Marine Oligo-Fucoidan as a Safe Functional Food for Managing Uterine Fibroids: Results from a Pilot Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
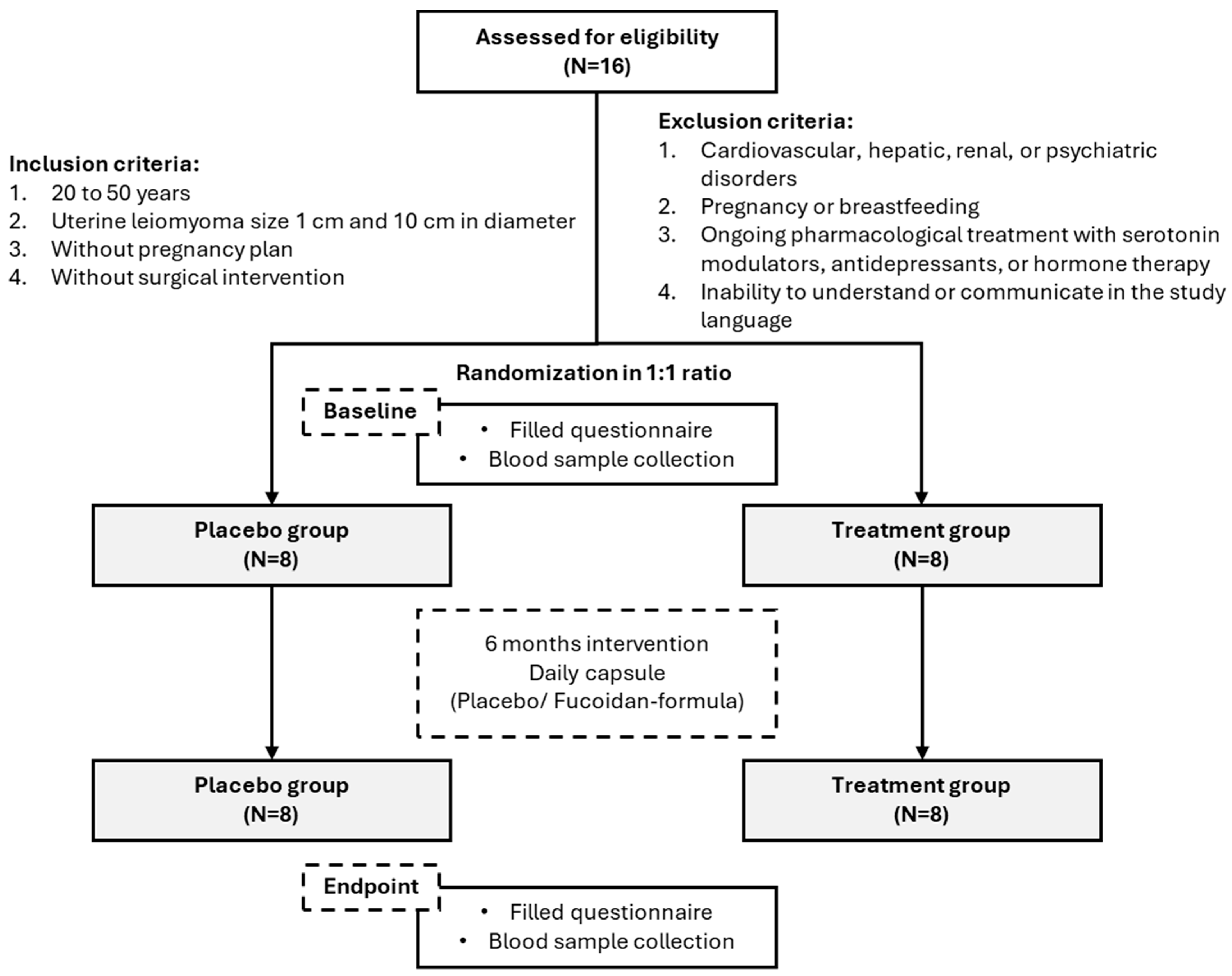
2.1. Study Design and Ethical Approval
2.2. Participant Recruitment and Eligibility Criteria
2.3. Biochemical Evaluation
2.4. Quality of Life Assessment (WHOQOL-BREF)
2.5. Pain Type and Symptom Evaluation
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics at Baseline
3.2. Blood Parameters and Biochemical Markers at Baseline and Endpoint
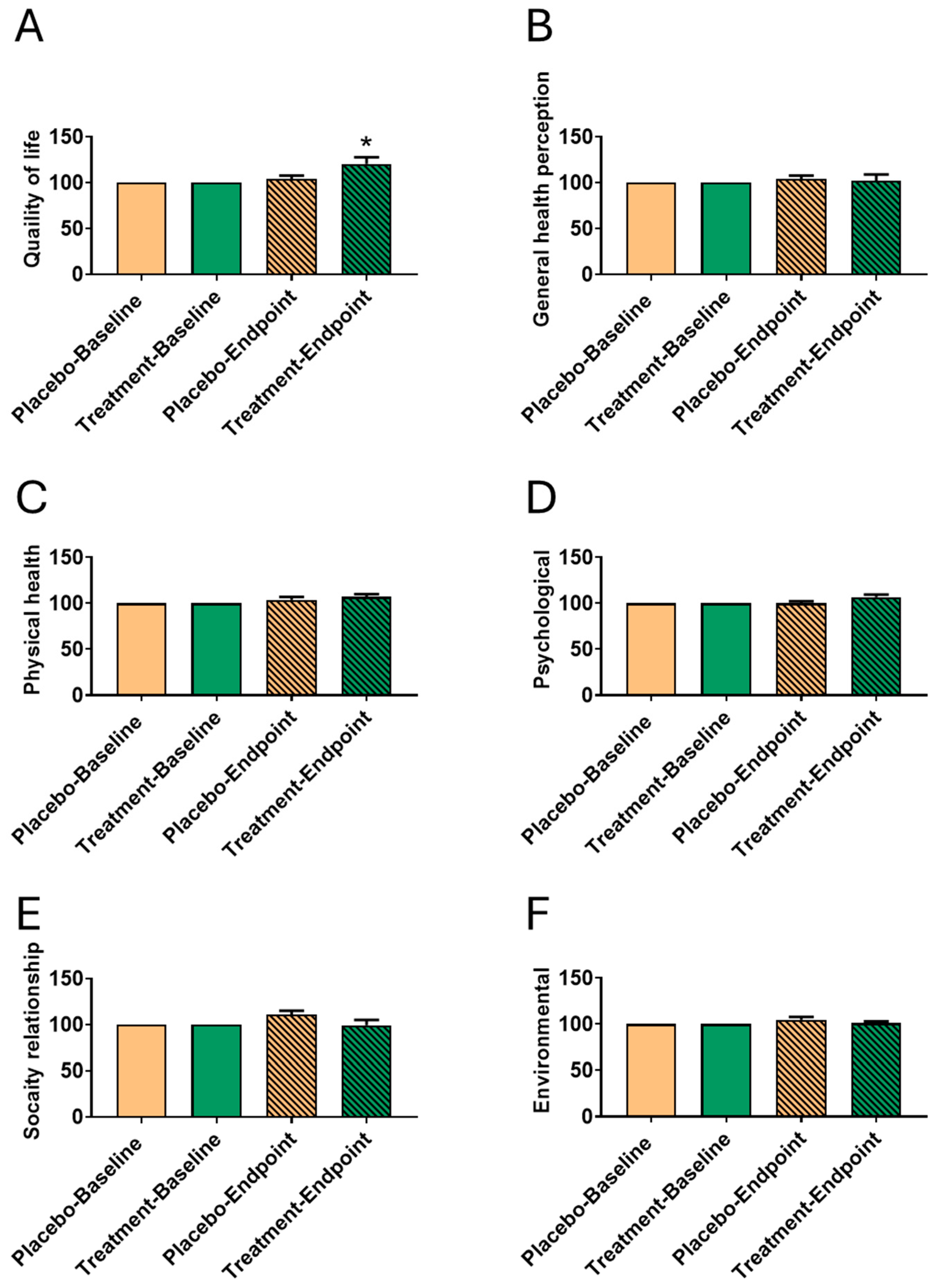
3.3. Impact of Fucoidan Formula on WHOQOL After 6-Month Intervention
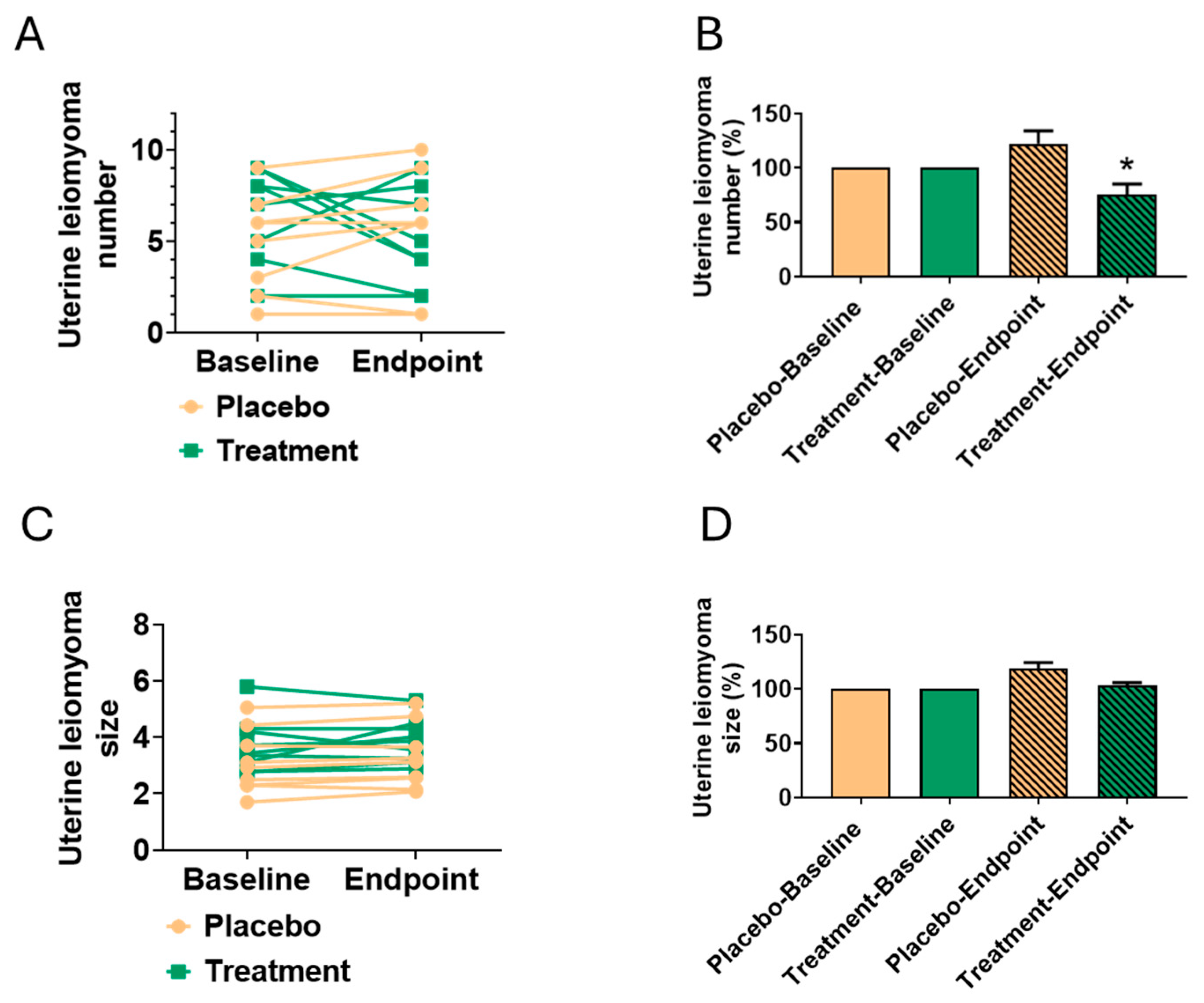
3.4. Reduction in Leiomyoma Number
3.5. Pain Type Assessment at Baseline and Endpoint
3.6. Period Discomfort and Symptom Changes from Baseline to Endpoint in Treatment and Placebo Groups
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALB | Albumin |
| ASD | Androstenedione |
| α-SMA | Alpha-Smooth Muscle Actin |
| Baso | Basophil Percentage |
| BUN | Blood Urea Nitrogen |
| COL1A1 | Collagen Type I Alpha 1 Chain |
| E2 | Estradiol |
| ECM | Extracellular Matrix |
| EGCG | Epigallocatechin-3-Gallate |
| Eosin | Eosinophil Percentage |
| ERK1/2 | Extracellular Signal-Regulated Kinase 1/2 |
| FSH | Follicle-Stimulating Hormone |
| GPT | Glutamate Pyruvate Transaminase |
| GOT | Glutamate Oxaloacetate Transaminase |
| HDL-C | High-Density Lipoprotein Cholesterol |
| Hct | Hematocrit |
| HGB | Hemoglobin |
| HS-CRP | High-Sensitivity C-Reactive Protein |
| LDL-C | Low-Density Lipoprotein Cholesterol |
| Lym-L | Lymphocyte Percentage |
| MCH | Mean Corpuscular Hemoglobin |
| MCHC | Mean Corpuscular Hemoglobin Concentration |
| MCV | Mean Corpuscular Volume |
| Mono | Monocyte Percentage |
| Net-s | Neutrophil Percentage |
| PCOS | Polycystic Ovary Syndrome |
| PGTR | Progesterone |
| Platel | Platelet Count |
| QOL | Quality of Life |
| RBC | Red Blood Cell Count |
| Smad2 | SMAD Family Member 2 |
| T-CHO | Total Cholesterol |
| TG | Triglycerides |
| UA | Uric Acid |
| UTI | Urinary Tract Infection |
| VIT-D | Vitamin D |
| WBC | White Blood Cell Count |
References
- Hervé, F.; Katty, A.; Isabelle, Q.; Céline, S. Impact of uterine fibroids on quality of life: A national cross-sectional survey. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 229, 32–37. [Google Scholar] [CrossRef]
- Becker, S.; Dolmans, M.; Herrera, F.C.; Petraglia, F.; Renner, S.P.; Ionescu-Ittu, R.; St-Pierre, J.; Boolell, M.; Bestel, E.; Hori, S.; et al. Pain Reduction in Linzagolix-Treated Patients With Uterine Fibroids: A Secondary Mediation Analysis of the PRIMROSE 1 and 2 Phase 3 Trials. Bjog 2025, 132, 1297–1306. [Google Scholar] [CrossRef]
- Marsh, E.E.; Ekpo, G.E.; Cardozo, E.R.; Brocks, M.; Dune, T.; Cohen, L.S. Racial differences in fibroid prevalence and ultrasound findings in asymptomatic young women (18–30 years old): A pilot study. Fertil. Steril. 2013, 99, 1951–1957. [Google Scholar] [CrossRef]
- Lippman, S.A.; Warner, M.; Samuels, S.; Olive, D.; Vercellini, P.; Eskenazi, B. Uterine fibroids and gynecologic pain symptoms in a population-based study. Fertil. Steril. 2003, 80, 1488–1494. [Google Scholar] [CrossRef]
- Chiang, Y.F.; Huang, K.C.; Huang, T.C.; Chen, H.Y.; Ali, M.; Al-Hendy, A.; Hsia, S.M. Regulatory roles of NAMPT and NAD(+) metabolism in uterine leiomyoma progression: Implications for ECM accumulation, stemness, and microenvironment. Redox Biol. 2024, 78, 103411. [Google Scholar] [CrossRef]
- Ciebiera, M.; Ali, M.; Prince, L.; Jackson-Bey, T.; Atabiekov, I.; Zgliczyński, S.; Al-Hendy, A. The Evolving Role of Natural Compounds in the Medical Treatment of Uterine Fibroids. J. Clin. Med. 2020, 9, 1479. [Google Scholar] [CrossRef]
- Huang, K.C.; Chiang, Y.F.; Ali, M.; Hsia, S.M. Cisplatin-Induced Muscle Wasting and Atrophy: Molecular Mechanism and Potential Therapeutic Interventions. J. Cachexia Sarcopenia Muscle 2025, 16, e13817. [Google Scholar] [CrossRef]
- Apostolova, E.; Lukova, P.; Baldzhieva, A.; Katsarov, P.; Nikolova, M.; Iliev, I.; Peychev, L.; Trica, B.; Oancea, F.; Delattre, C.; et al. Immunomodulatory and Anti-Inflammatory Effects of Fucoidan: A Review. Polymers 2020, 12, 2338. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.-F.; Huang, K.-C.; Wang, K.-L.; Huang, Y.-J.; Chen, H.-Y.; Ali, M.; Shieh, T.-M.; Hsia, S.-M. Protective Effects of an Oligo-Fucoidan-Based Formula against Osteoarthritis Development via iNOS and COX-2 Suppression following Monosodium Iodoacetate Injection. Mar. Drugs 2024, 22, 211. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.-F.; Chen, H.-Y.; Chang, Y.-J.; Shih, Y.-H.; Shieh, T.-M.; Wang, K.-L.; Hsia, S.-M. Protective Effects of Fucoxanthin on High Glucose- and 4-Hydroxynonenal (4-HNE)-Induced Injury in Human Retinal Pigment Epithelial Cells. Antioxidants 2020, 9, 1176. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Mishra, Y.; Kumar, A. Marine-derived bioactive compounds for neuropathic pain: Pharmacology and therapeutic potential. Naunyn Schmiedebergs Arch Pharmacol. 2025, 398, 6193–6220. [Google Scholar] [CrossRef]
- Shiau, J.-P.; Chuang, Y.-T.; Cheng, Y.-B.; Tang, J.-Y.; Hou, M.-F.; Yen, C.-Y.; Chang, H.-W. Impacts of Oxidative Stress and PI3K/AKT/mTOR on Metabolism and the Future Direction of Investigating Fucoidan-Modulated Metabolism. Antioxidants 2022, 11, 911. [Google Scholar] [CrossRef]
- Saad, E.E.; Michel, R.; Borahay, M.A. Immunosuppressive tumor microenvironment and uterine fibroids: Role in collagen synthesis. Cytokine Growth Factor Rev. 2024, 75, 93–100. [Google Scholar] [CrossRef]
- Belda-Antolí, M.; Ros Bernal, F.A.; Vicente-Mampel, J. From Sea to Relief: The Therapeutic Potential of Marine Algal Antioxidants in Pain Alleviation. Mar. Drugs. 2025, 23, 270. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Huang, T.-C.; Lin, L.-C.; Shieh, T.-M.; Wu, C.-H.; Wang, K.-L.; Hong, Y.-H.; Hsia, S.-M. Fucoidan Inhibits the Proliferation of Leiomyoma Cells and Decreases Extracellular Matrix-Associated Protein Expression. Cell. Physiol. Biochem. 2018, 49, 1970–1986. [Google Scholar] [CrossRef]
- Moore, A.B.; Yu, L.; Swartz, C.D.; Zheng, X.; Wang, L.; Castro, L.; E Kissling, G.; Walmer, D.K.; Robboy, S.J.; Dixon, D. Human uterine leiomyoma-derived fibroblasts stimulate uterine leiomyoma cell proliferation and collagen type I production, and activate RTKs and TGF beta receptor signaling in coculture. Cell Commun. Signal. 2010, 8, 10. [Google Scholar] [CrossRef]
- El Sabeh, M.; Saha, S.K.; Afrin, S.; Islam, S.; Borahay, M.A. Wnt/β-catenin signaling pathway in uterine leiomyoma: Role in tumor biology and targeting opportunities. Mol. Cell Biochem. 2021, 476, 3513–3536. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.L.; Tai, C.J.; Huang, C.W.; Chang, F.R.; Wang, J.Y. Efficacy of Low-Molecular-Weight Fucoidan as a Supplemental Therapy in Metastatic Colorectal Cancer Patients: A Double-Blind Randomized Controlled Trial. Mar. Drugs 2017, 15, 122. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Pandey, C.M.; Singh, U.; Gupta, A.; Sahu, C.; Keshri, A. Descriptive statistics and normality tests for statistical data. Ann. Card Anaesth. 2019, 22, 67–72. [Google Scholar]
- Gelman, A.; Hill, J. Data Analysis Using Regression and Multilevel/Hierarchical Models; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- David, M.; Pitz, C.M.; Mihaylova, A.; Siedentopf, F. Myoma-associated pain frequency and intensity: A retrospective evaluation of 1548 myoma patients. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 199, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Borah, B.J.; Nicholson, W.K.; Bradley, L.; Stewart, E.A. The impact of uterine leiomyomas: A national survey of affected women. Am. J. Obstet. Gynecol. 2013, 209, 319.e1–319.e20. [Google Scholar] [CrossRef]
- Arjeh, S.; Darsareh, F.; Asl, Z.A.; Azizi Kutenaei, M. Effect of oral consumption of vitamin D on uterine fibroids: A randomized clinical trial. Complement. Ther. Clin. Pract. 2020, 39, 101159. [Google Scholar] [CrossRef] [PubMed]
- Porcaro, G.; Santamaria, A.; Giordano, D.; Angelozzi, P. Vitamin D plus epigallocatechin gallate: A novel promising approach for uterine myomas. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 3344–3351. [Google Scholar]
- Ali, M.; Shahin, S.M.; Sabri, N.A.; Al-Hendy, A.; Yang, Q. 1,25 Dihydroxyvitamin D3 Enhances the Antifibroid Effects of Ulipristal Acetate in Human Uterine Fibroids. Reprod. Sci. 2019, 26, 812–828. [Google Scholar] [CrossRef] [PubMed]
- Ciebiera, M.; Męczekalski, B.; Łukaszuk, K.; Jakiel, G. Potential synergism between ulipristal acetate and vitamin D3 in uterine fibroid pharmacotherapy-2 case studies. Gynecol. Endocrinol. 2019, 35, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Hajhashemi, M.; Ansari, M.; Haghollahi, F.; Eslami, B. The effect of vitamin D supplementation on the size of uterine leiomyoma in women with vitamin D deficiency. Caspian J. Intern. Med. 2019, 10, 125–131. [Google Scholar] [PubMed]
- Corachán, A.; Ferrero, H.; Escrig, J.; Monleon, J.; Faus, A.; Cervelló, I.; Pellicer, A. Long-term vitamin D treatment decreases human uterine leiomyoma size in a xenograft animal model. Fertil. Steril. 2020, 113, 205–216.e204. [Google Scholar] [CrossRef]
- Zhang, D.; Al-Hendy, M.; Richard-Davis, G.; Montgomery-Rice, V.; Sharan, C.; Rajaratnam, V.; Khurana, A.; Al-Hendy, A. Green tea extract inhibits proliferation of uterine leiomyoma cells in vitro and in nude mice. Am. J. Obstet. Gynecol. 2010, 202, 289.e1–289.e9. [Google Scholar] [CrossRef]
- Włodarczyk, M.; Ciebiera, M.; Nowicka, G.; Łoziński, T.; Ali, M.; Al-Hendy, A. Epigallocatechin Gallate for the Treatment of Benign and Malignant Gynecological Diseases-Focus on Epigenetic Mechanisms. Nutrients 2024, 16, 559. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Parish, M.; Brennan, J.T.; Winer, B.L.; Segars, J.H. Targeting fibrotic signaling pathways by EGCG as a therapeutic strategy for uterine fibroids. Sci. Rep. 2023, 13, 8492. [Google Scholar] [CrossRef] [PubMed]
- Roshdy, E.; Rajaratnam, V.; Maitra, S.; Sabry, M.; Allah, A.S.; Al-Hendy, A. Treatment of symptomatic uterine fibroids with green tea extract: A pilot randomized controlled clinical study. Int. J. Womens Health 2013, 5, 477–486. [Google Scholar]
- Parsanezhad, M.E.; Azmoon, M.; Alborzi, S.; Rajaeefard, A.; Zarei, A.; Kazerooni, T.; Frank, V.; Schmidt, E.H. A randomized, controlled clinical trial comparing the effects of aromatase inhibitor (letrozole) and gonadotropin-releasing hormone agonist (triptorelin) on uterine leiomyoma volume and hormonal status. Fertil. Steril. 2010, 93, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.C.; Chiang, Y.F.; Chen, H.Y.; Huang, Y.J.; Liu, A.C.; Hsia, S.M. The Potential Effect of Fucoidan on Inhibiting Epithelial-to-Mesenchymal Transition, Proliferation, and Increase in Apoptosis for Endometriosis Treatment: In Vivo and In Vitro Study. Biomedicines 2020, 8, 528. [Google Scholar] [CrossRef]
- Zhang, J.; Riby, J.E.; Conde, L.; Grizzle, W.E.; Cui, X.; Skibola, C.F. A Fucus vesiculosus extract inhibits estrogen receptor activation and induces cell death in female cancer cell lines. BMC Complement. Altern. Med. 2016, 16, 151. [Google Scholar] [CrossRef]
- Nagata, C.; Kaneda, N.; Kabuto, M.; Shimizu, H. Factors associated with serum levels of estradiol and sex hormone-binding globulin among premenopausal Japanese women. Environ. Health Perspect. 1997, 105, 994–997. [Google Scholar] [CrossRef]
- Sriprasert, I.; Kono, N.; Karim, R.; Hodis, H.N.; Stanczyk, F.Z.; Shoupe, D.; Mack, W.J. Factors Associated With Serum Estradiol Levels Among Postmenopausal Women Using Hormone Therapy. Obstet. Gynecol. 2020, 136, 675–684. [Google Scholar] [CrossRef]
- Pataky, M.W.; Young, W.F.; Nair, K.S. Hormonal and Metabolic Changes of Aging and the Influence of Lifestyle Modifications. Mayo Clin. Proc. 2021, 96, 788–814. [Google Scholar] [CrossRef]
- Neumann, B.; Singh, B.; Brennan, J.; Blanck, J.; Segars, J.H. The impact of fibroid treatments on quality of life and mental health: A systematic review. Fertil. Steril. 2024, 121, 400–425. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Zahariev, N.; Katsarov, P.; Lukova, P.; Pilicheva, B. Novel Fucoidan Pharmaceutical Formulations and Their Potential Application in Oncology—A Review. Polymers 2023, 15, 3242. [Google Scholar] [CrossRef] [PubMed]

| Characteristic/Condition | Treatment Group (n = 8) | Placebo Group (n = 8) | p-Value |
|---|---|---|---|
| Age (years) | 42.88 ± 3.64 | 44.88 ± 2.80 | 0.242 |
| Menarche age (years) | 10.25 ± 6.39 | 11.00 ± 4.72 | 0.794 |
| Height (cm) | 158.88 ± 4.70 | 158.50 ± 2.93 | 0.851 |
| Weight (kg) | 55.00 ± 10.25 | 54.88 ± 6.92 | 0.978 |
| Number of pregnancies | 0.88 ± 1.25 | 0.50 ± 0.76 | 0.559 |
| Number of deliveries | 0.63 ± 0.92 | 0.38 ± 0.74 | 0.473 |
| Smoking (n, %) | 0 (0%) | 1 (12.5%) | - |
| Average cycle length (days) | 29–31 | 29–31 | 0.473 |
| Menstrual duration (days) | 4–5 | 4–5 | 1.000 |
| First dysmenorrhea onset | After menarche (>3 years) | After menarche (>3 years) | 0.737 |
| Dysmenorrhea timing | 1–3 days during menstruation | 1–3 days during menstruation | 0.662 |
| Dysmenorrhea persistence (days) | 1–2 days | 2–3 days | 0.324 |
| Impact severity of dysmenorrhea | Mild to moderate | Mild to moderate | 0.849 |
| Uterine Leiomyoma | 8/8 (100%) | 8/8 (100%) | - |
| Endometriosis | 3/8 (37.5%) | 1/8 (12.5%) | - |
| PCOS | 0/8 (0%) | 1/8 (12.5%) | - |
| Gynecological Cancer | 0/8 (0%) | 0/8 (0%) | - |
| Epilepsy | 0/8 (0%) | 0/8 (0%) | - |
| Diabetes | 0/8 (0%) | 1/8 (12.5%) | - |
| UTI | 0/8 (0%) | 1/8 (12.5%) | - |
| Pregnancy | 0/8 (0%) | 0/8 (0%) | - |
| Pregnancy Plan in 3 Months | 0/8 (0%) | 0/8 (0%) | - |
| Parameter (Unit) | Mean ± SD | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | Placebo | Baseline vs. Endpoint | Treatment vs. Placebo | |||||
| Baseline | Endpoint | Baseline | Endpoint | Treatment | Placebo | Baseline | Endpoint | |
| WBC 103/μL) | 6.21 ± 1.47 | 7.48 ± 2.61 | 5.11 ± 1.08 | 6.06 ± 1.90 | 0.999 | 0.999 | 0.999 | 0.999 |
| RBC (106/μL) | 4.23 ± 0.33 | 4.25 ± 0.30 | 4.25 ± 0.53 | 4.37 ± 0.55 | 0.999 | 0.999 | 0.999 | 0.999 |
| HGB (g/dL) | 12.45 ± 1.65 | 13.02 ± 0.88 | 12.13 ± 1.49 | 11.46 ± 2.73 | 0.999 | 0.999 | 0.999 | 0.999 |
| Hct (%) | 37.78 ± 4.72 | 38.53 ± 2.28 | 36.81 ± 3.90 | 35.66 ± 6.26 | 0.999 | 0.999 | 0.999 | 0.999 |
| Net-s (%) | 61.24 ± 6.25 | 58.00 ± 11.24 | 47.66 ± 18.73 | 58.76 ± 10.26 | 0.999 | 0.814 | 0.692 | 0.999 |
| Lym-L (%) | 26.36 ± 6.27 | 29.48 ± 8.13 | 39.25 ± 11.94 | 31.74 ± 9.77 | 0.999 | 0.932 | 0.724 | 0.999 |
| Mono (%) | 9.14 ± 1.97 | 8.85 ± 2.39 | 8.73 ± 4.81 | 6.48 ± 2.01 | 0.999 | 0.999 | 0.999 | 0.999 |
| Baso (%) | 0.84 ± 0.24 | 0.82 ± 0.31 | 0.93 ± 0.44 | 1.00 ± 0.45 | 0.999 | 0.999 | 0.999 | 0.999 |
| Eosin (%) | 2.43 ± 1.51 | 2.85 ± 3.19 | 3.44 ± 3.66 | 2.02 ± 1.55 | 0.999 | 0.999 | 0.999 | 0.999 |
| Platel (103/μL) | 313.63 ± 71.77 | 314.17 ± 99.72 | 281.13 ± 71.55 | 304.20 ± 68.67 | 0.999 | 0.242 | 0.999 | 0.852 |
| MCH (pg) | 29.30 ± 2.32 | 30.67 ± 1.11 | 28.31 ± 3.89 | 26.38 ± 5.79 | 0.999 | 0.999 | 0.999 | 0.999 |
| MCV (fL) | 89.10 ± 6.32 | 90.75 ± 2.18 | 85.66 ± 9.57 | 82.14 ± 13.18 | 0.999 | 0.999 | 0.999 | 0.909 |
| MCHC (g/dL) | 32.89 ± 0.85 | 33.80 ± 0.60 | 32.93 ± 1.31 | 31.78 ± 2.43 | 0.999 | 0.999 | 0.999 | 0.999 |
| Glucose (mg/dL) | 83.88 ± 3.98 | 83.67 ± 5.61 | 93.88 ± 15.72 | 93.20 ± 12.44 | 0.999 | 0.999 | 0.852 | 0.863 |
| TG (mg/dL) | 72.13 ± 21.33 | 76.83 ± 21.42 | 85.50 ± 28.19 | 94.00 ± 41.79 | 0.983 | 0.901 | 0.703 | 0.512 |
| T-CHO (mg/dL) | 181.75 ± 29.90 | 206.00 ± 34.17 | 183.00 ± 40.80 | 187.20 ± 49.82 | 0.200 | 0.987 | 0.999 | 0.423 |
| HDL-C (mg/dL) | 65.13 ± 16.99 | 66.33 ± 11.69 | 66.00 ± 11.03 | 61.60 ± 18.85 | 0.999 | 0.999 | 0.999 | 0.999 |
| LDL-C (mg/dL) | 108.75 ± 23.71 | 129.00 ± 28.71 | 108.63 ± 41.73 | 120.60 ± 39.45 | 0.352 | 0.773 | 0.999 | 0.902 |
| HS-CRP (mg/dL) | 2.45 ± 2.76 | 1.89 ± 3.09 | 1.16 ± 1.44 | 1.10 ± 1.76 | 0.999 | 0.999 | 0.999 | 0.999 |
| GOT (U/L) | 18.25 ± 5.26 | 18.50 ± 6.09 | 17.63 ± 3.81 | 18.20 ± 3.19 | 0.999 | 0.999 | 0.999 | 0.999 |
| GPT (U/L) | 16.00 ± 8.35 | 14.50 ± 4.28 | 15.25 ± 4.68 | 12.40 ± 1.82 | 0.999 | 0.999 | 0.999 | 0.999 |
| BUN (mg/dL) | 11.63 ± 2.62 | 12.33 ± 1.86 | 12.50 ± 2.62 | 11.40 ± 2.51 | 0.999 | 0.999 | 0.999 | 0.999 |
| UA (mg/dL) | 4.50 ± 1.31 | 4.68 ± 0.94 | 4.43 ± 0.96 | 4.30 ± 1.00 | 0.999 | 0.999 | 0.999 | 0.999 |
| ALB (g/dL) | 4.23 ± 0.25 | 4.27 ± 0.15 | 4.30 ± 0.17 | 4.42 ± 0.13 | 0.999 | 0.999 | 0.999 | 0.999 |
| FSH (mIU/mL) | 6.58 ± 3.04 | 17.91 ± 23.80 | 10.05 ± 11.24 | 6.50 ± 2.94 | 0.794 | 0.999 | 0.999 | 0.792 |
| PGTR (ng/mL) | 5.41 ± 3.89 | 3.35 ± 3.37 | 7.01 ± 7.95 | 9.67 ± 12.70 | 0.999 | 0.999 | 0.999 | 0.999 |
| VIT-D (ng/mL) | 22.09 ± 13.66 | 22.20 ± 8.20 | 21.74 ± 5.97 | 23.70 ± 1.52 | 0.999 | 0.999 | 0.999 | 0.999 |
| ASD (ng/mL) | 1.10 ± 0.63 | 1.29 ± 0.71 | 0.94 ± 0.46 | 0.69 ± 0.28 | 0.999 | 0.999 | 0.999 | 0.999 |
| E2 (pg/mL) | 151.13 ± 98.13 | 108.67 ± 54.61 | 140.14 ± 106.15 | 93.40 ± 76.00 | 0.003 * | 0.001 * | 0.812 | 0.602 |
| Mean ± SD | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|
| WHOQOL-BREF | Treatment | Placebo | Baseline vs. Endpoint | Treatment vs. Placebo | ||||
| Baseline | Endpoint | Baseline | Endpoint | Treatment | Placebo | Baseline | Endpoint | |
| Quality of life | 2.75 ± 0.45 | 3.25 ± 0.45 | 3.63 ± 0.50 | 3.75 ± 0.45 | 0.026 * | 0.572 | 0.001 * | 0.026 * |
| General health perception | 2.50 ± 0.73 | 2.50 ± 0.73 | 3.50 ± 0.50 | 3.63 ± 0.50 | 1.000 | 0.597 | 0.003 * | 0.001 * |
| Physical health | 2.98 ± 0.42 | 2.98 ± 0.35 | 3.20 ± 0.38 | 3.36 ± 0.25 | 1.000 | 0.354 | 0.303 | 0.031 * |
| Psychological | 3.06 ± 0.23 | 3.17 ± 0.42 | 3.40 ± 0.42 | 3.60 ± 0.33 | 0.503 | 0.274 | 0.062 | 0.021 * |
| Social relationships | 3.51 ± 0.42 | 3.43 ± 0.55 | 3.47 ± 0.47 | 3.81 ± 0.39 | 0.758 | 0.106 | 0.881 | 0.086 |
| Environmental | 3.74 ± 0.47 | 3.61 ± 0.48 | 3.83 ± 0.40 | 3.99 ± 0.39 | 0.580 | 0.458 | 0.669 | 0.078 |
| Treatment | Placebo | Baseline vs. Endpoint | Treatment vs. Placebo | |||||
|---|---|---|---|---|---|---|---|---|
| Leiomyoma Tissue | Baseline | Endpoint | Baseline | Endpoint | Treatment | Placebo | Baseline | Endpoint |
| Number | 6.00 ± 3.00 | 4.78 ± 2.31 | 4.44 ± 2.87 | 5.22 ± 3.28 | 0.361 | 0.609 | 0.261 | 0.764 |
| Size | 3.72 ± 1.02 | 3.86 ± 0.82 | 3.11 ± 1.17 | 3.26 ± 1.18 | 0.757 | 0.801 | 0.286 | 0.254 |
| Mean ± SD | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|
| Pain Type | Treatment | Placebo | Baseline vs. Endpoint | Treatment vs. Placebo | ||||
| Baseline | Endpoint | Baseline | Endpoint | Treatment | Placebo | Baseline | Endpoint | |
| Throbbing Pain | 0.75 ± 0.71 | 0.86 ± 0.90 | 0.63 ± 0.52 | 1.14 ± 0.90 | 0.800 | 0.187 | 0.692 | 0.563 |
| Sudden Sharp Pain | 0.38 ± 0.74 | 0.71 ± 0.76 | 0.50 ± 0.53 | 0.86 ± 1.07 | 0.397 | 0.418 | 0.705 | 0.777 |
| Stabbing Pain | 0.13 ± 0.35 | 0.43 ± 0.79 | 0.63 ± 0.52 | 0.57 ± 0.79 | 0.341 | 0.894 | 0.040 * | 0.768 |
| Cutting Pain | 0.13 ± 0.35 | 0.14 ± 0.38 | 0.00 ± 0.00 | 0.43 ± 0.79 | 0.926 | 0.145 | 0.334 | 0.403 |
| Cramping Pain | 1.00 ± 0.93 | 1.14 ± 1.07 | 0.63 ± 1.19 | 1.00 ± 1.15 | 0.785 | 0.028 * | 0.008 * | 0.814 |
| Gnawing Pain | 0.00 ± 0.00 | 0.29 ± 0.49 | 0.63 ± 1.18 | 0.57 ± 0.79 | 0.119 | 0.930 | 0.158 | 0.551 |
| Burning Pain | 0.13 ± 0.35 | 0.29 ± 0.49 | 0.00 ± 0.00 | 1.00 ± 1.07 | 0.598 | 0.028 * | 0.333 | 0.196 |
| Persistent Pain | 0.50 ± 0.76 | 0.57 ± 0.72 | 0.25 ± 0.46 | 1.29 ± 0.76 | 0.838 | 0.031 * | 0.505 | 0.108 |
| Dull Pain | 1.13 ± 0.64 | 1.43 ± 0.62 | 1.25 ± 0.46 | 1.14 ± 0.38 | 0.424 | 0.856 | 0.808 | 0.539 |
| Pain with Touch | 0.00 ± 0.00 | 0.00 ± 0.00 | 1.63 ± 0.74 | 0.29 ± 0.53 | - | 0.015 * | 0.001 * | 0.147 |
| Tearing Pain | 0.13 ± 0.35 | 0.57 ± 0.71 | 0.00 ± 0.00 | 0.43 ± 0.79 | 0.368 | 0.145 | 0.334 | 0.175 |
| Exhaustion Feeling | 0.25 ± 0.46 | 0.57 ± 0.79 | 0.50 ± 0.53 | 0.71 ± 0.95 | 0.473 | 0.052 | 0.334 | 0.786 |
| Nausea/Vomiting Feeling | 0.50 ± 0.71 | 0.00 ± 0.00 | 0.50 ± 0.53 | 0.86 ± 0.98 | 0.838 | 0.418 | 1.000 | 0.299 |
| Fear/Anxiety Feeling | 0.25 ± 0.46 | 0.00 ± 0.00 | 0.50 ± 0.53 | 0.43 ± 0.79 | 0.368 | 0.532 | 0.505 | 0.147 |
| Excruciating Pain | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.43 ± 0.79 | - | 0.145 | - | 0.175 |
| Symptom | Mean ± SD | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | Placebo | Baseline vs. Endpoint | Treatment vs. Placebo | |||||
| Baseline | Endpoint | Baseline | Endpoint | Treatment | Placebo | Baseline | Endpoint | |
| Dizziness | 1.50 ± 0.53 | 1.71 ± 0.76 | 1.13 ± 0.35 | 1.14 ± 0.38 | 0.705 | 1.000 | 0.12 | 0.108 |
| Muscle Pain | 1.75 ± 0.71 | 2.14 ± 1.07 | 1.50 ± 0.53 | 1.86 ± 0.69 | 0.809 | 0.438 | 0.438 | 0.809 |
| Breast Pain or Swelling | 2.00 ± 0.76 | 2.57 ± 0.79 | 1.88 ± 0.83 | 1.86 ± 0.38 | 0.619 | 0.717 | 0.758 | 0.278 |
| Lower Abdominal Swelling | 2.00 ± 0.76 | 2.43 ± 0.79 | 2.50 ± 1.20 | 2.14 ± 0.90 | 0.798 | 0.828 | 0.334 | 0.655 |
| General Body Pain | 1.75 ± 0.89 | 2.00 ± 1.15 | 1.50 ± 0.76 | 1.71 ± 0.76 | 1.000 | 0.744 | 0.554 | 0.815 |
| Back Pain | 1.88 ± 0.64 | 2.14 ± 0.69 | 2.25 ± 0.71 | 2.00 ± 0.82 | 1.000 | 0.506 | 0.285 | 0.781 |
| Headache | 1.75 ± 0.71 | 2.00 ± 1.15 | 2.38 ± 0.92 | 2.00 ± 1.00 | 1.000 | 0.672 | 0.149 | 0.579 |
| Skin Issues | 1.38 ± 1.06 | 1.71 ± 0.76 | 1.38 ± 0.52 | 1.14 ± 0.38 | 0.805 | 0.619 | 1.000 | 0.506 |
| Nausea or Vomiting | 1.00 ± 0.00 | 1.14 ± 0.38 | 1.38 ± 0.52 | 1.14 ± 0.38 | 1.000 | 0.278 | 0.060 | 0.590 |
| Diarrhea | 1.75 ± 1.16 | 2.29 ± 0.76 | 2.62 ± 1.06 | 2.29 ± 0.76 | 1.000 | 0.805 | 0.139 | 0.176 |
| Fatigue | 2.38 ± 0.92 | 2.29 ± 0.76 | 2.13 ± 0.99 | 1.71 ± 0.76 | 0.464 | 0.227 | 0.609 | 0.387 |
| Weight Gain | 1.50 ± 0.53 | 1.14 ± 0.38 | 1.13 ± 0.35 | 1.14 ± 0.38 | 0.438 | 0.705 | 1.000 | 0.693 |
| Facial Flushing | 1.00 ± 0.00 | 1.29 ± 0.49 | 1.13 ± 0.35 | 1.14 ± 0.38 | 1.000 | 1.000 | 0.334 | 0.590 |
| Chills | 1.25 ± 0.71 | 1.29 ± 0.49 | 1.00 ± 0.00 | 1.00 ± 0.00 | 0.334 | 1.000 | 0.554 | 0.590 |
| Palpitations | 1.25 ± 0.71 | 1.29 ± 0.49 | 1.00 ± 0.00 | 1.00 ± 0.00 | 0.717 | - | 0.334 | 0.590 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiang, Y.-F.; Huang, K.-C.; Huang, P.-S.; Ali, M.; Hsia, S.-M. Marine Oligo-Fucoidan as a Safe Functional Food for Managing Uterine Fibroids: Results from a Pilot Randomized Controlled Trial. Biomedicines 2025, 13, 1970. https://doi.org/10.3390/biomedicines13081970
Chiang Y-F, Huang K-C, Huang P-S, Ali M, Hsia S-M. Marine Oligo-Fucoidan as a Safe Functional Food for Managing Uterine Fibroids: Results from a Pilot Randomized Controlled Trial. Biomedicines. 2025; 13(8):1970. https://doi.org/10.3390/biomedicines13081970
Chicago/Turabian StyleChiang, Yi-Fen, Ko-Chieh Huang, Pei-Shen Huang, Mohamed Ali, and Shih-Min Hsia. 2025. "Marine Oligo-Fucoidan as a Safe Functional Food for Managing Uterine Fibroids: Results from a Pilot Randomized Controlled Trial" Biomedicines 13, no. 8: 1970. https://doi.org/10.3390/biomedicines13081970
APA StyleChiang, Y.-F., Huang, K.-C., Huang, P.-S., Ali, M., & Hsia, S.-M. (2025). Marine Oligo-Fucoidan as a Safe Functional Food for Managing Uterine Fibroids: Results from a Pilot Randomized Controlled Trial. Biomedicines, 13(8), 1970. https://doi.org/10.3390/biomedicines13081970









