Clinical Evaluation of COVID-19 Survivors at a Public Multidisciplinary Health Clinic
Abstract
1. Introduction
2. Materials and Methods
2.1. Population and Study Design
2.2. Variables
2.2.1. Baseline Variables
2.2.2. Follow-Up Variables
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Acute COVID-19 Characteristics
3.3. Post-COVID-19 Characteristics
3.4. Factors Associated with Post-Acute COVID-19 Syndrome
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Honigsbaum, M.; Krishnan, L. Taking pandemic sequelae seriously: From the Russian influenza to COVID-19 long-haulers. Lancet 2020, 396, 1389–1391. [Google Scholar] [CrossRef]
- Covid, W. Dashboard; World Health Organization: Geneva, Switzerland, 2020; Available online: https://covid19.who.int/ (accessed on 4 June 2025).
- Greenhalgh, T.; Sivan, M.; Perlowski, A.; Nikolich, J. Long COVID: A clinical update. Lancet 2024, 404, 10453. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. A Clinical Case Definition of Post COVID-19 Condition by a Delphi Consensus. 2021. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1 (accessed on 2 May 2025).
- Mahase, E. Long covid could be four different syndromes, review suggests. BMJ 2020, 371, 3981. [Google Scholar] [CrossRef]
- Akbarialiabad, H.; Taghrir, M.H.; Abdollahi, A.; Ghahramani, N.; Kumar, M.; Paydar, S.; Razani, B.; Mwangi, J.; Asadi-Pooya, A.A.; Malekmakan, L.; et al. Long COVID, a comprehensive systematic scoping review. Infection 2021, 49, 1163–1186. [Google Scholar] [CrossRef]
- Miller, B.; Sleat, D. The Hidden Pandemic: Long COVID. 2021, p. 3. Available online: https://institute.global/insights/public-services/hidden-pandemic-long-covid. (accessed on 2 May 2025).
- Huang, L.; Yao, Q.; Gu, X.; Wang, Q.; Ren, L.; Wang, Y.; Hu, P.; Guo, L.; Liu, M.; Xu, J.; et al. 1-year outcomes in hospital survivors with COVID-19: A longitudinal cohort study. Lancet 2021, 398, 747–758. [Google Scholar] [CrossRef]
- de Oliveira, J.F.; de Ávila, R.E.; de Oliveira, N.R.; Sampaio, N.d.C.S.; Botelho, M.; Gonçalves, F.A.; Neto, C.J.F.; Milagres, A.C.d.A.; Gomes, T.C.C.; Pereira, T.L.; et al. Persistent symptoms, quality of life, and risk factors in long COVID: A cross-sectional study of hospitalized patients in Brazil. Int. J. Infect. Dis. 2022, 122, 1044–1051. [Google Scholar] [CrossRef]
- Carfì, A.; Bernabei, R.; Landi, F. Persistent Symptoms in Patients After Acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Arnold, D.T.; Hamilton, F.W.; Milne, A.; Morley, A.J.; Viner, J.; Attwood, M.; Noel, A.; Gunning, S.; Hatrick, J.; Hamilton, S.; et al. Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: Results from a prospective UK cohort. Thorax 2020, 76, 399–401. [Google Scholar] [CrossRef] [PubMed]
- Carvalho-Schneider, C.; Laurent, E.; Lemaignen, A.; Beaufils, E.; Bourbao-Tournois, C.; Laribi, S.; Flament, T.; Ferreira-Maldent, N.; Bruyère, F.; Stefic, K.; et al. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin. Microbiol. Infect. 2021, 27, 258–263. [Google Scholar] [CrossRef]
- Goërtz, Y.M.J.; Van Herck, M.; Delbressine, J.M.; Vaes, A.W.; Meys, R.; Machado, F.V.C.; Houben-Wilke, S.; Burtin, C.; Posthuma, R.; Franssen, F.M.E.; et al. Persistent symptoms 3 months after a SARS-CoV-2 infection: The post-COVID-19 syndrome? ERJ Open Res. 2020, 6, 00542–2020. [Google Scholar] [CrossRef]
- Chopra, V.; Flanders, S.A.; O’mAlley, M.; Malani, A.N.; Prescott, H.C. Sixty-Day Outcomes Among Patients Hospitalized With COVID-19. Ann. Intern. Med. 2021, 174, 576–578. [Google Scholar] [CrossRef]
- Moreno-Pérez, O.; Merino, E.; Leon-Ramirez, J.-M.; Andres, M.; Ramos, J.M.; Arenas-Jiménez, J.; Asensio, S.; Sanchez, R.; Ruiz-Torregrosa, P.; Galan, I.; et al. Post-acute COVID-19 syndrome. Incidence and risk factors: A Mediterranean cohort study. J. Infect. 2021, 82, 378–383. [Google Scholar] [CrossRef]
- Mendelson, M.; Nel, J.; Blumberg, L.; Madhi, S.A.; Dryden, M.; Stevens, W.; Venter, F.W.D. Long-COVID: An evolving problem with an ex-tensive impact. S. Afr. Med. J. 2020, 111, 10–12. [Google Scholar] [CrossRef]
- O’MAhoney, L.L.; Routen, A.; Gillies, C.; Ekezie, W.; Welford, A.; Zhang, A.; Karamchandani, U.; Simms-Williams, N.; Cassambai, S.; Ardavani, A.; et al. The prevalence and long-term health effects of Long Covid among hospitalised and non-hospitalised populations: A systematic review and meta-analysis. eClinicalMedicine 2022, 55, 101762. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.B.; Medeiros, A.P.V.; de Melo, S.S.; Fonseca, C.S.; Jacob-Filho, W.; Avelino-Silva, T.J.; Aliberti, M.J.R.; for the CO-FRAIL Study Group. The long and winding road of COVID-19 in survivors of hospitalisation: Symptoms trajectory and predictors of long COVID. J. Intern. Med. 2022, 293, 264–268. [Google Scholar] [CrossRef]
- de Miranda, D.A.P.; Gomes, S.V.C.; Filgueiras, P.S.; Corsini, C.A.; Almeida, N.B.F.; Silva, R.A.; Medeiros, M.I.V.A.R.C.; Vilela, R.V.R.; Fernandes, G.R.; Grenfell, R.F.Q. Long COVID-19 syndrome: A 14-months longitudinal study during the two first epidemic peaks in Southeast Brazil. Trans. R. Soc. Trop. Med. Hyg. 2022, 116, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Mahler, D.A.; Wells, C.K. Evaluation of Clinical Methods for Rating Dyspnea. Chest 1988, 93, 580–586. [Google Scholar] [CrossRef]
- Devenney, E.; Hodges, J.R. The Mini-Mental State Examination: Pitfalls and limitations. Pract. Neurol. 2017, 17, 79–80. [Google Scholar] [CrossRef] [PubMed]
- Council, M.R. Aids to the Examination of the Peripheral Nervous System; Her Majesty’s Stationary Office: London, UK, 1976; pp. 1–70. [Google Scholar]
- Dourado, V.Z. Equações de referência para o teste de caminhada de seis minutos em indivíduos saudáveis. Arq. Bras. Cardiol. 2011, 96, e128–e138. [Google Scholar] [CrossRef]
- Reis, M.M.; Arantes, P.M.M. Medida da força de preensão manual- validade e confiabilidade do dinamômetro saehan. Fisioter. Pesqui. 2011, 18, 176–181. [Google Scholar] [CrossRef]
- Montemezzo, D.; Velloso, M.; Britto, R.R.; Parreira, V.F. Pressões respiratórias máximas: Equipamentos e procedimentos usados por fisioterapeutas brasileiros TT–Maximal respiratory pressures: Devices and procedures used by Brazilian physical therapists. Fisioter. Pesqui. 2010, 17, 147–152. [Google Scholar] [CrossRef]
- Neder, J.; Andreoni, S.; Lerario, M.; Nery, L. Reference values for lung function tests: II. Maximal respiratory pressures and voluntary ventilation. Braz. J. Med Biol. Res. 1999, 32, 719–727. [Google Scholar] [CrossRef]
- Sarmento, G.J.V. ABC da Fisioterapia Respiratoria, 2nd ed.; Manole: Barueri, Brasil, 2009. [Google Scholar]
- Nuttall, F.Q. Body mass index: Obesity, BMI, and health: A critical review. Nutr. Today 2015, 50, 117–128. [Google Scholar] [CrossRef]
- Bonifácio, L.P.; Csizmar, V.N.; Barbosa-Júnior, F.; Pereira, A.P.; Koenigkam-Santos, M.; Wada, D.T.; Gaspar, G.G.; Carvalho, F.S.; Bollela, V.R.; Santana, R.C.; et al. Long-Term Symptoms among COVID-19 Survivors in Prospective Cohort Study, Brazil. Emerg. Infect. Dis. 2022, 28, 730–733. [Google Scholar] [CrossRef]
- Taboada, M.; Moreno, E.; Cariñena, A.; Rey, T.; Pita-Romero, R.; Leal, S.; Sanduende, Y.; Rodríguez, A.; Nieto, C.; Vilas, E.; et al. Quality of life, functional status, and persistent symptoms after intensive care of COVID-19 patients. Br. J. Anaesth. 2021, 126, e110–e113. [Google Scholar] [CrossRef]
- Lundberg-Morris, L.; Leach, S.; Xu, Y.; Martikainen, J.; Santosa, A.; Gisslén, M.; Li, H.; Nyberg, F.; Bygdell, M. Covid-19 vaccine effectiveness against post-COVID-19 condition among 589 722 individuals in Sweden: Population based cohort study. BMJ 2023, 383, e076990. [Google Scholar] [CrossRef] [PubMed]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef] [PubMed]
- WHO. Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus: Abbreviated Report of a WHO Consul-tation. Approved by the Guidelines Review Committee. World Health Organization. 2011. Available online: https://iris.who.int/bitstream/handle/10665/70523/WHO_NMH_CHP_CPM_11.1_eng.pdf (accessed on 20 May 2025).
- Marin, B.G.; Aghagoli, G.; Lavine, K.; Yang, L.; Siff, E.J.; Chiang, S.S.; Salazar-Mather, T.P.; Dumenco, L.; Savaria, M.C.; Aung, S.N.; et al. Predictors of COVID-19 severity: A literature review. Rev. Med. Virol. 2021, 31, 1–10. [Google Scholar] [CrossRef]
- Muhamad, S.A.; Ugusman, A.; Kumar, J.; Skiba, D.; Hamid, A.A.; Aminuddin, A. COVID-19 and Hypertension: The What, the Why, and the How. Front. Physiol. 2021, 12, 589. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.S.; Wong, A.W.; Hague, C.J.; Murphy, D.T.; Johnston, J.C.; Ryerson, C.J.; Carlsten, C. A prospective study of 12-week respiratory outcomes in COVID-19-related hospitalisations. Thorax 2020, 76, 402–404. [Google Scholar] [CrossRef]
- Bildirici, Y.; Metintas, S.; Ucan, A.; Ak, G.; Eker Guvenc, S.; Metintas, M. Post-COVID-19 syndrome in a cohort of hospitalized COVID-19 patients. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 8291–8300. [Google Scholar] [PubMed]
- Vrettou, C.S.; Jolley, S.E.; Mantziou, V.; Dimopoulou, I. Clinical Comparison of Post-intensive Care Syndrome and Long Coro-navirus Disease. Criti. Care Clin. 2025, 41, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann-Struzek, C.; Joost, F.E.A.; Pletz, M.W.; Weiß, B.; Paul, N.; Ely, E.W.; Reinhart, K.; Rose, N. How are Long-Covid, Post-Sepsis-Syndrome and Post-Intensive-Care-Syndrome related? A conceptional approach based on the current research literature. Crit. Care 2024, 28, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, A.-F.; Minguet, P.; Colson, C.; Kellens, I.; Chaabane, S.; Delanaye, P.; Cavalier, E.; Chase, J.G.; Lambermont, B.; Misset, B. Post-intensive care syndrome after a critical COVID-19: Cohort study from a Belgian follow-up clinic. Ann. Intensiv. Care 2021, 11, 1–9. [Google Scholar] [CrossRef]

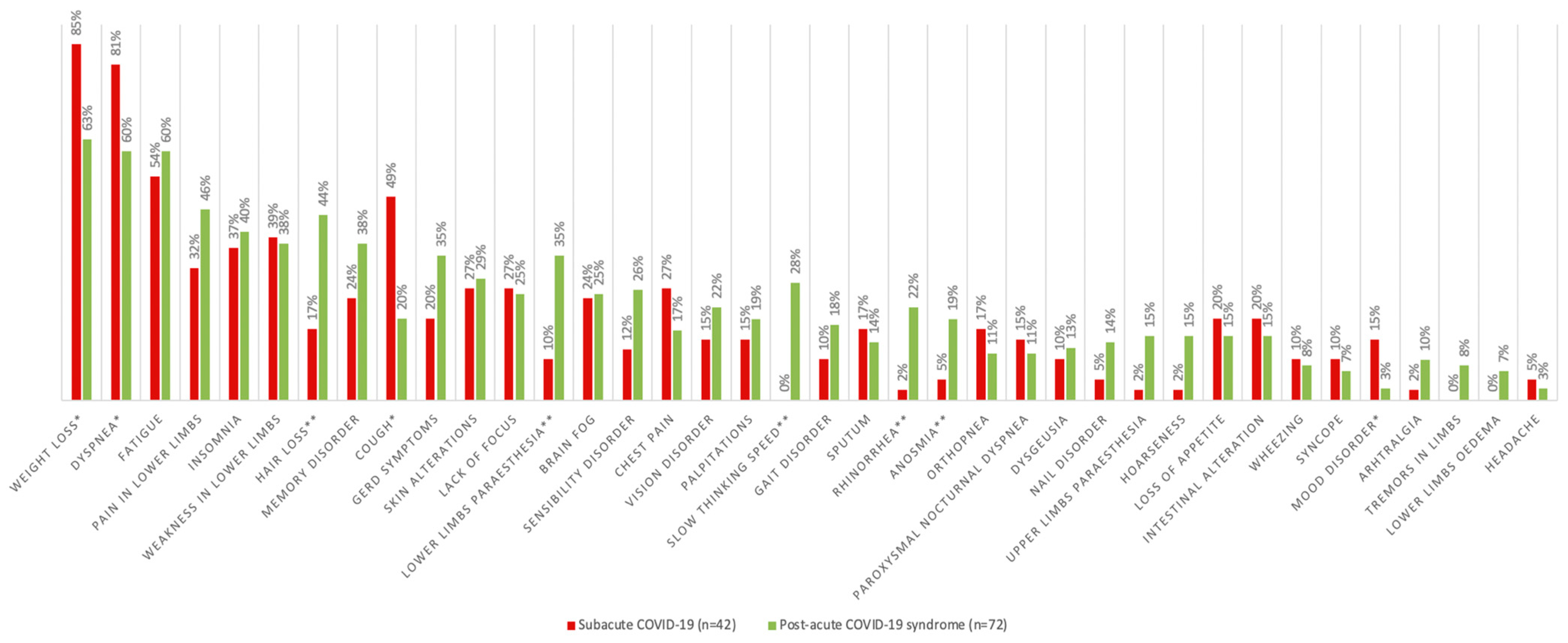
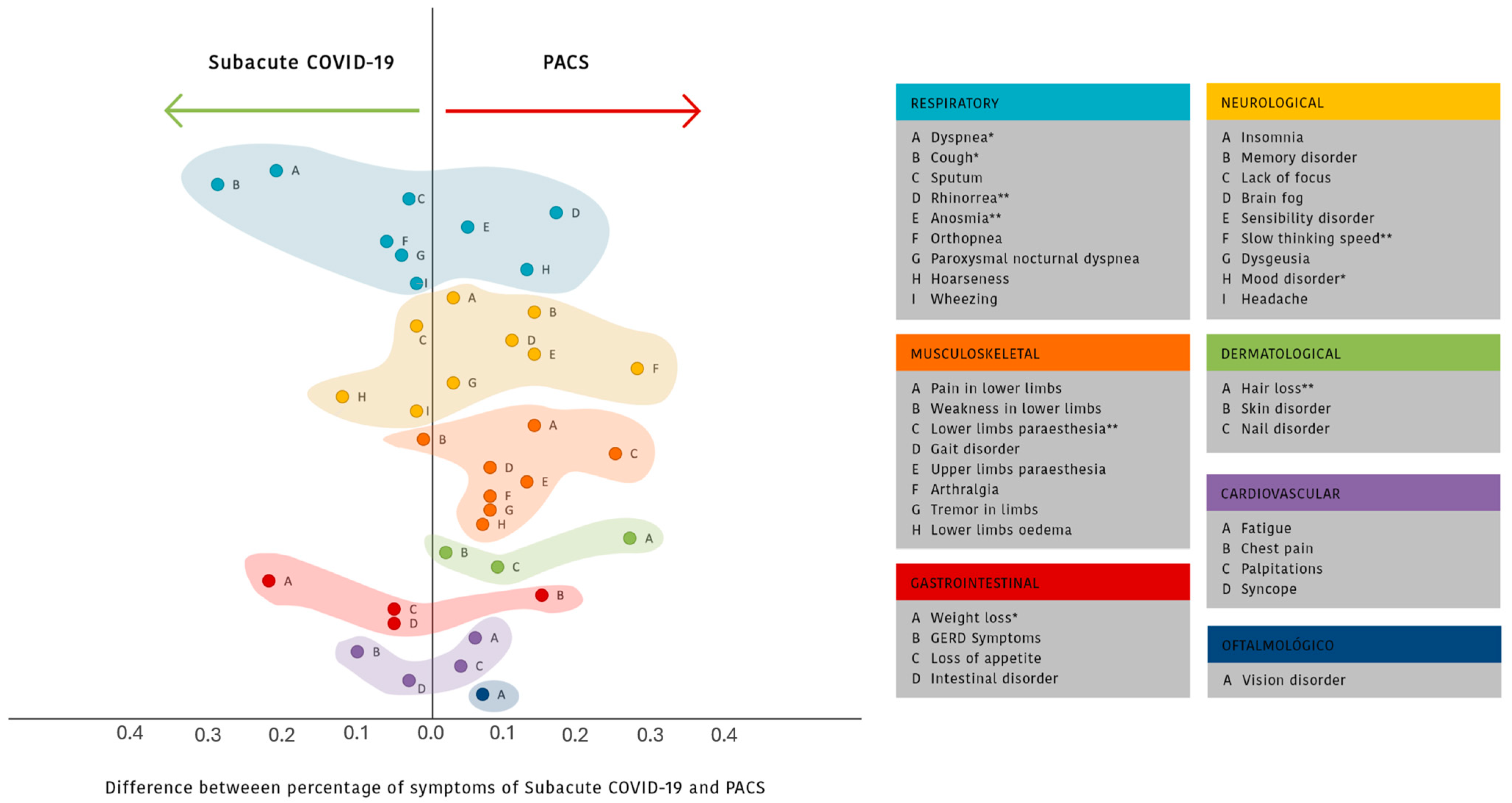

| Total (n = 113) | Subacute COVID-19 (n = 41) | Post-Acute COVID-19 Syndrome (n = 72) | p * | |
|---|---|---|---|---|
| Age (years), mean ± SD | 51.7 ± 13.2 | 52.9 ± 13.6 | 51.1 ± 13.0 | 0.484 |
| Sex, n (%) | 0.171 | |||
| Male | 53 (46.9) | 23 (56.1) | 30 (41.7) | |
| Female | 60 (53.1) | 18 (43.9) | 42 (58.3) | |
| Smoking status, n (%) | 0.891 | |||
| Never smoked | 76 (67.3) | 28 (68.3) | 48 (66.7) | |
| Smoker | 4 (3.5) | 1 (2.4) | 3 (4.2) | |
| Past smoker | 33 (29.2) | 12 (29.3) | 21 (29.2) | |
| Alcohol consumption, n (%) | 0.256 | |||
| Never drank alcohol | 86 (76.1) | 30 (73.2) | 56 (76.1) | |
| Occasionally drinks alcohol | 24 (21.2) | 11 (26.8) | 13 (18.1) | |
| Frequently drinks alcohol | 3 (2.7) | 0 (0.0) | 3 (4.2) | |
| Allergies, n (%) | 11 (9.7) | 4 (9.8) | 7 (9.7) | 1.000 |
| Presence of at least one comorbidity, n (%) | 92 (81.4) | 38 (92.7) | 54 (75.0) | 0.024 |
| Number of comorbidities, median (IQR) | 2.0 (1.0–4.0) | 3.0 (1.5–4.5) | 2.0 (0.2–4.0) | 0.156 |
| Comorbidities, n (%) | ||||
| Arterial hypertension | 69 (61.1) | 23 (56.1) | 46 (63.9) | 0.430 |
| Dyslipidemia | 35 (31.0) | 10 (24.4) | 25 (34.7) | 0.295 |
| Diabetes | 29 (25.7) | 11 (26.8) | 18 (25.0) | 0.827 |
| Congestive heart failure | 25 (22.1) | 11 (26.8) | 14 (19.4) | 0.480 |
| Hypothyroidism | 15 (13.3) | 4 (9.8) | 11 (15.3) | 0.299 |
| Coronary heart disease | 14 (12.4) | 5 (12.2) | 9 (12.5) | 1.000 |
| Previous deep vein thrombosis | 10 (8.8) | 5 (12.2) | 5 (6.9) | 0.492 |
| Peripheral venous insufficiency | 6 (5.4) | 3 (7.5) | 3 (4.2) | 0.665 |
| Arrhythmia | 6 (5.3) | 3 (7.3) | 3 (4.2) | 0.666 |
| Chronic obstructive pulmonary disease | 6 (5.3) | 3 (7.3) | 3 (4.2) | 0.666 |
| Depression | 6 (5.3) | 5 (12.2) | 1 (1.4) | 0.023 |
| Malignancy | 5 (4.4) | 2 (4.9) | 3 (4.2) | 1.000 |
| Chronic renal failure | 5 (4.4) | 1 (2.4) | 4 (5.6) | 0.662 |
| Rhinitis | 5 (4.4) | 2 (4.9) | 3 (4.2) | 1.000 |
| Asthma | 5 (4.4) | 2 (4.9) | 3 (4.2) | 1.000 |
| Previous pulmonary embolism | 4 (3.5) | 4 (9.8) | 0 (0.0) | 0.016 |
| Peripheral arterial disease | 3 (2.7) | 0 (0.0) | 3 (4.2) | 0.552 |
| Cerebrovascular disease | 3 (2.7) | 1 (2.5) | 2 (2.8) | 1.000 |
| Autoimmune disease | 2 (1.8) | 1 (2.4) | 1 (1.4) | 1.000 |
| Anemia | 1 (0.9) | 0 (0.0) | 1 (1.4) | 1.000 |
| Dementia | 1 (0.9) | 0 (0.0) | 1 (1.4) | 1.000 |
| Number of regular use medications, median (IQR) | 1.0 (0.0–4.0) | 2.0 (0.0–4.0) | 1.0 (0.0–5.0) | 0.925 |
| Previous hospitalization, n (%) | 55 (48.7) | 24 (58.5) | 31 (43.1) | 0.123 |
| Number of previous hospitalizations, median (IQR) | 0.0 (0.0–1.0) | 1.0 (0.0–2.0) | 0.0 (0.0–1.0) | 0.172 |
| Previous surgery, n (%) | 64 (56.6) | 25 (61.0) | 39 (54.2) | 0.556 |
| Number of previous surgeries, median (IQR) | 1.0 (0.0–2.0) | 1.0 (0.0–2.0) | 1.0 (0.0–2.0) | 0.617 |
| First dose of COVID-19 vaccination, n (%) | 57 (78.1) | 7 (53.8) | 50 (83.3) | 0.030 |
| Second dose of COVID-19 vaccination, n (%) | 49 (67.1) | 5 (38.5) | 44 (73.3) | 0.023 |
| Close contact contamination with COVID-19, n (%) | 60 (67.4) | 16 (61.5) | 44 (69.8) | 0.465 |
| Number of close contacts contaminated, median (IQR) | 1.0 (0.0–2.0) | 1.0 (0.0–2.0) | 1.0 (0.0–3.0) | 0.122 |
| Number of deaths of close contacts, median (IQR) | 0.0 (0.0–0.0) | 0.0 (0.0–0.2) | 0.0 (0.0–0.0) | 0.176 |
| Total (n = 113) | Subacute COVID-19 (n = 41) | Post-Acute COVID-19 Syndrome (n = 72) | * p | |
|---|---|---|---|---|
| Number of symptoms during acute infection, median (IQR) | 4.0 (0.0–12.0) | 4.0 (1.0–9.0) | 4.0 (0.0–12.0) | 0.442 |
| Signs and symptoms during acute infection, n (%) | ||||
| Dyspnea | 84 (74.3) | 29 (70.7) | 55 (76.4) | 0.511 |
| Fatigue | 63 (55.8) | 23 (56.1) | 40 (55.6) | 1.000 |
| Fever | 57 (50.4) | 20 (48.8) | 37 (51.4) | 0.846 |
| Myalgia | 53 (46.9) | 19 (46.3) | 34 (47.2) | 1.000 |
| Cough | 50 (44.2) | 14 (34.1) | 36 (50.0) | 0.118 |
| Anosmia | 35 (31.0) | 15 (36.6) | 20 (27.8) | 0.399 |
| Headache | 32 (28.3) | 7 (17.1) | 25 (34.7) | 0.053 |
| Dysgeusia | 21 (18.6) | 8 (19.5) | 13 (18.1) | 1.000 |
| Diarrhea | 15 (13.3) | 6 (14.6) | 9 (12.5) | 0.778 |
| Nausea and vomits | 14 (12.4) | 4 (9.8) | 10 (13.9) | 0.767 |
| Shivers | 10 (8.8) | 3 (7.3) | 7 (9.7) | 0.745 |
| Sputum | 8 (7.1) | 1 (2.4) | 7 (9.7) | 0.255 |
| Excessive sweating | 4 (3.5) | 1 (2.4) | 3 (4.2) | 1.000 |
| Hospitalization, n (%) | 106 (93.8) | 40 (97.6) | 66 (91.7) | 0.419 |
| Hospitalization characteristics | ||||
| Time from acute onset to hospitalization (days), median (IQR) | 8.0 (–22.0–20.0) | 8.0 (–22.0–20.0) | 8.0 (0.0–19.0) | 0.922 |
| Length of hospitalization (days), median (IQR) | 13.0 (0.0–62.0) | 9.0 (0.0–42.0) | 14.5 (0.0–62.0) | 0.062 |
| ICU admission, n (%) | 81 (76.4) | 26 (65.0) | 55 (83.3) | 0.037 |
| Length of ICU admission (days), median (IQR) | 9.0 (1.0–60.0) | 8.0 (1.0–25.0) | 9.0 (1.0–60.0) | 0.426 |
| Use of nasal cannula, n (%) | 84 (80.0) | 27 (67.5) | 57 (87.7) | 0.022 |
| Use of non-invasive mechanical ventilation, n (%) | 65 (61.3) | 19 (47.5) | 46 (69.7) | 0.026 |
| Length of non-invasive ventilation (days), median (IQR) | 4.0 (1.0–14.0) | 1.0 (1.0–3.0) | 7.5 (1.0–14.0) | 0.095 |
| Orotracheal intubation, n (%) | 24 (22.6) | 7 (17.5) | 17 (25.8) | 0.351 |
| Length of orotracheal intubation (days), mean ± SD | 14.7 ± 10.9 | 12.5 ± 5.2 | 15.7 ± 12.8 | 0.638 |
| Number of complications during hospitalization, median (IQR) | 0.0 (0.0–3.0) | 0.0 (0.0–3.0) | 0.0 (0.0–2.0) | 0.653 |
| Pulmonary embolism during hospitalization, n (%) | 5 (4.7) | 3 (7.5) | 2 (3.1) | 0.363 |
| Deep vein thrombosis during hospitalization, n (%) | 5 (4.7) | 2 (5.0) | 3 (4.5) | 1.000 |
| Readmission into hospital after discharge, n (%) | 3 (2.9) | 1 (2.6) | 2 (3.2) | 1.000 |
| Total (n = 113) | Subacute COVID-19 (n = 41) | Post-Acute COVID-19 Syndrome (n = 72) | p * | |
|---|---|---|---|---|
| BMI, median (IQR) | 31.3 (16.7–47.2) | 31.6 (16.7–37.7) | 31.2 (18.8–47.2) | 0.703 |
| BMI categories, n (%) | 0.361 | |||
| Underweight or normal | 10/107 (9.3) | 5/38 (13.2) | 5/69 (7.2) | |
| Overweight | 30/107 (28.0) | 8/38 (21.1) | 22/69 (31.9) | |
| Obese | 67/107 (62.6) | 25/38 (65.8) | 42/69 (60.9) | |
| Abdominal circumference (cm), mean ± SD | 103.8 ± 12.9 | 102.5 ± 14.9 | 104.1 ± 2.5 | 0.677 |
| Altered abdominal circumference according to sex, n (%) | 55/83 (66.3) | 10/14 (71.4) | 45/69 (62.5) | 0.764 |
| Heart rate (bpm), mean ± SD | 82.7 ± 14.7 | 87.4 ± 15.4 | 80.0 ± 13.8 | 0.010 |
| Elevated respiratory rate, n (%) | 39 (35.8) | 19 (50.0) | 20 (28.2) | 0.035 |
| Oxygen saturation at rest (%), median (IQR) | 96.0 (84–100) | 96.0 (85–100) | 96 (84–99) | 0.132 |
| Elevated blood pressure, n (%) | 50 (45.0) | 15 (38.5) | 35 (48.6) | 0.325 |
| 6-min walking test | ||||
| Predicted walking distance (PWD) (meters), mean ± SD | 490.5 ± 39.6 | 487.4 ± 41.1 | 492.6 ± 39.1 | 0.635 |
| Distance walked (meters), mean ± SD | 393.8 ± 99.3 | 444.0 ± 86.0 | 357.2 ± 95.1 | 0.005 |
| % of PWD, mean ± SD | 80.3 ± 21.0 | 89.9 ± 20.2 | 71.9 ± 18.1 | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haagsma, A.B.; Otto, F.G.; de Sá Vianna, M.L.G.; Maingue, P.M.; Muller, A.P.; de Oliveira, N.H.d.S.; Abbott, L.A.; da Silva, F.P.G.; Klein, C.K.; Herzog, D.M.; et al. Clinical Evaluation of COVID-19 Survivors at a Public Multidisciplinary Health Clinic. Biomedicines 2025, 13, 1888. https://doi.org/10.3390/biomedicines13081888
Haagsma AB, Otto FG, de Sá Vianna MLG, Maingue PM, Muller AP, de Oliveira NHdS, Abbott LA, da Silva FPG, Klein CK, Herzog DM, et al. Clinical Evaluation of COVID-19 Survivors at a Public Multidisciplinary Health Clinic. Biomedicines. 2025; 13(8):1888. https://doi.org/10.3390/biomedicines13081888
Chicago/Turabian StyleHaagsma, Ariele Barreto, Felipe Giaretta Otto, Maria Leonor Gomes de Sá Vianna, Paula Muller Maingue, Andréa Pires Muller, Nayanne Hevelin dos Santos de Oliveira, Luísa Arcoverde Abbott, Felipe Paes Gomes da Silva, Carolline Konzen Klein, Débora Marques Herzog, and et al. 2025. "Clinical Evaluation of COVID-19 Survivors at a Public Multidisciplinary Health Clinic" Biomedicines 13, no. 8: 1888. https://doi.org/10.3390/biomedicines13081888
APA StyleHaagsma, A. B., Otto, F. G., de Sá Vianna, M. L. G., Maingue, P. M., Muller, A. P., de Oliveira, N. H. d. S., Abbott, L. A., da Silva, F. P. G., Klein, C. K., Herzog, D. M., Fantin Unruh, J. C. B., Schoeler, L., Miyasaki, D., Soni, J. F., Stival, R. S. M., & Baena, C. P. (2025). Clinical Evaluation of COVID-19 Survivors at a Public Multidisciplinary Health Clinic. Biomedicines, 13(8), 1888. https://doi.org/10.3390/biomedicines13081888








