Glioblastoma: A Multidisciplinary Approach to Its Pathophysiology, Treatment, and Innovative Therapeutic Strategies
Abstract
1. Introduction
2. GBM Pathophysiology
2.1. Neuroinflammation
2.2. Genetics
2.3. Microbiome
2.4. Microenvironment
2.5. The Immunologic Role of GBM
2.6. Molecular Mechanisms Implicated in GBM
2.7. The Role of Gangliosides in GBM
2.8. The Role of Ceramides and Sphingolipids in GBM
3. Diagnostic Methods of GBM
3.1. Clinical Diagnosis
3.2. Imaging
3.3. Histopathology
3.4. Immunohistochemistry and Biomarkers
3.5. Liquid Biopsy
4. Treatment
4.1. Conventional Treatment
4.1.1. Surgical Approach
4.1.2. Pharmacological Approach
TMZ and Alkylating Agents
Paclitaxel
Exportin 1
Carmustine Wafers
Curcumin
Ceramide Therapeutics
4.1.3. Radiotherapy
4.2. Non-Conventional Treatments
4.2.1. Palliative Care
4.2.2. Diet Implementation
4.3. Future Perspectives
4.3.1. Optune
4.3.2. Immunological Therapy
4.3.3. Challenges and Future Directions
4.3.4. Immune Checkpoint Inhibitors (ICIs)
4.3.5. Peptide Vaccines
4.3.6. Dendritic Cell Vaccines
4.3.7. Chimeric Antigen Receptor T Cell (CAR-T) Therapy
4.3.8. Stem Cells
4.3.9. Virology
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Louis, D.N.; Perry, A.; Reifenberger, R.G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W.; et al. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Patil, N.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2013–2017. Neuro Oncol. 2020, 22 (Suppl. S2), iv1–iv96. [Google Scholar] [CrossRef]
- Thakkar, J.; Dolecek, T.A.; Horbinski, C.; Ostrom, Q.T.; Lightner, D.D.; Barnholtz-Sloan, J.S.; Aldape, K.; Davis, F.G.; de-Lima, E.K.; Xie, Y.; et al. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol. Biomarkers Prev. 2014, 23, 1985–1996. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E. Glioblastoma: Overview of Disease and Treatment. Clin. J. Oncol. Nurs. 2016, 20 (Suppl. S5), S2–S8. [Google Scholar] [CrossRef] [PubMed]
- Nakase, T.; Guerra, G.A.; Ostrom, Q.T.; Ge, T.; Melin, B.S.; Wrensch, M.; Wiencke, J.K.; Jenkins, R.B.; Eckel-Passow, J.E.; Glioma International Case-Control Study (GICC); et al. Genome-wide polygenic risk scores predict risk of glioma and molecular subtypes. Neuro Oncol. 2024, 26, 1933–1944. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.K.; Chen, Y.; Guan, X.; Nousome, D.; Sharma, C.; Canoll, P.; Bruce, J.; Sloan, A.E.; Cortes, E.; Vonsattel, J.P.; et al. Genome-wide methylation analyses in glioblastoma multiforme. PLoS ONE 2014, 9, e89376. [Google Scholar] [CrossRef]
- Chakrabarti, I.; Cockburn, M.; Cozen, W.; Wang, Y.P.; Preston-Martin, S. A Population-Based Description of Glioblastoma Multiforme in Los Angeles County, 1974–1999. Cancer 2005, 104, 2798–2806. [Google Scholar] [CrossRef]
- Luan, X.Z.; Wang, H.R.; Xiang, W.; Li, S.J.; He, H.; Chen, L.G. Extracranial Multiorgan Metastasis from Primary Glioblastoma: A Case Report. World J. Clin. Cases 2021, 9, 10300–10307. [Google Scholar] [CrossRef]
- Hata, N.; Katsuta, T.; Inoue, T.; Arikawa, K.; Yano, T.; Takeshita, M. Extracranial Metastasis of Glioblastoma to the Lung and Heart with a Histological Resemblance to Small Cell Carcinoma of the Lung: An Autopsy Case. No Shinkei Geka. Neurol. Surg. 2001, 29, 433–438. [Google Scholar]
- Hanif, F.; Muzaffar, K.; Perveen, K.; Malhi, S.M.; Simjee, S.U. Glioblastoma Multiforme: A Review of Its Epidemiology and Pathogenesis through Clinical Presentation and Treatment. Asian Pac. J. Cancer Prev. 2017, 18, 3–9. [Google Scholar]
- Braganza, M.Z.; Kitahara, C.M.; Berrington de Gonzalez, A.; Inskip, P.D.; Johnson, K.J.; Rajaraman, P. Ionizing radiation and the risk of brain and central nervous system tumors: A systematic review. Neuro Oncol. 2012, 14, 1316–1324. [Google Scholar] [CrossRef]
- Vaz-Salgado, M.A.; Villamayor, M.; Albarrán, V.; Alía, V.; Sotoca, P.; Chamorro, J.; Rosero, D.; Barrill, A.M.; Martín, M.; Fernandez, E.; et al. Recurrent glioblastoma: A review of the treatment options. Cancers 2023, 15, 4279. [Google Scholar] [CrossRef]
- Schirrmacher, V. Cancer vaccines and oncolytic viruses exert profoundly lower side effects in cancer patients than other systemic therapies: A comparative analysis. Biomedicines 2020, 8, 61. [Google Scholar] [CrossRef]
- Schirrmacher, V.; Lorenzen, D.; Van Gool, S.W.; Stuecker, W. A new strategy of cancer immunotherapy combining hyperthermia/oncolytic virus pretreatment with specific autologous anti-tumor vaccination—A review. Austin Oncol. Case Rep. 2017, 2, 1006. [Google Scholar]
- Koshy, M.; Villano, J.L.; Dolecek, T.A.; Howard, A.; Mahmood, U.; Chmura, S.J.; Markert, J.M.; Curran, W.J., Jr.; Wagner, L.; Beeler, D.K.; et al. Improved survival time trends of glioblastoma using the SEER 17 population-based registries. J. Neurooncol. 2012, 107, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Nóbrega, A.H.L.; Pimentel, R.S.; Prado, A.P.; Garcia, J.; Frozza, R.L.; Bernardi, A. Neuroinflammation in glioblastoma: The role of the microenvironment in tumour progression. Curr. Cancer Drug Targets 2024, 24, 579–594. [Google Scholar] [CrossRef] [PubMed]
- Roesler, R.; Dini, S.A.; Isolan, G.R. Neuroinflammation and immunoregulation in glioblastoma and brain metastases: Recent developments in imaging approaches. Clin. Exp. Immunol. 2021, 206, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gou, W.; Zhang, X. Neuroinflamación en el glioblastoma: Avances y perspectivas. Brain Sci. 2024, 14, 687. [Google Scholar] [CrossRef]
- Cugurra, A.; Mamuladze, T.; Rustenhoven, J.; Dykstra, T.; Beroshvili, G.; Greenberg, Z.J.; Baker, W.; Papadopoulos, Z.; Drieu, A.; Blackburn, S.; et al. Skull and vertebral bone marrow are myeloid cell reservoirs for the meninges and CNS parenchyma. Science 2021, 373, eabf7844. [Google Scholar] [CrossRef]
- Mitchell, D.; Shireman, J.; Sierra Potchanant, E.A.; Lara-Velazquez, M.; Dey, M. Neuroinflammation in autoimmune disease and primary brain tumors: The quest for striking the right balance. Front. Cell. Neurosci. 2021, 15, 716947. [Google Scholar] [CrossRef]
- Steeg, P.S. La barrera hematoencefálica en la biología y la terapia del cáncer. Nat. Rev. Clin. Oncol. 2021, 18, 696–714. [Google Scholar] [CrossRef] [PubMed]
- Crommentuijn, M.H.W.; Schetters, S.T.T.; Dusoswa, S.A.; Kruijssen, L.J.W.; Garcia-Vallejo, J.J.; van Kooyk, Y. Immune involvement of the contralateral hemisphere in a glioblastoma mouse model. J. Immunother. Cancer 2020, 8, e000323. [Google Scholar] [CrossRef] [PubMed]
- Bartos, L.M.; Quach, S.; Zenatti, V.; Kirchleitner, S.V.; Blobner, J.; Wind-Mark, K.; Kolabas, Z.I.; Ulukaya, S.; Holzgreve, A.; Ruf, V.C.; et al. Remote neuroinflammation in newly diagnosed glioblastoma correlates with unfavorable clinical outcome. Clin. Cancer Res. 2024, 30, 4618–4634. [Google Scholar] [CrossRef] [PubMed]
- Bueno-Martínez, E.; Lara-Almunia, M.; Rodríguez-Arias, C.; Otero-Rodríguez, A.; Garfias-Arajona, S.; González-Sarmiento, R. Polymorphisms in autophagy genes are genetic susceptibility factors in glioblastoma development. BMC Cancer 2022, 22, 146. [Google Scholar] [CrossRef]
- Pan, P.C.; Magge, R.S. Mechanisms of EGFR Resistance in Glioblastoma. Int. J. Mol. Sci. 2020, 21, 8471. [Google Scholar] [CrossRef]
- Gai, Q.J.; Fu, Z.; He, J.; Mao, M.; Yao, X.X.; Qin, Y.; Lan, X.; Zhang, L.; Miao, J.Y.; Wang, Y.X.; et al. EPHA2 mediates PDGFA activity and functions together with PDGFRA as prognostic marker and therapeutic target in glioblastoma. Signal Transduct. Target. Ther. 2022, 7, 33. [Google Scholar] [CrossRef]
- Arnoff, T.E.; El-Deiry, W.S. MDM2/MDM4 amplification and CDKN2A deletion in metastatic melanoma and glioblastoma multiforme may have implications for targeted therapeutics and immunotherapy. Am. J. Cancer Res. 2022, 12, 2102–2117. [Google Scholar]
- González-Tablas, M.; Arandia, D.; Jara-Acevedo, M.; Otero, Á.; Vital, A.L.; Prieto, C.; González-Garcia, N.; Nieto-Librero, A.B.; Tao, H.; Pascual, D.; et al. Heterogeneous EGFR, CDK4, MDM4, and PDGFRA Gene Expression Profiles in Primary GBM: No Association with Patient Survival. Cancers 2020, 12, 231. [Google Scholar] [CrossRef]
- Filippova, N.; Grimes, J.M.; Leavenworth, J.W.; Namkoong, D.; Yang, X.; King, P.H.; Crowley, M.; Crossman, D.K.; Nabors, L.B. Targeting the TREM1-positive myeloid microenvironment in glioblastoma. Neurooncol. Adv. 2022, 5, vdac189. [Google Scholar] [CrossRef]
- Kong, Y.; Feng, Z.C.; Zhang, Y.L.; Liu, X.F.; Ma, Y.; Zhao, Z.M.; Huang, B.; Chen, A.J.; Zhang, D.; Thorsen, F.; et al. Identification of Immune-Related Genes Contributing to the Development of Glioblastoma Using Weighted Gene Co-expression Network Analysis. Front. Immunol. 2020, 11, 1281. [Google Scholar] [CrossRef]
- Liu, N.Q.; Paassen, I.; Custers, L.; Zeller, P.; Teunissen, H.; Ayyildiz, D.; He, J.; Buhl, J.L.; Hoving, E.W.; van Oudenaarden, A.; et al. SMARCB1 Loss Activates Patient-Specific Distal Oncogenic Enhancers in Malignant Rhabdoid Tumors. Nat. Commun. 2023, 14, 7762. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Stroberg, E.; Wang, F.; Morales, L.; Shan, Y.; Rao, A.; Huang, J.H.; Wu, E.; Fonkem, E. SMARCB1 Gene Mutation Predisposes to Earlier Development of Glioblastoma: A Case Report of Familial GBM. J. Neuropathol. Exp. Neurol. 2020, 79, 562–565. [Google Scholar] [CrossRef]
- Liu, L.; Huh, J.R.; Shah, K. Microbiota and the gut-brain-axis: Implications for new therapeutic design in the CNS. EBioMedicine 2022, 77, 103908. [Google Scholar] [CrossRef]
- Aljarrah, D.; Chalour, N.; Zorgani, A.; Nissan, T.; Pranjol, M.Z.I. Exploring the gut microbiota and its potential as a biomarker in gliomas. Biomed. Pharmacother. 2024, 173, 116420. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Alam, M.T.; Dey, J.; Sasidharan, B.C.P.; Ray, U.; Srivastava, A.K.; Gandhi, S.; Tripathi, P.P. Healthy Gut, Healthy Brain: The Gut Microbiome in Neurodegenerative Disorders. Curr. Top. Med. Chem. 2020, 20, 1142–1153. [Google Scholar] [CrossRef]
- Zeng, C.; Zhang, C.; He, C.; Song, H. Investigating the causal impact of gut microbiota on glioblastoma: A bidirectional Mendelian randomization study. BMC Genomics 2023, 24, 784. [Google Scholar] [CrossRef]
- Patrizz, A.; Dono, A.; Zorofchian, S.; Hines, G.; Takayasu, T.; Husein, N.; Otani, Y.; Arevalo, O.; Choi, H.A.; Savarraj, J.; et al. Glioma and temozolomide induced alterations in gut microbiome. Sci. Rep. 2020, 10, 21002. [Google Scholar] [CrossRef]
- Wen, C.; Wei, S.; Zong, X.; Wang, Y.; Jin, M. Microbiota-gut-brain axis and nutritional strategy under heat stress. Anim. Nutr. 2021, 7, 1329–1336. [Google Scholar] [CrossRef]
- Heidor, R.; Furtado, K.S.; Ortega, J.F.; de Oliveira, T.F.; Tavares, P.E.; Vieira, A.; Miranda, M.L.; Purgatto, E.; Moreno, F.S. The chemopreventive activity of the histone deacetylase inhibitor tributyrin in colon carcinogenesis involves the induction of apoptosis and reduction of DNA damage. Toxicol. Appl. Pharmacol. 2014, 276, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Kuefer, R.; Hofer, M.D.; Altug, V.; Zorn, C.; Genze, F.; Kunzi-Rapp, K.; Hautmann, R.E.; Gschwend, J.E. Sodium butyrate and tributyrin induce in vivo growth inhibition and apoptosis in human prostate cancer. Br. J. Cancer 2004, 90, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Kuroiwa-Trzmielina, J.; de Conti, A.; Scolastici, C.; Pereira, D.; Horst, M.A.; Purgatto, E.; Ong, T.P.; Moreno, F.S. Chemoprevention of rat hepatocarcinogenesis with histone deacetylase inhibitors: Efficacy of tributyrin, a butyric acid prodrug. Int. J. Cancer 2009, 124, 2520–2527. [Google Scholar] [CrossRef] [PubMed]
- Nejman, D.; Livyatan, I.; Fuks, G.; Gavert, N.; Zwang, Y.; Geller, L.T.; Rotter-Maskowitz, A.; Weiser, R.; Mallel, G.; Gigi, E.; et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science 2020, 368, 973–980. [Google Scholar] [CrossRef]
- Zhang, Z.; Tang, H.; Chen, P.; Xie, H.; Tao, Y. Demystifying the manipulation of host immunity, metabolism, and extraintestinal tumors by the gut microbiome. Signal Transduct. Target. Ther. 2019, 4, 41. [Google Scholar] [CrossRef]
- Jiang, H.; Zeng, W.; Zhang, X.; Pei, Y.; Zhang, H.; Li, Y. The role of gut microbiota in patients with benign and malignant brain tumors: A pilot study. Bioengineered 2022, 13, 7847–7859. [Google Scholar] [CrossRef]
- Tripathy, D.K.; Panda, L.P.; Biswal, S.; Barhwal, K. Insights into the glioblastoma tumor microenvironment: Current and emerging therapeutic approaches. Front. Pharmacol. 2024, 15, 1355242. [Google Scholar] [CrossRef]
- Codrici, E.; Popescu, I.D.; Tanase, C.; Enciu, A.M. Friends with Benefits: Chemokines, Glioblastoma-Associated Microglia/Macrophages, and Tumor Microenvironment. Int. J. Mol. Sci. 2022, 23, 2509. [Google Scholar] [CrossRef]
- Read, R.D.; Tapp, Z.M.; Rajappa, P.; Hambardzumyan, D. Glioblastoma microenvironment-from biology to therapy. Genes Dev. 2024, 38, 360–379. [Google Scholar] [CrossRef]
- Strepkos, D.; Markouli, M.; Klonou, A.; Piperi, C.; Papavassiliou, A.G. Insights in the immunobiology of glioblastoma. J. Mol. Med. 2020, 98, 1–10. [Google Scholar] [CrossRef]
- Faisal, S.M.; Comba, A.; Varela, M.L.; Argento, A.E.; Brumley, E.; Abel, C., 2nd; Castro, M.G.; Lowenstein, P.R. The complex interactions between the cellular and non-cellular components of the brain tumor microenvironmental landscape and their therapeutic implications. Front. Oncol. 2022, 12, 1005069. [Google Scholar] [CrossRef]
- Markwell, S.M.; Ross, J.L.; Olson, C.L.; Brat, D.J. Necrotic reshaping of the glioma microenvironment drives disease progression. Acta Neuropathol. 2022, 143, 291–310. [Google Scholar] [CrossRef]
- Erices, J.I.; Bizama, C.; Niechi, I.; Uribe, D.; Rosales, A.; Fabres, K.; Navarro-Martínez, G.; Torres, Á.; San Martín, R.; Roa, J.C.; et al. Glioblastoma Microenvironment and Invasiveness: New Insights and Therapeutic Targets. Int. J. Mol. Sci. 2023, 24, 7047. [Google Scholar] [CrossRef] [PubMed]
- Domènech, M.; Hernández, A.; Plaja, A.; Martínez-Balibrea, E.; Balañà, C. Hypoxia: The Cornerstone of Glioblastoma. Int. J. Mol. Sci. 2021, 22, 12608. [Google Scholar] [CrossRef] [PubMed]
- Boyd, N.H.; Tran, A.N.; Bernstock, J.D.; Etminan, T.; Jones, A.B.; Gillespie, G.Y.; Friedman, G.K.; Hjelmeland, A.B. Glioma stem cells and their roles within the hypoxic tumor microenvironment. Theranostics 2021, 11, 665–683. [Google Scholar] [CrossRef]
- Feldman, L. Hypoxia within the glioblastoma tumor microenvironment: A master saboteur of novel treatments. Front. Immunol. 2024, 15, 1384249. [Google Scholar] [CrossRef]
- Abou Khouzam, R.; Janji, B.; Thiery, J.; Zaarour, R.F.; Chamseddine, A.N.; Mayr, H.; Savagner, P.; Kieda, C.; Gad, S.; Buart, S.; et al. Hypoxia as a potential inducer of immune tolerance, tumor plasticity and a driver of tumor mutational burden: Impact on cancer immunotherapy. Semin. Cancer Biol. 2023, 97, 104–123. [Google Scholar] [CrossRef]
- Wang, G.; Zhong, K.; Wang, Z.; Zhang, Z.; Tang, X.; Tong, A.; Zhou, L. Tumor-associated microglia and macrophages in glioblastoma: From basic insights to therapeutic opportunities. Front. Immunol. 2022, 13, 964898. [Google Scholar] [CrossRef]
- White, K.; Connor, K.; Meylan, M.; Bougoüin, A.; Salvucci, M.; Bielle, F.; O’Farrell, A.C.; Sweeney, K.; Weng, L.; Bergers, G.; et al. Identification, validation and biological characterisation of novel glioblastoma tumour microenvironment subtypes: Implications for precision immunotherapy. Ann. Oncol. 2023, 34, 300–314. [Google Scholar] [CrossRef]
- Yu, M.W.; Quail, D.F. Immunotherapy for Glioblastoma: Current Progress and Challenges. Front. Immunol. 2021, 12, 676301. [Google Scholar] [CrossRef]
- Ng, A.T.; Assi, T.; Tyler, S.; Jamous, K.T.; Arham, A.; Kawtharani, S.; Hazem, I. The challenges and clinical landscape of glioblastoma immunotherapy. CNS Oncol. 2024, 13, 2415878. [Google Scholar] [CrossRef]
- Bausart, M.; Préat, V.; Malfanti, A. Immunotherapy for glioblastoma: The promise of combination strategies. J. Exp. Clin. Cancer Res. 2022, 41, 35. [Google Scholar] [CrossRef]
- Norollahi, S.E.; Yousefi, B.; Nejatifar, F.; Yousefzadeh-Chabok, S.; Rashidy-Pour, A.; Samadani, A.A. Practical immunomodulatory landscape of glioblastoma multiforme (GBM) therapy. J. Egypt. Natl. Cancer Inst. 2024, 36, 33. [Google Scholar] [CrossRef]
- Yeh, S.-C.; Wang, P.-Y.; Lou, Y.-W.; Khoo, K.-H.; Hsiao, M.; Hsu, T.-L.; Wong, C.-H. Glycolipid GD3 and GD3 Synthase Are Key Drivers for Glioblastoma Stem Cells and Tumorigenicity. Proc. Natl. Acad. Sci. USA 2016, 113, 5592–5597. [Google Scholar] [CrossRef]
- Hein, V.; Baeza-Kallee, N.; Bergès, R.; Essakhi, N.; Soubéran, A.; Colin, C.; Morando, P.; Appay, R.; Graillon, T.; Tchoghandjian, A.; et al. The GD3 Ganglioside Promotes Cell Growth, Plasticity and Chemotherapy Resistance of Human Glioblastoma Cancer Stem Cells. Cancer Cell Int. 2025, 25, 246. [Google Scholar] [CrossRef]
- Ohkawa, Y.; Momota, H.; Kato, A.; Hashimoto, N.; Tsuda, Y.; Kotani, N.; Honke, K.; Suzumura, A.; Furukawa, K.; Ohmi, Y.; et al. Ganglioside GD3 Enhances Invasiveness of Gliomas by Forming a Complex with Platelet-Derived Growth Factor Receptor α and Yes Kinase. J. Biol. Chem. 2015, 290, 16043–16058. [Google Scholar] [CrossRef]
- Iwasawa, T.; Zhang, P.; Ohkawa, Y.; Momota, H.; Wakabayashi, T.; Ohmi, Y.; Bhuiyan, R.; Furukawa, K.; Furukawa, K. Enhancement of Malignant Properties of Human Glioma Cells by Ganglioside GD3/GD2. Int. J. Oncol. 2018, 52, 1255–1266. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, N.; Toyoda, M.; Ishiwata, T. Gangliosides as Signaling Regulators in Cancer. Int. J. Mol. Sci. 2021, 22, 5076. [Google Scholar] [CrossRef] [PubMed]
- Fabris, D.; Rožman, M.; Sajko, T.; Vukelić, Ž. Aberrant Ganglioside Composition in Glioblastoma Multiforme and Peritumoral Tissue: A Mass Spectrometry Characterization. Biochimie 2017, 137, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Sarbu, M.; Petrica, L.; Clemmer, D.E.; Vukelić, Ž.; Zamfir, A.D. Gangliosides of Human Glioblastoma Multiforme: A Comprehensive Mapping and Structural Analysis by Ion Mobility Tandem Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2021, 32, 1249–1257. [Google Scholar] [CrossRef]
- Sarkar, A.; Banerjee, S.; Biswas, K. Multi-Dimensional Role of Gangliosides in Modulating Cancer Hallmarks and Their Prospects in Targeted Cancer Therapy. Front. Pharmacol. 2023, 14, 1282572. [Google Scholar] [CrossRef]
- Karmelić, I.; Jurilj Sajko, M.; Sajko, T.; Rotim, K.; Fabris, D. The Role of Sphingolipid Rheostat in the Adult-Type Diffuse Glioma Pathogenesis. Front. Cell Dev. Biol. 2024, 12, 1466141. [Google Scholar] [CrossRef]
- Zaibaq, F.; Dowdy, T.; Larion, M. Targeting the Sphingolipid Rheostat in Gliomas. Int. J. Mol. Sci. 2022, 23, 9255. [Google Scholar] [CrossRef]
- Abuhusain, H.J.; Matin, A.; Qiao, Q.; Shen, H.; Kain, N.; Day, B.W.; Stringer, B.W.; Daniels, B.; Laaksonen, M.A.; Teo, C.; et al. A Metabolic Shift Favoring Sphingosine 1-Phosphate at the Expense of Ceramide Controls Glioblastoma Angiogenesis. J. Biol. Chem. 2013, 288, 37355–37364. [Google Scholar] [CrossRef]
- Tea, M.N.; Poonnoose, S.I.; Pitson, S.M. Targeting the Sphingolipid System as a Therapeutic Direction for Glioblastoma. Cancers 2020, 12, 111. [Google Scholar] [CrossRef]
- Czarnywojtek, A.; Borowska, M.; Dyrka, K.; Van Gool, S.; Sawicka-Gutaj, N.; Moskal, J.; Kościński, J.; Graczyk, P.; Hałas, T.; Lewandowska, A.M.; et al. Glioblastoma Multiforme: The Latest Diagnostics and Treatment Techniques. Pharmacology 2023, 108, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Omuro, A.; DeAngelis, L.M. Glioblastoma and other malignant gliomas: A clinical review. JAMA 2013, 310, 1842–1850. [Google Scholar] [CrossRef] [PubMed]
- Hadidchi, S.; Surento, W.; Lerner, A.; Liu, C.J.; Gibbs, W.N.; Kim, P.E.; Shiroishi, M.S. Headache and Brain Tumor. Neuroimaging Clin. N. Am. 2019, 29, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Schaff, L.R.; Mellinghoff, I.K. Glioblastoma and Other Primary Brain Malignancies in Adults: A Review. JAMA 2023, 329, 574–587. [Google Scholar] [CrossRef]
- Neugut, A.I.; Sackstein, P.; Hillyer, G.C.; Jacobson, J.S.; Bruce, J.; Lassman, A.B.; Stieg, P.A. Magnetic Resonance Imaging-Based Screening for Asymptomatic Brain Tumors: A Review. Oncologist 2019, 24, 375–384. [Google Scholar] [CrossRef]
- Moton, S.; Elbanan, M.; Zinn, P.O.; Colen, R.R. Imaging Genomics of Glioblastoma: Biology, Biomarkers, and Breakthroughs. Top. Magn. Reson. Imaging 2015, 24, 155–163. [Google Scholar] [CrossRef]
- Alexander, B.M.; Cloughesy, T.F. Adult Glioblastoma. J. Clin. Oncol. 2017, 35, 2402–2409. [Google Scholar] [CrossRef]
- Wirsching, H.G.; Galanis, E.; Weller, M. Glioblastoma. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2016; Volume 134, pp. 381–397. [Google Scholar] [CrossRef]
- Khandwala, K.; Mubarak, F.; Minhas, K. The many faces of glioblastoma: Pictorial review of atypical imaging features. Neuroradiol. J. 2021, 34, 33–41. [Google Scholar] [CrossRef]
- Galbraith, K.; Snuderl, M. Molecular Pathology of Gliomas. Surg. Pathol. Clin. 2021, 14, 379–386. [Google Scholar] [CrossRef]
- Martin, D.H.; Bianchi, E.; Ben Mustapha, S.; Frères, P. Le glioblastome [Glioblastoma]. Rev. Med. Liege 2021, 76, 419–424. [Google Scholar] [PubMed]
- González, A.; Pérez, J.; Hernández, M. Aspectos inmunohistoquímicos en glioblastomas. Neurol. Y Neurocir. 2017, 38, 287–295. [Google Scholar]
- Melhem, J.M.; Detsky, J.; Lim-Fat, M.J.; Perry, J.R. Updates in IDH-Wildtype Glioblastoma. Neurotherapeutics 2022, 19, 1705–1723. [Google Scholar] [CrossRef]
- Lan, Z.; Li, X.; Zhang, X. Glioblastoma: An Update in Pathology, Molecular Mechanisms and Biomarkers. Int. J. Mol. Sci. 2024, 25, 3040. [Google Scholar] [CrossRef]
- Butler, M.; Pongor, L.; Su, Y.T.; Xi, L.; Raffeld, M.; Quezado, M.; Trepel, J.; Aldape, K.; Pommier, Y.; Wu, J. MGMT Status as a Clinical Biomarker in Glioblastoma. Trends Cancer 2020, 6, 380–391. [Google Scholar] [CrossRef]
- Tomar, M.S.; Kumar, A.; Srivastava, C.; Shrivastava, A. Elucidating the mechanisms of Temozolomide resistance in gliomas and the strategies to overcome the resistance. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188616. [Google Scholar] [CrossRef]
- Senhaji, N.; Squalli Houssaini, A.; Lamrabet, S.; Louati, S.; Bennis, S. Molecular and Circulating Biomarkers in Patients with Glioblastoma. Int. J. Mol. Sci. 2022, 23, 7474. [Google Scholar] [CrossRef]
- Kanderi, T.; Munakomi, S.; Gupta, V. Glioblastoma Multiforme. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Nikanjam, M.; Kato, S.; Kurzrock, R. Liquid biopsy: Current technology and clinical applications. J. Hematol. Oncol. 2022, 15, 131. [Google Scholar] [CrossRef] [PubMed]
- Poulet, G.; Massias, J.; Taly, V. Liquid Biopsy: General Concepts. Acta Cytol. 2019, 63, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Ronvaux, L.; Riva, M.; Coosemans, A.; Herzog, M.; Rommelaere, G.; Donis, N.; D’Hondt, L.; Douxfils, J. Liquid Biopsy in Glioblastoma. Cancers 2022, 14, 3394. [Google Scholar] [CrossRef] [PubMed]
- Seyhan, A.A. Circulating Liquid Biopsy Biomarkers in Glioblastoma: Advances and Challenges. Int. J. Mol. Sci. 2024, 25, 7974. [Google Scholar] [CrossRef]
- Saenz-Antoñanzas, A.; Auzmendi-Iriarte, J.; Carrasco-Garcia, E.; Moreno-Cugnon, L.; Ruiz, I.; Villanua, J.; Egaña, L.; Otaegui, D.; Samprón, N.; Matheu, A. Liquid Biopsy in Glioblastoma: Opportunities, Applications and Challenges. Cancers 2019, 11, 950. [Google Scholar] [CrossRef]
- Shenoy, G.; Mrowczynski, O.; Rizk, E.; Zacharia, B.; Connor, J. Tumor-Derived Biomarkers in Liquid Biopsy of Glioblastoma. World Neurosurg. 2023, 170, 182–194. [Google Scholar] [CrossRef]
- Gatto, L.; Franceschi, E.; Di Nunno, V.; Tosoni, A.; Lodi, R.; Brandes, A.A. Liquid Biopsy in Glioblastoma Management: From Current Research to Future Perspectives. Oncologist 2021, 26, 865–878. [Google Scholar] [CrossRef]
- Eibl, R.H.; Schneemann, M. Liquid biopsy and glioblastoma. Explor. Target Antitumor Ther. 2023, 4, 28–41. [Google Scholar] [CrossRef]
- Batash, R.; Asna, N.; Schaffer, P.; Francis, N.; Schaffer, M. Glioblastoma Multiforme, Diagnosis and Treatment; Recent Literature Review. Curr. Med. Chem. 2017, 24, 3002–3009. [Google Scholar] [CrossRef]
- Kishwar Jafri, S.K.; Bukhari, S.S.; Shamim, S.M. Role of surgery in multifocal glioblastoma. J. Pak. Med. Assoc. 2022, 72, 576–578. [Google Scholar] [CrossRef]
- Conti Nibali, M.; Gay, L.G.; Sciortino, T.; Rossi, M.; Caroli, M.; Bello, L.; Riva, M. Surgery for Glioblastoma in Elderly Patients. Neurosurg. Clin. N. Am. 2021, 32, 137–148. [Google Scholar] [CrossRef]
- Minniti, G.; Niyazi, M.; Alongi, F.; Navarria, P.; Belka, C. Current status and recent advances in reirradiation of glioblastoma. Radiat. Oncol. 2021, 16, 36. [Google Scholar] [CrossRef]
- Wolbers, J.G. Novel strategies in glioblastoma surgery aim at safe, supra-maximum resection in conjunction with local therapies. Chin. J. Cancer 2014, 33, 8–15. [Google Scholar] [CrossRef]
- Nelson, T.A.; de Groot, J. Investigational treatment strategies in glioblastoma: Progress made and barriers to success. Expert Opin. Investig. Drugs 2023, 32, 921–930. [Google Scholar] [CrossRef]
- Weathers, S.P.; de Groot, J. VEGF Manipulation in Glioblastoma. Oncology 2015, 29, 720–727. [Google Scholar]
- Alifieris, C.; Trafalis, D.T. Glioblastoma multiforme: Pathogenesis and treatment. Pharmacol. Ther. 2015, 152, 63–82. [Google Scholar] [CrossRef]
- Herrlinger, U.; Tzaridis, T.; Mack, F.; Steinbach, J.P.; Schlegel, U.; Sabel, M.; Wick, W.; Meisner, C.; Platten, M.; Weller, M.; et al. Lomustine-temozolomide combination therapy versus standard temozolomide. N. Engl. J. Med. 2016, 374, 1530–1540. [Google Scholar] [CrossRef]
- Wang, F.; Huang, Q.; Su, H.; Sun, M.; Wang, Z.; Chen, Z.; Li, J.; Zhang, Y.; He, H.; Xu, J.; et al. Self-assembling paclitaxel-mediated stimulation of tumor-associated macrophages for postoperative treatment of glioblastoma. Proc. Natl. Acad. Sci. USA 2023, 120, e2204621120. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Qi, S.; Zhang, H.; Li, Z.; Wang, K.; Zhu, T.; Yang, Y.; Liu, X.; Liu, J.; Chen, L.; et al. Albumin-bound paclitaxel augment temozolomide treatment sensitivity of glioblastoma cells by disrupting DNA damage repair and promoting ferroptosis. J. Exp. Clin. Cancer Res. 2023, 42, 285. [Google Scholar] [PubMed]
- Green, A.L.; Ramkissoon, S.H.; McCauley, D.; Jones, K.; Perry, J.A.; Hsu, J.H.; Wang, Y.; Platten, M.; Wick, W.; Phillips, H.S.; et al. Preclinical antitumor efficacy of selective exportin 1 inhibitors in glioblastoma. Neuro Oncol. 2015, 17, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Lassman, A.B.; Wen, P.Y.; van den Bent, M.J.; Plotkin, S.R.; Walenkamp, A.M.E.; Green, A.L.; Ramkissoon, S.H.; Perry, J.A.; Hsu, J.H.; Phillips, H.S.; et al. A phase II study of the efficacy and safety of oral selinexor in recurrent glioblastoma. Clin. Cancer Res. 2022, 28, 452–460. [Google Scholar] [CrossRef]
- Okada, M.; Miyake, K.; Tamiya, T. Glioblastoma Treatment in the Elderly. Neurol. Med. Chir. 2017, 57, 667–676. [Google Scholar] [CrossRef]
- Zhang, Y.D.; Dai, R.Y.; Chen, Z.; Zhang, Y.H.; He, X.Z.; Zhou, J. Efficacy and safety of carmustine wafers in the treatment of rochaglioblastoma multiforme: A systematic review. Turk. Neurosurg. 2014, 24, 639–645. [Google Scholar]
- Walker, B.C.; Mittal, S. Antitumor Activity of Curcumin in Glioblastoma. Int. J. Mol. Sci. 2020, 21, 9435. [Google Scholar] [CrossRef]
- Zoi, V.; Galani, V.; Tsekeris, P.; Kyritsis, A.P.; Alexiou, G.A. Radiosensitization and Radioprotection by Curcumin in Glioblastoma and Other Cancers. Biomedicines 2022, 10, 312. [Google Scholar] [CrossRef]
- Bassi, R.; Dei Cas, M.; Tringali, C.; Compostella, F.; Paroni, R.; Giussani, P. Ceramide Is Involved in Temozolomide Resistance in Human Glioblastoma U87MG Overexpressing EGFR. Int. J. Mol. Sci. 2023, 24, 15394. [Google Scholar] [CrossRef]
- Giussani, P.; Brioschi, L.; Gjoni, E.; Riccitelli, E.; Viani, P. Sphingosine 1-Phosphate Stimulates ER to Golgi Ceramide Traffic to Promote Survival in T98G Glioma Cells. Int. J. Mol. Sci. 2024, 25, 8270. [Google Scholar] [CrossRef] [PubMed]
- Giussani, P.; Brioschi, L.; Bassi, R.; Riboni, L.; Viani, P. Phosphatidylinositol 3-Kinase/AKT Pathway Regulates the Endoplasmic Reticulum to Golgi Traffic of Ceramide in Glioma Cells. J. Biol. Chem. 2009, 284, 5088–5096. [Google Scholar] [CrossRef] [PubMed]
- Hau, E.; Shen, H.; Clark, C.; Graham, P.H.; Koh, E.S.; McDonald, K.L. The evolving roles and controversies of radiotherapy in the treatment of glioblastoma. J. Med. Radiat. Sci. 2016, 63, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Rocha Pinheiro, S.L.L.; Lemos, F.F.B.; Marques, H.S.S.; Silva Luz, M.; de Oliveira Silva, L.G.; Faria Souza Mendes Dos Santos, C.; da Silva, R.L.; Rocha-Antunes, C.; Pereira-Filho, G.A.A.; da Silva, N.S.; et al. Immunotherapy in glioblastoma treatment: Current state and future prospects. World J. Clin. Oncol. 2023, 14, 138–159. [Google Scholar] [CrossRef]
- Huang, P.; Li, L.; Qiao, J.; Li, X.; Zhang, P. Radiotherapy for glioblastoma in the elderly: A protocol for systematic review and meta-analysis. Medicine 2020, 99, e23890. [Google Scholar] [CrossRef]
- Berthold, D.; Carrasco, A.P.; Uhl, E.; Müller, H.; Dumitrascu, R.; Sibelius, U.; Böhme, M.; Neumann, H.; Schneider, F.; Heßler, V.; et al. Palliative care of older glioblastoma patients in neurosurgery. J. Neurooncol. 2022, 157, 297–305. [Google Scholar] [CrossRef]
- Rivoirard, R.; Vallard, A.; Boutet, C.; Falk, A.T.; Garin, C.; Adjabi, A.; Jouvet, A.; Delmas, P.; Cantaloube, G.; Larroque, B.; et al. A retrospective survey of the last 3 months of life in patients carrying glioblastoma: Clinical treatments and profiles. Mol. Clin. Oncol. 2018, 8, 115–120. [Google Scholar] [CrossRef]
- Okon, I.I.; Osama, M.; Akpan, A.; Fabrini Paleare, L.F.; Ferreira, M.Y.; Shafqat, M.D.; Javed, F.; Khan, O.; Ahmad, Z.; Hussain, S.; et al. The evolving role of palliative care in older people with glioblastoma. World Neurosurg. 2024, 192, 140–149. [Google Scholar] [CrossRef]
- Yalamarty, S.S.K.; Filipczak, N.; Li, X.; Subhan, M.A.; Parveen, F.; Ataide, J.A.; Smith, A.J.; Chen, Q.; Patel, R.; Zhao, W.; et al. Mechanisms of resistance and current treatment options for glioblastoma multiforme (GBM). Cancers 2023, 15, 2116. [Google Scholar] [CrossRef] [PubMed]
- Steindorf, K.; Depenbusch, J.; Haussmann, A.; Tsiouris, A.; Schmidt, L.; Hermann, S.; Sieverding, M.; Wiskemann, J.; Ungar, N. Change patterns and determinants of physical activity differ between breast, prostate, and colorectal cancer patients. Support. Care Cancer 2020, 28, 3207–3221. [Google Scholar] [CrossRef] [PubMed]
- Ayotte, S.L.; Harro, C.C. Effects of an individualized aerobic exercise program in individuals with a brain tumor undergoing inpatient rehabilitation. Rehabil. Oncol. 2017, 35, 163–171. [Google Scholar] [CrossRef]
- Capozzi, L.C.; Boldt, K.R.; Easaw, J.; Bultz, B.; Culos-Reed, S.N. Evaluating a 12-week exercise program for brain cancer patients. Psychooncology 2016, 25, 354–358. [Google Scholar] [CrossRef]
- Gehring, K.; Kloek, C.J.; Aaronson, N.K.; Janssen, K.W.; Jones, L.W.; Sitskoorn, M.M.; Weller, M.; Taphoorn, M.J.B.; Gijtenbeek, J.M.; Klein, M.; et al. Feasibility of a home-based exercise intervention with remote guidance for patients with stable grade II and III gliomas: A pilot randomized controlled trial. Clin. Rehabil. 2018, 32, 352–366. [Google Scholar] [CrossRef]
- Li, X.; Yu, X.; Dai, D.; Song, X.; Xu, W. The altered glucose metabolism in tumor and a tumor acidic microenvironment associated with extracellular matrix metalloproteinase inducer and monocarboxylate transporters. Oncotarget 2016, 7, 23141–23155. [Google Scholar] [CrossRef]
- Montella, L.; Sarno, F.; Altucci, L.; Cioffi, V.; Sigona, L.; Di Colandrea, S.; Capone, M.; D’Alessandro, A.; Liguori, G.; Carafa, V.; et al. A root in synapsis and the other one in the gut microbiome-brain axis: Are the two poles of ketogenic diet enough to challenge glioblastoma? Front. Nutr. 2021, 8, 703392. [Google Scholar] [CrossRef]
- Abdelwahab, M.G.; Fenton, K.E.; Preul, M.C.; Rho, J.M.; Lynch, A.; Stafford, P.; Eberhart, C.G.; Gilbert, M.R.; Shaw, E.G.; Sherman, J.H.; et al. The ketogenic diet is an effective adjuvant to radiation therapy for the treatment of malignant glioma. PLoS ONE 2012, 7, e36197. [Google Scholar] [CrossRef]
- Sperry, J.; Condro, M.C.; Guo, L.; Braas, D.; Vanderveer-Harris, N.; Kim, K.K.O.; Amin, S.B.; Mellinghoff, I.K.; de Groot, J.F.; Kunos, C.; et al. Glioblastoma utilizes fatty acids and ketone bodies for growth allowing progression during ketogenic diet therapy. iScience 2020, 23, 101453. [Google Scholar] [CrossRef] [PubMed]
- De Feyter, H.M.; Behar, K.L.; Rao, J.U.; Madden-Hennessey, K.; Ip, K.L.; Hyder, F.; Rothman, D.L.; Petroff, O.A.; de Graaf, R.; Bhattacharya, P.; et al. A ketogenic diet increases transport and oxidation of ketone bodies in RG2 and 9L gliomas without affecting tumor growth. Neuro Oncol. 2016, 18, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Rieger, J.; Bahr, O.; Maurer, G.D.; Hattingen, E.; Franz, K.; Brucker, D.; Kluge, A.; Schnell, O.; Tonn, J.C.; Wick, W.; et al. ERGO: A pilot study of ketogenic diet in recurrent glioblastoma. Int. J. Oncol. 2014, 44, 1843–1852. [Google Scholar] [CrossRef] [PubMed]
- Ghiaseddin, A.P.; Shin, D.; Melnick, K.; Tran, D.; Tam, H.H.; Bosnyak, C.; de Groot, J.F.; Chang, S.M.; Ma, Q.; Clarke, J.L.; et al. Tumor Treating Fields in the management of patients with malignant gliomas. Curr. Treat. Options Oncol. 2020, 21, 76. [Google Scholar] [CrossRef]
- Fabian, D.; Dong, D.; Fujii, T.; Glicksman, M.A.; Landry, J.C.; Shim, H.; Andrews, D.W.; Ly, M.; Judeh, A.S.; Claxton, J.; et al. Treatment of glioblastoma (GBM) with the addition of tumor-treating fields (TTF): A review. Cancers 2019, 11, 174. [Google Scholar] [CrossRef]
- Song, A.; Bar-Ad, V.; Martinez, N.; Glass, J.; Andrews, D.W.; Judy, K.; Evans, J.J.; Farrell, C.J.; Werner-Wasik, M.; Chervoneva, I.; et al. Initial Experience with Scalp Sparing Radiation with Concurrent Temozolomide and Tumor Treatment Fields (SPARE) for Patients with Newly Diagnosed Glioblastoma. J. Neurooncol. 2020, 147, 653–661. [Google Scholar] [CrossRef]
- Bagley, S.J.; Logun, M.; Fraietta, J.A.; Wang, X.; Desai, A.S.; Bagley, L.J.; Nabavizadeh, A.; Jarocha, D.; Martins, R.; Maloney, E.; et al. Intrathecal Bivalent CAR T Cells Targeting EGFR and IL13Rα2 in Recurrent Glioblastoma: Phase 1 Trial Interim Results. Nat. Med. 2024, 30, 1320–1329. [Google Scholar] [CrossRef]
- Brown, C.E.; Alizadeh, D.; Starr, R.; Weng, L.; Wagner, J.R.; Naranjo, A.; Ostberg, J.R.; Blanchard, M.S.; Kilpatrick, J.; Simpson, J.; et al. Regression of Glioblastoma after Chimeric Antigen Receptor T-Cell Therapy. N. Engl. J. Med. 2016, 375, 2561–2569. [Google Scholar] [CrossRef]
- Blanchard, R.; Adjei, I. Engineering the glioblastoma microenvironment with bioactive nanoparticles for effective immunotherapy. RSC Adv. 2023, 13, 31411–31425. [Google Scholar] [CrossRef]
- Wei, R.; Li, J.; Lin, W.; Pang, X.; Yang, H.; Lai, S.; Zhang, Y.; Wang, J.; Liu, X.; Shen, M.; et al. Nanoparticle-mediated blockade of CXCL12/CXCR4 signaling enhances glioblastoma immunotherapy: Monitoring early responses with MRI radiomics. Acta Biomater. 2024, 177, 414–430. [Google Scholar] [CrossRef]
- Chen, S.F.; Kau, M.; Wang, Y.C.; Chen, M.H.; Tung, F.I.; Chen, M.H.; Chang, S.J.; Wu, S.C.; Lin, Y.S.; Lee, T.C.; et al. Synergistically enhancing immunotherapy efficacy in glioblastoma with gold-core silica-shell nanoparticles and radiation. Int. J. Nanomed. 2023, 18, 7677–7693. [Google Scholar] [CrossRef]
- Agosti, E.; Zeppieri, M.; De Maria, L.; Tedeschi, C.; Fontanella, M.M.; Panciani, P.P.; Bello, L.; Giombini, S.; Cirillo, S.; Curtò, L.; et al. Glioblastoma immunotherapy: A systematic review of the present strategies and prospects for advancements. Int. J. Mol. Sci. 2023, 24, 15037. [Google Scholar] [CrossRef]
- Liau, L.M.; Ashkan, K.; Brem, S.; Campian, J.L.; Trusheim, J.E.; Iwamoto, F.M.; Tran, D.D.; Ansstas, G.; Cobbs, C.S.; Heth, J.A.; et al. Association of Autologous Tumor Lysate-Loaded Dendritic Cell Vaccination with Extension of Survival among Patients with Newly Diagnosed and Recurrent Glioblastoma. JAMA Oncol. 2022, 9, 112–121. [Google Scholar] [CrossRef]
- Rong, L.; Li, N.; Zhang, Z. Emerging therapies for glioblastoma: Current state and future directions. J. Exp. Clin. Cancer Res. 2022, 41, 142. [Google Scholar] [CrossRef]
- Arrieta, V.A.; Dmello, C.; McGrail, D.J.; Brat, D.J.; Lee-Chang, C.; Heimberger, A.B.; Ahmed, A.U.; Rostomily, R.C.; Giles, A.J.; Rodriguez, F.J.; et al. Immune checkpoint blockade in glioblastoma: From tumor heterogeneity to personalized treatment. J. Clin. Investig. 2023, 133, e163447. [Google Scholar] [CrossRef] [PubMed]
- Ueda, Y.; Usuki, K.; Fujita, J.; Matsumura, I.; Aotsuka, N.; Sekiguchi, N.; Suzuki, N.; Kondo, T.; Kobayashi, T.; Saito, M.; et al. Phase 1/2 study evaluating the safety and efficacy of DSP-7888 dosing emulsion in myelodysplastic syndromes. Cancer Sci. 2022, 113, 1377–1388. [Google Scholar] [CrossRef]
- Luksik, A.S.; Yazigi, E.; Shah, P.; Jackson, C.M. CAR T cell therapy in glioblastoma: Overcoming challenges related to antigen expression. Cancers 2023, 15, 1414. [Google Scholar] [CrossRef] [PubMed]
- Montoya, M.; Gallus, M.; Phyu, S.; Haegelin, J.; De Groot, J.; Okada, H. A roadmap of CAR-T-cell therapy in glioblastoma: Challenges and future perspectives. Cells 2024, 13, 726. [Google Scholar] [CrossRef] [PubMed]
- Abadi, B.; Akbari, M.; Sharifnia, Z.; Moradi, A.; Torabi, N.; Ganjalikhani-Hakemi, M.; Hojjat-Farsangi, M.; Ghaderi, A.; Hashemzadeh, S.; Zeinali, S.; et al. Stem cell-based therapy treating glioblastoma multiforme. Hematol. Oncol. Stem Cell Ther. 2021, 14, 1–15. [Google Scholar] [CrossRef]
- Herrera-Perez, M.; Voytik-Harbin, S.L.; Rickus, J.L. Extracellular matrix properties regulate the migratory response of glioblastoma stem cells in three-dimensional culture. Tissue Eng. Part A 2015, 21, 2572–2582. [Google Scholar] [CrossRef] [PubMed]
- Miska, J.; Lesniak, M.S. Neural stem cell carriers for the treatment of glioblastoma multiforme. EBioMedicine 2015, 2, 774–775. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Dai, Q.; Morshed, R.; Auffinger, B.; Han, Y.; Shlyakhtina, Y.; Sontheimer, H.; Snyder, E.; Harper, J.; Duckworth, E.; et al. Nanoparticle-programmed self-destructive neural stem cells for glioblastoma targeting and therapy. Small 2013, 9, 4123–4129. [Google Scholar] [CrossRef] [PubMed]
- Mooney, R.; Roma, L.; Zhao, D.; Van Haute, D.; Garcia, E.; Kim, S.U.; Monfils, A.; Lee, J.; Wang, J.; Shah, K.; et al. Neural stem cell-mediated intratumoral delivery of gold nanorods improves photothermal therapy. ACS Nano. 2014, 8, 12450–12460. [Google Scholar] [CrossRef]
- Bryukhovetskiy, I.S.; Shevchenko, V.E.; Khotimchenko, Y.S.; Baklaushev, V.P. Hematopoietic stem cells as a tool for the treatment of glioblastoma multiforme. Mol. Med. Rep. 2016, 14, 4511–4520. [Google Scholar] [CrossRef]
- Pacioni, S.; D’Alessandris, Q.G.; Giannetti, S.; Morgante, L.; Coccè, V.; Bonomi, A.; De Bonis, P.; Salvati, M.; Pallini, R.; Larocca, L.M.; et al. Human mesenchymal stromal cells inhibit tumor growth in orthotopic glioblastoma xenografts. Stem Cell Res. Ther. 2017, 8, 53. [Google Scholar] [CrossRef]
- Gujar, S.; Pol, J.; Kroemer, G.; Dammeijer, F.; Redondo-Pedraza, J.; Galluzzi, L. SnapShot: Cancer Immunotherapy with Oncolytic Viruses. Cell 2019, 176, 1240.e1. [Google Scholar] [CrossRef]
- Hamad, A.; Myles, R.; Menard, L.; Keating, A. Recent developments in glioblastoma therapy: Oncolytic viruses and emerging future strategies. Viruses 2023, 15, 547. [Google Scholar] [CrossRef]
- Ahmed, A.U.; Tyler, M.A.; Thaci, B.; Alexiades, N.G.; Han, Y.; Ulasov, I.V.; Lesniak, M.S.; Pierce, S.A.; Lowenstein, P.R.; Castro, M.G.; et al. A preclinical evaluation of neural stem cell-based cell carrier for targeted antiglioma oncolytic virotherapy. J. Natl. Cancer Inst. 2013, 105, 968–977. [Google Scholar] [CrossRef]
- Geletneky, K.; Hajda, J.; Angelova, A.L.; Leuchs, B.; Capper, D.; Bartsch, A.J.; Neumann, J.-O.; Schöning, T.; Hüsing, J.; Beelte, B.; et al. Oncolytic H-1 Parvovirus Shows Safety and Signs of Immunogenic Activity in a First Phase I/IIa Glioblastoma Trial. Mol. Ther. 2017, 25, 2620–2634. [Google Scholar] [CrossRef]

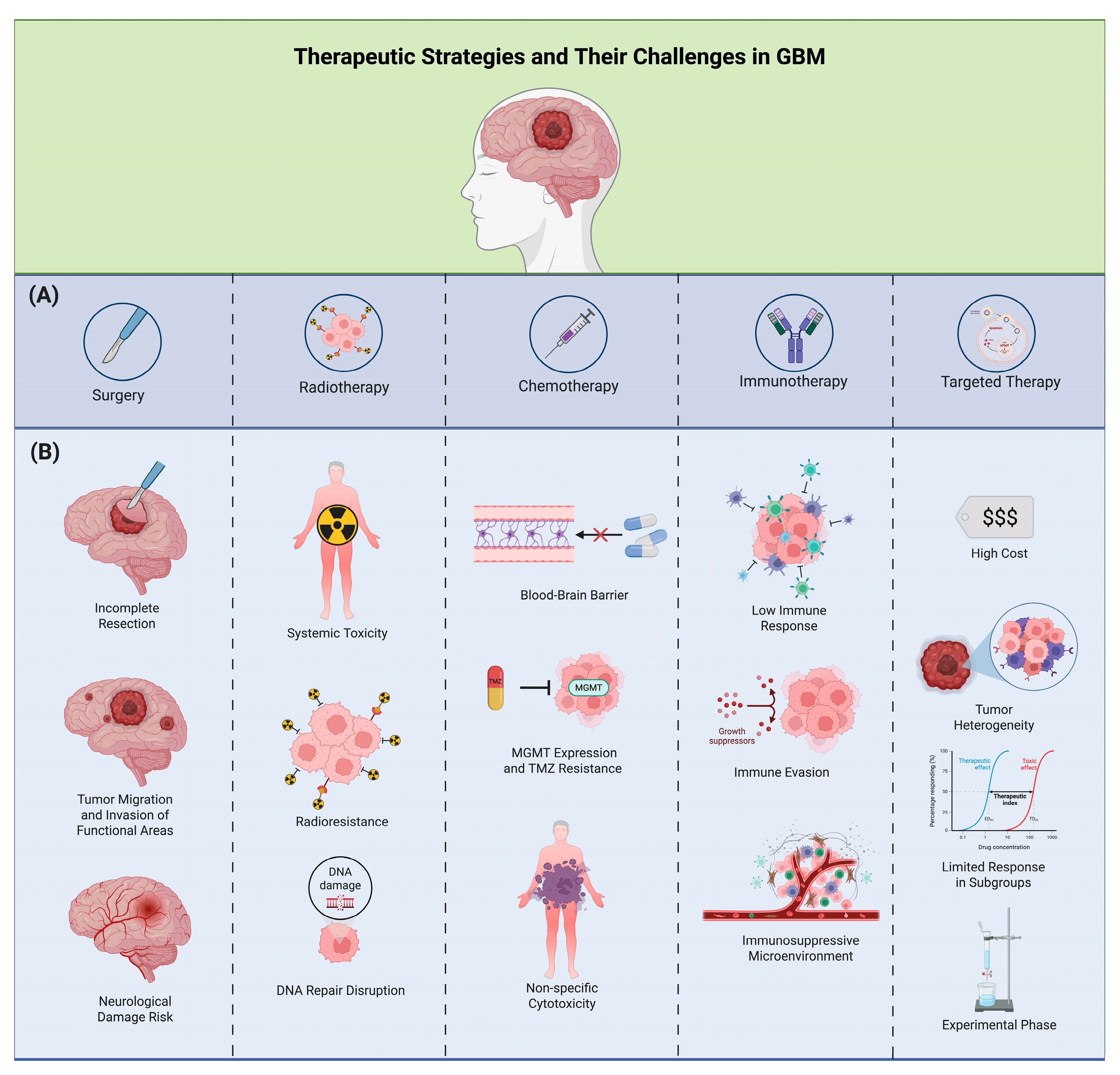
| Treatment | Outcomes | References |
|---|---|---|
| Conventional treatment | ||
| Surgical resection | The Stupp Protocol (maximal resection plus radiotherapy) remains the standard, with better outcomes when extensive resection is possible. Fluorescence-guided surgery with 5-ALA increases complete resection rates to about 65%. | Batash et al., 2017; Kishwar Jafri et al., 2022. [100,101]. |
| TMZ | Concurrent TMZ and RT increase 2-year survival to 27.2% versus 10.9% with RT alone. TMZ with chemotherapy further improves survival. | Alifieris et al., 2015, Schaff et al., 2023, Herrlinger et al., 2019. [77,107,108]. |
| PTX | Targets proliferating cells and can trigger a macrophage-mediated immune response against recurrent GBM via CD47 blockade. Albumin-bound PTX combined with TMZ shows a synergistic effect, suppressing GBM progression and improving survival in experimental models. | Wang et al., 2022; Qu et al., 2023. [56,110]. |
| XPO1 | XPO1 exports tumor suppressor proteins like Rb1, p27, and P53 from the nucleus. Selinexor, a selective XPO1 inhibitor, has shown efficacy in preclinical GBM models by promoting apoptosis. | Green et al., 2015; Lassman et al., 2022. [111,112]. |
| Carmustine wafers | Biodegradable implants that may extend GBM survival, with one study reporting an increase in overall survival from 5.5 to 8.7 months. While a study found improved survival without significant adverse effects, further studies are needed. | Okada et al., 2017; Zhang et al., 2014. [113,114]. |
| Curcumin | Curcumin has anti-inflammatory and antitumor properties. It reduces GBM cell growth, promotes apoptosis, and enhances the effects of RT and chemotherapy. | Walker et al., 2020; Zoi et al., 2022. [115,116]. |
| Ceramide | Ceramides induce apoptosis, while S1P promotes tumor aggressiveness. An imbalance favoring S1P, regulated by enzymes SPHK1 and SGPP2, is linked to higher tumor grade. Inhibiting SPHK1 and increasing ceramide levels can reduce angiogenesis and improve response to TMZ. | Karmelic, 2024; Zaibaq, 2024; Abuhusain 2013; Tea 2020. [70,71,72,73]. |
| RT | RT has been a GBM standard, typically delivered in 2 Gy fractions over 6 weeks (total 60 Gy). Its role in recurrent gliomas is mainly palliative, with limitations based on prior RT, tumor location, dose limits, and patient functional status. | Hau et al., 2016; Rocha et al., 2023; Huang et al., 2020. [120,121,122]. |
| Non-pharmacological treatment | ||
| Palliative care | Palliative care is crucial, but its timing varies due to many external and internal factors. Physical activity, though challenging due to symptoms like fatigue, may improve quality of life and mood in early stages, but evidence is limited, and its application is difficult as the disease progresses. | Berthold, D. 2022; Rivoirard, R. 2018; Okon II, 2024; Ayotte, 2017, Capozzi 2016; Gehring K, 2018. [123,124,125,126,127,128,129,130,131,132,133]. |
| Diet | The ketogenic diet reduces glucose, forcing the brain to use ketones, which tumor cells cannot efficiently metabolize. Animal studies suggest this diet may enhance the effects of RT by reducing tumor angiogenesis. However, evidence on its benefits in GBM patients is contradictory, with some studies questioning its impact on prognosis or suggesting it could be ineffective. | Li X, 2016; Montella L, 2021; Abdelwahab MG, 2012. [131,132,133]. |
| Future perspectives: immunological therapy stem cells and virology | ||
| Optune | Interferes with mitosis by destabilizing the plasma membrane, breaking chromosomes, and causing apoptosis, as well as disrupting septin fibers and inducing autophagy. Although promising, further research is needed to understand its effects on other cellular proteins in both cancerous and normal cells. | Davis et al., 2016. [4]. |
| Immune checkpoint inhibitors | ICIs show limited efficacy in GBM due to its immunosuppressive microenvironment. The CheckMate 143 trial found no overall survival benefit of nivolumab (anti-PD-1) compared to bevacizumab in recurrent GBM. | Ng Andrew et al., 2024. [59]. |
| Peptide vaccines | Vaccines like Rindopepimut (targeting EGFRvIII) and DSP-788 (targeting WT1) have been developed for GBM. Although phase III trials showed slight improvements in progression-free survival or median overall survival, neither vaccine demonstrated a significant survival benefit compared to controls. | Rong, et al, 2022; Ueda et al, 2022. [147,149]. |
| Dendritic cell vaccines | These vaccines activate T cells to trigger an immune response against GBM. Patients treated with these vaccines showed a median overall survival of 19.3 months versus 16.5 months in controls. | Rocha et al., 2023. [121]. |
| CAR-T therapy (chimeric antigen receptor T cells) | Uses modified T cells to target GBM antigens like IL-13Ra2, HER2, and EGFRvIII. Early-phase trials show promising results, but a lack of phase III studies means more research is needed to confirm its efficacy. | Rocha et al., 2023; Luksik et al., 2023; Montoya et al., 2024. [121,150,151]. |
| Stem cells | GBM’s hypoxic environment supports stem cell survival and function, while stem cells can cross the BBB, enabling targeted drug delivery directly to the tumor. Studies in animal models show stem cells loaded with chemotherapy or nanoparticles can improve survival and locally destroy tumors. However, interactions between physiological stem cells and glioma-specific CSCs are complex; NSCs may transform into CSCs and aid tumor growth, while HSCs show less risk of transformation, suggesting they could offer safer therapeutic options. | Abadi, 2021; Herrera-Perez, 2015, Miska, 2015; Bryukhovetskiy, 2016; Pacioni, 2017. [152,153,154,157,158]. |
| Virology | Oncolytic viruses selectively infect and kill GBM cells while stimulating a targeted immune response. These viruses trigger cancer cell death through tumor antigens and immune signals, with stem cells acting as delivery vehicles that cross the BBB and control viral action. This combined approach shows great promise as a potentially effective GBM treatment. | Gujar, 2019; Hamad, 2023. [159,160]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esparza-Salazar, F.; Murguiondo-Pérez, R.; Cano-Herrera, G.; Bautista-Gonzalez, M.F.; Loza-López, E.C.; Méndez-Vionet, A.; Van-Tienhoven, X.A.; Chumaceiro-Natera, A.; Simental-Aldaba, E.; Ibarra, A. Glioblastoma: A Multidisciplinary Approach to Its Pathophysiology, Treatment, and Innovative Therapeutic Strategies. Biomedicines 2025, 13, 1882. https://doi.org/10.3390/biomedicines13081882
Esparza-Salazar F, Murguiondo-Pérez R, Cano-Herrera G, Bautista-Gonzalez MF, Loza-López EC, Méndez-Vionet A, Van-Tienhoven XA, Chumaceiro-Natera A, Simental-Aldaba E, Ibarra A. Glioblastoma: A Multidisciplinary Approach to Its Pathophysiology, Treatment, and Innovative Therapeutic Strategies. Biomedicines. 2025; 13(8):1882. https://doi.org/10.3390/biomedicines13081882
Chicago/Turabian StyleEsparza-Salazar, Felipe, Renata Murguiondo-Pérez, Gabriela Cano-Herrera, Maria F. Bautista-Gonzalez, Ericka C. Loza-López, Amairani Méndez-Vionet, Ximena A. Van-Tienhoven, Alejandro Chumaceiro-Natera, Emmanuel Simental-Aldaba, and Antonio Ibarra. 2025. "Glioblastoma: A Multidisciplinary Approach to Its Pathophysiology, Treatment, and Innovative Therapeutic Strategies" Biomedicines 13, no. 8: 1882. https://doi.org/10.3390/biomedicines13081882
APA StyleEsparza-Salazar, F., Murguiondo-Pérez, R., Cano-Herrera, G., Bautista-Gonzalez, M. F., Loza-López, E. C., Méndez-Vionet, A., Van-Tienhoven, X. A., Chumaceiro-Natera, A., Simental-Aldaba, E., & Ibarra, A. (2025). Glioblastoma: A Multidisciplinary Approach to Its Pathophysiology, Treatment, and Innovative Therapeutic Strategies. Biomedicines, 13(8), 1882. https://doi.org/10.3390/biomedicines13081882







