Obesity and COVID-19: Pathophysiological Insights and Pulmonary Complications in a Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Collection
- The severity of COVID-19 (mild, moderate, or severe), as defined by the WHO criteria [9];
- Pulmonary involvement, based on chest imaging (X-ray or Computed Tomography scan), classified as mild (<25%), moderate (25–50%), or severe (>50%);
- Evolution during hospitalization (recovered, improved, stable, deteriorated, or death);
- ICU (intensive care unit) admission;
- The length of hospital stay (days).
2.3. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
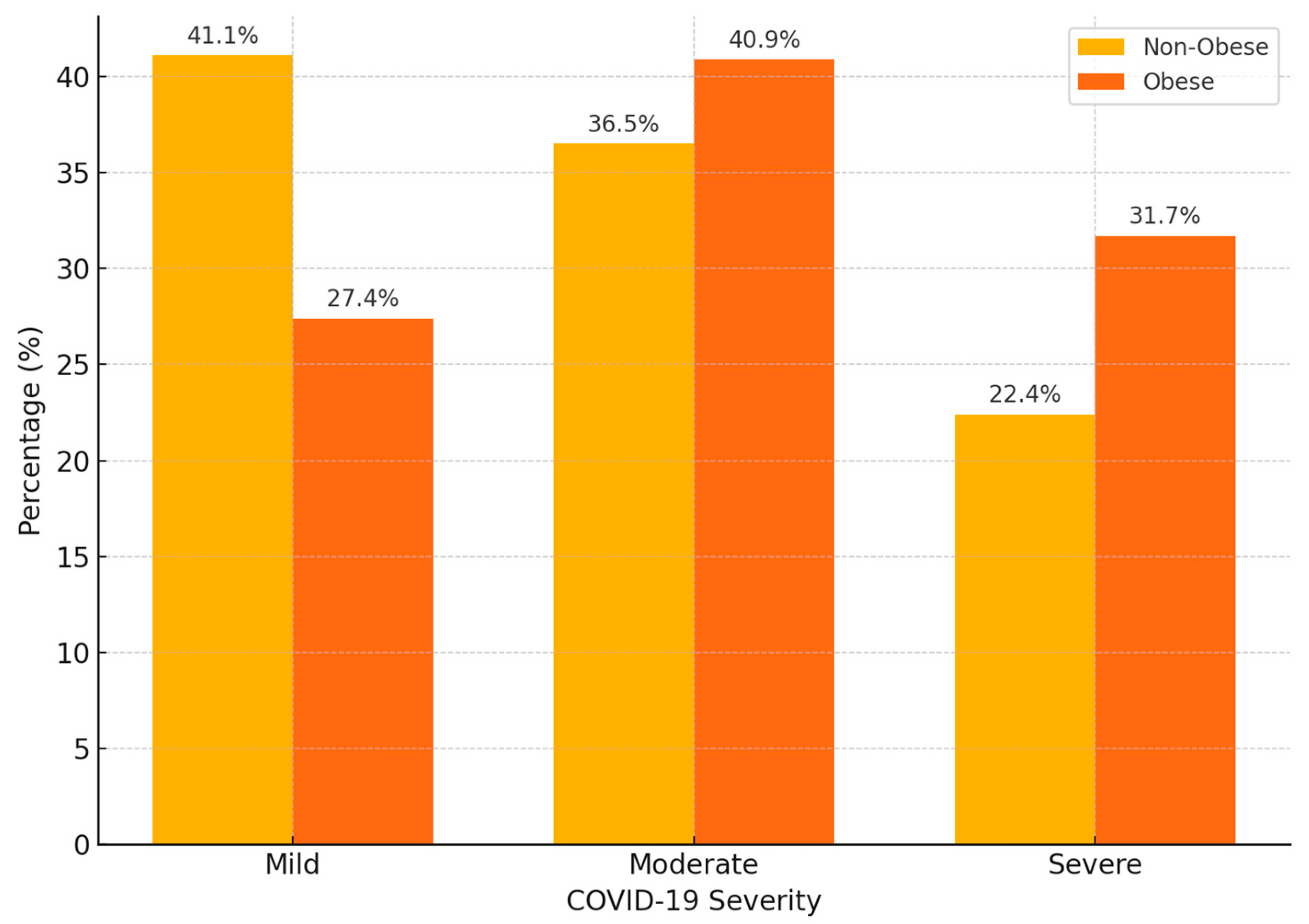
3.2. Association Between Obesity and COVID-19 Severity
3.3. Pulmonary Involvement According to Obesity Status
3.4. Clinical Course During Hospitalization
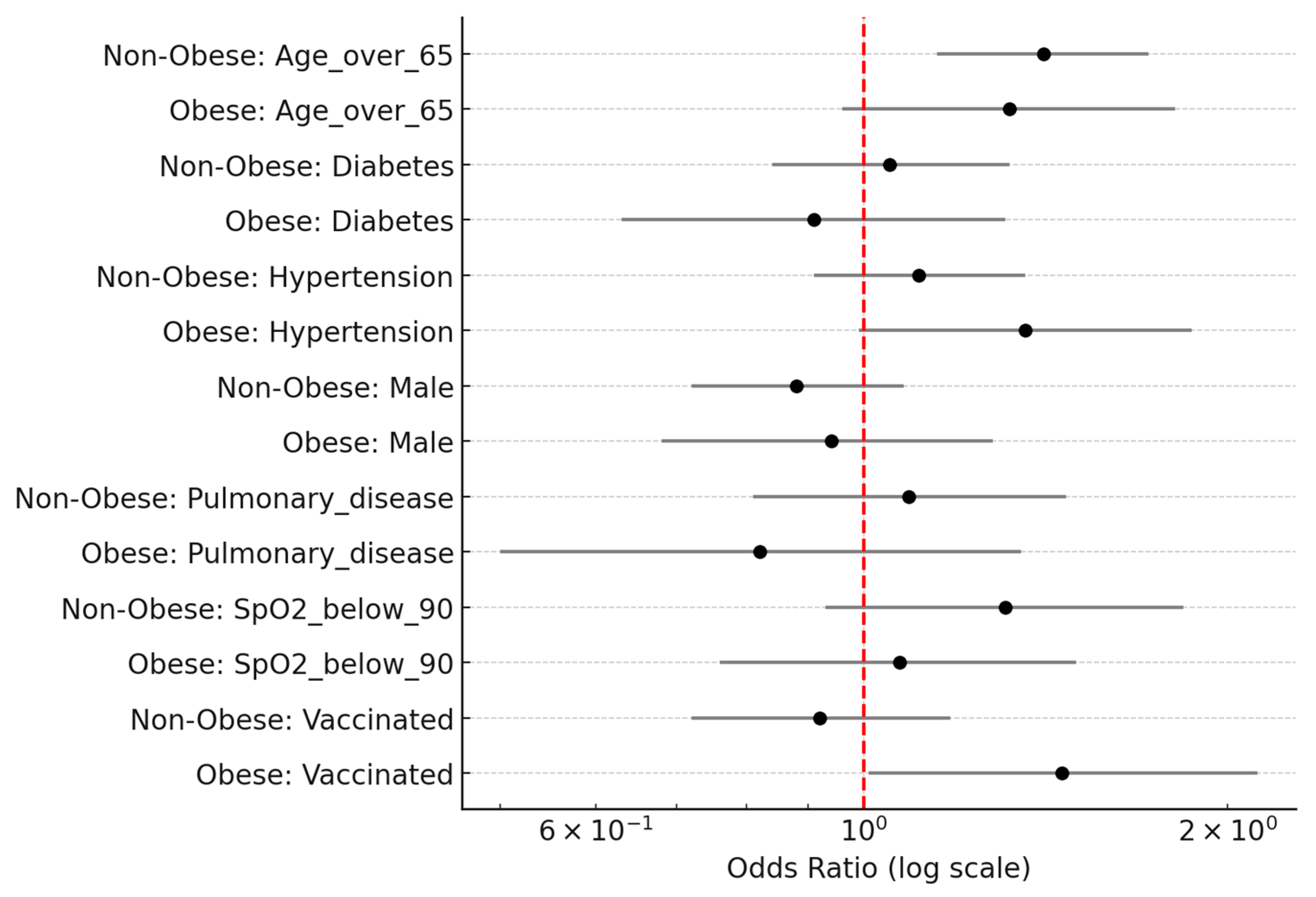
3.5. Multivariate Analysis: Predictors of Severe COVID-19
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hacker, K. The Burden of Chronic Disease. Mayo Clin. Proc. Innov. Qual. Outcomes 2024, 8, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Griera, J.L.; Manzanares, J.M.; Barbany, M.; Contreras, J.; Amigó, P.; Salas-Salvadó, J. Physical Activity, Energy Balance and Obesity. Public Health Nutr. 2007, 10, 1194–1199. [Google Scholar] [CrossRef] [PubMed]
- Manchia, M.; Gathier, A.W.; Yapici-Eser, H.; Schmidt, M.V.; de Quervain, D.; van Amelsvoort, T.; Bisson, J.I.; Cryan, J.F.; Howes, O.D.; Pinto, L.; et al. The Impact of the Prolonged COVID-19 Pandemic on Stress Resilience and Mental Health: A Critical Review across Waves. Eur. Neuropsychopharmacol. 2022, 55, 22–83. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Bettini, S.; Boschetti, M.; Barrea, L.; Savastano, S.; Colao, A.; OPERA Group. Low-Grade Inflammation, COVID-19, and Obesity: Clinical Aspect and Molecular Insights in Childhood and Adulthood. Int. J. Obes. 2022, 46, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Demeulemeester, F.; de Punder, K.; van Heijningen, M.; van Doesburg, F. Obesity as a Risk Factor for Severe COVID-19 and Complications: A Review. Cells 2021, 10, 933. [Google Scholar] [CrossRef]
- Zhu, X.; Yang, L.; Huang, K. COVID-19 and Obesity: Epidemiology, Pathogenesis and Treatment. Diabetes Metab. Syndr. Obes. 2020, 13, 4953–4959. [Google Scholar] [CrossRef]
- Pearce, C.; Rychetnik, L.; Wutzke, S.; Wilson, A.; Duggan, R.; Pegram, R.; Phillips, C.; Taylor, J. Obesity Prevention and the Role of Hospital and Community-Based Health Services: A Scoping Review. BMC Health Serv. Res. 2019, 19, 453. [Google Scholar] [CrossRef] [PubMed]
- Weir, C.B.; Jan, A. BMI Classification Percentile and Cut Off Points. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK541070/ (accessed on 3 July 2025).
- Guérin, P.J.; McLean, A.R.D.; Rashan, S.; Lawal, A.; Watson, J.A.; Strub-Wourgaft, N.; White, N.J. Definitions Matter: Heterogeneity of COVID-19 Disease Severity Criteria and Incomplete Reporting Compromise Meta-Analysis. PLOS Glob. Public Health 2022, 2, e0000561. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, S.M.; O’Rahilly, S. When Two Pandemics Meet: Why Is Obesity Associated with Increased COVID-19 Mortality? Med 2020, 1, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Petrakis, D.; Margină, D.; Tsarouhas, K.; Tekos, F.; Stan, M.; Nikitovic, D.; Kouretas, D.; Spandidos, D.A.; Tsatsakis, A. Obesity—A Risk Factor for Increased COVID-19 Prevalence, Severity and Lethality (Review). Mol. Med. Rep. 2020, 22, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Khwatenge, C.N.; Pate, M.; Miller, L.C.; Sang, Y. Immunometabolic Dysregulation at the Intersection of Obesity and COVID-19. Front. Immunol. 2021, 12, 732913. [Google Scholar] [CrossRef] [PubMed]
- Maurya, R.; Sebastian, P.; Namdeo, M.; Devender, M.; Gertler, A. COVID-19 Severity in Obesity: Leptin and Inflammatory Cytokine Interplay in the Link Between High Morbidity and Mortality. Front. Immunol. 2021, 12, 649359. [Google Scholar] [CrossRef] [PubMed]
- Ritter, A.; Kreis, N.N.; Louwen, F.; Yuan, J. Obesity and COVID-19: Molecular Mechanisms Linking Both Pandemics. Int. J. Mol. Sci. 2020, 21, 5793. [Google Scholar] [CrossRef] [PubMed]
- Abumweis, S.; Alrefai, W.; Alzoughool, F. Association of Obesity with COVID-19 Disease Severity and Mortality: A Meta-Analysis of Studies. Obes. Med. 2022, 33, 100431. [Google Scholar] [CrossRef] [PubMed]
- Paravidino, V.B.; Leite, T.H.; Mediano, M.F.F.; Silva, S.O.; Gomes, N.N.F.; de Oliveira, E.A.; Carvalho, R.M.; de Araújo, C.F.; de Castro, M.L.; de Souza, A.R.; et al. Association Between Obesity and COVID-19 Mortality and Length of Stay in Intensive Care Unit Patients in Brazil: A Retrospective Cohort Study. Sci. Rep. 2022, 12, 13737. [Google Scholar] [CrossRef] [PubMed]
- Mesas, A.E.; Cavero-Redondo, I.; Álvarez-Bueno, C.; Sarriá Cabrera, M.A.; Maffei de Andrade, S.; Sequí-Dominguez, I.; Martínez-Vizcaíno, V. Predictors of In-Hospital COVID-19 Mortality: A Comprehensive Systematic Review and Meta-Analysis Exploring Differences by Age, Sex and Health Conditions. PLoS ONE 2020, 15, e0241742. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose Tissue Inflammation and Metabolic Dysfunction in Obesity. Am. J. Physiol. Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Suárez, V.J.; Peris-Ramos, H.C.; Redondo-Flórez, L.; Beltrán-Velasco, A.I.; Martín-Rodríguez, A.; David-Fernandez, S.; Yáñez-Sepúlveda, R.; Tornero-Aguilera, J.F. Personalizing Nutrition Strategies: Bridging Research and Public Health. J. Pers. Med. 2024, 14, 305. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Kunugi, H. Approaches to Nutritional Screening in Patients with Coronavirus Disease 2019 (COVID-19). Int. J. Environ. Res. Public Health 2021, 18, 2772. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. An Overview of Obesity-Related Complications: The Epidemiological Evidence Linking Body Weight and Other Markers of Obesity to Adverse Health Outcomes. Diabetes Obes. Metab. 2025, 27 (Suppl. S2), 3–19. [Google Scholar] [CrossRef] [PubMed]


| Variable | Non-Obese | Obese | p-Value |
|---|---|---|---|
| Male | 1074 (50.9%) | 437 (48.9%) | 0.337 |
| Female | 1037 (49.1%) | 457 (51.1%) | 0.337 |
| Environment: Urban | 1270 (60.2%) | 508 (56.8%) | 0.031 |
| Environment: Rural | 769 (36.4%) | 339 (37.9%) | 0.031 |
| Age (years) | 63.51 ± 14.75 | 62.77 ± 15.57 | 0.198 |
| Smokers | 584 (27.7%) | 279 (31.2%) | 0.055 |
| Variable | OR | 95% CI Lower | 95% CI Upper | p-Value |
|---|---|---|---|---|
| Age > 65 | 1.32 | 0.96 | 1.81 | 0.083 |
| SpO2 < 90 | 1.07 | 0.76 | 1.5 | 0.706 |
| Male | 0.94 | 0.68 | 1.28 | 0.681 |
| Diabetes | 0.91 | 0.63 | 1.31 | 0.617 |
| Hypertension | 1.36 | 0.99 | 1.87 | 0.056 |
| Pulmonary disease | 0.82 | 0.5 | 1.35 | 0.431 |
| Vaccinated | 1.46 | 1.01 | 2.12 | 0.045 |
| Variable | OR | 95% CI Lower | 95% CI Upper | p-Value |
|---|---|---|---|---|
| Age > 65 | 1.41 | 1.15 | 1.72 | < 0.001 |
| SpO2 < 90 | 1.31 | 0.93 | 1.84 | 0.119 |
| Male | 0.88 | 0.72 | 1.08 | 0.210 |
| Diabetes | 1.05 | 0.84 | 1.32 | 0.648 |
| Hypertension | 1.11 | 0.91 | 1.36 | 0.311 |
| Pulmonary disease | 1.09 | 0.81 | 1.47 | 0.586 |
| Vaccinated | 0.92 | 0.72 | 1.18 | 0.517 |
| Variable | Adjusted OR | 95% CI Lower | 95% CI Upper | p-Value |
|---|---|---|---|---|
| Age > 65 years | 1.45 | 1.24 | 1.69 | <0.001 |
| Male sex | 1.18 | 1.01 | 1.38 | 0.041 |
| Obesity | 1.12 | 0.92 | 1.36 | 0.260 |
| Vaccinated | 0.88 | 0.74 | 1.05 | 0.160 |
| Diabetes | 1.21 | 1.03 | 1.43 | 0.022 |
| Hypertension | 1.09 | 0.93 | 1.27 | 0.280 |
| Chronic pulmonary disease | 1.15 | 0.92 | 1.44 | 0.220 |
| SpO2 < 90% at admission | 1.64 | 1.38 | 1.95 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumitru, C.S.; Patrascu, R.; Manole, A.; Capraru, I.D.; Corneluta, F.-M.; Manole, F.; Novacescu, D.; Zara, F. Obesity and COVID-19: Pathophysiological Insights and Pulmonary Complications in a Retrospective Cohort Study. Biomedicines 2025, 13, 2009. https://doi.org/10.3390/biomedicines13082009
Dumitru CS, Patrascu R, Manole A, Capraru ID, Corneluta F-M, Manole F, Novacescu D, Zara F. Obesity and COVID-19: Pathophysiological Insights and Pulmonary Complications in a Retrospective Cohort Study. Biomedicines. 2025; 13(8):2009. https://doi.org/10.3390/biomedicines13082009
Chicago/Turabian StyleDumitru, Cristina Stefania, Raul Patrascu, Alexia Manole, Ionut Dragos Capraru, Fira-Mladinescu Corneluta, Felicia Manole, Dorin Novacescu, and Flavia Zara. 2025. "Obesity and COVID-19: Pathophysiological Insights and Pulmonary Complications in a Retrospective Cohort Study" Biomedicines 13, no. 8: 2009. https://doi.org/10.3390/biomedicines13082009
APA StyleDumitru, C. S., Patrascu, R., Manole, A., Capraru, I. D., Corneluta, F.-M., Manole, F., Novacescu, D., & Zara, F. (2025). Obesity and COVID-19: Pathophysiological Insights and Pulmonary Complications in a Retrospective Cohort Study. Biomedicines, 13(8), 2009. https://doi.org/10.3390/biomedicines13082009








