Focused Modulation of Brain Activity: A Narrative Review
Abstract
1. Introduction
2. Biophysical Methods
2.1. Deep Brain Stimulation
| Method | Clinical Indication | Target Brain Region | Approval Year |
|---|---|---|---|
| Deep brain stimulation | Essential tremor, Parkinsonian tremor | Ventral intermediate nucleus of thalamus | 1997 [17] |
| Parkinson’s disease | Internal globus pallidus, subthalamic nucleus | 2002 [18], 2025 [19]—aDBS 1 | |
| Primary dystonia (under HDE 2) | Internal globus pallidus, subthalamic nucleus | 2003 [20] | |
| Obsessive–compulsive disorder (under HDE 2) | Anterior limb of the internal capsule | 2009 [21] | |
| Epilepsy | Anterior nucleus of thalamus | 2018 [22] | |
| Transcranial direct current stimulation | Major depressive disorder | Prefrontal cortex | 2015 [23] |
| Chronic pain syndromes such as fibromyalgia and migraine | Primary motor cortex | 2016 [24] | |
| Transcranial magnetic stimulation | Major depressive disorder | Cerebral cortex | 2008 [25,26], 2021 [27]—with comorbid anxiety |
| Headache (migraine with aura) | Occipital cortex | 2013 [28] | |
| Obsessive–compulsive disorder | Prefrontal cortex | 2017 [29] | |
| Smoking cessation | Prefrontal cortex, insula | 2020 [30] | |
| Transcranial focused ultrasound stimulation | Essential tremor | Ventral intermediate nucleus of the thalamus | 2016 [31] |
| Parkinson’s disease | Ventral intermediate nucleus of the thalamus | 2018 [32]—tremor; MRgFUS 3 2021 [33]—mobility, rigidity, or dyskinesia; MRgFUS 3 |
2.2. Transcranial Direct Current Stimulation
2.3. Transcranial Magnetic Stimulation
2.4. Focused Ultrasound Stimulation
2.5. Multi-Physical-Factor Stimulation Techniques
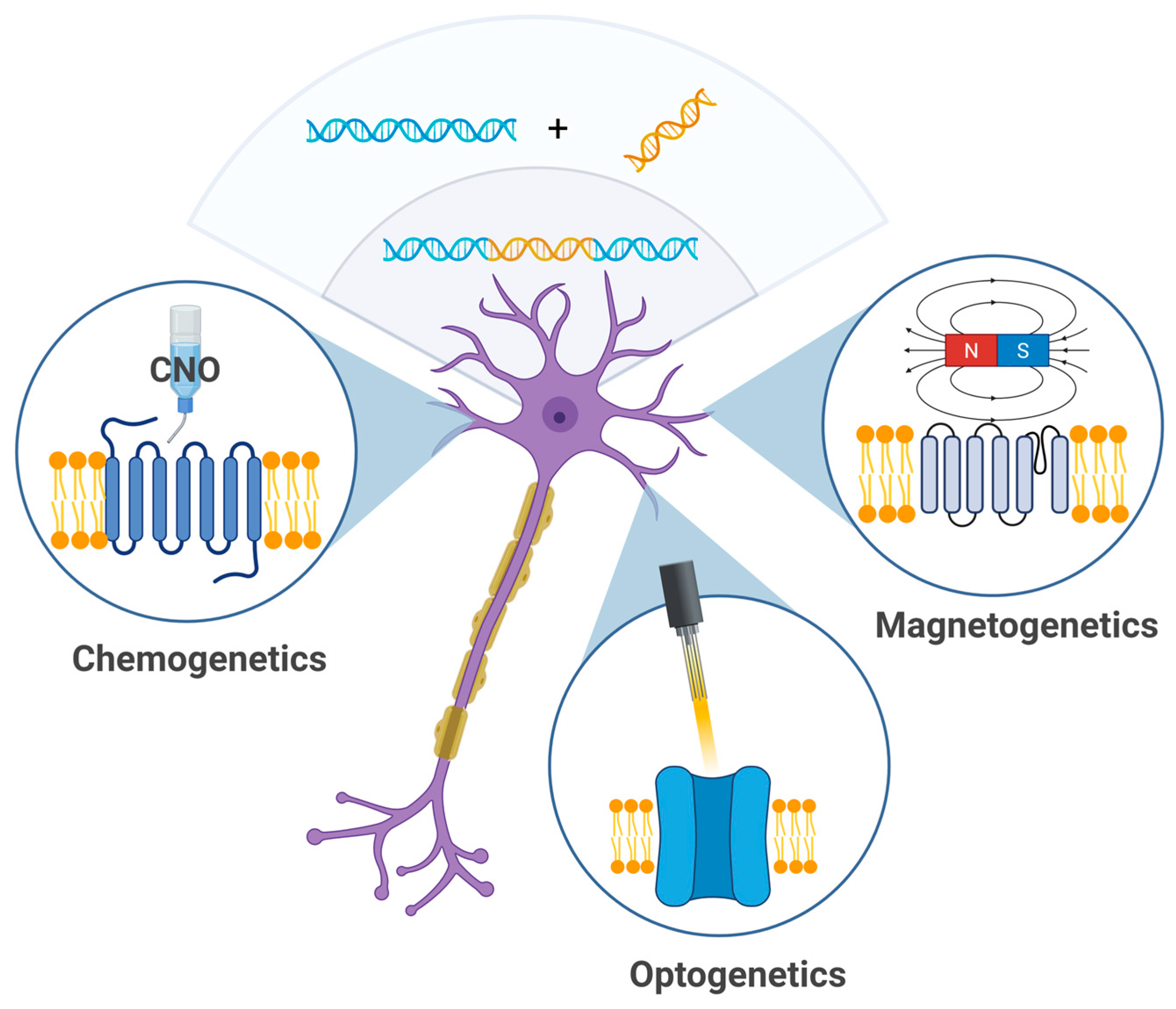
3. Genetic Methods
3.1. Chemogenetics
3.2. Magnetogenetics
3.3. Optogenetics
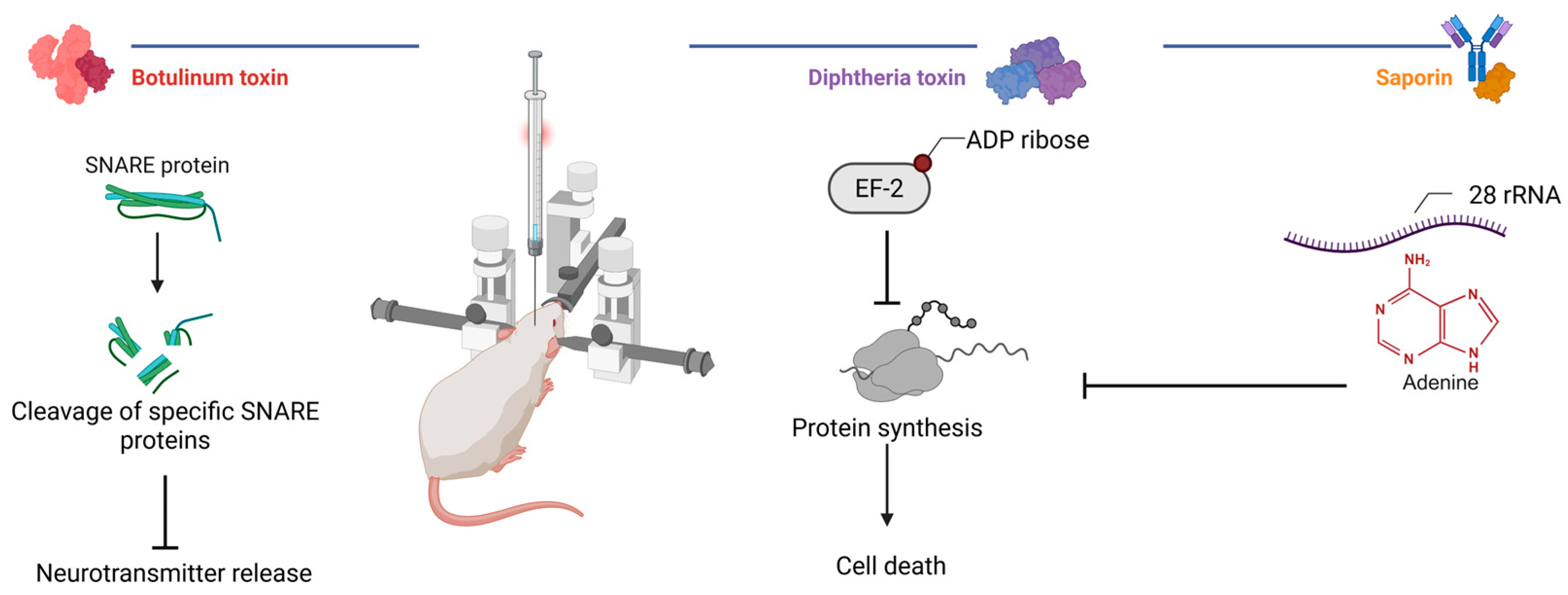
4. Biological Methods
4.1. Protein Synthesis Inhibiting Toxins: Saporin and Diphtheria Toxin
4.2. Neurotransmission Inhibiting Toxins: Botulinum Neurotoxins
5. Future Directions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 6-OHDA | 6-hydroxydopamine |
| ADAPT-PD | Adaptive DBS Algorithm for Personalized Therapy in Parkinson’s Disease |
| aDBS | Adaptive deep brain stimulation |
| ADP | Adenosine diphosphate |
| BBB | Blood–brain barrier |
| BCIs | Brain–computer interfaces |
| BDNF | Brain-derived neurotrophic factor |
| BoNTs | Botulinum neurotoxins |
| CFA | Complete Freund’s Adjuvant |
| CNO | Clozapine-N-oxide |
| DBS | Deep brain stimulation |
| DCZ | Deschloroclozapine |
| Derm-Bot | Dermorphin-botulinum neurotoxin |
| DLPFC | Dorsolateral prefrontal cortex |
| DREADDs | Designer Receptors Exclusively Activated by Designer Drugs |
| DT | Diphtheria toxin |
| EEG | Electroencephalogram |
| EF-2 | Elongation factor 2 |
| EPG | Electromagnetic perceptive gene |
| FDA | US Food and Drug Administration |
| fMRI | Functional magnetic resonance imaging |
| fNIRS | Functional near-infrared spectroscopy |
| FUS | Focused ultrasound stimulation |
| GABA | Gamma-aminobutyric acid |
| GDNF | Glial cell line-derived neurotrophic factor |
| HDE | Humanitarian Device Exemption |
| LF-TMS | Low-frequency transcranial magnetic stimulation |
| lOFC | Lateral orbitofrontal cortex |
| MRgFUS | Magnetic resonance image-guided focused ultrasound stimulation |
| MRI | Magnetic resonance imaging |
| rRNA | Ribosomal ribonucleic acid |
| rTMS | Repetitive transcranial magnetic stimulation |
| SNAP-25 | Synaptosomal-associated protein 25 |
| SP-Bot | Substance P–botulinum neurotoxin |
| sTMS | Single pulse transcranial magnetic stimulation |
| tDCS | Transcranial direct current stimulation |
| TMAS | Transcranial magnetoacoustic stimulation |
| TMS | Transcranial magnetic stimulation |
References
- Kuncel, A.M.; Grill, W.M. Selection of stimulus parameters for deep brain stimulation. Clin. Neurophysiol. 2004, 115, 2431–2441. [Google Scholar] [CrossRef]
- Butson, C.R.; McIntyre, C.C. Role of electrode design on the volume of tissue activated during deep brain stimulation. J. Neural. Eng. 2005, 3, 1. [Google Scholar] [CrossRef]
- Jakobs, M.; Fomenko, A.; Lozano, A.M.; Kiening, K.L. Cellular, molecular, and clinical mechanisms of action of deep brain stimulation—A systematic review on established indications and outlook on future developments. EMBO Mol. Med. 2019, 11, e9575. [Google Scholar] [CrossRef] [PubMed]
- Krauss, J.K.; Lipsman, N.; Aziz, T.; Boutet, A.; Brown, P.; Chang, J.W.; Davidson, B.; Grill, W.M.; Hariz, M.I.; Horn, A.; et al. Technology of deep brain stimulation: Current status and future directions. Nat. Rev. Neurol. 2021, 17, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Marín, G.; Castillo-Rangel, C.; Salomón-Lara, L.; Vega-Quesada, L.A.; Calderon, C.Z.; Borda-Low, C.D.; Soto-Abraham, J.E.; Coria-Avila, G.A.; Manzo, J.; García-Hernández, L.I. Deep brain stimulation in neurological diseases and other pathologies. Neurol. Perspect. 2022, 2, 151–159. [Google Scholar] [CrossRef]
- Ramasubbu, R.; Clark, D.L.; Golding, S.; Dobson, K.S.; Mackie, A.; Haffenden, A.; Kiss, Z.H. Long versus short pulse width subcallosal cingulate stimulation for treatment-resistant depression: A randomised, double-blind, crossover trial. Lancet Psychiatry 2020, 7, 29–40. [Google Scholar] [CrossRef]
- Nowacki, A.; Schober, M.; Nader, L.; Saryyeva, A.; Nguyen, T.A.K.; Green, A.L.; Polo, C.; Krauss, J.K.; Fontaine, D.; Aziz, T.Z. Deep brain stimulation for chronic cluster headache: Meta-analysis of individual patient data. Ann. Neurol. 2020, 88, 956–969. [Google Scholar] [CrossRef]
- Aibar-Durán, J.Á.; González, N.; Mirapeix, R.M.; Sánchez-Mateos, N.M.; Arsequell, C.R.; Pichot, M.B.; Nieto, R.B.; Fenoy, G.P.; Schmidt, C.Q.; Hernandez, F.M.; et al. Deep brain stimulation for chronic refractory cluster headache: A case series about long-term outcomes and connectivity analysis. Headache 2025, 65, 473–483. [Google Scholar] [CrossRef]
- Bishay, A.E.; Lyons, A.T.; Koester, S.W.; Paulo, D.L.; Liles, C.; Dambrino, R.J.; Feldman, F.J.; Ball, T.J.; Bick, S.K.; Englot, D.J.; et al. Global economic evaluation of the reported costs of deep brain stimulation. Stereotact. Funct. Neurosurg. 2024, 102, 257–274. [Google Scholar] [CrossRef]
- Fenoy, A.J.; Simpson, R.K. Risks of common complications in deep brain stimulation surgery: Management and avoidance. J. Neurosurg. 2014, 120, 132–139. [Google Scholar] [CrossRef]
- Hariz, M.I. Complications of deep brain stimulation surgery. Mov. Disord. 2002, 17, S162–S166. [Google Scholar] [CrossRef]
- Castelli, L.; Perozzo, P.; Zibetti, M.; Crivelli, B.; Morabito, U.; Lanotte, M.; Cossa, F.; Bergamasco, B.; Lopiano, L. Chronic deep brain stimulation of the subthalamic nucleus for Parkinson’s disease: Effects on cognition, mood, anxiety and personality traits. Eur. Neurol. 2006, 55, 136–144. [Google Scholar] [CrossRef]
- Voon, V.; Kubu, C.; Krack, P.; Houeto, J.L.; Tröster, A.I. Deep brain stimulation: Neuropsychological and neuropsychiatric issues. Mov. Disord. 2006, 21, S305–S327. [Google Scholar] [CrossRef]
- Priori, A.; Maiorana, N.; Dini, M.; Guidetti, M.; Marceglia, S.; Ferrucci, R. Adaptive deep brain stimulation (aDBS). Int. Rev. Neurobiol. 2021, 159, 111–127. [Google Scholar] [PubMed]
- Oehrn, C.R.; Cernera, S.; Hammer, L.H.; Shcherbakova, M.; Yao, J.; Hahn, A.; Wang, S.; Ostrem, J.L.; Little, S.; Starr, P.A. Chronic adaptive deep brain stimulation versus conventional stimulation in Parkinson’s disease: A blinded randomized feasibility trial. Nat. Med. 2024, 30, 3345–3356. [Google Scholar] [CrossRef] [PubMed]
- Adee, S. Smart brain-zapping implants could revolutionize Parkinson’s treatment. Nature 2006, 643, 625–627. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administratiom. PMA P960009: Medtronic Activa Tremor Control System. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMA/pma.cfm?id=P960009 (accessed on 15 June 2025).
- U.S. Food and Drug Administration. PMA P960009/S007: Medtronic Activa Tremor Control System-Supplement 007. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P960009S007 (accessed on 15 June 2025).
- U.S. Food and Drug Administration. PMA P960009/S478: Medtronic Activa System-Supplement 478. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P960009S478 (accessed on 15 June 2025).
- U.S. Food and Drug Administration. HDE H020007: Medtronic Activa Deep Brain Stimulation (DBS) System. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfhde/hde.cfm?id=375511 (accessed on 16 June 2025).
- U.S. Food and Drug Administration. HDE H050003: Medtronic (Activa) Deep Brain Stimulation for OCD Therapy. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfhde/hde.cfm?id=375533 (accessed on 16 June 2025).
- U.S. Food and Drug Administration. PMA P960009/S219: Medtronic DBS Therapy for Epilepsy-Supplement 219. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=p960009s219 (accessed on 16 June 2025).
- Soterix Medical, Inc. Receives CE Mark Approval for 1 × 1 tDCS Depression Therapy. Available online: https://soterixmedical.com/newsroom/press/2015/12/soterix-medical-inc-receives-ce-mark-approval-for-1x1/22 (accessed on 17 June 2025).
- Soterix Medical, Inc. Soterix Medical Launches PainX tDCS Treatment in EU with CE Approval. Available online: https://soterixmedical.com/newsroom/press/2016/01/soterix-medical-launches-painx-tdcs-treatment-in-eu-with/23 (accessed on 17 June 2025).
- U.S. Food and Drug Administration. Device Classification Under Section 513(f)(2)(De Novo): NeuroStar TMS System. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/denovo.cfm?ID=DEN070003 (accessed on 17 June 2025).
- U.S. Food and Drug Administration. Product Classification: Transcranial Magnetic Stimulator. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpcd/classification.cfm?id=4054 (accessed on 17 June 2025).
- U.S. Food and Drug Administration. K210201: Deep Transcranial Magnetic Stimulation (DTMS) System. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf21/K210201.pdf (accessed on 18 June 2025).
- U.S. Food and Drug Administration. K130556: Cerena Transcranial Magnetic Stimulator (TMS) Device. Available online: https://www.accessdata.fda.gov/cdrh_docs/reviews/k130556.pdf (accessed on 18 June 2025).
- U.S. Food and Drug Administration. DEN170078: Brainsway Deep Transcranial Magnetic Stimulation System. Available online: https://www.accessdata.fda.gov/cdrh_docs/reviews/DEN170078.pdf (accessed on 18 June 2025).
- U.S. Food and Drug Administration. K200957: Transcranial Magnetic Stimulation System for Neurological and Psychiatric Disorders and Conditions. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf20/K200957.pdf (accessed on 18 June 2025).
- U.S. Food and Drug Administration. PMA P150038: Magnetic Resonance Guided Focused Ultrasound Surgery System (MRgFUS). Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf15/P150038B.pdf (accessed on 19 June 2025).
- U.S. Food and Drug Administration. PMA P150038: Magnetic Resonance Guided Focused Ultrasound Surgery System (MRgFUS) Supplement. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf15/P150038S006B.pdf (accessed on 19 June 2025).
- Focused Ultrasound Foundation. FDA Approves FUS for Parkinson’s Disease. Available online: https://www.fusfoundation.org/newsletters/november-11-2021-fda-approves-fus-for-parkinsons-disease-and-more-news/ (accessed on 18 June 2025).
- Nitsche, M.A.; Bikson, M. Extending the parameter range for tDCS: Safety and tolerability of 4 mA stimulation. Brain Stimul. 2017, 10, 541–542. [Google Scholar] [CrossRef]
- Brunoni, A.R.; Nitsche, M.A.; Bolognini, N.; Bikson, M.; Wagner, T.; Merabet, L.; Edwards, D.J.; Valero-Cabre, A.; Rotenberg, A.; Pascual-Leone, A.; et al. Clinical research with transcranial direct current stimulation (tDCS): Challenges and future directions. Brain Stimul. 2012, 5, 175–195. [Google Scholar] [CrossRef]
- Lefaucheur, J.P.; Wendling, F. Mechanisms of action of tDCS: A brief and practical overview. Neurophysiol. Clin. 2019, 49, 269–275. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Fricke, K.; Henschke, U.; Schlitterlau, A.; Liebetanz, D.; Lang, N.; Henning, S.; Tergau, F.; Paulus, W. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J. Physiol. 2003, 553, 293–301. [Google Scholar] [CrossRef]
- Kronberg, G.; Bridi, M.; Abel, T.; Bikson, M.; Parra, L.C. Direct current stimulation modulates LTP and LTD: Activity dependence and dendritic effects. Brain Stimul. 2017, 10, 51–58. [Google Scholar] [CrossRef]
- Palm, U.; Hasan, A.; Strube, W.; Padberg, F. tDCS for the treatment of depression: A comprehensive review. Eur. Arch. Psychiatry Clin. Neurosci. 2016, 266, 681–694. [Google Scholar] [CrossRef]
- Grimm, S.; Beck, J.; Schuepbach, D.; Hell, D.; Boesiger, P.; Bermpohl, F.; Niehaus, L.; Boeker, H.; Northoff, G. Imbalance between left and right dorsolateral prefrontal cortex in major depression is linked to negative emotional judgment: An fMRI study in severe major depressive disorder. Biol. Psychiatry 2008, 63, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Kustubayeva, A.; Kamzanova, A.; Kudaibergenova, S.; Pivkina, V.; Matthews, G. Major depression and brain asymmetry in a decision-making task with negative and positive feedback. Symmetry 2020, 12, 2118. [Google Scholar] [CrossRef]
- Kustubayeva, A.; Eliassen, J.; Matthews, G.; Nelson, E. FMRI study of implicit emotional face processing in patients with MDD with melancholic subtype. Front. Hum. Neurosci. 2023, 17, 1029789. [Google Scholar] [CrossRef] [PubMed]
- Boggio, P.S.; Bermpohl, F.; Vergara, A.O.; Muniz, A.L.; Nahas, F.H.; Leme, P.B.; Rigonatti, S.P.; Fregni, F. Go-no-go task performance improvement after anodal transcranial DC stimulation of the left dorsolateral prefrontal cortex in major depression. J. Affect. Disord. 2007, 101, 91–98. [Google Scholar] [CrossRef]
- Keeser, D.; Meindl, T.; Bor, J.; Palm, U.; Pogarell, O.; Mulert, C.; Brunelin, J.; Möller, H.-J.; Reiser, M.; Padberg, F. Prefrontal transcranial direct current stimulation changes connectivity of resting-state networks during fMRI. J. Neurosci. 2011, 31, 15284–15293. [Google Scholar] [CrossRef]
- Mayberg, H.S.; Lozano, A.M.; Voon, V.; McNeely, H.E.; Seminowicz, D.; Hamani, C.; Schwalb, J.M.; Kennedy, S.H. Deep brain stimulation for treatment-resistant depression. Neuron 2005, 45, 651–660. [Google Scholar] [CrossRef]
- Sudbrack-Oliveira, P.; Barbosa, M.Z.; Thome-Souza, S.; Razza, L.B.; Gallucci-Neto, J.; Valiengo, L.D.C.L.; Brunoni, A.R. Transcranial direct current stimulation (tDCS) in the management of epilepsy: A systematic review. Seizure-Eur. J. Epilep. 2021, 86, 85–95. [Google Scholar] [CrossRef]
- Abuhaiba, S.I.; Duarte, I.C.; Castelhano, J.; Dionísio, A.; Sales, F.; Edden, R.; Castelo-Branco, M. The impact of cathodal tDCS on the GABAergic system in the epileptogenic zone: A multimodal imaging study. Front. Neurol. 2022, 13, 935029. [Google Scholar] [CrossRef]
- Klomjai, W.; Siripornpanich, V.; Aneksan, B.; Vimolratana, O.; Permpoonputtana, K.; Tretriluxana, J.; Thichanpiang, P. Effects of cathodal transcranial direct current stimulation on inhibitory and attention control in children and adolescents with attention-deficit hyperactivity disorder: A pilot randomized sham-controlled crossover study. J. Psychiatr. Res. 2022, 150, 130–141. [Google Scholar] [CrossRef]
- Duan, Z.; Zhang, C. Transcranial direct current stimulation for Parkinson’s disease: Systematic review and meta-analysis of motor and cognitive effects. NPJ Park. Dis. 2024, 10, 214. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, Y.; Liu, J.; Li, L.; Wang, Y.; Wu, X.; Bai, Y.; Li, J.; Zhang, Q.; Hui, Y. Transcranial direct current stimulation of the prefrontal cortex improves depression-like behaviors in rats with Parkinson’s disease. Brain Res. 2024, 1822, 148649. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Dawidziuk, A.; Darzi, A.; Singh, H.; Leff, D.R. Systematic review of combined functional near-infrared spectroscopy and transcranial direct-current stimulation studies. Neurophotonics 2020, 7, 020901. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, Y.; Song, P.; Yu, H.; Du, J.; Zhang, Y.; Sun, C. Varied response of EEG rhythm to different tDCS protocols and lesion hemispheres in stroke subjects with upper limb dysfunction. Neural. Plast. 2022, 2022, 7790730. [Google Scholar] [CrossRef]
- Sauvaget, A.; Tostivint, A.; Etcheverrigaray, F.; Pichot, A.; Dert, C.; Schirr-Bonnais, S.; Clouet, J.; Sellal, O.; Mauduit, N.; Leux, C.; et al. Hospital production cost of transcranial direct current stimulation (tDCS) in the treatment of depression. Neurophysiol. Clin. 2019, 49, 11–18. [Google Scholar] [CrossRef]
- Datta, A.; Bansal, V.; Diaz, J.; Patel, J.; Reato, D.; Bikson, M. Gyri-precise head model of transcranial direct current stimulation: Improved spatial focality using a ring electrode versus conventional rectangular pad. Brain Stimul. 2009, 2, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Caulfield, K.A.; George, M.S. Optimized APPS-tDCS electrode position, size, and distance doubles the on-target stimulation magnitude in 3000 electric field models. Sci. Rep. 2022, 12, 20116. [Google Scholar] [CrossRef]
- Lloyd, J.; Biloshytska, M.; Andreou, A.P.; Lambru, G. Noninvasive neuromodulation in headache: An update. Neurol. India 2021, 69 (Suppl. S1), S183–S193. [Google Scholar] [CrossRef]
- Lipton, R.B.; Dodick, D.W.; Silberstein, S.D.; Saper, J.R.; Aurora, S.K.; Pearlman, S.H.; Fischell, R.E.; Ruppel, P.L.; Goadsby, P.J. Single-pulse transcranial magnetic stimulation for acute treatment of migraine with aura: A randomised, double-blind, parallel-group, sham-controlled trial. Lancet Neurol. 2010, 9, 373–380. [Google Scholar] [CrossRef]
- Lloyd, J.O.; Hill, B.; Murphy, M.; Al-Kaisy, A.; Andreou, A.P.; Lambru, G. Single-Pulse Transcranial Magnetic Stimulation for the preventive treatment of difficult-to-treat migraine: A 12-month prospective analysis. Headache 2022, 23, 63. [Google Scholar] [CrossRef] [PubMed]
- Andreou, A.P.; Holland, P.R.; Akerman, S.; Summ, O.; Fredrick, J.; Goadsby, P.J. Transcranial magnetic stimulation and potential cortical and trigeminothalamic mechanisms in migraine. Brain 2016, 139, 2002–2014. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, J.O.; Chisholm, K.I.; Oehle, B.; Jones, M.G.; Okine, B.N.; Al-Kaisy, A.; Lambru, G.; McMahpn, S.B.; Andreou, A.P. Cortical mechanisms of single-pulse transcranial magnetic stimulation in migraine. Neurotherapeutics 2020, 17, 1973–1987. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, J.P. Transcranial magnetic stimulation. Handb. Clin. Neurol. 2019, 160, 559–580. [Google Scholar]
- Baeken, C.; Brem, A.K.; Arns, M.; Brunoni, A.R.; Filipcic, I.; Ganho-Ávila, A.; Langguth, B.; Padberg, F.; Poulet, E.; Rachid, F.; et al. Repetitive transcranial magnetic stimulation treatment for depressive disorders: Current knowledge and future directions. Curr. Opin. Psychiatry 2019, 32, 409–415. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, W.; Yang, S.; Dai, P.; Shen, L.; Wang, C. Repetitive transcranial magnetic stimulation for hallucination in schizophrenia spectrum disorders: A meta-analysis. Neural. Regen. Res. 2013, 8, 2666–2676. [Google Scholar]
- Mantovani, A.; Lisanby, S.H.; Pieraccini, F.; Ulivelli, M.; Castrogiovanni, P.; Rossi, S. Repetitive Transcranial Magnetic Stimulation (rTMS) in the treatment of panic disorder (PD) with comorbid major depression. J. Affect. Disord. 2007, 102, 277–280. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, C.; Wu, B.; Wang, Y.; Li, Q.; Wei, Y.; Yang, D.; Mu, J.; Zhu, D.; Zou, D.; et al. Left versus right repetitive transcranial magnetic stimulation in treating major depression: A meta-analysis of randomised controlled trials. Psychiatry Res. 2013, 210, 1260–1264. [Google Scholar] [CrossRef]
- Liu, C.; Li, L.; Li, B.; Liu, Z.; Xing, W.; Zhu, K.; Jin, W.; Lin, S.; Tan, W.; Ren, L.; et al. Efficacy and safety of theta burst versus repetitive transcranial magnetic stimulation for the treatment of depression: A meta-analysis of randomized controlled trials. Neuromodulation 2024, 27, 701–710. [Google Scholar] [CrossRef]
- Zrenner, B.; Gordon, P.C.; Belardinelli, P.; McDermott, E.J.; Soekadar, S.R.; Fallgatter, A.J.; Zrenner, C.; Ziemann, U.; Müller-Dahlhaus, F. Alpha-Synchronized Stimulation of the Left Dorsolateral Prefrontal Cortex in Depression Using Real-Time EEG-Triggered TMS. Biol. Psychiatry 2019, 85, S41. [Google Scholar] [CrossRef]
- Curtin, A.; Tong, S.; Sun, J.; Wang, J.; Onaral, B.; Ayaz, H. A systematic review of integrated functional near-infrared spectroscopy (fNIRS) and transcranial magnetic stimulation (TMS) studies. Front. Neurosci. 2019, 13, 84. [Google Scholar] [CrossRef]
- Plewnia, C.; Brendel, B.; Schwippel, T.; Nieratschker, V.; Ethofer, T.; Kammer, T.; Padberg, F.; Martus, P.; Fallgatter, A.J. Treatment of major depressive disorder with bilateral theta burst stimulation: Study protocol for a randomized, double-blind, placebo-controlled multicenter trial (TBS-D). Eur. Arch. Psychiatry Clin. Neurosci. 2021, 271, 1231–1243. [Google Scholar] [CrossRef]
- Goodman, M.S.; Vila-Rodriguez, F.; Barwick, M.; Burke, M.J.; Downar, J.; Hunter, J.; Kaster, T.S.; Knyahnytska, Y.; Kurdyak, P.; Maunder, R.; et al. A randomized sham-controlled trial of high-dosage accelerated intermittent theta burst rTMS in major depression: Study protocol. BMC Psychiatry 2024, 24, 28. [Google Scholar] [CrossRef]
- Simpson, K.N.; Welch, M.J.; Kozel, F.A.; Demitrack, M.A.; Nahas, Z. Cost-effectiveness of transcranial magnetic stimulation in the treatment of major depression: A health economics analysis. Adv. Ther. 2009, 26, 346–368. [Google Scholar] [CrossRef] [PubMed]
- Schulze, L.; Feffer, K.; Lozano, C.; Giacobbe, P.; Daskalakis, Z.J.; Blumberger, D.M.; Downar, J. Number of pulses or number of sessions? An open-label study of trajectories of improvement for once-vs. twice-daily dorsomedial prefrontal rTMS in major depression. Brain Stimul. 2018, 11, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Weigand, A.; Horn, A.; Caballero, R.; Cooke, D.; Stern, A.P.; Taylor, S.F.; Press, D.; Pascual-Leone, A.; Fox, M.D. Prospective validation that subgenual connectivity predicts antidepressant efficacy of transcranial magnetic stimulation sites. Biol. Psychiatry 2018, 84, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Park, T.Y.; Lee, W.; Kim, H. A review of functional neuromodulation in humans using low-intensity transcranial focused ultrasound. Biomed. Eng. Lett. 2024, 14, 407–438. [Google Scholar] [CrossRef]
- Keihani, A.; Sanguineti, C.; Chaichian, O.; Huston, C.A.; Moore, C.; Cheng, C.; Janssen, S.A.; Donati, F.L.; Mayeli, A.; Moussawi, K.; et al. Transcranial Focused Ultrasound Neuromodulation in Psychiatry: Main Characteristics, Current Evidence, and Future Directions. Brain Sci. 2024, 14, 1095. [Google Scholar] [CrossRef]
- Kubanek, J. Neuromodulation with transcranial focused ultrasound. Neurosurg. Focus 2018, 44, E14. [Google Scholar] [CrossRef]
- Zhang, T.; Pan, N.; Wang, Y.; Liu, C.; Hu, S. Transcranial focused ultrasound neuromodulation: A review of the excitatory and inhibitory effects on brain activity in human and animals. Front. Hum. Neurosci. 2021, 15, 749162. [Google Scholar] [CrossRef]
- Liu, X.; Naomi, S.S.M.; Sharon, W.L.; Russell, E.J. The applications of focused ultrasound (FUS) in Alzheimer’s disease treatment: A systematic review on both animal and human studies. Aging Dis. 2021, 12, 1977. [Google Scholar] [CrossRef] [PubMed]
- Rezai, A.R.; D’Haese, P.F.; Finomore, V.; Carpenter, J.; Ranjan, M.; Wilhelmsen, K.; Mehta, R.I.; Wang, P.; Najib, U.; Teixeira, C.V.L.; et al. Ultrasound blood–brain barrier opening and aducanumab in Alzheimer’s disease. N. Eng. J. Med. 2024, 390, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Padliya, T. Breaking Barriers with Sound: Focused Ultrasound in the Brain. IEEE Pulse 2025, 16, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.Y.; Lin, Y.S.; Kang, K.H.; Chao, T.K. Reversible blood–brain barrier disruption by repeated transcranial focused ultrasound allows enhanced extravasation. J. Control. Release 2011, 150, 111–116. [Google Scholar] [CrossRef]
- Dasgupta, A.; Liu, M.; Ojha, T.; Storm, G.; Kiessling, F.; Lammers, T. Ultrasound-mediated drug delivery to the brain: Principles, progress and prospects. Drug Discov. Today Technol. 2016, 20, 41–48. [Google Scholar] [CrossRef]
- Pan, Y.; Yoon, S.; Sun, J.; Huang, Z.; Lee, C.; Allen, M.; Wu, Y.; Chang, Y.; Sadelain, M.; Shung, K.K.; et al. Mechanogenetics for the remote and noninvasive control of cancer immunotherapy. Proc. Natl. Acad. Sci. USA 2018, 115, 992–997. [Google Scholar] [CrossRef]
- Shen, X.; Song, Z.; Xu, E.; Zhou, J.; Yan, F. Sensitization of nerve cells to ultrasound stimulation through Piezo1-targeted microbubbles. Ultrason. Sonochem. 2021, 73, 105494. [Google Scholar] [CrossRef]
- Xu, L.; Gong, Y.; Chien, C.Y.; Leuthardt, E.; Chen, H. Transcranial focused ultrasound-induced blood‒brain barrier opening in mice without shaving hairs. Sci. Rep. 2023, 13, 13500. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, L.; Liu, S.; Zhou, X.; Yin, T.; Liu, Z.; Yang, Z. Transcranial magneto-acoustic stimulation improves neuroplasticity in hippocampus of Parkinson’s disease model mice. Neurotherapeutics 2019, 16, 1210–1224. [Google Scholar] [CrossRef]
- Han, T.; Xu, Z.; Liu, C.; Li, S.; Song, P.; Huang, Q.; Zhou, Q.; Lin, Y.; Wang, Y. Simultaneously applying cathodal tDCS with low frequency rTMS at the motor cortex boosts inhibitory aftereffects. J. Neurosci. Methods 2019, 324, 108308. [Google Scholar] [CrossRef]
- Neuralink. PRIME Study Progress Update—Second Participant. Available online: https://neuralink.com/blog/prime-study-progress-update-second-participant/ (accessed on 15 June 2025).
- Han, J.J. Synchron Receives FDA Approval to Begin Early Feasibility Study of Their Endovascular, Brain-Computer Interface Device. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/aor.14049?casa_token=4LFQsYx0ZAUAAAAA%3Am0sM2YdirQjEzHzCU34UUtnFCiH5AADu_Kn6ulPiRtOihPLW70Q16mgourRiG2WHBbD16AbAkKfoU8I (accessed on 19 June 2025).
- Rotenberg, A.; Gunning, M.; Nadler, R.M.; Kiss, Z.H.T.; Illes, J. A liability framework for high-risk neural devices. Science 2025, 388, 1136–1138. [Google Scholar] [CrossRef]
- Keifer, O.; Kambara, K.; Lau, A.; Makinson, S.; Bertrand, D. Chemogenetics a robust approach to pharmacology and gene therapy. Biochem. Pharmacol. 2020, 175, 113889. [Google Scholar] [CrossRef]
- Yuan, P.; Grutzendler, J. Attenuation of β-amyloid deposition and neurotoxicity by chemogenetic modulation of neural activity. J. Neurosci. 2016, 36, 632–641. [Google Scholar] [CrossRef]
- Song, J.; Patel, R.V.; Sharif, M.; Ashokan, A.; Michaelides, M. Chemogenetics as a neuromodulatory approach to treating neuropsychiatric diseases and disorders. Mol. Ther. 2022, 30, 990–1005. [Google Scholar] [CrossRef]
- Nagai, Y.; Miyakawa, N.; Takuwa, H.; Hori, Y.; Oyama, K.; Ji, B.; Takahashi, M.; Huang, X.P.; Slocum, S.T.; DiBerto, J.F.; et al. Deschloroclozapine, a potent and selective chemogenetic actuator enables rapid neuronal and behavioral modulations in mice and monkeys. Nat. Neurosci. 2020, 23, 1157–1167. [Google Scholar] [CrossRef] [PubMed]
- Raper, J.; Galvan, A. Applications of chemogenetics in non-human primates. Curr. Opin. Pharmacol. 2022, 64, 102204. [Google Scholar] [CrossRef] [PubMed]
- Metto, A.C.; Telgkamp, P.; McLane-Svoboda, A.K.; Gilad, A.A.; Pelled, G. Closed-loop neurostimulation via expression of magnetogenetics-sensitive protein in inhibitory neurons leads to reduction of seizure activity in a rat model of epilepsy. Brain Res. 2023, 1820, 148591. [Google Scholar] [CrossRef]
- Zhi, W.; Li, Y.; Wang, L.; Hu, X. Advancing Neuroscience and Therapy: Insights into Genetic and Non-Genetic Neuromodulation Approaches. Cells 2025, 14, 122. [Google Scholar] [CrossRef]
- Kolesov, D.V.; Sokolinskaya, E.L.; Lukyanov, K.A.; Bogdanov, A.M. Molecular tools for targeted control of nerve cell electrical activity. Part I. Acta Nat. 2021, 13, 17–32. [Google Scholar] [CrossRef]
- Burguière, E.; Monteiro, P.; Feng, G.; Graybiel, A.M. Optogenetic stimulation of lateral orbitofronto-striatal pathway suppresses compulsive behaviors. Science 2013, 340, 1243–1246. [Google Scholar] [CrossRef]
- Jiang, L.U.; Li, W.; Mamtilahun, M.; Song, Y.; Ma, Y.; Qu, M.; Lu, Y.; He, X.; Zheng, J.; Fu, Z.; et al. Optogenetic inhibition of striatal GABAergic neuronal activity improves outcomes after ischemic brain injury. Stroke 2017, 48, 3375–3383. [Google Scholar] [CrossRef]
- Yoon, H.H.; Park, J.H.; Kim, Y.H.; Min, J.; Hwang, E.; Lee, C.J.; Suh, J.K.; Hwang, O.; Jeon, S.R. Optogenetic inactivation of the subthalamic nucleus improves forelimb akinesia in a rat model of Parkinson disease. Neurosurgery 2014, 74, 533–541. [Google Scholar] [CrossRef]
- Arslan, I.; Akgul, H.; Kara, M. Saporin, a polynucleotide–adenosine nucleosidase, may be an efficacious therapeutic agent for SARS-CoV-2 infection. SLAS Discov. 2021, 26, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Van Ness, B.G.; Howard, J.B.; Bodley, J.W. ADP-ribosylation of elongation factor 2 by diphtheria toxin. Isolation and properties of the novel ribosyl-amino acid and its hydrolysis products. J. Biol. Chem. 1980, 255, 10717–10720. [Google Scholar] [CrossRef] [PubMed]
- Wiley, R.G.; Oeltmann, T.N.; Lappi, D.A. Immunolesioning: Selective destruction of neurons using immunotoxin to rat NGF receptor. Brain Res. 1991, 562, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Schliebs, R.; Roβner, S.; Bigl, V. Immunolesion by 192IgG-saporin of rat basal forebrain cholinergic system: A useful tool to produce cortical cholinergic dysfunction. Prog. Brain Res. 1996, 109, 253–264. [Google Scholar]
- Leanza, G.; Muir, J.; Nilsson, O.G.; Wiley, R.G.; Dunnett, S.B.; Björklund, A. Selective immunolesioning of the basal forebrain cholinergic system disrupts short-term memory in rats. Eur. J. Neurosci. 1996, 8, 1535–1544. [Google Scholar] [CrossRef]
- Wiley, R.G.; Harrison, M.B.; Levey, A.I.; Lappi, D.A. Destruction of midbrain dopaminergic neurons by using immunotoxin to dopamine transporter. Cell Mol. Neurobiol. 2003, 23, 839–850. [Google Scholar] [CrossRef]
- Landrigan, J.; Dwyer, Z.; Beauchamp, S.; Rodriguez, R.; Fortin, T.; Hayley, S. Quantum dot conjugated saporin activates microglia and induces selective substantia nigra degeneration. Neurotoxicology 2020, 76, 153–161. [Google Scholar] [CrossRef]
- Gerashchenko, D.; Kohls, M.D.; Greco, M.; Waleh, N.S.; Salin-Pascual, R.; Kilduff, T.S.; Lappi, D.A.; Shiromani, P.J. Hypocretin-2-saporin lesions of the lateral hypothalamus produce narcoleptic-like sleep behavior in the rat. J. Neurosci. 2001, 21, 7273–7283. [Google Scholar] [CrossRef]
- Gerashchenko, D.; Blanco-Centurion, C.A.; Miller, J.D.; Shiromani, P.J. Insomnia following hypocretin2-saporin lesions of the substantia nigra. Neuroscience 2006, 137, 29–36. [Google Scholar] [CrossRef]
- Eikermann, M.; Vetrivelan, R.; Grosse-Sundrup, M.; Henry, M.E.; Hoffmann, U.; Yokota, S.; Saper, C.B.; Chamberlin, N.L. The ventrolateral preoptic nucleus is not required for isoflurane general anesthesia. Brain Res. 2011, 1426, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.J.; Yang, W.J.; Guo, C.N.; Qiu, M.H.; Kroeger, D.; Niu, J.G.; Zhan, S.Q.; Yang, X.F.; Gisabella, B.; Vetrivelan, R.; et al. Pontine control of rapid eye movement sleep and fear memory. CNS Neurosci. Ther. 2023, 29, 1602–1614. [Google Scholar] [CrossRef] [PubMed]
- Kudo, Y.; Shiosaka, S.; Matsuda, M.; Tohyama, M. An attempt to cause the selective loss of the cholinergic neurons in the basal forebrain of the rat: A new animal model of Alzheimer’s disease. Neurosci. Lett. 1989, 102, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.D.; Alderson, H.L.; Winn, P.; Latimer, M.P.; Nothacker, H.P.; Civelli, O. Fusion of diphtheria toxin and urotensin II produces a neurotoxin selective for cholinergic neurons in the rat mesopontine tegmentum. J. Neurochem. 2007, 102, 112–120. [Google Scholar] [CrossRef]
- MacLaren, D.A.; Santini, J.A.; Russell, A.L.; Markovic, T.; Clark, S.D. Deficits in motor performance after pedunculopontine lesions in rats–impairment depends on demands of task. Eur. J. Neurosci. 2014, 40, 3224–3236. [Google Scholar] [CrossRef]
- Steidl, S.; Wang, H.; Wise, R.A. Lesions of cholinergic pedunculopontine tegmental nucleus neurons fail to affect cocaine or heroin self-administration or conditioned place preference in rats. PLoS ONE 2014, 9, e84412. [Google Scholar] [CrossRef]
- MacLaren, D.A.; Wilson, D.I.; Winn, P. Selective lesions of the cholinergic neurons within the posterior pedunculopontine do not alter operant learning or nicotine sensitization. Brain Struct. Funct. 2016, 221, 1481–1497. [Google Scholar] [CrossRef]
- Hagihara, N.; Walbridge, S.; Olson, A.W.; Oldfield, E.H.; Youle, R.J. Vascular protection by chloroquine during brain tumor therapy with Tf-CRM107. Cancer Res. 2000, 60, 230–234. [Google Scholar]
- Oh, S.; Ohlfest, J.R.; Todhunter, D.A.; Vallera, V.D.; Hall, W.A.; Chen, H.; Vallera, D.A. Intracranial elimination of human glioblastoma brain tumors in nude rats using the bispecific ligand-directed toxin, DTEGF13 and convection enhanced delivery. Neuro-Oncol. 2009, 95, 331–342. [Google Scholar] [CrossRef]
- Yoon, D.J.; Kwan, B.H.; Chao, F.C.; Nicolaides, T.P.; Phillips, J.J.; Lam, G.Y.; Mason, A.B.; Weiss, W.A.; Kamei, D.T. Intratumoral therapy of glioblastoma multiforme using genetically engineered transferrin for drug delivery. Cancer Res. 2010, 70, 4520–4527. [Google Scholar] [CrossRef]
- Monheit, G.D.; Pickett, A. AbobotulinumtoxinA: A 25-year history. Aesthetic Surg. J. 2017, 37 (Suppl. S1), S4–S11. [Google Scholar] [CrossRef]
- Pantano, S.; Montecucco, C. The blockade of the neurotransmitter release apparatus by botulinum neurotoxins. Cell Mol. Life Sci. 2014, 71, 793–811. [Google Scholar] [CrossRef] [PubMed]
- Ando, S.; Kobayashi, S.; Waki, H.; Kon, K.; Fukui, F.; Tadenuma, T.; Iwamoto, M.; Takeda, Y.; Izumiyama, N.; Watanabe, K.; et al. Animal model of dementia induced by entorhinal synaptic damage and partial restoration of cognitive deficits by BDNF and carnitine. J. Neurosci. Res. 2002, 70, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Lacković, Z.; Rebić, V.; Riederer, P.F. Single intracerebroventricular injection of botulinum toxin type A produces slow onset and long-term memory impairment in rats. J. Neural. Transm. 2009, 116, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Holzmann, C.; Dräger, D.; Mix, E.; Hawlitschka, A.; Antipova, V.; Benecke, R.; Wree, A. Effects of intrastriatal botulinum neurotoxin A on the behavior of Wistar rats. Behav. Brain Res. 2012, 234, 107–116. [Google Scholar] [CrossRef]
- Bröer, S.; Zolkowska, D.; Gernert, M.; Rogawski, M.A. Proconvulsant actions of intrahippocampal botulinum neurotoxin B in the rat. Neuroscience 2013, 252, 253–261. [Google Scholar] [CrossRef]
- Costantin, L.; Bozzi, Y.; Richichi, C.; Viegi, A.; Antonucci, F.; Funicello, M.; Gobbi, M.; Mennini, T.; Rossetto, O.; Montecucco, C.; et al. Antiepileptic effects of botulinum neurotoxin E. J. Neurosci. 2005, 25, 1943–1951. [Google Scholar] [CrossRef]
- Manno, I.; Antonucci, F.; Caleo, M.; Bozzi, Y. BoNT/E prevents seizure-induced activation of caspase 3 in the rat hippocampus. Neuroreport 2007, 18, 373–376. [Google Scholar] [CrossRef]
- Gasior, M.; Tang, R.; Rogawski, M.A. Long-lasting attenuation of amygdala-kindled seizures after convection-enhanced delivery of botulinum neurotoxins A and B into the amygdala in rats. J. Pharmacol. Exp. Ther. 2013, 346, 528–534. [Google Scholar] [CrossRef]
- Ferrari, E.; Maywood, E.S.; Restani, L.; Caleo, M.; Pirazzini, M.; Rossetto, O.; Hastings, M.H.; Niranjan, D.; Schiavo, G.; Davletov, B. Re-assembled botulinum neurotoxin inhibits CNS functions without systemic toxicity. Toxins 2011, 3, 345–355. [Google Scholar] [CrossRef]
- Wree, A.; Mix, E.; Hawlitschka, A.; Antipova, V.; Witt, M.; Schmitt, O.; Benecke, R. Intrastriatal botulinum toxin abolishes pathologic rotational behaviour and induces axonal varicosities in the 6-OHDA rat model of Parkinson’s disease. Neurobiol. Dis. 2011, 41, 291–298. [Google Scholar] [CrossRef]
- Antipova, V.; Hawlitschka, A.; Mix, E.; Schmitt, O.; Dräger, D.; Benecke, R.; Wree, A. Behavioral and structural effects of unilateral intrastriatal injections of botulinum neurotoxin a in the rat model of Parkinson’s disease. J. Neurosci. Res. 2013, 91, 838–847. [Google Scholar] [CrossRef]
- Itakura, M.; Kohda, T.; Kubo, T.; Semi, Y.; Azuma, Y.T.; Nakajima, H.; Kozaki, S.; Takeuchi, T. Botulinum neurotoxin A subtype 2 reduces pathological behaviors more effectively than subtype 1 in a rat Parkinson’s disease model. Biochem. Biophys. Res. Commun. 2014, 447, 311–314. [Google Scholar] [CrossRef]
- Itakura, M.; Kohda, T.; Kubo, T.; Semi, Y.; Nishiyama, K.; Azuma, Y.T.; Nakajima, H.; Kozaki, S.; Takeuchi, T. Botulinum neurotoxin type A subtype 2 confers greater safety than subtype 1 in a rat Parkinson’s disease model. J. Vet. Med. Sci. 2014, 76, 1189–1193. [Google Scholar] [CrossRef] [PubMed]
- Hawlitschka, A.; Holzmann, C.; Wree, A.; Antipova, V. Repeated intrastriatal botulinum neurotoxin-A injection in hemiparkinsonian rats increased the beneficial effect on rotational behavior. Toxins 2018, 10, 368. [Google Scholar] [CrossRef] [PubMed]
- Tsang, A.R.; Rajakumar, N.; Jog, M.S. Intrapallidal injection of botulinum toxin A recovers gait deficits in a parkinsonian rodent model. Acta Physiol. 2019, 226, e13230. [Google Scholar] [CrossRef]
- Tsang, A.R.; Rajakumar, N.; Jog, M.S. Botulinum toxin A injection into the entopeduncular nucleus improves dynamic locomotory parameters in hemiparkinsonian rats. PLoS ONE 2019, 14, e0223450. [Google Scholar] [CrossRef]
- Antipova, V.; Holzmann, C.; Hawlitschka, A.; Wree, A. Botulinum neurotoxin-a injected intrastriatally into hemiparkinsonian rats improves the initiation time for left and right forelimbs in both forehand and backhand directions. Int. J. Mol. Sci. 2019, 20, 992. [Google Scholar] [CrossRef]
- Antipova, V.; Holzmann, C.; Hawlitschka, A.; Witt, M.; Wree, A. Antidepressant-like properties of intrastriatal botulinum neurotoxin-a injection in a unilateral 6-ohda rat model of parkinson’s disease. Toxins 2021, 13, 505. [Google Scholar] [CrossRef]
- Alberts, T.; Antipova, V.; Holzmann, C.; Hawlitschka, A.; Schmitt, O.; Kurth, J.; Stenzel, J.; Lindner, T.; Krause, B.J.; Wree, A.; et al. Olfactory bulb D2/D3 receptor availability after intrastriatal botulinum neurotoxin-a injection in a unilateral 6-OHDA rat model of Parkinson’s disease. Toxins 2022, 14, 94. [Google Scholar] [CrossRef]
- Wedekind, F.; Oskamp, A.; Lang, M.; Hawlitschka, A.; Zilles, K.; Wree, A.; Bauer, A. Intrastriatal administration of botulinum neurotoxin A normalizes striatal D2R binding and reduces striatal D1R binding in male hemiparkinsonian rats. J. Neurosci. Res. 2018, 96, 75–86. [Google Scholar] [CrossRef]
- Mann, T.; Zilles, K.; Dikow, H.; Hellfritsch, A.; Cremer, M.; Piel, M.; Rösch, F.; Hawlitschka, A.; Schmitt, O.; Wree, A. Dopamine, noradrenaline and serotonin receptor densities in the striatum of hemiparkinsonian rats following botulinum neurotoxin-A injection. Neuroscience 2018, 374, 187–204. [Google Scholar] [CrossRef] [PubMed]
- Mann, T.; Kurth, J.; Hawlitschka, A.; Stenzel, J.; Lindner, T.; Polei, S.; Hohn, A.; Krause, B.J.; Wree, A. [18F] fallypride-PET/CT analysis of the dopamine D2/D3 receptor in the hemiparkinsonian rat brain following intrastriatal botulinum neurotoxin A injection. Molecules 2018, 23, 587. [Google Scholar] [CrossRef] [PubMed]
- Mann, T.; Zilles, K.; Klawitter, F.; Cremer, M.; Hawlitschka, A.; Palomero-Gallagher, N.; Schmitt, O.; Wree, A. Acetylcholine neurotransmitter receptor densities in the striatum of hemiparkinsonian rats following Botulinum neurotoxin-A injection. Front. Neuroanat. 2018, 12, 65. [Google Scholar] [CrossRef] [PubMed]
- Thepsoparn, M.; Anukoolwittaya, P.; Toeypromthong, P.; Thanaboriboon, C. Efficacy and safety profile of Onabotulinum toxin-A injection at sphenopalatine ganglion in trigeminal neuralgia: A prospective observational study. J. Headache Pain 2024, 25, 1–7. [Google Scholar] [CrossRef]
- Simmonds, L.; Jamtøy, K.A.; Aschehoug, I.; Hara, S.; Meisingset, T.W.; Matharu, M.S.; Tronvik, E.; Bratbak, D.F. Open label experience of repeated OnabotulinumtoxinA injections towards the sphenopalatine ganglion in patients with chronic cluster headache and chronic migraine. Cephalalgia 2024, 44, 03331024241273967. [Google Scholar] [CrossRef]
- Antonucci, F.; Cerri, C.; Vetencourt, J.M.; Caleo, M. Acute neuroprotection by the synaptic blocker botulinum neurotoxin E in a rat model of focal cerebral ischaemia. Neuroscience 2010, 169, 395–401. [Google Scholar] [CrossRef]
- Caleo, M.; Restani, L.; Gianfranceschi, L.; Costantin, L.; Rossi, C.; Rossetto, O.; Montecucco, C.; Maffei, L. Transient synaptic silencing of developing striate cortex has persistent effects on visual function and plasticity. J. Neurosci. 2007, 27, 4530–4540. [Google Scholar] [CrossRef]
- Ferrari, E.; Gu, C.; Niranjan, D.; Restani, L.; Rasetti-Escargueil, C.; Obara, I.; Geranton, S.M.; Arsenault, J.; Goetze, T.A.; Harpe, C.B.; et al. Synthetic self-assembling clostridial chimera for modulation of sensory functions. Bioconjug. Chem. 2013, 24, 1750–1759. [Google Scholar] [CrossRef]
- Mehlan, J.; Brosig, H.; Schmitt, O.; Mix, E.; Wree, A.; Hawlitschka, A. Intrastriatal injection of botulinum neurotoxin-A is not cytotoxic in rat brain–a histological and stereological analysis. Brain Res. 2016, 1630, 18–24. [Google Scholar] [CrossRef]
- Schümann, F.; Schmitt, O.; Wree, A.; Hawlitschka, A. Distribution of Cleaved SNAP-25 in the Rat Brain, following Unilateral Injection of Botulinum Neurotoxin-A into the Striatum. Int. J. Mol. Sci. 2023, 24, 1685. [Google Scholar] [CrossRef]
- Maiarù, M.; Leese, C.; Certo, M.; Echeverria-Altuna, I.; Mangione, A.S.; Arsenault, J.; Davletov, B.; Hunt, S.P. Selective neuronal silencing using synthetic botulinum molecules alleviates chronic pain in mice. Sci. Transl. Med. 2018, 10, eaar7384. [Google Scholar] [CrossRef]
- Maiarù, M.; Leese, C.; Silva-Hucha, S.; Fontana-Giusti, S.; Tait, L.; Tamagnini, F.; Davletov, B.; Hunt, S.P. Substance P-botulinum mediates long-term silencing of pain pathways that can be re-instated with a second injection of the construct in mice. J. Pain 2024, 25, 104466. [Google Scholar] [CrossRef]

| Method | Advantage | Disadvantage | |
|---|---|---|---|
| Biophysical | Deep brain stimulation | Adjustable and reversible effects | Invasive procedure with surgical risks; high financial cost and maintenance burden; potential side effects |
| Transcranial direct current stimulation | Non-invasive; portable; safe | Low spatial resolution; shallow penetration; variable efficacy | |
| Transcranial magnetic stimulation | Non-invasive; protocol flexibility | Limited depth and spatial precision; high inter-individual variability; potential side effects | |
| Transcranial focused ultrasound stimulation | Non-invasive; high spatial precision; capable of deep brain targeting | Low temporal accuracy; early clinical stage | |
| Genetic | Chemogenetics | High cell-type specificity; no need for external devices | Requires viral vector delivery; high ligand doses may cause off-target effects; low time precision |
| Magnetogenetics | Good targeting precision | Unclear molecular mechanisms; technical complexity; requires genetic modification via viral vectors | |
| Optogenetics | Extremely high temporal and spatial precision | Requires genetic modification; light delivery via implanted optical fibers; potential phototoxicity; heterogeneous opsin expression | |
| Biological | Protein synthesis-inhibiting toxins | Flexible targeting via conjugation with ligands or antibodies | Requires stereotaxic injection; irreversible neuronal loss |
| Neurotransmission-inhibiting toxins | Reversible inhibition; modifiable duration | Requires stereotaxic injection; potential off-target transport | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhantleuova, A.; Karimova, A.; Andreou, A.P.; Kustubayeva, A.M.; Giniatullin, R.; Davletov, B. Focused Modulation of Brain Activity: A Narrative Review. Biomedicines 2025, 13, 1889. https://doi.org/10.3390/biomedicines13081889
Zhantleuova A, Karimova A, Andreou AP, Kustubayeva AM, Giniatullin R, Davletov B. Focused Modulation of Brain Activity: A Narrative Review. Biomedicines. 2025; 13(8):1889. https://doi.org/10.3390/biomedicines13081889
Chicago/Turabian StyleZhantleuova, Aisha, Altynay Karimova, Anna P. Andreou, Almira M. Kustubayeva, Rashid Giniatullin, and Bazbek Davletov. 2025. "Focused Modulation of Brain Activity: A Narrative Review" Biomedicines 13, no. 8: 1889. https://doi.org/10.3390/biomedicines13081889
APA StyleZhantleuova, A., Karimova, A., Andreou, A. P., Kustubayeva, A. M., Giniatullin, R., & Davletov, B. (2025). Focused Modulation of Brain Activity: A Narrative Review. Biomedicines, 13(8), 1889. https://doi.org/10.3390/biomedicines13081889






