Actinic Cheilitis: A Systematic Review and Meta-Analysis of Interventions, Treatment Outcomes, and Adverse Events
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol, Registration, Eligibility Criteria, and Outcomes of Interest
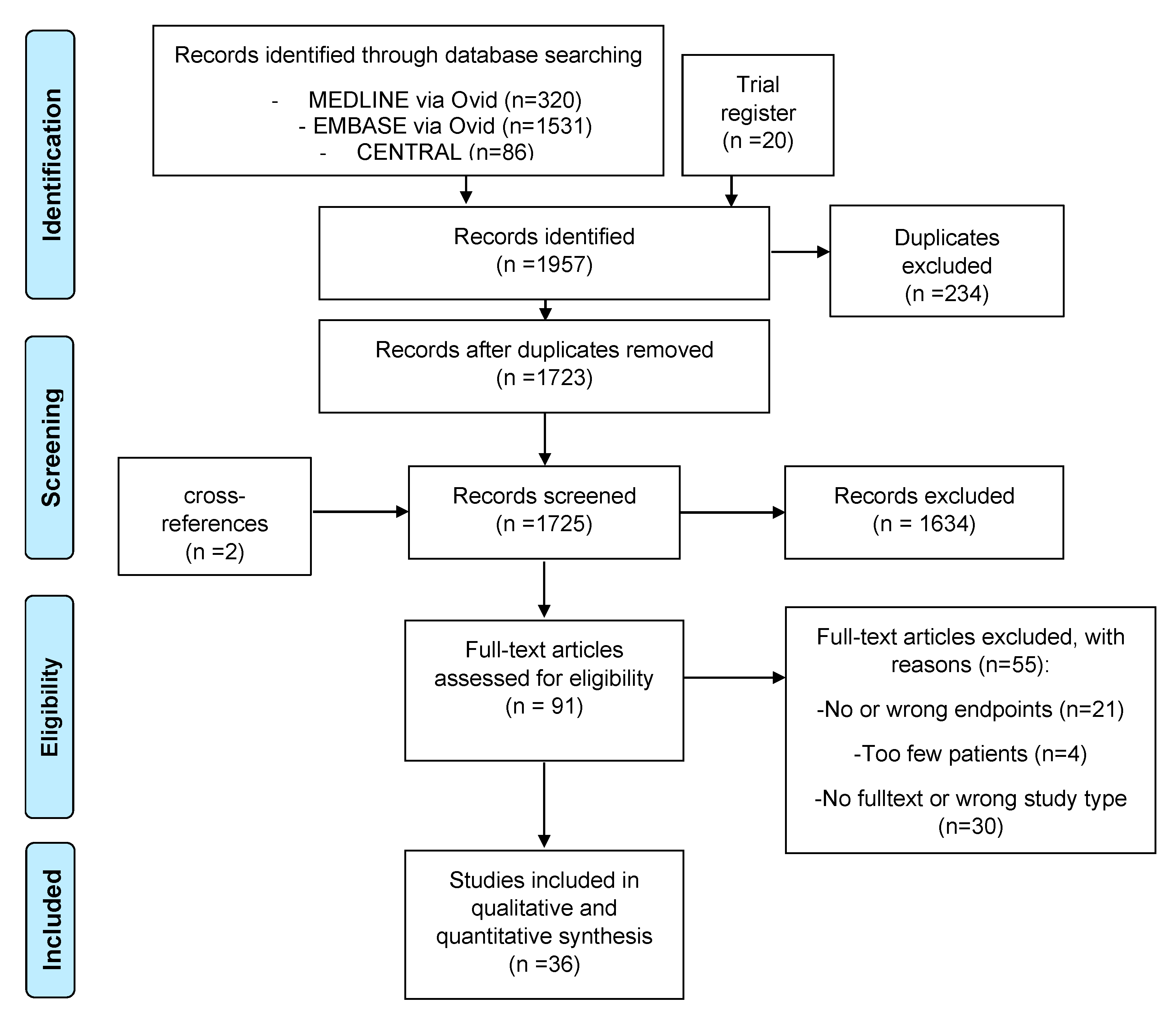
2.2. Literature Search and Study Selection
2.3. Data Collection, Synthesis, and Management
2.4. Assessment of Study Quality, Heterogeneity, Sensitivity Analysis, and Risk of Bias
- (1)
- sample selection and representativeness;
- (2)
- clarity and reliability of exposure and outcome measures;
- (3)
- temporal sequence of intervention and outcome;
- (4)
- presence and handling of confounding variables;
- (5)
- appropriateness of statistical analysis; and
- (6)
- completeness and transparency of result reporting.
3. Results
3.1. Identification and Characteristics of Included Studies
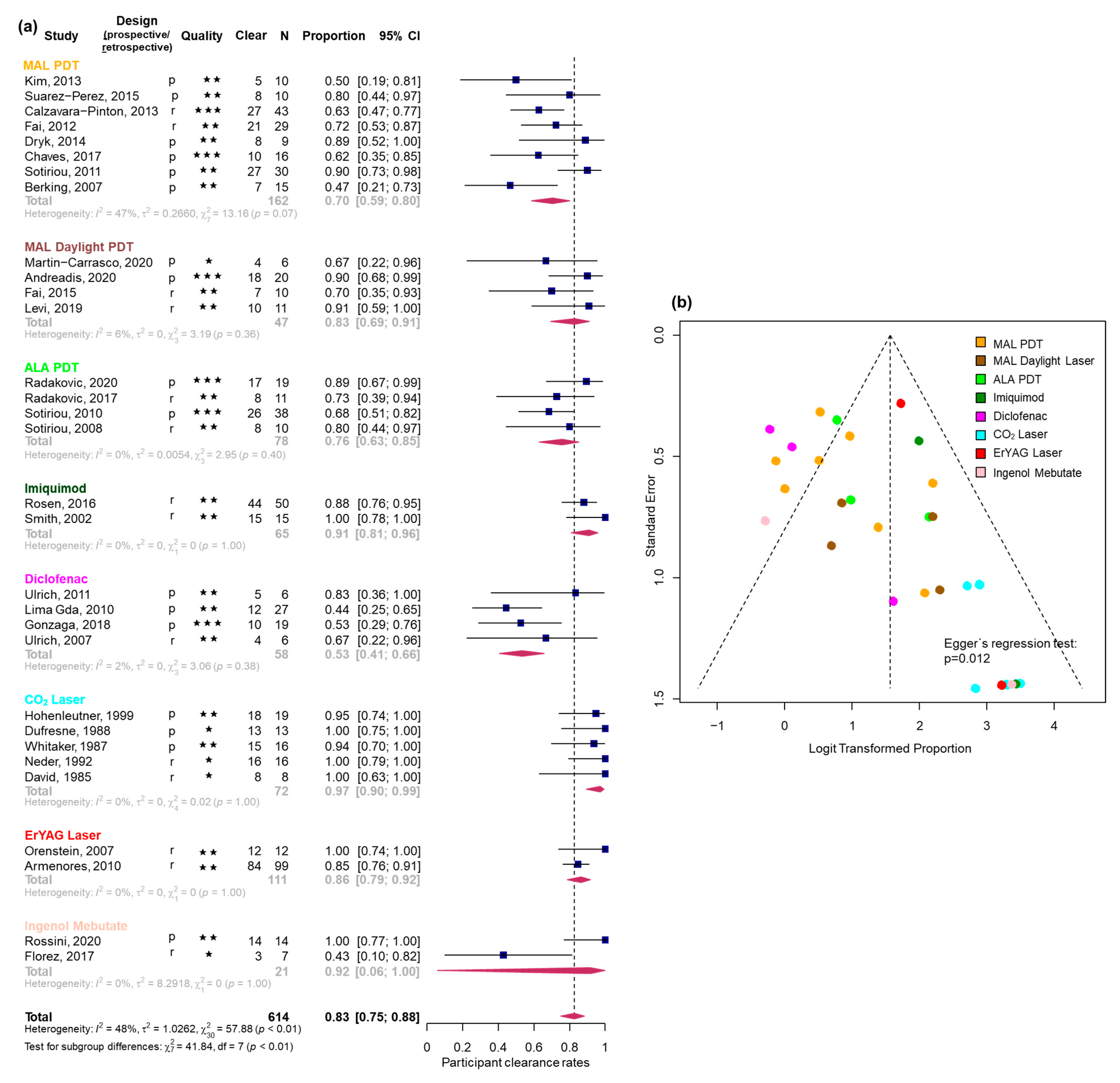
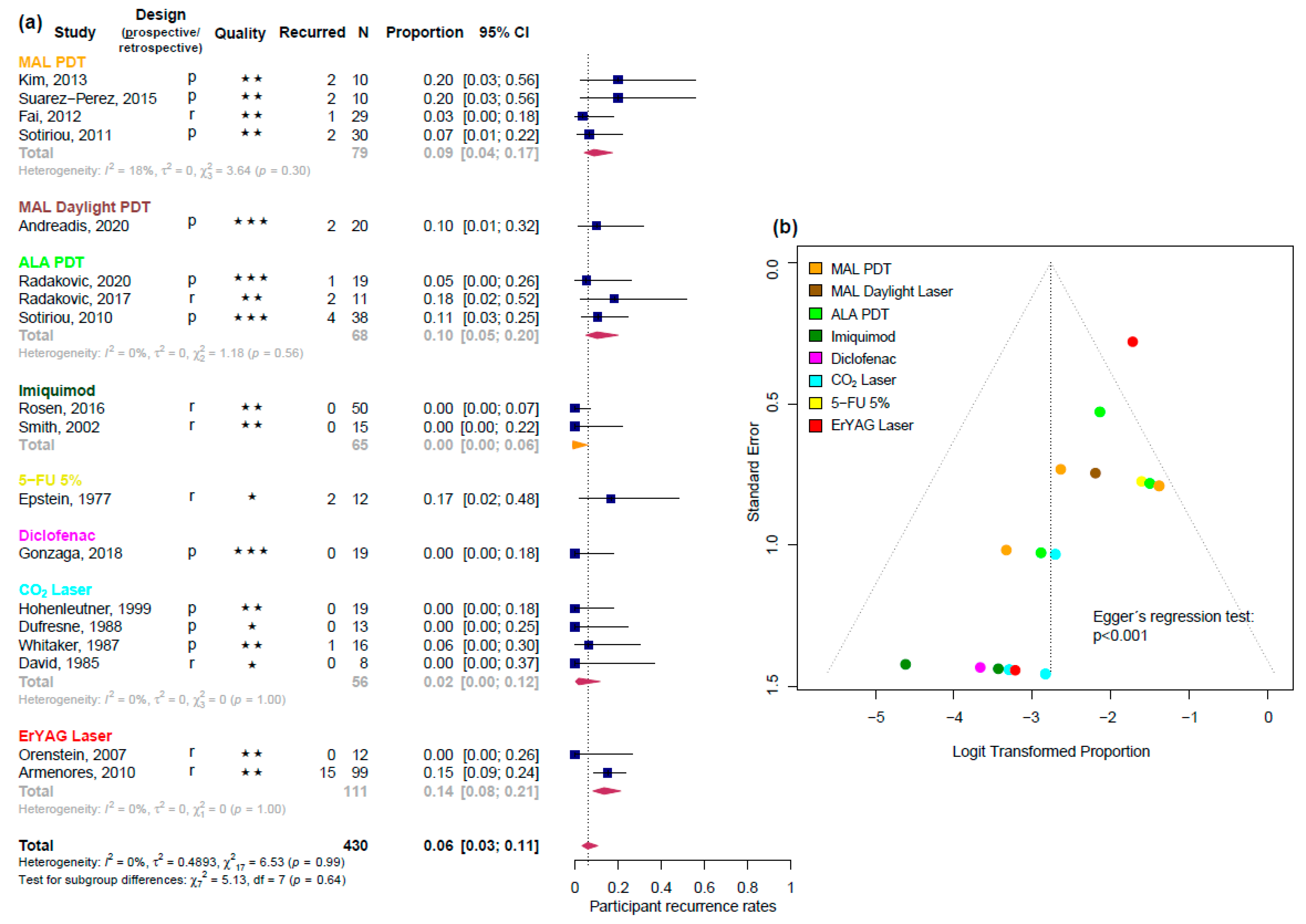
3.2. Efficacy Estimates Derived from Uncontrolled Studies
3.2.1. Participant Clearance Rate
3.2.2. Participant Recurrence Rate
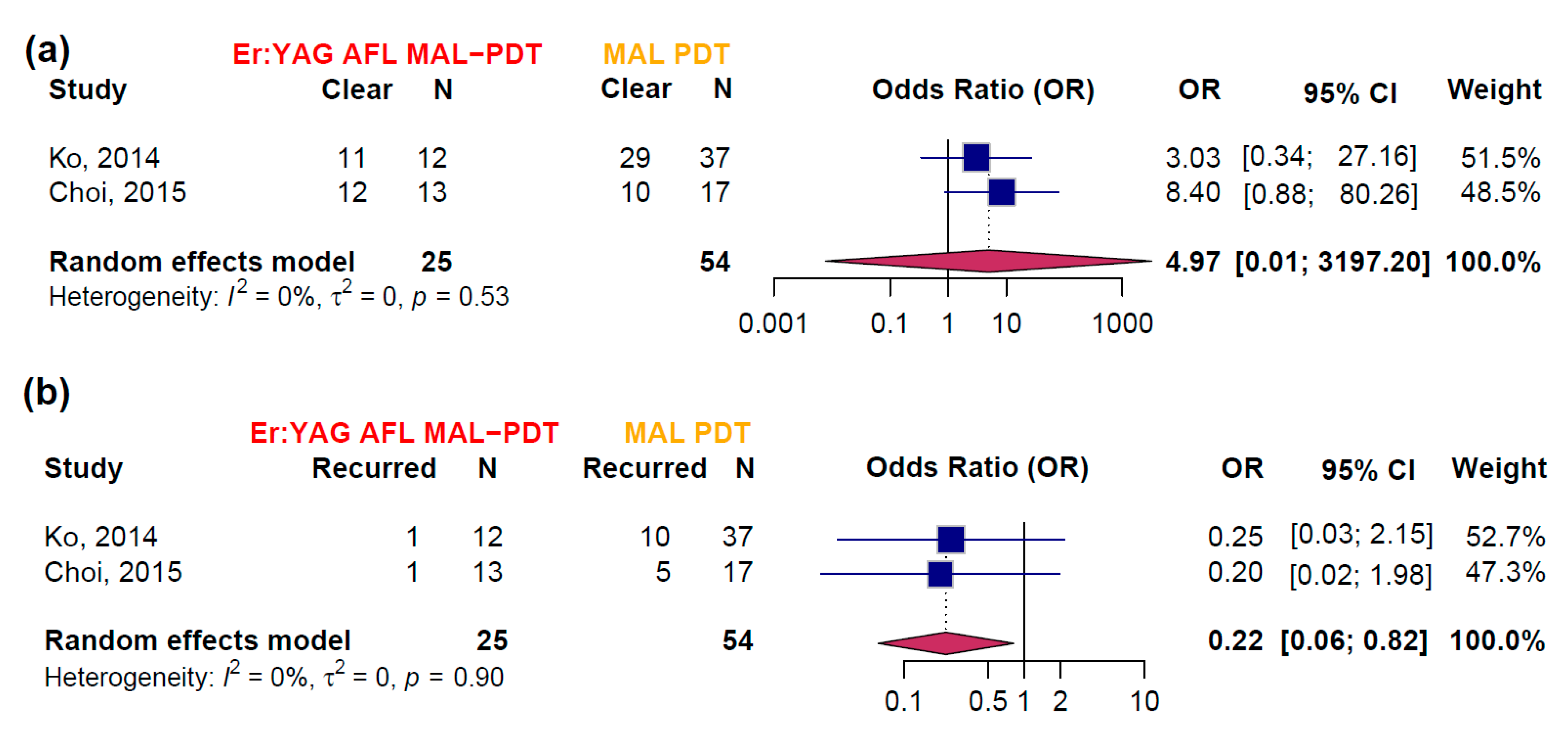
3.3. Efficacy Estimates Derived from RCTs
3.4. Adverse Events
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chaves, Y.N.; Torezan, L.A.; Lourenço, S.V.; Neto, C.F. Evaluation of the efficacy of photodynamic therapy for the treatment of actinic cheilitis. Photodermatol. Photoimmunol. Photomed. 2017, 33, 14–21. [Google Scholar] [CrossRef]
- Berking, C.; Herzinger, T.; Flaig, M.J.; Brenner, M.; Borelli, C.; Degitz, K. The efficacy of photodynamic therapy in actinic cheilitis of the lower lip: A prospective study of 15 patients. Dermatol. Surg. 2007, 33, 825–830. [Google Scholar] [CrossRef]
- Andreadis, D.; Pavlou, A.-M.; Vakirlis, E.; Anagnostou, E.; Vrani, F.; Poulopoulos, A.; Kolokotronis, A.; Ioannidis, D.; Sotiriou, E. Daylight photodynamic therapy for the management of actinic cheilitis. Arch. Dermatol. Res. 2020, 312, 731–737. [Google Scholar] [CrossRef]
- Main, J.H.; Pavone, M. Actinic cheilitis and carcinoma of the lip. J. Can. Dent. Assoc. 1994, 60, 113–116. [Google Scholar] [PubMed]
- Câmara, P.R.; Dutra, S.N.; Takahama, A., Jr.; Fontes, K.; Azevedo, R.S. A comparative study using WHO and binary oral epithelial dysplasia grading systems in actinic cheilitis. Oral Dis. 2016, 22, 523–529. [Google Scholar] [CrossRef]
- Konopka; Goslinski, T. Photodynamic Therapy in Dentistry. J. Dent. Res. 2007, 86, 694–707. [Google Scholar] [CrossRef]
- Armenores, P.; James, C.L.; Walker, P.C.; Huilgol, S.C. Treatment of actinic cheilitis with the Er:YAG laser. J. Am. Acad. Dermatol. 2010, 63, 642–646. [Google Scholar] [CrossRef]
- Bakirtzi, K.; Papadimitriou, I.; Andreadis, D.; Sotiriou, E. Treatment Options and Post-Treatment Malignant Transformation Rate of Actinic Cheilitis: A Systematic Review. Cancers 2021, 13, 3354. [Google Scholar] [CrossRef]
- Trager, M.H.; Farmer, K.; Ulrich, C.; Basset-Seguin, N.; Herms, F.; Geskin, L.J.; Bouaziz, J.; Lebbé, C.; de Masson, A.; Bagot, M.; et al. Actinic Cheilitis: A Systematic Review of Treatment Options. J. Eur. Acad. Dermatol. Venereol. 2020, 30, 815–823. [Google Scholar] [CrossRef]
- Ko, D.Y.; Choi, S.H.; Kim, K.H.; Song, K.H. Ablative fractional laser-assisted methyl aminolaevulinate photodynamic therapy (MAL-PDT) vs. conventional MAL-PDT for the treatment of actinic cheilitis: A randomized, comparative study. Br. J. Dermatol. 2014, 171, 51–52. [Google Scholar] [CrossRef][Green Version]
- Choi, S.H.; Kim, K.H.; Song, K.H. Efficacy of ablative fractional laser-assisted photodynamic therapy for the treatment of actinic cheilitis: 12-month follow-up results of a prospective, randomized, comparative trial. Br. J. Dermatol. 2015, 173, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Chu, H. Meta-analysis of Proportions Using Generalized Linear Mixed Models. Epidemiology 2020, 31, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Stijnen, T.; Hamza, T.H.; Özdemir, P. Random effects meta-analysis of event outcome in the framework of the generalized linear mixed model with applications in sparse data. Stat. Med. 2010, 29, 3046–3067. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Calzavara-Pinton, P.G.; Rossi, M.T.; Sala, R.; The Italian Group for Photodynamic Therapy. A retrospective analysis of real-life practice of off-label photodynamic therapy using methyl aminolevulinate (MAL-PDT) in 20 Italian dermatology departments. Part 2: Oncologic and infectious indications. Photochem. Photobiol. Sci. 2013, 12, 158–165. [Google Scholar] [CrossRef]
- Radakovic, S.; Dangl, M.; Tanew, A. 5-Aminolevulinic acid patch (Alacare) photodynamic therapy for actinic cheilitis: Data from a prospective 12-month follow-up study on 21 patients. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2011–2015. [Google Scholar] [CrossRef]
- Sotiriou, E.; Apalla, Z.; Chovarda, E.; Panagiotidou, D.; Ioannides, D. Photodynamic therapy with 5-aminolevulinic acid in actinic cheilitis: An 18-month clinical and histological follow-up. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 916–920. [Google Scholar] [CrossRef]
- Gonzaga, A.K.G.; de Oliveira, P.T.; da Silveira, É.J.D.; Queiroz, L.M.G.; de Medeiros, A.M.C. Diclofenac sodium gel therapy as an alternative to actinic cheilitis. Clin. Oral Investig. 2018, 22, 1319–1325. [Google Scholar] [CrossRef]
- Kim, S.K.; Song, H.S.; Kim, Y.C. Topical photodynamic therapy may not be effective for actinic cheilitis despite repeated treatments. Eur. J. Dermatol. 2013, 23, 917–918. [Google Scholar] [CrossRef]
- Suárez-Pérez, J.A.; López-Navarro, N.; Herrera-Acosta, E.; Aguilera, J.; Gallego, E.; Bosch, R.; Herrera, E. Treatment of actinic cheilitis with methyl aminolevulinate photodynamic therapy and light fractionation: A prospective study of 10 patients. Eur. J. Dermatol. 2015, 25, 623–624. [Google Scholar] [CrossRef]
- Fai, D.; Romano, I.; Cassano, N.; Vena, G.A. Methyl-aminolevulinate photodynamic therapy for the treatment of actinic cheilitis: A retrospective evaluation of 29 patients. G. Ital. Dermatol. Venereol. 2012, 147, 99–101. [Google Scholar] [PubMed]
- Dryk, K.; Gallego, E.; Herrera-Ceballos, E.; Alonso-Suarez, J.; Lopez-Navarro, N.; Bosch-Garcia, R.; Castillo-Munoz, R. The efficacy of fractionated photodynamic therapy in actinic cheilitis of the lower lip. J. Am. Acad. Dermatol. 2014, 70, AB157. [Google Scholar] [CrossRef]
- Sotiriou, E.; Lallas, A.; Goussi, C.; Apalla, Z.; Trigoni, A.; Chovarda, E.; Ioannides, D. Sequential use of photodynamic therapy and imiquimod 5% cream for the treatment of actinic cheilitis: A 12-month follow-up study. Br. J. Dermatol. 2011, 165, 888–892. [Google Scholar] [CrossRef]
- Fai, D.; Romanello, E.; Brumana, M.B.; Fai, C.; Vena, G.A.; Cassano, N.; Piaserico, S. Daylight photodynamic therapy with methyl-aminolevulinate for the treatment of actinic cheilitis. Dermatol. Ther. 2015, 28, 355–368. [Google Scholar] [CrossRef]
- Levi, A.; Hodak, E.; Enk, C.D.; Snast, I.; Slodownik, D.; Lapidoth, M. Daylight photodynamic therapy for the treatment of actinic cheilitis. Photodermatol. Photoimmunol. Photomed. 2019, 35, 11–16. [Google Scholar] [CrossRef]
- Radakovic, S.; Tanew, A. 5-aminolaevulinic acid patch-photodynamic therapy in the treatment of actinic cheilitis. Photodermatol. Photoimmunol. Photomed. 2017, 33, 306–310. [Google Scholar] [CrossRef]
- Sotiriou, E.; Apalla, Z.; Koussidou-Erremonti, T.; Ioannides, D. Actinic cheilitis treated with one cycle of 5-aminolaevulinic acid-based photodynamic therapy: Report of 10 cases. Br. J. Dermatol. 2008, 159, 261–262. [Google Scholar] [CrossRef]
- Rosen, T. Actinic cheilitis: Is Imiquimod 5% a viable treatment? A clinical study. Australas. J. Dermatol. 2016, 57 (Suppl. 2), 5. [Google Scholar]
- Smith, K.J.; Germain, M.; Yeager, J.; Skelton, H. Topical 5% imiquimod for the therapy of actinic cheilitis. J. Am. Acad. Dermatol. 2002, 47, 497–501. [Google Scholar] [CrossRef]
- Ulrich, M.; González, S.; Lange-Asschenfeldt, B.; Roewert-Huber, J.; Sterry, W.; Stockfleth, E.; Astner, S. Non-invasive diagnosis and monitoring of actinic cheilitis with reflectance confocal microscopy. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 276–284. [Google Scholar] [CrossRef]
- Lima, G.d.S.; Silva, G.F.; Gomes, A.P.; de Araújo, L.M.; Salum, F.G. Diclofenac in hyaluronic acid gel: An alternative treatment for actinic cheilitis. J. Appl. Oral Sci. 2010, 18, 533–537. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ulrich, C.; Forschner, T.; Ulrich, M.; Stockfleth, E.; Sterry, W.; Termeer, C. Management of actinic cheilitis using diclofenac 3% gel: A report of six cases. Br. J. Dermatol. 2007, 156 (Suppl. 3), 43–46. [Google Scholar] [CrossRef] [PubMed]
- Hohenleutner, S.; Landthaler, M.; Hohenleutner, U. CO(2) laser vaporisation of actinic cheilitis. Hautarzt 1999, 50, 562–565. [Google Scholar] [CrossRef]
- Whitaker, D.C. Microscopically proven cure of actinic cheilitis by CO2 laser. Lasers Surg. Med. 1987, 7, 520–523. [Google Scholar] [CrossRef]
- Orenstein, A.; Goldan, O.; Weissman, O.; Winkler, E.; Haik, J. A new modality in the treatment of actinic cheilitis using the Er:YAG laser. J. Cosmet. Laser Ther. 2007, 9, 23–25. [Google Scholar] [CrossRef]
- Rossini, R.d.C.; Dellatorre, G.; Mesquita, L.A.d.F.; Tarlé, R.G. Ingenol mebutate treatment for actinic cheilitis: Clinical, histopathological and p53 profile of 14 cases. J. Dermatol. Treat. 2020, 32, 1049–1052. [Google Scholar] [CrossRef]
- Martín-Carrasco, P.; Sendín-Martín, M.; Domínguez-Cruz, J.; Bernabeu-Wittel, J. Actinic Cheilitis Treated with Daylight Photodynamic Therapy. Actas Dermo-Sifiliogr. 2020, 111, 883–885. [Google Scholar] [CrossRef]
- Dufresne, R.G., Jr.; Garrett, A.B.; Bailin, P.L.; Ratz, J.L. Carbon dioxide laser treatment of chronic actinic cheilitis. J. Am. Acad. Dermatol. 1988, 19 Pt 1, 876–878. [Google Scholar] [CrossRef]
- Neder, A.; Nahlieli, O.; Kaplan, I. CO2 laser used in surgical treatment of actinic cheilitis. J. Clin. Laser Med. Surg. 1992, 10, 373–375. [Google Scholar] [CrossRef]
- David, L.M. Laser vermilion ablation for actinic cheilitis. J. Dermatol. Surg. Oncol. 1985, 11, 605–608. [Google Scholar] [CrossRef] [PubMed]
- Florez, A.; Batalla, A.; de la Torre, C. Management of actinic cheilitis using ingenol mebutate gel: A report of seven cases. J. Dermatol. Treat. 2017, 28, 149–151. [Google Scholar] [CrossRef] [PubMed]
- Epstein, E. Treatment of lip keratoses (actinic cheilitis) with topical fluorouracil. Arch. Dermatol. 1977, 113, 906–908. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.K. Actinic cheilitis. A prospective study comparing four treatment methods. Arch. Otolaryngol.—Head Neck Surg. 1989, 115, 848–852. [Google Scholar] [CrossRef]
- Husein-ElAhmed, H.; Almazan-Fernandez, F.M.; Husein-ElAhmed, S. Ingenol mebutate versus imiquimod versus diclofenac for actinic cheilitis: A 6-month follow-up clinical study. Clin. Exp. Dermatol. 2019, 44, 231–234. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). Risks of Picato for Actinic Keratosis Outweigh Benefits. 2020. Available online: https://www.ema.europa.eu/en/documents/press-release/risks-picato-actinic-keratosis-outweigh-benefits_en.pdf (accessed on 28 July 2025).
- Lai, M.; Pampena, R.; Cornacchia, L.; Pellacani, G.; Peris, K.; Longo, C. Treatments of actinic cheilitis: A systematic review of the literature. J. Am. Acad. Dermatol. 2020, 83, 876–887. [Google Scholar] [CrossRef]
- Salgueiro, A.P.; de Jesus, L.H.; de Souza, I.F.; Rados, P.V.; Visioli, F. Treatment of actinic cheilitis: A systematic review. Clin. Oral Investig. 2019, 23, 2041–2053. [Google Scholar] [CrossRef]
- Ayen-Rodriguez, A.; Naranjo-Diaz, M.J.; Ruiz-Villaverde, R. Laser Therapy for the Treatment of Actinic Cheilitis: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 4593. [Google Scholar] [CrossRef]
- Medeiros, C.K.S.; de França, G.M.; Lima, J.G.d.C.; Pinheiro, J.C.; Almeida, D.R.d.M.F.; Santos, P.P.d.A. Use of topical anti-inflammatory and antineoplastic agents in the treatment of young-aged actinic cheilitis: A systematic review. J. Cosmet. Dermatol. 2022, 21, 473–481. [Google Scholar] [CrossRef]
| Population | Intervention | Comparison | Outcome | Trials |
|---|---|---|---|---|
| Patients with actinic cheilitis (clinical or histological confirmed) with all grades of dysplasia subgroups (grade 1,2,3) | Any relevant therapy including:
| placebo, vehicle-controlled, active control (in RCTs) no control in uncontrolled or retrospective studies/case series | At least one of the following efficacy outcomes:
|
|
| Treatment | PCR | 95% CI | I2 | Number of Studies |
|---|---|---|---|---|
| CO2 laser | 0.97 | [0.90; 0.99] | 0% | 5 |
| Imiquimod | 0.91 | [0.81; 0.96] | 0% | 2 |
| Er:YAG laser | 0.86 | [0.79; 0.92] | 0% | 2 |
| MAL daylight PDT | 0.83 | [0.69; 0.91] | 6% | 4 |
| ALA red light PDT | 0.76 | [0.63; 0.85] | 0% | 4 |
| MAL red light PDT | 0.7 | [0.59; 0.80] | 47% | 8 |
| Diclofenac | 0.53 | [0.41; 0.66] | 2% | 4 |
| IMB | 0.92 | [0.06; 1.00] | 0% | 2 |
| Treatment | PRR | 95% CI | I2 | Number of Studies |
|---|---|---|---|---|
| Imiquimod | 0.0 | [0.00; 0.60] | na | 2 |
| CO2 laser | 0.02 | [0.00; 0.12] | 0% | 4 |
| MAL red light PDT | 0.09 | [0.04; 0.17] | 18% | 4 |
| ALA red light PDT | 0.1 | [0.05; 0.20] | 0% | 3 |
| Er:YAG laser | 0.14 | [0.08; 0.21] | 0% | 2 |
| Diclofenac | 0.0 | [0.00; 0.18] | na | 1 |
| MAL daylight PDT | 0.1 | [0.01; 0.32] | na | 1 |
| 5-FU 5% | 0.17 | [0.02; 0.48] | na | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Fartwsi, M.; Petzold, A.; Steeb, T.; Djawher, L.A.; Wessely, A.; Leppert, A.; Berking, C.; Heppt, M.V. Actinic Cheilitis: A Systematic Review and Meta-Analysis of Interventions, Treatment Outcomes, and Adverse Events. Biomedicines 2025, 13, 1896. https://doi.org/10.3390/biomedicines13081896
Al-Fartwsi M, Petzold A, Steeb T, Djawher LA, Wessely A, Leppert A, Berking C, Heppt MV. Actinic Cheilitis: A Systematic Review and Meta-Analysis of Interventions, Treatment Outcomes, and Adverse Events. Biomedicines. 2025; 13(8):1896. https://doi.org/10.3390/biomedicines13081896
Chicago/Turabian StyleAl-Fartwsi, Matthäus, Anne Petzold, Theresa Steeb, Lina Amin Djawher, Anja Wessely, Anett Leppert, Carola Berking, and Markus V. Heppt. 2025. "Actinic Cheilitis: A Systematic Review and Meta-Analysis of Interventions, Treatment Outcomes, and Adverse Events" Biomedicines 13, no. 8: 1896. https://doi.org/10.3390/biomedicines13081896
APA StyleAl-Fartwsi, M., Petzold, A., Steeb, T., Djawher, L. A., Wessely, A., Leppert, A., Berking, C., & Heppt, M. V. (2025). Actinic Cheilitis: A Systematic Review and Meta-Analysis of Interventions, Treatment Outcomes, and Adverse Events. Biomedicines, 13(8), 1896. https://doi.org/10.3390/biomedicines13081896







