Sensitivity and Specificity of a Revised Version of the TRACK-MS Screening Battery for Early Detection of Cognitive Impairment in Patients with Multiple Sclerosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Participants
2.2. Procedure
2.3. Detailed Neuropsychological Assessment
2.4. TRACK-MS and TRACK-MS-R
2.5. MS-Specific Cognitive Screening Batteries
2.6. Depression and Anxiety
2.7. Fatigue
2.8. Statistical Analysis
3. Results
3.1. Demographics and Clinical Data
3.2. Neuropsychological Assessment
3.3. Affective State and Fatigue
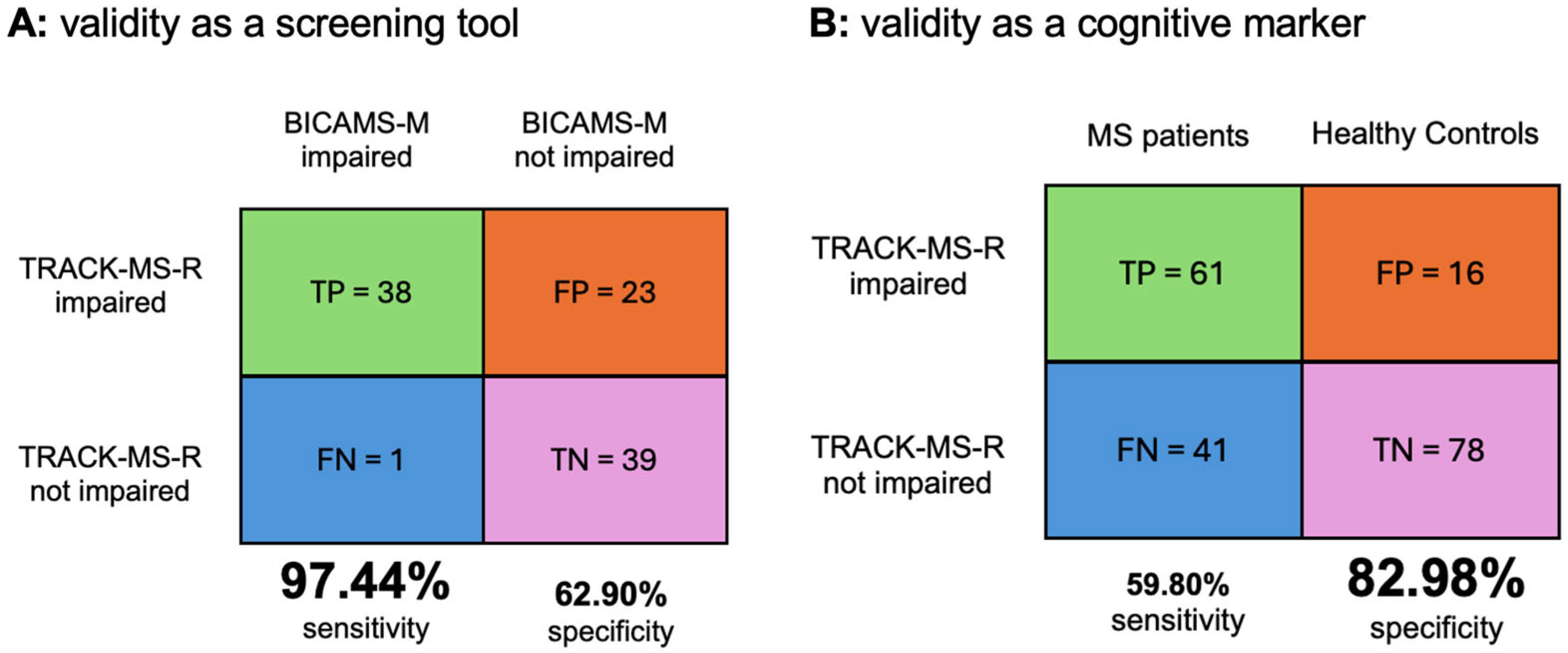
3.4. Sensitivity and Specificity of TRACK-MS-R vs. Gold Standard BICAMS-M
3.5. Sensitivity and Specificity of TRACK-MS-R as a Cognitive Marker of MS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MS | Multiple Sclerosis |
| RRMS | Relapsing-Remitting Multiple Sclerosis |
| PPMS | Primary Progressive Multiple Sclerosis |
| SPMS | Secondary Progressive Multiple Sclerosis |
| EDSS | Expanded Disability Status Scale |
| HC | Healthy Controls |
| BICAMS | Brief International Cognitive Assessment for MS |
| VLMT | Verbal Learning and Memory Test |
| BVMT-R | Brief Visuospatial Memory Test—Revised Version |
| SDMT | Symbol Digit Modalities Test |
| COWAT | Controlled Oral Word Association Test |
| WAIS | Wechsler Adult Intelligence Scale |
| RWT | Regensburger Wortflüssigkeitstest |
| TAP | Testbatterie zur Aufmerksamkeitsprüfung |
| WMS-R | Wechsler Memory Scale-Revised |
| HADS | Hospital Anxiety and Depression Scale |
| FSMC | Fatigue Scale for Motor and Cognitive Functions |
| TN | True negative |
| TP | True positive |
| FP | False positive |
| FN | False negative |
References
- Chiaravalloti, N.D.; De Luca, J. Cognitive impairment in multiple sclerosis. Lancet Neurol. 2008, 7, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Benedict, R.H.B.; Amato, M.P.; DeLuca, J.; Geurts, J.J.G. Cognitive impairment in multiple sclerosis: Clinical management, MRI, and therapeutic avenues. Lancet Neurol. 2020, 19, 860–871. [Google Scholar] [CrossRef]
- Amato, M.P.; Zipoli, V.; Portaccio, E. Multiple sclerosis-related cognitive changes: A review of cross-sectional and longitudinal studies. J. Neurol. Sci. 2006, 245, 41–46. [Google Scholar] [CrossRef]
- Eijlers, A.J.C.; van Geest, Q.; Dekker, I.; Steenwijk, M.D.; Meijer, K.A.; Hulst, H.E.; Barkhof, F.; Uitdehaag, B.M.J.; Schoonheim, M.M.; Geurts, J.J.G. Predicting cognitive decline in multiple sclerosis: A 5-year follow-up study. Brain 2018, 141, 2605–2618. [Google Scholar] [CrossRef]
- Macias Islas, M.A.; Ciampi, E. Assessment and Impact of Cognitive Impairment in Multiple Sclerosis: An Overview. Biomedicines 2019, 7, 22. [Google Scholar] [CrossRef]
- Kalb, R.; Beier, M.; Benedict, R.H.; Charvet, L.; Costello, K.; Feinstein, A.; Gingold, J.; Goverover, Y.; Halper, J.; Harris, C.; et al. Recommendations for cognitive screening and management in multiple sclerosis care. Mult. Scler. 2018, 24, 1665–1680. [Google Scholar] [CrossRef]
- Benito-Leon, J.; Morales, J.M.; Rivera-Navarro, J.; Mitchell, A. A review about the impact of multiple sclerosis on health-related quality of life. Disabil. Rehabil. 2003, 25, 1291–1303. [Google Scholar] [CrossRef]
- Morrow, S.A.; Rosehart, H.; Pantazopoulos, K. Anxiety and Depressive Symptoms Are Associated with Worse Performance on Objective Cognitive Tests in MS. J. Neuropsychiatry Clin. Neurosci. 2016, 28, 118–123. [Google Scholar] [CrossRef]
- Ruet, A.; Deloire, M.; Hamel, D.; Ouallet, J.C.; Petry, K.; Brochet, B. Cognitive impairment, health-related quality of life and vocational status at early stages of multiple sclerosis: A 7-year longitudinal study. J. Neurol. 2013, 260, 776–784. [Google Scholar] [CrossRef]
- De Meo, E.; Portaccio, E.; Giorgio, A.; Ruano, L.; Goretti, B.; Niccolai, C.; Patti, F.; Chisari, C.G.; Gallo, P.; Grossi, P.; et al. Identifying the Distinct Cognitive Phenotypes in Multiple Sclerosis. JAMA Neurol. 2021, 78, 414–425. [Google Scholar] [CrossRef]
- Wills, O.; Probst, Y. Towards new perspectives: A scoping review and meta-synthesis to redefine brain health for multiple sclerosis. Eur. J. Neurol. 2024, 31, e16210. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Alvarez, A.; Delgado-Alonso, C.; Goudsmit, M.; Gil, M.J.; Diez-Cirarda, M.; Valles-Salgado, M.; Montero-Escribano, P.; Hernandez-Lorenzo, L.; Matias-Guiu, J.; Matias-Guiu, J.A. Validation of a brief cross-cultural cognitive screening test in Multiple Sclerosis. Mult. Scler. Relat. Disord. 2022, 67, 104091. [Google Scholar] [CrossRef] [PubMed]
- Lechner-Scott, J.; Agland, S.; Allan, M.; Darby, D.; Diamond, K.; Merlo, D.; van der Walt, A. Managing cognitive impairment and its impact in multiple sclerosis: An Australian multidisciplinary perspective. Mult. Scler. Relat. Disord. 2023, 79, 104952. [Google Scholar] [CrossRef] [PubMed]
- Sumowski, J.F.; Benedict, R.; Enzinger, C.; Filippi, M.; Geurts, J.J.; Hamalainen, P.; Hulst, H.; Inglese, M.; Leavitt, V.M.; Rocca, M.A.; et al. Cognition in multiple sclerosis: State of the field and priorities for the future. Neurology 2018, 90, 278–288. [Google Scholar] [CrossRef]
- van Dongen, L.; Westerik, B.; van der Hiele, K.; Visser, L.H.; Schoonheim, M.M.; Douw, L.; Twisk, J.W.R.; de Jong, B.A.; Geurts, J.J.G.; Hulst, H.E. Introducing Multiple Screener: An unsupervised digital screening tool for cognitive deficits in MS. Mult. Scler. Relat. Disord. 2020, 38, 101479. [Google Scholar] [CrossRef]
- Wojcik, C.M.; Beier, M.; Costello, K.; DeLuca, J.; Feinstein, A.; Goverover, Y.; Gudesblatt, M.; Jaworski, M., 3rd; Kalb, R.; Kostich, L.; et al. Computerized neuropsychological assessment devices in multiple sclerosis: A systematic review. Mult. Scler. 2019, 25, 1848–1869. [Google Scholar] [CrossRef]
- Langdon, D.W.; Amato, M.P.; Boringa, J.; Brochet, B.; Foley, F.; Fredrikson, S.; Hamalainen, P.; Hartung, H.P.; Krupp, L.; Penner, I.K.; et al. Recommendations for a Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS). Mult. Scler. 2012, 18, 891–898. [Google Scholar] [CrossRef]
- Potticary, H.; Langdon, D. A Systematic Review and Meta-Analysis of the Brief Cognitive Assessment for Multiple Sclerosis (BICAMS) International Validations. J. Clin. Med. 2023, 12, 703. [Google Scholar] [CrossRef]
- Helmstaedter, C.; Lendt, M.; Lux, S. Verbaler Lern-Und Merk-Fähigkeitstest: VLMT: Manual, 1st ed.; Hogrefe Verlag: Göttingen, Germany, 2001. [Google Scholar]
- Benedict, R.H.B.; Schretlen, D.S.; Groninger, L.; Dobraski, M. Revision of the brief Visuospatial Memory Test: Studies of normal performance, reliability and validity. Psychol. Assess. 1996, 8, 145–153. [Google Scholar] [CrossRef]
- Benedict, R.H.B.; DeLuca, J.; Phillips, G.; LaRocca, N.; Hudson, L.D.; Rudick, R.; Consortium, M.S.O.A. Validity of the Symbol Digit Modalities Test as a cognition performance outcome measure for multiple sclerosis. Mult. Scler. 2017, 23, 721–733. [Google Scholar] [CrossRef]
- Henry, J.D.; Beatty, W.W. Verbal fluency deficits in multiple sclerosis. Neuropsychologia 2006, 44, 1166–1174. [Google Scholar] [CrossRef]
- Godefroy, O.; Azouvi, P.; Robert, P.; Roussel, M.; LeGall, D.; Meulemans, T.; Groupe de Réflexion sur l’Evaluation des Fonctions Exécutives Study Group. Dysexecutive syndrome: Diagnostic criteria and validation study. Ann. Neurol. 2010, 68, 855–864. [Google Scholar] [CrossRef]
- Cerezo Garcia, M.; Martin Plasencia, P.; Aladro Benito, Y. Alteration profile of executive functions in multiple sclerosis. Acta Neurol. Scand. 2015, 131, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Meca-Lallana, V.; Gascon-Gimenez, F.; Ginestal-Lopez, R.C.; Higueras, Y.; Tellez-Lara, N.; Carreres-Polo, J.; Eichau-Madueno, S.; Romero-Imbroda, J.; Vidal-Jordana, A.; Perez-Miralles, F. Cognitive impairment in multiple sclerosis: Diagnosis and monitoring. Neurol. Sci. 2021, 42, 5183–5193. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Alvarez, A.; Matias-Guiu, J.A.; Delgado-Alonso, C.; Hernandez-Lorenzo, L.; Cortes-Martinez, A.; Vidorreta, L.; Montero-Escribano, P.; Pytel, V.; Matias-Guiu, J. Cognitive Processes Underlying Verbal Fluency in Multiple Sclerosis. Front. Neurol. 2020, 11, 629183. [Google Scholar] [CrossRef] [PubMed]
- Blecher, T.; Miron, S.; Schneider, G.G.; Achiron, A.; Ben-Shachar, M. Association Between White Matter Microstructure and Verbal Fluency in Patients with Multiple Sclerosis. Front. Psychol. 2019, 10, 1607. [Google Scholar] [CrossRef] [PubMed]
- Robinson, G.; Shallice, T.; Bozzali, M.; Cipolotti, L. The differing roles of the frontal cortex in fluency tests. Brain 2012, 135, 2202–2214. [Google Scholar] [CrossRef]
- Taranu, D.; Tumani, H.; Holbrook, J.; Tumani, V.; Uttner, I.; Fissler, P. The TRACK-MS Test Battery: A Very Brief Tool to Track Multiple Sclerosis-Related Cognitive Impairment. Biomedicines 2022, 10, 2975. [Google Scholar] [CrossRef]
- Ross, T.P.; Calhoun, E.; Cox, T.; Wenner, C.; Kono, W.; Pleasant, M. The reliability and validity of qualitative scores for the Controlled Oral Word Association Test. Arch. Clin. Neuropsychol. 2007, 22, 475–488. [Google Scholar] [CrossRef]
- Aschenbrenner, S.; Tucha, O.; Lange, K.W. Regensburger Wortflüssigkeits-Test (RWT); Hogrefe Verlag: Göttingen, Germany, 2000. [Google Scholar]
- Shao, Z.; Janse, E.; Visser, K.; Meyer, A.S. What do verbal fluency tasks measure? Predictors of verbal fluency performance in older adults. Front. Psychol. 2014, 5, 772. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet. Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Cao, H.; Peyrodie, L.; Agnani, O.; Cavillon, F.; Hautecoeur, P.; Donzé, C. Evaluation of an Expanded Disability Status Scale (EDSS) modeling strategy in multiple sclerosis. Med. Biol. Eng. Comput. 2015, 53, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Elwood, R.W. The Wechsler Memory Scale-Revised: Psychometric characteristics and clinical application. Neuropsychol. Rev. 1991, 2, 179–201. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Fimm, B. Testbatterie Zur Aufmerksamkeitsprufung (TAP); Psytest: Freiburg, Germany, 1992. [Google Scholar]
- Petermann, F. Wechsler Adult Intelligence Scale—Fourth Edition (WAIS-IV), 4th ed.; Pearson Assessment: Frankfurt am Main, Germany, 2012. [Google Scholar]
- Filser, M.; Schreiber, H.; Pottgen, J.; Ullrich, S.; Lang, M.; Penner, I.K. The Brief International Cognitive Assessment in Multiple Sclerosis (BICAMS): Results from the German validation study. J. Neurol. 2018, 265, 2587–2593. [Google Scholar] [CrossRef]
- Snaith, R.P. The Hospital Anxiety And Depression Scale. Health Qual. Life Outcomes 2003, 1, 29. [Google Scholar] [CrossRef] [PubMed]
- Penner, I.K.; Raselli, C.; Stöcklin, M.; Opwis, K.; Kappos, L.; Calabrese, P. The Fatigue Scale for Motor and Cognition Functions (FSMC): Validation of a new instrument to assess multiple sclerosis-related fatigue. Mult. Scler. 2009, 15, 1509–1517. [Google Scholar] [CrossRef]
- Lix, L.M.; Keselman, J.C.; Keselman, H.J. Consequences of Assumption Violations Revisited: A Quantitative Review of Alternatives to the One-Way Analysis of Variance F Test. Rev. Educ. Res. 1996, 66, 579–619. [Google Scholar] [CrossRef]
- Conradsson, D.; Ytterberg, C.; von Koch, L.; Johansson, S. Changes in disability in people with multiple sclerosis: A 10-year prospective study. J. Neurol. 2018, 265, 119–126. [Google Scholar] [CrossRef]
- Brochet, B.; Ruet, A. Cognitive Impairment in Multiple Sclerosis with Regards to Disease Duration and Clinical Phenotypes. Front. Neurol. 2019, 10, 261. [Google Scholar] [CrossRef]
- Portaccio, E.; Amato, M.P. Cognitive Impairment in Multiple Sclerosis: An Update on Assessment and Management. NeuroSci 2022, 3, 667–676. [Google Scholar] [CrossRef]
- Louapre, C.; Perlbarg, V.; Garcia-Lorenzo, D.; Urbanski, M.; Benali, H.; Assouad, R.; Galanaud, D.; Freeman, L.; Bodini, B.; Papeix, C.; et al. Brain networks disconnection in early multiple sclerosis cognitive deficits: An anatomofunctional study. Hum. Brain Mapp. 2014, 35, 4706–4717. [Google Scholar] [CrossRef]
- Rocca, M.A.; Valsasina, P.; Hulst, H.E.; Abdel-Aziz, K.; Enzinger, C.; Gallo, A.; Pareto, D.; Riccitelli, G.; Muhlert, N.; Ciccarelli, O.; et al. Functional correlates of cognitive dysfunction in multiple sclerosis: A multicenter fMRI Study. Hum. Brain Mapp. 2014, 35, 5799–5814. [Google Scholar] [CrossRef]
- Akaike, S.; Okamoto, T.; Kurosawa, R.; Onodera, N.; Lin, Y.; Sato, W.; Yamamura, T.; Takahashi, Y. Exploring the Potential of the Corpus Callosum Area as a Predictive Marker for Impaired Information Processing in Multiple Sclerosis. J. Clin. Med. 2023, 12, 6948. [Google Scholar] [CrossRef] [PubMed]
- Gregorio, F.D.; Battaglia, S. The intricate brain-body interaction in psychiatric and neurological diseases. Adv. Clin. Exp. Med. 2024, 33, 321–326. [Google Scholar] [CrossRef]
- Calabrese, M.; Agosta, F.; Rinaldi, F.; Mattisi, I.; Grossi, P.; Favaretto, A.; Atzori, M.; Bernardi, V.; Barachino, L.; Rinaldi, L.; et al. Cortical lesions and atrophy associated with cognitive impairment in relapsing-remitting multiple sclerosis. Arch. Neurol. 2009, 66, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Petracca, M.; Pontillo, G.; Moccia, M.; Carotenuto, A.; Cocozza, S.; Lanzillo, R.; Brunetti, A.; Brescia Morra, V. Neuroimaging Correlates of Cognitive Dysfunction in Adults with Multiple Sclerosis. Brain Sci. 2021, 11, 346. [Google Scholar] [CrossRef] [PubMed]
- Burggraaff, J.; Liu, Y.; Prieto, J.C.; Simoes, J.; de Sitter, A.; Ruggieri, S.; Brouwer, I.; Lissenberg-Witte, B.I.; Rocca, M.A.; Valsasina, P.; et al. Manual and automated tissue segmentation confirm the impact of thalamus atrophy on cognition in multiple sclerosis: A multicenter study. Neuroimage Clin. 2021, 29, 102549. [Google Scholar] [CrossRef]
- Rocca, M.A.; Amato, M.P.; De Stefano, N.; Enzinger, C.; Geurts, J.J.; Penner, I.K.; Rovira, A.; Sumowski, J.F.; Valsasina, P.; Filippi, M.; et al. Clinical and imaging assessment of cognitive dysfunction in multiple sclerosis. Lancet Neurol. 2015, 14, 302–317. [Google Scholar] [CrossRef]
- Piacentini, C.; Argento, O.; Nocentini, U. Cognitive impairment in multiple sclerosis: “classic” knowledge and recent acquisitions. Arq. Neuropsiquiatr. 2023, 81, 585–596. [Google Scholar] [CrossRef]
- Elwick, H.; Topcu, G.; Allen, C.M.; Drummond, A.; Evangelou, N.; Nair, R.D. Cognitive measures used in adults with multiple sclerosis: A systematic review. Neuropsychol. Rehabil. 2022, 32, 2464–2481. [Google Scholar] [CrossRef]
- Oset, M.; Stasiolek, M.; Matysiak, M. Cognitive Dysfunction in the Early Stages of Multiple Sclerosis-How Much and How Important? Curr. Neurol. Neurosci. Rep. 2020, 20, 22. [Google Scholar] [CrossRef]
- Elwick, H.; Smith, L.; Mhizha-Murira, J.R.; Topcu, G.; Leighton, P.; Drummond, A.; Evangelou, N.; Das Nair, R. Cognitive assessment in multiple sclerosis clinical care: A qualitative evaluation of stakeholder perceptions and preferences. Neuropsychol. Rehabil. 2021, 32, 1456–1474. [Google Scholar] [CrossRef]
- Stavrogianni, K.; Giannopapas, V.; Kitsos, D.K.; Christouli, N.; Smyrni, V.; Chasiotis, A.K.; Akrivaki, A.; Dimitriadou, E.M.; Tzartos, J.S.; Tsivgoulis, G.; et al. Cognitive Impairment in Newly Diagnosed Patients with Multiple Sclerosis: A Systematic Review of Related Molecular Biomarkers and a Meta-Analysis of Associated Demographic and Disease-Related Characteristics. J. Clin. Med. 2025, 14, 2630. [Google Scholar] [CrossRef] [PubMed]
- Pless, S.; Woelfle, T.; Naegelin, Y.; Lorscheider, J.; Wiencierz, A.; Reyes, O.; Calabrese, P.; Kappos, L. Assessment of cognitive performance in multiple sclerosis using smartphone-based training games: A feasibility study. J. Neurol. 2023, 270, 3451–3463. [Google Scholar] [CrossRef] [PubMed]
- Ruano, L.; Branco, M.; Severo, M.; Sousa, A.; Castelo, J.; Araujo, I.; Pais, J.; Cerqueira, J.; Amato, M.P.; Lunet, N.; et al. Tracking cognitive impairment in multiple sclerosis using the Brain on Track test: A validation study. Neurol. Sci. 2020, 41, 183–191. [Google Scholar] [CrossRef]
- Meca-Lallana, J.E.; Prieto-Gonzalez, J.M.; Jimenez-Veiga, J.; Carreon-Guarnizo, E.; Jimenez-Martin, I.; Hernandez-Clares, R.; Sistiaga-Berrondo, A.; Carles-Dies, R.; Garcia-Molina, E.; Cerdan-Sanchez, M.; et al. Development and validation of a brief electronic screening test for cognitive impairment in multiple sclerosis (SCI-MS Test). Mult. Scler. Relat. Disord. 2019, 28, 50–56. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence (NICE). Multiple Sclerosis in Adults: Management; NICE Guideline NG220; NICE: London, UK, 2022; Available online: https://www.nice.org.uk/guidance/ng220 (accessed on 29 July 2025).
- Carotenuto, A.; Costabile, T.; Pontillo, G.; Moccia, M.; Falco, F.; Petracca, M.; Petruzzo, M.; Russo, C.V.; Di Stasi, M.; Paolella, C.; et al. Cognitive trajectories in multiple sclerosis: A long-term follow-up study. Neurol. Sci. 2022, 43, 1215–1222. [Google Scholar] [CrossRef]
- Chen, O.Y.; Lipsmeier, F.; Phan, H.; Dondelinger, F.; Creagh, A.; Gossens, C.; Lindemann, M.; de Vos, M. Personalized Longitudinal Assessment of Multiple Sclerosis Using Smartphones. IEEE J. Biomed. Health Inform. 2023, 27, 3633–3644. [Google Scholar] [CrossRef]
- Podda, J.; Tacchino, A.; Ponzio, M.; Di Antonio, F.; Susini, A.; Pedulla, L.; Battaglia, M.A.; Brichetto, G. Mobile Health App (DIGICOG-MS) for Self-Assessment of Cognitive Impairment in People with Multiple Sclerosis: Instrument Validation and Usability Study. JMIR Form. Res. 2024, 8, e56074. [Google Scholar] [CrossRef]
- Patti, F.; Amato, M.P.; Trojano, M.; Bastianello, S.; Tola, M.R.; Goretti, B.; Caniatti, L.; Di Monte, E.; Ferrazza, P.; Brescia Morra, V.; et al. Cognitive impairment and its relation with disease measures in mildly disabled patients with relapsing-remitting multiple sclerosis: Baseline results from the Cognitive Impairment in Multiple Sclerosis (COGIMUS) study. Mult. Scler. J. 2009, 15, 779–788. [Google Scholar] [CrossRef]
- DeLuca, J.; Chiaravalloti, N.D.; Sandroff, B.M. Treatment and management of cognitive dysfunction in patients with multiple sclerosis. Nat. Rev. Neurol. 2020, 16, 319–332. [Google Scholar] [CrossRef]
- Crowe, S.F. Decrease in performance on the verbal fluency test as a function of time: Evaluation in a young healthy sample. J. Clin. Exp. Neuropsychol. 1998, 20, 391–401. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | MS Patients (N = 102) | HC * (N = 94) | Statistics a | ||
|---|---|---|---|---|---|
| Mean (SD) | N (%) | Mean (SD) | N (%) | ||
| Age (years) | 45.49 (13.29) | 47.13 (14.11) | F(1, 194) = 0.70, p = 0.404 | ||
| Female/Male | 61/41 (59.8/40.2) | 69/25 (73.4/26.6) | χ2(1) = 4.05, p = 0.044 | ||
| Years of education | 14.61 (2.92) | 14.91 (2.85) | F(1, 194) = 0.56, p = 0.457 | ||
| EDSS * | 2.79 (1.82) | ||||
| Time since diagnosis (years) | 9.97 (8.09) | ||||
| Phenotype | |||||
| RRMS * | 73 (71.6) | ||||
| PPMS * | 11 (10.8) | ||||
| SPMS * | 17 (16.7) | ||||
| Cognitive Domains/PROMs * | MS Patients | HC * | Statistics a | |||||
|---|---|---|---|---|---|---|---|---|
| Tests | N (Cohort) | Mean (SD) | N (%) | Mean (SD) | N (%) | F | p-Value | Partial η2 |
| Verbal short-term memory | ||||||||
| digit span forward | 196 | 7.00 (1.85) | 8.11 (1.65) | 9.77 | <0.001 | 0.092 | ||
| Nonverbal short-term memory | ||||||||
| block-tapping-test forward | 196 | 8.17 (1.83) | 9.39 (1.77) | 11.82 | <0.001 | 0.109 | ||
| Verbal working memory | ||||||||
| digit span backwards | 196 | 5.75 (1.97) | 6.81 (1.86) | 8.15 | <0.001 | 0.78 | ||
| Nonverbal working memory | ||||||||
| block-tapping-test backwards | 196 | 8.45 (6.70) | 10.59 (10.89) | 1.40 | 0.248 | 0.014 | ||
| Verbal episodic memory | ||||||||
| VLMT * total | 196 | 50.55 (11.45) | 59.31 (8.05) | 30.16 | <0.001 | 0.238 | ||
| VLMT * delayed recall | 196 | 10.14 (3.94) | 12.78 (2.64) | 21.10 | <0.001 | 0.179 | ||
| VLMT * recognition | 196 | 13.37 (2.60) | 14.48 (0.86) | 7.89 | <0.001 | 0.076 | ||
| Visual episodic memory | ||||||||
| BVMT-R * total | 196 | 24.55 (8.04) | 28.20 (5.49) | 6.94 | 0.001 | 0.067 | ||
| BVMT-R * delayed recall | 196 | 9.73 (2.88) | 10.85 (1.73) | 5.96 | 0.003 | 0.058 | ||
| BVMT-R * recognition | 196 | 5.65 (0.94) | 5.87 (0.42) | 2.56 | 0.080 | 0.026 | ||
| Attentional functions | ||||||||
| TAP * divided attention (auditory) | 190 | 657.82 (159.65) | 613.09 (111.74) | 2.47 | 0.087 | 0.026 | ||
| TAP * divided attention (visual) | 190 | 871.54 (214.88) | 757.03 (95.60) | 11.41 | <0.001 | 0.109 | ||
| TAP * incompatibility | 190 | 573.99 (180.83) | 501.68 (109.98) | 5.47 | 0.005 | 0.055 | ||
| SDMT * | 196 | 50.13 (13.31) | 59.77 (9.33) | 17.05 | <0.001 | 0.150 | ||
| Executive functions | ||||||||
| phonemic verbal fluency (RWT * S) | 196 | 18.99 (7.16) | 24.47 (7.01) | 14.95 | <0.001 | 0.135 | ||
| phonemic verbal fluency (COWAT *) | 196 | 34.25 (9.95) | 43.77 (12.11) | 19.22 | <0.001 | 0.166 | ||
| matrices (WAIS *) | 193 | 17.16 (4.81) | 19.51 (3.13) | 8.38 | <0.001 | 0.081 | ||
| Cognitive fatigue (FSMC *) | 196 | 30.12 (12.05) | 18.46 (7.50) | 32.46 | <0.001 | 0.253 | ||
| mild | 13 (12.7) | 18 (19.1) | ||||||
| moderate | 17 (16.7) | 9 (9.6) | ||||||
| severe | 44 (43.1) | 3 (3.2) | ||||||
| Motor fatigue (FSMC *) | 196 | 31.83 (11.60) | 18.34 (7.31) | 46.19 | <0.001 | 0.325 | ||
| mild | 10 (9.8) | 16 (17) | ||||||
| moderate | 11 (10.8) | 10 (10.6) | ||||||
| severe | 57 (55.9) | 4 (4.3) | ||||||
| Depression (HADS-D *) | 196 | 5.81 (4.20) | 2.96 (2.64) | 16.26 | <0.001 | 0.144 | ||
| mild | 20 (19.6) | 7 (7.4) | ||||||
| moderate | 14 (13.7) | 0 (0) | ||||||
| severe | 2 (2) | 0 (0) | ||||||
| Anxiety (HADS-A *) | 196 | 7.19 (4.48) | 4.29 (2.55) | 15.06 | <0.001 | 0.135 | ||
| mild | 19 (18.6) | 7 (7.4) | ||||||
| moderate | 15 (14.7) | 0 (0) | ||||||
| severe | 9 (8.8) | 0 (0) | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balz, L.T.; Uttner, I.; Taranu, D.; Erhart, D.K.; Fangerau, T.; Jung, S.; Schreiber, H.; Senel, M.; Vardakas, I.; Lulé, D.E.; et al. Sensitivity and Specificity of a Revised Version of the TRACK-MS Screening Battery for Early Detection of Cognitive Impairment in Patients with Multiple Sclerosis. Biomedicines 2025, 13, 1902. https://doi.org/10.3390/biomedicines13081902
Balz LT, Uttner I, Taranu D, Erhart DK, Fangerau T, Jung S, Schreiber H, Senel M, Vardakas I, Lulé DE, et al. Sensitivity and Specificity of a Revised Version of the TRACK-MS Screening Battery for Early Detection of Cognitive Impairment in Patients with Multiple Sclerosis. Biomedicines. 2025; 13(8):1902. https://doi.org/10.3390/biomedicines13081902
Chicago/Turabian StyleBalz, Luisa T., Ingo Uttner, Daniela Taranu, Deborah K. Erhart, Tanja Fangerau, Stefanie Jung, Herbert Schreiber, Makbule Senel, Ioannis Vardakas, Dorothée E. Lulé, and et al. 2025. "Sensitivity and Specificity of a Revised Version of the TRACK-MS Screening Battery for Early Detection of Cognitive Impairment in Patients with Multiple Sclerosis" Biomedicines 13, no. 8: 1902. https://doi.org/10.3390/biomedicines13081902
APA StyleBalz, L. T., Uttner, I., Taranu, D., Erhart, D. K., Fangerau, T., Jung, S., Schreiber, H., Senel, M., Vardakas, I., Lulé, D. E., & Tumani, H. (2025). Sensitivity and Specificity of a Revised Version of the TRACK-MS Screening Battery for Early Detection of Cognitive Impairment in Patients with Multiple Sclerosis. Biomedicines, 13(8), 1902. https://doi.org/10.3390/biomedicines13081902







