Cytokine Networks in Triple-Negative Breast Cancer: Mechanisms, Therapeutic Targets, and Emerging Strategies
Abstract
1. Introduction
2. Tumor Proliferation and Survival
2.1. IL-6
2.2. IL-8
2.3. TGF-β
3. Dissemination: EMT, Invasion, Migration, and Metastasis
3.1. TGF-β
3.2. TNF-α
3.3. IL-1β
3.4. IL-6
3.5. IL-8
3.6. CXCL12
4. Angiogenesis
4.1. IL-6 and IL-8
4.2. IL-1β
4.3. TNF-α
4.4. VEGF
4.5. Implications of Anti-Angiogenic Approaches for Clinical Practice
5. Cytokine-Mediated Modulation of TME and Immune Landscape
5.1. TAMs
5.1.1. IL-1β
5.1.2. IL-6
5.1.3. CCL2
5.1.4. CCL5
5.1.5. TNF-α
5.1.6. TGF-β
5.1.7. IFN-α
5.2. TILs in TNBC: The Janus-Face of Anti-Tumor and Pro-Tumor Forces
5.2.1. IL-2
5.2.2. IL-12
5.2.3. IFN-γ
5.2.4. IL-10
5.2.5. TGF-β
6. Future Perspectives
6.1. Bispecific Antibodies
6.2. Multi-Cytokine Targeting
6.3. Cytokine-Induced Killer Cells
6.4. TRUCKs: Activating Cytokines Secreted by CAR-T
6.5. Next-Generation CAR-NK Cells
7. Materials and Methods
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Akt | Protein Kinase B |
| APCs | Antigen-Presenting Cells |
| BC | Breast Cancer |
| Bcl-2 | B-cell leukemia/lymphoma 2 protein |
| BCSCs | Breast Cancer Stem Cells |
| CAFs | Cancer-associated fibroblasts |
| CAR | Chimeric Antigen Receptor |
| CCLX | C-C motif chemokine ligand type X |
| CCRX | C-C chemokine receptor type X |
| CDX | Cluster of Differentiation type X |
| CIK | Cytokine-induced killer |
| CSCs | Cancer Stem Cells |
| CTLs | Cytotoxic T lymphocytes |
| CTX | Cetuximab |
| CXCL12 | C-X-C motif chemokine ligand 12 |
| CXCRX | C-X-C motif chemokine receptor type X |
| DC | Dendritic Cells |
| DCR | Disease Control Rate |
| DOX | Doxorubicin |
| DP | Disease Progression |
| DPP | Dipeptidyl Peptidase |
| EGFR | Epidermal Growth Factor Receptor |
| EMT | Epithelial-to-Mesenchymal Transition |
| EP | Electroporation |
| EVs | Extracellular vesicles |
| FAK | Focal Adhesion Kinase |
| FN-EDA | Fibronectin containing the Extra Domain A |
| HER2 | Human Epidermal Growth Factor Receptor 2 |
| IBC | Inflammatory BC |
| ICI | Immune checkpoint inhibitors |
| ICIs | Immune Checkpoint Inhibitors |
| IFN-γ | Interferon gamma |
| IL-X | Interleukin-type X |
| IrAEs | Immune-related Adverse Events |
| JAK | Janus Kinase |
| KIR | Killer immunoglobulin-like receptor |
| KLF4 | Krüppel-like factor 4 |
| mAb | monoclonal Antibody |
| MAPK | Mitogen-Activated Protein Kinase |
| mBC | Metastatic Breast Cancer |
| MCL-1 | Myeloid Cell Leukemia-1 |
| MDR | Multi-drug Resistance |
| MDSC | Myeloid-Derived Suppressor Cells |
| MET | Mesenchymal–Epithelial Transition |
| MMP9 | Matrix Metalloproteinase-9 |
| MMPs | Metalloproteinases |
| MTD | Maximum Tolerated Dose |
| MVD | Microvascular Density |
| mTNBC | Metastatic Triple Negative Breast Cancer |
| mTOR | Mammalian Target of Rapamycin |
| NACT | Neoadjuvant Chemotherapy |
| NF-κB | Factor Nuclear Kappa B |
| NK | Natural Killer |
| OS | Overall Survival |
| ORR | Objective Response Rate |
| OV | Oncolytic Virus |
| PARP | Poly (ADP-ribose) Polymerase |
| PBMCs | Peripheral Blood Mononuclear Cells |
| pCR | Pathologic Complete Response |
| PD-1 | Programmed Death Protein 1 |
| PD-L1 | Programmed Death-Ligand 1 |
| PFS | Progression-Free Survival |
| PIGF | Placental Growth Factor |
| PI3K | PhosphatidylInositol-3 Kinase |
| pIL12 | Il-12 plasmid |
| PLC | Phospholipase C |
| PR | Partial disease Responses |
| PTEN | Phosphatase and Tensin homolog |
| Rac1 | Ras-related C3 botulinum toxin substrate 1 |
| Rho | Rhodopsin |
| sIL-6R | Soluble form of IL-6R |
| SMADX | Suppressor of Mother Against Decapentaplegic Type X |
| SOC | Standard of Care |
| STAT | Signal Transducer and Activator of Transcription |
| STING | Stimulator of Interferon Genes |
| TAMs | Tumor-Associated Macrophages |
| TF | Transcription Factors |
| TGF-β | Transforming growth factor-beta |
| ThX | T-helper type X |
| TIBs | Tumor-Infiltrating B cells |
| TILs | Tumor Infiltrating Lymphocytes |
| TLR | Toll-like Receptor |
| TME | Tumor Microenvironment |
| TNBC | Triple Negative Breast Cancer |
| TNF-α | Tumor Necrosis Factor Alpha |
| Treg | Regulatory T cell |
| TRUCKs | T cells Redirected for Universal Cytokine Killing |
| VEGF | Vascular Endothelial Growth Factor |
References
- Howard, F.M.; Olopade, O.I. Epidemiology of Triple-Negative Breast Cancer. Cancer J. 2021, 27, 8–16. [Google Scholar] [CrossRef]
- Almansour, N.M. Triple-Negative Breast Cancer: A Brief Review About Epidemiology, Risk Factors, Signaling Pathways, Treatment and Role of Artificial Intelligence. Front. Mol. Biosci. 2022, 9, 836417. [Google Scholar] [CrossRef]
- Yin, L.; Duan, J.-J.; Bian, X.-W.; Yu, S. Triple-Negative Breast Cancer Molecular Subtyping and Treatment Progress. Breast Cancer Res. 2020, 22, 61. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, P.; Scatena, C.; Ghilli, M.; Bargagna, I.; Lorenzini, G.; Nicolini, A. Molecular Mechanisms, Biomarkers and Emerging Therapies for Chemotherapy Resistant TNBC. Int. J. Mol. Sci. 2022, 23, 1665. [Google Scholar] [CrossRef] [PubMed]
- Longley, D.B.; Johnston, P.G. Molecular Mechanisms of Drug Resistance. J. Pathol. 2005, 205, 275–292. [Google Scholar] [CrossRef] [PubMed]
- Zagami, P.; Carey, L.A. Triple Negative Breast Cancer: Pitfalls and Progress. npj Breast Cancer 2022, 8, 95. [Google Scholar] [CrossRef]
- Kureshi, C.T.; Dougan, S.K. Cytokines in Cancer. Cancer Cell 2025, 43, 15–35. [Google Scholar] [CrossRef]
- Guo, Z.; Zhu, Z.; Lin, X.; Wang, S.; Wen, Y.; Wang, L.; Zhi, L.; Zhou, J. Tumor Microenvironment and Immunotherapy for Triple-Negative Breast Cancer. Biomark. Res. 2024, 12, 166. [Google Scholar] [CrossRef]
- Malone, M.K.; Smrekar, K.; Park, S.; Blakely, B.; Walter, A.; Nasta, N.; Park, J.; Considine, M.; Danilova, L.V.; Pandey, N.B.; et al. Cytokines Secreted by Stromal Cells in TNBC Microenvironment as Potential Targets for Cancer Therapy. Cancer Biol. Ther. 2020, 21, 560–569. [Google Scholar] [CrossRef]
- Korkaya, H.; Liu, S.; Wicha, M.S. Breast Cancer Stem Cells, Cytokine Networks, and the Tumor Microenvironment. J. Clin. Investig. 2011, 121, 3804–3809. [Google Scholar] [CrossRef]
- Habanjar, O.; Bingula, R.; Decombat, C.; Diab-Assaf, M.; Caldefie-Chezet, F.; Delort, L. Crosstalk of Inflammatory Cytokines within the Breast Tumor Microenvironment. Int. J. Mol. Sci. 2023, 24, 4002. [Google Scholar] [CrossRef]
- Costa, R.L.B.; Han, H.S.; Gradishar, W.J. Targeting the PI3K/AKT/mTOR Pathway in Triple-Negative Breast Cancer: A Review. Breast Cancer Res. Treat. 2018, 169, 397–406. [Google Scholar] [CrossRef]
- LoRusso, P.M. Inhibition of the PI3K/AKT/mTOR Pathway in Solid Tumors. J. Clin. Oncol. 2016, 34, 3803–3815. [Google Scholar] [CrossRef] [PubMed]
- Rodon, J.; Curigliano, G.; Delord, J.-P.; Harb, W.; Azaro, A.; Han, Y.; Wilke, C.; Donnet, V.; Sellami, D.; Beck, T. A Phase Ib, Open-Label, Dose-Finding Study of Alpelisib in Combination with Paclitaxel in Patients with Advanced Solid Tumors. Oncotarget 2018, 9, 31709–31718. [Google Scholar] [CrossRef] [PubMed]
- Masuda, H.; Zhang, D.; Bartholomeusz, C.; Doihara, H.; Hortobagyi, G.N.; Ueno, N.T. Role of Epidermal Growth Factor Receptor in Breast Cancer. Breast Cancer Res. Treat. 2012, 136, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Carey, L.A.; Rugo, H.S.; Marcom, P.K.; Mayer, E.L.; Esteva, F.J.; Ma, C.X.; Liu, M.C.; Storniolo, A.M.; Rimawi, M.F.; Forero-Torres, A.; et al. TBCRC 001: Randomized Phase II Study of Cetuximab in Combination with Carboplatin in Stage IV Triple-Negative Breast Cancer. J. Clin. Oncol. 2012, 30, 2615–2623. [Google Scholar] [CrossRef]
- Johnson, D.E.; O’Keefe, R.A.; Grandis, J.R. Targeting the IL-6/JAK/STAT3 Signalling Axis in Cancer. Nat. Rev. Clin. Oncol. 2018, 15, 234–248. [Google Scholar] [CrossRef]
- Manore, S.G.; Doheny, D.L.; Wong, G.L.; Lo, H.-W. IL-6/JAK/STAT3 Signaling in Breast Cancer Metastasis: Biology and Treatment. Front. Oncol. 2022, 12, 866014. [Google Scholar] [CrossRef]
- Qin, W.; Chen, B.; Li, X.; Zhao, W.; Wang, L.; Zhang, N.; Wang, X.; Luo, D.; Liang, Y.; Li, Y.; et al. Cancer-Associated Fibroblasts Secrete CSF3 to Promote TNBC Progression via Enhancing PGM2L1-Dependent Glycolysis Reprogramming. Cell Death Dis. 2025, 16, 249. [Google Scholar] [CrossRef]
- Phi, L.T.H.; Sari, I.N.; Yang, Y.-G.; Lee, S.-H.; Jun, N.; Kim, K.S.; Lee, Y.K.; Kwon, H.Y. Cancer Stem Cells (CSCs) in Drug Resistance and Their Therapeutic Implications in Cancer Treatment. Stem Cells Int. 2018, 2018, 5416923. [Google Scholar] [CrossRef]
- Siegel, P.M.; Massagué, J. Cytostatic and Apoptotic Actions of TGF-β in Homeostasis and Cancer. Nat. Rev. Cancer 2003, 3, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Hartman, Z.C.; Poage, G.M.; den Hollander, P.; Tsimelzon, A.; Hill, J.; Panupinthu, N.; Zhang, Y.; Mazumdar, A.; Hilsenbeck, S.G.; Mills, G.B.; et al. Growth of Triple-Negative Breast Cancer Cells Relies upon Coordinate Autocrine Expression of the Proinflammatory Cytokines IL-6 and IL-8. Cancer Res. 2013, 73, 3470–3480. [Google Scholar] [CrossRef] [PubMed]
- Rose-John, S. IL-6 Trans-Signaling via the Soluble IL-6 Receptor: Importance for the pro-Inflammatory Activities of IL-6. Int. J. Biol. Sci. 2012, 8, 1237–1247. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Lang, X.; Li, X. The Role of IL-6/JAK2/STAT3 Signaling Pathway in Cancers. Front. Oncol. 2022, 12, 1023177. [Google Scholar] [CrossRef]
- Chang, Q.; Bournazou, E.; Sansone, P.; Berishaj, M.; Gao, S.P.; Daly, L.; Wels, J.; Theilen, T.; Granitto, S.; Zhang, X.; et al. The IL-6/JAK/Stat3 Feed-Forward Loop Drives Tumorigenesis and Metastasis. Neoplasia 2013, 15, 848–862. [Google Scholar] [CrossRef]
- Chen, S.; Li, L. Degradation Strategy of Cyclin D1 in Cancer Cells and the Potential Clinical Application. Front. Oncol. 2022, 12, 949688. [Google Scholar] [CrossRef]
- Bournazou, E.; Bromberg, J. Targeting the Tumor Microenvironment: JAK-STAT3 Signaling. JAK-STAT 2013, 2, e23828. [Google Scholar] [CrossRef]
- Yu, H.; Pardoll, D.; Jove, R. STATs in Cancer Inflammation and Immunity: A Leading Role for STAT3. Nat. Rev. Cancer 2009, 9, 798–809. [Google Scholar] [CrossRef]
- Qin, J.-J.; Yan, L.; Zhang, J.; Zhang, W.-D. STAT3 as a Potential Therapeutic Target in Triple Negative Breast Cancer: A Systematic Review. J. Exp. Clin. Cancer Res. 2019, 38, 195. [Google Scholar] [CrossRef]
- Guo, Z.; Han, S. Targeting Cancer Stem Cell Plasticity in Triple-Negative Breast Cancer. Explor. Target. Antitumor Ther. 2023, 4, 1165–1181. [Google Scholar] [CrossRef]
- Pavitra, E.; Kancharla, J.; Gupta, V.K.; Prasad, K.; Sung, J.Y.; Kim, J.; Tej, M.B.; Choi, R.; Lee, J.-H.; Han, Y.-K.; et al. The Role of NF-κB in Breast Cancer Initiation, Growth, Metastasis, and Resistance to Chemotherapy. Biomed. Pharmacother. 2023, 163, 114822. [Google Scholar] [CrossRef] [PubMed]
- Hsu, E.-C.; Kulp, S.K.; Huang, H.-L.; Tu, H.-J.; Chao, M.-W.; Tseng, Y.-C.; Yang, M.-C.; Salunke, S.B.; Sullivan, N.J.; Chen, W.-C.; et al. Integrin-Linked Kinase as a Novel Molecular Switch of the IL-6-NF-κB Signaling Loop in Breast Cancer. Carcinogenesis 2016, 37, 430–442. [Google Scholar] [CrossRef] [PubMed]
- Felcher, C.M.; Bogni, E.S.; Kordon, E.C. IL-6 Cytokine Family: A Putative Target for Breast Cancer Prevention and Treatment. Int. J. Mol. Sci. 2022, 23, 1809. [Google Scholar] [CrossRef] [PubMed]
- Chung, A.W.; Kozielski, A.J.; Qian, W.; Zhou, J.; Anselme, A.C.; Chan, A.A.; Pan, P.-Y.; Lee, D.J.; Chang, J.C. Tocilizumab Overcomes Chemotherapy Resistance in Mesenchymal Stem-like Breast Cancer by Negating Autocrine IL-1A Induction of IL-6. npj Breast Cancer 2022, 8, 30. [Google Scholar] [CrossRef]
- Alraouji, N.N.; Aboussekhra, A. Tocilizumab Inhibits IL-8 and the Proangiogenic Potential of Triple Negative Breast Cancer Cells. Mol. Carcinog. 2021, 60, 51–59. [Google Scholar] [CrossRef]
- Waugh, D.J.J.; Wilson, C. The Interleukin-8 Pathway in Cancer. Clin. Cancer Res. 2008, 14, 6735–6741. [Google Scholar] [CrossRef]
- Liu, Q.; Li, A.; Tian, Y.; Wu, J.D.; Liu, Y.; Li, T.; Chen, Y.; Han, X.; Wu, K. The CXCL8-CXCR1/2 Pathways in Cancer. Cytokine Growth Factor. Rev. 2016, 31, 61–71. [Google Scholar] [CrossRef]
- Brew, R.; Erikson, J.S.; West, D.C.; Kinsella, A.R.; Slavin, J.; Christmas, S.E. Interleukin-8 as an Autocrine Growth Factor for Human Colon Carcinoma Cells in Vitro. Cytokine 2000, 12, 78–85. [Google Scholar] [CrossRef]
- Kamohara, H.; Takahashi, M.; Ishiko, T.; Ogawa, M.; Baba, H. Induction of Interleukin-8 (CXCL-8) by Tumor Necrosis Factor-Alpha and Leukemia Inhibitory Factor in Pancreatic Carcinoma Cells: Impact of CXCL-8 as an Autocrine Growth Factor. Int. J. Oncol. 2007, 31, 627–632. [Google Scholar]
- Deng, F.; Weng, Y.; Li, X.; Wang, T.; Fan, M.; Shi, Q. Overexpression of IL-8 Promotes Cell Migration via PI3K-Akt Signaling Pathway and EMT in Triple-Negative Breast Cancer. Pathol. Res. Pract. 2020, 216, 152902. [Google Scholar] [CrossRef]
- Fousek, K.; Horn, L.A.; Palena, C. Interleukin-8: A Chemokine at the Intersection of Cancer Plasticity, Angiogenesis, and Immune Suppression. Pharmacol. Ther. 2021, 219, 107692. [Google Scholar] [CrossRef]
- Xiong, X.; Liao, X.; Qiu, S.; Xu, H.; Zhang, S.; Wang, S.; Ai, J.; Yang, L. CXCL8 in Tumor Biology and Its Implications for Clinical Translation. Front. Mol. Biosci. 2022, 9, 723846. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Long, L.; Wang, J.; Wang, Y.; Liu, Y.; Wang, L.; Luo, F. Insights on CXC Chemokine Receptor 2 in Breast Cancer: An Emerging Target for Oncotherapy. Oncol. Lett. 2019, 18, 5699–5708. [Google Scholar] [CrossRef] [PubMed]
- McClelland, S.; Maxwell, P.J.; Branco, C.; Barry, S.T.; Eberlein, C.; LaBonte, M.J. Targeting IL-8 and Its Receptors in Prostate Cancer: Inflammation, Stress Response, and Treatment Resistance. Cancers 2024, 16, 2797. [Google Scholar] [CrossRef] [PubMed]
- Kahraman, D.C.; Kahraman, T.; Cetin-Atalay, R. Targeting PI3K/Akt/mTOR Pathway Identifies Differential Expression and Functional Role of IL8 in Liver Cancer Stem Cell Enrichment. Mol. Cancer Ther. 2019, 18, 2146–2157. [Google Scholar] [CrossRef]
- Huang, R.; Wang, Z.; Hong, J.; Wu, J.; Huang, O.; He, J.; Chen, W.; Li, Y.; Chen, X.; Shen, K. Targeting Cancer-Associated Adipocyte-Derived CXCL8 Inhibits Triple-Negative Breast Cancer Progression and Enhances the Efficacy of Anti-PD-1 Immunotherapy. Cell Death Dis. 2023, 14, 703. [Google Scholar] [CrossRef]
- Houthuijzen, J.M.; Daenen, L.G.M.; Roodhart, J.M.L.; Voest, E.E. The Role of Mesenchymal Stem Cells in Anti-Cancer Drug Resistance and Tumour Progression. Br. J. Cancer 2012, 106, 1901–1906. [Google Scholar] [CrossRef]
- Chen, D.-R.; Lu, D.-Y.; Lin, H.-Y.; Yeh, W.-L. Mesenchymal Stem Cell-Induced Doxorubicin Resistance in Triple Negative Breast Cancer. Biomed. Res. Int. 2014, 2014, 532161. [Google Scholar] [CrossRef]
- Xie, K. Interleukin-8 and Human Cancer Biology. Cytokine Growth Factor. Rev. 2001, 12, 375–391. [Google Scholar] [CrossRef]
- Ginestier, C.; Liu, S.; Diebel, M.E.; Korkaya, H.; Luo, M.; Brown, M.; Wicinski, J.; Cabaud, O.; Charafe-Jauffret, E.; Birnbaum, D.; et al. CXCR1 Blockade Selectively Targets Human Breast Cancer Stem Cells in Vitro and in Xenografts. J. Clin. Investig. 2010, 120, 485–497. [Google Scholar] [CrossRef]
- Tang, B.; Yoo, N.; Vu, M.; Mamura, M.; Nam, J.-S.; Ooshima, A.; Du, Z.; Desprez, P.-Y.; Anver, M.R.; Michalowska, A.M.; et al. TGF-β Can Suppress Tumorigenesis through Effects on the Putative Cancer Stem or Early Progenitor Cell and Committed Progeny in a Breast Cancer Xenograft Model. Cancer Res. 2007, 67, 8643–8652. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Wu, Z.; Qi, Y.; Wu, Q.; Yang, F. Expression of TGF-β1 and the mechanism of invasiveness and metastasis induced by TGF-β1 in breast cancer. Zhonghua Zhong Liu Za Zhi 2009, 31, 679–682. [Google Scholar] [PubMed]
- Vikram, R.; Chou, W.C.; Hung, S.-C.; Shen, C.-Y. Tumorigenic and Metastatic Role of CD44−/Low/CD24−/Low Cells in Luminal Breast Cancer. Cancers 2020, 12, 1239. [Google Scholar] [CrossRef] [PubMed]
- Aashaq, S.; Batool, A.; Mir, S.A.; Beigh, M.A.; Andrabi, K.I.; Shah, Z.A. TGF-β Signaling: A Recap of SMAD-Independent and SMAD-Dependent Pathways. J. Cell. Physiol. 2022, 237, 59–85. [Google Scholar] [CrossRef]
- Aleksakhina, S.N.; Kashyap, A.; Imyanitov, E.N. Mechanisms of Acquired Tumor Drug Resistance. Biochim. Biophys. Acta Rev. Cancer 2019, 1872, 188310. [Google Scholar] [CrossRef]
- Murayama, T.; Gotoh, N. Drug Resistance Mechanisms of Cancer Stem-like Cells and Their Therapeutic Potential as Drug Targets. Cancer Drug Resist. 2019, 2, 457–470. [Google Scholar] [CrossRef]
- Derynck, R.; Turley, S.J.; Akhurst, R.J. TGFβ Biology in Cancer Progression and Immunotherapy. Nat. Rev. Clin. Oncol. 2021, 18, 9–34. [Google Scholar] [CrossRef]
- Liu, S.; Ren, J.; Ten Dijke, P. Targeting TGFβ Signal Transduction for Cancer Therapy. Signal Transduct. Target. Ther. 2021, 6, 8. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, B.P. Epithelial-Mesenchymal Transition in Breast Cancer Progression and Metastasis. Chin. J. Cancer 2011, 30, 603–611. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The Basics of Epithelial-Mesenchymal Transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef]
- Christiansen, J.J.; Rajasekaran, A.K. Reassessing Epithelial to Mesenchymal Transition as a Prerequisite for Carcinoma Invasion and Metastasis. Cancer Res. 2006, 66, 8319–8326. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.; Shyanti, R.K.; Mishra, M.K. Targeted Therapy Approaches for Epithelial-Mesenchymal Transition in Triple Negative Breast Cancer. Front. Oncol. 2024, 14, 1431418. [Google Scholar] [CrossRef] [PubMed]
- Garg, M. Epithelial-Mesenchymal Transition—Activating Transcription Factors—Multifunctional Regulators in Cancer. World J. Stem Cells 2013, 5, 188–195. [Google Scholar] [CrossRef]
- Bhat-Nakshatri, P.; Appaiah, H.; Ballas, C.; Pick-Franke, P.; Goulet, R.; Badve, S.; Srour, E.F.; Nakshatri, H. SLUG/SNAI2 and Tumor Necrosis Factor Generate Breast Cells with CD44+/CD24− Phenotype. BMC Cancer 2010, 10, 411. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.L.; Robin, T.P.; Ford, H.L. Molecular Pathways: Targeting the TGF-β Pathway for Cancer Therapy. Clin. Cancer Res. 2012, 18, 4514–4521. [Google Scholar] [CrossRef]
- Papageorgis, P.; Stylianopoulos, T. Role of TGFβ in Regulation of the Tumor Microenvironment and Drug Delivery (Review). Int. J. Oncol. 2015, 46, 933–943. [Google Scholar] [CrossRef]
- Neuzillet, C.; Tijeras-Raballand, A.; Cohen, R.; Cros, J.; Faivre, S.; Raymond, E.; de Gramont, A. Targeting the TGFβ Pathway for Cancer Therapy. Pharmacol. Ther. 2015, 147, 22–31. [Google Scholar] [CrossRef]
- Muraoka, R.S.; Koh, Y.; Roebuck, L.R.; Sanders, M.E.; Brantley-Sieders, D.; Gorska, A.E.; Moses, H.L.; Arteaga, C.L. Increased Malignancy of Neu-Induced Mammary Tumors Overexpressing Active Transforming Growth Factor β1. Mol. Cell. Biol. 2003, 23, 8691–8703. [Google Scholar] [CrossRef]
- Muraoka, R.S.; Dumont, N.; Ritter, C.A.; Dugger, T.C.; Brantley, D.M.; Chen, J.; Easterly, E.; Roebuck, L.R.; Ryan, S.; Gotwals, P.J.; et al. Blockade of TGF-β Inhibits Mammary Tumor Cell Viability, Migration, and Metastases. J. Clin. Investig. 2002, 109, 1551–1559. [Google Scholar] [CrossRef]
- Nam, J.-S.; Terabe, M.; Mamura, M.; Kang, M.-J.; Chae, H.; Stuelten, C.; Kohn, E.; Tang, B.; Sabzevari, H.; Anver, M.R.; et al. An Anti-Transforming Growth Factor Beta Antibody Suppresses Metastasis via Cooperative Effects on Multiple Cell Compartments. Cancer Res. 2008, 68, 3835–3843. [Google Scholar] [CrossRef]
- Ehata, S.; Hanyu, A.; Hayashi, M.; Aburatani, H.; Kato, Y.; Fujime, M.; Saitoh, M.; Miyazawa, K.; Imamura, T.; Miyazono, K. Transforming Growth Factor-β Promotes Survival of Mammary Carcinoma Cells through Induction of Antiapoptotic Transcription Factor DEC1. Cancer Res. 2007, 67, 9694–9703. [Google Scholar] [CrossRef]
- Yadav, P.; Shankar, B.S. Radio Resistance in Breast Cancer Cells Is Mediated through TGF-β Signalling, Hybrid Epithelial-Mesenchymal Phenotype and Cancer Stem Cells. Biomed. Pharmacother. 2019, 111, 119–130. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, L.; He, X.; Zhang, P.; Sun, C.; Xu, X.; Lu, Y.; Li, F. TGF-β Plays a Vital Role in Triple-Negative Breast Cancer (TNBC) Drug-Resistance through Regulating Stemness, EMT and Apoptosis. Biochem. Biophys. Res. Commun. 2018, 502, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Bagati, A.; Kumar, S.; Jiang, P.; Pyrdol, J.; Zou, A.E.; Godicelj, A.; Mathewson, N.D.; Cartwright, A.N.R.; Cejas, P.; Brown, M.; et al. Integrin Avβ6-TGFβ-SOX4 Pathway Drives Immune Evasion in Triple-Negative Breast Cancer. Cancer Cell 2021, 39, 54–67.e9. [Google Scholar] [CrossRef] [PubMed]
- Mariathasan, S.; Turley, S.J.; Nickles, D.; Castiglioni, A.; Yuen, K.; Wang, Y.; Kadel, E.E.; Koeppen, H.; Astarita, J.L.; Cubas, R.; et al. TGFβ Attenuates Tumour Response to PD-L1 Blockade by Contributing to Exclusion of T Cells. Nature 2018, 554, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Batlle, E.; Massagué, J. Transforming Growth Factor-β Signaling in Immunity and Cancer. Immunity 2019, 50, 924–940. [Google Scholar] [CrossRef]
- Bai, X.; Yi, M.; Jiao, Y.; Chu, Q.; Wu, K. Blocking TGF-β Signaling to Enhance the Efficacy of Immune Checkpoint Inhibitor. OncoTargets Ther. 2019, 12, 9527–9538. [Google Scholar] [CrossRef]
- Bauer, T.M.; Santoro, A.; Lin, C.-C.; Garrido-Laguna, I.; Joerger, M.; Greil, R.; Spreafico, A.; Yau, T.; Goebeler, M.-E.; Hütter-Krönke, M.L.; et al. Phase I/Ib, Open-Label, Multicenter, Dose-Escalation Study of the Anti-TGF-β Monoclonal Antibody, NIS793, in Combination with Spartalizumab in Adult Patients with Advanced Tumors. J. Immunother. Cancer 2023, 11, e007353. [Google Scholar] [CrossRef]
- Barve, M.; Aaron, P.; Manning, L.; Bognar, E.; Wallraven, G.; Horvath, S.; Stanbery, L.; Nemunaitis, J. Pilot Study of Combination Gemogenovatucel-T (Vigil) and Durvalumab in Women with Relapsed BRCA-Wt Triple-Negative Breast or Ovarian Cancer. Clin. Med. Insights Oncol. 2022, 16, 11795549221110501. [Google Scholar] [CrossRef]
- Cruceriu, D.; Baldasici, O.; Balacescu, O.; Berindan-Neagoe, I. The Dual Role of Tumor Necrosis Factor-Alpha (TNF-α) in Breast Cancer: Molecular Insights and Therapeutic Approaches. Cell. Oncol. 2020, 43, 1–18. [Google Scholar] [CrossRef]
- Yang, F.; Zhao, Z.; Zhao, N. Clinical Implications of Tumor Necrosis Factor Receptor 2 in Breast Cancer. Oncol. Lett. 2017, 14, 2393–2398. [Google Scholar] [CrossRef][Green Version]
- Qiao, Y.; He, H.; Jonsson, P.; Sinha, I.; Zhao, C.; Dahlman-Wright, K. AP-1 Is a Key Regulator of Proinflammatory Cytokine TNFα-Mediated Triple-Negative Breast Cancer Progression. J. Biol. Chem. 2016, 291, 5068–5079. [Google Scholar] [CrossRef]
- Cai, X.; Cao, C.; Li, J.; Chen, F.; Zhang, S.; Liu, B.; Zhang, W.; Zhang, X.; Ye, L. Inflammatory Factor TNF-α Promotes the Growth of Breast Cancer via the Positive Feedback Loop of TNFR1/NF-κB (and/or P38)/p-STAT3/HBXIP/TNFR1. Oncotarget 2017, 8, 58338–58352. [Google Scholar] [CrossRef]
- Pileczki, V.; Braicu, C.; Gherman, C.D.; Berindan-Neagoe, I. TNF-α Gene Knockout in Triple Negative Breast Cancer Cell Line Induces Apoptosis. Int. J. Mol. Sci. 2012, 14, 411–420. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, Z.; Horta, C.A.; Yang, J. Regulation of Epithelial-Mesenchymal Transition by Tumor Microenvironmental Signals and Its Implication in Cancer Therapeutics. Semin. Cancer Biol. 2023, 88, 46–66. [Google Scholar] [CrossRef]
- Mercogliano, M.F.; Bruni, S.; Elizalde, P.V.; Schillaci, R. Tumor Necrosis Factor α Blockade: An Opportunity to Tackle Breast Cancer. Front. Oncol. 2020, 10, 584. [Google Scholar] [CrossRef]
- Bilir, C.; Engin, H.; Can, M.; Likhan, S.; Demirtas, D.; Kuzu, F.; Bayraktaroglu, T. Increased Serum Tumor Necrosis Factor Receptor-Associated Factor-6 Expression in Patients with Non-Metastatic Triple-Negative Breast Cancer. Oncol. Lett. 2015, 9, 2819–2824. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Asiedu, M.K.; Ingle, J.N.; Behrens, M.D.; Radisky, D.C.; Knutson, K.L. TGFβ/TNFα-Mediated Epithelial-Mesenchymal Transition Generates Breast Cancer Stem Cells with a Claudin-Low Phenotype. Cancer Res. 2011, 71, 4707–4719. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Feng, Z.; Jia, S.; Wang, W.; Du, Z.; Chen, N.; Chen, Z. Daintain/AIF-1 Promotes Breast Cancer Cell Migration by up-Regulated TNF-α via Activate P38 MAPK Signaling Pathway. Breast Cancer Res. Treat. 2012, 131, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-W.; Xia, W.; Huo, L.; Lim, S.-O.; Wu, Y.; Hsu, J.L.; Chao, C.-H.; Yamaguchi, H.; Yang, N.-K.; Ding, Q.; et al. Epithelial-Mesenchymal Transition Induced by TNF-α Requires NF-κB-Mediated Transcriptional Upregulation of Twist1. Cancer Res. 2012, 72, 1290–1300. [Google Scholar] [CrossRef]
- Storci, G.; Sansone, P.; Mari, S.; D’Uva, G.; Tavolari, S.; Guarnieri, T.; Taffurelli, M.; Ceccarelli, C.; Santini, D.; Chieco, P.; et al. TNFα Up-Regulates SLUG via the NF-κB/HIF1α Axis, Which Imparts Breast Cancer Cells with a Stem Cell-like Phenotype. J. Cell. Physiol. 2010, 225, 682–691. [Google Scholar] [CrossRef]
- Liu, W.; Lu, X.; Shi, P.; Yang, G.; Zhou, Z.; Li, W.; He, S.; Hu, H.; Liao, S.; He, J. TNF-α Increases Breast Cancer Stem-Like Cells through Up-Regulating TAZ Expression via the Non-Canonical NF-κB Pathway. Sci. Rep. 2020, 10, 1804. [Google Scholar] [CrossRef]
- Yamamoto, M.; Taguchi, Y.; Ito-Kureha, T.; Semba, K.; Yamaguchi, N.; Inoue, J. NF-κB Non-Cell-Autonomously Regulates Cancer Stem Cell Populations in the Basal-like Breast Cancer Subtype. Nat. Commun. 2013, 4, 2299. [Google Scholar] [CrossRef]
- Wu, Y.; Deng, J.; Rychahou, P.G.; Qiu, S.; Evers, B.M.; Zhou, B.P. Stabilization of Snail by NF-κB Is Required for Inflammation-Induced Cell Migration and Invasion. Cancer Cell 2009, 15, 416–428. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Chen, X.; Shu, Y. Gene Expression of the Invasive Phenotype of TNF-α-Treated MCF-7 Cells. Biomed. Pharmacother. 2009, 63, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Spriggs, D.R.; Sherman, M.L.; Frei, E.; Kufe, D.W. Clinical Studies with Tumour Necrosis Factor. Ciba Found. Symp. 1987, 131, 206–227. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, T.; Wakabayashi, H.; Matsumine, A.; Sudo, A.; Uchida, A. TNF Inhibitor Suppresses Bone Metastasis in a Breast Cancer Cell Line. Biochem. Biophys. Res. Commun. 2011, 407, 525–530. [Google Scholar] [CrossRef]
- Madhusudan, S.; Foster, M.; Muthuramalingam, S.R.; Braybrooke, J.P.; Wilner, S.; Kaur, K.; Han, C.; Hoare, S.; Balkwill, F.; Talbot, D.C.; et al. A Phase II Study of Etanercept (Enbrel), a Tumor Necrosis Factor Alpha Inhibitor in Patients with Metastatic Breast Cancer. Clin. Cancer Res. 2004, 10, 6528–6534. [Google Scholar] [CrossRef]
- Wilson, B.E.; Shen, Q.; Cescon, D.W.; Reedijk, M. Exploring Immune Interactions in Triple Negative Breast Cancer: IL-1β Inhibition and Its Therapeutic Potential. Front. Genet. 2023, 14, 1086163. [Google Scholar] [CrossRef]
- Malik, A.; Kanneganti, T.-D. Function and Regulation of IL-1α in Inflammatory Diseases and Cancer. Immunol. Rev. 2018, 281, 124–137. [Google Scholar] [CrossRef]
- Voronov, E.; Dotan, S.; Krelin, Y.; Song, X.; Elkabets, M.; Carmi, Y.; Rider, P.; Cohen, I.; Romzova, M.; Kaplanov, I.; et al. Unique Versus Redundant Functions of IL-1α and IL-1β in the Tumor Microenvironment. Front. Immunol. 2013, 4, 177. [Google Scholar] [CrossRef]
- Naldini, A.; Filippi, I.; Miglietta, D.; Moschetta, M.; Giavazzi, R.; Carraro, F. Interleukin-1β Regulates the Migratory Potential of MDAMB231 Breast Cancer Cells through the Hypoxia-Inducible Factor-1α. Eur. J. Cancer 2010, 46, 3400–3408. [Google Scholar] [CrossRef]
- Filippi, I.; Carraro, F.; Naldini, A. Interleukin-1β Affects MDAMB231 Breast Cancer Cell Migration under Hypoxia: Role of HIF-1α and NFκB Transcription Factors. Mediat. Inflamm. 2015, 2015, 789414. [Google Scholar] [CrossRef]
- Storr, S.J.; Safuan, S.; Ahmad, N.; El-Refaee, M.; Jackson, A.M.; Martin, S.G. Macrophage-Derived Interleukin-1β Promotes Human Breast Cancer Cell Migration and Lymphatic Adhesion in Vitro. Cancer Immunol. Immunother. 2017, 66, 1287–1294. [Google Scholar] [CrossRef]
- Shen, Q.; Cohen, B.; Zheng, W.; Rahbar, R.; Martin, B.; Murakami, K.; Lamorte, S.; Thompson, P.; Berman, H.; Zúñiga-Pflücker, J.C.; et al. Notch Shapes the Innate Immunophenotype in Breast Cancer. Cancer Discov. 2017, 7, 1320–1335. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Fu, S.; Zhang, J.; Liu, B.; Li, Z. Targeting Inflammasome/IL-1 Pathways for Cancer Immunotherapy. Sci. Rep. 2016, 6, 36107. [Google Scholar] [CrossRef]
- Oh, K.; Lee, O.-Y.; Park, Y.; Seo, M.W.; Lee, D.-S. IL-1β Induces IL-6 Production and Increases Invasiveness and Estrogen-Independent Growth in a TG2-Dependent Manner in Human Breast Cancer Cells. BMC Cancer 2016, 16, 724. [Google Scholar] [CrossRef] [PubMed]
- Soria, G.; Ofri-Shahak, M.; Haas, I.; Yaal-Hahoshen, N.; Leider-Trejo, L.; Leibovich-Rivkin, T.; Weitzenfeld, P.; Meshel, T.; Shabtai, E.; Gutman, M.; et al. Inflammatory Mediators in Breast Cancer: Coordinated Expression of TNFα & IL-1β with CCL2 & CCL5 and Effects on Epithelial-to-Mesenchymal Transition. BMC Cancer 2011, 11, 130. [Google Scholar] [CrossRef]
- Holen, I.; Lefley, D.V.; Francis, S.E.; Rennicks, S.; Bradbury, S.; Coleman, R.E.; Ottewell, P. IL-1 Drives Breast Cancer Growth and Bone Metastasis in Vivo. Oncotarget 2016, 7, 75571–75584. [Google Scholar] [CrossRef]
- Kaplanov, I.; Carmi, Y.; Kornetsky, R.; Shemesh, A.; Shurin, G.V.; Shurin, M.R.; Dinarello, C.A.; Voronov, E.; Apte, R.N. Blocking IL-1β Reverses the Immunosuppression in Mouse Breast Cancer and Synergizes with Anti–PD-1 for Tumor Abrogation. Proc. Natl. Acad. Sci. USA 2019, 116, 1361–1369. [Google Scholar] [CrossRef]
- Chen, X.; Yang, W.; Deng, X.; Ye, S.; Xiao, W. Interleukin-6 Promotes Proliferative Vitreoretinopathy by Inducing Epithelial-Mesenchymal Transition via the JAK1/STAT3 Signaling Pathway. Mol. Vis. 2020, 26, 517–529. [Google Scholar]
- Kumari, N.; Dwarakanath, B.S.; Das, A.; Bhatt, A.N. Role of Interleukin-6 in Cancer Progression and Therapeutic Resistance. Tumour Biol. 2016, 37, 11553–11572. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Wang, X.; Liu, X.; Li, H.; Lv, Q.; Zhu, J.; Wei, B.; Tang, Y. STAT3, a Poor Survival Predicator, Is Associated with Lymph Node Metastasis from Breast Cancer. J. Breast Cancer 2013, 16, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.; Liang, S.; Ghosh, S.; Hornsby, P.J.; Li, R. Interleukin 6 Secreted from Adipose Stromal Cells Promotes Migration and Invasion of Breast Cancer Cells. Oncogene 2009, 28, 2745–2755. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, N.J.; Sasser, A.K.; Axel, A.E.; Vesuna, F.; Raman, V.; Ramirez, N.; Oberyszyn, T.M.; Hall, B.M. Interleukin-6 Induces an Epithelial-Mesenchymal Transition Phenotype in Human Breast Cancer Cells. Oncogene 2009, 28, 2940–2947. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil Extracellular Traps Kill Bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Meier, C.; Brieger, A. The Role of IL-8 in Cancer Development and Its Impact on Immunotherapy Resistance. Eur. J. Cancer 2025, 218, 115267. [Google Scholar] [CrossRef]
- Alassaf, E.; Mueller, A. The Role of PKC in CXCL8 and CXCL10 Directed Prostate, Breast and Leukemic Cancer Cell Migration. Eur. J. Pharmacol. 2020, 886, 173453. [Google Scholar] [CrossRef]
- Long, X.; Ye, Y.; Zhang, L.; Liu, P.; Yu, W.; Wei, F.; Ren, X.; Yu, J. IL-8, a Novel Messenger to Cross-Link Inflammation and Tumor EMT via Autocrine and Paracrine Pathways (Review). Int. J. Oncol. 2016, 48, 5–12. [Google Scholar] [CrossRef]
- Ma, Y.; Fu, Y.; Fan, X.; Ji, Q.; Duan, X.; Wang, Y.; Zhang, Y.; Wang, Z.; Hao, H. FAK/IL-8 Axis Promotes the Proliferation and Migration of Gastric Cancer Cells. Gastric Cancer 2023, 26, 528–541. [Google Scholar] [CrossRef]
- Rueda, P.; Richart, A.; Récalde, A.; Gasse, P.; Vilar, J.; Guérin, C.; Lortat-Jacob, H.; Vieira, P.; Baleux, F.; Chretien, F.; et al. Homeostatic and Tissue Reparation Defaults in Mice Carrying Selective Genetic Invalidation of CXCL12/Proteoglycan Interactions. Circulation 2012, 126, 1882–1895. [Google Scholar] [CrossRef] [PubMed]
- Rossi, E.; Sanz-Rodriguez, F.; Eleno, N.; Düwell, A.; Blanco, F.J.; Langa, C.; Botella, L.M.; Cabañas, C.; Lopez-Novoa, J.M.; Bernabeu, C. Endothelial Endoglin Is Involved in Inflammation: Role in Leukocyte Adhesion and Transmigration. Blood 2013, 121, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Zhan, T.; Cao, C.; Li, L.; Gu, N.; Civin, C.I.; Zhan, X. MIM Regulates the Trafficking of Bone Marrow Cells via Modulating Surface Expression of CXCR4. Leukemia 2016, 30, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Ivins, S.; Chappell, J.; Vernay, B.; Suntharalingham, J.; Martineau, A.; Mohun, T.J.; Scambler, P.J. The CXCL12/CXCR4 Axis Plays a Critical Role in Coronary Artery Development. Dev. Cell 2015, 33, 455–468. [Google Scholar] [CrossRef]
- Zhu, Y.; Yu, T.; Zhang, X.-C.; Nagasawa, T.; Wu, J.Y.; Rao, Y. Role of the Chemokine SDF-1 as the Meningeal Attractant for Embryonic Cerebellar Neurons. Nat. Neurosci. 2002, 5, 719–720. [Google Scholar] [CrossRef]
- Daniel, S.K.; Seo, Y.D.; Pillarisetty, V.G. The CXCL12-CXCR4/CXCR7 Axis as a Mechanism of Immune Resistance in Gastrointestinal Malignancies. Semin. Cancer Biol. 2020, 65, 176–188. [Google Scholar] [CrossRef]
- Pillarisetti, K.; Gupta, S.K. Cloning and Relative Expression Analysis of Rat Stromal Cell Derived Factor-1 (SDF-1)1: SDF-1α mRNA Is Selectively Induced in Rat Model of Myocardial Infarction. Inflammation 2001, 25, 293–300. [Google Scholar] [CrossRef]
- Laguri, C.; Sadir, R.; Rueda, P.; Baleux, F.; Gans, P.; Arenzana-Seisdedos, F.; Lortat-Jacob, H. The Novel CXCL12γ Isoform Encodes an Unstructured Cationic Domain Which Regulates Bioactivity and Interaction with Both Glycosaminoglycans and CXCR4. PLoS ONE 2007, 2, e1110. [Google Scholar] [CrossRef]
- Ray, P.; Stacer, A.C.; Fenner, J.; Cavnar, S.P.; Meguiar, K.; Brown, M.; Luker, K.E.; Luker, G.D. CXCL12-γ in Primary Tumors Drives Breast Cancer Metastasis. Oncogene 2015, 34, 2043–2051. [Google Scholar] [CrossRef]
- Yang, Y.; Li, J.; Lei, W.; Wang, H.; Ni, Y.; Liu, Y.; Yan, H.; Tian, Y.; Wang, Z.; Yang, Z.; et al. CXCL12-CXCR4/CXCR7 Axis in Cancer: From Mechanisms to Clinical Applications. Int. J. Biol. Sci. 2023, 19, 3341–3359. [Google Scholar] [CrossRef]
- Pozzobon, T.; Goldoni, G.; Viola, A.; Molon, B. CXCR4 Signaling in Health and Disease. Immunol. Lett. 2016, 177, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Scala, S. Molecular Pathways: Targeting the CXCR4-CXCL12 Axis--Untapped Potential in the Tumor Microenvironment. Clin. Cancer Res. 2015, 21, 4278–4285. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, E.L.; Lee, W.; Lu, J.; Lowy, A.M.; Kim, J. Chemokine CXCL12 Activates Dual CXCR4 and CXCR7-Mediated Signaling Pathways in Pancreatic Cancer Cells. J. Transl. Med. 2012, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Tang, T.; Zhu, J.; Tang, Y.; Sun, H.; Li, S. CXCL12 Has Therapeutic Value in Facial Nerve Injury and Promotes Schwann Cells Autophagy and Migration via PI3K-AKT-mTOR Signal Pathway. Int. J. Biol. Macromol. 2019, 124, 460–468. [Google Scholar] [CrossRef]
- Strazza, M.; Azoulay-Alfaguter, I.; Peled, M.; Smrcka, A.V.; Skolnik, E.Y.; Srivastava, S.; Mor, A. PLCε1 Regulates SDF-1α-Induced Lymphocyte Adhesion and Migration to Sites of Inflammation. Proc. Natl. Acad. Sci. USA 2017, 114, 2693–2698. [Google Scholar] [CrossRef]
- Hu, X.-M.; Zhang, H.; Xu, H.; Zhang, H.-L.; Chen, L.-P.; Cui, W.-Q.; Yang, W.; Shen, W. Chemokine Receptor CXCR4 Regulates CaMKII/CREB Pathway in Spinal Neurons That Underlies Cancer-Induced Bone Pain. Sci. Rep. 2017, 7, 4005. [Google Scholar] [CrossRef]
- Zhang, X.F.; Wang, J.F.; Matczak, E.; Proper, J.A.; Groopman, J.E. Janus Kinase 2 Is Involved in Stromal Cell-Derived Factor-1α-Induced Tyrosine Phosphorylation of Focal Adhesion Proteins and Migration of Hematopoietic Progenitor Cells. Blood 2001, 97, 3342–3348. [Google Scholar] [CrossRef]
- Song, Z.-Y.; Gao, Z.-H.; Chu, J.-H.; Han, X.-Z.; Qu, X.-J. Downregulation of the CXCR4/CXCL12 Axis Blocks the Activation of the Wnt/β-Catenin Pathway in Human Colon Cancer Cells. Biomed. Pharmacother. 2015, 71, 46–52. [Google Scholar] [CrossRef]
- Rajagopal, S.; Kim, J.; Ahn, S.; Craig, S.; Lam, C.M.; Gerard, N.P.; Gerard, C.; Lefkowitz, R.J. β-Arrestin- but Not G Protein-Mediated Signaling by the “Decoy” Receptor CXCR7. Proc. Natl. Acad. Sci. USA 2010, 107, 628–632. [Google Scholar] [CrossRef]
- Khare, T.; Bissonnette, M.; Khare, S. CXCL12-CXCR4/CXCR7 Axis in Colorectal Cancer: Therapeutic Target in Preclinical and Clinical Studies. Int. J. Mol. Sci. 2021, 22, 7371. [Google Scholar] [CrossRef]
- Murad, H.A.S.; Rafeeq, M.M.; Alqurashi, T.M.A. Role and Implications of the CXCL12/CXCR4/CXCR7 Axis in Atherosclerosis: Still a Debate. Ann. Med. 2021, 53, 1598–1612. [Google Scholar] [CrossRef]
- Feng, W.; Huang, W.; Chen, J.; Qiao, C.; Liu, D.; Ji, X.; Xie, M.; Zhang, T.; Wang, Y.; Sun, M.; et al. CXCL12-Mediated HOXB5 Overexpression Facilitates Colorectal Cancer Metastasis through Transactivating CXCR4 and ITGB3. Theranostics 2021, 11, 2612–2633. [Google Scholar] [CrossRef] [PubMed]
- Ahirwar, D.K.; Nasser, M.W.; Ouseph, M.M.; Elbaz, M.; Cuitiño, M.C.; Kladney, R.D.; Varikuti, S.; Kaul, K.; Satoskar, A.R.; Ramaswamy, B.; et al. Fibroblast-Derived CXCL12 Promotes Breast Cancer Metastasis by Facilitating Tumor Cell Intravasation. Oncogene 2018, 37, 4428–4442. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Zhao, X.; Zhu, L.; Li, S.; Xiao, Q.; He, W.; Yin, L. Targeting Intracellular MMPs Efficiently Inhibits Tumor Metastasis and Angiogenesis. Theranostics 2018, 8, 2830–2845. [Google Scholar] [CrossRef] [PubMed]
- Teng, F.; Tian, W.-Y.; Wang, Y.-M.; Zhang, Y.-F.; Guo, F.; Zhao, J.; Gao, C.; Xue, F.-X. Cancer-Associated Fibroblasts Promote the Progression of Endometrial Cancer via the SDF-1/CXCR4 Axis. J. Hematol. Oncol. 2016, 9, 8. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, L.; Han, X.; Wang, Y.; Mi, J.; Wang, C.; Sun, D.; Fu, Y.; Zhao, X.; Guo, H.; et al. Saikosaponin A Inhibits Triple-Negative Breast Cancer Growth and Metastasis Through Downregulation of CXCR4. Front. Oncol. 2019, 9, 1487. [Google Scholar] [CrossRef]
- Kawakita, E.; Yang, F.; Kumagai, A.; Takagaki, Y.; Kitada, M.; Yoshitomi, Y.; Ikeda, T.; Nakamura, Y.; Ishigaki, Y.; Kanasaki, K.; et al. Metformin Mitigates DPP-4 Inhibitor-Induced Breast Cancer Metastasis via Suppression of mTOR Signaling. Mol. Cancer Res. 2021, 19, 61–73. [Google Scholar] [CrossRef]
- Luker, G.D.; Yang, J.; Richmond, A.; Scala, S.; Festuccia, C.; Schottelius, M.; Wester, H.-J.; Zimmermann, J. At the Bench: Pre-Clinical Evidence for Multiple Functions of CXCR4 in Cancer. J. Leukoc. Biol. 2021, 109, 969–989. [Google Scholar] [CrossRef]
- Mezzapelle, R.; Leo, M.; Caprioglio, F.; Colley, L.S.; Lamarca, A.; Sabatino, L.; Colantuoni, V.; Crippa, M.P.; Bianchi, M.E. CXCR4/CXCL12 Activities in the Tumor Microenvironment and Implications for Tumor Immunotherapy. Cancers 2022, 14, 2314. [Google Scholar] [CrossRef]
- Veglia, F.; Sanseviero, E.; Gabrilovich, D.I. Myeloid-Derived Suppressor Cells in the Era of Increasing Myeloid Cell Diversity. Nat. Rev. Immunol. 2021, 21, 485–498. [Google Scholar] [CrossRef]
- Santagata, S.; Napolitano, M.; D’Alterio, C.; Desicato, S.; Maro, S.D.; Marinelli, L.; Fragale, A.; Buoncervello, M.; Persico, F.; Gabriele, L.; et al. Targeting CXCR4 Reverts the Suppressive Activity of T-Regulatory Cells in Renal Cancer. Oncotarget 2017, 8, 77110–77120. [Google Scholar] [CrossRef]
- Mehdizadeh, R.; Shariatpanahi, S.P.; Goliaei, B.; Rüegg, C. Targeting Myeloid-Derived Suppressor Cells in Combination with Tumor Cell Vaccination Predicts Anti-Tumor Immunity and Breast Cancer Dormancy: An in Silico Experiment. Sci. Rep. 2023, 13, 5875. [Google Scholar] [CrossRef]
- Chen, I.X.; Chauhan, V.P.; Posada, J.; Ng, M.R.; Wu, M.W.; Adstamongkonkul, P.; Huang, P.; Lindeman, N.; Langer, R.; Jain, R.K. Blocking CXCR4 Alleviates Desmoplasia, Increases T-Lymphocyte Infiltration, and Improves Immunotherapy in Metastatic Breast Cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 4558–4566. [Google Scholar] [CrossRef]
- Ciavattone, N.G.; Bevoor, A.; Farfel, A.; Rehman, A.; Ho, K.K.Y.; Rock, E.C.; Chen, Y.-C.; Luker, K.E.; Humphries, B.A.; Luker, G.D. Inhibiting CXCR4 Reduces Immunosuppressive Effects of Myeloid Cells in Breast Cancer Immunotherapy. Sci. Rep. 2025, 15, 5204. [Google Scholar] [CrossRef]
- Wani, N.; Nasser, M.W.; Ahirwar, D.K.; Zhao, H.; Miao, Z.; Shilo, K.; Ganju, R.K. C-X-C Motif Chemokine 12/C-X-C Chemokine Receptor Type 7 Signaling Regulates Breast Cancer Growth and Metastasis by Modulating the Tumor Microenvironment. Breast Cancer Res. 2014, 16, R54. [Google Scholar] [CrossRef]
- Eelen, G.; Treps, L.; Li, X.; Carmeliet, P. Basic and Therapeutic Aspects of Angiogenesis Updated. Circ. Res. 2020, 127, 310–329. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.; Dwivedi, S.K.D.; Bhattacharya, R.; Mukherjee, P.; Rao, G. VEGF Signaling: Role in Angiogenesis and Beyond. Biochim. Biophys. Acta (BBA) Rev. Cancer 2024, 1879, 189079. [Google Scholar] [CrossRef] [PubMed]
- D’Alessio, A.; Moccia, F.; Li, J.-H.; Micera, A.; Kyriakides, T.R. Angiogenesis and Vasculogenesis in Health and Disease. Biomed. Res. Int. 2015, 2015, 126582. [Google Scholar] [CrossRef] [PubMed]
- Wautier, J.-L.; Wautier, M.-P. Vascular Permeability in Diseases. Int. J. Mol. Sci. 2022, 23, 3645. [Google Scholar] [CrossRef]
- Liu, Z.-L.; Chen, H.-H.; Zheng, L.-L.; Sun, L.-P.; Shi, L. Angiogenic Signaling Pathways and Anti-Angiogenic Therapy for Cancer. Signal Transduct. Target. Ther. 2023, 8, 198. [Google Scholar] [CrossRef]
- Adams, R.H.; Alitalo, K. Molecular Regulation of Angiogenesis and Lymphangiogenesis. Nat. Rev. Mol. Cell Biol. 2007, 8, 464–478. [Google Scholar] [CrossRef]
- Elayat, G.; Selim, A. Angiogenesis in Breast Cancer: Insights and Innovations. Clin. Exp. Med. 2024, 24, 178. [Google Scholar] [CrossRef]
- Bahrami, A.; Khalaji, A.; Bahri Najafi, M.; Sadati, S.; Raisi, A.; Abolhassani, A.; Eshraghi, R.; Khaksary Mahabady, M.; Rahimian, N.; Mirzaei, H. NF-κB Pathway and Angiogenesis: Insights into Colorectal Cancer Development and Therapeutic Targets. Eur. J. Med. Res. 2024, 29, 610. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Chen, J. Anti-angiogenesis in Lung Cancer: Current Situation, Progress and Confusion. Zhongguo Fei Ai Za Zhi 2022, 25, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.; Do, Y.; Kwon, B.S.; Chang, W.; Lee, M.-S.; Kim, J.; Cho, J.G. Angiogenesis and Vasculogenic Mimicry as Therapeutic Targets in Ovarian Cancer. BMB Rep. 2020, 53, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Canino, C.; Perrone, L.; Bosco, E.; Saltalamacchia, G.; Mosca, A.; Rizzo, M.; Porta, C. Targeting Angiogenesis in Metastatic Renal Cell Carcinoma. Expert Rev. Anticancer Ther. 2019, 19, 245–257. [Google Scholar] [CrossRef]
- Yao, C.; Wu, S.; Kong, J.; Sun, Y.; Bai, Y.; Zhu, R.; Li, Z.; Sun, W.; Zheng, L. Angiogenesis in Hepatocellular Carcinoma: Mechanisms and Anti-Angiogenic Therapies. Cancer Biol. Med. 2023, 20, 25–43. [Google Scholar] [CrossRef]
- Cho, W.C.; Jour, G.; Aung, P.P. Role of Angiogenesis in Melanoma Progression: Update on Key Angiogenic Mechanisms and Other Associated Components. Semin. Cancer Biol. 2019, 59, 175–186. [Google Scholar] [CrossRef]
- Ahir, B.K.; Engelhard, H.H.; Lakka, S.S. Tumor Development and Angiogenesis in Adult Brain Tumor: Glioblastoma. Mol. Neurobiol. 2020, 57, 2461–2478. [Google Scholar] [CrossRef]
- Mohammed, R.A.A.; Ellis, I.O.; Mahmmod, A.M.; Hawkes, E.C.; Green, A.R.; Rakha, E.A.; Martin, S.G. Lymphatic and Blood Vessels in Basal and Triple-Negative Breast Cancers: Characteristics and Prognostic Significance. Mod. Pathol. 2011, 24, 774–785. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, F.; Liu, P.; Han, R. Relationship of Vascular Endothelial Growth Factor Expression and Microvessel Density with Clinicopathological Features of Triple-Negative Breast Cancer. Zhongguo Linchuang Yanjiu 2024, 37, 1511–1515. [Google Scholar] [CrossRef]
- De Brot, M.; Rocha, R.M.; Soares, F.A.; Gobbi, H. Microvessel Density as Determined by Computerized Image Analysis of CD34 and CD105 Expression Correlates with Poor Outcome in Triple-Negative Breast Cancer. Cancer Res. 2012, 72, P1-06-03. [Google Scholar] [CrossRef]
- Nalwoga, H.; Arnes, J.B.; Stefansson, I.M.; Wabinga, H.; Foulkes, W.D.; Akslen, L.A. Vascular Proliferation Is Increased in Basal-like Breast Cancer. Breast Cancer Res. Treat. 2011, 130, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Linderholm, B.K.; Hellborg, H.; Johansson, U.; Elmberger, G.; Skoog, L.; Lehtiö, J.; Lewensohn, R. Significantly Higher Levels of Vascular Endothelial Growth Factor (VEGF) and Shorter Survival Times for Patients with Primary Operable Triple-Negative Breast Cancer. Ann. Oncol. 2009, 20, 1639–1646. [Google Scholar] [CrossRef] [PubMed]
- Al-Khalaf, H.H.; Al-Harbi, B.; Al-Sayed, A.; Arafah, M.; Tulbah, A.; Jarman, A.; Al-Mohanna, F.; Aboussekhra, A. Interleukin-8 Activates Breast Cancer-Associated Adipocytes and Promotes Their Angiogenesis- and Tumorigenesis-Promoting Effects. Mol. Cell. Biol. 2019, 39, e00332-18. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-S.; Nam, S.-M.; Song, H.K.; Lee, S.; Kim, K.; Lim, H.K.; Lee, H.; Kang, K.-T.; Kwon, Y.-J.; Chun, Y.-J.; et al. CCL8 Mediates Crosstalk between Endothelial Colony Forming Cells and Triple-Negative Breast Cancer Cells through IL-8, Aggravating Invasion and Tumorigenicity. Oncogene 2021, 40, 3245–3259. [Google Scholar] [CrossRef]
- Potente, M.; Gerhardt, H.; Carmeliet, P. Basic and Therapeutic Aspects of Angiogenesis. Cell 2011, 146, 873–887. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, J.; Deng, X.; Xiong, F.; Zhang, S.; Gong, Z.; Li, X.; Cao, K.; Deng, H.; He, Y.; et al. The Role of Microenvironment in Tumor Angiogenesis. J. Exp. Clin. Cancer Res. 2020, 39, 204. [Google Scholar] [CrossRef]
- Wei, L.-H.; Kuo, M.-L.; Chen, C.-A.; Chou, C.-H.; Lai, K.-B.; Lee, C.-N.; Hsieh, C.-Y. Interleukin-6 Promotes Cervical Tumor Growth by VEGF-Dependent Angiogenesis via a STAT3 Pathway. Oncogene 2003, 22, 1517–1527. [Google Scholar] [CrossRef]
- Jin, K.; Pandey, N.B.; Popel, A.S. Simultaneous Blockade of IL-6 and CCL5 Signaling for Synergistic Inhibition of Triple-Negative Breast Cancer Growth and Metastasis. Breast Cancer Res. 2018, 20, 54. [Google Scholar] [CrossRef]
- Liang, S.; Chen, Z.; Jiang, G.; Zhou, Y.; Liu, Q.; Su, Q.; Wei, W.; Du, J.; Wang, H. Activation of GPER Suppresses Migration and Angiogenesis of Triple Negative Breast Cancer via Inhibition of NF-κB/IL-6 Signals. Cancer Lett. 2017, 386, 12–23. [Google Scholar] [CrossRef]
- Schraufstatter, I.U.; Chung, J.; Burger, M. IL-8 Activates Endothelial Cell CXCR1 and CXCR2 through Rho and Rac Signaling Pathways. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001, 280, L1094–L1103. [Google Scholar] [CrossRef]
- Lai, Y.; Shen, Y.; Liu, X.-H.; Zhang, Y.; Zeng, Y.; Liu, Y.-F. Interleukin-8 Induces the Endothelial Cell Migration through the Activation of Phosphoinositide 3-Kinase-Rac1/RhoA Pathway. Int. J. Biol. Sci. 2011, 7, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Dubey, S.; Varney, M.L.; Dave, B.J.; Singh, R.K. IL-8 Directly Enhanced Endothelial Cell Survival, Proliferation, and Matrix Metalloproteinases Production and Regulated Angiogenesis. J. Immunol. 2003, 170, 3369–3376. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.; Galisteo, R.; Gutkind, J.S. CXCL8/IL8 Stimulates Vascular Endothelial Growth Factor (VEGF) Expression and the Autocrine Activation of VEGFR2 in Endothelial Cells by Activating NFκB through the CBM (Carma3/Bcl10/Malt1) Complex. J. Biol. Chem. 2009, 284, 6038–6042. [Google Scholar] [CrossRef] [PubMed]
- Toney, N.J.; Opdenaker, L.M.; Frerichs, L.; Modarai, S.R.; Ma, A.; Archinal, H.; Ajayi, G.O.; Sims-Mourtada, J. B Cells Enhance IL-1β Driven Invasiveness in Triple Negative Breast Cancer. Sci. Rep. 2025, 15, 2211. [Google Scholar] [CrossRef]
- Fan, X.; He, L.; Dai, Q.; He, J.; Chen, X.; Dai, X.; Zhang, C.; Sun, D.; Meng, X.; Sun, S.; et al. Interleukin-1β Augments the Angiogenesis of Endothelial Progenitor Cells in an NF-κB/CXCR7-Dependent Manner. J. Cell. Mol. Med. 2020, 24, 5605–5614. [Google Scholar] [CrossRef]
- Tanaka, T.; Kanai, H.; Sekiguchi, K.; Aihara, Y.; Yokoyama, T.; Arai, M.; Kanda, T.; Nagai, R.; Kurabayashi, M. Induction of VEGF Gene Transcription by IL-1β Is Mediated through Stress-Activated MAP Kinases and Sp1 Sites in Cardiac Myocytes. J. Mol. Cell. Cardiol. 2000, 32, 1955–1967. [Google Scholar] [CrossRef]
- Fan, F.; Stoeltzing, O.; Liu, W.; McCarty, M.F.; Jung, Y.D.; Reinmuth, N.; Ellis, L.M. Interleukin-1β Regulates Angiopoietin-1 Expression in Human Endothelial Cells. Cancer Res. 2004, 64, 3186–3190. [Google Scholar] [CrossRef]
- Balkwill, F. Tumour Necrosis Factor and Cancer. Nat. Rev. Cancer 2009, 9, 361–371. [Google Scholar] [CrossRef]
- Shin, M.R.; Kang, S.K.; Kim, Y.S.; Lee, S.Y.; Hong, S.C.; Kim, E.-C. TNF-α and LPS Activate Angiogenesis via VEGF and SIRT1 Signalling in Human Dental Pulp Cells. Int. Endod. J. 2015, 48, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ji, X.; Kang, N.; Zhou, J.; Liang, X.; Li, J.; Han, T.; Zhao, C.; Yang, T. Tumor Necrosis Factor α Inhibition Overcomes Immunosuppressive M2b Macrophage-Induced Bevacizumab Resistance in Triple-Negative Breast Cancer. Cell Death Dis. 2020, 11, 993. [Google Scholar] [CrossRef]
- Dakowicz, D.; Zajkowska, M.; Mroczko, B. Relationship between VEGF Family Members, Their Receptors and Cell Death in the Neoplastic Transformation of Colorectal Cancer. Int. J. Mol. Sci. 2022, 23, 3375. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y.; Hosaka, K.; Andersson, P.; Wang, J.; Tholander, F.; Cao, Z.; Morikawa, H.; Tegnér, J.; Yang, Y.; et al. VEGF-B Promotes Cancer Metastasis through a VEGF-A-Independent Mechanism and Serves as a Marker of Poor Prognosis for Cancer Patients. Proc. Natl. Acad. Sci. USA 2015, 112, E2900–E2909. [Google Scholar] [CrossRef]
- Zhang, L.; Ye, C.; Li, P.; Li, C.; Shu, W.; Zhao, Y.; Wang, X. ADSCs Stimulated by VEGF-C Alleviate Intestinal Inflammation via Dual Mechanisms of Enhancing Lymphatic Drainage by a VEGF-C/VEGFR-3-Dependent Mechanism and Inhibiting the NF-κB Pathway by the Secretome. Stem Cell Res. Ther. 2022, 13, 448. [Google Scholar] [CrossRef]
- Li, Y.-L.; Zhao, H.; Ren, X.-B. Relationship of VEGF/VEGFR with Immune and Cancer Cells: Staggering or Forward? Cancer Biol. Med. 2016, 13, 206–214. [Google Scholar] [CrossRef]
- Rahma, O.E.; Hodi, F.S. The Intersection between Tumor Angiogenesis and Immune Suppression. Clin. Cancer Res. 2019, 25, 5449–5457. [Google Scholar] [CrossRef]
- Garcia, J.; Hurwitz, H.I.; Sandler, A.B.; Miles, D.; Coleman, R.L.; Deurloo, R.; Chinot, O.L. Bevacizumab (Avastin®) in Cancer Treatment: A Review of 15 Years of Clinical Experience and Future Outlook. Cancer Treat. Rev. 2020, 86, 102017. [Google Scholar] [CrossRef]
- Brufsky, A.; Valero, V.; Tiangco, B.; Dakhil, S.; Brize, A.; Rugo, H.S.; Rivera, R.; Duenne, A.; Bousfoul, N.; Yardley, D.A. Second-Line Bevacizumab-Containing Therapy in Patients with Triple-Negative Breast Cancer: Subgroup Analysis of the RIBBON-2 Trial. Breast Cancer Res. Treat. 2012, 133, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Saloustros, E.; Nikolaou, M.; Kalbakis, K.; Polyzos, A.; Christofillakis, C.; Kentepozidis, N.; Pistamaltzian, N.; Kourousis, C.; Vamvakas, L.; Georgoulias, V.; et al. Weekly Paclitaxel and Carboplatin Plus Bevacizumab as First-Line Treatment of Metastatic Triple-Negative Breast Cancer. A Multicenter Phase II Trial by the Hellenic Oncology Research Group. Clin. Breast Cancer 2018, 18, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, R.F.; Bakri, H.M.; Abdelfattah, O.N.; Eid, S. Does Bevacizumab Carry a Hope for Metastatic Triple-Negative Breast Cancer in the Era of Immunotherapy? Anticancer Drugs 2022, 33, e604–e609. [Google Scholar] [CrossRef] [PubMed]
- Cameron, D.; Brown, J.; Dent, R.; Jackisch, C.; Mackey, J.; Pivot, X.; Steger, G.G.; Suter, T.M.; Toi, M.; Parmar, M.; et al. Adjuvant Bevacizumab-Containing Therapy in Triple-Negative Breast Cancer (BEATRICE): Primary Results of a Randomised, Phase 3 Trial. Lancet Oncol. 2013, 14, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, J.H.; Ballman, K.; Polley, M.-Y.C.; Campbell, J.D.; Fan, C.; Selitsky, S.; Fernandez-Martinez, A.; Parker, J.S.; Hoadley, K.A.; Hu, Z.; et al. CALGB 40603 (Alliance): Long-Term Outcomes and Genomic Correlates of Response and Survival After Neoadjuvant Chemotherapy With or Without Carboplatin and Bevacizumab in Triple-Negative Breast Cancer. J. Clin. Oncol. 2022, 40, 1323–1334. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Q.; Li, Y.; Li, Q.; Su, F.; Yao, H.; Su, S.; Wang, Q.; Jin, L.; Wang, Y.; et al. Efficacy and Safety of Camrelizumab Combined with Apatinib in Advanced Triple-Negative Breast Cancer: An Open-Label Phase II Trial. J. Immunother. Cancer 2020, 8, e000696. [Google Scholar] [CrossRef]
- Fan, M.; Zhang, J.; Wang, Z.; Wang, B.; Zhang, Q.; Zheng, C.; Li, T.; Ni, C.; Wu, Z.; Shao, Z.; et al. Phosphorylated VEGFR2 and Hypertension: Potential Biomarkers to Indicate VEGF-Dependency of Advanced Breast Cancer in Anti-Angiogenic Therapy. Breast Cancer Res. Treat. 2014, 143, 141–151. [Google Scholar] [CrossRef]
- Zhang, Q.; Shao, B.; Tong, Z.; Ouyang, Q.; Wang, Y.; Xu, G.; Li, S.; Li, H. A Phase Ib Study of Camrelizumab in Combination with Apatinib and Fuzuloparib in Patients with Recurrent or Metastatic Triple-Negative Breast Cancer. BMC Med. 2022, 20, 321. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Tian, Z.; Lin, Y.; Li, H.; Zhu, Z.; Liu, Q.; Su, S.; Zeng, Y.; Jia, W.; et al. Multicenter Phase II Trial of Camrelizumab Combined with Apatinib and Eribulin in Heavily Pretreated Patients with Advanced Triple-Negative Breast Cancer. Nat. Commun. 2022, 13, 3011. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, J.; Zhang, Y.; Ji, F.; Chen, Y.; Zhu, T.; Zhang, L.; Gao, H.; Yang, M.; Li, J.; et al. Low-Dose Apatinib Combined with Neoadjuvant Chemotherapy in the Treatment of Early-Stage Triple-Negative Breast Cancer (LANCET): A Single-Center, Single-Arm, Phase II Trial. Ther. Adv. Med. Oncol. 2022, 14, 17588359221118053. [Google Scholar] [CrossRef]
- Liu, J.; He, M.; Ou, K.; Wang, X.; Wang, Y.; Qi, L.; Chai, Y.; Jiang, M.; Ma, F.; Luo, Y.; et al. Efficacy and Safety of Apatinib Combined with Dose-Dense Paclitaxel and Carboplatin in Neoadjuvant Therapy for Locally Advanced Triple-Negative Breast Cancer: A Prospective Cohort Study with Propensity-Matched Analysis. Int. J. Cancer 2024, 154, 133–144. [Google Scholar] [CrossRef]
- Chung, H.C.; Saada-Bouzid, E.; Longo, F.; Yanez, E.; Im, S.-A.; Castanon, E.; Desautels, D.N.; Graham, D.M.; Garcia-Corbacho, J.; Lopez, J.; et al. Lenvatinib plus Pembrolizumab for Patients with Previously Treated, Advanced, Triple-Negative Breast Cancer: Results from the Triple-Negative Breast Cancer Cohort of the Phase 2 LEAP-005 Study. Cancer 2024, 130, 3278–3288. [Google Scholar] [CrossRef]
- Curigliano, G.; Pivot, X.; Cortés, J.; Elias, A.; Cesari, R.; Khosravan, R.; Collier, M.; Huang, X.; Cataruozolo, P.E.; Kern, K.A.; et al. Randomized Phase II Study of Sunitinib versus Standard of Care for Patients with Previously Treated Advanced Triple-Negative Breast Cancer. Breast 2013, 22, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Shen, L.; Jiang, J.; Zhang, L.; Zhang, Z.; Pan, J.; Ni, C.; Chen, Z. Antiangiogenic Therapy Reverses the Immunosuppressive Breast Cancer Microenvironment. Biomark. Res. 2021, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, H.; Jamiyan, T.; Yamaguchi, R.; Kakumoto, A.; Abe, A.; Harada, O.; Masunaga, A. Tumor Microenvironment in Triple-Negative Breast Cancer: The Correlation of Tumor-Associated Macrophages and Tumor-Infiltrating Lymphocytes. Clin. Transl. Oncol. 2021, 23, 2513–2525. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Zhao, T.; Luo, R.; Qiu, R.; Li, Z. Tumor-Associated Macrophages: Key Players in Triple-Negative Breast Cancer. Front. Oncol. 2022, 12, 772615. [Google Scholar] [CrossRef]
- Sami, E.; Paul, B.T.; Koziol, J.A.; ElShamy, W.M. The Immunosuppressive Microenvironment in BRCA1-IRIS–Overexpressing TNBC Tumors Is Induced by Bidirectional Interaction with Tumor-Associated Macrophages. Cancer Res. 2020, 80, 1102–1117. [Google Scholar] [CrossRef]
- Sawa-Wejksza, K.; Kandefer-Szerszeń, M. Tumor-Associated Macrophages as Target for Antitumor Therapy. Arch. Immunol. Ther. Exp. 2018, 66, 97–111. [Google Scholar] [CrossRef]
- Roumenina, L.; Daugan, M.V.; Noe, R.; Petitprez, F.; Vano, Y.A.; Sanchez-Salas, R.; Becht, E.; Meilleroux, J.; Clec’H, B.L.; Giraldo, N.A.; et al. Tumor Cells Hijack Macrophage-Produced Complement C1q to Promote Tumor Growth. Cancer Immunol. Res. 2019, 7, 1091–1105. [Google Scholar] [CrossRef]
- Lu, C.; Liu, Y.; Miao, L.; Kong, X.; Li, H.; Chen, H.; Zhao, X.; Zhang, B.; Cui, X. Research Progress on the Role of Tumor-associated Macrophages in Tumor Development and Their Use as Molecular Targets (Review). Int. J. Oncol. 2024, 64, 11. [Google Scholar] [CrossRef]
- Mantovani, A.; Marchesi, F.; Malesci, A.; Laghi, L.; Allavena, P. Tumor-Associated Macrophages as Treatment Targets in Oncology. Nat. Rev. Clin. Oncol. 2017, 14, 399–416. [Google Scholar] [CrossRef]
- Jaiswal, A.; Murakami, K.; Elia, A.; Shibahara, Y.; Done, S.J.; Wood, S.A.; Donato, N.J.; Ohashi, P.S.; Reedijk, M. Therapeutic Inhibition of USP9x-Mediated Notch Signaling in Triple-Negative Breast Cancer. Proc. Natl. Acad. Sci. USA 2021, 118, e2101592118. [Google Scholar] [CrossRef]
- Gelfo, V.; Romaniello, D.; Mazzeschi, M.; Sgarzi, M.; Grilli, G.; Morselli, A.; Manzan, B.; Rihawi, K.; Lauriola, M. Roles of IL-1 in Cancer: From Tumor Progression to Resistance to Targeted Therapies. Int. J. Mol. Sci. 2020, 21, 6009. [Google Scholar] [CrossRef] [PubMed]
- Pe, K.C.S.; Saetung, R.; Yodsurang, V.; Chaotham, C.; Suppipat, K.; Chanvorachote, P.; Tawinwung, S. Triple-Negative Breast Cancer Influences a Mixed M1/M2 Macrophage Phenotype Associated with Tumor Aggressiveness. PLoS ONE 2022, 17, e0273044. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and Tumor Progression: Signaling Pathways and Targeted Intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef] [PubMed]
- Caronni, N.; Terza, F.L.; Frosio, L.; Ostuni, R. IL-1β+ Macrophages and the Control of Pathogenic Inflammation in Cancer. Trends Immunol. 2025, 46, 403–415. [Google Scholar] [CrossRef]
- Tunali, G.; Yanik, H.; Ozturk, S.C.; Demirkol-Canli, S.; Efthymiou, G.; Yilmaz, K.B.; Van Obberghen-Schilling, E.; Esendagli, G. A Positive Feedback Loop Driven by Fibronectin and IL-1β Sustains the Inflammatory Microenvironment in Breast Cancer. Breast Cancer Res. 2023, 25, 27. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Lee, S.O.; Yang, X.; Duan, S.; Tsai, Y.; Strojny, L.R.; Keng, P.; Chen, Y. IL-6 Promotes Growth and Epithelial-Mesenchymal Transition of CD133+ Cells of Non-Small Cell Lung Cancer. Oncotarget 2015, 7, 6626–6638. [Google Scholar] [CrossRef]
- Vecchi, L.; Mota, S.T.S.; Zóia, M.A.P.; Martins, I.C.; de Souza, J.B.; Santos, T.G.; Beserra, A.d.O.; de Andrade, V.P.; Goulart, L.R.; Araújo, T.G. Interleukin-6 Signaling in Triple Negative Breast Cancer Cells Elicits the Annexin A1/Formyl Peptide Receptor 1 Axis and Affects the Tumor Microenvironment. Cells 2022, 11, 1705. [Google Scholar] [CrossRef]
- Tang, X.; Mo, C.; Wang, Y.; Wei, D.; Xiao, H. Anti-Tumour Strategies Aiming to Target Tumour-Associated Macrophages. Immunology 2013, 138, 93–104. [Google Scholar] [CrossRef]
- Saleh, L.; Wilson, C.; Holen, I. CDK4/6 Inhibitors: A Potential Therapeutic Approach for Triple Negative Breast Cancer. MedComm 2021, 2, 514–530. [Google Scholar] [CrossRef]
- Larsson, P.; Pettersson, D.; Olsson, M.; Sarathchandra, S.; Abramsson, A.; Zetterberg, H.; Ittner, E.; Forssell-Aronsson, E.; Kovács, A.; Karlsson, P.; et al. Repurposing Proteasome Inhibitors for Improved Treatment of Triple-Negative Breast Cancer. Cell Death Discov. 2024, 10, 57. [Google Scholar] [CrossRef]
- Hamid, R.; Alaziz, M.; Mahal, A.S.; Ashton, A.W.; Halama, N.; Jaeger, D.; Jiao, X.; Pestell, R.G. The Role and Therapeutic Targeting of CCR5 in Breast Cancer. Cells 2023, 12, 2237. [Google Scholar] [CrossRef] [PubMed]
- Rabe, D.C.; Walker, N.D.; Rustandy, F.D.; Wallace, J.; Lee, J.; Stott, S.L.; Rosner, M.R. Tumor Extracellular Vesicles Regulate Macrophage-Driven Metastasis through CCL5. Cancers 2021, 13, 3459. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.L.; Raghavakaimal, A.; Tang, C.-M.; Gardner, K.P.; Kelly, S.; Pourhassan, N.; Ray, N. Safety, Efficacy, and Clinical Outcomes of the Anti-CCR5 Inhibitor (Leronlimab): A Pooled Analysis of Three Clinical Trials in Patients with mTNBC. J. Clin. Oncol. 2022, 40, e13062. [Google Scholar] [CrossRef]
- Narasimhan, H.; Ferraro, F.; Bleilevens, A.; Weiskirchen, R.; Stickeler, E.; Maurer, J. Tumor Necrosis Factor-α (TNFα) Stimulate Triple-Negative Breast Cancer Stem Cells to Promote Intratumoral Invasion and Neovasculogenesis in the Liver of a Xenograft Model. Biology 2022, 11, 1481. [Google Scholar] [CrossRef]
- Deepak, K.G.K.; Vempati, R.; Nagaraju, G.P.; Dasari, V.R.; Nagini, S.; Rao, D.N.; Malla, R.R. Tumor Microenvironment: Challenges and Opportunities in Targeting Metastasis of Triple Negative Breast Cancer. Pharmacol. Res. 2020, 153, 104683. [Google Scholar] [CrossRef]
- Yu, M.; Zhou, X.; Niu, L.; Lin, G.; Huang, J.; Zhou, W.; Gan, H.; Wang, J.; Jiang, X.; Yin, B.; et al. Targeting Transmembrane TNF-α Suppresses Breast Cancer Growth. Cancer Res. 2013, 73, 4061–4074. [Google Scholar] [CrossRef]
- Wysocki, P.J.; Ostrowski, A.; Segal, R.; Potocki, P.M.; Kwinta, L.; Konopka, K.; Florin, L.B.; Prince, S.M. Abstract CT135: Extracorporeal Pulldown of Soluble TNFRs to Unleash the Activity of Endogenous TNFα in Chemorefractory Triple-Negative Breast Cancer Patients—First-in-Human Experience. Cancer Res. 2021, 81, CT135. [Google Scholar] [CrossRef]
- Hourani, T.; Sharma, A.; Luwor, R.B.; Achuthan, A.A. Transforming Growth Factor-β in Tumor Microenvironment: Understanding Its Impact on Monocytes and Macrophages for Its Targeting. Int. Rev. Immunol. 2025, 44, 82–97. [Google Scholar] [CrossRef]
- Fan, Y.; He, S. The Characteristics of Tumor Microenvironment in Triple Negative Breast Cancer. Cancer Manag. Res. 2022, 14, 1–17. [Google Scholar] [CrossRef]
- Arwert, E.N.; Harney, A.S.; Entenberg, D.; Wang, Y.; Sahai, E.; Pollard, J.W.; Condeelis, J.S. A Unidirectional Transition from Migratory to Perivascular Macrophage Is Required for Tumor Cell Intravasation. Cell Rep. 2018, 23, 1239–1248. [Google Scholar] [CrossRef]
- Yi, M.; Li, T.; Niu, M.; Zhang, H.; Wu, Y.; Wu, K.; Dai, Z. Targeting Cytokine and Chemokine Signaling Pathways for Cancer Therapy. Signal Transduct. Target. Ther. 2024, 9, 176. [Google Scholar] [CrossRef]
- Chernosky, N.M.; Tamagno, I.; Polak, K.L.; Chan, E.R.; Yuan, X.; Jackson, M.W. Toll-Like Receptor 3-Mediated Interferon-β Production Is Suppressed by Oncostatin m and a Broader Epithelial-Mesenchymal Transition Program. Breast Cancer Res. 2024, 26, 167. [Google Scholar] [CrossRef]
- Doherty, M.R.; Cheon, H.; Junk, D.J.; Vinayak, S.; Varadan, V.; Telli, M.L.; Ford, J.M.; Stark, G.R.; Jackson, M.W. Interferon-Beta Represses Cancer Stem Cell Properties in Triple-Negative Breast Cancer. Proc. Natl. Acad. Sci. USA 2017, 114, 13792–13797. [Google Scholar] [CrossRef]
- Loi, S.; Michiels, S.; Salgado, R.; Sirtaine, N.; Jose, V.; Fumagalli, D.; Kellokumpu-Lehtinen, P.-L.; Bono, P.; Kataja, V.; Desmedt, C.; et al. Tumor Infiltrating Lymphocytes Are Prognostic in Triple Negative Breast Cancer and Predictive for Trastuzumab Benefit in Early Breast Cancer: Results from the FinHER Trial. Ann. Oncol. 2014, 25, 1544–1550. [Google Scholar] [CrossRef]
- Denkert, C.; Von Minckwitz, G.; Darb-Esfahani, S.; Lederer, B.; Heppner, B.I.; Weber, K.E.; Budczies, J.; Huober, J.; Klauschen, F.; Furlanetto, J.; et al. Tumour-Infiltrating Lymphocytes and Prognosis in Different Subtypes of Breast Cancer: A Pooled Analysis of 3771 Patients Treated with Neoadjuvant Therapy. Lancet Oncol. 2018, 19, 40–50. [Google Scholar] [CrossRef]
- Kathleen Cuningham Foundation Consortium for Research into Familial Breast Cancer (kConFab); Savas, P.; Virassamy, B.; Ye, C.; Salim, A.; Mintoff, C.P.; Caramia, F.; Salgado, R.; Byrne, D.J.; Teo, Z.L.; et al. Single-Cell Profiling of Breast Cancer T Cells Reveals a Tissue-Resident Memory Subset Associated with Improved Prognosis. Nat. Med. 2018, 24, 986–993. [Google Scholar] [CrossRef]
- Cai, B.; Ma, P.; Ding, P.; Sun, D.; Bu, Q.; Zhang, J. Composition and Plasticity of Triple-negative Breast Carcinoma-infiltrating Regulatory T Cells. APMIS 2020, 128, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Dieci, M.V.; Criscitiello, C.; Goubar, A.; Viale, G.; Conte, P.; Guarneri, V.; Ficarra, G.; Mathieu, M.C.; Delaloge, S.; Curigliano, G.; et al. Prognostic Value of Tumor-Infiltrating Lymphocytes on Residual Disease after Primary Chemotherapy for Triple-Negative Breast Cancer: A Retrospective Multicenter Study. Ann. Oncol. 2014, 25, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.G.; Jeong, J.; Hong, S.; Jung, W.H. Current Issues and Clinical Evidence in Tumor-Infiltrating Lymphocytes in Breast Cancer. J. Pathol. Transl. Med. 2015, 49, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Fyfe, G.; Fisher, R.I.; Rosenberg, S.A.; Sznol, M.; Parkinson, D.R.; Louie, A.C. Results of Treatment of 255 Patients with Metastatic Renal Cell Carcinoma Who Received High-Dose Recombinant Interleukin-2 Therapy. J. Clin. Oncol. 1995, 13, 688–696. [Google Scholar] [CrossRef]
- Atkins, M.B.; Lotze, M.T.; Dutcher, J.P.; Fisher, R.I.; Weiss, G.; Margolin, K.; Abrams, J.; Sznol, M.; Parkinson, D.; Hawkins, M.; et al. High-Dose Recombinant Interleukin 2 Therapy for Patients with Metastatic Melanoma: Analysis of 270 Patients Treated between 1985 and 1993. J. Clin. Oncol. 1999, 17, 2105–2116. [Google Scholar] [CrossRef]
- Raeber, M.E.; Sahin, D.; Karakus, U.; Boyman, O. A Systematic Review of Interleukin-2-Based Immunotherapies in Clinical Trials for Cancer and Autoimmune Diseases. EBioMedicine 2023, 90, 104539. [Google Scholar] [CrossRef] [PubMed]
- Parisi, G.; Saco, J.D.; Salazar, F.B.; Tsoi, J.; Krystofinski, P.; Puig-Saus, C.; Zhang, R.; Zhou, J.; Cheung-Lau, G.C.; Garcia, A.J.; et al. Persistence of Adoptively Transferred T Cells with a Kinetically Engineered IL-2 Receptor Agonist. Nat. Commun. 2020, 11, 660. [Google Scholar] [CrossRef] [PubMed]
- Diab, A.; Tannir, N.M.; Bentebibel, S.-E.; Hwu, P.; Papadimitrakopoulou, V.; Haymaker, C.; Kluger, H.M.; Gettinger, S.N.; Sznol, M.; Tykodi, S.S.; et al. Bempegaldesleukin (NKTR-214) plus Nivolumab in Patients with Advanced Solid Tumors: Phase I Dose-Escalation Study of Safety, Efficacy, and Immune Activation (PIVOT-02). Cancer Discov. 2020, 10, 1158–1173. [Google Scholar] [CrossRef] [PubMed]
- Feils, A.S.; Erbe, A.K.; Birstler, J.; Kim, K.; Hoch, U.; Currie, S.L.; Nguyen, T.; Yu, D.; Siefker-Radtke, A.O.; Tannir, N.; et al. Associations between KIR/KIR-Ligand Genotypes and Clinical Outcome for Patients with Advanced Solid Tumors Receiving BEMPEG plus Nivolumab Combination Therapy in the PIVOT-02 Trial. Cancer Immunol. Immunother. 2023, 72, 2099–2111. [Google Scholar] [CrossRef]
- Rolig, A.S.; Rose, D.C.; McGee, G.H.; Rubas, W.; Kivimäe, S.; Redmond, W.L. Combining Bempegaldesleukin (CD122-Preferential IL-2 Pathway Agonist) and NKTR-262 (TLR7/8 Agonist) Improves Systemic Antitumor CD8+ T Cell Cytotoxicity over BEMPEG+RT. J. Immunother. Cancer 2022, 10, e004218. [Google Scholar] [CrossRef]
- Tugues, S.; Burkhard, S.H.; Ohs, I.; Vrohlings, M.; Nussbaum, K.; Vom Berg, J.; Kulig, P.; Becher, B. New Insights into IL-12-Mediated Tumor Suppression. Cell Death Differ. 2015, 22, 237–246. [Google Scholar] [CrossRef]
- Aste-Amezaga, M.; D’Andrea, A.; Kubin, M.; Trinchieri, G. Cooperation of Natural Killer Cell Stimulatory Factor/Interleukin-12 with Other Stimuli in the Induction of Cytokines and Cytotoxic Cell-Associated Molecules in Human T and NK Cells. Cell. Immunol. 1994, 156, 480–492. [Google Scholar] [CrossRef]
- Prochazkova, J.; Pokorna, K.; Holan, V. IL-12 Inhibits the TGF-β-Dependent T Cell Developmental Programs and Skews the TGF-β-Induced Differentiation into a Th1-like Direction. Immunobiology 2012, 217, 74–82. [Google Scholar] [CrossRef]
- Wang, H.; Ruan, G.; Li, Y.; Liu, X. The Role and Potential Application of IL-12 in the Immune Regulation of Tuberculosis. Int. J. Mol. Sci. 2025, 26, 3106. [Google Scholar] [CrossRef]
- Divino, C.M.; Chen, S.H.; Yang, W.; Thung, S.; Brower, S.T.; Woo, S.L. Anti-Tumor Immunity Induced by Interleukin-12 Gene Therapy in a Metastatic Model of Breast Cancer Is Mediated by Natural Killer Cells. Breast Cancer Res. Treat. 2000, 60, 129–134. [Google Scholar] [CrossRef]
- Berraondo, P.; Sanmamed, M.F.; Ochoa, M.C.; Etxeberria, I.; Aznar, M.A.; Pérez-Gracia, J.L.; Rodríguez-Ruiz, M.E.; Ponz-Sarvise, M.; Castañón, E.; Melero, I. Cytokines in Clinical Cancer Immunotherapy. Br. J. Cancer 2019, 120, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Tan, D.; Sun, H.; Li, Z.; Zhang, L.; Zheng, Y.; Liu, S.; Zhang, Y.; He, Q. Interleukin-12 Delivery Strategies and Advances in Tumor Immunotherapy. Curr. Issues Mol. Biol. 2024, 46, 11548–11579. [Google Scholar] [CrossRef] [PubMed]
- Ghouse, S.M.; Nguyen, H.-M.; Bommareddy, P.K.; Guz-Montgomery, K.; Saha, D. Oncolytic Herpes Simplex Virus Encoding IL12 Controls Triple-Negative Breast Cancer Growth and Metastasis. Front. Oncol. 2020, 10, 384. [Google Scholar] [CrossRef] [PubMed]
- Telli, M.L.; Nagata, H.; Wapnir, I.; Acharya, C.R.; Zablotsky, K.; Fox, B.A.; Bifulco, C.B.; Jensen, S.M.; Ballesteros-Merino, C.; Le, M.H.; et al. Intratumoral Plasmid IL-12 Expands CD8+ T Cells and Induces a CXCR3 Gene Signature in Triple-Negative Breast Tumors That Sensitizes Patients to Anti-PD-1 Therapy. Clin. Cancer Res. 2021, 27, 2481–2493. [Google Scholar] [CrossRef]
- Telli, M.L.; Wapnir, I.; Devitt, B.; Cuff, K.; Soliman, H.; Vinayak, S.; Canton, D.A.; Twitty, C.; Foerter, K.M.; Joshi, R. Abstract P3-09-04: Phase 2, Open-Label Study of Intratumoral Tavokinogene Telseplasmid (Tavo) plus Electroporation in Combination with Intravenous Pembrolizumab Therapy in Patients with Inoperable Locally Advanced or Metastatic Triple-Negative Breast Cancer (mTNBC) (KEYNOTE-890/OMS-I141). Cancer Res. 2020, 80, P3-09-04. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, L.; Zhou, L.; Yu, S.; Lan, Y.; Liang, Q.; Liu, J.; Cao, A.; Liu, Y. Tumor Microenvironment-Triggered Charge Reversal Polymetformin-Based Nanosystem Co-Delivered Doxorubicin and IL-12 Cytokine Gene for Chemo-Gene Combination Therapy on Metastatic Breast Cancer. ACS Appl. Mater. Interfaces 2020, 12, 45873–45890. [Google Scholar] [CrossRef]
- Schwarz, E.; Savardekar, H.; Zelinskas, S.; Mouse, A.; Lapurga, G.; Lyberger, J.; Rivaldi, A.; Ringwalt, E.M.; Miller, K.E.; Yu, L.; et al. Trabectedin Enhances the Antitumor Effects of IL-12 in Triple-Negative Breast Cancer. Cancer Immunol. Res. 2025, 13, 560–576. [Google Scholar] [CrossRef]
- Jorgovanovic, D.; Song, M.; Wang, L.; Zhang, Y. Roles of IFN-γ in Tumor Progression and Regression: A Review. Biomark. Res. 2020, 8, 49. [Google Scholar] [CrossRef]
- Garcia-Diaz, A.; Shin, D.S.; Moreno, B.H.; Saco, J.; Escuin-Ordinas, H.; Rodriguez, G.A.; Zaretsky, J.M.; Sun, L.; Hugo, W.; Wang, X.; et al. Interferon Receptor Signaling Pathways Regulating PD-L1 and PD-L2 Expression. Cell Rep. 2017, 19, 1189–1201. [Google Scholar] [CrossRef]
- Nederlof, I.; Isaeva, O.I.; De Graaf, M.; Gielen, R.C.A.M.; Bakker, N.A.M.; Rolfes, A.L.; Garner, H.; Boeckx, B.; Traets, J.J.H.; Mandjes, I.A.M.; et al. Neoadjuvant Nivolumab or Nivolumab plus Ipilimumab in Early-Stage Triple-Negative Breast Cancer: A Phase 2 Adaptive Trial. Nat. Med. 2024, 30, 3223–3235. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; Sinn, B.; Karn, T.; Untch, M.; Treue, D.; Sinn, H.-P.; Weber, K.; Hanusch, C.; Fasching, P.; Huober, J.; et al. Abstract PD2-07: mRNA Signatures Predict Response to Durvalumab Therapy in Triple Negative Breast Cancer (TNBC)—Results of the Translational Biomarker Programme of the Neoadjuvant Double-Blind Placebo Controlled GeparNuevo Trial. Cancer Res. 2019, 79, PD2-07. [Google Scholar] [CrossRef]
- Sceneay, J.; Goreczny, G.J.; Wilson, K.; Morrow, S.; DeCristo, M.J.; Ubellacker, J.M.; Qin, Y.; Laszewski, T.; Stover, D.G.; Barrera, V.; et al. Interferon Signaling Is Diminished with Age and Is Associated with Immune Checkpoint Blockade Efficacy in Triple-Negative Breast Cancer. Cancer Discov. 2019, 9, 1208–1227. [Google Scholar] [CrossRef] [PubMed]
- Zibelman, M.; MacFarlane, A.W.; Costello, K.; McGowan, T.; O’Neill, J.; Kokate, R.; Borghaei, H.; Denlinger, C.S.; Dotan, E.; Geynisman, D.M.; et al. A Phase 1 Study of Nivolumab in Combination with Interferon-Gamma for Patients with Advanced Solid Tumors. Nat. Commun. 2023, 14, 4513. [Google Scholar] [CrossRef]
- Zimmerli, D.; Brambillasca, C.S.; Talens, F.; Bhin, J.; Linstra, R.; Romanens, L.; Bhattacharya, A.; Joosten, S.E.P.; Da Silva, A.M.; Padrao, N.; et al. MYC Promotes Immune-Suppression in Triple-Negative Breast Cancer via Inhibition of Interferon Signaling. Nat. Commun. 2022, 13, 6579. [Google Scholar] [CrossRef]
- Lee, J.V.; Housley, F.; Yau, C.; Nakagawa, R.; Winkler, J.; Anttila, J.M.; Munne, P.M.; Savelius, M.; Houlahan, K.E.; Van De Mark, D.; et al. Combinatorial Immunotherapies Overcome MYC-Driven Immune Evasion in Triple Negative Breast Cancer. Nat. Commun. 2022, 13, 3671. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, S.; Srivastava, R.K.; Nandi, A.; Thacker, G.; Murali, H.; Kim, S.; Baldeon, M.; Tobias, J.; Blanco, M.A.; et al. Loss of ELF5-FBXW7 Stabilizes IFNGR1 to Promote the Growth and Metastasis of Triple-Negative Breast Cancer through Interferon-γ Signalling. Nat. Cell Biol. 2020, 22, 591–602. [Google Scholar] [CrossRef]
- Mannino, M.H.; Zhu, Z.; Xiao, H.; Bai, Q.; Wakefield, M.R.; Fang, Y. The Paradoxical Role of IL-10 in Immunity and Cancer. Cancer Lett. 2015, 367, 103–107. [Google Scholar] [CrossRef]
- Kozłowski, L.; Zakrzewska, I.; Tokajuk, P.; Wojtukiewicz, M.Z. Concentration of Interleukin-6 (IL-6), Interleukin-8 (IL-8) and Interleukin-10 (IL-10) in Blood Serum of Breast Cancer Patients. Rocz. Akad. Med. Bialymst. 2003, 48, 82–84. [Google Scholar]
- Li, Y.; Yu, H.; Jiao, S.; Yang, J. Prognostic value of IL-10 expression in tumor tissues of breast cancer patients. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2014, 30, 517–520. [Google Scholar]
- Toney, N.J.; Opdenaker, L.M.; Cicek, K.; Frerichs, L.; Kennington, C.R.; Oberly, S.; Archinal, H.; Somasundaram, R.; Sims-Mourtada, J. Tumor-B-Cell Interactions Promote Isotype Switching to an Immunosuppressive IgG4 Antibody Response through Upregulation of IL-10 in Triple Negative Breast Cancers. J. Transl. Med. 2022, 20, 112. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Ammar, A.; Storr, S.J.; Green, A.R.; Rakha, E.; Ellis, I.O.; Martin, S.G. IL-6 and IL-10 Are Associated with Good Prognosis in Early Stage Invasive Breast Cancer Patients. Cancer Immunol. Immunother. 2018, 67, 537–549. [Google Scholar] [CrossRef]
- Emmerich, J.; Mumm, J.B.; Chan, I.H.; LaFace, D.; Truong, H.; McClanahan, T.; Gorman, D.M.; Oft, M. IL-10 Directly Activates and Expands Tumor-Resident CD8(+) T Cells without de Novo Infiltration from Secondary Lymphoid Organs. Cancer Res. 2012, 72, 3570–3581. [Google Scholar] [CrossRef] [PubMed]
- Kundu, N.; Beaty, T.L.; Jackson, M.J.; Fulton, A.M. Antimetastatic and Antitumor Activities of Interleukin 10 in a Murine Model of Breast Cancer. J. Natl. Cancer Inst. 1996, 88, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Naing, A.; Infante, J.R.; Papadopoulos, K.P.; Chan, I.H.; Shen, C.; Ratti, N.P.; Rojo, B.; Autio, K.A.; Wong, D.J.; Patel, M.R.; et al. PEGylated IL-10 (Pegilodecakin) Induces Systemic Immune Activation, CD8+ T Cell Invigoration and Polyclonal T Cell Expansion in Cancer Patients. Cancer Cell 2018, 34, 775–791.e3. [Google Scholar] [CrossRef]
- Naing, A.; Wong, D.J.; Infante, J.R.; Korn, W.M.; Aljumaily, R.; Papadopoulos, K.P.; Autio, K.A.; Pant, S.; Bauer, T.M.; Drakaki, A.; et al. Pegilodecakin Combined with Pembrolizumab or Nivolumab for Patients with Advanced Solid Tumours (IVY): A Multicentre, Multicohort, Open-Label, Phase 1b Trial. Lancet Oncol. 2019, 20, 1544–1555. [Google Scholar] [CrossRef]
- Ratti, N.; Shifrin, N.; McCauley, S.; Verma, R.; Van Vlasselaer, P.; Oft, M.; Leveque, J. Combination of Pegilodecakin and Docetaxel Shows Synergy in Tumor Rejection in Immune Resistant TNBC Model. Ann. Oncol. 2018, 29, viii421. [Google Scholar] [CrossRef]
- Formenti, S.C.; Lee, P.; Adams, S.; Goldberg, J.D.; Li, X.; Xie, M.W.; Ratikan, J.A.; Felix, C.; Hwang, L.; Faull, K.F.; et al. Focal Irradiation and Systemic TGFβ Blockade in Metastatic Breast Cancer. Clin. Cancer Res. 2018, 24, 2493–2504. [Google Scholar] [CrossRef]
- Yi, M.; Wu, Y.; Niu, M.; Zhu, S.; Zhang, J.; Yan, Y.; Zhou, P.; Dai, Z.; Wu, K. Anti-TGF-β/PD-L1 Bispecific Antibody Promotes T Cell Infiltration and Exhibits Enhanced Antitumor Activity in Triple-Negative Breast Cancer. J. Immunother. Cancer 2022, 10, e005543. [Google Scholar] [CrossRef]
- Lan, Y.; Yeung, T.-L.; Huang, H.; Wegener, A.A.; Saha, S.; Toister-Achituv, M.; Jenkins, M.H.; Chiu, L.-Y.; Lazorchak, A.; Tarcic, O.; et al. Colocalized Targeting of TGF-β and PD-L1 by Bintrafusp Alfa Elicits Distinct Antitumor Responses. J. Immunother. Cancer 2022, 10, e004122. [Google Scholar] [CrossRef]
- Kment, J.; Newsted, D.; Young, S.; Vermeulen, M.C.; Laight, B.J.; Greer, P.A.; Lan, Y.; Craig, A.W. Blockade of TGF-β and PD-L1 by Bintrafusp Alfa Promotes Survival in Preclinical Ovarian Cancer Models by Promoting T Effector and NK Cell Responses. Br. J. Cancer 2024, 130, 2003–2015. [Google Scholar] [CrossRef]
- Spira, A.; Awada, A.; Isambert, N.; Lorente, D.; Penel, N.; Zhang, Y.; Ojalvo, L.S.; Hicking, C.; Rolfe, P.A.; Ihling, C.; et al. Identification of HMGA2 as a Predictive Biomarker of Response to Bintrafusp Alfa in a Phase 1 Trial in Patients with Advanced Triple-Negative Breast Cancer. Front. Oncol. 2022, 12, 981940. [Google Scholar] [CrossRef]
- Egan, J.E.; Quadrini, K.J.; Santiago-Schwarz, F.; Hadden, J.W.; Brandwein, H.J.; Signorelli, K.L. IRX-2, a Novel in Vivo Immunotherapeutic, Induces Maturation and Activation of Human Dendritic Cells in Vitro. J. Immunother. 2007, 30, 624–633. [Google Scholar] [CrossRef]
- Czystowska, M.; Han, J.; Szczepanski, M.J.; Szajnik, M.; Quadrini, K.; Brandwein, H.; Hadden, J.W.; Signorelli, K.; Whiteside, T.L. IRX-2, a Novel Immunotherapeutic, Protects Human T Cells from Tumor-Induced Cell Death. Cell Death Differ. 2009, 16, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Schilling, B.; Halstead, E.S.; Schuler, P.; Harasymczuk, M.; Egan, J.E.; Whiteside, T.L. IRX-2, a Novel Immunotherapeutic, Enhances and Protects NK-Cell Functions in Cancer Patients. Cancer Immunol. Immunother. 2012, 61, 1395–1405. [Google Scholar] [CrossRef] [PubMed]
- Schilling, B.; Harasymczuk, M.; Schuler, P.; Egan, J.E.; Whiteside, T.L. IRX-2, a Novel Biologic, Favors the Expansion of T Effector over T Regulatory Cells in a Human Tumor Microenvironment Model. J. Mol. Med. 2012, 90, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Page, D.B.; Su, A.; Moxon, N.; Mellinger, S.L.; Kelly, T.; Conlin, A.K.; Topp, Z.Z.; Newell, P.; Fredrich, N.; Massimino, K.P.; et al. NeoIRX Trial: Immunologic Induction with Peri-Lymphatic Cytokines to Enhance Pembrolizumab (Pembro) Response in Stage II/III Triple-Negative Breast Cancer (TNBC). J. Clin. Oncol. 2023, 41, 604. [Google Scholar] [CrossRef]
- Jiang, Y.; Qiu, J.; Ye, N.; Xu, Y. Current Status of Cytokine-Induced Killer Cells and Combination Regimens in Breast Cancer. Front. Immunol. 2025, 16, 1476644. [Google Scholar] [CrossRef]
- Pievani, A.; Borleri, G.; Pende, D.; Moretta, L.; Rambaldi, A.; Golay, J.; Introna, M. Dual-Functional Capability of CD3+CD56+ CIK Cells, a T-Cell Subset That Acquires NK Function and Retains TCR-Mediated Specific Cytotoxicity. Blood 2011, 118, 3301–3310. [Google Scholar] [CrossRef]
- Guo, Y.; Han, W. Cytokine-Induced Killer (CIK) Cells: From Basic Research to Clinical Translation. Chin. J. Cancer 2015, 34, 6. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.; Yang, B.; Lu, S.; Du, Y.; Liu, H. Adjuvant Treatment for Triple-Negative Breast Cancer: A Retrospective Study of Immunotherapy with Autologous Cytokine-Induced Killer Cells in 294 Patients. Cancer Biol. Med. 2019, 16, 350–360. [Google Scholar] [CrossRef]
- Li, M.; Wang, Y.; Wei, F.; An, X.; Zhang, N.; Cao, S.; Ren, B.; Zhang, X.; Ren, X. Efficiency of Cytokine-Induced Killer Cells in Combination with Chemotherapy for Triple-Negative Breast Cancer. J. Breast Cancer 2018, 21, 150–157. [Google Scholar] [CrossRef]
- Ngo, H.T.; Phamb, P.V. Breast Cancer Treatment by Transplantations of Dendritic Cells and Cytokine-Induced Killer Cells: An Update on Clinical Trials. Biomed. Res. Ther. 2021, 8, 4700–4717. [Google Scholar] [CrossRef]
- Wang, X.; Ren, J.; Zhang, J.; Yan, Y.; Jiang, N.; Yu, J.; Di, L.; Song, G.; Che, L.; Jia, J.; et al. Prospective Study of Cyclophosphamide, Thiotepa, Carboplatin Combined with Adoptive DC-CIK Followed by Metronomic Cyclophosphamide Therapy as Salvage Treatment for Triple Negative Metastatic Breast Cancers Patients (Aged < 45). Clin. Transl. Oncol. 2016, 18, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Weng, D.; Pan, Q.; Xiang, T.; Yang, C.; Wu, Z.; Li, M.; Xie, S.; Tang, Y.; Xia, J.; et al. Adjuvant Alternative Cytokine-Induced Killer Cell Combined with Natural Killer Cell Immunotherapy Improves the Prognosis of Post-Mastectomy Breast Cancer. Front. Immunol. 2022, 13, 974487. [Google Scholar] [CrossRef] [PubMed]
- Sommaggio, R.; Cappuzzello, E.; Dalla Pietà, A.; Tosi, A.; Palmerini, P.; Carpanese, D.; Nicolè, L.; Rosato, A. Adoptive Cell Therapy of Triple Negative Breast Cancer with Redirected Cytokine-Induced Killer Cells. Oncoimmunology 2020, 9, 1777046. [Google Scholar] [CrossRef]
- Wu, C.-C.; Pan, M.-R.; Shih, S.-L.; Shiau, J.-P.; Wu, C.-C.; Chang, S.-J.; Kao, C.-N.; Chen, F.-M.; Hou, M.-F.; Luo, C.-W. Combination of FAK Inhibitor and Cytokine-Induced Killer Cell Therapy: An Alternative Therapeutic Strategy for Patients with Triple-Negative Breast Cancer. Biomed. Pharmacother. 2023, 163, 114732. [Google Scholar] [CrossRef]
- Silveira, C.R.F.; Corveloni, A.C.; Caruso, S.R.; Macêdo, N.A.; Brussolo, N.M.; Haddad, F.; Fernandes, T.R.; de Andrade, P.V.; Orellana, M.D.; Guerino-Cunha, R.L. Cytokines as an Important Player in the Context of CAR-T Cell Therapy for Cancer: Their Role in Tumor Immunomodulation, Manufacture, and Clinical Implications. Front. Immunol. 2022, 13, 947648. [Google Scholar] [CrossRef]
- Chamorro, D.F.; Somes, L.K.; Hoyos, V. Engineered Adoptive T-Cell Therapies for Breast Cancer: Current Progress, Challenges, and Potential. Cancers 2023, 16, 124. [Google Scholar] [CrossRef]
- Bui, T.A.; Mei, H.; Sang, R.; Ortega, D.G.; Deng, W. Advancements and Challenges in Developing in Vivo CAR T Cell Therapies for Cancer Treatment. eBioMedicine 2024, 106, 105266. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, Y.; Liu, W.; Li, X. Engineered IL-7 Receptor Enhances the Therapeutic Effect of AXL-CAR-T Cells on Triple-Negative Breast Cancer. Biomed. Res. Int. 2020, 2020, 4795171. [Google Scholar] [CrossRef] [PubMed]
- Marei, H.E.; Bedair, K.; Hasan, A.; Al-Mansoori, L.; Caratelli, S.; Sconocchia, G.; Gaiba, A.; Cenciarelli, C. Current Status and Innovative Developments of CAR-T-Cell Therapy for the Treatment of Breast Cancer. Cancer Cell Int. 2025, 25, 3. [Google Scholar] [CrossRef] [PubMed]
- Stüber, T.; Monjezi, R.; Wallstabe, L.; Kühnemundt, J.; Nietzer, S.L.; Dandekar, G.; Wöckel, A.; Einsele, H.; Wischhusen, J.; Hudecek, M. Inhibition of TGF-β-Receptor Signaling Augments the Antitumor Function of ROR1-Specific CAR T-Cells against Triple-Negative Breast Cancer. J. Immunother. Cancer 2020, 8, e000676. [Google Scholar] [CrossRef]
- Nasiri, F.; Kazemi, M.; Mirarefin, S.M.J.; Mahboubi Kancha, M.; Ahmadi Najafabadi, M.; Salem, F.; Dashti Shokoohi, S.; Evazi Bakhshi, S.; Safarzadeh Kozani, P.; Safarzadeh Kozani, P. CAR-T Cell Therapy in Triple-Negative Breast Cancer: Hunting the Invisible Devil. Front. Immunol. 2022, 13, 1018786. [Google Scholar] [CrossRef]
- Balkhi, S.; Zuccolotto, G.; Di Spirito, A.; Rosato, A.; Mortara, L. CAR-NK Cell Therapy: Promise and Challenges in Solid Tumors. Front. Immunol. 2025, 16, 1574742. [Google Scholar] [CrossRef]
- Raftery, M.J.; Franzén, A.S.; Radecke, C.; Boulifa, A.; Schönrich, G.; Stintzing, S.; Blohmer, J.-U.; Pecher, G. Next Generation CD44v6-Specific CAR-NK Cells Effective against Triple Negative Breast Cancer. Int. J. Mol. Sci. 2023, 24, 9038. [Google Scholar] [CrossRef]
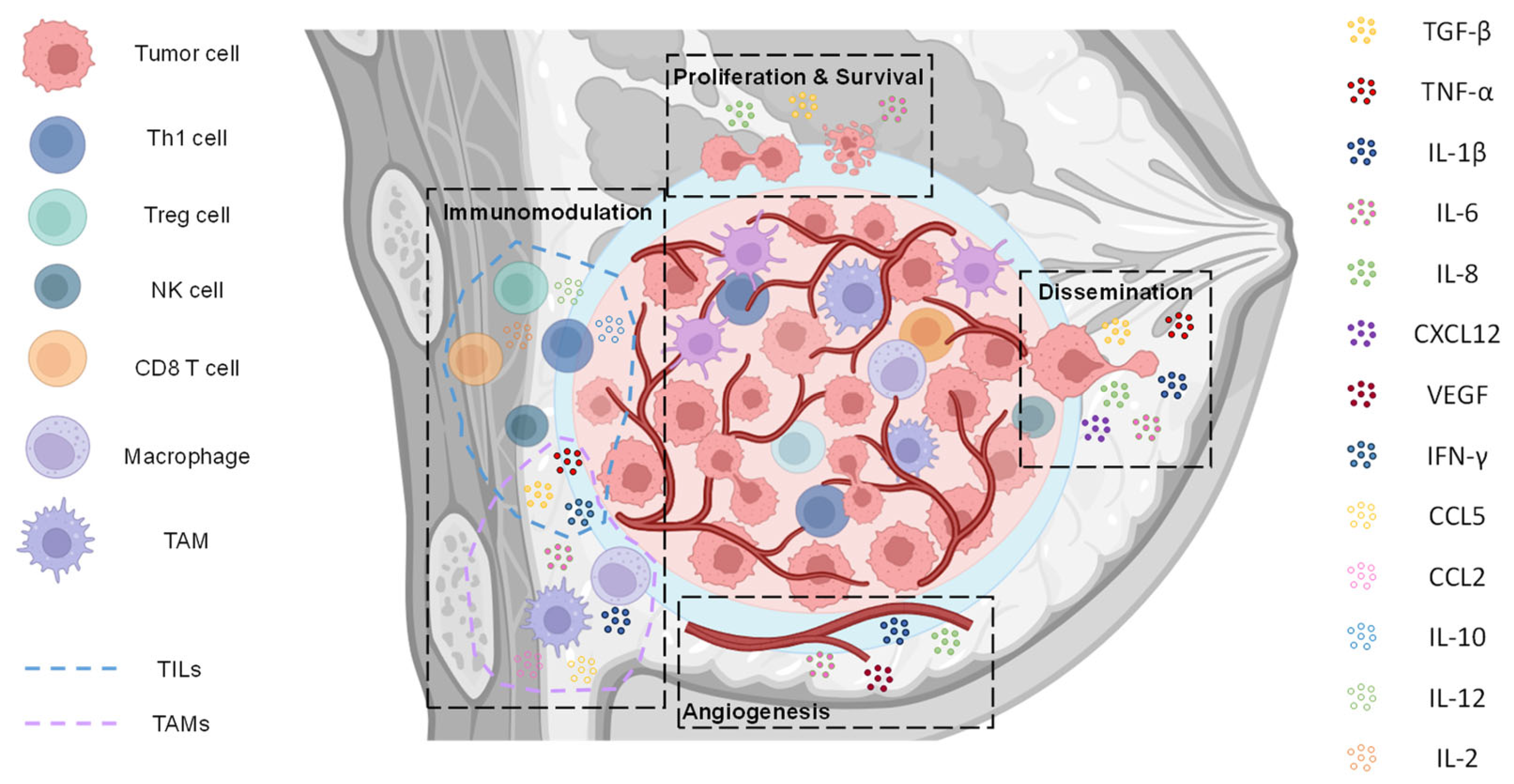
| Cytokine | Clinical Trial ID | Treatment | Phase Trial Stage | Status | Results | Disease Setting |
|---|---|---|---|---|---|---|
| IL-6 | NCT05846789 | SOC chemotherapy ± Tocilizumab | Phase II | Recruiting | No results posted | mTNBC or ER low BC |
| NCT06008275 | Neratinib + Ruxolitinib | Phase I | Recruiting | No results posted | mTNBC | |
| NCT02876302 | Ruxolitinib ± Paclitaxel ± Doxorubicin ± Cyclophosphamide | Phase II | Active, not recruiting | 1st R + 2nd P pCR: 83.3%; DFS: 66.7%; OS:50% R + P pCR: 94.1%; DFS: 53.3%; OS: 29.4% | TNBC | |
| NCT02041429 | Ruxolitinib + Paclitaxel | Phase I/II | Completed | R + P: MTD: 25 mg bid RP2D: 15 mg bid 68% of patients required dose reductions; PR: 21%; SD: 63%; DP: 16% | HER2-negative mBC | |
| IL-8 | NCT02001974 | Paclitaxel + Reparixin | Phase I | Completed | Group 1: P + R oral 400 mg tid; CBR: 25.0%; 6-mths PFS: 25%; TTP: 58 Group 2: P + R oral increase to 800 mg tid; CBR: 33.3%; 6-mths PFS: 0%; TTP: 67 Group 3: P + R increase to 1200 mg tid; CBR: 56.5%; 6-mths PFS: 17.4%; TTP: 170 | HER2-negative mBC |
| NCT02370238 | Paclitaxel + Reparixin | Phase II | Completed | Weekly administration is safe and tolerable | mTNBC | |
| TGF-β | NCT03012230 | Ruxolitinib + Pembrolizumab | Phase I | Completed | No results posted | TNBC |
| NCT02672475 | Galunisertib + Paclitaxel | Phase I | Completed | No results posted | Metastatic AR-negative TNBC | |
| NCT02725489 | Vigil + Durvalumab | Phase II | Completed | No results posted | Advanced/metastatic women’s cancers | |
| NCT01401062 | Fresolimumab + radiotherapy | Phase II | Completed | Good immune response, increase in CD8 T cells, and longer median OS | mBC | |
| NCT02947165 | NIS793 + PDR001 | Phase I/Ib | Completed | No results posted | Advanced/metastatic tumors, including TNBC | |
| IL-1 | NCT02900664 | PDR001 in combination with CJM112, EGF816, Canakinumab or Trametinib | Phase Ib | Completed | No results posted | Advanced/metastatic cancer, including TNBC |
| NCT01802970 | Anakinra ± Nab-paclitaxel ± Capecitabine ± Eribulin ± Vinorelbine | Phase Ib | Completed | No results posted | HER2-negative mBC | |
| NCT03742349 | Spartalizumab + LAG525 ± NIR178 ± Capmatinib ± MCS110 ± Canakinumab | Phase Ib | Terminated | No results posted | Advanced or mTNBC | |
| NCT06710197 | Anakinra | Phase II | Recruiting | No results posted | Early-stage TNBC and ER-low BC | |
| CXCL12 | NCT05103917 | X4P-001 + Toriplimab | Phase I/II | Unknown status | No results posted | Locally advanced/mTNBC/ |
| Clinical Trial ID | Treatment | Phase Trial Stage | Status | Results | Disease Setting |
|---|---|---|---|---|---|
| NCT00281697 | Bevacizumab + Taxane vs. Taxane | Phase III | Completed | PFS 6 mths vs. 2.7 mths; OS 17.9 mths vs. 12.6 mths; ORR 41% vs. 18% | mTNBC |
| NCT00691379 | Bevacizumab + Paclitaxel + Carboplatin | Phase I/II | Completed | ORR 65.2%; PFS 10.3 mths; OS 25.7 mths | mTNBC |
| NCT03577743 | Bevacizumab + Carboplatin + Paclitaxel | Phase II | Completed | PFS 27 mths; OS 55 mths; | mTNBC |
| NCT00479674 | Bevacizumab + Nab-paclitaxel + Carboplatin | Phase II | Completed | CR 18%; PR 69%; PFS 15 mths | mTNBC (Stage IV or inoperable Stage III) |
| NCT00618657 | Bevacizumab + Carboplatin + Nab-paclitaxel | Phase II | Completed | PFS 93% 2 years; 17.6% pCR | Stages I–III TNBC |
| NCT01094184 | Bevacizumab + Paclitaxel + Docetaxel | Phase IV | Completed | OS 11.6 mths (10 mg/kg) 18.9 mths (15 mg/kg); PFS 6.7 mths (10 mg/kg) 7.9 mths (15 mg/kg) | Advanced TNBC |
| NCT00733408 | Bevacizumab + Nab-Paclitaxel + Erlotinib | Phase II | Completed | PFS 9.1 mths; OS 18.1 mths; PR 74% | mTNBC |
| NCT00528567 | Bevacizumab + Chemotherapy vs. Chemotherapy | Phase III | Completed | IDFS: 31.5 mths vs. 21 mths; no difference OS | Operable primary invasive TNBC |
| NCT00861705 | Bevacizumab ± Doxorubicin + Cyclophosphamide Paclitaxel ± Carboplatin | Phase II | Active | ORR 32%; PFS 5.1 mths; DR 12.1 mths; OS 11.4 mths | Previously treated TNBC |
| NCT03394287 | Apatinib (daily or intermittent) + Camrelizumab | Phase II | Completed | ORR 43.3% (daily), PFS 3.7 mths vs. 1.9 mths; DCR 63.3% vs. 40% | Advanced TNBC |
| NCT01176669 | Apatinib | Phase II | Completed | PFS 3.8 mths; OS 10.6 mths; OR 17.5% | Pretreated mTNBC |
| NCT03945604 | Apatinib + Camrelizumab + Fuzuloparib | Phase Ib | Completed | ORR 6.9%; PFS 5.2 mths; OS 64.2% 12 mths | Recurrent and mTNBC |
| NCT04303741 | Apatinib + Camrelizumab + Eribulin | Phase II | Completed | ORR 37%; PFS 8.1 mths; | Advanced TNBC |
| NCT03243838 | Apatinib + Docetaxel + Epirubicin + Cyclophosphamide | Phase II | Completed | pCR 54.8%; OR: 93.5%; PFS 90.9% 24 mths; OS 94.4% 24 mths | Untreated stages IIA-IIIB TNBC |
| NCT03735082 | Apatinib ± Paclitaxel ± Carboplatin | Phase II | Completed | ORR 88%; pCR: 60.9% vs. 30.4% | Locally advanced TNBC |
| NCT03797326 | Lenvatinib + Pembrolizumab | Phase II | Completed | ORR 32%; PFS 5.1 mths; DR 12.1 mths; OS 11.4 mths | Advanced previously treated TNBC |
| NCT04427293 | Lenvatinib + Pembrolizumab | Phase I | Recruiting | / | Untreated TNBC |
| NCT06849492 | Lenvatinib + Camrelizumab + Nab-paclitaxel | Phase II | Active | / | Advanced first-Line TNBC |
| NCT06140576 | Lenvatinib + Sindilimab + Nab-paclitaxel | Phase Ib/IIa | Active | / | Recurrent and mTNBC |
| NCT03251378 | Fruquintinib | Phase I | Completed | PFS 38.46% 29 mths | mTNBC |
| NCT04577963 | Fruquintinib + Tislelizumab | Phase Ib/2 | Completed | No results posted | Advanced/mTNBC |
| NCT00246571 | Sunitinib vs. chemotherapy (Capecitabine/Vinorelbine/Docetaxel/Paclitaxel/Gemcitabine) | Phase II | Completed | PFS 1.7 mths vs. 2.5 mths SOC; ORR 8.8 vs. 11.5%; OS 9.4 vs. 10.5 mths | Previously advanced treated TNBC |
| Cytokine | Clinical Trial ID | Treatment | Phase Trial Stage | Status | Results | Disease Setting |
|---|---|---|---|---|---|---|
| CCL2 | NCT04265872 | Bortezomib + Pembrolizumab + Cisplatin | Phase I | Recruiting | / | mTNBC |
| CCL5 | NCT03838367 | Leronlimab + Carboplatin | Phase Ib/II | Active, not recruiting | Results submitted, not reviewed | mTNBC |
| NCT04313075 | Leronlimab + TPC | Compassionate Use | No longer available | No results posted | mTNBC | |
| NCT04504942 | Leronlimab | Phase II | Unknown status | No results posted | Locally advanced/mTNBC | |
| TNF-α | NCT04004910 | Immunopheresis® LW-02 column | Phase I/II | Unknown status | No results posted | Advanced/refractory BC |
| IFN-α | NCT05756166 | Rintatolimod, Celecoxib, and IFN-α 2b + Pembrolizumab | Phase I/IIa | Terminated | No results posted | mTNBC/unresectable TNBC |
| IFN-γ | NCT02614456 | Interferon-gamma and Nivolumab | Phase I | Completed | No results posted | mTNBC |
| IL-2 | NCT02983045 | Bempegal desleukin + Nivolumab | PIVOT-02, Phase I | Terminated | ORR of 21% in the >2/3L metastatic patients, with an ORR of 23% in >2/3L metastatic patients with PD-L1 negative baseline. Patients with known pre-treatment PD-L1 status: ORR 14% in PD-L1 negative patients and 17% in PD-L1 positive patients. DCR 45% | Locally advanced/mTNBC |
| NCT03435640 | Bempegal desleukin + NKTR-262 TLR 7/8 agonist + Nivolumab | REVEAL, Phase Ib | Terminated | ORR 14.3% significant expansion of activated CD8+ T cells in BEMPEG + NKTR-262 | Locally advanced/mTNBC | |
| IL-12 | NCT02531425 | IT-pIL12-EP | OMS-I140, Phase I | Completed | No results posted | Locally advanced, inoperable, mTNBC/or treatment-refractory TNBC |
| NCT03567720 | IT-pIL12-EP + Pembrolizumab | KEYNOTE-890, Phase II | Unknown | ORR 17.4%, median DCR 16.6 mths, median OS 11 mths | Locally advanced/mTNBC | |
| IL-10 | NCT02009449 | Pegilodecakin + Platinum and taxane-based chemotherapy1 or platinum and gemcitabine chemotherapy2 | Phase I/Ib | Completed | ORR 28.6% plus chemotherapy1; DCR 25% monotherapy vs. 71.4% chemotherapy1 vs. 55.6 chemotherapy2; mPFS 2 mths vs. 3.9 mths chemotherapy1 vs. 3.7 mths chemotherapy2; mOS 5.3 mths vs. 11.2 mths chemotherapy1 vs. 6.8 mths chemotherapy2 | Advanced solid tumors |
| Cytokine/s | Clinical Trial ID | Treatment | Phase Trial Stage | Status | Results | Disease Setting |
|---|---|---|---|---|---|---|
| TGF-β | NCT04489940 | Bintrafusp Alfa | Phase II | Terminated | No results posted | mTNBC |
| NCT04296942 | Bintrafusp Alfa + BN-brachyury | BrEAsT, Phase Ib | Terminated | No results posted | Advanced BC | |
| NCT03579472 | Bintrafusp Alfa + Eribulin mesylate | Phase I | Terminated | No results posted | mTNBC | |
| NCT04789668 | Bintrafusp Alfa + pimasertib | Phase I/II | Completed | No results posted | Brain metastatic, including TNBC | |
| VEGF | NCT06449222 | BNT327 + Nab-paclitaxel ± Carboplatin ± Gemcitabine ± Paclitaxel ± Eribulin | Phase II | Recruiting | / | Locally advanced/mTNBC |
| NCT06724263 | B1962 Injection | Phase II | Active | / | Locally advanced/mTNBC | |
| IL2, IL1β, IFN-γ, and TNF-α | NCT04373031 | IRX-2 + Pembrolizumab | neoIRX, Phase II | Active, not recruiting | pCR 83% vs. 33% pembro alone | Stages II/III TNBC |
| CIK | NCT01395056 | CIK + Chemotherapy | Observational | Completed | Prevented disease recurrence and prolonged survival | mTNBC |
| NCT01232062 | CIK + High-Dose Chemotherapy | Observational | Completed | Time to disease progression 6 to 12 months; survival rates 1 year | mTNBC | |
| TRUCK | NCT04099797 | C7R-GD2.CAR T cells + Lymphodepletion | Phase I | Completed | PR 29%; SD 71%; cytokine release syndrome Grade 1–Grade 4 (75%); TIAN 88%; neurological improvement 90% | GD2-expressing brain tumors (GAIL-B) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosado-Sanz, M.; Martínez-Alarcón, N.; Abellán-Soriano, A.; Golfe, R.; Trinidad, E.M.; Font de Mora, J. Cytokine Networks in Triple-Negative Breast Cancer: Mechanisms, Therapeutic Targets, and Emerging Strategies. Biomedicines 2025, 13, 1945. https://doi.org/10.3390/biomedicines13081945
Rosado-Sanz M, Martínez-Alarcón N, Abellán-Soriano A, Golfe R, Trinidad EM, Font de Mora J. Cytokine Networks in Triple-Negative Breast Cancer: Mechanisms, Therapeutic Targets, and Emerging Strategies. Biomedicines. 2025; 13(8):1945. https://doi.org/10.3390/biomedicines13081945
Chicago/Turabian StyleRosado-Sanz, María, Nuria Martínez-Alarcón, Adrián Abellán-Soriano, Raúl Golfe, Eva M. Trinidad, and Jaime Font de Mora. 2025. "Cytokine Networks in Triple-Negative Breast Cancer: Mechanisms, Therapeutic Targets, and Emerging Strategies" Biomedicines 13, no. 8: 1945. https://doi.org/10.3390/biomedicines13081945
APA StyleRosado-Sanz, M., Martínez-Alarcón, N., Abellán-Soriano, A., Golfe, R., Trinidad, E. M., & Font de Mora, J. (2025). Cytokine Networks in Triple-Negative Breast Cancer: Mechanisms, Therapeutic Targets, and Emerging Strategies. Biomedicines, 13(8), 1945. https://doi.org/10.3390/biomedicines13081945





