Molecular Testing in Thyroid Nodules: How Much Does It Change Clinical Practice?
Abstract
1. Introduction
2. Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.; Wang, X.; Hu, H.; Qu, H.; Xu, Y.; Li, Q. Prevalence and Trends of Thyroid Disease Among Adults, 1999–2018. Endocr. Pract. 2023, 29, 875–880. [Google Scholar] [CrossRef]
- Tessler, F.N.; Middleton, W.D.; Grant, E.G. Thyroid Imaging Reporting and Data System (TI-RADS): A User’s Guide. Radiology 2018, 287, 29–36, Erratum in Radiology 2018, 287, 1082. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.Z.; Baloch, Z.W.; Cochand-Priollet, B.; Schmitt, F.C.; Vielh, P.; VanderLaan, P.A. The 2023 Bethesda System for Reporting Thyroid Cytopathology. Thyroid 2023, 33, 1039–1044. [Google Scholar] [CrossRef]
- Cooper, D.S.; Doherty, G.M.; Haugen, B.R.; Kloos, R.T.; Lee, S.L.; Mandel, S.J.; Mazzaferri, E.L.; McIver, B.; Pacini, F.; Schlumberger, M.; et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009, 19, 1167–1214, Erratum in Thyroid 2010, 20, 942. Hauger, Bryan R [corrected to Haugen, Bryan R]. Erratum in Thyroid 2010, 20, 674–675. [Google Scholar] [CrossRef]
- Schumm, M.A.; Shu, M.L.; Hughes, E.G.; Nikiforov, Y.E.; Nikiforova, M.N.; Wald, A.I.; Lechner, M.G.; Tseng, C.H.; Sajed, D.P.; Wu, J.X.; et al. Prognostic Value of Preoperative Molecular Testing and Implications for Initial Surgical Management in Thyroid Nodules Harboring Suspected (Bethesda V) or Known (Bethesda VI) Papillary Thyroid Cancer. JAMA Otolaryngol. Head Neck Surg. 2023, 149, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.; Yip, L. Decision Making in Indeterminate Thyroid Nodules and the Role of Molecular Testing. Surg. Clin. N. Am. 2019, 99, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Welschmeyer, A.; Kligerman, M.; Noel, J. Management of Indeterminate Thyroid Nodules: A Model Comparing Surgery, Molecular Testing, and Observation. Otolaryngol. Head Neck Surg. 2024, 171, 1349–1354. [Google Scholar] [CrossRef]
- Chowdhury, R.; Hier, J.; Payne, K.E.; Abdulhaleem, M.; Dimitstein, O.; Eisenbach, N.; Forest, V.I.; Payne, R.J. Impact of Molecular Testing on Surgical Decision-Making in Indeterminate Thyroid Nodules: A Systematic Review and Meta-Analysis of Recent Advancements. Cancers 2025, 17, 1156. [Google Scholar] [CrossRef]
- Ramonell, K.M.; Yip, L. The Landmark Series: Testing in Thyroid Nodule Fine Needle Aspiration Cytology. Ann. Surg. Oncol. 2025, 32, 2323–2328. [Google Scholar] [CrossRef]
- Noureldine, S.I.; Olson, M.T.; Agrawal, N.; Prescott, J.D.; Zeiger, M.A.; Tufano, R.P. Effect of Gene Expression Classifier Molecular Testing on the Surgical Decision-Making Process for Patients With Thyroid Nodules. JAMA Otolaryngol. Head Neck Surg. 2015, 141, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Fallahi, M.M.; Koulaian, S.; Mardani, P.; Malekhosseini, S.A.; Shahriarirad, R. The diagnostic role of FNA based on clinicopathological features in thyroid malignancy. BMC Endocr. Disord. 2025, 25, 119. [Google Scholar] [CrossRef]
- Abraham, P.J.; Lindeman, B.M. Management of Incidental Thyroid Nodules. Surg. Clin. N. Am. 2024, 104, 711–723. [Google Scholar] [CrossRef]
- Song, Z.; Wu, C.; Kasmirski, J.; Gillis, A.; Fazendin, J.; Lindeman, B.; Chen, H. Incidental Thyroid Nodules on Computed Tomography: A Systematic Review and Meta-Analysis Examining Prevalence, Follow-Up, and Risk of Malignancy. Thyroid 2024, 34, 1389–1400. [Google Scholar] [CrossRef]
- Patel, K.N.; Yip, L.; Lubitz, C.C.; Grubbs, E.G.; Miller, B.S.; Shen, W.; Angelos, P.; Chen, H.; Doherty, G.M.; Fahey, T.J., 3rd; et al. The American Association of Endocrine Surgeons Guidelines for the Definitive Surgical Management of Thyroid Disease in Adults. Ann. Surg. 2020, 271, e21–e93. [Google Scholar] [CrossRef]
- Hannoush, Z.C.; Ruiz-Cordero, R.; Jara, M.; Kargi, A.Y. Current State of Molecular Cytology in Thyroid Nodules: Platforms and Their Diagnostic and Theranostic Utility. J Clin. Med. 2024, 13, 1759. [Google Scholar] [CrossRef] [PubMed]
- Dawkins, A.; Richards, D.; Sabujan, A.; Nair, R. The Routine Collection of Just-in-Case Thyroid Aspirates for Molecular Testing at the Time of Initial Fine Needle Aspiration. Our Experience. Ultrasound Q. 2025, 41, e00704. [Google Scholar] [CrossRef] [PubMed]
- Noureldine, S.I.; Najafian, A.; Aragon Han, P.; Olson, M.T.; Genther, D.J.; Schneider, E.B.; Prescott, J.D.; Agrawal, N.; Mathur, A.; Zeiger, M.A.; et al. Evaluation of the Effect of Diagnostic Molecular Testing on the Surgical Decision-Making Process for Patients With Thyroid Nodules. JAMA Otolaryngol. Head Neck Surg. 2016, 142, 676–682. [Google Scholar] [CrossRef]
- Nikiforov, Y.E.; Steward, D.L.; Robinson-Smith, T.M.; Haugen, B.R.; Klopper, J.P.; Zhu, Z.; Fagin, J.A.; Falciglia, M.; Weber, K.; Nikiforova, M.N. Molecular testing for mutations in improving the fine-needle aspiration diagnosis of thyroid nodules. J. Clin. Endocrinol. Metab. 2009, 94, 2092–2098. [Google Scholar] [CrossRef]
- Jug, R.; Parajuli, S.; Ahmadi, S.; Jiang, X.S. Negative Results on Thyroid Molecular Testing Decrease Rates of Surgery for Indeterminate Thyroid Nodules. Endocr. Pathol. 2019, 30, 134–137. [Google Scholar] [CrossRef]
- Fung, M.H.M.; Tang, C.; Kwok, G.W.; Chan, T.H.; Luk, Y.; Lui, D.T.W.; Wong, C.K.H.; Lang, B.H.H. High Rates of Unnecessary Surgery for Indeterminate Thyroid Nodules in the Absence of Molecular Test and the Cost-Effectiveness of Utilizing Molecular Test in an Asian Population: A Decision Analysis. Thyroid 2025, 35, 166–176. [Google Scholar] [CrossRef]
- Steward, D.L.; Carty, S.E.; Sippel, R.S.; Yang, S.P.; Sosa, J.A.; Sipos, J.A.; Figge, J.J.; Mandel, S.; Haugen, B.R.; Burman, K.D.; et al. Performance of a Multigene Genomic Classifier in Thyroid Nodules With Indeterminate Cytology: A Prospective Blinded Multicenter Study. JAMA Oncol. 2019, 5, 204–212. [Google Scholar] [CrossRef]
- Zhu, C.Y.; Donangelo, I.; Gupta, D.; Nguyen, D.T.; Ochoa, J.E.; Yeh, M.W.; Livhits, M.J. Outcomes of Indeterminate Thyroid Nodules Managed Nonoperatively after Molecular Testing. J. Clin. Endocr. Metab. 2021, 106, e1240–e1247. [Google Scholar] [CrossRef]
- Barnes, A.B.; Justice-Clark, T.; Li, W.; Randle, R.W. Molecular Testing for Indeterminate Thyroid Nodules: Association of Negative Predictive Value With Nodule Size. Am. Surg. 2022, 88, 2745–2751. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Ijaz, K.; Esebua, M. Molecular Testing Using ThyGeNEXT/ThyraMIR in Thyroid Nodules With Indeterminate Cytology: A Single Medical Institute Experience. Diagn. Cytopathol. 2025, 53, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Nachum, S.; Tondi Resta, I.; Baloch, Z.; Mandel, S.J. Thyroid Nodules with Indeterminate Cytology and Negative Molecular Profile: Prevalence of Malignancy and Practice Paradigms for Surveillance. Thyroid 2025, 35, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, D.; Kim, M.; Choi, J.H.; Yeager, T.; Samuel, K.; Khajoueinejad, N.; Buseck, A.; Imtiaz, S.; Fernandez-Ranvier, G.; Lee, D.; et al. How Effective is the Use of Molecular Testing in Preoperative Decision Making for Management of Indeterminate Thyroid Nodules? World J. Surg. 2022, 46, 3043–3050. [Google Scholar] [CrossRef]
- Scappaticcio, L.; Di Martino, N.; Ferrazzano, P.; Lucà, S.; Clery, E.; Longo, M.; Paglionico, V.A.; Cozzolino, G.; Maiorino, M.I.; Docimo, G.; et al. Patient-specific factors, patient preference, and nodule size as implications in the initial surgery of high risk indeterminate thyroid nodules. Endocrine 2025, 28, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lau, L.W.; Ghaznavi, S.; Frolkis, A.D.; Stephenson, A.; Robertson, H.L.; Rabi, D.M.; Paschke, R. Malignancy risk of hyperfunctioning thyroid nodules compared with non-toxic nodules: Systematic review and a meta-analysis. Thyroid Res. 2021, 14, 3. [Google Scholar] [CrossRef]
- Ross, D.S.; Burch, H.B.; Cooper, D.S.; Greenlee, M.C.; Laurberg, P.; Maia, A.L.; Rivkees, S.A.; Samuels, M.; Sosa, J.A.; Stan, M.N.; et al. 2016 American Thyroid Association Guidelines for Diagnosis and Management of Hyperthyroidism and Other Causes of Thyrotoxicosis. Thyroid 2016, 26, 1343–1421, Erratum in: Thyroid 2017, 27, 1462. [Google Scholar] [CrossRef] [PubMed]
- Endo, M.; Porter, K.; Long, C.; Azaryan, I.; Phay, J.E.; Ringel, M.D.; Sipos, J.A.; Nabhan, F. Features of Cytologically Indeterminate Molecularly Benign Nodules Treated With Surgery. J. Clin. Endocrinol. Metab. 2020, 105, e3971–e3980. [Google Scholar] [CrossRef] [PubMed]
- Al-Qurayshi, Z.; Deniwar, A.; Thethi, T.; Mallik, T.; Srivastav, S.; Murad, F.; Bhatia, P.; Moroz, K.; Sholl, A.B.; Kandil, E. Association of Malignancy Prevalence With Test Properties and Performance of the Gene Expression Classifier in Indeterminate Thyroid Nodules. JAMA Otolaryngol. Head Neck Surg. 2017, 143, 403–408. [Google Scholar] [CrossRef]
- Raghunathan, R.; Longstaff, X.R.; Hughes, E.G.; Li, S.J.; Sant, V.R.; Tseng, C.H.; Rao, J.; Wu, J.X.; Yeh, M.W.; Livhits, M.J. Diagnostic performance of molecular testing in indeterminate (Bethesda III and IV) thyroid nodules with Hürthle cell cytology. Surgery 2024, 175, 221–227. [Google Scholar] [CrossRef]
- Brauner, E.; Holmes, B.J.; Krane, J.F.; Nishino, M.; Zurakowski, D.; Hennessey, J.V.; Faquin, W.C.; Parangi, S. Performance of the Afirma Gene Expression Classifier in Hürthle Cell Thyroid Nodules Differs from Other Indeterminate Thyroid Nodules. Thyroid 2015, 25, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.M.; Zeiger, M.A. Thyroid Nodule Molecular Testing: Is It Ready for Prime Time? Front. Endocrinol. (Lausanne) 2020, 11, 590128. [Google Scholar] [CrossRef] [PubMed]
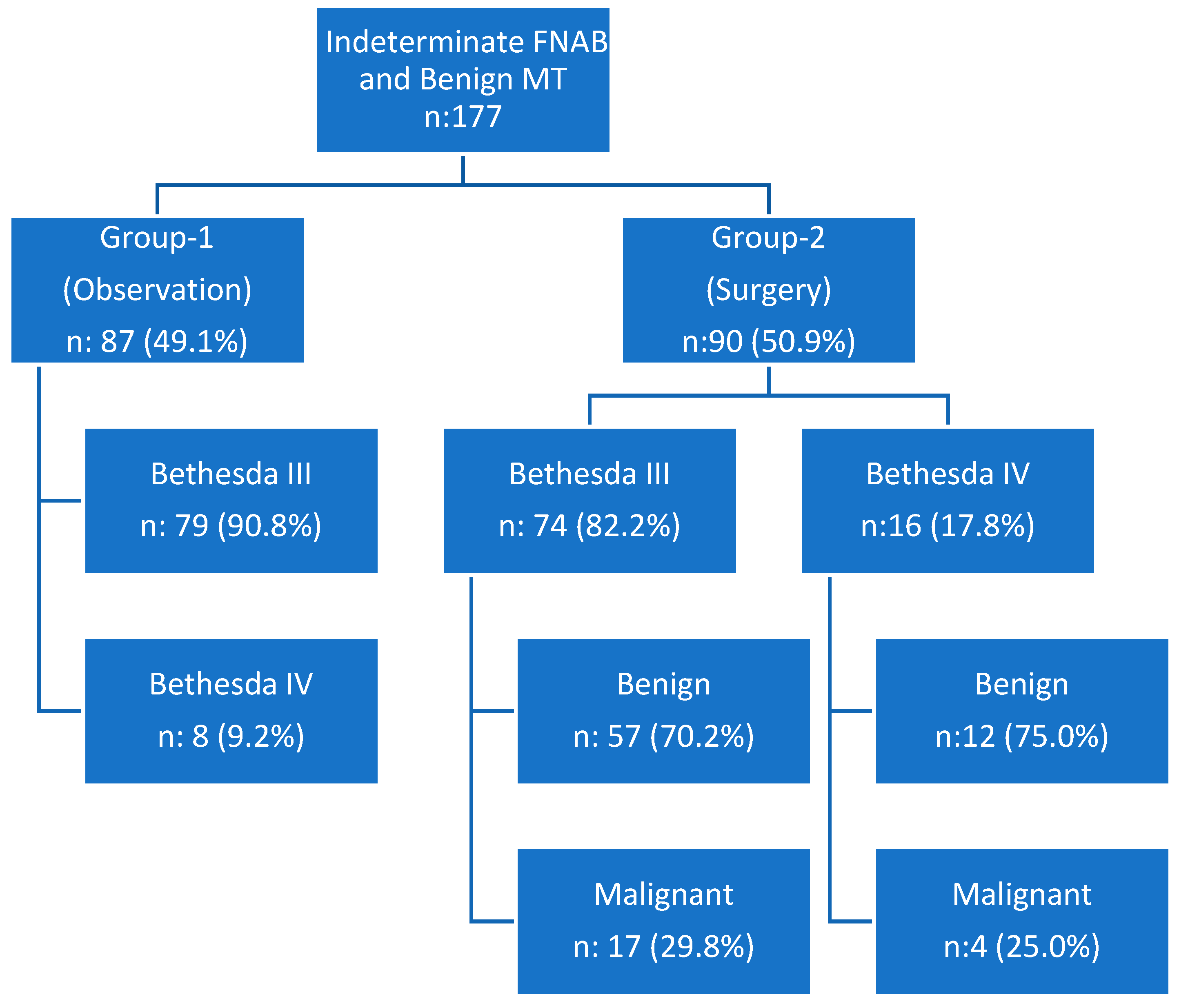
| Bivariate Analysis | All Patients (n: 177) | Group-1 (n: 87) | Group-2 (n: 90) | p-Value | |
|---|---|---|---|---|---|
| Age (years) (mean ± SD) | 55.9 ± 13.9 | 57.2 ± 13.3 | 54.7 ± 14.6 | 0.218 | |
| Gender | Female | 141 (79.7%) | 72 (82.8%) | 69 (76.7%) | 0.314 |
| Male | 36 (20.3%) | 15 (17.2%) | 21 (23.3%) | ||
| Ethnicity | White | 120 (67.8%) | 60 (69.0%) | 60 (66.7%) | 0.583 |
| Black | 52 (29.4%) | 26 (29.9%) | 26 (28.9%) | ||
| Asian | 4 (2.3%) | 1 (1.1%) | 3 (3.3%) | ||
| Hispanic | 1 (0.6%) | - | 1 (1.1%) | ||
| Preoperative Weight (kg) (median(IQR)) | 82.9 (70.45–101.3) | 83.5 (72.1–100.2) | 82.1 (70.3–102.3) | 0.840 | |
| Preoperative BMI (kg/m2) (median(IQR)) | 29.6 (25.4–36.6) | 29.6 (25.7–35.7) | 28.9 (25.4–36.9) | 0.824 | |
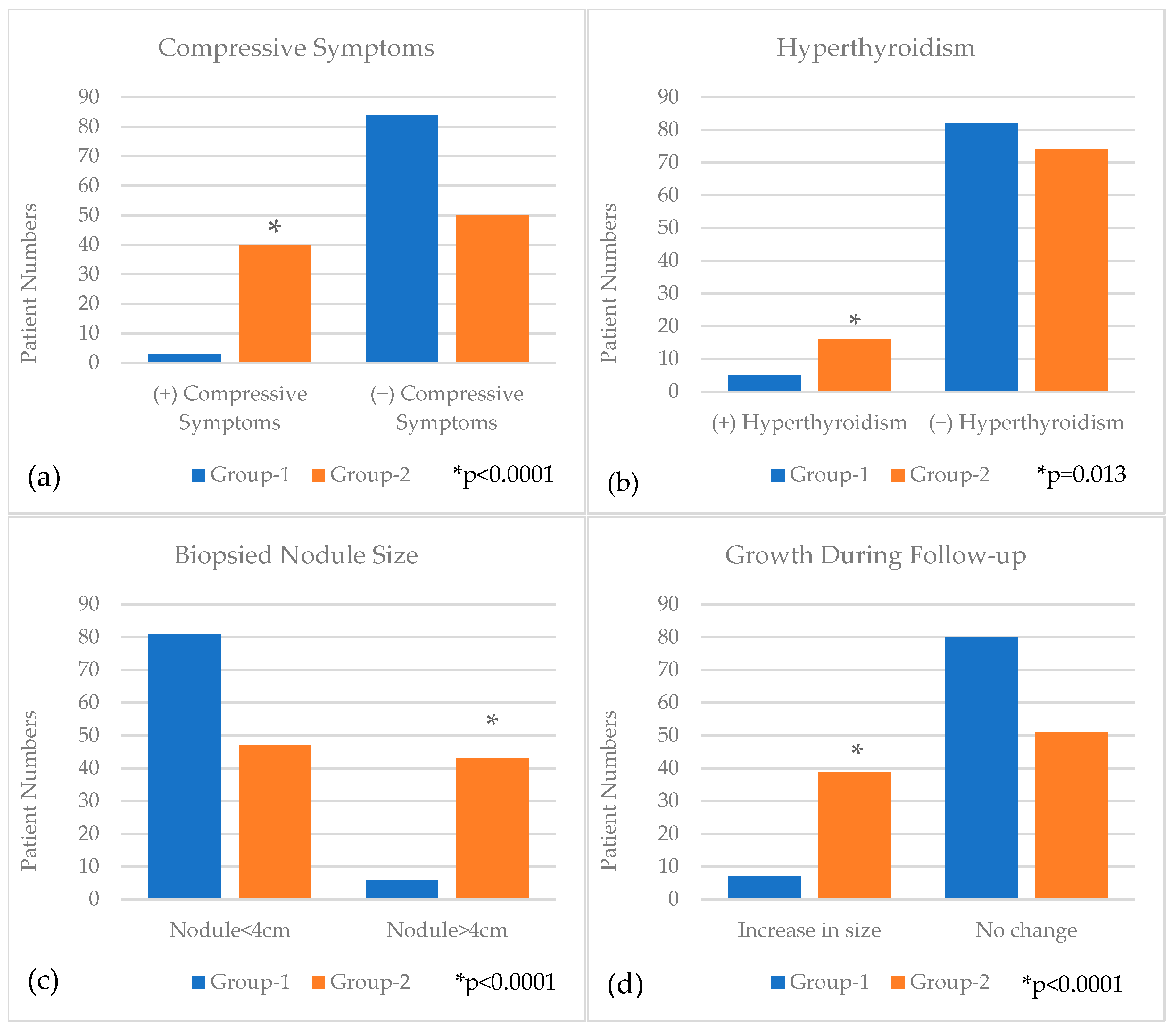
| Compressive Symptoms | Yes | 43 (24.3%) | 3 (3.4%) | 40 (44.4%) | <0.0001 |
| No | 134 (75.7%) | 84 (96.6%) | 50 (55.6%) | ||
| Radiation History | Yes | 7 (4.0%) | 2 (2.3%) | 5 (5.6%) | 0.444 |
| No | 170 (96.0%) | 85 (97.7%) | 85 (94.4%) | ||
| Hyperthyroidism | Yes | 21 (11.9%) | 5 (5.7%) | 16 (17.8%) | 0.013 |
| No | 156 (88.1%) | 82 (94.3%) | 74 (82.2%) | ||
| Hypothyroidism | Yes | 20 (11.3%) | 10 (11.5%) | 10 (11.1%) | 0.936 |
| No | 157 (88.7%) | 77 (88.5%) | 80 (88.9%) | ||
| Family History thyroid disease | Yes | 34 (19.2%) | 15 (17.2%) | 19 (21.1%) | 0.514 |
| No | 143 (80.8%) | 72 (82.8%) | 71 (78.9%) | ||
| Nodule Size (cm) (median(IQR)) | 2.8 (1.95–4) | 2.2 (1.7–2.9) | 3.7 (2.6–4.7) | <0.0001 | |
| Nodule Location | Isthmus | 8 (4.5%) | 4 (4.6%) | 4 (4.4%) | 0.779 |
| Right | 89 (50.3%) | 46 (52.9%) | 43 (47.8%) | ||
| Left | 80 (45.2%) | 37 (42.5%) | 43 (47.8%) | ||
| Nodule Size Category | <4 cm | 128 (72.3%) | 81 (93.1%) | 47 (52.2%) | <0.0001 |
| >4 cm | 49 (27.7%) | 6 (6.9%) | 43 (47.8%) | ||
| Number of Thyroid Nodules | Single | 76 (42.9%) | 41 (47.1%) | 35 (38.9%) | 0.268 |
| Multiple | 101 (57.1%) | 46 (52.9%) | 55 (61.1%) | ||
| Growth during Follow-up | Yes | 46 (26.0%) | 7 (8.0%) | 39 (43.3%) | <0.0001 |
| No | 131 (74.0%) | 80 (92.0%) | 51 (56.7%) | ||
| ACR-TIRADS Score | 2 | 2 (1.1%) | - | 2 (2.2%) | 0.098 |
| 3 | 63 (35.6%) | 25 (28.7%) | 38 (42.2%) | ||
| 4 | 96 (54.2%) | 52 (59.8%) | 44 (48.9%) | ||
| 5 | 16 (9.0%) | 10 (11.5%) | 6 (6.7%) | ||
| FNAB results | Bethesda 3 | 153 (86.4%) | 79 (90.8%) | 74 (82.2%) | 0.095 |
| Bethesda 4 | 24 (13.6%) | 8 (9.2%) | 16 (17.8%) | ||
| Multivariate Analysis | Odds Ratio | 95% Confidence Interval | p-Value | |
| Compressive Symptoms | No | Reference | <0.0001 | |
| Yes | 23.2 | 6.058–88.887 | ||
| Hyperthyroidism | No | Reference | 0.007 | |
| Yes | 5.874 | 1.628–21.197 | ||
| Biopsied Nodule Size | <4 cm | Reference | <0.0001 | |
| >4 cm | 11.359 | 3.896–33.117 | ||
| Increasing Size during Follow up | No | Reference | 0.0002 | |
| Yes | 7.850 | 2.721–22.649 | ||
| 7 Patients | 6 Patient | Incidental Papillary Microcarcinoma, 0.1–0.5 mm |
| 1 Patient | Papillary Thyroid Microcarcinoma, 7.5 mm | |
| 4 patients | Noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) | |
| 4 patients | Oncocytic carcinoma of the thyroid | |
| 3 patients | Follicular thyroid carcinoma | |
| 1 patient | Poorly differentiated thyroid carcinoma | |
| 1 patient | Carcinoid tumor metastasis | |
| 1 patient | Invasive follicular variant papillary thyroid carcinoma | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostek, M.; Swaminathan, N.; Izhar, A.; Gillis, A.; Chen, H.; Lindeman, B. Molecular Testing in Thyroid Nodules: How Much Does It Change Clinical Practice? Biomedicines 2025, 13, 1947. https://doi.org/10.3390/biomedicines13081947
Kostek M, Swaminathan N, Izhar A, Gillis A, Chen H, Lindeman B. Molecular Testing in Thyroid Nodules: How Much Does It Change Clinical Practice? Biomedicines. 2025; 13(8):1947. https://doi.org/10.3390/biomedicines13081947
Chicago/Turabian StyleKostek, Mehmet, Niranjna Swaminathan, Azeem Izhar, Andrea Gillis, Herbert Chen, and Brenessa Lindeman. 2025. "Molecular Testing in Thyroid Nodules: How Much Does It Change Clinical Practice?" Biomedicines 13, no. 8: 1947. https://doi.org/10.3390/biomedicines13081947
APA StyleKostek, M., Swaminathan, N., Izhar, A., Gillis, A., Chen, H., & Lindeman, B. (2025). Molecular Testing in Thyroid Nodules: How Much Does It Change Clinical Practice? Biomedicines, 13(8), 1947. https://doi.org/10.3390/biomedicines13081947





