Is the Activation of the Postsynaptic Ligand Gated Glycine- or GABAA Receptors Essential for the Receptor Clustering at Inhibitory Synapses?
Abstract
1. Introduction
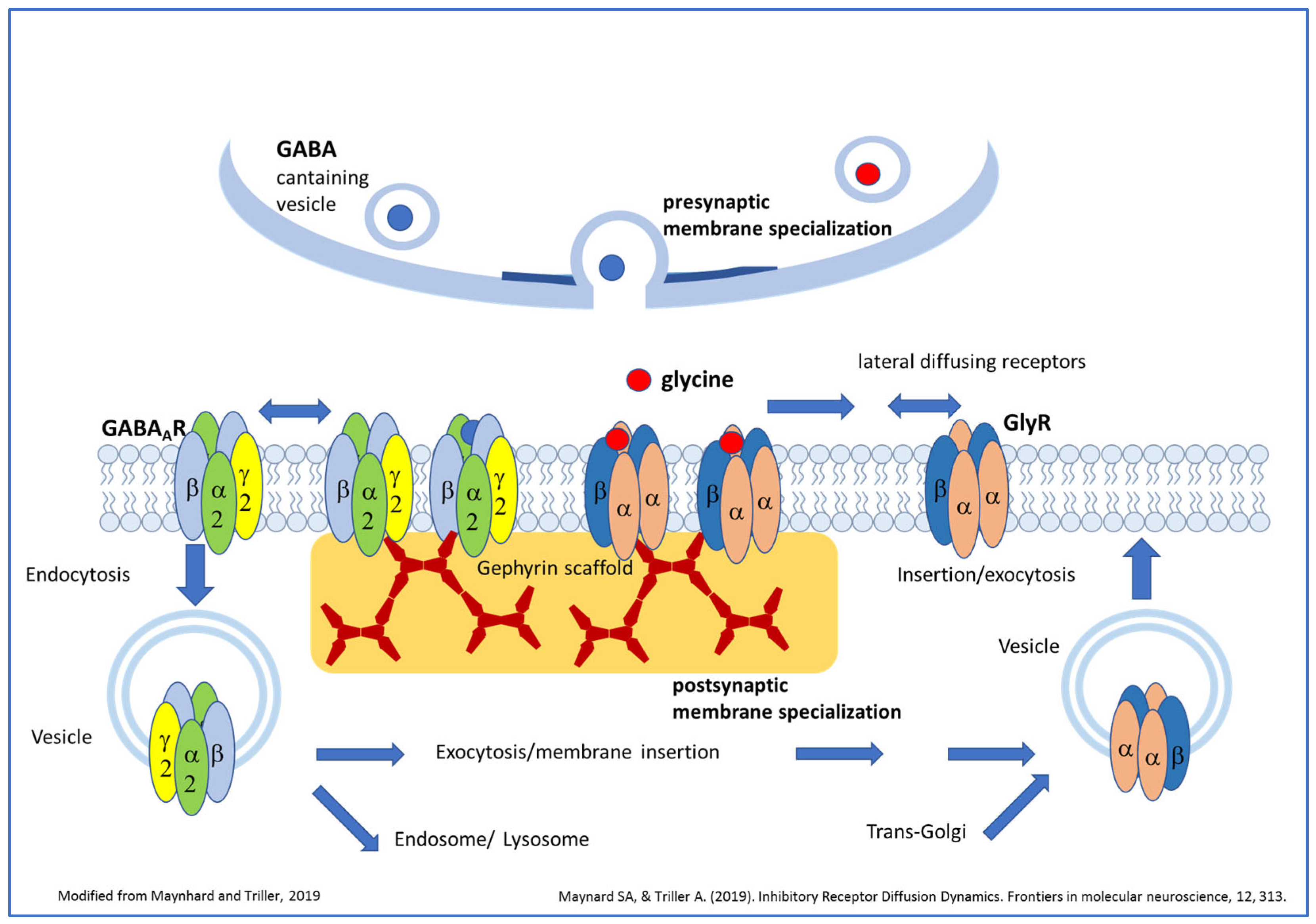
2. Postsynaptic Clustering of the Strychnine-Sensitive GlyRs in Early Developmental Stages In Vitro
3. Postsynaptic Clustering of GlyRs in Early Developmental Stages In Vivo
4. Postsynaptic GlyR Clustering in Mature Neurons In Vitro
5. Gephyrin Cluster Formation at Glycinergic Synapses
6. Postsynaptic Clustering of the GABAA Receptors In Vitro and In Vivo
7. Gephyrin Clustering at GABAergic Synapses
8. Limitations and Perspectives
9. Clinical Relevance
10. Conclusions
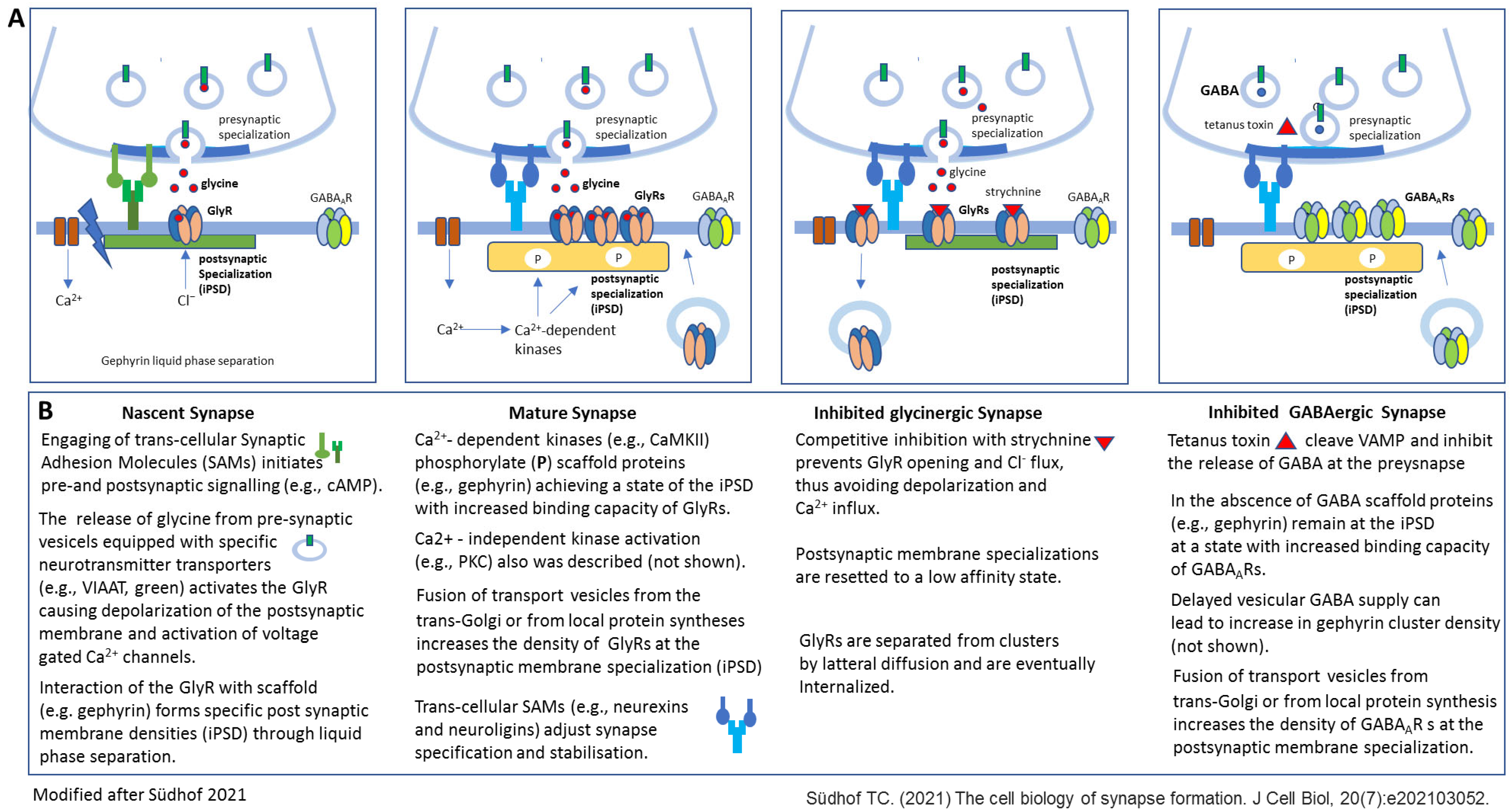
- The neurotransmitter-dependent activation of GlyRs seems to be essential for GlyR cluster formation, whereas several publications support that in the case of GABAAR cluster formation, the ligand-dependent activation is not essential for the initial postsynaptic clustering of these receptors (Figure 1 and Figure 2). Whether or not the capacity of GABAARs to form clusters independently from GABA binding is relevant under certain physiological conditions remains to be further elucidated.
- It is very likely that under physiological conditions, the release of the neurotransmitter on both synapse types could regulate the postsynaptic receptor cluster formation: both transmitters are able to induce the formation of new GlyR or GABAAR clusters, and both transmitters seem to be sufficient to modulate the size of existing GlyRs or GABAAR clusters, respectively.
- Glycine seems to be essential for the maintenance of existing GlyRs, whereas GABA seems to be dispensable for the maintenance of GABAAR clusters. Again, the interesting question of whether the independence of GABAAR clusters from continuous GABA activation is an important distinct feature of GABAergic synapses in comparison to GlyRs with relevancy in certain physiological conditions cannot be answered based on present knowledge.
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AMPA | α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| cAMP | cyclic adenosine monophosphate |
| CaMKII | Ca2+/calmodulin-dependent protein kinase type II |
| CDK5 | cyclin-dependent kinase 5 |
| Clptm1 | cleft lip and palate transmembrane protein 1 |
| 3D-SIM | 3D structured illumination microscope |
| dSTORM | direct Stochastic Optical Reconstruction Microscopy |
| EPSC | excitatory postsynaptic currents |
| GAD | glutamic acid decarboxylase |
| GABA | γ-aminobutyric acid |
| GABAAR | γ-aminobutyric acid A-receptors |
| GARLH | GABAAR regulatory LHPFL family protein |
| GyR | glycine receptor |
| GSK3β | glycogen Synthase Kinase 3 beta |
| HFU | High-frequency uncaging |
| iPSD | inhibitory postsynaptic density |
| IPSC | inhibitory postsynaptic currents |
| LHFPL4 | lipoma HMGIC fusion partner-like 4 |
| Munc18-1 | mammal unc-18-1 (Munc18-1 or n-Sec1; herein M18) |
| NLG2 | neuroligin 2 |
| NMDA | N-methyl D-aspartate |
| PKA | protein kinase A |
| PKC | protein kinase C |
| RER | rough endoplasmic reticulum |
| SIM | Structured Illumination Microscopy |
| Shisa-7 | Shisa family member 7 |
| SNARE | soluble N-ethylmaleimide-sensitive factor attachment protein receptor |
| SSD | subsynaptic domain |
| uIPSCs | uncaging-evoked inhibitory postsynaptic currents |
| VIAAT | Vesicular inhibitory amino acid transporter |
| VAMP2 | Vesicle-associated membrane protein 2 |
References
- Nusser, Z.; Hájos, N.; Somogyi, P.; Mody, I. Increased number of synaptic GABA(A) receptors underlies potentiation at hippocampal inhibitory synapses. Nature 1998, 395, 172–177. [Google Scholar] [CrossRef]
- Kilman, V.; van Rossum, M.C.; Turrigiano, G.G. Activity deprivation reduces miniature IPSC amplitude by decreasing the number of postsynaptic GABA(A) receptors clustered at neocortical synapses. J. Neurosci. 2002, 22, 1328–1337. [Google Scholar] [CrossRef]
- Barberis, A. Postsynaptic plasticity of GABAergic synapses. Neuropharmacology 2020, 169, 107643. [Google Scholar] [CrossRef]
- Chiu, C.Q.; Martenson, J.S.; Yamazaki, M.; Natsume, R.; Sakimura, K.; Tomita, S.; Tavalin, S.J.; Higley, M.J. Input-Specific NMDAR-Dependent Potentiation of Dendritic GABAergic Inhibition. Neuron 2018, 97, 368–377.e3. [Google Scholar] [CrossRef] [PubMed]
- Welle, T.M.; Rajgor, D.; Garcia, J.D.; Kareemo, D.; Zych, S.M.; Gookin, S.E.; Martinez, T.P.; Dell’Acqua, M.L.; Ford, C.P.; Kennedy, M.J.; et al. miRNA-mediated control of gephyrin synthesis drives sustained inhibitory synaptic plasticity. EMBO Rep. 2024, 25, 5141–5168. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, N.; Roemer, V.; Janzen, D.; Villmann, C. Impaired Glycine Receptor Trafficking in Neurological Diseases. Front. Mol. Neurosci. 2018, 11, 291. [Google Scholar] [CrossRef] [PubMed]
- Luscher, B.; Fuchs, T.; Kilpatrick, C.L. GABAA receptor trafficking-mediated plasticity of inhibitory synapses. Neuron 2011, 70, 385–409. [Google Scholar] [CrossRef]
- Südhof, T.C. The cell biology of synapse formation. J. Cell Biol. 2021, 220, e202103052. [Google Scholar] [CrossRef]
- Qi, C.; Luo, L.D.; Feng, I.; Ma, S. Molecular mechanisms of synaptogenesis. Front. Synaptic Neurosci. 2022, 14, 939793. [Google Scholar] [CrossRef]
- Welle, T.M.; Smith, K.R. Release your inhibitions: The cell biology of GABAergic postsynaptic plasticity. Curr. Opin. Neurobiol. 2025, 90, 102952. [Google Scholar] [CrossRef]
- Betz, H. Ligand-gated ion channels in the brain: The amino acid receptor superfamily. Neuron 1990, 5, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Mizzi, N.; Blundell, R. Glycine receptors: Structure, function, and therapeutic implications. Mol. Aspects Med. 2025, 103, 101360. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Sun, D. GABA receptors in brain development, function, and injury. Metab. Brain Dis. 2015, 30, 367–379. [Google Scholar] [CrossRef]
- Gravielle, M.C. Regulation of GABAA Receptors Induced by the Activation of L-Type Voltage-Gated Calcium Channels. Membranes 2021, 11, 486. [Google Scholar] [CrossRef] [PubMed]
- Rostas, J.A.P.; Skelding, K.A. Calcium/Calmodulin-Stimulated Protein Kinase II (CaMKII): Different Functional Outcomes from Activation, Depending on the Cellular Microenvironment. Cells 2023, 12, 401. [Google Scholar] [CrossRef]
- Maynard, S.A.; Triller, A. Inhibitory Receptor Diffusion Dynamics. Front. Mol. Neurosci. 2019, 12, 313. [Google Scholar] [CrossRef]
- Tyagarajan, S.K.; Fritschy, J.M. Gephyrin: A master regulator of neuronal function? Nat. Rev. Neurosci. 2014, 15, 141–156. [Google Scholar] [CrossRef]
- Groeneweg, F.L.; Trattnig, C.; Kuhse, J.; Nawrotzki, R.A.; Kirsch, J. Gephyrin: A key regulatory protein of inhibitory synapses and beyond. Histochem. Cell Biol. 2018, 150, 489–508. [Google Scholar] [CrossRef]
- Lévi, S.; Vannier, C.; Triller, A. Strychnine-sensitive stabilization of postsynaptic glycine receptor clusters. J. Cell Sci. 1998, 111 Pt 3, 335–345. [Google Scholar] [CrossRef]
- Kirsch, J.; Betz, H. Glycine-receptor activation is required for receptor clustering in spinal neurons. Nature 1998, 392, 717–720. [Google Scholar] [CrossRef]
- Du, J.; Lü, W.; Wu, S.; Cheng, Y.; Gouaux, E. Glycine receptor mechanism elucidated by electron cryo-microscopy. Nature 2015, 526, 224–229. [Google Scholar] [CrossRef]
- Zhu, H. Structure and Mechanism of Glycine Receptor Elucidated by Cryo-Electron Microscopy. Front. Pharmacol. 2022, 13, 925116. [Google Scholar] [CrossRef] [PubMed]
- Betz, H.; Kuhse, J.; Fischer, M.; Schmieden, V.; Laube, B.; Kuryatov, A.; Langosch, D.; Meyer, G.; Bormann, J.; Rundström, N.; et al. Structure, diversity and synaptic localization of inhibitory glycine receptors. J. Physiol. 1994, 88, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Barron, S.E.; Guth, P.S. Uses and limitations of strychnine as a probe in neurotransmission. Trends Pharmacol. Sci. 1987, 8, 204–206. [Google Scholar] [CrossRef]
- Rasmussen, H.; Rasmussen, T.; Triller, A.; Vannier, C. Strychnine-blocked glycine receptor is removed from synapses by a shift in insertion/degradation equilibrium. Mol. Cell. Neurosci. 2002, 19, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, I.; Miki, M.; Asakawa, K.; Kawakami, K.; Oda, Y.; Hirata, H. Glycinergic transmission and postsynaptic activation of CaMKII are required for glycine receptor clustering in vivo. Genes Cells Devoted Mol. Cell. Mech. 2013, 18, 211–224. [Google Scholar] [CrossRef]
- Flores, C.E.; Nikonenko, I.; Mendez, P.; Fritschy, J.M.; Tyagarajan, S.K.; Muller, D. Activity-dependent inhibitory synapse remodeling through gephyrin phosphorylation. Proc. Natl. Acad. Sci. USA 2015, 112, E65–E72. [Google Scholar] [CrossRef]
- Ogino, K.; Yamada, K.; Nishioka, T.; Oda, Y.; Kaibuchi, K.; Hirata, H. Phosphorylation of Gephyrin in Zebrafish Mauthner Cells Governs Glycine Receptor Clustering and Behavioral Desensitization to Sound. J. Neurosci. 2019, 39, 8988–8997. [Google Scholar] [CrossRef]
- Nakahata, Y.; Eto, K.; Murakoshi, H.; Watanabe, M.; Kuriu, T.; Hirata, H.; Moorhouse, A.J.; Ishibashi, H.; Nabekura, J. Activation-Dependent Rapid Postsynaptic Clustering of Glycine Receptors in Mature Spinal Cord Neurons. eNeuro 2017, 4, e0194-16.2017. [Google Scholar] [CrossRef]
- Lipp, P.; Reither, G. Protein kinase C: The “masters” of calcium and lipid. Cold Spring Harb. Perspect. Biol. 2011, 3, a004556. [Google Scholar] [CrossRef]
- Newton, A.C. Protein kinase C: Perfectly balanced. Crit. Rev. Biochem. Mol. Biol. 2018, 53, 208–230. [Google Scholar] [CrossRef]
- Breitinger, U.; Bahnassawy, L.M.; Janzen, D.; Roemer, V.; Becker, C.M.; Villmann, C.; Breitinger, H.G. PKA and PKC Modulators Affect Ion Channel Function and Internalization of Recombinant Alpha1 and Alpha1-Beta Glycine Receptors. Front. Mol. Neurosci. 2018, 11, 154. [Google Scholar] [CrossRef]
- Specht, C.G.; Grünewald, N.; Pascual, O.; Rostgaard, N.; Schwarz, G.; Triller, A. Regulation of glycine receptor diffusion properties gephyrin interactions by protein kinase C. EMBO J. 2011, 30, 3842–3853. [Google Scholar] [CrossRef] [PubMed]
- Uezu, A.; Kanak, D.J.; Bradshaw, T.W.; Soderblom, E.J.; Catavero, C.M.; Burette, A.C.; Weinberg, R.J.; Soderling, S.H. Identification of an elaborate complex mediating postsynaptic inhibition. Science 2016, 353, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Krueger-Burg, D. Understanding GABAergic synapse diversity and its implications for GABAergic pharmacotherapy. Trends Neurosci. 2025, 48, 47–61. [Google Scholar] [CrossRef]
- Kasaragod, V.B.; Schindelin, H. Structure-Function Relationships of Glycine and GABAA Receptors and Their Interplay With the Scaffolding Protein Gephyrin. Front. Mol. Neurosci. 2018, 11, 317. [Google Scholar] [CrossRef]
- Bai, G.; Wang, Y.; Zhang, M. Gephyrin-mediated formation of inhibitory postsynaptic density sheet via phase separation. Cell Res. 2021, 31, 312–325. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Kim, S.; Hwang, D.E.; Eom, Y.G.; Jang, G.; Park, H.Y.; Choi, J.M.; Ko, J.; Shin, Y. Thermodynamic modulation of gephyrin condensation by inhibitory synapse components. Proc. Natl. Acad. Sci. USA 2024, 121, e2313236121. [Google Scholar] [CrossRef]
- Zacchi, P.; Antonelli, R.; Cherubini, E. Gephyrin phosphorylation in the functional organization and plasticity of GABAergic synapses. Front. Cell. Neurosci. 2014, 8, 103. [Google Scholar] [CrossRef]
- Pizzarelli, R.; Griguoli, M.; Zacchi, P.; Petrini, E.M.; Barberis, A.; Cattaneo, A.; Cherubini, E. Tuning GABAergic Inhibition: Gephyrin Molecular Organization and Functions. Neuroscience 2020, 439, 125–136. [Google Scholar] [CrossRef]
- Zhou, L.; Kiss, E.; Demmig, R.; Kirsch, J.; Nawrotzki, R.A.; Kuhse, J. Binding of gephyrin to microtubules is regulated by its phosphorylation at Ser270. Histochem. Cell Biol. 2021, 156, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Kuhse, J.; Kalbouneh, H.; Schlicksupp, A.; Mükusch, S.; Nawrotzki, R.; Kirsch, J. Phosphorylation of gephyrin in hippocampal neurons by cyclin-dependent kinase CDK5 at Ser-270 is dependent on collybistin. J. Biol. Chem. 2012, 287, 30952–30966. [Google Scholar] [CrossRef]
- Maas, C.; Belgardt, D.; Lee, H.K.; Heisler, F.F.; Lappe-Siefke, C.; Magiera, M.M.; van Dijk, J.; Hausrat, T.J.; Janke, C.; Kneussel, M. Synaptic activation modifies microtubules underlying transport of postsynaptic cargo. Proc. Natl. Acad. Sci. USA 2009, 106, 8731–8736. [Google Scholar] [CrossRef]
- Rao, A.; Cha, E.M.; Craig, A.M. Mismatched appositions of presynaptic and postsynaptic components in isolated hippocampal neurons. J. Neurosci. 2000, 20, 8344–8353. [Google Scholar] [CrossRef] [PubMed]
- Verhage, M.; Maia, A.S.; Plomp, J.J.; Brussaard, A.B.; Heeroma, J.H.; Vermeer, H.; Toonen, R.F.; Hammer, R.E.; van den Berg, T.K.; Missler, M.; et al. Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science 2000, 287, 864–869. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ma, C. Neuronal SNARE complex assembly guided by Munc18-1 and Munc13-1. FEBS Open Bio 2022, 12, 1939–1957. [Google Scholar] [CrossRef]
- Gally, C.; Bessereau, J.L. GABA is dispensable for the formation of junctional GABA receptor clusters in Caenorhabditis elegans. J. Neurosci. 2003, 23, 2591–2599. [Google Scholar] [CrossRef]
- Jin, Y.; Jorgensen, E.; Hartwieg, E.; Horvitz, H.R. The Caenorhabditis elegans gene unc-25 encodes glutamic acid decarboxylase and is required for synaptic transmission but not synaptic development. J. Neurosci. 1999, 19, 539–548. [Google Scholar] [CrossRef]
- Harms, K.J.; Craig, A.M. Synapse composition and organization following chronic activity blockade in cultured hippocampal neurons. J. Comp. Neurol. 2005, 490, 72–84. [Google Scholar] [CrossRef]
- Ramsay, H.J.; Gookin, S.E.; Ramsey, A.M.; Kareemo, D.J.; Crosby, K.C.; Stich, D.G.; Olah, S.S.; Actor-Engel, H.S.; Smith, K.R.; Kennedy, M.J. AMPA and GABAA receptor nanodomains assemble in the absence of synaptic neurotransmitter release. Front. Mol. Neurosci. 2023, 16, 1232795. [Google Scholar] [CrossRef]
- Humeau, Y.; Doussau, F.; Grant, N.J.; Poulain, B. How botulinum and tetanus neurotoxins block neurotransmitter release. Biochimie 2000, 82, 427–446. [Google Scholar] [CrossRef]
- Benke, T.A.; Swann, J. The tetanus toxin model of chronic epilepsy. Adv. Exp. Med. Biol. 2004, 548, 226–238. [Google Scholar] [CrossRef]
- Louis, E.D.; Williamson, P.D.; Darcey, T.M. Experimental models of chronic focal epilepsy: A critical review of four models. Yale J. Biol. Med. 1987, 60, 255–272. [Google Scholar]
- Megighian, A.; Pirazzini, M.; Fabris, F.; Rossetto, O.; Montecucco, C. Tetanus and tetanus neurotoxin: From peripheral uptake to central nervous tissue targets. J. Neurochem. 2021, 158, 1244–1253. [Google Scholar] [CrossRef]
- Chen, H.; Tang, A.H.; Blanpied, T.A. Subsynaptic spatial organization as a regulator of synaptic strength and plasticity. Curr. Opin. Neurobiol. 2018, 51, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Crosby, K.C.; Gookin, S.E.; Garcia, J.D.; Hahm, K.M.; Dell’Acqua, M.L.; Smith, K.R. Nanoscale Subsynaptic Domains Underlie the Organization of the Inhibitory Synapse. Cell Rep. 2019, 26, 3284–3297.e3. [Google Scholar] [CrossRef]
- Held, R.G.; Liang, J.; Brunger, A.T. Nanoscale architecture of synaptic vesicles and scaffolding complexes revealed by cryo-electron tomography. Proc. Natl. Acad. Sci. USA 2024, 121, e2403136121. [Google Scholar] [CrossRef]
- Olah, S.S.; Kareemo, D.J.; Buchta, W.C.; Sinnen, B.L.; Miller, C.N.; Actor-Engel, H.S.; Gookin, S.E.; Winborn, C.S.; Kleinjan, M.S.; Crosby, K.C.; et al. Acute reorganization of postsynaptic GABAA receptors reveals the functional impact of molecular nanoarchitecture at inhibitory synapses. Cell Rep. 2023, 42, 113331. [Google Scholar] [CrossRef]
- Xu, N.; Chen, S.Y.; Tang, A.H. Tuning synapse strength by nanocolumn plasticity. Trends Neurosci. 2025, 48, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Lévi, S.; Schweizer, C.; Bannai, H.; Pascual, O.; Charrier, C.; Triller, A. Homeostatic regulation of synaptic GlyR numbers driven by lateral diffusion. Neuron 2008, 59, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Comenencia-Ortiz, E.; Moss, S.J.; Davies, P.A. Phosphorylation of GABAA receptors influences receptor trafficking and neurosteroid actions. Psychopharmacology 2014, 231, 3453–3465. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Morrow, D.H.; Nathanson, A.J.; Henley, J.M.; Wilkinson, K.A.; Moss, S.J. Phosphorylation on Ser-359 of the α2 subunit in GABA type A receptors down-regulates their density at inhibitory synapses. J. Biol. Chem. 2020, 295, 12330–12342. [Google Scholar] [CrossRef]
- Oh, W.C.; Lutzu, S.; Castillo, P.E.; Kwon, H.B. De novo synaptogenesis induced by GABA in the developing mouse cortex. Science 2016, 353, 1037–1040. [Google Scholar] [CrossRef]
- Burlingham, S.R.; Wong, N.F.; Peterkin, L.; Lubow, L.; Dos Santos Passos, C.; Benner, O.; Ghebrial, M.; Cast, T.P.; Xu-Friedman, M.A.; Südhof, T.C.; et al. Induction of synapse formation by de novo neurotransmitter synthesis. Nat. Commun. 2022, 13, 3060. [Google Scholar] [CrossRef]
- Christie, S.B.; Miralles, C.P.; De Blas, A.L. GABAergic innervation organizes synaptic and extrasynaptic GABAA receptor clustering in cultured hippocampal neurons. J. Neurosci. 2002, 22, 684–697. [Google Scholar] [CrossRef]
- Carricaburu, E.; Benner, O.; Burlingham, S.R.; Dos Santos Passos, C.; Hobaugh, N.; Karr, C.H.; Chanda, S. Gephyrin promotes autonomous assembly and synaptic localization of GABAergic postsynaptic components without presynaptic GABA release. Proc. Natl. Acad. Sci. USA 2024, 121, e2315100121. [Google Scholar] [CrossRef]
- Tyagarajan, S.K.; Ghosh, H.; Yévenes, G.E.; Nikonenko, I.; Ebeling, C.; Schwerdel, C.; Sidler, C.; Zeilhofer, H.U.; Gerrits, B.; Muller, D.; et al. Regulation of GABAergic synapse formation and plasticity by GSK3beta-dependent phosphorylation of gephyrin. Proc. Natl. Acad. Sci. USA 2011, 108, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Choii, G.; Ko, J. Gephyrin: A central GABAergic synapse organizer. Exp. Mol. Med. 2015, 47, e158. [Google Scholar] [CrossRef]
- Dong, N.; Qi, J.; Chen, G. Molecular reconstitution of functional GABAergic synapses with expression of neuroligin-2 and GABAA receptors. Mol. Cell. Neurosci. 2007, 35, 14–23. [Google Scholar] [CrossRef]
- Soykan, T.; Schneeberger, D.; Tria, G.; Buechner, C.; Bader, N.; Svergun, D.; Tessmer, I.; Poulopoulos, A.; Papadopoulos, T.; Varoqueaux, F.; et al. A conformational switch in collybistin determines the differentiation of inhibitory postsynapses. EMBO J. 2014, 33, 2113–2133. [Google Scholar] [CrossRef] [PubMed]
- Chubykin, A.A.; Atasoy, D.; Etherton, M.R.; Brose, N.; Kavalali, E.T.; Gibson, J.R.; Südhof, T.C. Activity-dependent validation of excitatory versus inhibitory synapses by neuroligin-1 versus neuroligin-2. Neuron 2007, 54, 919–931. [Google Scholar] [CrossRef] [PubMed]
- Halff, E.F.; Hannan, S.; Kwanthongdee, J.; Lesept, F.; Smart, T.G.; Kittler, J.T. Phosphorylation of neuroligin-2 by PKA regulates its cell surface abundance and synaptic stabilization. Sci. Signal. 2022, 15, eabg2505. [Google Scholar] [CrossRef]
- Gookin, S.E.; Taylor, M.R.; Schwartz, S.L.; Kennedy, M.J.; Dell’Acqua, M.L.; Crosby, K.C.; Smith, K.R. Complementary Use of Super-Resolution Imaging Modalities to Study the Nanoscale Architecture of Inhibitory Synapses. Front. Synaptic Neurosci. 2022, 14, 852227. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Shepard, R.D.; Lu, W. Regulation of GABAARs by Transmembrane Accessory Proteins. Trends Neurosci. 2021, 44, 152–165. [Google Scholar] [CrossRef]
- Sun, C.; Zhu, H.; Clark, S.; Gouaux, E. Cryo-EM structures reveal native GABAA receptor assemblies and pharmacology. Nature 2023, 622, 195–201. [Google Scholar] [CrossRef]
- Zhou, J.; Noviello, C.M.; Teng, J.; Moore, H.; Lega, B.; Hibbs, R.E. Resolving native GABAA receptor structures from the human brain. Nature 2025, 638, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Piro, I.; Eckes, A.L.; Kasaragod, V.B.; Sommer, C.; Harvey, R.J.; Schaefer, N.; Villmann, C. Novel Functional Properties of Missense Mutations in the Glycine Receptor β Subunit in Startle Disease. Front. Mol. Neurosci. 2021, 14, 745275. [Google Scholar] [CrossRef]
- Gallagher, C.I.; Ha, D.A.; Harvey, R.J.; Vandenberg, R.J. Positive Allosteric Modulators of Glycine Receptors and Their Potential Use in Pain Therapies. Pharmacol. Rev. 2022, 74, 933–961. [Google Scholar] [CrossRef]
- Zhang, W.; Xiong, B.R.; Zhang, L.Q.; Huang, X.; Yuan, X.; Tian, Y.K.; Tian, X.B. The role of the GABAergic system in diseases of the central nervous system. Neuroscience 2021, 470, 88–99. [Google Scholar] [CrossRef]
- Kim, J.J.; Hibbs, R.E. Direct Structural Insights into GABAA Receptor Pharmacology. Trends Biochem. Sci. 2021, 46, 502–517. [Google Scholar] [CrossRef]
- Greenfield, L.J., Jr. Molecular mechanisms of antiseizure drug activity at GABAA receptors. Seizure 2013, 22, 589–600. [Google Scholar] [CrossRef]
- Balon, R.; Starcevic, V. Role of Benzodiazepines in Anxiety Disorders. Adv. Exp. Med. Biol. 2020, 1191, 367–388. [Google Scholar] [CrossRef]
- Nicholson, M.W.; Sweeney, A.; Pekle, E.; Alam, S.; Ali, A.B.; Duchen, M.; Jovanovic, J.N. Diazepam-induced loss of inhibitory synapses mediated by PLCδ/Ca2+/calcineurin signalling downstream of GABAA receptors. Mol. Psychiatry 2018, 23, 1851–1867. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Tannenberg, R.K.; Dodd, P.R. Reduced expression of the inhibitory synapse scaffolding protein gephyrin in Alzheimer’s disease. J. Alzheimer’s Dis. 2008, 14, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Hales, C.M.; Rees, H.; Seyfried, N.T.; Dammer, E.B.; Duong, D.M.; Gearing, M.; Montine, T.J.; Troncoso, J.C.; Thambisetty, M.; Levey, A.I.; et al. Abnormal gephyrin immunoreactivity associated with Alzheimer disease pathologic changes. J. Neuropathol. Exp. Neurol. 2013, 72, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Shen, L.; Yin, H.; Pan, Y.M.; Wang, L.; Chen, D.; Xi, Z.Q.; Xiao, Z.; Wang, X.F.; Zhou, S.N. Downregulation of gephyrin in temporal lobe epilepsy neurons in humans and a rat model. Synapse 2011, 65, 1006–1014. [Google Scholar] [CrossRef]
- Kiss, E.; Gorgas, K.; Schlicksupp, A.; Groß, D.; Kins, S.; Kirsch, J.; Kuhse, J. Biphasic Alteration of the Inhibitory Synapse Scaffold Protein Gephyrin in Early and Late Stages of an Alzheimer Disease Model. Am. J. Pathol. 2016, 186, 2279–2291. [Google Scholar] [CrossRef]
- Kim, S.; Kang, M.; Park, D.; Lee, A.R.; Betz, H.; Ko, J.; Chang, I.; Um, J.W. Impaired formation of high-order gephyrin oligomers underlies gephyrin dysfunction-associated pathologies. iScience 2021, 24, 102037. [Google Scholar] [CrossRef]
- Li, J.; Casteels, T.; Frogne, T.; Ingvorsen, C.; Honoré, C.; Courtney, M.; Huber, K.V.M.; Schmitner, N.; Kimmel, R.A.; Romanov, R.A.; et al. Artemisinins Target GABAA Receptor Signaling and Impair α Cell Identity. Cell 2017, 168, 86–100.e15. [Google Scholar] [CrossRef]
- Kasaragod, V.B.; Hausrat, T.J.; Schaefer, N.; Kuhn, M.; Christensen, N.R.; Tessmer, I.; Maric, H.M.; Madsen, K.L.; Sotriffer, C.; Villmann, C.; et al. Elucidating the Molecular Basis for Inhibitory Neurotransmission Regulation by Artemisinins. Neuron 2019, 101, 673–689.e11. [Google Scholar] [CrossRef]
- Kiss, E.; Kins, S.; Zöller, Y.; Schilling, S.; Gorgas, K.; Groß, D.; Schlicksupp, A.; Rosner, R.; Kirsch, J.; Kuhse, J. Artesunate restores the levels of inhibitory synapse proteins and reduces amyloid-β and C-terminal fragments (CTFs) of the amyloid precursor protein in an AD-mouse model. Mol. Cell. Neurosci. 2021, 113, 103624. [Google Scholar] [CrossRef] [PubMed]
- Kuhse, J.; Groeneweg, F.; Kins, S.; Gorgas, K.; Nawrotzki, R.; Kirsch, J.; Kiss, E. Loss of Extrasynaptic Inhibitory Glycine Receptors in the Hippocampus of an AD Mouse Model Is Restored by Treatment with Artesunate. Int. J. Mol. Sci. 2023, 24, 4623. [Google Scholar] [CrossRef] [PubMed]
- Kiss, E.; Kins, S.; Gorgas, K.; Venczel Szakács, K.H.; Kirsch, J.; Kuhse, J. Another Use for a Proven Drug: Experimental Evidence for the Potential of Artemisinin and Its Derivatives to Treat Alzheimer’s Disease. Int. J. Mol. Sci. 2024, 25, 4165. [Google Scholar] [CrossRef] [PubMed]
| Year | Authors/Title | System | Conditions | Results | Author’s Main Conclusions | Reference |
| 1998 | Lévi S, Vannier C, and Triller A. Strychnine-sensitive stabilization of postsynaptic glycine receptor clusters | spinal cord neurons (rat) in vitro | Strychnine for 3, 7, 11 Div; 0.1, 1 and 10 uM Strychnine wash-out | GlyR number and size reduced, gephyrin clusters persisted at synapses | “…formation and/or stabilization of GlyR but not of gephyrin postsynaptic clusters depends upon functional GlyR.” “…the activation or conformational change of the GlyR is involved in the establishment and/or maintenance of the interaction between GlyR and gephyrin.” | [11] |
| 1998 | Kirsch J and Betz, H. Glycine-receptor activation is required for receptor clustering in spinal neurons | spinal cord neurons (rat) in vitro | Strychnine for 8 Div; 0.05, 0.1, and 0.5 μM block of L-type Ca2+ channels Strychnine wash-out | GlyR number reduced, gephyrin was removed from synapses | “…activation of GlyR is required for receptor clustering but not for the maintenance of differentiated postsynaptic sites.” “…gephyrin accumulation at glycinergic but not GABAergic synapses is prevented by strychnine.” | [12] |
| 2000 | Rao A, Cha EM, Craig AM. Mismatched appositions of presynaptic and postsynaptic components in isolated hippocampal neurons | hippocampal pyramidal cells (rat) deprived of GABA input in vitro (microisland cultures) | 16–29 Div no GABAergic input | 90% of pyramidal cells form GABAA receptor clusters | “…GABAergic input is not necessary for the formation of GABAA receptor clusters.” “…both presynaptic and postsynaptic precursors can form independently of each other; i.e., neither component is necessary for formation of the other, but both can form spontaneously and later become aligned to form a functional synapse.” | [20] |
| 2000 | Verhage M, Maia AS, Plomp JJ, Brussaard AB, Heeroma JH, Vermeer H, Toonen RF, Hammer RE, van den Berg TK, Missler M, Geuze HJ, Südhof TC. Synaptic Assembly of the Brain in the Absence of Neurotransmitter Secretion | mice | deletion of Munc18-1, complete loss of neurotransmitter secretion | normal synapse formation, normal postsynaptic response in the absence of GABA, but degeneration without activation | “…synaptic connectivity does not depend on neurotransmitter secretion, but its maintenance does. Neurotransmitter secretion probably functions to validate already established synaptic connections.” | [21] |
| 2002 | Rasmussen H, Rasmussen T, Triller A, Vannier C. Strychnine-blocked glycine receptor is removed from synapses by a shift in insertion/degradation equilibrium. | spinal cord ventral horn neurons (rat) in vitro | short-term exposure of neurons to strychnine 10 μM | perinuclear accumulation of GlyRs within few hours, preceding the decrease in GlyR clusters at the cell surface | “…the normal turnover of the receptor is maintained in the presence of activity blockade because strychnine does not modify the rate of GlyR removal from postsynaptic sites via endocytosis.” “…activity may be required for gephyrin-dependent, synaptic anchoring of GlyR, it is not essential for the maintenance of this localization, once acquired.” | [13] |
| 2003 | Gally C, Bessereau JL. GABA is dispensable for the formation of junctional GABA receptor clusters in Caenorhabditis elegans | Caenorhabditis elegans GABAergic neuromuscular junctions | unc-25 mutants that do not synthesize GABA | GABA receptor clustering in mutant is identical to that in the wild type | “…at GABAergic neuromuscular junctions, GABA receptor clustering requires nerve–muscle interaction but not GABA neurotransmission.” | [22] |
| 2005 | Harms KJ, Craig AM Synapse composition and organization following chronic activity blockade in cultured hippocampal neurons. | hippocampal neurons in vitro (rat) | 16–18 Div, chronic tetanus toxin to inhibit neurotransmitter release | postsynaptic recruitment of GABAARs, and gephyrin with normal density, size and distribution | “…activity and transmitter release are not necessary for the basics of glutamate or GABA synapse assembly.” | [23] |
| 2009 | Maas C, Belgardt D, Lee HK, Heisler FF, Lappe-Siefke C, Magiera MM, van Dijk J, Hausrat TJ, Janke C, and Kneussel M. Synaptic activation modifies microtubules underlying transport of postsynaptic cargo. | hippocampal neurons, (mouse and rat) mRFP-gephyrin | 10–14 Div, Strychnine over 8 hs | mRFP-gephyrin signal numbers, sizes, and intensities reduced in neurites on GlyR blockade, mRFP-gephyrin accumulated in cell body clusters, impairment of neurite transport | “…synaptic activity regulates tubulin posttranslational modification, underlying intracellular transport of synaptic cargo, to determine the number of proteins available for synaptic transmission.” | [19] |
| 2013 | Yamanaka I, Miki M, Asakawa K, Kawakami K, Oda Y, Hirata H. Glycinergic transmission and postsynaptic activation of CaMKII are required for glycine receptor clustering in vivo. | zebrafish Mauthner cells | - Strychnine bath at 20~800 μM for various lengths of time during zebra-fish development: between 22 hpf and 31 dpf - nifedipine | GlyR number and density reduced, GlyR clusters are not maintained when strychnine is given at later stages when GlyR clusters have been already formed. | “…the formation and maintenance of GlyR clusters in the M-cells in the developing animals are regulated in a synaptic transmission-dependent manner, and CaMKII activation at the postsynapse is essential for GlyR clustering”. | [14] |
| 2016 | Oh WC, Lutzu S, Castillo PE, Kwon HB. De novo synaptogenesis induced by GABA in the developing mouse corte x | organotypic slice cultures from mouse somatosensory cortex acute cortical slices newborn mice | - two-photon GABA photolysis to mimic local GABA release in dendrites - GABA uncaging on layer 2/3 pyramidal neurons in vivo -GABAAR blockade by GABAzine - optogenetics | new gephyrin clusters formed and the amplitudes of uIPSCs subsequently increased reduced gephyrin clustering after blockade new gephyrin puncta and dendritic spines in young neurons | “…GABA is sufficient to drive inhibitory synapse formation. “…early-developing GABAergic inputs from cortical interneurons control both inhibitory and excitatory circuitry during cortical development.” “…early-depolarizing GABA action appears to promote local synaptogenesis and shapes cortical circuitry during brain development.” | [26] |
| 2017 | Nakahata Y, Eto K, Murakoshi H, Watanabe M, Kuriu T, Hirata H, Moorhouse AJ, Ishibashi H, Nabekura J. Activation-dependent rapid post-synaptic clustering of glycine receptors in mature spinal cord neurons | spinal cord neurons (mouse) in vitro | Strychnine over 14 Div; 1 μM Strychnine wash-out Local glycine (1 M) for 1 h | GlyR clusters with reduced size at inhibitory synapses return to control levels within 1 h of strychnine washout GlyR clustering at gephyrin-positive postsynapse | “In contrast to the current depolarization-dependent model of GlyR clustering, …the activation of GlyRs in more mature neurons…elicits changes in diffusion and increases in the postsynaptic GlyR clusters.” “…this phenomenon is dependent on PKC, but neither Ca2+ nor CaMKII activity.” “GlyR activation is more important for the formation of synaptic clustering of GlyR than for maintenance in mature neurons.” | [16] |
| 2022 | Burlingham SR, Wong NF, Peterkin L, Lubow L, Dos Santos Passos C, Benner O, Ghebrial M, Cast TP, Xu-Friedman MA, Südhof TC, Chanda S. Induction of synapse formation by de novo neurotransmitter synthesis | stem cells-derived human neurons in vivo mouse neurons of purely glutamatergic identity | ectopic expression of vGAT, GAD65, and GAD (V57 factors) | elevated the numbers of gephyrin clusters without changes in their sizes | “…presynaptic release of a neurotransmitter itself can signal the organization of relevant postsynaptic apparatus…” | [27] |
| 2024 | Carricaburu E, Benner O, Burlingham SR, Dos Santos Passos C, Hobaugh N, Karr CH, Chanda S. Gephyrin promotes autonomous assembly and synaptic localization of GABAergic postsynaptic components without presynaptic GABA release | Induced pluripotent stem cells (iPSC) reprogrammed into human glutamatergic neurons by a single transcription factor, Neurogenin-2 | GABA-free cellular environment Gephyrin knock-out ectopic expression of V57 factors | postsynaptic GABAAR subunits and gephyrin clusters assembly elimination of GABAARs submembrane aggregation increased density of GAD-opposed gephyrin clusters | “…molecular organization of GABAergic postsynapses can initiate via a GABA-independent but Gephyrin-dependent intrinsic mechanism. “ “Gephyrin provides crucial structural support for postsynaptic assembly, regardless of GABA signals.” “Self-organizing GABAergic postsynaptic structures could be functionally stimulated by de novo biosynthesis and ectopic release of presynaptic GABA.” | [29] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiss, E.; Kirsch, J.; Kuhse, J.; Kins, S. Is the Activation of the Postsynaptic Ligand Gated Glycine- or GABAA Receptors Essential for the Receptor Clustering at Inhibitory Synapses? Biomedicines 2025, 13, 1905. https://doi.org/10.3390/biomedicines13081905
Kiss E, Kirsch J, Kuhse J, Kins S. Is the Activation of the Postsynaptic Ligand Gated Glycine- or GABAA Receptors Essential for the Receptor Clustering at Inhibitory Synapses? Biomedicines. 2025; 13(8):1905. https://doi.org/10.3390/biomedicines13081905
Chicago/Turabian StyleKiss, Eva, Joachim Kirsch, Jochen Kuhse, and Stefan Kins. 2025. "Is the Activation of the Postsynaptic Ligand Gated Glycine- or GABAA Receptors Essential for the Receptor Clustering at Inhibitory Synapses?" Biomedicines 13, no. 8: 1905. https://doi.org/10.3390/biomedicines13081905
APA StyleKiss, E., Kirsch, J., Kuhse, J., & Kins, S. (2025). Is the Activation of the Postsynaptic Ligand Gated Glycine- or GABAA Receptors Essential for the Receptor Clustering at Inhibitory Synapses? Biomedicines, 13(8), 1905. https://doi.org/10.3390/biomedicines13081905






