.000343 | −1.64547 | 1 |
| Bub3 | 0.000544 | −1.745 | 1 |
| H3f3c | 0.000553 | −1.2829 | 1 |
| ENSRNOG00000066033 | 0.000611 | −2.36167 | 1 |
| Ifitm2 | 0.000663 | 1.348301 | 1 |
| Ppp2ca | 0.0007 | −1.18861 | 1 |
| Ilf2 | 0.000741 | −1.2681 | 1 |
| Plcd1 | 0.000753 | −2.92558 | 1 |
| Zbtb46 | 0.000971 | 3.74685 | 1 |
| Rasgrp2 | 0.000971 | 3.74685 | 1 |
| ENSRNOG00000021395 | 0.001041 | 2.009885 | 1 |
| Ralb | 0.001146 | −2.83811 | 1 |
| Eif2d | 0.001146 | −2.83811 | 1 |
| Pafah1b2 | 0.001146 | −3.16004 | 1 |
| Ciapin1 | 0.001485 | −1.51994 | 1 |
| Gene ID | p-Value | Log2_FC | Adjusted p-Value |
|---|---|---|---|
| Igfbp6 | 3.65 × 10−19 | 4.5062336 | 4.49 × 10−15 |
| ENSRNOG00000066117 | 4.89 × 10−17 | 2.2258475 | 6.01 × 10−13 |
| Rpl39.4 | 6.60 × 10−12 | 1.0457567 | 8.12 × 10−8 |
| ENSRNOG00000021395 | 2.04 × 10−11 | 4.2120505 | 2.51 × 10−7 |
| Rpl35al1 | 7.40 × 10−11 | 1.5173244 | 9.10 × 10−7 |
| Snrpg.1 | 1.94 × 10−9 | 1.0166251 | 2.39 × 10−5 |
| Pllp | 3.17 × 10−9 | 1.0429563 | 3.8998 × 10−5 |
| S100a10 | 6.55 × 10−8 | 1.3552269 | 0.00080551 |
| Actn4 | 1.52 × 10−7 | −1.0014088 | 0.00187237 |
| Gng5 | 4.654 × 10−7 | −1.5479987 | 0.00572219 |
| Ckmt1 | 6.238 × 10−7 | −1.035877 | 0.00767044 |
| Arpp19.1 | 1.635 × 10−6 | 1.9381278 | 0.02009886 |
| Ccne1 | 3.284 × 10−6 | 1.4799837 | 0.04037549 |
| Ankrd11 | 3.403 × 10−6 | −1.3870625 | 0.04184274 |
| Eef1e1 | 5.506 × 10−6 | 1.0792953 | 0.06770111 |
| ENSRNOG00000066033 | 9.522 × 10−6 | −2.1043181 | 0.11708198 |
| Rbx1 | 4.511 × 10−5 | 2.2120505 | 0.55472283 |
| Rspry1 | 6.991 × 10−5 | −3.9172325 | 0.85964477 |
| Mrpl22 | 8.062 × 10−5 | 1.1104102 | 0.9912706 |
| Mtres1 | 0.0001019 | 1.320864 | 0.55238095 |
| Gene ID | p-Value | Log2_FC | Adjusted p-Value |
|---|---|---|---|
| Arf4 | 2.56 × 10−6 | 1.713882 | 0.03149491 |
| Rpl35al1 | 3.23 × 10−6 | 1.1983587 | 0.03976302 |
| Thumpd3 | 4.19 × 10−6 | 1.6076826 | 0.05146818 |
| S100a10 | 7.05 × 10−6 | 1.3335076 | 0.08665579 |
| Rac1 | 7.21 × 10−6 | 1.3608454 | 0.0886293 |
| Mt-nd3 | 1.03 × 10−5 | 1.5909069 | 0.12687993 |
| ENSRNOG00000067072 | 1.09 × 10−5 | 2 | 0.13350203 |
| Mt-nd6 | 3.36 × 10−5 | 2.2306129 | 0.41298922 |
| Bub3 | 5.29 × 10−5 | 1.6737718 | 0.6501446 |
| Polr1g | 5.8 × 10−5 | 1.3081223 | 0.71298689 |
| Arfrp1 | 9.56 × 10−5 | 1.4150375 | 1 |
| Phlda2 | 0.000132 | 3.1926451 | 1 |
| Klhdc10 | 0.000132 | 3.3081223 | 1 |
| Rpl9 | 0.000135 | −1.266787 | 1 |
| Naa20 | 0.000181 | 1.3225254 | 1 |
| Hspa14 | 0.000213 | 1.474416 | 1 |
| Ccz1b | 0.000238 | 1.2097186 | 1 |
| Atp1b1 | 0.000313 | 1.6265416 | 1 |
| Pfdn4 | 0.000318 | 1.0036113 | 1 |
| MGC116121 | 0.00047 | −2.714246 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klein, A.K.; Derenge, B.P.; Mukherjee, M.; Reddy, S.P.; Larsen, T.D.; Ayyappan, P.; Gandy, T.C.T.; Siemers, K.M.; Kareta, M.S.; Baack, M.L. The Transgenerational Impact of High-Fat Diet and Diabetic Pregnancy on Embryonic Transcriptomics and Mitochondrial Health. Biomedicines 2025, 13, 2019. https://doi.org/10.3390/biomedicines13082019
Klein AK, Derenge BP, Mukherjee M, Reddy SP, Larsen TD, Ayyappan P, Gandy TCT, Siemers KM, Kareta MS, Baack ML. The Transgenerational Impact of High-Fat Diet and Diabetic Pregnancy on Embryonic Transcriptomics and Mitochondrial Health. Biomedicines. 2025; 13(8):2019. https://doi.org/10.3390/biomedicines13082019
Chicago/Turabian StyleKlein, Abigail K., Benjamin P. Derenge, Malini Mukherjee, Srikrishna P. Reddy, Tricia D. Larsen, Prathapan Ayyappan, Tyler C. T. Gandy, Kyle M. Siemers, Michael S. Kareta, and Michelle L. Baack. 2025. "The Transgenerational Impact of High-Fat Diet and Diabetic Pregnancy on Embryonic Transcriptomics and Mitochondrial Health" Biomedicines 13, no. 8: 2019. https://doi.org/10.3390/biomedicines13082019
APA StyleKlein, A. K., Derenge, B. P., Mukherjee, M., Reddy, S. P., Larsen, T. D., Ayyappan, P., Gandy, T. C. T., Siemers, K. M., Kareta, M. S., & Baack, M. L. (2025). The Transgenerational Impact of High-Fat Diet and Diabetic Pregnancy on Embryonic Transcriptomics and Mitochondrial Health. Biomedicines, 13(8), 2019. https://doi.org/10.3390/biomedicines13082019








