Abstract
Neurodegenerative diseases (NDDs) pose an immense global health burden, and developing effective treatments is hindered by the blood–brain barrier (BBB). Intrathecal (IT) administration of therapeutics directly into the cerebrospinal fluid (CSF) bypasses the BBB, offering a promising avenue for antisense oligonucleotides (ASOs), gene therapies, antibodies, and stem cells for these disorders. This review synthesizes the current landscape of IT therapies for Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, and Amyotrophic Lateral Sclerosis based on the current literature and ClinicalTrials.gov. We highlight key trials and approaches, including the success of ASOs in spinal muscular atrophy and recent progress in other NDDs. However, the efficacy of these novel treatments is often constrained by the limitations of first-generation IT delivery systems, which struggle with uneven distribution, systemic leakage, and the demands of modern biologics. Drawing from recent analyses, we underscore the critical shortcomings of current devices and point out the innovations needed in shaping next-generation systems: subcutaneous access ports, CSF flow platforms, AI-driven adaptive dosing, nanoporous membranes, intrathecal pseudodelivery, and hydrogel scaffolds. We conclude by emphasizing the urgent need for these advanced IT drug delivery systems, alongside rigorous comparative assessments, cost–benefit analyses, and clear regulatory pathways to fully realize the potential of emerging CNS therapies and transform NDD management.
1. Introduction
Neurodegenerative diseases (NDDs) encompass a wide array of chronic, progressive disorders characterized by the gradual loss of neurons, and represent a growing burden for aging populations worldwide, posing significant challenges for healthcare systems due to their high morbidity, long disease course, and the lack of curative treatments [1]. This heterogeneous group of disorders comprises, among others, Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), and Amyotrophic Lateral Sclerosis (ALS). AD is the most common cause of dementia, affecting over 50 million individuals worldwide [2]. Clinically, it is characterized by progressive memory loss, cognitive dysfunction, and behavioral changes, primarily resulting from the accumulation of extracellular amyloid beta (Aβ) plaques and intracellular neurofibrillary tangles composed of hyperphosphorylated tau protein [3]. These aggregates disrupt synaptic function, trigger neuroinflammation, and promote neurodegeneration, starting in the hippocampus and ultimately affecting the entire cerebral cortex. PD, the second most frequent NDD, is a progressive movement disorder primarily resulting from the degeneration of dopaminergic neurons in the substantia nigra pars compacta [4]. Clinically, PD is characterized by motor symptoms, including tremors, bradykinesia, rigidity, and postural instability, as well as non-motor symptoms such as cognitive impairment, depression, and autonomic dysfunctions, which significantly impact the patient’s quality of life [5]. The pathological hallmarks of PD include the presence of Lewy bodies, composed of misfolded α-synuclein and extensive loss of dopaminergic neurons [6]. HD is a rare, autosomal dominant NDD caused by a CAG trinucleotide repeat expansion in the HTT gene, leading to the production of mutant huntingtin protein [7]. This toxic protein accumulates in neurons, causing progressive motor dysfunction, psychiatric symptoms, and cognitive decline [8]. Compared to the other NDDs, HD is unique in that the genetic cause is known and consistent across all affected individuals, making it a prime candidate for targeted molecular therapies. Finally, ALS is a rapidly progressive neurodegenerative disorder that affects both upper and lower motor neurons, leading to muscle weakness, atrophy, fasciculations, and ultimately respiratory failure [9]. Most cases are sporadic, but approximately 10% are familial, often linked to mutations in genes such as SOD1, C9orf72, and TARDBP [10].
Despite extensive research, treatment strategies for NDDs remain largely symptomatic, offering only modest improvements in quality of life and limited slowing of disease progression. The complexity of NDDs arises from the incompletely understood, diverse etiologies, as well as the co-existence of multifactorial pathogenic mechanisms, including protein misfolding and aggregation, mitochondrial dysfunction, oxidative stress, excitotoxicity, impaired axonal transport, and chronic neuroinflammation [11]. Moreover, the blood–brain barrier (BBB), the highly selective semipermeable border that protects the central nervous system (CNS), simultaneously restricts the entry of potentially therapeutic agents [12]. This has significantly hindered the effective delivery of emerging therapies, particularly those based on large or polar molecules such as antisense oligonucleotides (ASOs), monoclonal antibodies, viral vectors for gene therapy, and stem cells. With many promising agents failing to reach therapeutic concentrations in target CNS regions when administered systemically, it is currently a critical unmet need for delivery strategies that can bridge this gap.
In this context, intrathecal (IT) drug administration—the delivery of therapeutic agents directly into the cerebrospinal fluid (CSF)—addresses this need by bypassing the BBB and achieving immediate proximity to the CNS milieu [13]. This approach can yield markedly higher central drug concentrations with lower systemic exposure, enabling the use of modalities that would otherwise be pharmacologically inaccessible. In recent years, IT delivery has moved beyond niche indications and into the mainstream of experimental neurotherapeutics, underpinning trials of ASOs, gene therapy vectors, targeted immunotherapies, and regenerative cell-based products. Yet, the current literature remains fragmented: while individual studies demonstrate feasibility and preliminary efficacy, there is no comprehensive synthesis that critically appraises device technology, pharmacokinetics, and safety across NDDs. Addressing this knowledge gap is essential to accelerate translation, optimize delivery systems, and inform regulatory pathways for next-generation CNS therapies.
The aim of this review is to synthesize the current landscape of IT therapies for the aforementioned NDDs, based on the current literature and ClinicalTrials.gov. The most relevant trials and therapeutic approaches are highlighted, including the success of ASOs in Spinal muscular atrophy and recent progress in other NDDs. To offer a precise picture of the situation, the limitations of first-generation IT delivery systems are also presented, which struggle with uneven distribution, systemic leakage, and the demands of modern biologics. These critical shortcomings of current devices are detailed, along with the innovations shaping next-generation systems: subcutaneous access ports, CSF flow platforms, AI-driven adaptive dosing, nanoporous membranes, intrathecal pseudodelivery, and hydrogel scaffolds. The conclusion emphasizes the urgent need for the development of advanced IT drug delivery systems, alongside rigorous comparative assessments, cost–benefit analyses, and clear regulations to fully realize the potential of emerging CNS therapies, transform NDD management, and improve patients’ quality of life and lifespan.
2. Intrathecal Route: Principles and First-Generation Devices
The intrathecal (IT) route of drug administration involves delivering therapeutic agents directly into the CSF, bypassing the restrictive blood–brain barrier (BBB). This enables therapeutics, particularly large molecules such as biologics, ASOs, and stem cells, to reach target tissues in the CNS at therapeutic concentrations. Although the clinical application of IT delivery is still evolving, the great potential of this method depends primarily on understanding the anatomical and physiological principles that underlie the intrathecal route and the improvement in first-generation devices.
The relevant anatomical and physiological aspects from a therapeutic perspective are related to the meninges surrounding the CNS and the role of the CSF in providing mechanical cushioning, immunological protection, nutritional support, and waste exchange [14]. The CSF, summing approximately 150 mL, circulates through the ventricular system and subarachnoid space, undergoing complete turnover approximately four to five times per day via absorption at the arachnoid villi into the venous sinuses [15]. Moreover, when considering peripherally administered medication, the BBB acts as a highly selective barrier for substances entering the CNS from the systemic circulation [16]. Although this structure plays a crucial role in protecting the brain from toxins and maintaining homeostasis, it severely restricts the entry of potentially therapeutic macromolecules.
Intrathecal administration bypasses the BBB by directly accessing the CSF. From the CSF, therapeutic agents can distribute along the neuraxis, particularly targeting the periventricular, cortical, and spinal cord regions. IT administration follows distinct pharmacokinetic principles compared to systemic delivery [17]. Upon injection into the lumbar subarachnoid space, therapeutics distribute in a rostral direction due to pulsatile CSF flow driven by cardiac and respiratory cycles [18]. The distribution and clearance of drugs within the CSF are influenced by several factors, including drug-related factors such as molecular size, charge, or lipophilicity, as well as patient-related factors, including CSF flow dynamics [19]. Larger molecules (e.g., ASOs, proteins, viral vectors) exhibit limited diffusion and are more reliant on convective CSF flow. Charged and hydrophilic molecules tend to remain within the CSF compartment, whereas lipophilic compounds have an increased biodisponibility [20]. Factors related to administration protocols, such as injection volume, drug concentration, and excipients, can also affect CNS spread and penetration. When considering CSF dynamics, pathological conditions such as hydrocephalus or spinal stenosis can alter mechanically drug dispersion and lead to localized drug accumulation or reduced efficacy [21]. Clearance of IT-administered drugs occurs primarily via bulk flow into systemic circulation through arachnoid granulations, glymphatic drainage pathways, and, to a lesser extent, perivascular routes [22]. Or, in NDDs, particularly AD, there is a reduced rate of substance clearance from the CNS, influencing the drug concentration balance [23]. The half-life of IT-delivered substances in the CSF is typically longer than plasma administered drugs, enhancing the potential for sustained CNS exposure with appropriately designed delivery systems.
From a historical perspective, the concept of IT administration dates back to the early 20th century, when it was originally employed for spinal anesthesia and the treatment of infectious meningitis [24]. The use of the IT route for chronic neurological conditions, however, gained importance in the latter half of the 20th century. Among the key milestones worth mentioning are the 1970s and 1980s, when implantable pumps for the continuous IT administration of analgesics (e.g., morphine) and muscle relaxants (e.g., baclofen) were developed [25]. During the 1990s and 2000s, IT delivery was proposed for chemotherapy in CNS malignancies and leptomeningeal metastases [26]. In 2016, the Food and Drug Administration (FDA) approved Nusinersen, an ASOs administered via intrathecal injection for spinal muscular atrophy (SMA), marking the first intrathecal gene-targeted therapy in humans for broad clinical use [27]. More recently, in 2023, the FDA approved tofersen, an intrathecally administered ASO for SOD1-mutant ALS patients [28]. These milestones are a clear indication of the potential of IT routes in treating various NDDs, with the guidelines and recommendations expected to broaden in the next few years.
The success of IT therapies depends not only on the therapeutic molecule, which is expected to be improved thanks to the advancements in drug design, but also heavily relies on the reliability, safety, and reproducibility of the delivery system. First-generation intrathecal devices include spinal needles for repeated lumbar punctures, percutaneous catheters, and implantable infusion systems [29]. While each method has specific clinical applications and its particularities, they collectively represent the foundational tools for CNS-directed therapy.
For many IT-delivered biologics and ASOs, including nusinersen and tofersen, drug administration is currently achieved via lumbar puncture using a spinal needle. This technique, involving the percutaneous insertion of a needle (typically 20–25 gauge) between the L3 and L5 vertebrae to access the subarachnoid space, is preferred due to its simplicity and regulatory acceptance [30]. The advantages of this technique worth mentioning are the low initial costs, minimal invasiveness, and the absence of surgical implantation requirements. Still, procedural discomfort and anxiety, particularly challenging in patients with scoliosis, spinal degenerative disorders, or obesity, the risk of post-puncture headache, and the impracticality for frequent or long-term use are the most relevant limitations of this approach [31]. Additionally, there are factors such as steep rostrocaudal gradients after lumbar bolus injections and substantial sensitivity to injection volume, rate, and patient-specific CSF flow that limit reliable supratentorial/parenchymal exposure for large biologics (e.g., antibodies, ASOs) and make dosing less reproducible across subjects. Recent in vitro and in silico human models confirm that modifying parameters (volume, rate, injection site) can improve cranial transport, and even optimized bolus protocols usually give more heterogeneous brain exposure than continuous approaches [32].
For more frequent or continuous delivery, intrathecal catheters may be employed. These catheters are surgically placed into the lumbar or thoracic subarachnoid space and are often connected to an external port or reservoir. In daily clinical practice, they are currently used for the administration of intrathecal baclofen in cases of severe spasticity [33], when orally administered medication is insufficient, and for the delivery of chemotherapeutics in CNS tumors [34]. In the research field, they are used for experimental gene or cell therapy studies requiring repeated access. The most relevant challenges faced by clinicians in this procedure are the risk of infection and catheter dislodgement, the potential for fibrosis and catheter occlusion, and the necessity of routine monitoring and maintenance [35]. Although more invasive than the classical lumbar puncture, intrathecal catheters allow for controlled delivery, programmable dosing, and the possibility of combination therapies.
Finally, implantable intrathecal infusion pumps provide programmable, long-term delivery of IT medications and are particularly useful in managing chronic pain and spasticity [36]. Devices such as the Medtronic SynchroMed™ II system (Medtronic, Medtronic Parkway Minneapolis, MN, USA) consist of a subcutaneously implanted reservoir connected to an intrathecal catheter. The primary strengths of this method lie in its continuous steady-state drug delivery and the reduced need for frequent lumbar punctures, which is particularly convenient for patients with improved compliance [37]. Still, the approach requires surgical implantation, peri- and postprocedural risks such as pump malfunction, infections, and catheter-related complications [38]. While currently used primarily for small-molecule therapeutics, there is limited experience with large biomolecules (e.g., ASOs, viral vectors). Still, there is growing interest in adapting these systems for the delivery of biologics and gene therapies; however, compatibility and stability issues should be addressed, particularly when considering patients with NDD who require long-term follow-up.
Indwelling catheters with subcutaneous access ports and implantable pumps enable repeated bolus dosing, leading to improved adherence and cumulative exposure; however, they also carry risks of device-related infections and catheter malfunctions. Contemporary series report perioperative or device-related infection rates for intrathecal drug delivery systems in the 2–6% range, with long-term series indicating roughly 0.7% per patient-year in some cohorts. Programmable implantable pumps (continuous infusion) typically provide the steadiest CSF levels and, in preclinical and clinical examples, can produce more homogeneous CNS exposure for large molecules than isolated lumbar boluses, a potential pharmacokinetic advantage for large biologics or enzyme/gene therapies where sustained gradients improve parenchymal penetration.
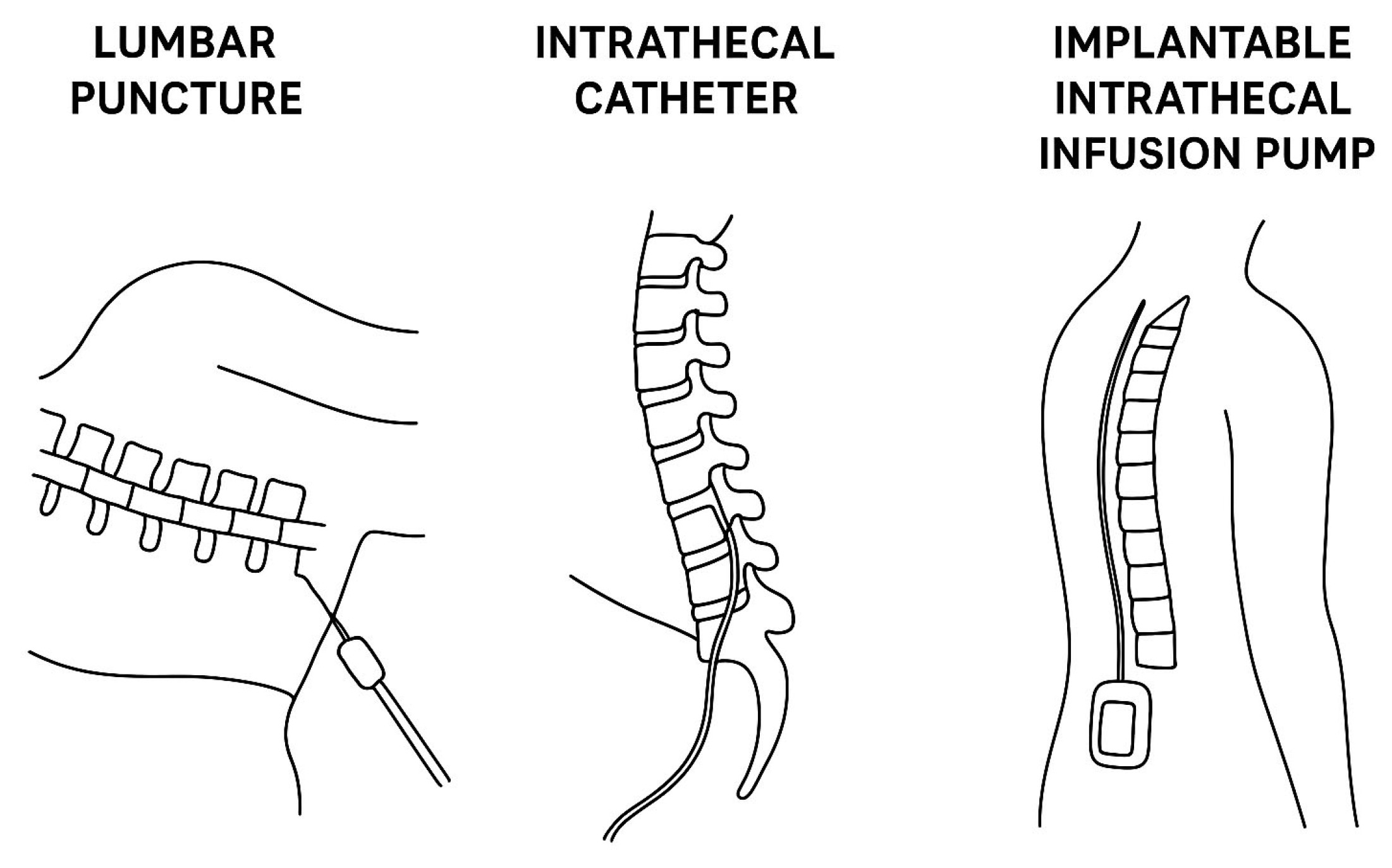
Table 1 summarizes the most relevant first-generation devices and common approaches for IT drug delivery, while Figure 1 reveals in a schematic manner the differences between the three approaches.

Table 1.
Advantages and limitations of the most relevant approaches and devices for intrathecal drug delivery.
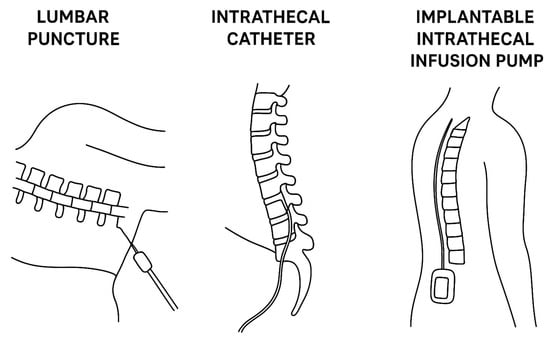
Figure 1.
Comparative illustration of the most frequent approaches for intrathecal therapy delivery (lumbar puncture versus intrathecal catheter versus implantable intrathecal infusion pump).
3. Intrathecal Therapies in NDDs: A Clinical Trial Landscape
We have structured this chapter in a systematic manner to cover the most significant clinical trials conducted in recent years, with a focus on the most relevant NDDs.
3.1. Alzheimer’s Disease
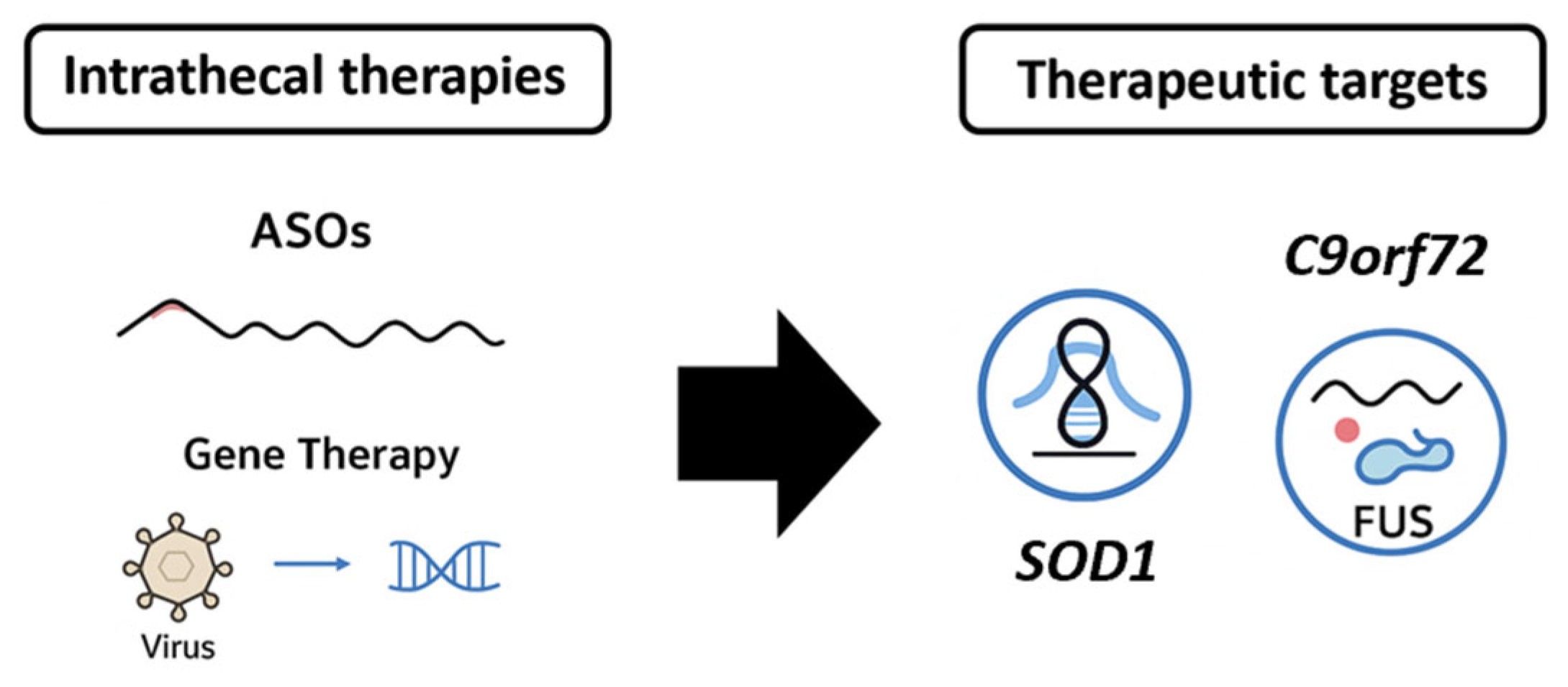
While AD remains the most prolific among NDDs in terms of clinical trials of novel therapeutic compounds [39], an incompletely explored part is related to IT-delivered anti-AD drugs. Based on the current literature and ClinicalTrials.gov, three main classes of pharmaceutics are relevant: ASOs targeting Tau protein and Amyloid Precursor Protein (APP), anti-amyloid beta (Aβ) antibodies, and gene therapies.
3.1.1. Asos in Alzheimer’s Disease
The development of ASOs that modulate RNA expression represents a transformative approach in the treatment of NDDs. In AD, ASOs target either tau pathology, with BIIB080 as a representative compound, or APP. Hyperphosphorylated tau remains one of the main pathological changes, strongly correlated with AD’s clinical progression, making it an attractive target for ASO therapy [40]. BIIB080 (also known as IONIS-MAPTRx) is a tau-targeted ASO developed through a collaboration between Ionis Pharmaceuticals and Biogen [41]. Administered intrathecally, BIIB080 binds to the pre-mRNA of the MAPT gene, promoting its degradation via RNase H-mediated cleavage, thereby reducing tau protein synthesis. Initial clinical evaluation of BIIB080 was the object of a Phase 1b randomized placebo-controlled trial (NCT03186989) [42]. This dose-escalation study enrolled patients with mild AD and demonstrated favorable safety and tolerability of up to 60 mg IT every 12 weeks. Notably, CSF total tau and phosphorylated tau levels decreased in a dose-dependent manner, with reductions sustained for several months post-injection. These findings were corroborated by exploratory neuroimaging endpoints, which suggested potential stabilization of disease-related atrophy patterns. The trial’s results laid the groundwork for a larger Phase 2 study (NCT04986707), which is currently enrolling participants to assess long-term efficacy and cognitive outcomes [43].
Although less developed than tau-targeted approaches, antisense oligonucleotides (ASOs) directed at APP mRNA represent an emerging strategy for reducing Aβ production, as Aβ is a key component of senile plaques. Preclinical models have demonstrated that APP-lowering ASOs can lead to substantial reductions in Aβ42 without perturbing essential physiological functions [44]. While no APP-targeted ASO has yet entered late-stage clinical trials for AD, early phase programs are in development, leveraging the IT route for precise CNS delivery. These programs aim to validate the concept of upstream mRNA intervention, potentially synergizing with or replacing anti-Aβ monoclonal antibodies [45]. Still, several challenges remain in the application of ASOs in AD management. Widespread tau expression requires regionally broad CNS distribution, which is currently limited by CSF flow heterogeneity. Dosing schedules involving IT injections every 12–16 weeks may present barriers to compliance in an elderly population [46]. Nevertheless, the preliminary promising data from BIIB080 clinical trials suggest that IT ASO therapy is biologically active and well-tolerated in AD, meriting continued investigation.
3.1.2. Monoclonal Antibodies in Alzheimer’s Disease
Monoclonal antibodies targeting Aβ (e.g., aducanumab, lecanemab) have demonstrated modest clinical benefits in early-stage AD when administered intravenously [47]. However, systemic delivery often results in limited CNS penetration, with only ~0.1–0.5% of the injected dose crossing the BBB [48]. This inefficiency not only increases the required dose and costs but also contributes to peripheral adverse effects, such as amyloid-related imaging abnormalities (ARIA) [49]. A potential strategy to overcome these limitations could be the IT administration of anti-Aβ antibodies. By delivering antibodies directly into the CSF, higher CNS concentrations may be achieved with lower total doses, potentially enhancing efficacy while reducing systemic exposure. One of the first IT antibody trials in AD is NCT03397506, a Phase 1 study evaluating the safety and pharmacokinetics of an investigational anti-Aβ monoclonal antibody administered via the intrathecal route [50]. This open-label, dose-escalation study enrolled subjects with early symptomatic AD and examined various dosing regimens to determine optimal CNS bioavailability. Although detailed results have not yet been published, preliminary data suggest that IT delivery results in significantly higher CSF-to-plasma concentration ratios compared to IV administration, confirming the pharmacological rationale of this approach. With many unknowns still remaining, future trials are expected to assess not only pharmacokinetics but also target engagement (e.g., CSF Aβ reduction, PET imaging) and cognitive outcomes. Even this medication might raise challenges and have associated risks. For example, CSF dynamics and antibody distribution can have individual variability, complicating dosing standardization and requiring tailored therapies. Immunogenicity and ARIA risk remain concerns; however, initial findings suggest that intrathecal administration, by avoiding peripheral accumulation, may reduce the incidence of ARIA.
3.1.3. Gene Therapy in Alzheimer’s Disease
Finally, an interesting and promising therapeutic avenue in NDDs comprises gene therapy, offering the potential to provide sustained, and in some cases, one-time disease-modifying interventions. Gene therapy in AD aims to either enhance neuroprotection or modulate pathogenic processes, such as amyloidogenesis and tau hyperphosphorylation [51]. The IT route provides a compelling delivery platform for gene therapies, offering direct access to the CNS, particularly for vector-based approaches. Historically, gene therapy in AD focused on targeted intracerebral delivery of neurotrophic factors such as nerve growth factor (NGF) [52] or brain-derived neurotrophic factor (BDNF) [53], based on the observation that basal forebrain cholinergic neurons are particularly vulnerable to degeneration in early AD. However, intracerebral injection is invasive, has limited distribution, and is logistically challenging. The IT route presents an alternative that is both less invasive and potentially capable of achieving more widespread CNS gene expression, particularly when using engineered viral vectors with enhanced neuronal tropism. Among the various vector platforms, adeno-associated virus (AAV) has emerged as the most widely used for CNS-directed gene therapy, due to its favorable safety profile, low immunogenicity, and capacity for long-term gene expression [54]. Specific serotypes, particularly AAV9 and AAVrh10, have demonstrated robust CNS tropism when administered via the IT route [55]. AAV9 has been shown to efficiently transduce neurons, astrocytes, and oligodendrocytes throughout the spinal cord and brain, especially in younger individuals and animal models [56]. AAVrh10, derived from non-human primates, offers similar advantages and may be less susceptible to neutralizing antibodies in the general population [57]. Preclinical studies have demonstrated that IT administration of these vectors can result in widespread CNS gene expression, particularly when administered at the lumbar cistern or via cisterna magna injection. The biodistribution achieved with IT delivery has been shown to encompass hippocampal, cortical, and thalamic regions—areas of high relevance in AD pathology.
The primary therapeutic strategies under investigation for IT gene therapy in AD can be categorized into three main areas: neurotrophic support, modulation of amyloid and tau, and support for synaptic and mitochondrial function [58]. With early-stage AD being characterized by a loss of cholinergic neurons in the basal forebrain, neurotrophic factors such as NGF and BDNF have been investigated for their potential to support these neurons. In early clinical studies (e.g., NCT00087789), NGF gene delivery via stereotactic injections has demonstrated long-term neuronal viability, as evidenced by the upregulation of choline acetyltransferase (ChAT) and persistent transgene expression for several years [59]. However, the limited distribution and surgical risk prompted the exploration of IT delivery as a less invasive, scalable approach. Preclinical IT-AAV-NGF studies have demonstrated successful transgene expression in hippocampal and cortical neurons, accompanied by improvements in memory tasks.
Another promising application is the delivery of genes that either suppress amyloidogenic pathways or promote tau clearance. Preclinical models have utilized AAV-mediated delivery of neprilysin, an enzyme that degrades Aβ, resulting in a reduced plaque burden and improved cognition [60]. Similarly, AAV vectors encoding tau-targeting intrabodies or microRNAs have shown efficacy in reducing tau pathology when delivered via the CSF [61]. More recent strategies aim to enhance synaptic plasticity and metabolic resilience in AD neurons. PGC-1α, a regulator of mitochondrial biogenesis, has been delivered via AAV to improve neuronal energy metabolism and reduce oxidative stress [62]. Similarly, overexpression of synaptic regulators such as PSD95 or Homer1a has been proposed to counteract synaptic failure; however, these remain primarily in early development [63].
While many IT gene therapy strategies for AD are in preclinical or early translational stages, several clinical trials and IND-enabling programs are underway. For example, a Phase 1/2 program (not yet publicly registered) is reportedly investigating IT-AAV-BDNF delivery in early-stage AD patients, building on animal data showing improved memory and synaptogenesis.
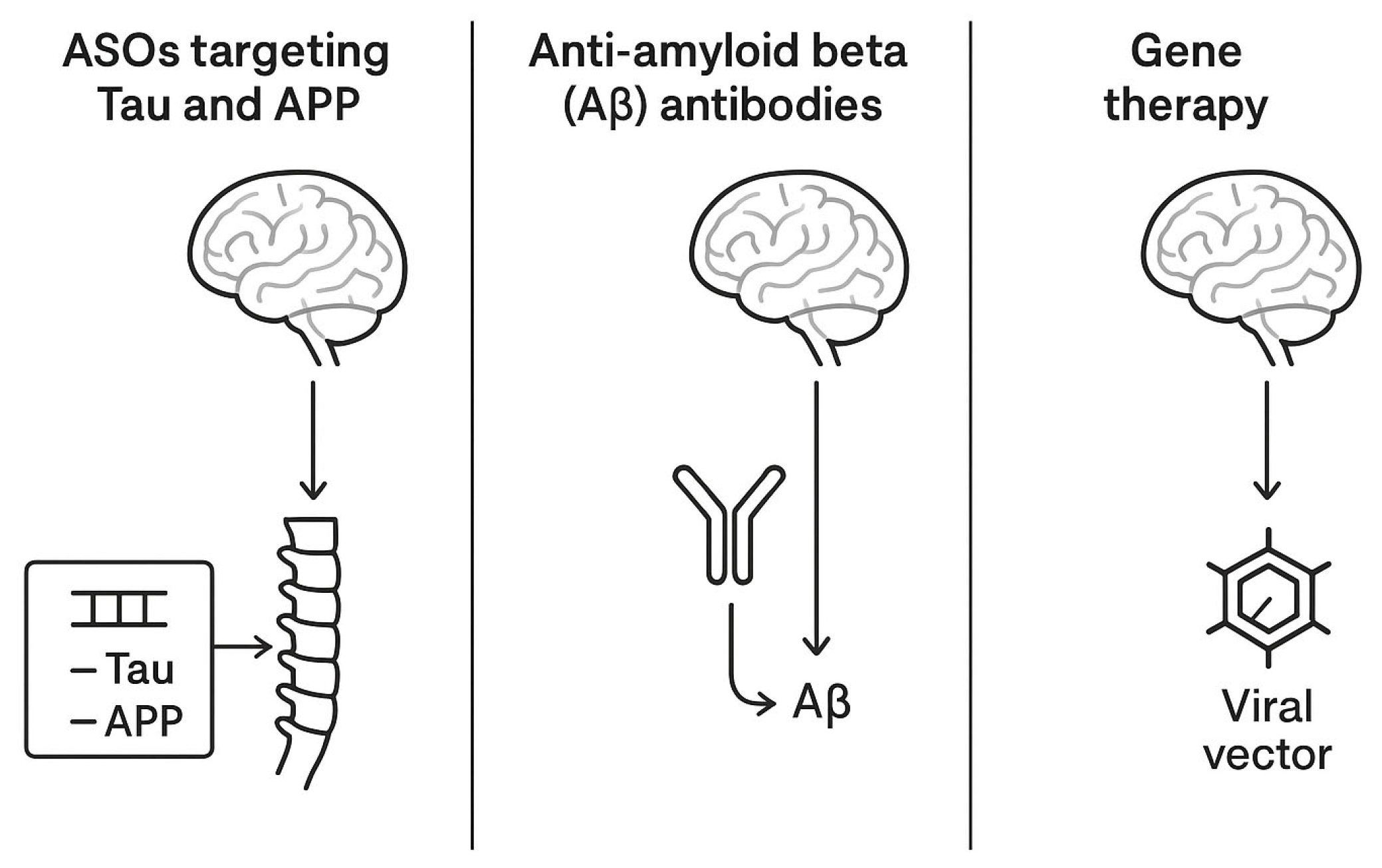
Other industry-sponsored programs are targeting tau expression modulation using gene therapy vectors encoding tau-lowering RNA interference sequences, administered intrathecally for maximal parenchymal access. Table 2 summarizes the most relevant aspects of the clinical trial landscape in AD, while Figure 2 offers an illustrative comparative overview of the therapies.

Table 2.
Current status of clinical trials in AD (↑—increase in value).
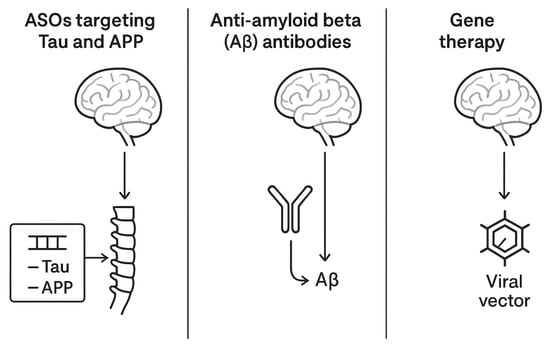
Figure 2.
A comparative schematic representation of intrathecal therapies for AD.
Although the Gene Therapy Advisory Committee (GTAC) and regulatory bodies have raised concerns about vector immunogenicity, long-term expression control, and off-target effects, advances in self-limiting vectors, inducible promoters, and immunosuppression regimens are helping mitigate these risks [64]. Despite the promise, several challenges limit the near-term translation of IT gene therapy in AD:
- -
- Nonuniform Vector Distribution: Even with intrathecal delivery, complete cortical and hippocampal coverage remains difficult, especially in older patients with altered CSF dynamics or brain atrophy.
- -
- Preexisting Immunity to AAV Serotypes: Up to 60% of the population harbors neutralizing antibodies to common AAVs, which may reduce efficacy or pose safety risks.
- -
- Dose Escalation Risks: High-dose IT vector administration, as seen in spinal muscular atrophy (SMA) gene therapy, carries risks of aseptic meningitis, liver toxicity, and sensory neuronopathy [65].
The coming years will be critical in determining whether gene therapy, along with the other aforementioned medication administered intrathecally, can transition from theoretical promise to daily clinical practice, improving the prognosis of AD patients.
3.2. Parkinson’s Disease
3.2.1. Gene Therapy in Parkinson’s Disease
When considering PD, although current pharmacotherapies are more diverse than anti-AD medications and provide satisfactory motor symptom relief in most patients, they do not modify disease progression [66]. Furthermore, their efficacy diminishes over time, with late-stage PD patients suffering from motor fluctuations, dyskinesias, and the exacerbation of non-motor symptoms [67]. Consequently, disease-modifying therapies that target the underlying neurodegenerative processes are needed. Gene therapy and neurotrophic factor delivery have emerged as promising strategies, particularly when administered via intrathecal or cisternal routes, having advantages over the peripheral delivery method. This section primarily focuses on gene therapy targeting GBA1 mutations, and neurotrophic factor delivery, highlighting both their mechanistic rationale and the translational challenges associated with them.
Mutations in the GBA1 gene, which encodes the lysosomal enzyme glucocerebrosidase (GCase), represent the most common genetic risk factor for PD [68]. Both homozygous and heterozygous GBA1 mutations reduce GCase activity, resulting in impaired lysosomal function, accumulation of α-synuclein, and increased neuronal vulnerability [69]. GBA1 mutation carriers tend to have earlier PD onset, faster progression, and more severe non-motor symptoms, particularly cognitive impairment [70]. Enhancing GCase activity has thus become a therapeutic strategy aimed at restoring lysosomal homeostasis, improving α-synuclein clearance, and slowing disease progression [71]. Small-molecule chaperones and enzyme replacement therapies have been tested with limited success due to insufficient CNS penetration. In contrast, AAV-mediated gene therapy provides a means to deliver the GBA1 gene directly to affected neurons, thereby enabling sustained enzymatic activity following a single administration.
PR001 is an investigational AAV9-based gene therapy that delivers a functional copy of the human GBA1 gene under a CAG promoter [72]. Developed by Prevail Therapeutics (now part of Eli Lilly), PR001 is designed to increase GCase expression throughout the CNS, following a single intrathecal administration into the cisterna magna. This delivery site, located at the cranial end of the spinal subarachnoid space, is advantageous for distributing viral vectors to both the brain and spinal cord through CSF circulation, with increased access to midbrain structures such as the substantia nigra. Preclinical studies in rodent and non-human primate models of GBA1-associated PD demonstrated that cisterna magna injection of PR001 leads to widespread vector biodistribution, robust transgene expression in cortical and subcortical regions, increased GCase activity and reductions in α-synuclein accumulation. Behavioral improvements and neuroprotection were also observed in these models, supporting the translational potential of this approach [73].
PR001 is currently under investigation in a Phase 1/2 clinical trial (PROPEL; NCT04127578) in patients with PD and biallelic or heterozygous GBA1 mutations. The trial is an open-label, dose-escalation study evaluating safety, tolerability, and exploratory efficacy of a one-time cisterna magna infusion of PR001. Early data from this trial have shown an acceptable safety profile at low and intermediate doses, with transient fever and headache as the most common adverse events, with dose-dependent increases in CSF GCase activity, sustained for several months post-administration. Preliminary biomarker signals suggested a reduction in CSF α-synuclein levels. Still, longer-term follow-up and efficacy data, including motor and cognitive assessments, are pending. A parallel natural history study (NCT04128245) is also being conducted to better understand disease progression in GBA1-PD and facilitate the interpretation of endpoints [74].
Several challenges and aspects should be addressed to improve the outcomes of this therapy. Firstly, patient selection should be more rigorous. While GBA1 mutations are a logical starting point, their clinical heterogeneity necessitates precise stratification. Further studies are needed to evaluate whether non-GBA1 PD patients may benefit from similar lysosomal-targeted therapies [75]. The risk of AAV-neutralizing antibodies and cellular immune responses still remains; therefore, pre-screening for anti-AAV9 titers and optimized immunosuppression protocols are essential [76]. Finally, vector engineering to enhance cell-specific tropism and optimize spread to the CSF and CNS remains a priority.
3.2.2. Neurotrofic Factors in Parkinson’s Disease
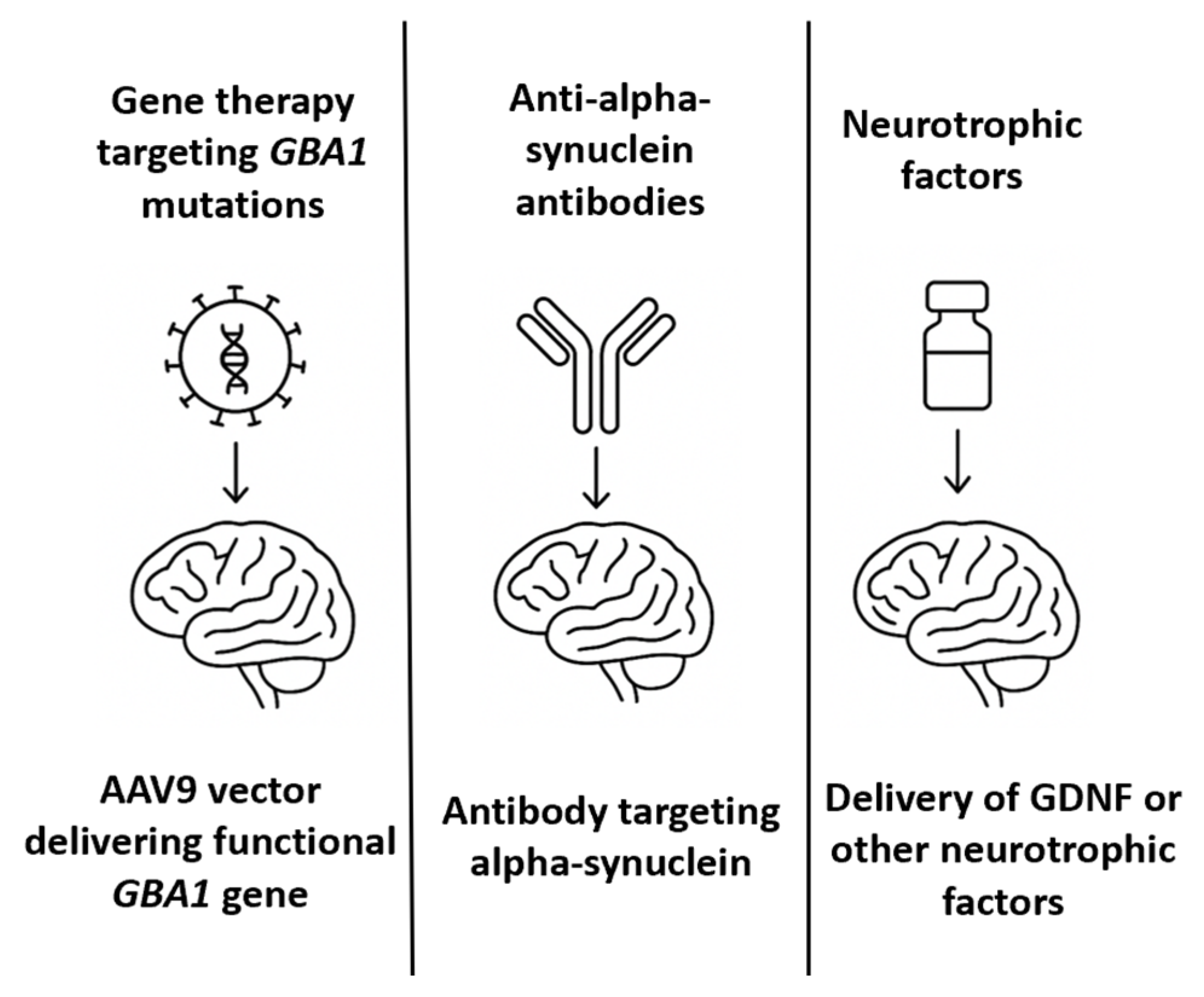
A long-standing therapeutic hypothesis in PD is that the exogenous administration of neurotrophic factors could support the survival of dopaminergic neurons, promote regeneration, and slow disease progression [77]. Among the most studied is glial cell line-derived neurotrophic factor (GDNF), which binds to the GFRα1/RET receptor complex to activate intracellular survival pathways in nigrostriatal neurons [78]. In PD patients, endogenous GDNF expression is significantly reduced in the substantia nigra [79]. Preclinical studies in 6-OHDA and MPTP models have demonstrated that GDNF delivery can reverse dopaminergic degeneration and restore motor function. Initial relevant clinical trials of GDNF in PD include an open-label Phase 1 study in which patients received continuous intraputaminal GDNF infusion, resulting in improved motor scores and PET-documented dopaminergic activity [80]. A subsequent randomized, placebo-controlled Phase 2 trial failed to meet primary endpoints, raising questions about efficacy, placebo response, and delivery variability. Considering the limitations associated with intracerebral infusion, alternative delivery routes—including intrathecal and intracisternal administration—have been explored to achieve broader CNS distribution. Preclinical studies have demonstrated that GDNF delivered intrathecally or via the cisterna magna can diffuse through perivascular spaces and reach midbrain targets; however, the concentrations achieved in the putamen are lower than those achieved via direct infusion [81]. To overcome these limitations, gene therapy vectors encoding GDNF have been developed. AAV2-GDNF was tested in Phase 1/2 trials via stereotactic injection into the putamen [82]. These trials demonstrated safety and target expression but were limited by the slow onset of clinical benefit and concerns about retrograde transport to the substantia nigra. Alternatively, several biotechnology companies and academic groups are now investigating next-generation neurotrophic factor therapies, including encapsulated cell therapies (e.g., NTCELL), which secrete GDNF locally after implantation into the striatum, synthetic mimetics of GDNF that are small enough to cross the BBB and bind GFRα1/RET receptors and mRNA therapies encoding neurotrophic factors [83]. Table 3 summarizes the most relevant milestones in PD therapeutic approaches, while Figure 3 offers an illustrative comparative overview of these therapies.

Table 3.
Current status of clinical trials in PD (→—determines the following process; ↑—increase in value).
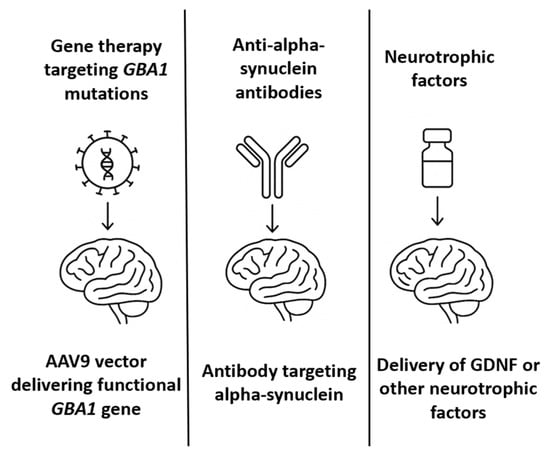
Figure 3.
A comparative schematic representation of intrathecal therapies for Parkinson’s disease.
It is clear that future strategies must integrate advanced delivery systems, such as convection-enhanced delivery (CED), nanoparticle carriers, or gene therapy vectors optimized for IT use. Biomarker-driven patient selection and real-time imaging of target engagement will be critical for trial success.
3.3. Huntington’s Disease
In the context of HD, ASOs are used to selectively degrade HTT mRNA, thereby reducing production of both mutants and, in some cases, wild-type huntingtin proteins [84]. Preclinical studies in HD mouse and non-human primate models have demonstrated that ASOs-mediated HTT suppression results in significant reductions in mHTT levels in the brain and CSF, restoration of transcriptional dysregulation, and improvements in motor performance and survival [85]. The pioneering molecule in this domain is Tominersen (formerly IONIS-HTTRx or RG6042), a fully phosphorothioate-modified 2′-O-methoxyethyl ASO developed by Ionis Pharmaceuticals and Roche, targeting both wild-type and mutant HTT mRNA transcripts [86]. It was the first HTT-lowering therapy to enter human trials and remains the most extensively studied ASOs in HD. to date. The phase 1/2a Trial (NCT02519036) was the first-in-human study demonstrating that monthly IT administration of tominersen led to dose-dependent reductions (up to 40%) of mHTT in CSF in early manifest HD patients [87]. The drug was well tolerated at lower doses, and exploratory cognitive and motor endpoints trended positively. A phase 3 randomized, placebo-controlled trial (GENERATION HD1 trial–NCT03761849) aimed to evaluate the efficacy of tominersen in 791 early manifest HD patients [88]. Participants received either 120 mg every 8 weeks or every 16 weeks via lumbar puncture. However, in March 2021, the study was halted early due to a lack of efficacy and worsening outcomes in the higher-frequency dosing arm. The data showed modest reductions in mHTT, but the every-8-weeks group experienced more adverse events, including ventricular enlargement, increased neurofilament light chain (NfL) levels, and clinical deterioration [89]. These findings prompted a need for reassessment of HTT-lowering strategies. Still, post hoc analyses suggested that certain subgroups (e.g., younger patients with lower disease burden) may still benefit from therapy. Currently, a new trial (GENERATION HD2, NCT05686551) is ongoing, with tominersen being administered in lower and less frequent doses to reduce toxicity and maintain target engagement [90].
Given the potential risks of lowering wild-type HTT, another interesting alternative approach is based on allele-selective ASOs that preferentially target mutant HTT mRNA. These designs exploit single-nucleotide polymorphisms (SNPs) in linkage disequilibrium with the CAG expansion to selectively degrade the mutant transcript [91]. Programs such as Wave Life Sciences’ WVE-120101 and WVE-120102, which targeted specific SNPs (rs362307 and rs362331), initially showed suboptimal efficacy and were discontinued [92]. However, newer backbones and improved chemistry (e.g., PN backbone) have revived interest in next-generation SNP-targeting ASOs. The WVE-003 program (NCT05032196) is currently under evaluation for safety, tolerability, and target engagement after IT administration [93].
Unlike ASOs, gene therapy approaches aim to permanently suppress or repair the HTT gene using viral vectors such as adeno-associated viruses (AAVs). These vectors can deliver short-hairpin RNAs (shRNAs), artificial microRNAs (miRNAs), or zinc finger repressors directly into the brain, leading to long-lasting mHTT knockdown [94]. However, the large size of the HTT gene (exceeding AAV capacity) and broad CNS involvement of HD make gene therapy delivery particularly challenging. Consequently, many programs have opted for region-specific intracranial delivery, especially targeting the striatum and thalamus. One example is the AMT-130 (NCT04120493) trial. Developed by uniQure, this AAV5-based gene therapy expresses an miRNA targeting exon 1 of the HTT transcript. Administered via bilateral intrastriatal infusion using convection-enhanced delivery (CED), AMT-130 has shown sustained mHTT knockdown in preclinical models [95]. An ongoing Phase 1/2 trial is assessing the long-term safety, reduction in mHTT in CSF, and imaging-based biomarkers. Voyager Therapeutics’ VY-HTT01 (now discontinued) utilized an AAV vector with an artificial miRNA delivered directly into the striatum [96]. Despite promising preclinical data, the program was halted due to concerns over delivery complexity and safety. Currently, no AAV-based HTT-lowering therapy has utilized the intrathecal route in clinical trials, primarily due to limitations in vector spread and tropism. However, advances in capsid engineering (e.g., AAV.PHP.B, AAV9 variants) and promoters may enable IT or cisternal delivery of HTT-targeting vectors in future iterations [97].
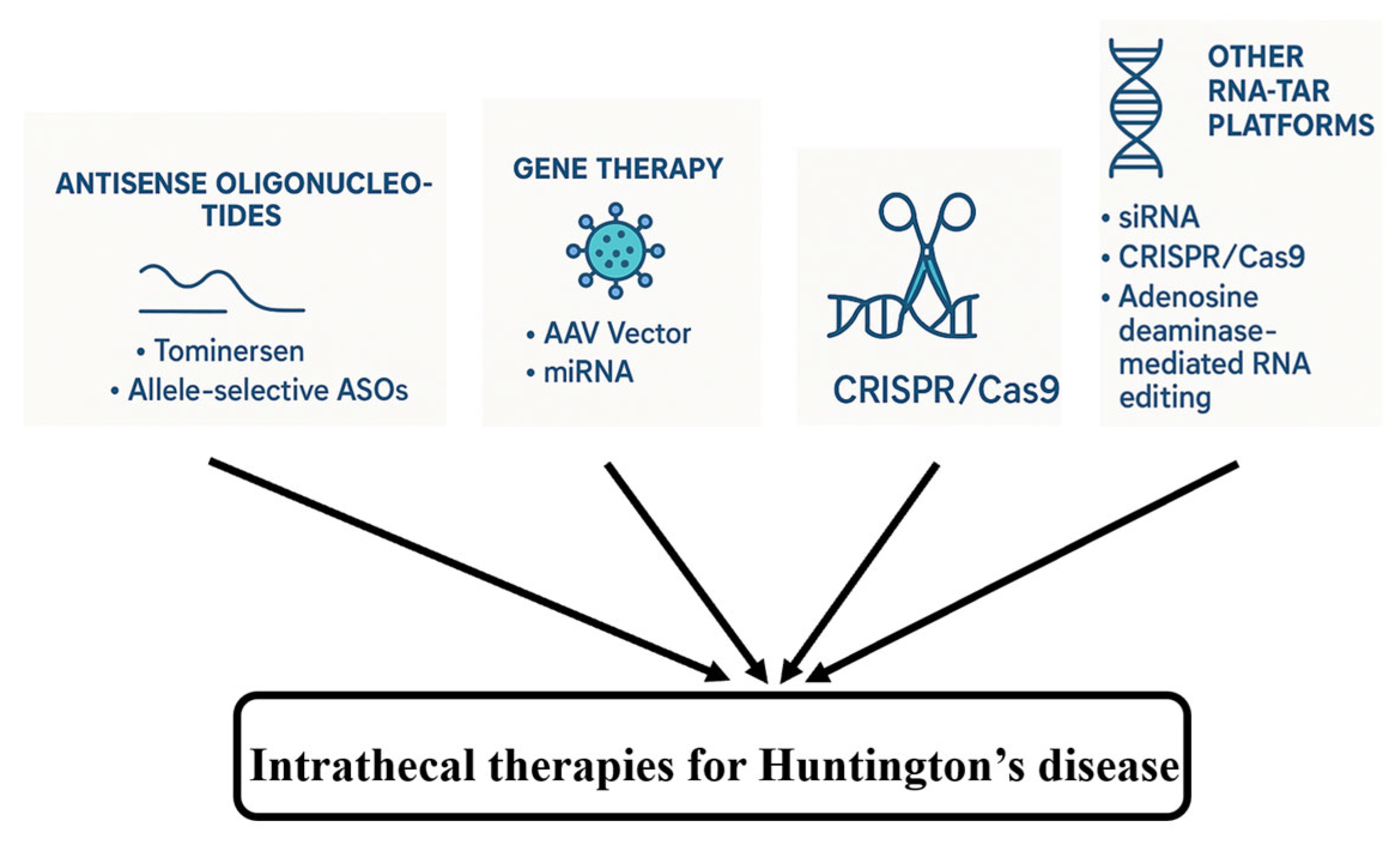
Beyond gene transfer, other RNA-targeted platforms are being explored for IT delivery. For example, similar to ASOs, small interfering RNAs (siRNAs) have been investigated for HTT suppression, though challenges related to stability and delivery persist. Preclinical studies have explored the use of CRISPR/Cas9 or CRISPR interference (CRISPRi) to silence or correct the HTT gene, but the delivery of large Cas9 constructs and potential adverse effects remain significant barriers [98]. Adenosine deaminase-mediated RNA editing offers a theoretical method to correct CAG expansions or disrupt mHTT translation. These techniques, summarized in Figure 4, are in early stages but may eventually complement IT delivery strategies.
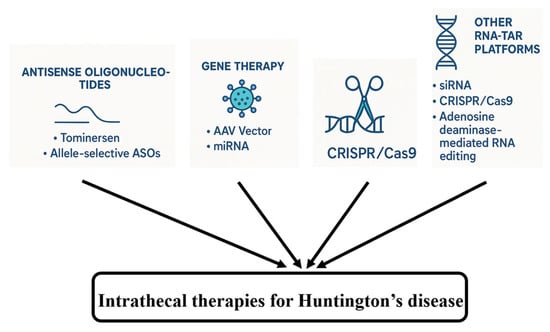
Figure 4.
Intrathecal therapies for HD–an overview.
3.4. Amyotrophic Lateral Sclerosis
Recent therapeutic advances, particularly in ASOs and gene therapy, offer new hope in the therapeutic landscape of ALS. IT delivery is central to their clinical deployment. ASOs can target several genes. One common target is SOD1, as SOD1 mutations are among the most frequent genetic causes of ALS, accounting for ~20% of familial ALS cases and ~2% of all ALS [99]. Tofersen (BIIB067) is a phosphorothioate-modified 2′-O-methoxyethyl (2′-MOE) ASO developed to selectively degrade SOD1 mRNA, thereby reducing toxic protein accumulation [100]. The Phase 1/2 Trial (NCT02623699) consisted of the ascending-dose administration of monthly IT injections of tofersen, which were generally well tolerated and resulted in dose-dependent reductions in CSF SOD1 and neurofilament light chain (NfL) [101]. Subsequently, in the phase 3 VALOR Trial (NCT02623699), a double-blind, placebo-controlled trial involving 108 participants with confirmed SOD1 mutations, tofersen failed to reach the primary endpoint. However, significant reductions in plasma NfL and CSF SOD1 were observed, and exploratory analyses suggested a potential long-term benefit in early treated patients [102]. In April 2023, the FDA granted accelerated approval for tofersen based on biomarker evidence of target engagement, marking the first approved therapy targeting a genetic form of ALS [28].
Another interesting target for ASOs is C9orf72, particularly the hexanucleotide (GGGGCC) repeat expansions in C9orf72, which are present in ~40% of familial ALS cases and 5–10% of sporadic ALS cases [103]. ASOs targeting C9orf72 mRNA or intronic repeat-containing RNAs are in active development. For example, BIIB078 (Ionis) was designed to selectively suppress the mutant C9orf72 allele. Despite strong preclinical rationale, a Phase 1 trial (NCT03626012) was discontinued after failing to demonstrate clinical benefit or favorable biomarker profiles [104]. Wave Life Sciences (WVE-004), targeting repeat-containing transcripts, is currently being evaluated in a Phase 1b/2a trial (FOCUS-C9, NCT04931862). Preliminary results show a dose-dependent reduction in poly(GP) DPRs in CSF, suggesting pharmacodynamic engagement, though clinical data are pending [105].
Finally, mutations in FUS, an RNA-binding protein, are implicated in juvenile-onset ALS and drive toxic cytoplasmic mislocalization of the protein [106]. A preclinical ASO program by Ionis and Biogen targeting FUS mRNA has entered IND-enabling studies, with IT delivery planned based on animal efficacy in spinal motor neurons.
In contrast to ASOs, gene therapy aims for more durable, potentially permanent, disease modification. In ALS, AAV vectors, particularly AAV9 and AAVrh10, are suitable for IT delivery. APB-102 is an AAVrh10 vector delivering an artificial microRNA targeting SOD1 mRNA [107]. It is administered via intrathecal injection to ensure widespread spinal cord delivery. A Phase 1/2 trial (NCT04833907) is ongoing to assess safety, tolerability, and target engagement. Preclinical studies demonstrated the efficient knockdown of SOD1 and preservation of motor function in murine models [108]. AAV9, the vector backbone for Zolgensma (approved for spinal muscular atrophy), is being adapted for ALS due to its demonstrated ability to cross the BBB when delivered systemically or via IT injection [109]. Passage Bio’s PBFT02, targeting GRN in frontotemporal dementia with TDP-43 pathology, utilizes cisterna magna administration—a route that may also benefit ALS gene therapies by providing superior CNS distribution [110]. TSHA-102 (Taysha Gene Therapies), a self-regulating gene therapy for Rett syndrome, is testing intrathecal dosing for sustained expression [111]. Similar regulatory switches could be used in future ALS constructs to fine-tune gene expression and reduce toxicity. Figure 5 summarizes, in an illustrative manner, the therapeutic directions in ALS.
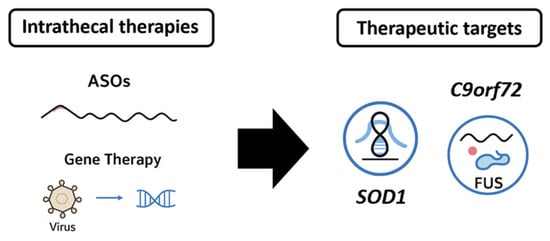
Figure 5.
A schematic overview of intrathecal therapies and the main therapeutic targets for ALS.
3.5. Spinal Muscular Atrophy (SMA)—A Success Story and Path Forward
SMA, although not a classical NDD, is important to consider when evaluating IT-administered therapies from several perspectives. This devastating autosomal recessive neuromuscular disorder is caused by bi allelic deletions or mutations in the survival motor neuron 1 (SMN1) gene [112]. The resultant deficiency in the SMN protein leads to progressive degeneration of anterior horn cells in the spinal cord, culminating in muscular weakness, atrophy, and in severe cases, early mortality [113]. Historically, SMA has been one of the leading genetic causes of infant death, and until 2016, SMA’s management was purely supportive [114].
The therapeutic revolution began with the approval of nusinersen, followed by onasemnogene abeparvovec (gene therapy) and risdiplam (oral splicing modulator). Among these, nusinersen has been a landmark, administered directly into the CSF via lumbar puncture [115]. As an ASOs, nusinersen modulates SMN2 pre-mRNA splicing to promote exon 7 inclusion, increasing functional SMN protein expression predominantly in the CNS [115]. Several reasons support the approach based on intrathecal administration: efficient delivery to spinal motor neurons, minimal systemic exposure, reduced peripheral side effects, and sustained therapeutic concentration in the CSF due to slow clearance.
Moreover, the results from clinical trials demonstrated the compelling efficacy of IT-delivered nusinersen. For example, the ENDEAR trial (2016), which included infantile-onset SMA, demonstrated that nusinersen significantly improved motor milestones and survival compared to the sham control [116]. Another trial, CHERISH (2017), included patients with later-onset (Type II) SMA who received nusinersen, and showed significant gains in motor function (HFMSE scores) [117]. The SHINE trial and long-term extensions continued to show durable benefits [118]. Additionally, real-world registries (e.g., SMArtCARE, RESTORE) have confirmed these findings across diverse clinical settings and genotypes. Importantly, early initiation—especially in pre-symptomatic individuals—yields the most profound benefits, aligning with the concept of a therapeutic window before irreversible motor neuron loss [119].
Despite favorable outcomes, IT nusinersen delivery has also brought technical and logistical challenges. Particularly in infants and young children, sedation and specialized expertise are required. Patients with severe scoliosis or spinal fusion, commonly encountered in Type II and III SMA, pose therapeutic challenges, as standard lumbar access may be impossible. Image-guided (fluoroscopic or CT) intrathecal delivery and implantable reservoirs (e.g., subcutaneous intrathecal catheters) could be potential solutions. Furthermore, studies are investigating modified ASOs with enhanced stability and tissue penetration, potentially allowing less frequent dosing or alternative routes (e.g., intraventricular).
SMA is a success story that exemplifies the triumph of precision medicine in neurology. Intrathecal therapeutic administration has been instrumental in offering a meaningful outcome to a once-fatal disease. Nusinersen, delivered intrathecally, has set a precedent for treating genetic CNS disorders at their source. Several factors could explain the contrast between the success of IT therapy in SMA and the more modest or uncertain outcomes in other NDDs. First, SMA is a monogenic disease with a well-defined molecular target (SMN2 splicing), allowing for precise ASOs design and a clear biomarker (SMN protein) for pharmacodynamic assessment. Second, the disease affects motor neurons throughout the CNS but spares much of the BBB integrity in early stages, enabling relatively homogeneous distribution of IT-delivered ASOs to relevant spinal cord regions. Third, SMA patients, particularly infants and young children, have faster CSF turnover and shorter neuraxial lengths, facilitating more even drug distribution compared to the heterogeneous CSF flow patterns in adult-onset NDDs, such as AD and PD. Finally, clinical trial design benefited from robust natural history data, sensitive functional endpoints, and early intervention in a rapidly progressive disease, all of which enhanced the ability to detect meaningful treatment effects. These lessons underscore that IT delivery is most likely to succeed when the therapeutic target is genetically validated, the distribution requirements match achievable CSF pharmacokinetics, and endpoints are sensitive enough to capture early functional gains. Still, there are challenges that need to be overcome, detailed in the following section, with current IT delivery techniques needing refinement.
4. Challenges in Intrathecal Drug Delivery and Potential Alternatives
Intrathecal administration of therapeutics has gained prominence as a viable route for delivering therapeutic agents in neurological diseases, with SMA being the first successful story in clinical practice. Still, besides the incontestable advantages of IT drug delivery, there are anatomical, pharmacological, procedural, and logistical challenges that need to be overcome. Table 4 summarizes the most relevant challenges and potential solutions to improve intrathecal drug delivery.

Table 4.
Challenges and potential solutions to improve intrathecal drug delivery.
From an anatomical perspective, the main challenges are related to accessing the intrathecal space, complications arising from repeated dosing, and the biological barriers of the human body. Lumbar puncture is the standard route for IT drug administration but becomes increasingly difficult in NDD patients due to age-related degeneration, comorbidities (e.g., osteoporosis), and disease-specific complications, including severe scoliosis, vertebral collapse, or spinal fusion surgeries [120]. In such cases, fluoroscopy-guided or CT-guided access is necessary, which increases costs, radiation exposure, and procedural time [121].
With most IT-administered therapies requiring chronic administration (e.g., every 4 months for nusinersen), this entails repetitive lumbar punctures, each procedure introducing procedural risks, including post-dural puncture headache, infections, CSF leaks, and decreased patient compliance. Thus, for long-term success, neuroanesthesia support is required.
Disease-specific conditions alter the already highly selective biological barriers, CSF dynamics, immunogenicity, and inflammatory responses. Many NDDs alter the CSF flow and composition: hydrocephalus ex vacuo in AD, spinal canal narrowing in ALS, and chronic inflammation affecting drug absorption and metabolism [126]. Additionally, repeated IT administration of biologics or oligonucleotides can lead to CSF pleocytosis, or more severe conditions such as sterile meningitis or choroid plexus inflammation [127].
Besides anatomical challenges, pharmacological aspects also need to be addressed to improve the outcome of IT-administered therapies. Uneven drug distribution in CSF remains a problem, being influenced by CSF dynamics and pulsatility, and also by molecular weight and charge of the drug [122]. Medication concentrations tend to decline rostrally, leading to subtherapeutic levels in brain regions. This is particularly problematic for diseases like HD or AD, where supraspinal targeting is essential. Another relevant aspect is the limited parenchymal penetration, particularly for large molecules, such as ASOs, which face significant penetration limitations through natural barriers. This results in periventricular or superficial CNS targeting, with limited effect on deeper nuclei [123].
Technological and device-related issues represent another critical limitation of IT drug delivery-based therapies. For chronic administration, implanted catheters or subcutaneous reservoirs (e.g., intrathecal pumps) have been proposed as good alternatives to repetitive lumbar punctures for IT medication administration [36,37]. Still, these devices carry a risk of infection, dislodgement, and fibrosis, while pump systems may malfunction, especially with viscous or particulate-loaded formulations. When considering NDD patients who need periodic imaging monitoring, device-related MRI compatibility and artifact generation remain a concern. Additionally, there is a lack of standardization, with significant variability in drug formulation (concentration), delivery volume, and rate, which results in inconsistent clinical outcomes and complicates regulatory approvals [124].
Even when addressing the abovementioned issues, the healthcare system and the economic burden remain relevant limitations. IT drug administration requires an adequate infrastructure, skilled proceduralists, sterile procedural environments, and long-term follow-up clinics, all of which are found in tertiary centers, making global generalized accessibility a significant concern [128]. The economic burden is generated not only by the cost of the drug, but mainly due to procedural costs, imaging, and monitoring requirements. These costs are magnified in chronic progressive diseases, creating sustainability concerns for health systems and payers [129].
Finally, in particular cases, ethical and psychosocial aspects could become serious limiting factors for IT administration of therapeutics. Special considerations should be taken when treating pediatric patients (special consents). Sedation risks should be more thoroughly explained to the patients. Additionally, the balance between disease progression (late-stage NDDs with associated comorbidities) and procedural benefits should be regularly assessed in chronic administration protocols [125].
Addressing all these factors is highly relevant, as it significantly impacts treatment adherence, improves patients’ quality of life, and ultimately leads to better long-term outcomes.
Ultimately, there are also alternative routes to the lumbar intrathecal administration, to deliver therapeutic agents into the CSF, with intracisternal (cisterna magna) and intraventricular approaches as two notable possibilities that deserve consideration due to their potential advantages in terms of biodistribution and CNS targeting. Several preclinical and translational studies have demonstrated that cisterna magna (CM) injections can achieve broader and more efficient rostral CNS distribution than lumbar puncture, with reduced procedural complexity compared to stereotactic intracerebral approaches. For example, Chen et al. (2023) provided a systematic comparison of CSF delivery routes for AAV-based gene therapy, highlighting significant variability in vector biodistribution between lumbar, intracisternal, and intracerebroventricular routes [130].
Moreover, Hinderer et al. (2018) reported that CM administration of AAV9 in dogs and non-human primates achieved widespread brain and spinal cord transduction, with a lower incidence of inflammatory responses compared to intraventricular injection [131]. In parallel, Taghian et al. (2020) described a minimally invasive CM delivery technique in sheep that demonstrated high CNS bioavailability and is now being translated into clinical protocols [132].
Intraventricular administration, although more invasive, has also been used successfully in various models, particularly for targeting ependymal cells as a source of continuous therapeutic protein secretion, as demonstrated in the work conducted by Yamazaki et al. (2014) [133]. Table 5 summarizes, in a comparative manner, the alternative routes for drug delivery to the CNS.

Table 5.
Comparative overview of the alternative routes for drug delivery to the central nervous system (CNS).
Collectively, these findings suggest that intracisternal and intraventricular routes may serve as viable or even preferable alternatives to lumbar IT administration for specific therapeutic modalities, especially those requiring widespread cortical or deep brain distribution. Future studies comparing these delivery strategies in terms of pharmacokinetics, biodistribution, safety, immunogenicity, and patient tolerability will be crucial to optimizing CSF-targeted therapies for neurodegenerative diseases.
5. Next-Generation Drug Delivery Systems for Intrathecal Therapies
Despite significant improvements in the field, the current drug delivery platforms still remain limited by poor CSF and parenchymal distribution, infection and mechanical complication rates, fixed-rate or manually adjusted dosing, and an inability to clear pathogenic proteins or precisely control biologic exposure over long timescales. Next-generation drug delivery systems must address these gaps, and innovations are needed in all aspects of the devices. Subcutaneous access ports and refined catheter architectures should be optimized to reduce the risk of infection and enable safer, repeated CSF access for bolus or programmable therapy administration. A relevant example is the minimally invasive lumbar port (MILP) system, which was successfully implanted and maintained in animal models by MacAllister et al. [134]. The study describes the development of an MILP model for serial CSF collection in rhesus macaques as an alternative to surgically invasive ventricular reservoir and lumbar laminectomy models. Unlike existing systems, MILP implantation does not require stereotaxic placement, MRI guidance, or extensive postoperative care, and was well tolerated without neurologic deficits, infection, or skin erosion. The model achieved a 70% successful establishment rate over 3 months, with 57% of devices maintaining long-term function (average 19.2 months), reliably yielding 0.5–1.0 mL of CSF in unanesthetized but restrained macaques for pharmacokinetic/pharmacodynamic studies [134]. While complications included port failure and nonpatency, the MILP provides a closed, reliable, and less invasive system for frequent, atraumatic lumbar CSF sampling, serving as a practical alternative to temporary catheterization and, in some studies, to ventricular access when lumbar CSF is an acceptable surrogate [134].
CSF flow platforms and extracorporeal/convective exchange approaches are being developed specifically to homogenize distribution and overcome the stagnation zones that limit lumbar-to-ventricular transport [135]. Next-generation intrathecal systems should center on implantable, programmable infusion pumps and surgically implanted access ports that enable repeat, precise CSF dosing. Starting from currently available pumps such as Medtronic’s SynchroMed line [136], it is clear that there is a need to improve the programmable parameters of bolus infusion via intrathecal catheters. Clinical and bench data should confirm the high delivery accuracy and the ability to tailor dosing over time.
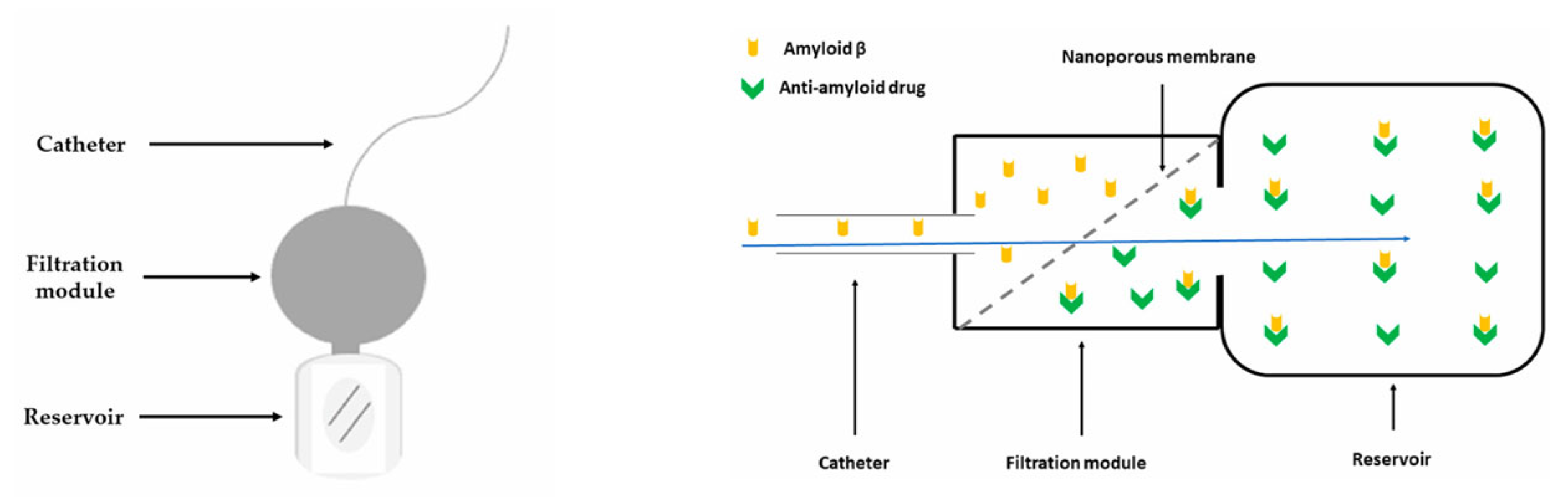
Concurrently, “nanoporous membrane”-based implantable reservoirs and intrathecal pseudodelivery devices (which present affinity-based sinks or size-selective membranes that permit molecular exchange without systemic exposure) offer a way to capture or slowly release large biologics directly in CSF while minimizing peripheral toxicity. One relevant example is an experimental device capable of filtering Aβ from the CSF developed and tested in an AD mouse model by Schreiner et al. [137]. The authors presented in their paper the multistep process of developing a functionalized biocompatible nanoporous ceramic membrane, followed by a complex evaluation of the membrane’s structural and functional integrity, and finally its use in mice. The nanoporous ceramic filter-based system, which selectively filters Aβ from the CSF, seemed to be a feasible and safe treatment modality in the AD mouse model, with the prototype having a functional lifespan of around four weeks (see Figure 6). With future research focusing on the development of advanced nanoporous ceramic filters with anti-biofouling properties, which ensure long-term therapeutic action, these devices could be promising tools for antibodies and aggregate-clearing strategies.
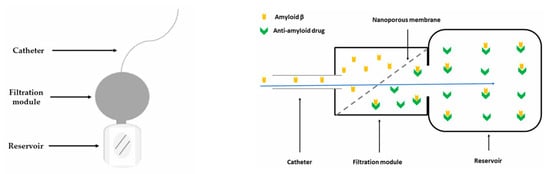
Figure 6.
Schematic representation of an experimental CSF filtering device (left) and of the filtration module, adapted from Schreiner et al. [137], where the Aβ-rich CSF travels through the nanoporous ceramic filter (blue arrow), the size-based filtration takes place, and subsequently, via a specific antigen–antibody reaction, Aβ is permanently sequestered in the reservoir (right).
Simultaneously, the field is moving toward innovative adaptive systems: artificial intelligence (AI)-enabled closed-loop pumps and sensor-guided platforms can tailor intrathecal dosing in real time based on physiological or biomarker feedback, thereby improving efficacy, lowering dose, and diminishing side effects. Initial variants of flow sensors have been tested in recent years, with a particular focus on those that can operate within the clinical range for CSF flow. One example is the pressure-sensitive capacitor sensor developed by Raj et al., which is suitable for long-term implantation and features a wireless external spectrometer for measuring passive subcutaneous components [138]. The study describes the development of an MEMS-based capacitive pressure and flow sensor designed for integration into CSF shunt systems to monitor intracranial pressure and shunt function. The device is fabricated on silicon wafers with carefully engineered capacitor membranes (520 μm wide, 0.5 μm plate spacing) optimized for high sensitivity to the very small pressure and flow ranges relevant to CSF dynamics. The sensor chip is mounted on a biocompatible PMMA carrier with a fluid channel, sealed, and coupled with inductors to enable wireless readout of the resonant frequency. In a “twin capacitor” configuration, the device simultaneously measures flow (via pressure drop between sensors) and intracranial pressure (via averaged signal), with correction for gravitational effects using a tilt sensor. Bench testing demonstrated highly accurate quadratic relationships between frequency and both flow and pressure, with resolutions of ~0.6 mL/h for flow and 0.01 cm H2O for pressure, minimal drift, and sensitivity sufficient for clinical application. Compared with existing devices, this sensor offers miniaturization, wireless readout, simultaneous flow and pressure monitoring, and incorporation into standard shunt systems, providing a robust tool for early detection of shunt malfunction or occlusion while minimizing invasiveness [138].
Advancements in “smart” and sustained-release IT platforms, which reduce hardware demands or add responsive control, are mandatory for future improved devices. Bioactive hydrogel scaffolds offer local, sustained release, and trophic support, enhancing tissue retention and regenerative potential. Several research groups have already tested bioactive hydrogels in spinal cord injury models. For example, Sun et al. fabricated composite hydrogels (CRP) based on chitosan, RADA16 nanofibers, and nerve-promoted, with excellent injectability, superior biodegradability, and biocompatibility. CRP promote the proliferation and migration of bone marrow mesenchymal stem cells and also induce the proliferation and differentiation of neural stem cells into neurons [139]. In addition, nano- and microparticle carriers delivered intrathecally are being engineered to navigate CSF flow, improve rostral distribution, and modulate pharmacokinetics across the CNS—an area captured in recent comprehensive reviews of IT nanoparticle delivery for CNS disease [140]. The literature indicates that the brain penetration by nanoparticles is influenced by their properties and functionalization. IT delivery of nanoparticles achieves higher and prolonged drug concentrations in the brain, improving distribution and minimizing systemic exposure. Various types, including lipid-based, cell-derived biomimetic, inorganic, and polymeric types, show promise in current studies [140], with liposomal nanoparticles being particularly effective. Engineered nanocarriers, including polymeric nanoparticles, solid lipid nanoparticles, liposomes, micelles, dendrimers, and more advanced systems like nanogels and nanotubes, offer benefits such as enhanced CNS penetration, extended circulation, and sustained release. However, their efficacy depends on critical physicochemical properties (size, shape, charge, biodegradability, and biocompatibility), while concerns remain about solubility, toxicity, clearance, and limited deep parenchymal penetration [141]. Uptake across the blood–cerebrospinal fluid barrier primarily occurs via receptor-mediated transcytosis (RMT), utilizing pathways such as transferrin, insulin, LDL receptors, LDL receptor-related proteins (LRP1, LRP2, LRP8) [142], folate receptors, and plasma protein transport mechanisms, with the choroid plexus epithelium playing a crucial role [143]. Although RMT and novel strategies, such as folate receptor-mediated exosome transport, show great promise, optimizing ligand–receptor interactions, minimizing toxicity, and standardizing safety assessments remain essential for the clinical translation of NP-based CNS drug delivery [144].
Integrating these technologies is crucial for providing a path to safer and more effective intrathecal therapies for neurodegenerative diseases. However, clinical translation will require rigorous comparative pharmacokinetic/pharmacodynamic studies, standardized safety endpoints, and multidisciplinary device–drug regulatory strategies [145].
6. Conclusions
With the increasing incidence and prevalence of NDDs worldwide, and yet no curative treatment for general use, IT-administered drug therapies offer a promising avenue in this context. The IT route has several advantages when pharmacologically targeting the CNS, including the ability to bypass the BBB; however, it also poses significant challenges to its adequate implementation. Lumbar puncture, intrathecal catheters, and implantable intrathecal infusion pumps are the primary approaches in IT drug delivery, each with its own strengths and drawbacks.
Considering the most relevant NDDs, IT treatments include mainly ASOs, gene therapy using viral vectors such as AAVs, and small interfering RNAs (siRNAs). With a small number of phase 1 and 2 clinical trials currently in progress, it is difficult to draw definitive conclusions yet. The only medication already approved and with a verified positive impact based on real-world data is nusinersen, used in the treatment of SMA. Still, this success story also demonstrates the multiple anatomical, pharmacological, economic, and sometimes ethical challenges that need to be addressed and overcome before realizing similar IT-administered drugs on the market.
With these great perspectives, there are a couple of directions that need to be addressed in order to improve the outcomes of IT-delivered drugs. Firstly, there is a pressing need to improve delivery techniques and resolve device-related limitations. Secondly, there is a high need for standardization of administration protocols and drug formulations. Finally, the economic burden related to the healthcare system necessitates rethinking the infrastructure to respond to the needs of both the administration and follow-up.
Author Contributions
Conceptualization, methodology, T.G.S., R.C.C. and M.M.-G.; formal analysis, investigation, data curation, T.G.S., R.C.C. and O.D.S.; writing—original draft preparation, T.G.S. and O.D.S.; writing—review and editing, M.M.-G. and R.C.C.; supervision, M.M.-G. All authors have read and agreed to the published version of the manuscript.
Funding
The research has been funded by the Instituto de Salud Carlos III (ISCIII) by project DTS22/00071 and co-funded by the Boosting Ingenium for Excellence (BI4E) project, funded by the European Union’s HORIZON-WIDERA-2021-ACCESS-05-01-European Excellence Initiative under the Grant Agreement No. 101071321.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available in the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AAV | Adeno-associated viruses |
| AD | Alzheimer’s disease |
| ALS | Amyotrophic lateral sclerosis |
| ARIA | Amyloid-related imaging abnormalities |
| Aβ | Amyloid beta |
| CM | Cisterna magna |
| CSF | Cerebrospinal fluid |
| HD | Huntington disease |
| IT | Intrathecal |
| NDD | Neurodegenerative disorder |
| PD | Parkinson’s disease |
| siRNA | Small interfering RNA |
References
- Van Schependom, J.; D’haeseleer, M. Advances in Neurodegenerative Diseases. J. Clin. Med. 2023, 12, 1709. [Google Scholar] [CrossRef] [PubMed]
- Safiri, S.; Jolfayi, A.G.; Fazlollahi, A.; Morsali, S.; Sarkesh, A.; Sorkhabi, A.D.; Golabi, B.; Aletaha, R.; Asghari, K.M.; Hamidi, S.; et al. Alzheimer’s disease: A comprehensive review of epidemiology, risk factors, symptoms diagnosis, management, caregiving, advanced treatments and associated challenges. Front. Med. 2024, 11, 1474043. [Google Scholar] [CrossRef] [PubMed]
- Kamatham, P.T.; Shukla, R.; Khatri, D.K.; Vora, L.K. Pathogenesis, diagnostics, and therapeutics for Alzheimer’s disease: Breaking the memory barrier. Ageing Res. Rev. 2024, 101, 102481. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.D.; Yi, L.X.; Wang, D.Q.; Lim, T.M.; Tan, E.K. Role of dopamine in the pathophysiology of Parkinson’s disease. Transl. Neurodegener. 2023, 12, 44. [Google Scholar] [CrossRef]
- Váradi, C. Clinical Features of Parkinson’s Disease: The Evolution of Critical Symptoms. Biology 2020, 9, 103. [Google Scholar] [CrossRef]
- Dickson, D.W. Neuropathology of Parkinson disease. Parkinsonism. Relat. Disord. 2018, 46, S30–S33. [Google Scholar] [CrossRef]
- Stoker, T.B.; Mason, S.L.; Greenland, J.C.; Holden, S.T.; Santini, H.; Barker, R.A. Huntington’s disease: Diagnosis and management. Pract. Neurol. 2021, 22, 32–41. [Google Scholar] [CrossRef]
- McColgan, P.; Tabrizi, S.J. Huntington’s disease: A clinical review. Eur. J. Neurol. 2017, 25, 24–34. [Google Scholar] [CrossRef]
- Segura, T.; Medrano, I.H.; Collazo, S.; Maté, C.; Sguera, C.; Del Rio-Bermudez, C.; Casero, H.; Salcedo, I.; García-García, J.; Alcahut-Rodríguez, C.; et al. Symptoms timeline and outcomes in amyotrophic lateral sclerosis using artificial intelligence. Sci. Rep. 2023, 13, 702. [Google Scholar] [CrossRef]
- Wang, H.; Guan, L.; Deng, M. Recent progress of the genetics of amyotrophic lateral sclerosis and challenges of gene therapy. Front. Neurosci. 2023, 17, 1170996. [Google Scholar] [CrossRef]
- Wareham, L.K.; Liddelow, S.A.; Temple, S.; Benowitz, L.I.; Di Polo, A.; Wellington, C.; Goldberg, J.L.; He, Z.; Duan, X.; Bu, G.; et al. Solving neurodegeneration: Common mechanisms and strategies for new treatments. Mol. Neurodegener. 2022, 17, 23. [Google Scholar] [CrossRef]
- Kadry, H.; Noorani, B.; Cucullo, L. A blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef]
- De Andres, J.; Hayek, S.; Perruchoud, C.; Lawrence, M.M.; Reina, M.A.; De Andres-Serrano, C.; Rubio-Haro, R.; Hunt, M.; Yaksh, T.L. Intrathecal Drug Delivery: Advances and Applications in the Management of Chronic Pain Patient. Front. Pain Res. 2022, 3, 900566. [Google Scholar] [CrossRef]
- Wichmann, T.O.; Damkier, H.H.; Pedersen, M. A Brief Overview of the Cerebrospinal Fluid System and Its Implications for Brain and Spinal Cord Diseases. Front. Hum. Neurosci. 2022, 15, 737217. [Google Scholar] [CrossRef] [PubMed]
- Sakka, L.; Coll, G.; Chazal, J. Anatomy and physiology of cerebrospinal fluid. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2011, 128, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Gan, L.; Ren, L.; Lin, Y.; Ma, C.; Lin, X. Factors influencing the blood-brain barrier permeability. Brain Res. 2022, 1788, 147937. [Google Scholar] [CrossRef]
- Prager, J.; Deer, T.; Levy, R.; Bruel, B.; Buchser, E.; Caraway, D.; Cousins, M.; Jacobs, M.; McGlothlen, G.; Rauck, R.; et al. Best Practices for Intrathecal Drug Delivery for Pain. Neuromodul. Technol. Neural Interface 2014, 17, 354–372. [Google Scholar] [CrossRef]
- Hablitz, L.M.; Plá, V.; Giannetto, M.; Vinitsky, H.S.; Stæger, F.F.; Metcalfe, T.; Nguyen, R.; Benrais, A.; Nedergaard, M. Circadian control of brain glymphatic and lymphatic fluid flow. Nat. Commun. 2020, 11, 4411. [Google Scholar] [CrossRef]
- Hornkjøl, M.; Valnes, L.M.; Ringstad, G.; Rognes, M.E.; Eide, P.-K.; Mardal, K.-A.; Vinje, V. CSF circulation and dispersion yield rapid clearance from intracranial compartments. Front. Bioeng. Biotechnol. 2022, 10, 932469. [Google Scholar] [CrossRef]
- Stielow, M.; Witczyńska, A.; Kubryń, N.; Fijałkowski, Ł.; Nowaczyk, J.; Nowaczyk, A. The Bioavailability of Drugs—The Current State of Knowledge. Molecules 2023, 28, 8038. [Google Scholar] [CrossRef]
- Yamada, S.; Kelly, E. Cerebrospinal Fluid Dynamics and the Pathophysiology of Hydrocephalus: New Concepts. Semin. Ultrasound, CT MRI 2016, 37, 84–91. [Google Scholar] [CrossRef]
- Burman, R.; Alperin, N. CSF-to-blood toxins clearance is modulated by breathing through cranio–spinal CSF oscillation. J. Sleep Res. 2023, 33, e14029. [Google Scholar] [CrossRef]
- Li, Y.; Rusinek, H.; Butler, T.; Glodzik, L.; Pirraglia, E.; Babich, J.; Mozley, P.D.; Nehmeh, S.; Pahlajani, S.; Wang, X.; et al. Decreased CSF clearance and increased brain amyloid in Alzheimer’s disease. Fluids Barriers CNS 2022, 19, 21. [Google Scholar] [CrossRef]
- Hay, R.; Gupta, A. Continuous spinal anaesthesia. BJA Educ. 2022, 22, 295–297. [Google Scholar] [CrossRef] [PubMed]
- Rawal, N.; Tandon, B. Epidural and intrathecal morphine in intensive care units. Intensiv. Care Med. 1985, 11, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Dupoiron, D.; Autier, L.; Lebrec, N.; Seegers, V.; Folliard, C.; Patsouris, A.; Campone, M.; Augereau, P. Intrathecal Catheter for Chemotherapy in Leptomeningeal Carcinomatosis From HER2-Negative Metastatic Breast Cancer. J. Breast Cancer 2023, 26, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Wu, L.; Qu, R.; Jiang, T.; Bai, J.; Sheng, L.; Feng, P.; Sun, J. History of development of the life-saving drug “Nusinersen” in spinal muscular atrophy. Front. Cell. Neurosci. 2022, 16, 942976. [Google Scholar] [CrossRef]
- Blair, H.A. Tofersen: First Approval. Drugs 2023, 83, 1039–1043. [Google Scholar] [CrossRef]
- Ericson, T.; Singla, P.; Kohan, L. Intrathecal Pumps. Phys. Med. Rehabil. Clin. N. Am. 2022, 33, 409–424. [Google Scholar] [CrossRef]
- Jane, L.A.; Wray, A.A. Lumbar Puncture. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557553/ (accessed on 21 April 2025).
- Hudgins, P.; Fountain, A.; Chapman, P.; Shah, L. Difficult Lumbar Puncture: Pitfalls and Tips from the Trenches. Am. J. Neuroradiol. 2017, 38, 1276–1283. [Google Scholar] [CrossRef]
- Burla, G.K.R.; Shrestha, D.; Bowen, M.; Horvath, J.D.; Martin, B.A. Evaluating the effect of injection protocols on intrathecal solute dispersion in non-human primates: An in vitro study using a cynomolgus cerebrospinal fluid system. Fluids Barriers CNS 2024, 21, 61. [Google Scholar] [CrossRef]
- Boster, A.L.; Adair, R.L.; Gooch, J.L.; Nelson, M.E.S.; Toomer, A.; Urquidez, J.; Saulino, M. Best Practices for Intrathecal Baclofen Therapy: Dosing and Long-Term Management. Neuromodulation Technol. Neural Interface 2016, 19, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Singh, E.; Gurses, M.E.; Costello, M.C.; Berke, C.; Lu, V.M.; Daggubati, L.; Komotar, R.J.; Ivan, M.E.; Shah, A.H. Intrathecal chemotherapy for leptomeningeal disease in high-grade gliomas: A systematic review. J. Neurooncol. 2024, 167, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Binyamin, Y.; Azem, K.; Heesen, M.; Gruzman, I.; Frenkel, A.; Fein, S.; Eidelman, L.A.; Garren, A.; Frank, D.; Orbach-Zinger, S. The effect of placement and management of intrathecal catheters following accidental dural puncture on the incidence of postdural puncture headache and severity: A retrospective real-world study. Anaesthesia 2023, 78, 1256–1261. [Google Scholar] [CrossRef]
- Knight, K.H.; Brand, F.M.; Mchaourab, A.S.; Veneziano, G. Implantable intrathecal pumps for chronic pain: Highlights and updates. Croat. Med. J. 2007, 48, 22–34. [Google Scholar]
- Bolash, R.; Mekhail, N. Intrathecal Pain Pumps. Neurosurg. Clin. N. Am. 2014, 25, 735–742. [Google Scholar] [CrossRef]
- Delhaas, E.; Huygen, F. Complications associated with intrathecal drug delivery systems. BJA Educ. 2020, 20, 51–57. [Google Scholar] [CrossRef]
- Passeri, E.; Elkhoury, K.; Morsink, M.; Broersen, K.; Linder, M.; Tamayol, A.; Malaplate, C.; Yen, F.T.; Arab-Tehrany, E. Alzheimer’s Disease: Treatment Strategies and Their Limitations. Int. J. Mol. Sci. 2022, 23, 13954. [Google Scholar] [CrossRef]
- Rawat, P.; Sehar, U.; Bisht, J.; Selman, A.; Culberson, J.; Reddy, P.H. Phosphorylated Tau in Alzheimer’s Disease and Other Tauopathies. Int. J. Mol. Sci. 2022, 23, 12841. [Google Scholar] [CrossRef]
- Edwards, A.L.; Collins, J.A.; Junge, C.; Kordasiewicz, H.; Mignon, L.; Wu, S.; Li, Y.; Lin, L.; DuBois, J.; Hutchison, R.M.; et al. Exploratory Tau Biomarker Results from a Multiple Ascending-Dose Study of BIIB080 in Alzheimer Disease. JAMA Neurol. 2023, 80, 1344–1352. [Google Scholar] [CrossRef]
- Mummery, C.J.; Börjesson-Hanson, A.; Blackburn, D.J.; Vijverberg, E.G.B.; De Deyn, P.P.; Ducharme, S.; Jonsson, M.; Schneider, A.; Rinne, J.O.; Ludolph, A.C.; et al. Tau-targeting antisense oligonucleotide MAPTRx in mild Alzheimer’s disease: A phase 1b, randomized, placebo-controlled trial. Nat. Med. 2023, 29, 1437–1447. [Google Scholar] [CrossRef]
- Lozupone, M.; Dibello, V.; Sardone, R.; Castellana, F.; Zupo, R.; Lampignano, L.; Bortone, I.; Stallone, R.; Altamura, M.; Bellomo, A.; et al. The development of peptide- and oligonucleotide-based drugs to prevent the formation of abnormal tau in tauopathies. Expert Opin. Drug Discov. 2023, 18, 515–526. [Google Scholar] [CrossRef]
- Hung, C.; Fertan, E.; Livesey, F.J.; Klenerman, D.; Patani, R. APP antisense oligonucleotides reduce amyloid-β aggregation and rescue endolysosomal dysfunction in Alzheimer’s disease. Brain 2024, 147, 2325–2333. [Google Scholar] [CrossRef] [PubMed]
- Klinkovskij, A.; Shepelev, M.; Isaakyan, Y.; Aniskin, D.; Ulasov, I. Advances of Genome Editing with CRISPR/Cas9 in Neurodegeneration: The Right Path towards Therapy. Biomedicines 2023, 11, 3333. [Google Scholar] [CrossRef] [PubMed]
- Baryakova, T.H.; Pogostin, B.H.; Langer, R.; McHugh, K.J. Overcoming barriers to patient adherence: The case for developing innovative drug delivery systems. Nat. Rev. Drug Discov. 2023, 22, 387–409. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-H.; Kim, S.; Nam, Y.; Park, Y.H.; Shin, S.M.; Moon, M. Second-generation anti-amyloid monoclonal antibodies for Alzheimer’s disease: Current landscape and future perspectives. Transl. Neurodegener. 2025, 14, 6. [Google Scholar] [CrossRef]
- Pardridge, W.M. Blood-Brain Barrier and Delivery of Protein and Gene Therapeutics to Brain. Front. Aging Neurosci. 2020, 11, 373. [Google Scholar] [CrossRef]
- Hampel, H.; Elhage, A.; Cho, M.; Apostolova, L.G.; Nicoll, J.A.R.; Atri, A. Amyloid-related imaging abnormalities (ARIA): Radiological, biological and clinical characteristics. Brain 2023, 146, 4414–4424. [Google Scholar] [CrossRef]
- Cummings, J.L. Maximizing the benefit and managing the risk of anti-amyloid monoclonal antibody therapy for Alzheimer’s disease: Strategies and research directions. Neurotherapeutics 2025, 22, e00570. [Google Scholar] [CrossRef]
- Ataei, B.; Hokmabadi, M.; Asadi, S.; Asadifard, E.; Zarch, S.M.A.; Najafi, S.; Bagheri-Mohammadi, S. A review of the advances, insights, and prospects of gene therapy for Alzheimer’s disease: A novel target for therapeutic medicine. Gene 2024, 912, 148368. [Google Scholar] [CrossRef]
- Rodrigues, B.d.S.; Kanekiyo, T.; Singh, J. Nerve Growth Factor Gene Delivery across the Blood–Brain Barrier to Reduce Beta Amyloid Accumulation in AD Mice. Mol. Pharm. 2020, 17, 2054–2063. [Google Scholar] [CrossRef]
- Arora, S.; Kanekiyo, T.; Singh, J. Functionalized nanoparticles for brain targeted BDNF gene therapy to rescue Alzheimer’s disease pathology in transgenic mouse model. Int. J. Biol. Macromol. 2022, 208, 901–911. [Google Scholar] [CrossRef]
- Wang, D.; Tai, P.W.L.; Gao, G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov. 2019, 18, 358–378. [Google Scholar] [CrossRef] [PubMed]
- Issa, S.S.; Shaimardanova, A.A.; Solovyeva, V.V.; Rizvanov, A.A. Various AAV Serotypes and Their Applications in Gene Therapy: An Overview. Cells 2023, 12, 785. [Google Scholar] [CrossRef] [PubMed]
- Pattali, R.; Mou, Y.; Li, X.-J. AAV9 Vector: A Novel modality in gene therapy for spinal muscular atrophy. Gene Ther. 2019, 26, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, A.M.; Rafi, M.A.; Bagel, J.H.; Brisson, B.K.; Marshall, M.S.; Salvador, J.P.; Jiang, X.; Swain, G.P.; Prociuk, M.L.; Odonnell, P.A.; et al. AAVrh10 Gene Therapy Ameliorates Central and Peripheral Nervous System Disease in Canine Globoid Cell Leukodystrophy (Krabbe Disease). Hum. Gene Ther. 2018, 29, 785–801. [Google Scholar] [CrossRef]
- Wang, L.; Ma, L.; Gao, Z.; Wang, Y.; Qiu, J. Significance of gene therapy in neurodegenerative diseases. Front. Neurosci. 2025, 19, 1515255. [Google Scholar] [CrossRef]
- Amadoro, G.; Latina, V.; Balzamino, B.O.; Squitti, R.; Varano, M.; Calissano, P.; Micera, A. Nerve Growth Factor-Based Therapy in Alzheimer’s Disease and Age-Related Macular Degeneration. Front. Neurosci. 2021, 15, 735928. [Google Scholar] [CrossRef]
- Saxena, S.K.; Ansari, S.; Maurya, V.K.; Kumar, S.; Sharma, D.; Malhotra, H.S.; Tiwari, S.; Srivastava, C.; Paweska, J.T.; Abdel-Moneim, A.S.; et al. Neprilysin-Mediated Amyloid Beta Clearance and Its Therapeutic Implications in Neurodegenerative Disorders. ACS Pharmacol. Transl. Sci. 2024, 7, 3645–3657. [Google Scholar] [CrossRef]
- Marino, M.; Holt, M.G. AAV Vector-Mediated Antibody Delivery (A-MAD) in the Central Nervous System. Front. Neurol. 2022, 13, 870799. [Google Scholar] [CrossRef]
- Shi, H.-Z.; Wang, Y.-J.; Wang, Y.-X.; Xu, L.-F.; Pan, W.; Shi, L.; Wang, J. The potential benefits of PGC-1α in treating Alzheimer’s disease are dependent on the integrity of the LLKYL L3 motif: Effect of regulating mitochondrial axonal transportation. Exp. Gerontol. 2024, 194, 112514. [Google Scholar] [CrossRef]
- Shao, C.Y.; Mirra, S.S.; Sait, H.B.R.; Sacktor, T.C.; Sigurdsson, E.M. Postsynaptic degeneration as revealed by PSD-95 reduction occurs after advanced Aβ and tau pathology in transgenic mouse models of Alzheimer’s disease. Acta Neuropathol. 2011, 122, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Ewaisha, R.; Anderson, K.S. Immunogenicity of CRISPR therapeutics—Critical considerations for clinical translation. Front. Bioeng. Biotechnol. 2023, 11, 1138596. [Google Scholar] [CrossRef] [PubMed]
- García-González, N.; Gonçalves-Sánchez, J.; Gómez-Nieto, R.; Gonçalves-Estella, J.M.; López, D.E. Advances and Challenges in Gene Therapy for Neurodegenerative Diseases: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 12485. [Google Scholar] [CrossRef] [PubMed]
- Poewe, W.; Seppi, K.; Marini, K.; Mahlknecht, P. New hopes for disease modification in Parkinson’s Disease. Neuropharmacology 2020, 171, 108085. [Google Scholar] [CrossRef]
- Freitas, M.E.; Hess, C.W.; Fox, S.H. Motor Complications of Dopaminergic Medications in Parkinson’s Disease. Semin. Neurol. 2017, 37, 147–157. [Google Scholar] [CrossRef]
- Huh, Y.E.; Usnich, T.; Scherzer, C.R.; Klein, C.; Chung, S.J. GBA1 Variants and Parkinson’s Disease: Paving the Way for Targeted Therapy. J. Mov. Disord. 2023, 16, 261–278. [Google Scholar] [CrossRef]
- Vieira, S.R.L.; Schapira, A.H.V. Glucocerebrosidase mutations and Parkinson disease. J. Neural Transm. 2022, 129, 1105–1117. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, H.; Tang, B.; Guo, J. Clinical, mechanistic, biomarker, and therapeutic advances in GBA1-associated Parkinson’s disease. Transl. Neurodegener. 2024, 13, 48. [Google Scholar] [CrossRef]
- Fregno, I.; Pérez-Carmona, N.; Rudinskiy, M.; Soldà, T.; Bergmann, T.J.; Ruano, A.; Delgado, A.; Cubero, E.; Bellotto, M.; García-Collazo, A.M.; et al. Allosteric Modulation of GCase Enhances Lysosomal Activity and Reduces ER Stress in GCase-Related Disorders. Int. J. Mol. Sci. 2025, 26, 4392. [Google Scholar] [CrossRef]
- Abeliovich, A.; Hefti, F.; Sevigny, J.; Björklund, A.; Bloem, B.R.; Brundin, P.; Federoff, H. Gene Therapy for Parkinson’s Disease Associated with GBA1 Mutations. J. Park. Dis. 2021, 11, S183–S188. [Google Scholar] [CrossRef]
- Patel, R.V.; Nanda, P.; Richardson, R.M. Neurosurgical gene therapy for central nervous system diseases. Neurotherapeutics 2024, 21, e00434. [Google Scholar] [CrossRef]
- Cavallieri, F.; Cury, R.G.; Guimarães, T.; Fioravanti, V.; Grisanti, S.; Rossi, J.; Monfrini, E.; Zedde, M.; Di Fonzo, A.; Valzania, F.; et al. Recent Advances in the Treatment of Genetic Forms of Parkinson’s Disease: Hype or Hope? Cells 2023, 12, 764. [Google Scholar] [CrossRef]
- Menozzi, E.; Toffoli, M.; Schapira, A.H. Targeting the GBA1 pathway to slow Parkinson disease: Insights into clinical aspects, pathogenic mechanisms and new therapeutic avenues. Pharmacol. Ther. 2023, 246, 108419. [Google Scholar] [CrossRef]
- Mendell, J.R.; Connolly, A.M.; Lehman, K.J.; Griffin, D.A.; Khan, S.Z.; Dharia, S.D.; Quintana-Gallardo, L.; Rodino-Klapac, L.R. Testing preexisting antibodies prior to AAV gene transfer therapy: Rationale, lessons and future considerations. Mol. Ther.–Methods Clin. Dev. 2022, 25, 74–83. [Google Scholar] [CrossRef]
- El Ouaamari, Y.; Bos, J.V.D.; Willekens, B.; Cools, N.; Wens, I. Neurotrophic Factors as Regenerative Therapy for Neurodegenerative Diseases: Current Status, Challenges and Future Perspectives. Int. J. Mol. Sci. 2023, 24, 3866. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Mukhida, K.; Mendez, I. GDNF therapy for Parkinson’s disease. Expert Rev. Neurother. 2008, 8, 1125–1139. [Google Scholar] [CrossRef] [PubMed]
- Barker, R.A.; Saarma, M.; Svendsen, C.N.; Morgan, C.; Whone, A.; Fiandaca, M.S.; Luz, M.; Bankiewicz, K.S.; Fiske, B.; Isaacs, L.; et al. Neurotrophic factors for Parkinson’s disease: Current status, progress, and remaining questions. Conclusions from a 2023 workshop. J. Park. Dis. 2024, 14, 1659–1676. [Google Scholar] [CrossRef]
- Van Laar, A.D.; Christine, C.W.; Phielipp, N.; Larson, P.S.; Elder, J.B.; Merola, A.; Sebastian, W.S.; Fiandaca, M.S.; Kells, A.P.; Wisniewski, M.E.; et al. Intraputaminal Delivery of Adeno-Associated Virus Serotype 2–Glial Cell Line–Derived Neurotrophic Factor in Mild or Moderate Parkinson’s Disease. Mov. Disord. 2025, 40, 1297–1306. [Google Scholar] [CrossRef]
- Pardridge, W.M. A Historical Review of Brain Drug Delivery. Pharmaceutics 2022, 14, 1283. [Google Scholar] [CrossRef]
- Richardson, R.M.; Kells, A.P.; Rosenbluth, K.H.; Salegio, E.A.; Fiandaca, M.S.; Larson, P.S.; Starr, P.A.; Martin, A.J.; Lonser, R.R.; Federoff, H.J.; et al. Interventional MRI-guided Putaminal Delivery of AAV2-GDNF for a Planned Clinical Trial in Parkinson’s Disease. Mol. Ther. 2011, 19, 1048–1057. [Google Scholar] [CrossRef]
- Stefani, A.; Pierantozzi, M.; Cardarelli, S.; Stefani, L.; Cerroni, R.; Conti, M.; Garasto, E.; Mercuri, N.B.; Marini, C.; Sucapane, P. Neurotrophins as Therapeutic Agents for Parkinson’s Disease; New Chances From Focused Ultrasound? Front. Neurosci. 2022, 16, 846681. [Google Scholar] [CrossRef]
- Yao, J.-Y.; Liu, T.; Hu, X.-R.; Sheng, H.; Chen, Z.-H.; Zhao, H.-Y.; Li, X.-J.; Wang, Y.; Hao, L. An insight into allele-selective approaches to lowering mutant huntingtin protein for Huntington’s disease treatment. Biomed. Pharmacother. 2024, 180, 117557. [Google Scholar] [CrossRef] [PubMed]
- Caron, N.S.; Banos, R.; Aly, A.E.; Xie, Y.; Ko, S.; Potluri, N.; Anderson, C.; Black, H.F.; Anderson, L.M.; Gordon, B.; et al. Cerebrospinal fluid mutant huntingtin is a biomarker for huntingtin lowering in the striatum of Huntington disease mice. Neurobiol. Dis. 2022, 166, 105652. [Google Scholar] [CrossRef] [PubMed]
- McColgan, P.; Thobhani, A.; Boak, L.; Schobel, S.A.; Nicotra, A.; Palermo, G.; Trundell, D.; Zhou, J.; Schlegel, V.; Ducray, P.S.; et al. Tominersen in Adults with Manifest Huntington’s Disease. N. Engl. J. Med. 2023, 389, 2203–2205. [Google Scholar] [CrossRef]
- Tabrizi, S.J.; Estevez-Fraga, C.; van Roon-Mom, W.M.C.; Flower, M.D.; Scahill, R.I.; Wild, E.J.; Muñoz-Sanjuan, I.; Sampaio, C.; Rosser, A.E.; Leavitt, B.R. Potential disease-modifying therapies for Huntington’s disease: Lessons learned and future opportunities. Lancet Neurol. 2022, 21, 645–658. [Google Scholar] [CrossRef]
- Boareto, M.; Yamamoto, Y.; Long, J.D.; Sampaio, C.; McColgan, P.; Diack, C.; Ducray, P.S. Modeling Disease Progression and Placebo Response in Huntington Disease. Neurology 2025, 104, e213646. [Google Scholar] [CrossRef]
- Liddy, V.; West, D.; Nguyen, M.; Boak, L.; Schobel, S.; Olivier, N.; Hodge, K.; HD-COPE; Townhill, J. F14 Collaborating with the community to conduct clinical trials in huntington’s disease: Lessons from the tominersen phase iii generation HD1 study. J. Neurol. Neurosurg. Psychiatry 2021, 92, A24–A25. [Google Scholar] [CrossRef]
- Estevez-Fraga, C.; Tabrizi, S.J.; Wild, E.J.; Morton, J. Huntington’s Disease Clinical Trials Corner: August 2023. J. Huntington’s Dis. 2023, 12, 169–185. [Google Scholar] [CrossRef]
- Claassen, D.O.; Corey-Bloom, J.; Dorsey, E.R.; Edmondson, M.; Kostyk, S.K.; LeDoux, M.S.; Reilmann, R.; Rosas, H.D.; Walker, F.; Wheelock, V.; et al. Genotyping single nucleotide polymorphisms for allele-selective therapy in Huntington disease. Neurol. Genet. 2020, 6, e430. [Google Scholar] [CrossRef]
- Svrzikapa, N.; Longo, K.A.; Prasad, N.; Boyanapalli, R.; Brown, J.M.; Dorset, D.; Yourstone, S.; Powers, J.; Levy, S.E.; Morris, A.J.; et al. Investigational Assay for Haplotype Phasing of the Huntingtin Gene. Mol. Ther.–Methods Clin. Dev. 2020, 19, 162–173. [Google Scholar] [CrossRef]
- Tabrizi, S.J.; Leavitt, B.R.; Landwehrmeyer, G.B.; Wild, E.J.; Saft, C.; Barker, R.A.; Blair, N.F.; Craufurd, D.; Priller, J.; Rickards, H.; et al. Targeting huntingtin expression in patients with Huntington’s disease. N. Engl. J. Med. 2019, 380, 2307–2316. [Google Scholar] [CrossRef]
- Byun, S.; Lee, M.; Kim, M. Gene Therapy for Huntington’s Disease: The Final Strategy for a Cure? J. Mov. Disord. 2022, 15, 15–20. [Google Scholar] [CrossRef]
- Kotowska-Zimmer, A.; Przybyl, L.; Pewinska, M.; Suszynska-Zajczyk, J.; Wronka, D.; Figiel, M.; Olejniczak, M. A CAG repeat-targeting artificial miRNA lowers the mutant huntingtin level in the YAC128 model of Huntington’s disease. Mol. Ther.–Nucleic Acids 2022, 28, 702–715. [Google Scholar] [CrossRef]
- Ferguson, M.W.; Kennedy, C.J.; Palpagama, T.H.; Waldvogel, H.J.; Faull, R.L.M.; Kwakowsky, A. Current and Possible Future Therapeutic Options for Huntington’s Disease. J. Central Nerv. Syst. Dis. 2022, 14. [Google Scholar] [CrossRef]
- Li, L.; Vasan, L.; Kartono, B.; Clifford, K.; Attarpour, A.; Sharma, R.; Mandrozos, M.; Kim, A.; Zhao, W.; Belotserkovsky, A.; et al. Advances in Recombinant Adeno-Associated Virus Vectors for Neurodegenerative Diseases. Biomedicines 2023, 11, 2725. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Sharma, A.R.; Bhattacharya, M.; Lee, S.-S.; Chakraborty, C. CRISPR-Cas9: A Preclinical and Clinical Perspective for the Treatment of Human Diseases. Mol. Ther. 2021, 29, 571–586. [Google Scholar] [CrossRef] [PubMed]
- Berdyński, M.; Miszta, P.; Safranow, K.; Andersen, P.M.; Morita, M.; Filipek, S.; Żekanowski, C.; Kuźma-Kozakiewicz, M. SOD1 mutations associated with amyotrophic lateral sclerosis analysis of variant severity. Sci. Rep. 2022, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Saini, A.; Chawla, P.A. Breaking barriers with tofersen: Enhancing therapeutic opportunities in amyotrophic lateral sclerosis. Eur. J. Neurol. 2023, 31, 103. [Google Scholar] [CrossRef]
- Miller, T.; Cudkowicz, M.; Shaw, P.J.; Andersen, P.M.; Atassi, N.; Bucelli, R.C.; Genge, A.; Glass, J.; Ladha, S.; Ludolph, A.L.; et al. Phase 1–2 Trial of Antisense Oligonucleotide Tofersen for SOD1 ALS. N. Engl. J. Med. 2020, 383, 109–119. [Google Scholar] [CrossRef]
- Miller, T.M.; Cudkowicz, M.E.; Genge, A.; Shaw, P.J.; Sobue, G.; Bucelli, R.C.; Chiò, A.; Van Damme, P.; Ludolph, A.C.; Glass, J.D.; et al. Trial of Antisense Oligonucleotide Tofersen for SOD1 ALS. N. Engl. J. Med. 2022, 387, 1099–1110. [Google Scholar] [CrossRef]
- Smeyers, J.; Banchi, E.-G.; Latouche, M. C9ORF72: What It Is, What It Does, and Why It Matters. Front. Cell. Neurosci. 2021, 15, 661447. [Google Scholar] [CrossRef]
- Berg, L.H.v.D.; Rothstein, J.D.; Shaw, P.J.; Babu, S.; Benatar, M.; Bucelli, R.C.; Genge, A.; Glass, J.D.; Hardiman, O.; Libri, V.; et al. Safety, tolerability, and pharmacokinetics of antisense oligonucleotide BIIB078 in adults with C9orf72-associated amyotrophic lateral sclerosis: A phase 1, randomised, double blinded, placebo-controlled, multiple ascending dose study. Lancet Neurol. 2024, 23, 901–912. [Google Scholar] [CrossRef]
- Chamakioti, M.; Karantzelis, N.; Taraviras, S. Advanced Gene-Targeting Therapies for Motor Neuron Diseases and Muscular Dystrophies. Int. J. Mol. Sci. 2022, 23, 4824. [Google Scholar] [CrossRef]
- Xiao, X.; Li, M.; Ye, Z.; He, X.; Wei, J.; Zha, Y. FUS gene mutation in amyotrophic lateral sclerosis: A new case report and systematic review. Amyotroph. Lateral Scler. Front. Degener. 2023, 25, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Meijboom, K.E.; Brown, R.H. Approaches to Gene Modulation Therapy for ALS. Neurotherapeutics 2022, 19, 1159–1179. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Li, S.; Li, X.-J.; Yin, P. Pathological insights from amyotrophic lateral sclerosis animal models: Comparisons, limitations, and challenges. Transl. Neurodegener. 2023, 12, 46. [Google Scholar] [CrossRef]
- Lejman, J.; Panuciak, K.; Nowicka, E.; Mastalerczyk, A.; Wojciechowska, K.; Lejman, M. Gene Therapy in ALS and SMA: Advances, Challenges and Perspectives. Int. J. Mol. Sci. 2023, 24, 1130. [Google Scholar] [CrossRef]
- Buccellato, F.R.; D’aNca, M.; Tartaglia, G.M.; Del Fabbro, M.; Galimberti, D. Frontotemporal dementia: From genetics to therapeutic approaches. Expert Opin. Investig. Drugs 2024, 33, 561–573. [Google Scholar] [CrossRef]
- Sadhu, C.; Lyons, C.; Oh, J.; Jagadeeswaran, I.; Gray, S.J.; Sinnett, S.E. The Efficacy of a Human-Ready miniMECP2 Gene Therapy in a Pre-Clinical Model of Rett Syndrome. Genes 2023, 15, 31. [Google Scholar] [CrossRef]
- Keinath, M.C.; Prior, D.E.; Prior, T.W. Spinal Muscular Atrophy: Mutations, Testing, and Clinical Relevance. Appl. Clin. Genet. 2021, 14, 11–25. [Google Scholar] [CrossRef]
- Cancès, C.; Richelme, C.; Barnerias, C.; Espil, C. Clinical features of spinal muscular atrophy (SMA) type 2. Arch. Pediatr. 2020, 27, 7S18–7S22. [Google Scholar] [CrossRef]
- Boardman, F.K.; Young, P.J.; Griffiths, F.E. Newborn screening for spinal muscular atrophy: The views of affected families and adults. Am. J. Med. Genet. Part A 2017, 173, 1546–1561. [Google Scholar] [CrossRef] [PubMed]
- Günther, R.; Wurster, C.D.; Brakemeier, S.; Osmanovic, A.; Schreiber-Katz, O.; Petri, S.; Uzelac, Z.; Hiebeler, M.; Thiele, S.; Walter, M.C.; et al. Long-term efficacy and safety of nusinersen in adults with 5q spinal muscular atrophy: A prospective European multinational observational study. Lancet Reg. Health Eur. 2024, 39, 100862. [Google Scholar] [CrossRef] [PubMed]
- Finkel, R.S.; Mercuri, E.; Darras, B.T.; Connolly, A.M.; Kuntz, N.L.; Kirschner, J.; Chiriboga, C.A.; Saito, K.; Servais, L.; Tizzano, E.; et al. Nusinersen versus Sham Control in Infantile-Onset Spinal Muscular Atrophy. N. Engl. J. Med. 2017, 377, 1723–1732. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, E.; Darras, B.T.; Chiriboga, C.A.; Day, J.W.; Campbell, C.; Connolly, A.M.; Iannaccone, S.T.; Kirschner, J.; Kuntz, N.L.; Saito, K.; et al. Nusinersen versus Sham Control in Later-Onset Spinal Muscular Atrophy. N. Engl. J. Med. 2018, 378, 625–635. [Google Scholar] [CrossRef]
- Pearson, S.D.; Thokala, P.; Stevenson, M.; Rind, D. The Effectiveness and Value of Treatments for Spinal Muscular Atrophy. J. Manag. Care Spec. Pharm. 2019, 25, 1300–1306. [Google Scholar] [CrossRef]
- Abbas, K.S.; Eltaras, M.M.; El-Shahat, N.A.; Abdelazeem, B.; Shaqfeh, M.; Brašić, J.R. The Safety and Efficacy of Nusinersen in the Treatment of Spinal Muscular Atrophy: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Medicina 2022, 58, 213. [Google Scholar] [CrossRef]
- Engelborghs, S.; Niemantsverdriet, E.; Struyfs, H.; Blennow, K.; Brouns, R.; Comabella, M.; Dujmovic, I.; Van Der Flier, W.; Frölich, L.; Galimberti, D.; et al. Consensus guidelines for lumbar puncture in patients with neurological diseases. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2017, 8, 111–126. [Google Scholar] [CrossRef]
- Ökçesiz, I.; Dönmez, H.; Herdem, N.; Sarilar, A.Ç. Computed Tomography–Guided Lumbar Puncture: Advantages and Disadvantages. J. Comput. Assist. Tomogr. 2022, 46, 269–273. [Google Scholar] [CrossRef]
- Kouzehgarani, G.N.; Feldsien, T.; Engelhard, H.H.; Mirakhur, K.K.; Phipps, C.; Nimmrich, V.; Clausznitzer, D.; Lefebvre, D.R. Harnessing cerebrospinal fluid circulation for drug delivery to brain tissues. Adv. Drug Deliv. Rev. 2021, 173, 20–59. [Google Scholar] [CrossRef]
- Khoury, N.; Pizzo, M.E.; Discenza, C.B.; Joy, D.; Tatarakis, D.; Todorov, M.I.; Negwer, M.; Ha, C.; De Melo, G.L.; Sarrafha, L.; et al. Fc-engineered large molecules targeting blood-brain barrier transferrin receptor and CD98hc have distinct central nervous system and peripheral biodistribution. Nat. Commun. 2025, 16, 1822. [Google Scholar] [CrossRef] [PubMed]
- Manuel, M.-G.; Tamba, B.-I.; Leclere, M.; Mabrouk, M.; Schreiner, T.-G.; Ciobanu, R.; Cristina, T.-Z. Intrathecal Pseudodelivery of Drugs in the Therapy of Neurodegenerative Diseases: Rationale, Basis and Potential Applications. Pharmaceutics 2023, 15, 768. [Google Scholar] [CrossRef]
- Capozza, M.A.; Triarico, S.; Mastrangelo, S.; Attinà, G.; Maurizi, P.; Ruggiero, A. Narrative review of intrathecal drug delivery (IDD): Indications, devices and potential complications. Ann. Transl. Med. 2021, 9, 186. [Google Scholar] [CrossRef]
- Prasuhn, J.; Xu, J.; Hua, J.; van Zijl, P.; Knutsson, L. Exploring neurodegenerative disorders using advanced magnetic resonance imaging of the glymphatic system. Front. Psychiatry 2024, 15, 1368489. [Google Scholar] [CrossRef] [PubMed]
- Alhamadani, F.; Zhang, K.; Parikh, R.; Wu, H.; Rasmussen, T.P.; Bahal, R.; Zhong, X.-B.; Manautou, J.E. Adverse Drug Reactions and Toxicity of the Food and Drug Administration–Approved Antisense Oligonucleotide Drugs. Drug Metab. Dispos. 2022, 50, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Morganroth, J.; Bardakjian, T.M.; Dratch, L.; Quinn, C.C.; Elman, L.B. Enhancing Clinical Infrastructure for the Delivery of Intrathecal and Genetic Therapies. Neurol. Clin. Pract. 2024, 14, e200303. [Google Scholar] [CrossRef]
- Nandi, A.; Counts, N.; Chen, S.; Seligman, B.; Tortorice, D.; Vigo, D.; Bloom, D.E. Global and regional projections of the economic burden of Alzheimer’s disease and related dementias from 2019 to 2050: A value of statistical life approach. eClinicalMedicine 2022, 51, 101580. [Google Scholar] [CrossRef]
- Chen, X.; Lim, D.A.; Lawlor, M.W.; Dimmock, D.; Vite, C.H.; Lester, T.; Tavakkoli, F.; Sadhu, C.; Prasad, S.; Gray, S.J. Biodistribution of Adeno-Associated Virus Gene Therapy Following Cerebrospinal Fluid-Directed Administration. Hum. Gene Ther. 2023, 34, 94–111. [Google Scholar] [CrossRef]
- Hinderer, C.; Bell, P.; Katz, N.; Vite, C.H.; Louboutin, J.-P.; Bote, E.; Yu, H.; Zhu, Y.; Casal, M.L.; Bagel, J.; et al. Evaluation of Intrathecal Routes of Administration for Adeno-Associated Viral Vectors in Large Animals. Hum. Gene Ther. 2018, 29, 15–24. [Google Scholar] [CrossRef]
- Taghian, T.; Marosfoi, M.G.; Puri, A.S.; Cataltepe, O.; King, R.M.; Diffie, E.B.; Maguire, A.S.; Martin, D.R.; Fernau, D.; Batista, A.R.; et al. A Safe and Reliable Technique for CNS Delivery of AAV Vectors in the Cisterna Magna. Mol. Ther. 2020, 28, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Hirai, Y.; Miyake, K.; Shimada, T. Targeted gene transfer into ependymal cells through intraventricular injection of AAV1 vector and long-term enzyme replacement via the CSF. Sci. Rep. 2014, 4, 5506. [Google Scholar] [CrossRef] [PubMed]
- MacAllister, R.P.; McCully, C.M.L.; Bacher, J.; Thomas, M.L., III.; Cruz, R.; Wangari, S.; Warren, K.E. Minimally Invasive Lumbar Port System for the Collection of Cerebrospinal Fluid from Rhesus Macaques (Macaca mulatta). Comp. Med. 2016, 66, 349–352. [Google Scholar]
- González, M.M. Preparing for the new wave of intrathecal CNS therapies: Innovations in drug delivery devices. Expert. Rev. Med. Devices 2025, 22, 525–528. [Google Scholar] [CrossRef]
- Wesemann, K.; Coffey, R.J.; Wallace, M.S.; Tan, Y.; Broste, S.; Buvanendran, A. Clinical Accuracy and Safety Using the SynchroMed II Intrathecal Drug Infusion Pump. Reg. Anesthesia Pain Med. 2014, 39, 341–346. [Google Scholar] [CrossRef]
- Jamet, E. An eye-tracking study of cueing effects in multimedia learning. Comput. Human Behav. 2014, 32, 47–53. [Google Scholar] [CrossRef]
- Raj, R.; Lakshmanan, S.; Apigo, D.; Kanwal, A.; Liu, S.; Russell, T.; Madsen, J.R.; Thomas, G.A.; Farrow, R.C. Demonstration that a new flow sensor can operate in the clinical range for cerebrospinal fluid flow. Sens. Actuators A Phys. 2015, 234, 223–231. [Google Scholar] [CrossRef]
- Sun, Z.; Luan, X.; Sun, Z.; Li, D.; Hu, H.; Xue, Q.; Liu, B.; Yu, Q.; Wei, G.; Zhang, X.; et al. Bioactive Peptide Hydrogel Scaffold with High Fluidity, Thermosensitivity, and Neurotropism in 3D Spatial Structure for Promoted Repair of Spinal Cord Injury. Small 2024, 21, e2406990. [Google Scholar] [CrossRef]
- Madadi, A.K.; Sohn, M.-J. Advances in Intrathecal Nanoparticle Delivery: Targeting the Blood–Cerebrospinal Fluid Barrier for Enhanced CNS Drug Delivery. Pharmaceuticals 2024, 17, 1070. [Google Scholar] [CrossRef]
- Demeule, M.; Régina, A.; Ché, C.; Poirier, J.; Nguyen, T.; Gabathuler, R.; Castaigne, J.-P.; Béliveau, R. Identification and Design of Peptides as a New Drug Delivery System for the Brain. J. Pharmacol. Exp. Ther. 2008, 324, 1064–1072. [Google Scholar] [CrossRef]
- May, P.; Woldt, E.; Matz, R.L.; Boucher, P. The LDL receptor-related protein (LRP) family: An old family of proteins with new physiological functions. Ann. Med. 2007, 39, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Marzolo, M.-P.; Farfán, P. New Insights into the Roles of Megalin/LRP2 and the Regulation of its Functional Expression. Biol. Res. 2011, 44, 89–105. [Google Scholar] [CrossRef]
- Strazielle, N.; Ghersi-Egea, J.-F. Potential Pathways for CNS Drug Delivery Across the Blood-Cerebrospinal Fluid Barrier. Curr. Pharm. Des. 2016, 22, 5463–5476. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Xie, H. Application of stimuli-responsive hydrogel in brain disease treatment. Front. Bioeng. Biotechnol. 2024, 12, 1450267. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).