Glioblastoma: From Pathophysiology to Novel Therapeutic Approaches
Abstract
1. Introduction
2. Glioblastoma Pathophysiology
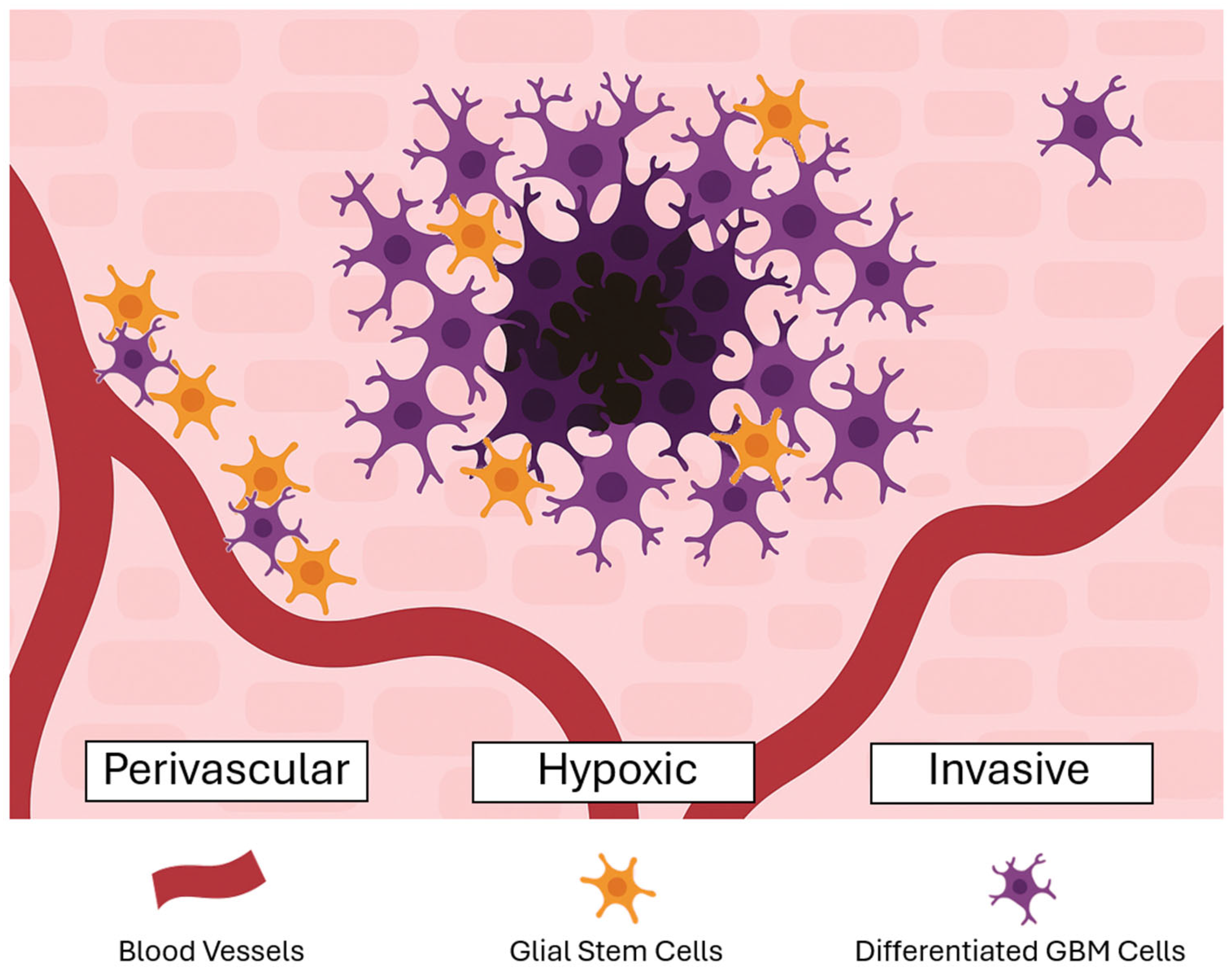
2.1. Glioma Stem Cells
2.2. Tumor Microenvironment
2.3. Angiogenesis
2.4. Epigenetics
3. Glioblastoma Diagnostic Challenges
3.1. 2-Deoxy-2-[18F]fluoro-D-glucose
3.2. 18F-Floretyrosine (FET PET)
3.3. [68Ga]Ga-PSMA-617
4. Newly Diagnosed Glioblastoma Therapies and Challenges
4.1. Current Therapies
4.1.1. Surgery
4.1.2. Stupp Protocol
4.1.3. Tumor Treating Fields
4.2. Abandoned Therapies
4.2.1. Carmustine Wafers
4.2.2. Anti-Angiogenic Therapy
5. Recurrent Glioblastoma Therapies and Challenges
5.1. Current Therapies
5.1.1. Re-Resection
5.1.2. Reirradiation
5.1.3. Chemotherapy
5.1.4. Anti-Angiogenic Therapy
5.1.5. Tumor Treating Fields
5.2. Abandoned Therapies
6. Novel Therapeutics
6.1. Lomustine
6.2. Laser Interstitial Thermal Therapy
6.3. GammaTile®
6.4. Immunotherapy
6.5. Targeted Radionuclide Therapy
6.6. Vaccines
6.6.1. Dendritic Cell Vaccines
6.6.2. Peptide Vaccines
6.7. CAR T-Cell Therapy
6.7.1. IL-13Rα2
6.7.2. EGFR
6.7.3. HER2
6.7.4. CAR Natural Killer-Cell Therapy
7. Future Directions
7.1. Diagnostics
7.2. Gene Therapy
7.3. Light-Based Therapy
7.3.1. Photodynamic Therapy
7.3.2. Sonodynamic Therapy
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| (18F)FET | O-(2-[18F]fluoroethyl)-L-tyrosine |
| 5-FC | 5-fluorocytosine |
| 5-FU | 5-fluorouracil |
| APC | antigen-presenting cell |
| BBB | blood–brain barrier |
| BCNU | carmustine |
| BELOB | bevacizumab and lomustine in recurrent glioblastoma |
| BEV | bevacizumab |
| CAR | chimeric antigen receptor |
| CCNU | lomustine |
| CNS | central nervous system |
| CRCs | Chromatin-remodeling complexes |
| CpG | cytosine–guanine–dinucleotide |
| DC | dendritic cell |
| DD | dose-dense |
| EGFR | epidermal growth factor receptor |
| EOR | extent of resection |
| FISH | fluorescence in situ hybridization |
| GBM | glioblastoma |
| GSC | glioma stem cells |
| H-1PV | H-1 parvovirus |
| HER2 | human epidermal growth factor 2 |
| HIF-1α | hypoxia-inducible transcription-factor-1α |
| IDH | isocitrate dehydrogenase |
| IL-13 | interleukin-13 |
| LAT1 | L-type amino acid transporter |
| LITT | laser interstitial thermal therapy |
| MGMT | O6-methylguanine-DNA methyltransferase |
| MRI | magnetic resonance imaging |
| ndGBM | newly diagnosed glioblastoma |
| NK | natural killer |
| OS | overall survival |
| PD-1 | programmed death-1 |
| PD-L1 | programmed cell death ligand-1 |
| PET | positron emission tomography |
| PFS | progression-free survival |
| PSMA | prostate-specific membrane antigen |
| PTEN | phosphatase and tensin homolog |
| QoL | quality of life |
| REGAL | REcentin™ in Glioblastoma Alone and with Lomustine |
| REGOMA | regorafenib glioblastoma |
| rGBM | recurrent glioblastoma |
| SOC | standard of care |
| TAMS | tumor-associated macrophages |
| TERT | telomerase reverse transcriptase |
| TME | tumor microenvironment |
| TMZ | temozolomide |
| TP53 | tumor protein p53 |
| TTF | tumor treating fields |
| reRT | reirradiation |
| WHO | World Health Organization |
References
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Taillibert, S.; Kanner, A.; Read, W.; Steinberg, D.M.; Lhermitte, B.; Toms, S.; Idbaih, A.; Ahluwalia, M.S.; Fink, K.; et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma: A Randomized Clinical Trial. JAMA 2017, 318, 2306–2316. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, F.; Ali, H.; Lathia, J.D.; Chen, P. Immunotherapy for glioblastoma: Current state, challenges, and future perspectives. Cell. Mol. Immunol. 2024, 21, 1354–1375. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Dey, D.; Barik, D.; Mohapatra, I.; Kim, S.; Sharma, M.; Prasad, S.; Wang, P.; Singh, A.; Singh, G. Glioblastoma at the crossroads: Current understanding and future therapeutic horizons. Signal Transduct. Target. Ther. 2025, 10, 213. [Google Scholar] [CrossRef]
- Gömöri, É.; Pál, J.; Kovács, B.; Dóczi, T. Concurrent hypermethylation of DNMT1, MGMT and EGFR genes in progression of gliomas. Diagn. Pathol. 2012, 7, 8. [Google Scholar] [CrossRef]
- Etcheverry, A.; Aubry, M.; De Tayrac, M.; Vauleon, E.; Boniface, R.; Guenot, F.; Saikali, S.; Hamlat, A.; Riffaud, L.; Menei, P.; et al. DNA methylation in glioblastoma: Impact on gene expression and clinical outcome. BMC Genom. 2010, 11, 701. [Google Scholar] [CrossRef]
- Hegi, M.E.; Diserens, A.C.; Gorlia, T.; Hamou, M.F.; de Tribolet, N.; Weller, M.; Kros, J.M.; Hainfellner, J.A.; Mason, W.; Mariani, L.; et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. 2005, 352, 997–1003. [Google Scholar] [CrossRef]
- Hegi, M.E.; Genbrugge, E.; Gorlia, T.; Stupp, R.; Gilbert, M.R.; Chinot, O.L.; Nabors, L.B.; Jones, G.; Van Criekinge, W.; Straub, J.; et al. MGMT Promoter Methylation Cutoff with Safety Margin for Selecting Glioblastoma Patients into Trials Omitting Temozolomide: A Pooled Analysis of Four Clinical Trials. Clin. Cancer Res. 2019, 25, 1809–1816. [Google Scholar] [CrossRef]
- Hegi, M.E.; Oppong, F.B.; Perry, J.R.; Wick, W.; Henriksson, R.; Laperriere, N.J.; Gorlia, T.; Malmstrom, A.; Weller, M. No benefit from TMZ treatment in glioblastoma with truly unmethylated MGMT promoter: Reanalysis of the CE.6 and the pooled Nordic/NOA-08 trials in elderly glioblastoma patients. Neuro-Oncology 2024, 26, 1867–1875. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Wang, J.; Lan, Y.; Qi, H.Y.; Wang, L.H.; Wei, S.; Yuan, Y.; Ge, J.; Li, A.L.; Yan, Z.X.; Li, L.; et al. Comparison of Fluorescence In Situ Hybridization, Next-Generation Sequencing, and DNA Methylation Microarray for Copy Number Variation Assessment in Gliomas. Lab. Investig. 2025, 105, 104168. [Google Scholar] [CrossRef] [PubMed]
- Priesterbach-Ackley, L.P.; Cordier, F.; de Witt Hamer, P.; Snijders, T.J.; Robe, P.A.; Kusters, B.; de Leng, W.W.J.; den Dunnen, W.F.A.; Brandsma, D.; Jansen, C.; et al. Diffuse, IDH-wildtype gliomas in adults with minimal histological change and isolated TERT promoter mutation: Not simply CNS WHO grade 4. Acta Neuropathol. 2024, 148, 12. [Google Scholar] [CrossRef] [PubMed]
- Verhaak, R.G.; Hoadley, K.A.; Purdom, E.; Wang, V.; Qi, Y.; Wilkerson, M.D.; Miller, C.R.; Ding, L.; Golub, T.; Mesirov, J.P.; et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010, 17, 98–110. [Google Scholar] [CrossRef]
- Hovinga, K.E.; McCrea, H.J.; Brennan, C.; Huse, J.; Zheng, J.; Esquenazi, Y.; Panageas, K.S.; Tabar, V. EGFR amplification and classical subtype are associated with a poor response to bevacizumab in recurrent glioblastoma. J. Neuro-Oncol. 2019, 142, 337–345. [Google Scholar] [CrossRef]
- Brennan, C.W.; Verhaak, R.G.; McKenna, A.; Campos, B.; Noushmehr, H.; Salama, S.R.; Zheng, S.; Chakravarty, D.; Sanborn, J.Z.; Berman, S.H.; et al. The somatic genomic landscape of glioblastoma. Cell 2013, 155, 462–477. [Google Scholar] [CrossRef]
- Behnan, J.; Finocchiaro, G.; Hanna, G. The landscape of the mesenchymal signature in brain tumours. Brain 2019, 142, 847–866. [Google Scholar] [CrossRef]
- Cloughesy, T.F.; Mochizuki, A.Y.; Orpilla, J.R.; Hugo, W.; Lee, A.H.; Davidson, T.B.; Wang, A.C.; Ellingson, B.M.; Rytlewski, J.A.; Sanders, C.M.; et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat. Med. 2019, 25, 477–486. [Google Scholar] [CrossRef]
- Schonberg, D.L.; Lubelski, D.; Miller, T.E.; Rich, J.N. Brain tumor stem cells: Molecular characteristics and their impact on therapy. Mol. Asp. Med. 2014, 39, 82–101. [Google Scholar] [CrossRef]
- Zheng, H.; Ying, H.; Yan, H.; Kimmelman, A.C.; Hiller, D.J.; Chen, A.J.; Perry, S.R.; Tonon, G.; Chu, G.C.; Ding, Z.; et al. p53 and Pten control neural and glioma stem/progenitor cell renewal and differentiation. Nature 2008, 455, 1129–1133. [Google Scholar] [CrossRef]
- De Bacco, F.; Orzan, F.; Crisafulli, G.; Prelli, M.; Isella, C.; Casanova, E.; Albano, R.; Reato, G.; Erriquez, J.; D’Ambrosio, A.; et al. Coexisting cancer stem cells with heterogeneous gene amplifications, transcriptional profiles, and malignancy are isolated from single glioblastomas. Cell Rep. 2023, 42, 112816. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, J.W.; Lee, J.H. Genetic Architectures and Cell-of-Origin in Glioblastoma. Front. Oncol. 2020, 10, 615400. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Auffinger, B.; Guo, D.; Hasan, T.; Deheeger, M.; Tobias, A.L.; Kim, J.Y.; Atashi, F.; Zhang, L.; Lesniak, M.S.; et al. Dedifferentiation of Glioma Cells to Glioma Stem-like Cells By Therapeutic Stress-induced HIF Signaling in the Recurrent GBM Model. Mol. Cancer Ther. 2016, 15, 3064–3076. [Google Scholar] [CrossRef] [PubMed]
- Iranmanesh, Y.; Jiang, B.; Favour, O.C.; Dou, Z.; Wu, J.; Li, J.; Sun, C. Mitochondria’s Role in the Maintenance of Cancer Stem Cells in Glioblastoma. Front. Oncol. 2021, 11, 582694. [Google Scholar] [CrossRef] [PubMed]
- Scully, S.; Francescone, R.; Faibish, M.; Bentley, B.; Taylor, S.L.; Oh, D.; Schapiro, R.; Moral, L.; Yan, W.; Shao, R. Transdifferentiation of Glioblastoma Stem-Like Cells into Mural Cells Drives Vasculogenic Mimicry in Glioblastomas. J. Neurosci. 2012, 32, 12950–12960. [Google Scholar] [CrossRef]
- Hambardzumyan, D.; Bergers, G. Glioblastoma: Defining Tumor Niches. Trends Cancer 2015, 1, 252–265. [Google Scholar] [CrossRef]
- Charles, N.; Ozawa, T.; Squatrito, M.; Bleau, A.M.; Brennan, C.W.; Hambardzumyan, D.; Holland, E.C. Perivascular nitric oxide activates notch signaling and promotes stem-like character in PDGF-induced glioma cells. Cell Stem Cell 2010, 6, 141–152. [Google Scholar] [CrossRef]
- Calabrese, C.; Poppleton, H.; Kocak, M.; Hogg, T.L.; Fuller, C.; Hamner, B.; Oh, E.Y.; Gaber, M.W.; Finklestein, D.; Allen, M.; et al. A Perivascular Niche for Brain Tumor Stem Cells. Cancer Cell 2007, 11, 69–82. [Google Scholar] [CrossRef]
- Li, Z.; Bao, S.; Wu, Q.; Wang, H.; Eyler, C.; Sathornsumetee, S.; Shi, Q.; Cao, Y.; Lathia, J.; McLendon, R.E.; et al. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell 2009, 15, 501–513. [Google Scholar] [CrossRef]
- Boyd, N.H.; Tran, A.N.; Bernstock, J.D.; Etminan, T.; Jones, A.B.; Gillespie, G.Y.; Friedman, G.K.; Hjelmeland, A.B. Glioma stem cells and their roles within the hypoxic tumor microenvironment. Theranostics 2021, 11, 665–683. [Google Scholar] [CrossRef]
- Kaur, B.; Khwaja, F.W.; Severson, E.A.; Matheny, S.L.; Brat, D.J.; Van Meir, E.G. Hypoxia and the hypoxia-inducible-factor pathway in glioma growth and angiogenesis. Neuro-Oncology 2005, 7, 134–153. [Google Scholar] [CrossRef]
- Qiang, L.; Wu, T.; Zhang, H.W.; Lu, N.; Hu, R.; Wang, Y.J.; Zhao, L.; Chen, F.H.; Wang, X.T.; You, Q.D.; et al. HIF-1α is critical for hypoxia-mediated maintenance of glioblastoma stem cells by activating Notch signaling pathway. Cell Death Differ. 2012, 19, 284–294. [Google Scholar] [CrossRef]
- Ngo, M.T.; Sarkaria, J.N.; Harley, B.A.C. Perivascular Stromal Cells Instruct Glioblastoma Invasion, Proliferation, and Therapeutic Response within an Engineered Brain Perivascular Niche Model. Adv. Sci. 2022, 9, e2201888. [Google Scholar] [CrossRef]
- Pombero, A.; Garcia-Lopez, R.; Martínez, S. Pericyte-Glioblastoma Cell Interaction: A Key Target to Prevent Glioblastoma Progression. Cells 2023, 12, 1324. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.V.; Conroy, S.; Tomar, T.; Eggens-Meijer, E.; Bhat, K.; Copray, S.; Walenkamp, A.M.E.; Boddeke, E.; Balasubramanyian, V.; Wagemakers, M.; et al. TGF-β is an inducer of ZEB1-dependent mesenchymal transdifferentiation in glioblastoma that is associated with tumor invasion. Cell Death Dis. 2014, 5, e1443. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Huang, Z.; Zhou, W.; Wu, Q.; Sloan, A.E.; Ouyang, G.; McLendon, R.E.; Yu, J.S.; Rich, J.N.; Bao, S. The Zinc Finger Transcription Factor ZFX Is Required for Maintaining the Tumorigenic Potential of Glioblastoma Stem Cells. Stem Cells 2014, 32, 2033–2047. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Ke, S.Q.; Huang, Z.; Flavahan, W.; Fang, X.; Paul, J.; Wu, L.; Sloan, A.E.; Mclendon, R.E.; Li, X.; et al. Periostin secreted by glioblastoma stem cells recruits M2 tumour-associated macrophages and promotes malignant growth. Nat. Cell Biol. 2015, 17, 170–182. [Google Scholar] [CrossRef]
- Cavaleri, J.M.; Monaco, E.A.I. Periostin: A Potential Target for Glioblastoma Multiforme Treatment. Neurosurgery 2015, 76, N17–N19. [Google Scholar] [CrossRef][Green Version]
- Friebel, E.; Kapolou, K.; Unger, S.; Núñez, N.G.; Utz, S.; Rushing, E.J.; Regli, L.; Weller, M.; Greter, M.; Tugues, S.; et al. Single-Cell Mapping of Human Brain Cancer Reveals Tumor-Specific Instruction of Tissue-Invading Leukocytes. Cell 2020, 181, 1626–1642.e1620. [Google Scholar] [CrossRef]
- Rodriguez, S.M.B.; Staicu, G.-A.; Sevastre, A.-S.; Baloi, C.; Ciubotaru, V.; Dricu, A.; Tataranu, L.G. Glioblastoma Stem Cells—Useful Tools in the Battle against Cancer. Int. J. Mol. Sci. 2022, 23, 4602. [Google Scholar] [CrossRef]
- Ye, X.-Z.; Xu, S.-L.; Xin, Y.-H.; Yu, S.-C.; Ping, Y.-F.; Chen, L.; Xiao, H.-L.; Wang, B.; Yi, L.; Wang, Q.-L.; et al. Tumor-Associated Microglia/Macrophages Enhance the Invasion of Glioma Stem-like Cells via TGF-β1 Signaling Pathway. J. Immunol. 2012, 189, 444–453. [Google Scholar] [CrossRef]
- Liu, Z.; Kuang, W.; Zhou, Q.; Zhang, Y. TGF-β1 secreted by M2 phenotype macrophages enhances the stemness and migration of glioma cells via the SMAD2/3 signalling pathway. Int. J. Mol. Med. 2018, 42, 3395–3403. [Google Scholar] [CrossRef]
- Bergers, G.; Brekken, R.; Mcmahon, G.; Vu, T.H.; Itoh, T.; Tamaki, K.; Tanzawa, K.; Thorpe, P.; Itohara, S.; Werb, Z.; et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat. Cell Biol. 2000, 2, 737–744. [Google Scholar] [CrossRef]
- Nozawa, H.; Chiu, C.; Hanahan, D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc. Natl. Acad. Sci. USA 2006, 103, 12493–12498. [Google Scholar] [CrossRef]
- Guo, X.; Qiu, W.; Wang, C.; Qi, Y.; Li, B.; Wang, S.; Zhao, R.; Cheng, B.; Han, X.; Du, H.; et al. Neuronal Activity Promotes Glioma Progression by Inducing Proneural-to-Mesenchymal Transition in Glioma Stem Cells. Cancer Res. 2024, 84, 372–387. [Google Scholar] [CrossRef]
- Desai, A.; Webb, B.; Gerson, S.L. CD133+ cells contribute to radioresistance via altered regulation of DNA repair genes in human lung cancer cells. Radiother. Oncol. 2014, 110, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Yuan, X.; Zeng, Z.; Tunici, P.; Ng, H.; Abdulkadir, I.R.; Lu, L.; Irvin, D.; Black, K.L.; Yu, J.S. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol. Cancer 2006, 5, 67. [Google Scholar] [CrossRef] [PubMed]
- Malmström, A.; Grønberg, B.H.; Marosi, C.; Stupp, R.; Frappaz, D.; Schultz, H.; Abacioglu, U.; Tavelin, B.; Lhermitte, B.; Hegi, M.E.; et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: The Nordic randomised, phase 3 trial. Lancet Oncol. 2012, 13, 916–926. [Google Scholar] [CrossRef] [PubMed]
- Wick, W.; Platten, M.; Meisner, C.; Felsberg, J.; Tabatabai, G.; Simon, M.; Nikkhah, G.; Papsdorf, K.; Steinbach, J.P.; Sabel, M.; et al. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: The NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012, 13, 707–715. [Google Scholar] [CrossRef]
- Lee, E.J.; Rath, P.; Liu, J.; Ryu, D.; Pei, L.; Noonepalle, S.K.; Shull, A.Y.; Feng, Q.; Litofsky, N.S.; Miller, D.C.; et al. Identification of Global DNA Methylation Signatures in Glioblastoma-Derived Cancer Stem Cells. J. Genet. Genom. 2015, 42, 355–371. [Google Scholar] [CrossRef]
- Dréan, A.; Rosenberg, S.; Lejeune, F.-X.; Goli, L.; Nadaradjane, A.A.; Guehennec, J.; Schmitt, C.; Verreault, M.; Bielle, F.; Mokhtari, K.; et al. ATP binding cassette (ABC) transporters: Expression and clinical value in glioblastoma. J. Neuro-Oncol. 2018, 138, 479–486. [Google Scholar] [CrossRef]
- Hartz, A.M.; Bauer, B. ABC transporters in the CNS—An inventory. Curr. Pharm. Biotechnol. 2011, 12, 656–673. [Google Scholar] [CrossRef]
- Biserova, K.; Jakovlevs, A.; Uljanovs, R.; Strumfa, I. Cancer Stem Cells: Significance in Origin, Pathogenesis and Treatment of Glioblastoma. Cells 2021, 10, 621. [Google Scholar] [CrossRef]
- Yuen, C.A.; Asuthkar, S.; Guda, M.R.; Tsung, A.J.; Velpula, K.K. Cancer stem cell molecular reprogramming of the Warburg effect in glioblastomas: A new target gleaned from an old concept. CNS Oncol. 2016, 5, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.M.; Choi, J.; Lim, M. Mechanisms of immunotherapy resistance: Lessons from glioblastoma. Nat. Immunol. 2019, 20, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Aaroe, A.; Liang, J.; Puduvalli, V.K. Tumor microenvironment in glioblastoma: Current and emerging concepts. Neuro-Oncol. Adv. 2023, 5, vdad009. [Google Scholar] [CrossRef] [PubMed]
- Da Mesquita, S.; Fu, Z.; Kipnis, J. The Meningeal Lymphatic System: A New Player in Neurophysiology. Neuron 2018, 100, 375–388. [Google Scholar] [CrossRef]
- Yankova, G.; Bogomyakova, O.; Tulupov, A. The glymphatic system and meningeal lymphatics of the brain: New understanding of brain clearance. Rev. Neurosci. 2021, 32, 693–705. [Google Scholar] [CrossRef]
- Venkataramani, V.; Yang, Y.; Schubert, M.C.; Reyhan, E.; Tetzlaff, S.K.; Wißmann, N.; Botz, M.; Soyka, S.J.; Beretta, C.A.; Pramatarov, R.L.; et al. Glioblastoma hijacks neuronal mechanisms for brain invasion. Cell 2022, 185, 2899–2917.e2831. [Google Scholar] [CrossRef]
- Wu, C.Y.; Chen, Y.; Lin, Y.J.; Wei, K.C.; Chang, K.Y.; Feng, L.Y.; Chen, K.T.; Li, G.; Ren, A.L.; Nitta, R.T.; et al. Tumor-Associated Microglia Secrete Extracellular ATP to Support Glioblastoma Progression. Cancer Res. 2024, 84, 4017–4030. [Google Scholar] [CrossRef]
- Beltra, J.-C.; Manne, S.; Abdel-Hakeem, M.S.; Kurachi, M.; Giles, J.R.; Chen, Z.; Casella, V.; Ngiow, S.F.; Khan, O.; Huang, Y.J.; et al. Developmental Relationships of Four Exhausted CD8+ T Cell Subsets Reveals Underlying Transcriptional and Epigenetic Landscape Control Mechanisms. Immunity 2020, 52, 825–841.e828. [Google Scholar] [CrossRef]
- Batchu, S.; Hanafy, K.A.; Redjal, N.; Godil, S.S.; Thomas, A.J. Single-cell analysis reveals diversity of tumor-associated macrophages and their interactions with T lymphocytes in glioblastoma. Sci. Rep. 2023, 13, 20874. [Google Scholar] [CrossRef]
- Szulzewsky, F.; Pelz, A.; Feng, X.; Synowitz, M.; Markovic, D.; Langmann, T.; Holtman, I.R.; Wang, X.; Eggen, B.J.; Boddeke, H.W.; et al. Glioma-associated microglia/macrophages display an expression profile different from M1 and M2 polarization and highly express Gpnmb and Spp1. PLoS ONE 2015, 10, e0116644. [Google Scholar] [CrossRef]
- Didomenico, J.; Lamano, J.B.; Oyon, D.; Li, Y.; Veliceasa, D.; Kaur, G.; Ampie, L.; Choy, W.; Lamano, J.B.; Bloch, O. The immune checkpoint protein PD-L1 induces and maintains regulatory T cells in glioblastoma. OncoImmunology 2018, 7, e1448329. [Google Scholar] [CrossRef]
- Reuss, A.M.; Groos, D.; Buchfelder, M.; Savaskan, N. The Acidic Brain-Glycolytic Switch in the Microenvironment of Malignant Glioma. Int. J. Mol. Sci. 2021, 22, 5518. [Google Scholar] [CrossRef] [PubMed]
- Moeller, B.J.; Dreher, M.R.; Rabbani, Z.N.; Schroeder, T.; Cao, Y.; Li, C.Y.; Dewhirst, M.W. Pleiotropic effects of HIF-1 blockade on tumor radiosensitivity. Cancer Cell 2005, 8, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Gonçalves, V.; Gonçalves, C.S.; Granja, S.; Vieira De Castro, J.; Reis, R.M.; Costa, B.M.; Baltazar, F. MCT1 Is a New Prognostic Biomarker and Its Therapeutic Inhibition Boosts Response to Temozolomide in Human Glioblastoma. Cancers 2021, 13, 3468. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, Z.; Chen, Y.; Tian, H.; Chai, P.; Shen, Y.; Yao, Y.; Xu, S.; Ge, S.; Jia, R. Lactate and lactylation in cancer. Signal Transduct. Target. Ther. 2025, 10, 38. [Google Scholar] [CrossRef]
- Filatova, A.; Seidel, S.; Bogurcu, N.; Graf, S.; Garvalov, B.K.; Acker, T. Acidosis Acts through HSP90 in a PHD/VHL-Independent Manner to Promote HIF Function and Stem Cell Maintenance in Glioma. Cancer Res. 2016, 76, 5845–5856. [Google Scholar] [CrossRef]
- Raghunand, N.; Gillies, R.J. pH and drug resistance in tumors. Drug Resist. Updates 2000, 3, 39–47. [Google Scholar] [CrossRef]
- Faes, S.; Duval, A.P.; Planche, A.; Uldry, E.; Santoro, T.; Pythoud, C.; Stehle, J.C.; Horlbeck, J.; Letovanec, I.; Riggi, N.; et al. Acidic tumor microenvironment abrogates the efficacy of mTORC1 inhibitors. Mol. Cancer 2016, 15, 78. [Google Scholar] [CrossRef]
- Williams, C.H.; Neitzel, L.R.; Cornell, J.; Rea, S.; Mills, I.; Silver, M.S.; Ahmad, J.D.; Birukov, K.G.; Birukova, A.; Brem, H.; et al. GPR68-ATF4 signaling is a novel prosurvival pathway in glioblastoma activated by acidic extracellular microenvironment. Exp. Hematol. Oncol. 2024, 13, 13. [Google Scholar] [CrossRef]
- Estrella, V.; Chen, T.; Lloyd, M.; Wojtkowiak, J.; Cornnell, H.H.; Ibrahim-Hashim, A.; Bailey, K.; Balagurunathan, Y.; Rothberg, J.M.; Sloane, B.F.; et al. Acidity generated by the tumor microenvironment drives local invasion. Cancer Res. 2013, 73, 1524–1535. [Google Scholar] [CrossRef]
- Hjelmeland, A.B.; Wu, Q.; Heddleston, J.M.; Choudhary, G.S.; MacSwords, J.; Lathia, J.D.; McLendon, R.; Lindner, D.; Sloan, A.; Rich, J.N. Acidic stress promotes a glioma stem cell phenotype. Cell Death Differ. 2011, 18, 829–840. [Google Scholar] [CrossRef]
- Cortes Ballen, A.I.; Amosu, M.; Ravinder, S.; Chan, J.; Derin, E.; Slika, H.; Tyler, B. Metabolic Reprogramming in Glioblastoma Multiforme: A Review of Pathways and Therapeutic Targets. Cells 2024, 13, 1574. [Google Scholar] [CrossRef]
- Deberardinis, R.J.; Mancuso, A.; Daikhin, E.; Nissim, I.; Yudkoff, M.; Wehrli, S.; Thompson, C.B. Beyond aerobic glycolysis: Transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl. Acad. Sci. USA 2007, 104, 19345–19350. [Google Scholar] [CrossRef]
- Taïb, B.; Aboussalah, A.M.; Moniruzzaman, M.; Chen, S.; Haughey, N.J.; Kim, S.F.; Ahima, R.S. Lipid accumulation and oxidation in glioblastoma multiforme. Sci. Rep. 2019, 9, 19593. [Google Scholar] [CrossRef]
- Shakya, S.; Gromovsky, A.D.; Hale, J.S.; Knudsen, A.M.; Prager, B.; Wallace, L.C.; Penalva, L.O.F.; Brown, H.A.; Kristensen, B.W.; Rich, J.N.; et al. Altered lipid metabolism marks glioblastoma stem and non-stem cells in separate tumor niches. Acta Neuropathol. Commun. 2021, 9, 101. [Google Scholar] [CrossRef]
- Phillips, R.E.; Yang, Y.; Smith, R.C.; Thompson, B.M.; Yamasaki, T.; Soto-Feliciano, Y.M.; Funato, K.; Liang, Y.; Garcia-Bermudez, J.; Wang, X.; et al. Target identification reveals lanosterol synthase as a vulnerability in glioma. Proc. Natl. Acad. Sci. USA 2019, 116, 7957–7962. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, N.; El-Baba, C.; Araji, K.; El-Khoury, R.; Usta, J.; Darwiche, N. The Pentose Phosphate Pathway in Cancer: Regulation and Therapeutic Opportunities. Chemotherapy 2021, 66, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, L.; Zang, Q.; Li, S.; Li, L.; Wang, Z.; He, J.; Qiang, B.; Han, W.; Zhang, R.; et al. Rewiring of purine metabolism in response to acidosis stress in glioma stem cells. Cell Death Dis. 2021, 12, 277. [Google Scholar] [CrossRef] [PubMed]
- Kathagen-Buhmann, A.; Schulte, A.; Weller, J.; Holz, M.; Herold-Mende, C.; Glass, R.; Lamszus, K. Glycolysis and the pentose phosphate pathway are differentially associated with the dichotomous regulation of glioblastoma cell migration versus proliferation. Neuro-Oncology 2016, 18, 1219–1229. [Google Scholar] [CrossRef]
- Chinnaiyan, P.; Kensicki, E.; Bloom, G.; Prabhu, A.; Sarcar, B.; Kahali, S.; Eschrich, S.; Qu, X.; Forsyth, P.; Gillies, R. The Metabolomic Signature of Malignant Glioma Reflects Accelerated Anabolic Metabolism. Cancer Res. 2012, 72, 5878–5888. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, S.; Zadeh, G. Metabolic reprogramming in glioblastoma: The influence of cancer metabolism on epigenetics and unanswered questions. Neuro-Oncology 2016, 18, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Guarnaccia, L.; Navone, S.E.; Trombetta, E.; Cordiglieri, C.; Cherubini, A.; Crisà, F.M.; Rampini, P.; Miozzo, M.; Fontana, L.; Caroli, M.; et al. Angiogenesis in human brain tumors: Screening of drug response through a patient-specific cell platform for personalized therapy. Sci. Rep. 2018, 8, 8748. [Google Scholar] [CrossRef] [PubMed]
- Mangraviti, A.; Raghavan, T.; Volpin, F.; Skuli, N.; Gullotti, D.; Zhou, J.; Asnaghi, L.; Sankey, E.; Liu, A.; Wang, Y.; et al. HIF-1α- Targeting Acriflavine Provides Long Term Survival and Radiological Tumor Response in Brain Cancer Therapy. Sci. Rep. 2017, 7, 14978. [Google Scholar] [CrossRef]
- Jain, R.K. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science 2005, 307, 58–62. [Google Scholar] [CrossRef]
- Le Guelte, A.; Dwyer, J.; Gavard, J. Jumping the barrier: VE-cadherin, VEGF and other angiogenic modifiers in cancer. Biol. Cell 2011, 103, 593–605. [Google Scholar] [CrossRef]
- Ho, V.C.; Duan, L.-J.; Cronin, C.; Liang, B.T.; Fong, G.-H. Elevated Vascular Endothelial Growth Factor Receptor-2 Abundance Contributes to Increased Angiogenesis in Vascular Endothelial Growth Factor Receptor-1–Deficient Mice. Circulation 2012, 126, 741–752. [Google Scholar] [CrossRef]
- Hardee, M.E.; Zagzag, D. Mechanisms of glioma-associated neovascularization. Am. J. Pathol. 2012, 181, 1126–1141. [Google Scholar] [CrossRef]
- Study of the Safety and Efficacy of Dichloroacetate (DCA) in Glioblastoma and Other Recurrent Brain Tumors. Available online: https://www.clinicaltrials.gov/study/NCT01111097 (accessed on 9 August 2025).
- Liu, X.M.; Zhang, Q.P.; Mu, Y.G.; Zhang, X.H.; Sai, K.; Pang, J.C.; Ng, H.K.; Chen, Z.P. Clinical significance of vasculogenic mimicry in human gliomas. J. Neuro-Oncol. 2011, 105, 173–179. [Google Scholar] [CrossRef]
- El Hallani, S.; Boisselier, B.; Peglion, F.; Rousseau, A.; Colin, C.; Idbaih, A.; Marie, Y.; Mokhtari, K.; Thomas, J.L.; Eichmann, A.; et al. A new alternative mechanism in glioblastoma vascularization: Tubular vasculogenic mimicry. Brain 2010, 133, 973–982. [Google Scholar] [CrossRef]
- Shahani, A.; Slika, H.; Elbeltagy, A.; Lee, A.; Peters, C.; Dotson, T.; Raj, D.; Tyler, B. The epigenetic mechanisms involved in the treatment resistance of glioblastoma. Cancer Drug Resist. 2025, 8, 12. [Google Scholar] [CrossRef]
- López, V.; Tejedor, J.R.; Carella, A.; García, M.G.; Santamarina-Ojeda, P.; Pérez, R.F.; Mangas, C.; Urdinguio, R.G.; Aranburu, A.; De La Nava, D.; et al. Epigenetic Deregulation of the Histone Methyltransferase KMT5B Contributes to Malignant Transformation in Glioblastoma. Front. Cell Dev. Biol. 2021, 9, 671838. [Google Scholar] [CrossRef] [PubMed]
- Tong, F.; Zhao, J.X.; Fang, Z.Y.; Cui, X.T.; Su, D.Y.; Liu, X.; Zhou, J.H.; Wang, G.X.; Qiu, Z.J.; Liu, S.Z.; et al. MUC1 promotes glioblastoma progression and TMZ resistance by stabilizing EGFRvIII. Pharmacol. Res. 2023, 187, 106606. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Berglund, A.E.; Macaulay, R.J.; Etame, A.B. Epigenetic Activation of TUSC3 Sensitizes Glioblastoma to Temozolomide Independent of MGMT Promoter Methylation Status. Int. J. Mol. Sci. 2023, 24, 15179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Dong, Z.; Prager, B.C.; Kim, L.J.K.; Wu, Q.; Gimple, R.C.; Wang, X.; Bao, S.; Hamerlik, P.; Rich, J.N. Chromatin remodeler HELLS maintains glioma stem cells through E2F3 and MYC. JCI Insight 2019, 4, e126140. [Google Scholar] [CrossRef]
- Liau, B.B.; Sievers, C.; Donohue, L.K.; Gillespie, S.M.; Flavahan, W.A.; Miller, T.E.; Venteicher, A.S.; Hebert, C.H.; Carey, C.D.; Rodig, S.J.; et al. Adaptive Chromatin Remodeling Drives Glioblastoma Stem Cell Plasticity and Drug Tolerance. Cell Stem Cell 2017, 20, 233–246.e237. [Google Scholar] [CrossRef]
- Tolstorukov, M.Y.; Sansam, C.G.; Lu, P.; Koellhoffer, E.C.; Helming, K.C.; Alver, B.H.; Tillman, E.J.; Evans, J.A.; Wilson, B.G.; Park, P.J.; et al. Swi/Snf chromatin remodeling/tumor suppressor complex establishes nucleosome occupancy at target promoters. Proc. Natl. Acad. Sci. USA 2013, 110, 10165–10170. [Google Scholar] [CrossRef]
- Kitange, G.J.; Mladek, A.C.; Carlson, B.L.; Schroeder, M.A.; Pokorny, J.L.; Cen, L.; Decker, P.A.; Wu, W.; Lomberk, G.A.; Gupta, S.K.; et al. Inhibition of Histone Deacetylation Potentiates the Evolution of Acquired Temozolomide Resistance Linked to MGMT Upregulation in Glioblastoma Xenografts. Clin. Cancer Res. 2012, 18, 4070–4079. [Google Scholar] [CrossRef]
- Rhee, I.; Bachman, K.E.; Park, B.H.; Jair, K.W.; Yen, R.W.; Schuebel, K.E.; Cui, H.; Feinberg, A.P.; Lengauer, C.; Kinzler, K.W.; et al. DNMT1 and DNMT3b cooperate to silence genes in human cancer cells. Nature 2002, 416, 552–556. [Google Scholar] [CrossRef]
- Li, E.; Bestor, T.H.; Jaenisch, R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell 1992, 69, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Thon, N.; Thorsteinsdottir, J.; Eigenbrod, S.; Schüller, U.; Lutz, J.; Kreth, S.; Belka, C.; Tonn, J.-C.; Niyazi, M.; Kreth, F.W. Outcome in unresectable glioblastoma: MGMT promoter methylation makes the difference. J. Neurol. 2017, 264, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, A.; Hachem, L.D.; Mansouri, S.; Nassiri, F.; Laperriere, N.J.; Xia, D.; Lindeman, N.I.; Wen, P.Y.; Chakravarti, A.; Mehta, M.P.; et al. MGMT promoter methylation status testing to guide therapy for glioblastoma: Refining the approach based on emerging evidence and current challenges. Neuro-Oncology 2019, 21, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Spyropoulou, A.; Gargalionis, A.; Dalagiorgou, G.; Adamopoulos, C.; Papavassiliou, K.A.; Lea, R.W.; Piperi, C.; Papavassiliou, A.G. Role of Histone Lysine Methyltransferases SUV39H1 and SETDB1 in Gliomagenesis: Modulation of Cell Proliferation, Migration, and Colony Formation. NeuroMol. Med. 2014, 16, 70–82. [Google Scholar] [CrossRef]
- Shi, Y.; Lan, F.; Matson, C.; Mulligan, P.; Whetstine, J.R.; Cole, P.A.; Casero, R.A.; Shi, Y. Histone Demethylation Mediated by the Nuclear Amine Oxidase Homolog LSD1. Cell 2004, 119, 941–953. [Google Scholar] [CrossRef]
- Malatesta, M.; Steinhauer, C.; Mohammad, F.; Pandey, D.P.; Squatrito, M.; Helin, K. Histone acetyltransferase PCAF is required for Hedgehog-Gli-dependent transcription and cancer cell proliferation. Cancer Res. 2013, 73, 6323–6333. [Google Scholar] [CrossRef]
- Brandsma, D.; Stalpers, L.; Taal, W.; Sminia, P.; van den Bent, M.J. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008, 9, 453–461. [Google Scholar] [CrossRef]
- Cui, M.; Zorrilla-Veloz, R.I.; Hu, J.; Guan, B.; Ma, X. Diagnostic Accuracy of PET for Differentiating True Glioma Progression From Post Treatment-Related Changes: A Systematic Review and Meta-Analysis. Front. Neurol. 2021, 12, 671867. [Google Scholar] [CrossRef]
- Law, I.; Albert, N.L.; Arbizu, J.; Boellaard, R.; Drzezga, A.; Galldiks, N.; la Fougere, C.; Langen, K.J.; Lopci, E.; Lowe, V.; et al. Joint EANM/EANO/RANO practice guidelines/SNMMI procedure standards for imaging of gliomas using PET with radiolabelled amino acids and [18F]FDG: Version 1.0. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 540–557. [Google Scholar] [CrossRef]
- Parent, E.E.; Johnson, D.R.; Gleason, T.; Villanueva-Meyer, J.E. Neuro-Oncology Practice Clinical Debate: FDG PET to differentiate glioblastoma recurrence from treatment-related changes. Neuro-Oncol. Pract. 2021, 8, 518–525. [Google Scholar] [CrossRef]
- Cai, L.; Kirchleitner, S.V.; Zhao, D.; Li, M.; Tonn, J.C.; Glass, R.; Kalin, R.E. Glioblastoma Exhibits Inter-Individual Heterogeneity of TSPO and LAT1 Expression in Neoplastic and Parenchymal Cells. Int. J. Mol. Sci. 2020, 21, 612. [Google Scholar] [CrossRef]
- Werner, J.M.; Weller, J.; Ceccon, G.; Schaub, C.; Tscherpel, C.; Lohmann, P.; Bauer, E.K.; Schafer, N.; Stoffels, G.; Baues, C.; et al. Diagnosis of Pseudoprogression Following Lomustine-Temozolomide Chemoradiation in Newly Diagnosed Glioblastoma Patients Using FET-PET. Clin. Cancer Res. 2021, 27, 3704–3713. [Google Scholar] [CrossRef]
- Ceccon, G.; Lazaridis, L.; Stoffels, G.; Rapp, M.; Weber, M.; Blau, T.; Lohmann, P.; Kebir, S.; Herrmann, K.; Fink, G.R.; et al. Use of FET PET in glioblastoma patients undergoing neurooncological treatment including tumour-treating fields: Initial experience. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1626–1635. [Google Scholar] [CrossRef]
- Pauleit, D.; Floeth, F.; Hamacher, K.; Riemenschneider, M.J.; Reifenberger, G.; Muller, H.W.; Zilles, K.; Coenen, H.H.; Langen, K.J. O-(2-[18F]fluoroethyl)-L-tyrosine PET combined with MRI improves the diagnostic assessment of cerebral gliomas. Brain 2005, 128, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Singnurkar, A.; Poon, R.; Detsky, J. 18F-FET-PET imaging in high-grade gliomas and brain metastases: A systematic review and meta-analysis. J. Neuro-Oncol. 2023, 161, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Holzgreve, A.; Unterrainer, L.M.; Ruf, V.C.; Quach, S.; Bartos, L.M.; Suchorska, B.; Niyazi, M.; Wenter, V.; Herms, J.; et al. Combination of pre-treatment dynamic [18F]FET PET radiomics and conventional clinical parameters for the survival stratification in patients with IDH-wildtype glioblastoma. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Koh, E.S.; Gan, H.K.; Senko, C.; Francis, R.J.; Ebert, M.; Lee, S.T.; Lau, E.; Khasraw, M.; Nowak, A.K.; Bailey, D.L.; et al. [18F]-fluoroethyl-L-tyrosine (FET) in glioblastoma (FIG) TROG 18.06 study: Protocol for a prospective, multicentre PET/CT trial. BMJ Open 2023, 13, e071327. [Google Scholar] [CrossRef]
- Brighi, C.; Puttick, S.; Woods, A.; Keall, P.; Tooney, P.A.; Waddington, D.E.J.; Sproule, V.; Rose, S.; Fay, M. Comparison between [68Ga]Ga-PSMA-617 and [18F]FET PET as Imaging Biomarkers in Adult Recurrent Glioblastoma. Int. J. Mol. Sci. 2023, 24, 16208. [Google Scholar] [CrossRef]
- Verma, P.; Singh, B.K.; Sudhan, M.D.; Singh, R.K.; Bagul, S.D.; Chandak, A.R.; Soni, B.K.; Shelly, D.; Basu, S. 68 Ga-PSMA-11 PET/CT Imaging in Brain Gliomas and Its Correlation with Clinicopathological Prognostic Parameters. Clin. Nucl. Med. 2023, 48, e559–e563. [Google Scholar] [CrossRef]
- Kunikowska, J.; Kulinski, R.; Muylle, K.; Koziara, H.; Krolicki, L. 68Ga-Prostate-Specific Membrane Antigen-11 PET/CT: A New Imaging Option for Recurrent Glioblastoma Multiforme? Clin. Nucl. Med. 2020, 45, 11–18. [Google Scholar] [CrossRef]
- Eigenbrod, S.; Trabold, R.; Brucker, D.; Eros, C.; Egensperger, R.; La Fougere, C.; Gobel, W.; Ruhm, A.; Kretzschmar, H.A.; Tonn, J.C.; et al. Molecular stereotactic biopsy technique improves diagnostic accuracy and enables personalized treatment strategies in glioma patients. Acta Neurochir. 2014, 156, 1427–1440. [Google Scholar] [CrossRef]
- Hamisch, C.A.; Minartz, J.; Blau, T.; Hafkemeyer, V.; Ruess, D.; Hellerbach, A.; Grau, S.J.; Ruge, M.I. Frame-based stereotactic biopsy of deep-seated and midline structures in 511 procedures: Feasibility, risk profile, and diagnostic yield. Acta Neurochir. 2019, 161, 2065–2071. [Google Scholar] [CrossRef]
- Nabors, L.B.; Portnow, J.; Ahluwalia, M.; Baehring, J.; Brem, H.; Brem, S.; Butowski, N.; Campian, J.L.; Clark, S.W.; Fabiano, A.J.; et al. Central Nervous System Cancers, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2020, 18, 1537–1570. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, M.M.; Recinos, P.F.; Nowacki, A.S.; Schroeder, J.L.; Angelov, L.; Barnett, G.H.; Vogelbaum, M.A. Residual tumor volume versus extent of resection: Predictors of survival after surgery for glioblastoma. J. Neurosurg. 2014, 121, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Aiudi, D.; Iacoangeli, A.; Dobran, M.; Polonara, G.; Chiapponi, M.; Mattioli, A.; Gladi, M.; Iacoangeli, M. The Prognostic Role of Volumetric MRI Evaluation in the Surgical Treatment of Glioblastoma. J. Clin. Med. 2024, 13, 849. [Google Scholar] [CrossRef] [PubMed]
- Blomstergren, A.; Rydelius, A.; Abul-Kasim, K.; Latt, J.; Sundgren, P.C.; Bengzon, J. Evaluation of reproducibility in MRI quantitative volumetric assessment and its role in the prediction of overall survival and progression-free survival in glioblastoma. Acta Radiol. 2019, 60, 516–525. [Google Scholar] [CrossRef]
- Weller, M.; van den Bent, M.; Preusser, M.; Le Rhun, E.; Tonn, J.C.; Minniti, G.; Bendszus, M.; Balana, C.; Chinot, O.; Dirven, L.; et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat. Rev. Clin. Oncol. 2021, 18, 170–186. [Google Scholar] [CrossRef]
- Wen, P.Y.; Weller, M.; Lee, E.Q.; Alexander, B.M.; Barnholtz-Sloan, J.S.; Barthel, F.P.; Batchelor, T.T.; Bindra, R.S.; Chang, S.M.; Chiocca, E.A.; et al. Glioblastoma in adults: A Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro-Oncology 2020, 22, 1073–1113. [Google Scholar] [CrossRef]
- Karschnia, P.; Young, J.S.; Youssef, G.C.; Dono, A.; Hani, L.; Sciortino, T.; Bruno, F.; Juenger, S.T.; Teske, N.; Dietrich, J.; et al. Development and validation of a clinical risk model for postoperative outcome in newly diagnosed glioblastoma: A report of the RANO resect group. Neuro-Oncology 2025, 27, 1046–1060. [Google Scholar] [CrossRef]
- Brugada-Bellsola, F.; Rodriguez, P.T.; Gonzalez-Crespo, A.; Menendez-Giron, S.; Panisello, C.H.; Garcia-Armengol, R.; Alonso, C.J.D. Intraoperative ultrasound and magnetic resonance comparative analysis in brain tumor surgery: A valuable tool to flatten ultrasound’s learning curve. Acta Neurochir. 2024, 166, 337. [Google Scholar] [CrossRef]
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.J.; Group, A.L.-G.S. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 2006, 7, 392–401. [Google Scholar] [CrossRef]
- Balana, C.; Vaz, M.A.; Manuel Sepulveda, J.; Mesia, C.; Del Barco, S.; Pineda, E.; Munoz-Langa, J.; Estival, A.; de Las Penas, R.; Fuster, J.; et al. A phase II randomized, multicenter, open-label trial of continuing adjuvant temozolomide beyond 6 cycles in patients with glioblastoma (GEINO 14-01). Neuro-Oncology 2020, 22, 1851–1861. [Google Scholar] [CrossRef]
- Gilbert, M.R.; Wang, M.; Aldape, K.D.; Stupp, R.; Hegi, M.E.; Jaeckle, K.A.; Armstrong, T.S.; Wefel, J.S.; Won, M.; Blumenthal, D.T.; et al. Dose-Dense Temozolomide for Newly Diagnosed Glioblastoma: A Randomized Phase III Clinical Trial. J. Clin. Oncol. 2013, 31, 4085–4091. [Google Scholar] [CrossRef]
- Chowdhary, S.A.; Ryken, T.; Newton, H.B. Survival outcomes and safety of carmustine wafers in the treatment of high-grade gliomas: A meta-analysis. J. Neuro-Oncol. 2015, 122, 367–382. [Google Scholar] [CrossRef]
- Westphal, M.; Hilt, D.C.; Bortey, E.; Delavault, P.; Olivares, R.; Warnke, P.C.; Whittle, I.R.; Jaaskelainen, J.; Ram, Z. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro-Oncology 2003, 5, 79–88. [Google Scholar] [CrossRef]
- Hart, M.G.; Garside, R.; Rogers, G.; Somerville, M.; Stein, K.; Grant, R. Chemotherapy wafers for high grade glioma. Cochrane Database Syst. Rev. 2011, 2018, CD007294. [Google Scholar] [CrossRef]
- Chinot, O.L.; Wick, W.; Mason, W.; Henriksson, R.; Saran, F.; Nishikawa, R.; Carpentier, A.F.; Hoang-Xuan, K.; Kavan, P.; Cernea, D.; et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N. Engl. J. Med. 2014, 370, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.R.; Dignam, J.J.; Armstrong, T.S.; Wefel, J.S.; Blumenthal, D.T.; Vogelbaum, M.A.; Colman, H.; Chakravarti, A.; Pugh, S.; Won, M.; et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N. Engl. J. Med. 2014, 370, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Chauffert, B.; Feuvret, L.; Bonnetain, F.; Taillandier, L.; Frappaz, D.; Taillia, H.; Schott, R.; Honnorat, J.; Fabbro, M.; Tennevet, I.; et al. Randomized phase II trial of irinotecan and bevacizumab as neo-adjuvant and adjuvant to temozolomide-based chemoradiation compared with temozolomide-chemoradiation for unresectable glioblastoma: Final results of the TEMAVIR study from ANOCEF. Ann. Oncol. 2014, 25, 1442–1447. [Google Scholar] [CrossRef] [PubMed]
- Minniti, G.; Niyazi, M.; Alongi, F.; Navarria, P.; Belka, C. Current status and recent advances in reirradiation of glioblastoma. Radiat. Oncol. 2021, 16, 36. [Google Scholar] [CrossRef]
- McNamara, M.G.; Lwin, Z.; Jiang, H.; Templeton, A.J.; Zadeh, G.; Bernstein, M.; Chung, C.; Millar, B.A.; Laperriere, N.; Mason, W.P. Factors impacting survival following second surgery in patients with glioblastoma in the temozolomide treatment era, incorporating neutrophil/lymphocyte ratio and time to first progression. J. Neuro-Oncol. 2014, 117, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Woernle, C.M.; Peus, D.; Hofer, S.; Rushing, E.J.; Held, U.; Bozinov, O.; Krayenbuhl, N.; Weller, M.; Regli, L. Efficacy of Surgery and Further Treatment of Progressive Glioblastoma. World Neurosurg. 2015, 84, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Seystahl, K.; Wick, W.; Weller, M. Therapeutic options in recurrent glioblastoma—An update. Crit. Rev. Oncol. Hematol. 2016, 99, 389–408. [Google Scholar] [CrossRef] [PubMed]
- Dobi, A.; Darazs, B.; Fodor, E.; Cserhati, A.; Egyud, Z.; Maraz, A.; Laszlo, S.; Dodd, L.; Reisz, Z.; Barzo, P.; et al. Low Fraction Size Re-irradiation for Large Volume Recurrence of Glial Tumours. Pathol. Oncol. Res. 2020, 26, 2651–2658. [Google Scholar] [CrossRef]
- Chapman, C.H.; Hara, J.H.; Molinaro, A.M.; Clarke, J.L.; Oberheim Bush, N.A.; Taylor, J.W.; Butowski, N.A.; Chang, S.M.; Fogh, S.E.; Sneed, P.K.; et al. Reirradiation of recurrent high-grade glioma and development of prognostic scores for progression and survival. Neuro-Oncol. Pract. 2019, 6, 364–374. [Google Scholar] [CrossRef]
- Marwah, R.; Xing, D.; Squire, T.; Soon, Y.Y.; Gan, H.K.; Ng, S.P. Reirradiation versus systemic therapy versus combination therapy for recurrent high-grade glioma: A systematic review and meta-analysis of survival and toxicity. J. Neuro-Oncol. 2023, 164, 505–524. [Google Scholar] [CrossRef]
- Shi, W.; Scannell Bryan, M.; Gilbert, M.R.; Mehta, M.P.; Blumenthal, D.T.; Brown, P.D.; Valeinis, E.; Hopkins, K.; Souhami, L.; Andrews, D.W.; et al. Investigating the Effect of Reirradiation or Systemic Therapy in Patients With Glioblastoma After Tumor Progression: A Secondary Analysis of NRG Oncology/Radiation Therapy Oncology Group Trial 0525. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 38–44. [Google Scholar] [CrossRef]
- Scoccianti, S.; Francolini, G.; Carta, G.A.; Greto, D.; Detti, B.; Simontacchi, G.; Visani, L.; Baki, M.; Poggesi, L.; Bonomo, P.; et al. Re-irradiation as salvage treatment in recurrent glioblastoma: A comprehensive literature review to provide practical answers to frequently asked questions. Crit. Rev. Oncol. Hematol. 2018, 126, 80–91. [Google Scholar] [CrossRef]
- Weller, M.; Le Rhun, E. How did lomustine become standard of care in recurrent glioblastoma? Cancer Treat. Rev. 2020, 87, 102029. [Google Scholar] [CrossRef]
- Taal, W.; Oosterkamp, H.M.; Walenkamp, A.M.; Dubbink, H.J.; Beerepoot, L.V.; Hanse, M.C.; Buter, J.; Honkoop, A.H.; Boerman, D.; de Vos, F.Y.; et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): A randomised controlled phase 2 trial. Lancet Oncol. 2014, 15, 943–953. [Google Scholar] [CrossRef]
- Wick, W.; Gorlia, T.; Bendszus, M.; Taphoorn, M.; Sahm, F.; Harting, I.; Brandes, A.A.; Taal, W.; Domont, J.; Idbaih, A.; et al. Lomustine and Bevacizumab in Progressive Glioblastoma. N. Engl. J. Med. 2017, 377, 1954–1963. [Google Scholar] [CrossRef]
- Pope, W.B.; Xia, Q.; Paton, V.E.; Das, A.; Hambleton, J.; Kim, H.J.; Huo, J.; Brown, M.S.; Goldin, J.; Cloughesy, T. Patterns of progression in patients with recurrent glioblastoma treated with bevacizumab. Neurology 2011, 76, 432–437. [Google Scholar] [CrossRef]
- Stupp, R.; Wong, E.T.; Kanner, A.A.; Steinberg, D.; Engelhard, H.; Heidecke, V.; Kirson, E.D.; Taillibert, S.; Liebermann, F.; Dbaly, V.; et al. NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: A randomised phase III trial of a novel treatment modality. Eur. J. Cancer 2012, 48, 2192–2202. [Google Scholar] [CrossRef]
- Batchelor, T.T.; Mulholland, P.; Neyns, B.; Nabors, L.B.; Campone, M.; Wick, A.; Mason, W.; Mikkelsen, T.; Phuphanich, S.; Ashby, L.S.; et al. Phase III Randomized Trial Comparing the Efficacy of Cediranib As Monotherapy, and in Combination With Lomustine, Versus Lomustine Alone in Patients With Recurrent Glioblastoma. J. Clin. Oncol. 2013, 31, 3212–3218. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, G.; De Salvo, G.L.; Brandes, A.A.; Eoli, M.; Ruda, R.; Faedi, M.; Lolli, I.; Pace, A.; Daniele, B.; Pasqualetti, F.; et al. Regorafenib compared with lomustine in patients with relapsed glioblastoma (REGOMA): A multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Oncol. 2019, 20, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Caccese, M.; Desideri, I.; Villani, V.; Simonelli, M.; Buglione, M.; Chiesa, S.; Franceschi, E.; Gaviani, P.; Stasi, I.; Caserta, C.; et al. REGOMA-OSS: A large, Italian, multicenter, prospective, observational study evaluating the efficacy and safety of regorafenib in patients with recurrent glioblastoma. ESMO Open 2024, 9, 102943. [Google Scholar] [CrossRef]
- Herrlinger, U.; Tzaridis, T.; Mack, F.; Steinbach, J.P.; Schlegel, U.; Sabel, M.; Hau, P.; Kortmann, R.D.; Krex, D.; Grauer, O.; et al. Lomustine-temozolomide combination therapy versus standard temozolomide therapy in patients with newly diagnosed glioblastoma with methylated MGMT promoter (CeTeG/NOA-09): A randomised, open-label, phase 3 trial. Lancet 2019, 393, 678–688. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Chandla, A.; Mekonnen, M.; Hovis, G.E.A.; Teton, Z.E.; Patel, K.S.; Everson, R.G.; Wadehra, M.; Yang, I. Safety and Efficacy of Laser Interstitial Thermal Therapy as Upfront Therapy in Primary Glioblastoma and IDH-Mutant Astrocytoma: A Meta-Analysis. Cancers 2024, 16, 2131. [Google Scholar] [CrossRef]
- de Groot, J.F.; Kim, A.H.; Prabhu, S.; Rao, G.; Laxton, A.W.; Fecci, P.E.; O’Brien, B.J.; Sloan, A.; Chiang, V.; Tatter, S.B.; et al. Efficacy of laser interstitial thermal therapy (LITT) for newly diagnosed and recurrent IDH wild-type glioblastoma. Neuro-Oncol. Adv. 2022, 4, vdac040. [Google Scholar] [CrossRef]
- Yu, J.S.; Meade, S.M.; Zhao, R.; Wei, W.; Dashora, H.; Prayson, R.; Grabowski, M.M.; Stevens, G.; Lobbous, M.; Murphy, E.S.; et al. Expedited chemoradiation after laser interstitial thermal therapy (LITT) is feasible and safe in patients with newly diagnosed glioblastoma. Neuro-Oncol Adv. 2025, 7, vdaf038. [Google Scholar] [CrossRef]
- Salehi, A.; Paturu, M.R.; Patel, B.; Cain, M.D.; Mahlokozera, T.; Yang, A.B.; Lin, T.H.; Leuthardt, E.C.; Yano, H.; Song, S.K.; et al. Therapeutic enhancement of blood-brain and blood-tumor barriers permeability by laser interstitial thermal therapy. Neuro-Oncol Adv. 2020, 2, vdaa071. [Google Scholar] [CrossRef]
- Morello, A.; Bianconi, A.; Rizzo, F.; Bellomo, J.; Meyer, A.C.; Garbossa, D.; Regli, L.; Cofano, F. Laser Interstitial Thermotherapy (LITT) in Recurrent Glioblastoma: What Window of Opportunity for This Treatment? Technol. Cancer Res. Treat. 2024, 23, 15330338241249026. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.H.; Tatter, S.; Rao, G.; Prabhu, S.; Chen, C.; Fecci, P.; Chiang, V.; Smith, K.; Williams, B.J.; Mohammadi, A.M.; et al. Laser Ablation of Abnormal Neurological Tissue Using Robotic NeuroBlate System (LAANTERN): 12-Month Outcomes and Quality of Life After Brain Tumor Ablation. Neurosurgery 2020, 87, E338–E346. [Google Scholar] [CrossRef] [PubMed]
- Scharfen, C.O.; Sneed, P.K.; Wara, W.M.; Larson, D.A.; Phillips, T.L.; Prados, M.D.; Weaver, K.A.; Malec, M.; Acord, P.; Lamborn, K.R.; et al. High activity iodine-125 interstitial implant for gliomas. Int. J. Radiat. Oncol. Biol. Phys. 1992, 24, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.M.; Cornu, P.; Boisserie, G.; Hasboun, D.; Tep, B.; Hardiman, C.; Valery, C.A.; Delattre, J.Y.; Dormont, D.; Baillet, F.; et al. Brachytherapy of glioblastoma recurring in previously irradiated territory: Predictive value of tumor volume. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 67–74. [Google Scholar] [CrossRef]
- Chamberlain, M.C.; Barba, D.; Kormanik, P.; Berson, A.M.; Saunders, W.M.; Shea, M.C. Concurrent cisplatin therapy and iodine 125 brachytherapy for recurrent malignant brain tumors. Arch. Neurol. 1995, 52, 162–167. [Google Scholar] [CrossRef]
- Gessler, D.J.; Ferreira, C.; Dusenbery, K.; Chen, C.C. GammaTile®: Surgically targeted radiation therapy for glioblastomas. Future Oncol. 2020, 16, 2445–2455. [Google Scholar] [CrossRef]
- Gessler, D.J.; Neil, E.C.; Shah, R.; Levine, J.; Shanks, J.; Wilke, C.; Reynolds, M.; Zhang, S.; Ozutemiz, C.; Gencturk, M.; et al. GammaTile® brachytherapy in the treatment of recurrent glioblastomas. Neuro-Oncol Adv. 2022, 4, vdab185. [Google Scholar] [CrossRef]
- Garcia, M.A.; Turner, A.; Brachman, D.G. The role of GammaTile in the treatment of brain tumors: A technical and clinical overview. J. Neuro-Oncol. 2024, 166, 203–212. [Google Scholar] [CrossRef]
- Smith, K.; Nakaji, P.; Thomas, T.; Pinnaduwage, D.; Wallstrom, G.; Choi, M.; Zabramski, J.; Chen, C.; Brachman, D. Safety and patterns of survivorship in recurrent GBM following resection and surgically targeted radiation therapy: Results from a prospective trial. Neuro-Oncology 2022, 24, S4–S15. [Google Scholar] [CrossRef]
- Ishida, Y.; Agata, Y.; Shibahara, K.; Honjo, T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992, 11, 3887–3895. [Google Scholar] [CrossRef]
- Xue, S.; Song, G.; Yu, J. The prognostic significance of PD-L1 expression in patients with glioma: A meta-analysis. Sci. Rep. 2017, 7, 4231. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Forsyth, P.A.; Algazi, A.; Hamid, O.; Hodi, F.S.; Moschos, S.J.; Khushalani, N.I.; Lewis, K.; Lao, C.D.; Postow, M.A.; et al. Combined Nivolumab and Ipilimumab in Melanoma Metastatic to the Brain. N. Engl. J. Med. 2018, 379, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Gadgeel, S.; Rodriguez-Abreu, D.; Speranza, G.; Esteban, E.; Felip, E.; Domine, M.; Hui, R.; Hochmair, M.J.; Clingan, P.; Powell, S.F.; et al. Updated Analysis From KEYNOTE-189: Pembrolizumab or Placebo Plus Pemetrexed and Platinum for Previously Untreated Metastatic Nonsquamous Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2020, 38, 1505–1517. [Google Scholar] [CrossRef] [PubMed]
- Reardon, D.A.; Brandes, A.A.; Omuro, A.; Mulholland, P.; Lim, M.; Wick, A.; Baehring, J.; Ahluwalia, M.S.; Roth, P.; Bahr, O.; et al. Effect of Nivolumab vs Bevacizumab in Patients With Recurrent Glioblastoma: The CheckMate 143 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 1003–1010. [Google Scholar] [CrossRef]
- Omuro, A.; Brandes, A.A.; Carpentier, A.F.; Idbaih, A.; Reardon, D.A.; Cloughesy, T.; Sumrall, A.; Baehring, J.; van den Bent, M.; Bahr, O.; et al. Radiotherapy combined with nivolumab or temozolomide for newly diagnosed glioblastoma with unmethylated MGMT promoter: An international randomized phase III trial. Neuro-Oncology 2023, 25, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.; Weller, M.; Idbaih, A.; Steinbach, J.; Finocchiaro, G.; Raval, R.R.; Ansstas, G.; Baehring, J.; Taylor, J.W.; Honnorat, J.; et al. Phase III trial of chemoradiotherapy with temozolomide plus nivolumab or placebo for newly diagnosed glioblastoma with methylated MGMT promoter. Neuro-Oncology 2022, 24, 1935–1949. [Google Scholar] [CrossRef]
- Lim, M.; Xia, Y.; Bettegowda, C.; Weller, M. Current state of immunotherapy for glioblastoma. Nat. Rev. Clin. Oncol. 2018, 15, 422–442. [Google Scholar] [CrossRef]
- Yuen, C.; Reid, P.; Zhang, Z.; Soliven, B.; Luke, J.J.; Rezania, K. Facial Palsy Induced by Checkpoint Blockade: A Single Center Retrospective Study. J. Immunother. 2019, 42, 94–96. [Google Scholar] [CrossRef]
- Thompson, J.A.; Schneider, B.J.; Brahmer, J.; Andrews, S.; Armand, P.; Bhatia, S.; Budde, L.E.; Costa, L.; Davies, M.; Dunnington, D.; et al. NCCN Guidelines Insights: Management of Immunotherapy-Related Toxicities, Version 1.2020. J. Natl. Compr. Cancer Netw. 2020, 18, 230–241. [Google Scholar] [CrossRef]
- Habermeier, A.; Graf, J.; Sandhofer, B.F.; Boissel, J.P.; Roesch, F.; Closs, E.I. System L amino acid transporter LAT1 accumulates O-(2-fluoroethyl)-L-tyrosine (FET). Amino Acids 2015, 47, 335–344. [Google Scholar] [CrossRef]
- Pichler, J.; Traub-Weidinger, T.; Spiegl, K.; Imamovic, L.; Braat, A.; Snijders, T.J.; Verhoeff, J.J.C.; Flamen, P.; Tauchmanova, L.; Hayward, C.; et al. Results from a phase I study of 4-l-[131I]iodo-phenylalanine ([131I]IPA) with external radiation therapy in patients with recurrent glioblastoma (IPAX-1). Neuro-Oncol Adv. 2024, 6, vdae130. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.R.; Caplin, M.E.; Kunz, P.L.; Ruszniewski, P.B.; Bodei, L.; Hendifar, A.; Mittra, E.; Wolin, E.M.; Yao, J.C.; Pavel, M.E.; et al. (177)Lu-Dotatate plus long-acting octreotide versus high-dose long-acting octreotide in patients with midgut neuroendocrine tumours (NETTER-1): Final overall survival and long-term safety results from an open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021, 22, 1752–1763. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Halperin, D.; Myrehaug, S.; Herrmann, K.; Pavel, M.; Kunz, P.L.; Chasen, B.; Tafuto, S.; Lastoria, S.; Capdevila, J.; et al. [(177)Lu]Lu-DOTA-TATE plus long-acting octreotide versus high-dose long-acting octreotide for the treatment of newly diagnosed, advanced grade 2-3, well-differentiated, gastroenteropancreatic neuroendocrine tumours (NETTER-2): An open-label, randomised, phase 3 study. Lancet 2024, 403, 2807–2817. [Google Scholar] [CrossRef]
- ME, G.G.; De la Rosa-Herencia, A.S.; Flores-Martinez, A.; Ortega-Bellido, M.; Sanchez-Sanchez, R.; Blanco-Acevedo, C.; Gahete, M.D.; Solivera, J.; Luque, R.M.; Fuentes-Fayos, A.C. Assessing the diagnostic, prognostic, and therapeutic potential of the somatostatin/cortistatin system in glioblastoma. Cell. Mol. Life Sci. 2025, 82, 173. [Google Scholar] [CrossRef]
- Kiviniemi, A.; Gardberg, M.; Frantzen, J.; Pesola, M.; Vuorinen, V.; Parkkola, R.; Tolvanen, T.; Suilamo, S.; Johansson, J.; Luoto, P.; et al. Somatostatin receptor subtype 2 in high-grade gliomas: PET/CT with 68Ga-DOTA-peptides, correlation to prognostic markers, and implications for targeted radiotherapy. EJNMMI Res. 2015, 5, 25. [Google Scholar] [CrossRef]
- Lai, G.; Wu, H.; Yang, K.; Hu, K.; Zhou, Y.; Chen, X.; Fu, F.; Li, J.; Xie, G.; Wang, H.-F.; et al. Progress of nanoparticle drug delivery system for the treatment of glioma. Front. Bioeng. Biotechnol. 2024, 12, 1403511. [Google Scholar] [CrossRef]
- Wei, D.; Zhang, N.; Qu, S.; Wang, H.; Li, J. Advances in nanotechnology for the treatment of GBM. Front. Neurosci. 2023, 17, 1180943. [Google Scholar] [CrossRef]
- Alphandery, E. Nano-Therapies for Glioblastoma Treatment. Cancers 2020, 12, 242. [Google Scholar] [CrossRef]
- Gal, O.; Betzer, O.; Rousso-Noori, L.; Sadan, T.; Motiei, M.; Nikitin, M.; Friedmann-Morvinski, D.; Popovtzer, R.; Popovtzer, A. Antibody Delivery into the Brain by Radiosensitizer Nanoparticles for Targeted Glioblastoma Therapy. J. Nanotheranostics 2022, 3, 177–188. [Google Scholar] [CrossRef]
- Vaz-Salgado, M.A.; Villamayor, M.; Albarrán, V.; Alía, V.; Sotoca, P.; Chamorro, J.; Rosero, D.; Barrill, A.M.; Martín, M.; Fernandez, E.; et al. Recurrent Glioblastoma: A Review of the Treatment Options. Cancers 2023, 15, 4279. [Google Scholar] [CrossRef]
- Sampson, J.H.; Gunn, M.D.; Fecci, P.E.; Ashley, D.M. Brain immunology and immunotherapy in brain tumours. Nat. Rev. Cancer 2020, 20, 12–25. [Google Scholar] [CrossRef]
- Latzer, P.; Zelba, H.; Battke, F.; Reinhardt, A.; Shao, B.; Bartsch, O.; Rabsteyn, A.; Harter, J.; Schulze, M.; Okech, T.; et al. A real-world observation of patients with glioblastoma treated with a personalized peptide vaccine. Nat. Commun. 2024, 15, 6870. [Google Scholar] [CrossRef]
- Liau, L.M.; Ashkan, K.; Brem, S.; Campian, J.L.; Trusheim, J.E.; Iwamoto, F.M.; Tran, D.D.; Ansstas, G.; Cobbs, C.S.; Heth, J.A.; et al. Association of Autologous Tumor Lysate-Loaded Dendritic Cell Vaccination with Extension of Survival Among Patients With Newly Diagnosed and Recurrent Glioblastoma: A Phase 3 Prospective Externally Controlled Cohort Trial. JAMA Oncol. 2023, 9, 112–121. [Google Scholar] [CrossRef]
- Ng, A.T.; Steve, T.; Jamouss, K.T.; Arham, A.; Kawtharani, S.; Assi, H.I. The challenges and clinical landscape of glioblastoma immunotherapy. CNS Oncol. 2024, 13, 2415878. [Google Scholar] [CrossRef]
- Wen, P.Y.; Reardon, D.A.; Armstrong, T.S.; Phuphanich, S.; Aiken, R.D.; Landolfi, J.C.; Curry, W.T.; Zhu, J.J.; Glantz, M.; Peereboom, D.M.; et al. A Randomized Double-Blind Placebo-Controlled Phase II Trial of Dendritic Cell Vaccine ICT-107 in Newly Diagnosed Patients with Glioblastoma. Clin. Cancer Res. 2019, 25, 5799–5807. [Google Scholar] [CrossRef]
- Li, L.; Zhou, J.; Dong, X.; Liao, Q.; Zhou, D.; Zhou, Y. Dendritic cell vaccines for glioblastoma fail to complete clinical translation: Bottlenecks and potential countermeasures. Int. Immunopharmacol. 2022, 109, 108929. [Google Scholar] [CrossRef]
- Datsi, A.; Sorg, R.V. Dendritic Cell Vaccination of Glioblastoma: Road to Success or Dead End. Front. Immunol. 2021, 12, 770390. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, Y.; Lindstrom, A.R.; Lin, T.Y.; Lam, K.S.; Li, Y. Peptide-based materials for cancer immunotherapy. Theranostics 2019, 9, 7807–7825. [Google Scholar] [CrossRef]
- Ahluwalia, M.S.; Reardon, D.A.; Abad, A.P.; Curry, W.T.; Wong, E.T.; Figel, S.A.; Mechtler, L.L.; Peereboom, D.M.; Hutson, A.D.; Withers, H.G.; et al. Phase IIa Study of SurVaxM Plus Adjuvant Temozolomide for Newly Diagnosed Glioblastoma. J. Clin. Oncol. 2023, 41, 1453–1465. [Google Scholar] [CrossRef]
- An, Z.; Aksoy, O.; Zheng, T.; Fan, Q.-W.; Weiss, W.A. Epidermal growth factor receptor and EGFRvIII in glioblastoma: Signaling pathways and targeted therapies. Oncogene 2018, 37, 1561–1575. [Google Scholar] [CrossRef]
- Park, S.; Maus, M.V.; Choi, B.D. CAR-T cell therapy for the treatment of adult high-grade gliomas. npj Precis. Oncol. 2024, 8, 279. [Google Scholar] [CrossRef]
- Reardon, D.A.; Desjardins, A.; Vredenburgh, J.J.; O’Rourke, D.M.; Tran, D.D.; Fink, K.L.; Nabors, L.B.; Li, G.; Bota, D.A.; Lukas, R.V.; et al. Rindopepimut with Bevacizumab for Patients with Relapsed EGFRvIII-Expressing Glioblastoma (ReACT): Results of a Double-Blind Randomized Phase II Trial. Clin. Cancer Res. 2020, 26, 1586–1594. [Google Scholar] [CrossRef]
- Weller, M.; Butowski, N.; Tran, D.D.; Recht, L.D.; Lim, M.; Hirte, H.; Ashby, L.; Mechtler, L.; Goldlust, S.A.; Iwamoto, F.; et al. Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): A randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017, 18, 1373–1385. [Google Scholar] [CrossRef]
- June, C.H.; Sadelain, M. Chimeric Antigen Receptor Therapy. N. Engl. J. Med. 2018, 379, 64–73. [Google Scholar] [CrossRef]
- Schuster, S.J.; Bishop, M.R.; Tam, C.S.; Waller, E.K.; Borchmann, P.; McGuirk, J.P.; Jager, U.; Jaglowski, S.; Andreadis, C.; Westin, J.R.; et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2019, 380, 45–56. [Google Scholar] [CrossRef]
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.L.; Lekakis, L.J.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017, 377, 2531–2544. [Google Scholar] [CrossRef]
- Brown, C.E.; Alizadeh, D.; Starr, R.; Weng, L.; Wagner, J.R.; Naranjo, A.; Ostberg, J.R.; Blanchard, M.S.; Kilpatrick, J.; Simpson, J.; et al. Regression of Glioblastoma after Chimeric Antigen Receptor T-Cell Therapy. N. Engl. J. Med. 2016, 375, 2561–2569. [Google Scholar] [CrossRef]
- Montoya, M.; Gallus, M.; Phyu, S.; Haegelin, J.; de Groot, J.; Okada, H. A Roadmap of CAR-T-Cell Therapy in Glioblastoma: Challenges and Future Perspectives. Cells 2024, 13, 726. [Google Scholar] [CrossRef]
- Bagley, S.J.; Logun, M.; Fraietta, J.A.; Wang, X.; Desai, A.S.; Bagley, L.J.; Nabavizadeh, A.; Jarocha, D.; Martins, R.; Maloney, E.; et al. Intrathecal bivalent CAR T cells targeting EGFR and IL13Rα2 in recurrent glioblastoma: Phase 1 trial interim results. Nat. Med. 2024, 30, 1320–1329. [Google Scholar] [CrossRef]
- Schmidts, A.; Srivastava, A.A.; Ramapriyan, R.; Bailey, S.R.; Bouffard, A.A.; Cahill, D.P.; Carter, B.S.; Curry, W.T.; Dunn, G.P.; Frigault, M.J.; et al. Tandem chimeric antigen receptor (CAR) T cells targeting EGFRvIII and IL-13Rα2 are effective against heterogeneous glioblastoma. Neuro-Oncol Adv. 2023, 5, vdac185. [Google Scholar] [CrossRef]
- Bagley, S.J.; Binder, Z.A.; Lamrani, L.; Marinari, E.; Desai, A.S.; Nasrallah, M.P.; Maloney, E.; Brem, S.; Lustig, R.A.; Kurtz, G.; et al. Repeated peripheral infusions of anti-EGFRvIII CAR T cells in combination with pembrolizumab show no efficacy in glioblastoma: A phase 1 trial. Nat. Cancer 2024, 5, 517–531. [Google Scholar] [CrossRef]
- Strassheimer, F.; Elleringmann, P.; Ludmirski, G.; Roller, B.; Macas, J.; Alekseeva, T.; Cakmak, P.; Aliraj, B.; Krenzlin, H.; Demes, M.C.; et al. CAR-NK cell therapy combined with checkpoint inhibition induces an NKT cell response in glioblastoma. Br. J. Cancer 2025, 132, 849–860. [Google Scholar] [CrossRef]
- Kiefer, A.; Prufer, M.; Roder, J.; Pfeifer Serrahima, J.; Bodden, M.; Kuhnel, I.; Oberoi, P.; Wels, W.S. Dual Targeting of Glioblastoma Cells with Bispecific Killer Cell Engagers Directed to EGFR and ErbB2 (HER2) Facilitates Effective Elimination by NKG2D-CAR-Engineered NK Cells. Cells 2024, 13, 246. [Google Scholar] [CrossRef]
- Martins, T.A.; Kaymak, D.; Tatari, N.; Gerster, F.; Hogan, S.; Ritz, M.F.; Sabatino, V.; Wieboldt, R.; Bartoszek, E.M.; McDaid, M.; et al. Enhancing anti-EGFRvIII CAR T cell therapy against glioblastoma with a paracrine SIRPγ-derived CD47 blocker. Nat. Commun. 2024, 15, 9718. [Google Scholar] [CrossRef]
- Brown, C.E.; Hibbard, J.C.; Alizadeh, D.; Blanchard, M.S.; Natri, H.M.; Wang, D.; Ostberg, J.R.; Aguilar, B.; Wagner, J.R.; Paul, J.A.; et al. Locoregional delivery of IL-13Rα2-targeting CAR-T cells in recurrent high-grade glioma: A phase 1 trial. Nat. Med. 2024, 30, 1001–1012. [Google Scholar] [CrossRef]
- O’Rourke, D.M.; Nasrallah, M.P.; Desai, A.; Melenhorst, J.J.; Mansfield, K.; Morrissette, J.J.D.; Martinez-Lage, M.; Brem, S.; Maloney, E.; Shen, A.; et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci. Transl. Med. 2017, 9, 399. [Google Scholar] [CrossRef]
- Ahmed, N.; Brawley, V.; Hegde, M.; Bielamowicz, K.; Kalra, M.; Landi, D.; Robertson, C.; Gray, T.L.; Diouf, O.; Wakefield, A.; et al. HER2-Specific Chimeric Antigen Receptor-Modified Virus-Specific T Cells for Progressive Glioblastoma: A Phase 1 Dose-Escalation Trial. JAMA Oncol. 2017, 3, 1094–1101. [Google Scholar] [CrossRef]
- Burger, M.C.; Forster, M.T.; Romanski, A.; Strassheimer, F.; Macas, J.; Zeiner, P.S.; Steidl, E.; Herkt, S.; Weber, K.J.; Schupp, J.; et al. Intracranial injection of natural killer cells engineered with a HER2-targeted chimeric antigen receptor in patients with recurrent glioblastoma. Neuro-Oncology 2023, 25, 2058–2071. [Google Scholar] [CrossRef]
- Hegde, M.; Mukherjee, M.; Grada, Z.; Pignata, A.; Landi, D.; Navai, S.A.; Wakefield, A.; Fousek, K.; Bielamowicz, K.; Chow, K.K.; et al. Tandem CAR T cells targeting HER2 and IL13Rα2 mitigate tumor antigen escape. J. Clin. Investig. 2016, 126, 3036–3052. [Google Scholar] [CrossRef]
- Xiong, Q.; Zhu, J.; Zhang, Y.; Deng, H. CAR-NK cell therapy for glioblastoma: What to do next? Front. Oncol. 2023, 13, 1192128. [Google Scholar] [CrossRef]
- Sabahi, M.; Fathi Jouzdani, A.; Sadeghian, Z.; Dabbagh Ohadi, M.A.; Sultan, H.; Salehipour, A.; Maniakhina, L.; Rezaei, N.; Adada, B.; Mansouri, A.; et al. CAR-engineered NK cells versus CAR T cells in treatment of glioblastoma; strength and flaws. J. Neuro-Oncol. 2025, 171, 495–530. [Google Scholar] [CrossRef]
- Souza-Fonseca-Guimaraes, F.; Cursons, J.; Huntington, N.D. The Emergence of Natural Killer Cells as a Major Target in Cancer Immunotherapy. Trends Immunol. 2019, 40, 142–158. [Google Scholar] [CrossRef]
- Sheridan, C. Industry appetite for natural killer cells intensifies. Nat. Biotechnol. 2023, 41, 159–161. [Google Scholar] [CrossRef]
- Ma, R.; Lu, T.; Li, Z.; Teng, K.Y.; Mansour, A.G.; Yu, M.; Tian, L.; Xu, B.; Ma, S.; Zhang, J.; et al. An Oncolytic Virus Expressing IL15/IL15Rα Combined with Off-the-Shelf EGFR-CAR NK Cells Targets Glioblastoma. Cancer Res. 2021, 81, 3635–3648. [Google Scholar] [CrossRef]
- Kickingereder, P.; Bonekamp, D.; Nowosielski, M.; Kratz, A.; Sill, M.; Burth, S.; Wick, A.; Eidel, O.; Schlemmer, H.P.; Radbruch, A.; et al. Radiogenomics of Glioblastoma: Machine Learning-based Classification of Molecular Characteristics by Using Multiparametric and Multiregional MR Imaging Features. Radiology 2016, 281, 907–918. [Google Scholar] [CrossRef]
- Lee, M.H.; Kim, J.; Kim, S.T.; Shin, H.M.; You, H.J.; Choi, J.W.; Seol, H.J.; Nam, D.H.; Lee, J.I.; Kong, D.S. Prediction of IDH1 Mutation Status in Glioblastoma Using Machine Learning Technique Based on Quantitative Radiomic Data. World Neurosurg. 2019, 125, e688–e696. [Google Scholar] [CrossRef]
- Mair, R.; Mouliere, F. Cell-free DNA technologies for the analysis of brain cancer. Br. J. Cancer 2022, 126, 371–378. [Google Scholar] [CrossRef]
- Miller, A.M.; Shah, R.H.; Pentsova, E.I.; Pourmaleki, M.; Briggs, S.; Distefano, N.; Zheng, Y.; Skakodub, A.; Mehta, S.A.; Campos, C.; et al. Tracking tumour evolution in glioma through liquid biopsies of cerebrospinal fluid. Nature 2019, 565, 654–658. [Google Scholar] [CrossRef]
- Li, J.; Wang, W.; Wang, J.; Cao, Y.; Wang, S.; Zhao, J. Viral Gene Therapy for Glioblastoma Multiforme: A Promising Hope for the Current Dilemma. Front. Oncol. 2021, 11, 678226. [Google Scholar] [CrossRef]
- Rainov, N.G. A phase III clinical evaluation of herpes simplex virus type 1 thymidine kinase and ganciclovir gene therapy as an adjuvant to surgical resection and radiation in adults with previously untreated glioblastoma multiforme. Hum. Gene Ther. 2000, 11, 2389–2401. [Google Scholar] [CrossRef]
- Cloughesy, T.F.; Landolfi, J.; Vogelbaum, M.A.; Ostertag, D.; Elder, J.B.; Bloomfield, S.; Carter, B.; Chen, C.C.; Kalkanis, S.N.; Kesari, S.; et al. Durable complete responses in some recurrent high-grade glioma patients treated with Toca 511 + Toca FC. Neuro-Oncology 2018, 20, 1383–1392. [Google Scholar] [CrossRef]
- Schuelke, M.R.; Gundelach, J.H.; Coffey, M.; West, E.; Scott, K.; Johnson, D.R.; Samson, A.; Melcher, A.; Vile, R.G.; Bram, R.J. Phase I trial of sargramostim/pelareorep therapy in pediatric patients with recurrent or refractory high-grade brain tumors. Neuro-Oncol Adv. 2022, 4, vdac085. [Google Scholar] [CrossRef]
- Foloppe, J.; Kempf, J.; Futin, N.; Kintz, J.; Cordier, P.; Pichon, C.; Findeli, A.; Vorburger, F.; Quemeneur, E.; Erbs, P. The Enhanced Tumor Specificity of TG6002, an Armed Oncolytic Vaccinia Virus Deleted in Two Genes Involved in Nucleotide Metabolism. Mol. Ther. Oncolytics 2019, 14, 1–14. [Google Scholar] [CrossRef]
- Geletneky, K.; Hajda, J.; Angelova, A.L.; Leuchs, B.; Capper, D.; Bartsch, A.J.; Neumann, J.O.; Schoning, T.; Husing, J.; Beelte, B.; et al. Oncolytic H-1 Parvovirus Shows Safety and Signs of Immunogenic Activity in a First Phase I/IIa Glioblastoma Trial. Mol. Ther. 2017, 25, 2620–2634. [Google Scholar] [CrossRef]
- Desjardins, A.; Gromeier, M.; Herndon, J.E., II; Beaubier, N.; Bolognesi, D.P.; Friedman, A.H.; Friedman, H.S.; McSherry, F.; Muscat, A.M.; Nair, S.; et al. Recurrent Glioblastoma Treated with Recombinant Poliovirus. N. Engl. J. Med. 2018, 379, 150–161. [Google Scholar] [CrossRef]
- Behan, F.M.; Iorio, F.; Picco, G.; Gonçalves, E.; Beaver, C.M.; Migliardi, G.; Santos, R.; Rao, Y.; Sassi, F.; Pinnelli, M.; et al. Prioritization of cancer therapeutic targets using CRISPR-Cas9 screens. Nature 2019, 568, 511–516. [Google Scholar] [CrossRef]
- Begagic, E.; Beculic, H.; Duzic, N.; Dzidic-Krivic, A.; Pugonja, R.; Muharemovic, A.; Jaganjac, B.; Salkovic, N.; Sefo, H.; Pojskic, M. CRISPR/Cas9-Mediated Gene Therapy for Glioblastoma: A Scoping Review. Biomedicines 2024, 12, 238. [Google Scholar] [CrossRef]
- Huang, K.; Liu, X.; Li, Y.; Wang, Q.; Zhou, J.; Wang, Y.; Dong, F.; Yang, C.; Sun, Z.; Fang, C.; et al. Genome-Wide CRISPR-Cas9 Screening Identifies NF-κB/E2F6 Responsible for EGFRvIII-Associated Temozolomide Resistance in Glioblastoma. Adv. Sci. 2019, 6, 1900782. [Google Scholar] [CrossRef]
- Han, B.; Cai, J.; Gao, W.; Meng, X.; Gao, F.; Wu, P.; Duan, C.; Wang, R.; Dinislam, M.; Lin, L.; et al. Loss of ATRX suppresses ATM dependent DNA damage repair by modulating H3K9me3 to enhance temozolomide sensitivity in glioma. Cancer Lett. 2018, 419, 280–290. [Google Scholar] [CrossRef]
- Al-Sammarraie, N.; Ray, S.K. Applications of CRISPR-Cas9 Technology to Genome Editing in Glioblastoma Multiforme. Cells 2021, 10, 2342. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Li, J.; Liang, J.; Ren, X.; Yun, D.; Liu, J.; Fan, J.; Zhang, Y.; Zhang, J.; et al. PDPN contributes to constructing immunosuppressive microenvironment in IDH wildtype glioma. Cancer Gene Ther. 2023, 30, 345–357. [Google Scholar] [CrossRef]
- Chen, W.S.; Cao, Z.; Sugaya, S.; Lopez, M.J.; Sendra, V.G.; Laver, N.; Leffler, H.; Nilsson, U.J.; Fu, J.; Song, J.; et al. Pathological lymphangiogenesis is modulated by galectin-8-dependent crosstalk between podoplanin and integrin-associated VEGFR-3. Nat. Commun. 2016, 7, 11302. [Google Scholar] [CrossRef]
- Han, X.; Abdallah, M.O.E.; Breuer, P.; Stahl, F.; Bakhit, Y.; Potthoff, A.L.; Pregler, B.E.F.; Schneider, M.; Waha, A.; Wullner, U.; et al. Downregulation of MGMT expression by targeted editing of DNA methylation enhances temozolomide sensitivity in glioblastoma. Neoplasia 2023, 44, 100929. [Google Scholar] [CrossRef]
- Rosenblum, D.; Gutkin, A.; Dammes, N.; Peer, D. Progress and challenges towards CRISPR/Cas clinical translation. Adv. Drug Deliv. Rev. 2020, 154–155, 176–186. [Google Scholar] [CrossRef]
- Vasilev, A.; Sofi, R.; Rahman, R.; Smith, S.J.; Teschemacher, A.G.; Kasparov, S. Using Light for Therapy of Glioblastoma Multiforme (GBM). Brain Sci. 2020, 10, 75. [Google Scholar] [CrossRef]
- Domka, W.; Bartusik-Aebisher, D.; Rudy, I.; Dynarowicz, K.; Pięta, K.; Aebisher, D. Photodynamic therapy in brain cancer: Mechanisms, clinical and preclinical studies and therapeutic challenges. Front. Chem. 2023, 11, 1250621. [Google Scholar] [CrossRef]
- Aebisher, D.; Przygórzewska, A.; Myśliwiec, A.; Dynarowicz, K.; Krupka-Olek, M.; Bożek, A.; Kawczyk-Krupka, A.; Bartusik-Aebisher, D. Current Photodynamic Therapy for Glioma Treatment: An Update. Biomedicines 2024, 12, 375. [Google Scholar] [CrossRef]
- Lietke, S.; Schmutzer, M.; Schwartz, C.; Weller, J.; Siller, S.; Aumiller, M.; Heckl, C.; Forbrig, R.; Niyazi, M.; Egensperger, R.; et al. Interstitial Photodynamic Therapy Using 5-ALA for Malignant Glioma Recurrences. Cancers 2021, 13, 1767. [Google Scholar] [CrossRef]
- Cesca, B.A.; Pellicer San Martin, K.; Caverzan, M.D.; Oliveda, P.M.; Ibarra, L.E. State-of-the-art photodynamic therapy for malignant gliomas: Innovations in photosensitizers and combined therapeutic approaches. Explor. Target. Antitumor Ther. 2025, 6, 1002303. [Google Scholar] [CrossRef]
- Hutton, D.L.; Burns, T.C.; Hossain-Ibrahim, K. A review of sonodynamic therapy for brain tumors. Neurosurg. Focus 2024, 57, E7. [Google Scholar] [CrossRef] [PubMed]
- Marcus, S.L.; de Souza, M.P. Theranostic Uses of the Heme Pathway in Neuro-Oncology: Protoporphyrin IX (PpIX) and Its Journey from Photodynamic Therapy (PDT) through Photodynamic Diagnosis (PDD) to Sonodynamic Therapy (SDT). Cancers 2024, 16, 740. [Google Scholar] [CrossRef] [PubMed]
- Placantonakis, D.; Grabowski, M.; Burns, T.C.; Butowski, N.A.; Fenn, P.; Clanton, R.; Henry, L.; Potter, W.; Andresen, C.; Marcus, S.; et al. A phase 1/2 dose escalation and expansion study of sonodynamic therapy with SONALA-001 in combination with Exablate 4000 Type 2.0 MR-guided focused ultrasound in patients with progressive or recurrent glioblastoma (rGBM). J. Clin. Oncol. 2024, 42, TPS2101. [Google Scholar] [CrossRef]

| Resistance Mechanism | Description | Therapeutic Strategy |
|---|---|---|
| BBB | Limits penetration of therapeutic agents | Focused ultrasound BBB penetrable drug Nanoparticle-based delivery |
| Glioma stem cells | Subpopulation of self-renewing therapeutic-resistant cells | Notch, Wnt, or Hedgehog pathway inhibitors Anti-CD133 or CD44 therapies |
| The Warburg Effect | Glycolysis for ATP production | Glucose transporter 1 inhibitors Pyruvate kinase inhibitors Pyruvate dehydrogenase kinase inhibitors Hexokinase 2 inhibitors |
| Immune evasion | Immunosuppressive tumor microenvironment | Immune checkpoint inhibitors CAR-T cells Vaccines |
| Angiogenesis | Aberrant blood vessel formation | Anti-VEGF therapy |
| Epigenetics | MGMT promoter methylation | Alkylating therapy |
| Genomic alterations | Alterations that promote resistance, proliferation, and survival | Targeted therapy CRISPR-based gene editing |
| Clinical Trial Identification | Study Title | Diagnostic Test |
|---|---|---|
| NCT06451042 | FET-PET/MRI Based Treatment Planning for Glioblastoma Multiforme in Post-Surgical Patients (FET-TREAT) | FET-PET/MRI |
| NCT02902757 | FDG PET/CT in Monitoring Very Early Therapy Response in Patients with Glioblastoma | fludeoxyglucose F-18 |
| NCT06613841 | Multitracer [18F]Fluciclovine and 18F-FDG PET, and Advanced MRI for Metabolic Profiling of Glioblastoma | fluciclovine F18 |
| NCT07067905 | Clinical Evaluation of [68Ga]Ga-XT771 PET for Diagnosis in Patients with Glioblastoma and Clear Cell Renal Cell Carcinoma | 68Ga-XT771 |
| NCT06645808 | PET-imaging of Two Vartumabs in Patients with Solid Tumors | |
| NCT06797661 | Insights Into the Pathophysiology of Neurovascular Uncoupling in Patients with Brain Lesions. | FDG-PET |
| NCT06113705 | Imaging and Biological Markers for Prediction and Identification of Glioblastoma Pseudoprogression: a Prospective Study. | 18F-GE-180 PET |
| NCT06319027 | Identifying Findings on Brain Scans That Could Help Make Better Predictions About Brain Cancer Progression, The GABLE Trial | fluciclovine F18 |
| NCT06645808 | PET-imaging of Two Vartumabs in Patients with Solid Tumors | 89Zr-DFO-N-Suc-F8scFv 89Zr-DFO-N-Suc-C9scFv |
| NCT05781321 | Short Course Radiotherapy for the Treatment of Patients With Glioblastoma, SAGA Study | fluorodopa F 18 |
| NCT05386043 | Registering Genomics and Imaging of Tumors (ReGIT) | FET F-18 |
| Clinical Trial Identification | Primary Objective | Phase | Main Inclusion Criteria | Newly Diagnosed (N) or Recurrent (R) |
|---|---|---|---|---|
| NCT04699773 | Evaluate the effects of LITT combined with hypo-fractionated radiation therapy on newly diagnosed gliomas | NA | Glioma|Glioblastoma|Brain Tumor | N |
| NCT04181684 | Evaluate the efficacy of LITT combined with hypo-fractionated radiation therapy in treating recurrent gliomas | NA | Glioblastoma|Brain Tumor|Glioma|Neoplasms | R |
| NCT03277638 | Assess the safety and efficacy of combining LITT with pembrolizumab in patients with brain tumors | I/II | Glioblastoma, Adult | R |
| NCT06558214 | Evaluate the safety and feasibility of TTFields, MLA, and pembrolizumab combination therapy in recurrent or progressive WHO Grade IV gliomas | II | Recurrent Glioblastoma | R |
| Clinical Trial Identification | Primary Objective | Phase | Main Inclusion Criterion | Newly Diagnosed (N) or Recurrent (R) |
|---|---|---|---|---|
| NCT05342883 | Evaluate the feasibility and safety of adding GammaTile at resection with standard chemoradiation in newly diagnosed glioblastoma | IV | Glioblastoma | N |
| NCT04427384 | Evaluate real-world clinical and patient-reported outcomes to determine the effectiveness and safety of STaRT therapy | NA | Brain Tumor, Recurrent|Brain Tumor|Brain Tumor, Primary|Brain Tumor—Metastatic|Brain Tumor, Adult: Glioblastoma|Brain Tumor, Adult Meningioma | R |
| Clinical Trial Identification | Primary Objective | Phase | Main Inclusion Criterion | Newly Diagnosed (N) or Recurrent (R) |
|---|---|---|---|---|
| NCT02287428 | Evaluate NeoVax with radiation, pembrolizumab, and temozolomide in newly diagnosed glioblastoma. | I | Glioblastoma | N |
| NCT06805305 | Assess DOC1021 + pIFN with standard care in newly diagnosed glioblastoma. | II | Glioblastoma (glioblastoma) | N |
| NCT05743595 | Test a neoantigen DNA vaccine with PD-1 blockade in MGMT-unmethylated glioblastoma. | I | Unmethylated Glioblastoma | N |
| NCT04573140 | Determine safety and MTD of RNA-LP vaccines in adult glioblastoma and pediatric HGG. | I|II | Adult Glioblastoma|High Grade Glioma|WHO Grade III or IV Malignant Glioma | N |
| NCT06389591 | Evaluate safety and MTD of RNA-LP vaccines in recurrent glioblastoma. | I | Recurrent Glioblastoma | R |
| NCT04201873 | Assess pembrolizumab combined with ATL-DC vaccine in surgically accessible recurrent glioblastoma. | I | Recurrent Glioblastoma | R |
| NCT03382977 | Evaluate safety and tolerability of VBI-1901 in recurrent glioblastoma. | I|II | Glioblastoma | R |
| Clinical Trial Identification | Primary Objective | Phase | Main Inclusion Criterion | Newly Diagnosed (N) or Recurrent (R) |
|---|---|---|---|---|
| NCT06186401 | Evaluate the safety, side effects, and optimal dose of E-SYNC CAR T cells following lymphodepleting chemotherapy in EGFRvIII-positive glioblastoma. | I | EGFR Gene Mutation|Glioblastoma|MGMT-Unmethylated Glioblastoma|Recurrent Glioblastoma | R |
| NCT05660369 | Evaluate the safety and efficacy of IL15-enhanced GPC3-CAR T cells (GO-CART) in patients with GPC3-positive brain tumors. | I | Glioblastoma|Malignant Glioma|Recurrent Glioblastoma|Recurrent Glioma | R |
| NCT04003649 | Assess the safety and effectiveness of IL13Rα2-CAR T cells alone or with nivolumab and ipilimumab in recurrent or refractory glioblastoma. | I | Recurrent Glioblastoma|Refractory Glioblastoma | R |
| NCT06482905 | Evaluate the safety, tolerability, and antitumor activity of anti-B7-H3 CAR-T cells (TX103) in recurrent or progressive grade 4 glioma. | I | High-grade Glioma|WHO Grade IV Glioma | R |
| NCT06186401 | Assess the safety, side effects, and optimal dose of EGFRvIII-targeting CAR T cells (E-SYNC) following lymphodepleting chemotherapy in EGFRvIII+ glioblastoma. | I | EGFR Gene Mutation|Glioblastoma|MGMT-Unmethylated Glioblastoma|Recurrent Glioblastoma | R |
| NCT05835687 | Determine the maximum tolerated dose and safety of locoregionally delivered B7-H3-CAR T cells in children and young adults with primary CNS tumors or diffuse midline gliomas. | I | Central Nervous System Neoplasms|Atypical Teratoid/Rhabdoid Tumor|Diffuse Midline Glioma, H3 K27M-Mutant|Ependymoma|High Grade Glioma|Glioblastoma|Medulloblastoma | R |
| NCT05474378 | Evaluate the manufacturing feasibility and safety of intrathecal B7-H3 CAR T cell delivery in adults with recurrent IDH-wild-type glioblastoma. | I | Brain and Nervous System | R |
| NCT05366179 | Assess the safety of B7-H3 CAR T cells (CAR.B7-H3T) in patients with glioblastoma. | I | Glioblastoma Multiforme | R |
| NCT06815029 | Evaluate the safety, tolerability, and optimal dose of TGFβR2KO/IL13Rα2 CAR T cells delivered intracranially in recurrent or progressive glioblastoma or IDH-mutant astrocytoma. | I | Recurrent Astrocytoma, IDH-Mutant, Grade 3|Recurrent Astrocytoma, IDH-Mutant, Grade 4|Recurrent Glioblastoma | R |
| NCT05660369 | Determine the optimal dose and safety of CARv3-TEAM-E T cells for treating patients with glioblastoma. | I | Glioblastoma|Malignant Glioma|Recurrent Glioblastoma|Recurrent Glioma | R |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, A.; Fote, G.; Himstead, A.; Zheng, M.; Elliott, E.; Smith, S.M.; Lou, J.; Yuen, C.A. Glioblastoma: From Pathophysiology to Novel Therapeutic Approaches. Biomedicines 2025, 13, 1963. https://doi.org/10.3390/biomedicines13081963
Ribeiro A, Fote G, Himstead A, Zheng M, Elliott E, Smith SM, Lou J, Yuen CA. Glioblastoma: From Pathophysiology to Novel Therapeutic Approaches. Biomedicines. 2025; 13(8):1963. https://doi.org/10.3390/biomedicines13081963
Chicago/Turabian StyleRibeiro, Anatevka, Gianna Fote, Alexander Himstead, Michelle Zheng, Emma Elliott, Sara Mae Smith, Jerry Lou, and Carlen A. Yuen. 2025. "Glioblastoma: From Pathophysiology to Novel Therapeutic Approaches" Biomedicines 13, no. 8: 1963. https://doi.org/10.3390/biomedicines13081963
APA StyleRibeiro, A., Fote, G., Himstead, A., Zheng, M., Elliott, E., Smith, S. M., Lou, J., & Yuen, C. A. (2025). Glioblastoma: From Pathophysiology to Novel Therapeutic Approaches. Biomedicines, 13(8), 1963. https://doi.org/10.3390/biomedicines13081963






