Evaluation of the Effectiveness and Accuracy of Non-Invasive Preimplantation Genetic Testing (niPGT) Compared to Invasive Embryo Biopsy
Abstract
1. Introduction
1.1. Background and Significance
1.2. Cellular Pathways and Molecular Mechanisms of cfDNA Release
1.3. EV-Mediated DNA Secretion
1.4. Chromatin Remodeling and Nuclear Expulsion Events
2. Material and Methods
2.1. Study Design and Systematic Review Protocol
2.2. Search Strategies and Literature Selection
2.3. Eligibility Criteria
2.4. Data Extraction and Quality Assessment
2.5. Quality Assessment of Included Studies
2.6. cfDNA Collection, Processing, and Sequencing Analysis
3. Results
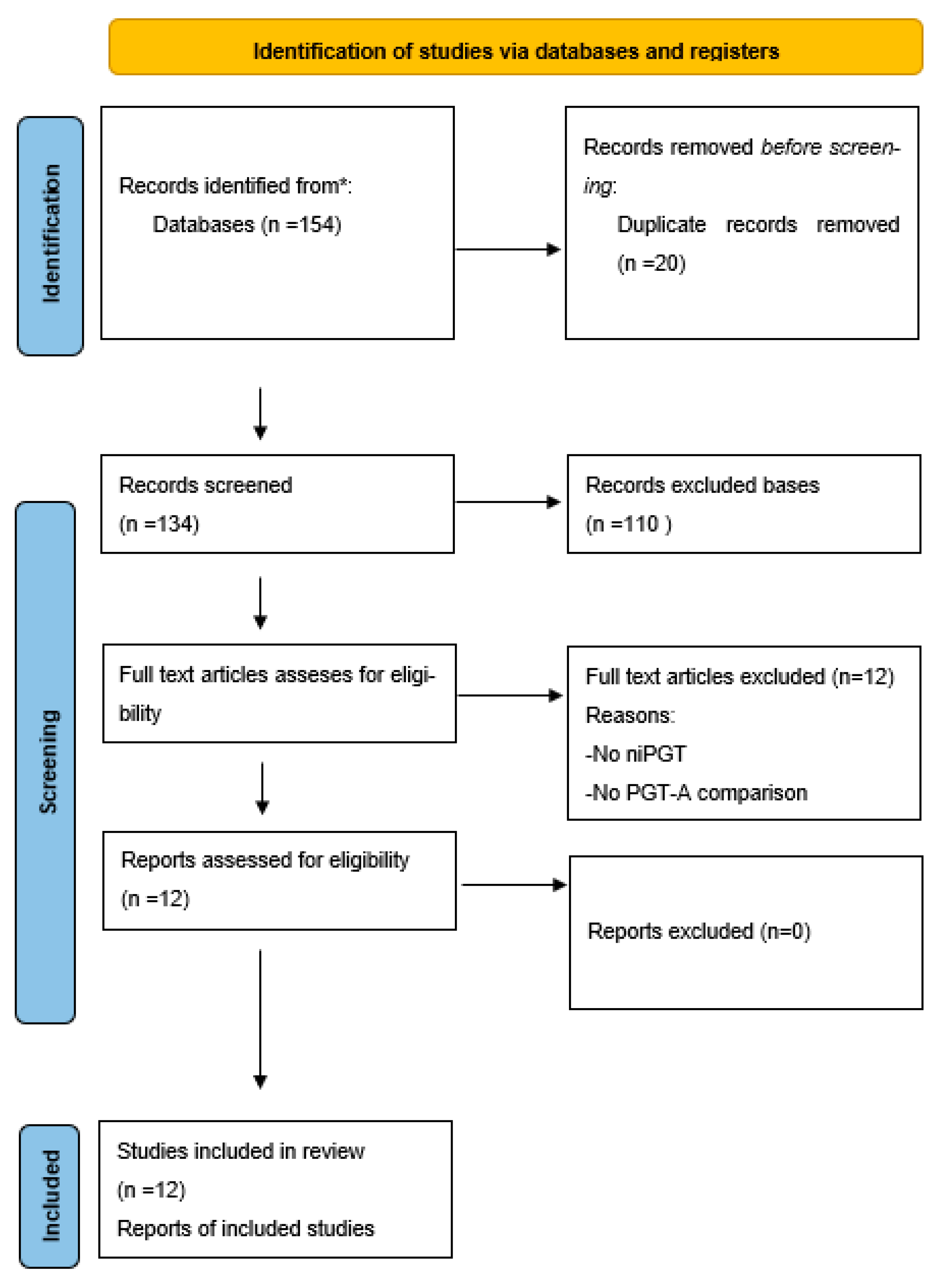
3.1. Study Selection and PRISMA Flow Diagram
3.2. Summery of Studies and Primary Outcomes
3.2.1. Study Design and Methodological Considerations: Assessing the Strengths and Weaknesses of Each Approach
3.2.2. Sample Size and Statistical Power: Impact on Diagnostic Performance and Clinical Generalizability
3.2.3. Variability in cfDNA-Collection Methods: Implications for DNA Integrity and Sequencing Accuracy
3.2.4. Primary Outcomes: Diagnostic Accuracy and Clinical Performance of niPGT
3.3. Clinical Outcomes Following niPGT-Based Embryo Selection
3.3.1. Implantation and Clinical Pregnancy Rates: Indicators of Embryo Viability
3.3.2. Live Birth and Miscarriage Rates Are the Ultimate Measure of niPGT Success
4. Discussion
4.1. Diagnostic Accuracy and Concordance Rates of niPGT: Molecular Mechanisms and Underlying Biological Factors
4.2. Clinical Utility and ART Outcomes: Molecular Mechanisms and Implications for niPGT Implementation
4.3. Molecular Factors Influencing niPGT Reliability
5. Future Directions and Unresolved Challenges
6. Limitations
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gudapati, S.; Chaudhari, K.; Shrivastava, D.; Yelne, S. Advancements and Applications of Preimplantation Genetic Testing in In Vitro Fertilization: A Comprehensive Review. Cureus 2024, 16, e57357. [Google Scholar] [CrossRef]
- Simopoulou, M.; Sfakianoudis, K.; Maziotis, E.; Tsioulou, P.; Grigoriadis, S.; Rapani, A.; Giannelou, P.; Asimakopoulou, M.; Kokkali, G.; Pantou, A.; et al. PGT-A: Who and when? A systematic review and network meta-analysis of RCTs. J. Assist. Reprod. Genet. 2021, 38, 1939–1957. [Google Scholar] [CrossRef]
- ESHRE Working Group on Chromosomal Mosaicism; De Rycke, M.; Capalbo, A.; Coonen, E.; Coticchio, G.; Fiorentino, F.; Goossens, V.; Mcheik, S.; Rubio, C.; Sermon, K.; et al. ESHRE survey results and good practice recommendations on managing chromosomal mosaicism. Hum. Reprod. Open 2022, 2022, hoac044. [Google Scholar] [CrossRef]
- Moustakli, E.; Zikopoulos, A.; Skentou, C.; Bouba, I.; Dafopoulos, K.; Georgiou, I. Evolution of Minimally Invasive and Non-Invasive Preimplantation Genetic Testing: An Overview. J. Clin. Med. 2024, 13, 2160. [Google Scholar] [CrossRef]
- Del Collado, M.; Andrade, G.M.; Gonçalves, N.J.N.; Fortini, S.; Perecin, F.; Carriero, M.M. The embryo non-invasive pre-implantation diagnosis era: How far are we? Anim Reprod. 2023, 20, e20230069. [Google Scholar] [CrossRef]
- Yang, S.; Xu, B.; Zhuang, Y.; Zhang, Q.; Li, J.; Fu, X. Current research status and clinical applications of noninvasive preimplantation genetic testing: A review. Medicine 2024, 103, e39964. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.R.; Arrach, N.; Rhodes-Long, K.; Ahmady, A.; Ingles, S.; Chung, K.; Bendikson, K.A.; Paulson, R.J.; McGinnis, L.K. Pushing the limits of detection: Investigation of cell-free DNA for aneuploidy screening in embryos. Fertil. Steril. 2018, 110, 467–475.e2. [Google Scholar] [CrossRef]
- Lu, S.; Chang, C.-J.; Guan, Y.; Szafer-Glusman, E.; Punnoose, E.; Do, A.; Suttmann, B.; Gagnon, R.; Rodriguez, A.; Landers, M.; et al. Genomic Analysis of Circulating Tumor Cells at the Single-Cell Level. J. Mol. Diagn. 2020, 22, 770–781. [Google Scholar] [CrossRef] [PubMed]
- Bronkhorst, A.J.; Ungerer, V.; Oberhofer, A.; Gabriel, S.; Polatoglou, E.; Randeu, H.; Uhlig, C.; Pfister, H.; Mayer, Z.; Holdenrieder, S. New Perspectives on the Importance of Cell-Free DNA Biology. Diagnostics 2022, 12, 2147. [Google Scholar] [CrossRef]
- Leaver, M.; Wells, D. Non-invasive preimplantation genetic testing (niPGT): The next revolution in reproductive genetics? Human Reprod. Update 2020, 26, 16–42. [Google Scholar] [CrossRef] [PubMed]
- Grabuschnig, S.; Bronkhorst, A.J.; Holdenrieder, S.; Rodriguez, I.R.; Schliep, K.P.; Schwendenwein, D.; Ungerer, V.; Sensen, C.W. Putative Origins of Cell-Free DNA in Humans: A Review of Active and Passive Nucleic Acid Release Mechanisms. Int. J. Mol. Sci. 2020, 21, 8062. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Kari, S.; Subramanian, K.; Altomonte, I.A.; Murugesan, A.; Yli-Harja, O.; Kandhavelu, M. Programmed cell death detection methods: A systematic review and a categorical comparison. Apoptosis 2022, 27, 482–508. [Google Scholar] [CrossRef]
- Sirajee, A.S.; Kabiraj, D.; De, S. Cell-free nucleic acid fragmentomics: A non-invasive window into cellular epigenomes. Transl. Oncol. 2024, 49, 102085. [Google Scholar] [CrossRef]
- Palini, S.; Galluzzi, L.; De Stefani, S.; Bianchi, M.; Wells, D.; Magnani, M.; Bulletti, C. Genomic DNA in human blastocoele fluid. Reprod. Biomed. Online 2013, 26, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Handayani, N.; Aubry, D.; Boediono, A.; Wiweko, B.; Sirait, B.; Sini, I.; Polim, A.A.; Dwiranti, A.; Bowolaksono, A. The origin and possible mechanism of embryonic cell-free DNA release in spent embryo culture media: A review. J. Assist. Reprod. Genet. 2023, 40, 1231–1242. [Google Scholar] [CrossRef]
- Tsering, T.; Nadeau, A.; Wu, T.; Dickinson, K.; Burnier, J.V. Extracellular vesicle-associated DNA: Ten years since its discovery in human blood. Cell Death Dis. 2024, 15, 668. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Song, Y.; Zhu, Z.; Huang, X.; Fan, J.; Qiao, J.; Mao, F. Cell–cell communication: New insights and clinical implications. Signal Transduct. Target. Ther. 2024, 9, 196. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Liang, J.; Qin, T.; Zhang, Y.; Chen, X.; Wang, Z. The Role of Extracellular Vesicles in Embryo Implantation. Front. Endocrinol. 2022, 13, 809596. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.A.; Baba, S.K.; Sadida, H.Q.; Marzooqi, S.A.; Jerobin, J.; Altemani, F.H.; Algehainy, N.; Alanazi, M.A.; Abou-Samra, A.-B.; Kumar, R.; et al. Extracellular vesicles as tools and targets in therapy for diseases. Signal Transduct. Target. Ther. 2024, 9, 27. [Google Scholar] [CrossRef]
- Bolumar, D.; Moncayo-Arlandi, J.; Gonzalez-Fernandez, J.; Ochando, A.; Moreno, I.; Monteagudo-Sanchez, A.; Marin, C.; Diez, A.; Fabra, P.; Checa, M.A.; et al. Vertical trans-mission of maternal DNA through extracellular vesicles associates with altered embryo bioenergetics during the periconception period. eLife 2023, 12, RP88008. [Google Scholar] [CrossRef]
- Trumpff, C.; Michelson, J.; Lagranha, C.J.; Taleon, V.; Karan, K.R.; Sturm, G.; Lindqvist, D.; Fernström, J.; Moser, D.; Kaufman, B.A.; et al. Stress and circulating cell-free mitochondrial DNA: A systematic review of human studies, physiological considerations, and technical recommendations. Mitochondrion 2021, 59, 225–245. [Google Scholar] [CrossRef]
- Sialakouma, A.; Karakasiliotis, I.; Ntala, V.; Nikolettos, N.; Asimakopoulos, B. Embryonic Cell-free DNA in Spent Culture Medium: A Non-invasive Tool for Aneuploidy Screening of the Corresponding Embryos. In Vivo 2021, 35, 3449–3457. [Google Scholar] [CrossRef]
- Caamaño, D.; Cabezas, J.; Aguilera, C.; Martinez, I.; Wong, Y.S.; Sagredo, D.S.; Ibañez, B.; Rodriguez, S.; Castro, F.O.; Rodriguez-Alvarez, L. DNA Content in Embryonic Extracellular Vesicles Is Independent of the Apoptotic Rate in Bovine Embryos Produced In Vitro. Animals 2024, 14, 1041. [Google Scholar] [CrossRef]
- Thakur, B.K.; Zhang, H.; Becker, A.; Matei, I.; Huang, Y.; Costa-Silva, B.; Zheng, Y.; Hoshino, A.; Brazier, H.; Xiang, J.; et al. Double-stranded DNA in exosomes: A novel biomarker in cancer detection. Cell Res. 2014, 24, 766–769. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Capra, E.; Lange-Consiglio, A. The Biological Function of Extracellular Vesicles during Fertilization, Early Embryo—Maternal Crosstalk and Their Involvement in Reproduction: Review and Overview. Biomolecules 2020, 10, 1510. [Google Scholar] [CrossRef]
- Hammond, E.R.; McGillivray, B.C.; Wicker, S.M.; Peek, J.C.; Shelling, A.N.; Stone, P.; Chamley, L.W.; Cree, L.M. Characterizing nuclear and mitochondrial DNA in spent embryo culture media: Genetic contamination identified. Fertil. Steril. 2017, 107, 220–228.e5. [Google Scholar] [CrossRef]
- Layek, S.S.; Kanani, S.; Doultani, S.; Gohil, T.; Patil, S.; Sudhakar, A.; Raval, K.B.; Kuppusamy, K.; Gorani, S.; Raj, S.; et al. Analyzing Cell-free Genomic DNA in Spent Culture Media: Noninvasive Insight into the Blastocysts. Glob. Med. Genet. 2024, 11, 227–232. [Google Scholar] [CrossRef]
- Elzanowska, J.; Semira, C.; Costa-Silva, B. DNA in extracellular vesicles: Biological and clinical aspects. Mol. Oncol. 2020, 15, 1701–1714. [Google Scholar] [CrossRef]
- Volovsky, M.; Scott, R.T.; Seli, E. Non-invasive preimplantation genetic testing for aneuploidy: Is the promise real? Hum. Reprod. Prod. 2024, 39, 1899–1908. [Google Scholar] [CrossRef]
- Müller, C.; Leutz, A. Chromatin remodeling in development and differentiation. Curr. Opin. Genet. Dev. 2001, 11, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Wang, H.; Wu, W.; Geng, S.; Zhong, G.; Li, Y.; Guo, H.; Long, G.; Ren, Q.; Luan, Y.; et al. Circulating cell-free DNA fragmentation is a stepwise and conserved process linked to apoptosis. BMC Biol. 2023, 21, 253. [Google Scholar] [CrossRef]
- Lee, M.T.; Bonneau, A.R.; Giraldez, A.J. Zygotic Genome Activation During the Maternal-to-Zygotic Transition. Annu. Rev. Cell Dev. Biol. 2014, 30, 581–613. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, A.L.; Zorzan, I.; Rugg-Gunn, P.J. Epigenetic regulation of early human embryo development. Cell Stem Cell 2023, 30, 1569–1584. [Google Scholar] [CrossRef] [PubMed]
- Bohers, E.; Viailly, P.-J.; Jardin, F. cfDNA Sequencing: Technological Approaches and Bioinformatic Issues. Pharmaceuticals 2021, 14, 596. [Google Scholar] [CrossRef]
- Mansisidor, A.R.; Risca, V.I. Chromatin accessibility: Methods, mechanisms, and biological insights. Nucleus 2022, 13, 238–278. [Google Scholar] [CrossRef]
- Hovhannisyan, G.; Harutyunyan, T.; Aroutiounian, R.; Liehr, T. The Diagnostic, Prognostic, and Therapeutic Potential of Cell-Free DNA with a Special Focus on COVID-19 and Other Viral Infections. Int. J. Mol. Sci. 2023, 24, 14163. [Google Scholar] [CrossRef]
- MacDonald, K.M.; Benguerfi, S.; Harding, S.M. Alerting the immune system to DNA damage: Micronuclei as mediators. Wu, Q.; editor. Essays Biochem. 2020, 64, 753–764. [Google Scholar]
- Di Bona, M.; Bakhoum, S.F. Micronuclei and Cancer. Cancer Discov. 2024, 14, 214–226. [Google Scholar] [CrossRef]
- Chesnokova, E.; Beletskiy, A.; Kolosov, P. The Role of Transposable Elements of the Human Genome in Neuronal Function and Pathology. Int. J. Mol. Sci. 2022, 23, 5847. [Google Scholar] [CrossRef]
- Poli, M.; Girardi, L.; Fabiani, M.; Moretto, M.; Romanelli, V.; Patassini, C.; Zuccarello, D.; Capalbo, A. Past, Present, and Future Strategies for Enhanced Assessment of Embryo’s Genome and Reproductive Competence in Women of Advanced Reproductive Age. Front. Endocrinol. 2019, 10, 154. [Google Scholar] [CrossRef] [PubMed]
- Rubio, C.; Navarro-Sánchez, L.; García-Pascual, C.M.; Ocali, O.; Cimadomo, D.; Venier, W.; Barroso, G.; Kopcow, L.; Bahçeci, M.; Kulmann, M.I.R.; et al. Multicenter prospective study of concordance between embryonic cell-free DNA and trophectoderm biopsies from 1301 human blastocysts. Am. J. Obstet. Gynecol. 2020, 223, 751.e1–751.e13. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, R.; Zeng, L.; Hu, L.; Shi, J.; Cai, L.; Yao, B.; Wang, X.-X.; Xu, Y.; Yao, Y.; et al. Embryo selection through non-invasive preimplantation genetic testing with cell-free DNA in spent culture media: A protocol for a multicentre, double-blind, randomised controlled trial. BMJ Open 2022, 12, e057254. [Google Scholar] [CrossRef] [PubMed]
- Lledo, B.; Morales, R.; Ortiz, J.A.; Rodriguez-Arnedo, A.; Ten, J.; Castillo, J.C.; Bernabeu, A.; Llacer, J.; Bernabeu, R. Consistent results of non-invasive PGT-A of human embryos using two different techniques for chromosomal analysis. Reprod. Biomed. Online 2021, 42, 555–563. [Google Scholar] [CrossRef]
- Xu, H.; Zhou, M.; Cao, Y.; Zhang, D.; Han, M.; Gao, X.; Xu, B.; Zhang, A. Genome-wide analysis of long noncoding RNAs, microRNAs, and mRNAs forming a competing endogenous RNA network in repeated implantation failure. Gene 2019, 720, 144056. [Google Scholar] [CrossRef]
- Kuznyetsov, V.; Madjunkova, S.; Antes, R.; Abramov, R.; Motamedi, G.; Ibarrientos, Z.; Librach, C.; Kelly, G.M. Evaluation of a novel non-invasive preimplantation genetic screening approach. PLoS ONE 2018, 13, e0197262. [Google Scholar] [CrossRef]
- Yin, B.; Zhang, H.; Xie, J.; Wei, Y.; Zhang, C.; Meng, L. Validation of preimplantation genetic tests for aneuploidy (PGT-A) with DNA from spent culture media (SCM): Concordance assessment and implication. Reprod. Biol. Endocrinol. 2021, 19, 41. [Google Scholar] [CrossRef]
- Sun, B.L.; Wang, Y.; Zhou, L.; Zhang, C.H.; Wu, Z.X.; Qiao, J.; Sun, Q.Y.; Yao, Y.X.; Wang, J.; Yi, Z.Y.; et al. Effectiveness of non-invasive chromosomal screening for normal karyotype and chromosomal rearrangements. Front. Genet. 2023, 14, 1036467. [Google Scholar] [CrossRef]
- Chen, S.; Wang, L.; Hu, Y.; Yao, Y.; Gao, F.; Chang, C.; Zhang, L.; Huang, H.; Lu, D.; Xu, C. Noninvasive preimplantation genetic testing for aneuploidy using blastocyst spent culture medium may serve as a backup of trophectoderm biopsy in conventional preimplantation genetic testing. BMC Med. Genom. 2025, 18, 34. [Google Scholar] [CrossRef]
- Chen, J.; Jia, L.; Li, T.; Guo, Y.; He, S.; Zhang, Z.; Su, W.; Zhang, S.; Fang, C. Diagnostic efficiency of blastocyst culture medium in noninvasive preimplantation genetic testing. F&S Rep. 2020, 15, 88–94. [Google Scholar]
- Yeung, Q.S.Y.; Zhang, Y.X.; Chung, J.P.W.; Lui, W.T.; Kwok, Y.K.Y.; Gui, B.; Kong, G.W.S.; Cao, Y.; Li, T.C.; Choy, K.W. A prospective study of non-invasive preimplantation genetic testing for aneuploidies (NiPGT-A) using next-generation sequencing (NGS) on spent culture media (SCM). J. Assist. Reprod. Genet. 2019, 36, 1609–1621. [Google Scholar] [CrossRef]
- Kulmann, M.I.R.; Riboldi, M.; Martello, C.; Bos-Mikich, A.; Frantz, G.; Dutra, C.; Donatti, L.M.; Oliveira, N.; Frantz, N. First Baby Born in Brazil after Simultaneous Diagnosis through Non-Invasive and Conventional PGT-A. Rev. Bras. Ginecol. Obs. 2021, 43, 878–882. [Google Scholar] [CrossRef]
- Xu, J.; Fang, R.; Chen, L.; Chen, D.; Xiao, J.-P.; Yang, W.; Wang, H.; Song, X.; Ma, T.; Bo, S.; et al. Noninvasive chromosome screening of human embryos by genome sequencing of embryo culture medium for in vitro fertilization. Proc. Natl. Acad. Sci. USA 2016, 113, 11907–11912. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, Y.; Huang, J.; Tang, F.; Wen, L.; Qiao, J. A computational DNA methylation method to remove contaminated DNA from spent embryo culture medium for noninvasive preimplantation genetic testing. EBioMedicine 2025, 114, 105669. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, F.; Zhang, X.; Yin, J.; Du, M.; Jiang, M.; Liu, L.; Li, J.; Huang, Y.; Wang, J. High-throughput single-cell whole-genome amplification through centrifugal emulsification and eMDA. Commun. Biol. 2019, 2, 147. [Google Scholar] [CrossRef] [PubMed]
- Bakalova, D.N.; Navarro-Sánchez, L.; Rubio, C. Non-Invasive Preimplantation Genetic Testing. Genes 2025, 16, 552. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chen, Z.; Li, M.; Sun, L. Biopsy vs. Comprehensive embryo/blastocyst analysis: A closer look at embryonic chromosome evaluation. Hum. Reprod. Open 2025, 2025, hoaf013. [Google Scholar] [CrossRef]
- Belandres, D.; Shamonki, M.; Arrach, N. Current status of spent embryo media research for preimplantation genetic testing. J. Assist. Reprod. Genet. 2019, 36, 819–826. [Google Scholar] [CrossRef]
- Rahimirad, S.; Derderian, S.; Hamel, L.; Scarlata, E.; McKercher, G.; Brimo, F.; Rajan, R.; Rompre-Brodeur, A.; Kassouf, W.; Sanchez-Salas, R.; et al. Refined Procedure to Purify and Sequence Circulating Cell-Free DNA in Prostate Cancer. Int. J. Mol. Sci. 2025, 26, 5839. [Google Scholar] [CrossRef]
- Logsdon, G.A.; Vollger, M.R.; Eichler, E.E. Long-read human genome sequencing and its applications. Nat. Rev. Genet. 2020, 21, 597–614. [Google Scholar] [CrossRef]
- Yu, S.C.Y.; Deng, J.; Qiao, R.; Cheng, S.H.; Peng, W.; Lau, S.L.; Choy, L.L.; Leung, T.Y.; Wong, J.; Wong, V.W.-S.; et al. Comparison of Single Molecule, Real-Time Sequencing and Nanopore Sequencing for Analysis of the Size, End-Motif, and Tissue-of-Origin of Long Cell-Free DNA in Plasma. Clin. Chem. 2022, 69, 168–179. [Google Scholar] [CrossRef]
- Peng, H.; Pan, M.; Zhou, Z.; Chen, C.; Xing, X.; Cheng, S.; Zhang, S.; Zheng, H.; Qian, K. The impact of preanalytical variables on the analysis of cell-free DNA from blood and urine samples. Front. Cell Dev. Biol. 2024, 12, 1385041. [Google Scholar] [CrossRef] [PubMed]
- Johansen, J.E.; Broberger, C.; Lavebratt, C.; Johansson, C.; Kuhar, M.J.; Hökfelt, T.; Schalling, M. Hypothalamic CART and serum leptin levels are reduced in the anorectic (anx/anx) mouse. Mol. Brain Res. 2000, 84, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Kallianidis, K.; Dimitroulia, E.; Mavrogianni, D.; Liokari, E.; Bletsa, R.; Anagnostou, E.; Sofikitis, N.; Loutradis, D.; Mavrogianni, D. Comparison of the Fetal Fraction of Cell-Free DNA in In-Vitro Fertilization (IVF) Versus Natural Conception Evaluation of the Fetal Fraction With IVF Parameters. Cureus 2022, 14, e24516. [Google Scholar] [CrossRef] [PubMed]
- de Miranda, F.S.; Barauna, V.G.; dos Santos, L.; Costa, G.; Vassallo, P.F.; Campos, L.C.G. Properties and Application of Cell-Free DNA as a Clinical Biomarker. Int. J. Mol. Sci. 2021, 22, 9110. [Google Scholar] [CrossRef]
- Lee, S.E.; Park, H.Y.; Hur, J.Y.; Kim, H.J.; Kim, I.A.; Kim, W.S.; Lee, K.Y. Genomic profiling of extracellular vesicle-derived DNA from bronchoalveolar lavage fluid of patients with lung adenocarcinoma. Transl. Lung Cancer Res. 2021, 10, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Bouba, I.; Hatzi, E.; Ladias, P.; Sakaloglou, P.; Kostoulas, C.; Georgiou, I. Biological and Clinical Significance of Mosaicism in Human Preimplantation Embryos. J. Dev. Biol. 2021, 9, 18. [Google Scholar] [CrossRef]
- Abedalthagafi, M.; Bawazeer, S.; Fawaz, R.I.; Heritage, A.M.; Alajaji, N.M.; Faqeih, E. Non-invasive prenatal testing: A revolutionary journey in prenatal testing. Front. Med. 2023, 10, 1265090. [Google Scholar] [CrossRef]
- Bednarska-Czerwińska, A.; Smoleń-Dzirba, J.; Strychalska, A.; Sierka, W.; Wróblewska, U.; Mermer, P.; Masarczyk, B.; Jodłowiec-Lubańska, N.; Kokot, A.; Simka-Lampa, K.; et al. Comparison of Non-Invasive and Minimally Invasive Preimplantation Genetic Testing for Aneuploidy Using Samples Derived from the Same Embryo Culture. J. Clin. Med. 2024, 14, 33. [Google Scholar] [CrossRef]
- Hanson, B.M.; Tao, X.; Hong, K.H.; Comito, C.E.; Pangasnan, R.; Seli, E.; Jalas, C.; Scott, R.T. Noninvasive preimplantation genetic testing for aneuploidy exhibits high rates of deoxyribonucleic acid amplification failure and poor correlation with results obtained using trophectoderm biopsy. Fertil. Steril. 2021, 115, 1461–1470. [Google Scholar] [CrossRef] [PubMed]
- Victor, A.R.; Griffin, D.K.; Brake, A.J.; Tyndall, J.C.; Murphy, A.E.; Lepkowsky, L.T.; Lal, A.; Zouves, C.G.; Barnes, F.L.; McCoy, R.C.; et al. Assessment of aneuploidy concordance between clinical trophectoderm biopsy and blastocyst. Hum. Reprod. 2018, 34, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Girardi, L.; Figliuzzi, M.; Poli, M.; Serdarogullari, M.; Patassini, C.; Caroselli, S.; Pergher, I.; Cogo, F.; Coban, O.; Boynukalin, F.K.; et al. The use of copy number loads to designate mosaicism in blastocyst stage PGT-A cycles: Fewer is better. Hum. Reprod. 2023, 38, 982–991. [Google Scholar] [CrossRef] [PubMed]
- Shitara, A.; Takahashi, K.; Goto, M.; Takahashi, H.; Iwasawa, T.; Onodera, Y.; Makino, K.; Miura, H.; Shirasawa, H.; Sato, W.; et al. Cell-free DNA in spent culture medium effectively reflects the chromosomal status of embryos following culturing beyond implantation compared to trophectoderm biopsy. PLoS ONE 2021, 16, e0246438. [Google Scholar] [CrossRef]
- Ma, L.; Guo, H.; Zhao, Y.; Liu, Z.; Wang, C.; Bu, J.; Sun, T.; Wei, J. Liquid biopsy in cancer: Current status, challenges and future prospects. Signal Transduct. Target. Ther. 2024, 9, 336. [Google Scholar] [CrossRef]
- Franco, J.G., Jr.; Vagnini, L.D.; Petersen, C.G.; Renzi, A.; Canas, M.C.T.; Petersen, B.; Ricci, J.; Nicoletti, A.; Zamara, C.; Dieamant, F.; et al. Noninvasive Preimplantation Genetic Testing for Aneuploidy (niPGT-A): The first Brazilian baby. JBRA Assist. Reprod. 2020, 24, 517. [Google Scholar] [CrossRef]
- Smith, J.; Kean, V.; Bianchi, D.W.; Feldman, G.; Petrucelli, N.; Simon, M.; Gonik, B. Cell-free DNA results lead to unexpected diagnosis. Clin. Case Rep. 2017, 5, 1323–1326. [Google Scholar] [CrossRef]
- Lacconi, V.; Massimiani, M.; Carriero, I.; Bianco, C.; Ticconi, C.; Pavone, V.; Alteri, A.; Muzii, L.; Rago, R.; Pisaturo, V.; et al. When the Embryo Meets the Endometrium: Iden-tifying the Features Required for Successful Embryo Implantation. Int. J. Mol. Sci. 2024, 25, 2834. [Google Scholar] [CrossRef]
- Sakkas, D.; Navarro-Sánchez, L.; Ardestani, G.; Barroso, G.; Bisioli, C.; Boynukalin, K.; Cimadomo, D.; Frantz, N.; Kopcow, L.; Andrade, G.M.; et al. The impact of implementing a non-invasive preimplantation genetic testing for aneuploidies (niPGT-A) embryo culture protocol on embryo viability and clinical outcomes. Hum. Reprod. 2024, 39, 1952–1959. [Google Scholar] [CrossRef]
- Giuliano, R.; Maione, A.; Vallefuoco, A.; Sorrentino, U.; Zuccarello, D. Preimplantation Genetic Testing for Genetic Diseases: Limits and Review of Current Literature. Genes 2023, 14, 2095. [Google Scholar] [CrossRef]
| Author/Year | Study Type | Cases (Number) | Groups | Interventions | Selection Bias | Comparability | Outcome Assessment | Total NOS Score (0–9) | Risk of Bias |
|---|---|---|---|---|---|---|---|---|---|
| Rubio et al., 2020, [42] | Cohort Study | 484 | niPGT-A vs. PGT-A | cfDNA from spent culture medium vs. Trophectoderm biopsy | 3/4 | 2/2 | 3/3 | 8/9 | Low |
| Huang et al., 2022, [43] | Observational | 246 | niPGT-A vs. PGT-A | Non-invasive cfDNA testing vs. TE biopsy | 3/4 | 2/2 | 3/3 | 8/9 | Low |
| Lledo et al., 2021, [44] | Retrospective Study | 178 | niPGT-A vs. PGT-A | cfDNA-based aneuploidy screening | 2/4 | 1/2 | 2/3 | 5/9 | Moderate |
| Xu et al., 2019, [45] | Observational | 150 | niPGT-A vs. PGT-A | cfDNA from embryo culture media | 3/4 | 2/2 | 3/3 | 8/9 | Low |
| Kuznyetsov et al., 2018, [46] | Pilot Study | 50 | niPGT-A vs. PGT-A | cfDNA analysis from blastocyst medium | 2/4 | 1/2 | 2/3 | 5/9 | High |
| Sialakouma et al., 2021, [23] | Multicenter Study | 325 | niPGT-A vs. PGT-A | NGS-based cfDNA testing | 3/4 | 2/2 | 3/3 | 8/9 | Low |
| Yin et al., 2021, [47] | Retrospective Study | 175 | niPGT-A vs. PGT-A | Embryonic cfDNA testing | 2/4 | 1/2 | 2/3 | 5/9 | Moderate |
| Sun et al., 2023, [48] | Retrospective Study | 85 | niPGT-A vs. PGT-A | Non-invasive PGT approaches | 2/4 | 1/2 | 2/3 | 5/9 | High |
| Chen et al., 2025, [49] | Cohort Study | 265 | niPGT-A vs. PGT-A | Blastocyst culture cfDNA vs. TE biopsy | 3/4 | 2/2 | 3/3 | 8/9 | Low |
| Chen et al., 2020, [50] | Cohort Study | 26 | niPGT-A vs. PGT-A | Review of cfDNA-based niPGT-A methods | 2/4 | 1/2 | 2/3 | 5/9 | Moderate |
| Yeung et al., 2019, [51] | Prospective Study | 14 | niPGT-A vs. PGT-A | Non-invasive cfDNA testing vs. TE biopsy | 3/4 | 2/2 | 3/3 | 8/9 | Low |
| Kulmann et al., 2021, [52] | Retrospective Study | 11 | niPGT-A vs. PGT-A | cfDNA from spent culture medium vs. Trophectoderm biopsy | 2/4 | 1/2 | 2/3 | 5/9 | Moderate |
| Author/Year | cfDNA Collection Method | cfDNA Yield | Maternal DNA Contamination | Sequencing Platform | Bioinformatics Analysis | False Positive/Negative Rates |
|---|---|---|---|---|---|---|
| Rubio et al., 2020, [42] | Spent Culture Medium | Low | High | NGS | CNV + SNP Filtering | Moderate |
| Huang et al., 2022, [43] | Spent Culture Medium | Moderate | High | NGS + WGA | Mosaicism Detection | Moderate |
| Lledo et al., 2021, [44] | Blastocoel Fluid | High | Low | qPCR | Copy Number Analysis | Low |
| Xu et al., 2019, [45] | Spent Culture Medium | Low | High | NGS + WGA | Read-Depth Assessment | High |
| Kuznyetsov et al., 2016, [46] | Blastocoel Fluid | High | Low | NGS + WGA | Variant Calling | Moderate |
| Sialakouma et al., 2021, [23] | Spent Culture Medium | Moderate | High | NGS + WGA | NGS-Based Filtering | Moderate |
| Yin et al., 2021, [47] | Embryonic cfDNA | Moderate | Moderate | NGS + WGA | CNV Analysis | Moderate |
| Sun et al., 2023, [48] | Spent Culture Medium | Low | High | NGS + WGA | General Review | Not Reported |
| Chen et al., 2025, [49] | Blastocyst Culture Medium | Moderate | Moderate | NGS + WGA | Genomic Integrity | Low |
| Chen et al., 2020, [50] | Review of cfDNA Methods | - | - | - | - | - |
| Yeung et al., 2019, [51] | Spent Culture Medium | Low | High | NGS + WGA | Basic Filtering | High |
| Kulmann et al., 2021, [52] | Spent Culture Medium | Low | High | NGS + WGA | NGS-Based Detection | High |
| Author/Year | Study Type | Cases (n) | Groups | Interventions | Primary Outcome |
|---|---|---|---|---|---|
| Rubio et al., 2020, [42] | Cohort Study | 484 | niPGT-A vs. PGT-A | cfDNA from spent culture medium vs. Trophectoderm biopsy | Concordance rate, diagnostic accuracy (sensitivity, specificity, PPV, NPV) |
| Huang et al., 2022, [43] | Observational | 246 | niPGT-A vs. PGT-A | Non-invasive cfDNA testing vs. TE biopsy | Sensitivity, specificity, clinical outcomes |
| Lledo et al., 2021, [44] | Retrospective Study | 178 | niPGT-A vs. PGT-A | cfDNA-based aneuploidy screening | Correlation with PGT-A, clinical pregnancy rates |
| Xu et al., 2019, [45] | Observational | 150 | niPGT-A vs. PGT-A | cfDNA from embryo culture media | Accuracy, false positive/negative rates |
| Kuznyetsov et al., 2016, [46] | Pilot Study | 50 | niPGT-A vs. PGT-A | cfDNA analysis from blastocyst medium | Diagnostic accuracy, feasibility |
| Sialakouma et al., 2021, [23] | Multicenter Study | 325 | niPGT-A vs. PGT-A | NGS-based cfDNA testing | Sensitivity, specificity, pregnancy outcomes |
| Yin et al., 2021, [47] | Retrospective Study | 175 | niPGT-A vs. PGT-A | Embryonic cfDNA testing | Concordance with invasive PGT-A, clinical outcomes |
| Sun et al., 2023, [48] | Retrospective Study | 85 | niPGT-A vs. PGT-A | Non-invasive PGT approaches | Overview of findings and future directions |
| Chen et al., 2025, [49] | Cohort Study | 265 | niPGT-A vs. PGT-A | Blastocyst culture cfDNA vs. TE biopsy | Reliability, clinical pregnancy rates |
| Chen et al., 2020, [50] | Review Article | - | niPGT-A vs. PGT-A | Review of cfDNA-based niPGT-A methods | Reliability, accuracy, and limitations of niPGT-A |
| Yeung et al., 2019, [51] | Prospective Study | 14 | niPGT-A vs. PGT-A | Non-invasive cfDNA testing vs. TE biopsy | Sensitivity, specificity, pregnancy outcomes |
| Kulmann et al., 2021, [52] | Retrospective Study | 11 | niPGT-A vs. PGT-A | cfDNA from spent culture medium vs. Trophectoderm biopsy | Accuracy, false-positive/-negative rates |
| Author/Year | Study Type | Cases (n) | Implantation Rate (%) | Clinical Pregnancy Rate (%) | Live Birth Rate (%) | Miscarriage Rate (%) |
|---|---|---|---|---|---|---|
| Rubio et al., 2020, [42] | Cohort Study | 484 | 57.3 | 68.4 | 45.1 | 15.7 |
| Huang et al., 2022, [43] | Observational | 246 | 53.6 | 64.1 | 42.3 | 17.9 |
| Lledo et al., 2021, [44] | Retrospective Study | 178 | 49.8 | 61.2 | 39.7 | 20.3 |
| Xu et al., 2019, [45] | Observational | 150 | 45.2 | 58.7 | 37.5 | 22.1 |
| Kuznyetsov et al., 2016, [46] | Pilot Study | 50 | 50.1 | 59.3 | 40.2 | 18.5 |
| Sialakouma et al., 2021, [23] | Multicenter Study | 325 | 58.4 | 70.1 | 47.8 | 14.2 |
| Yin et al., 2021, [47] | Retrospective Study | 175 | 52.7 | 62.4 | 41.5 | 19.6 |
| Sun et al., 2023, [48] | Retrospective Study | 85 | Not Reported | Not Reported | Not Reported | Not Reported |
| Chen et al., 2025, [49] | Cohort Study | 265 | 54.9 | 66.5 | 44.3 | 16.8 |
| Yeung et al., 2019, [51] | Prospective Study | 14 | 48.2 | 55.9 | 36.1 | 23.4 |
| Kulmann et al., 2021, [52] | Retrospective Study | 11 | 44.5 | 51.3 | 33.4 | 25.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voros, C.; Darlas, M.; Athanasiou, D.; Athanasiou, A.; Athanasiou, A.; Bananis, K.; Papadimas, G.; Tsimpoukelis, C.; Gkirgkinoudis, A.; Sapantzoglou, I.; et al. Evaluation of the Effectiveness and Accuracy of Non-Invasive Preimplantation Genetic Testing (niPGT) Compared to Invasive Embryo Biopsy. Biomedicines 2025, 13, 2010. https://doi.org/10.3390/biomedicines13082010
Voros C, Darlas M, Athanasiou D, Athanasiou A, Athanasiou A, Bananis K, Papadimas G, Tsimpoukelis C, Gkirgkinoudis A, Sapantzoglou I, et al. Evaluation of the Effectiveness and Accuracy of Non-Invasive Preimplantation Genetic Testing (niPGT) Compared to Invasive Embryo Biopsy. Biomedicines. 2025; 13(8):2010. https://doi.org/10.3390/biomedicines13082010
Chicago/Turabian StyleVoros, Charalampos, Menelaos Darlas, Diamantis Athanasiou, Antonia Athanasiou, Aikaterini Athanasiou, Kyriakos Bananis, Georgios Papadimas, Charalampos Tsimpoukelis, Athanasios Gkirgkinoudis, Ioakeim Sapantzoglou, and et al. 2025. "Evaluation of the Effectiveness and Accuracy of Non-Invasive Preimplantation Genetic Testing (niPGT) Compared to Invasive Embryo Biopsy" Biomedicines 13, no. 8: 2010. https://doi.org/10.3390/biomedicines13082010
APA StyleVoros, C., Darlas, M., Athanasiou, D., Athanasiou, A., Athanasiou, A., Bananis, K., Papadimas, G., Tsimpoukelis, C., Gkirgkinoudis, A., Sapantzoglou, I., Papapanagiotou, I., Vaitsis, D., Koulakmanidis, A.-M., Topalis, V., Thomakos, N., Theodora, M., Antsaklis, P., Chatzinikolaou, F., Dahl, H. A., ... Loutradis, D. (2025). Evaluation of the Effectiveness and Accuracy of Non-Invasive Preimplantation Genetic Testing (niPGT) Compared to Invasive Embryo Biopsy. Biomedicines, 13(8), 2010. https://doi.org/10.3390/biomedicines13082010










