The Clinical Spectrum of Acquired Hypomagnesemia: From Etiology to Therapeutic Approaches
Abstract
1. Introduction
Search Methodology
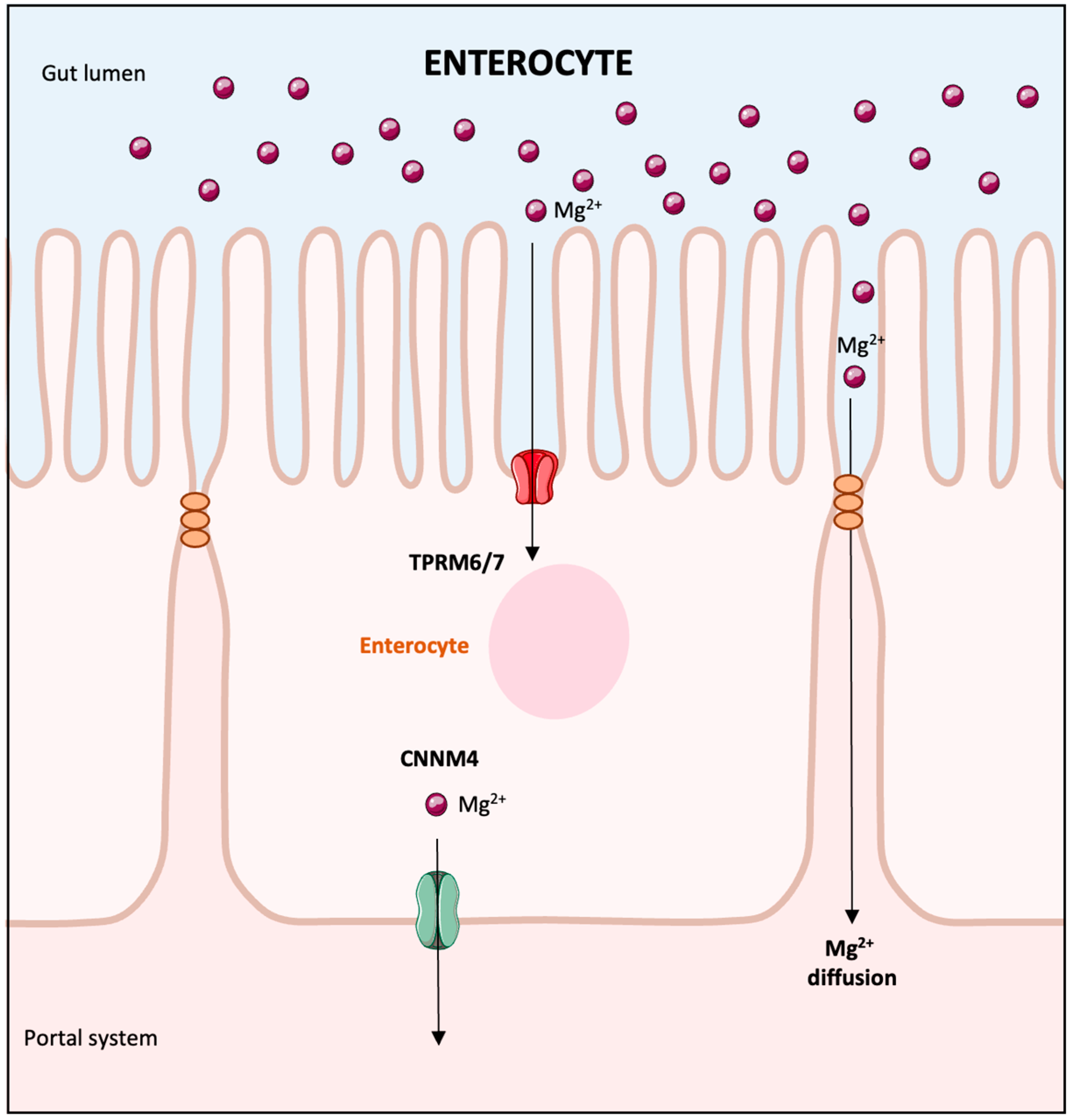
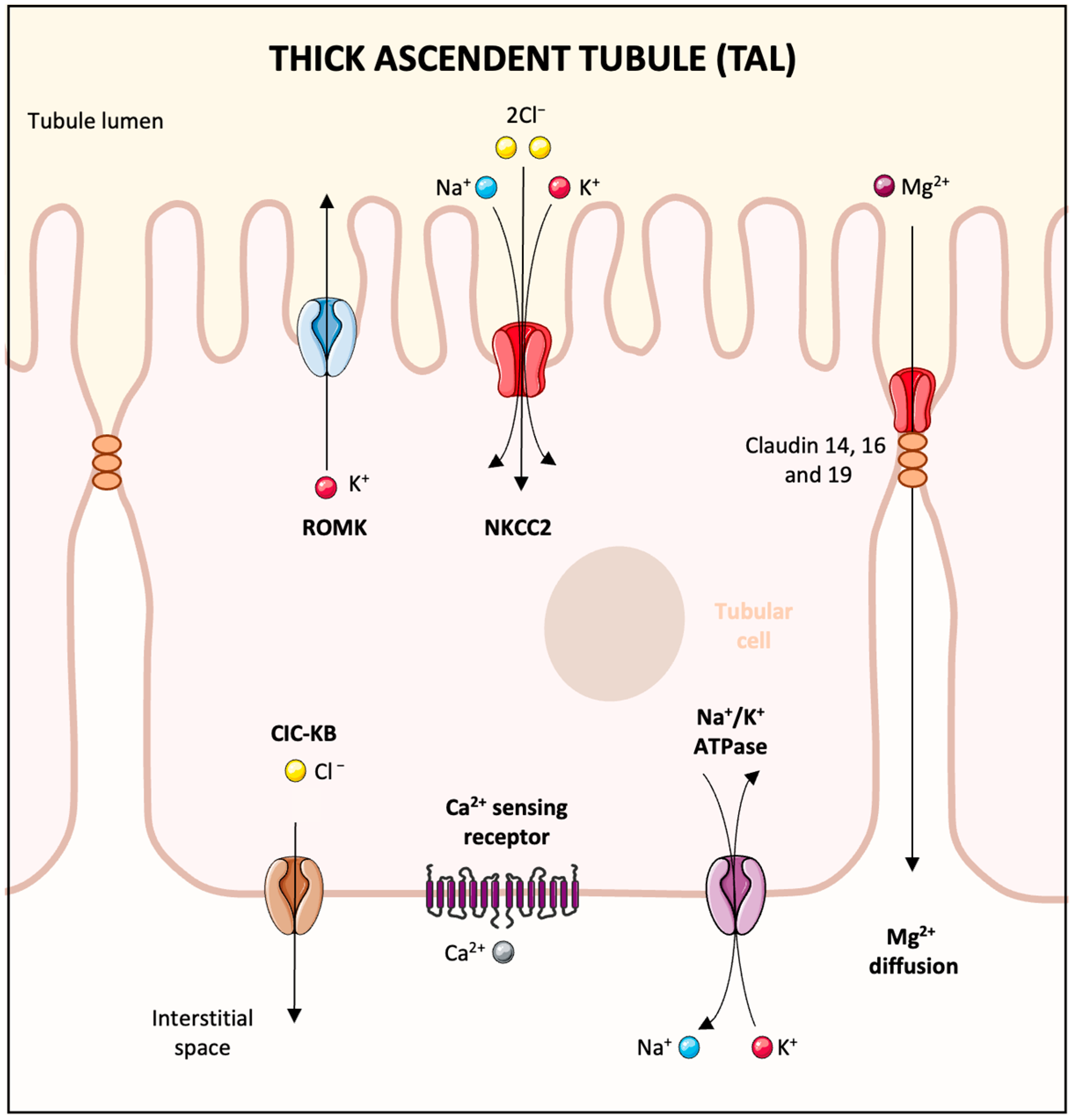
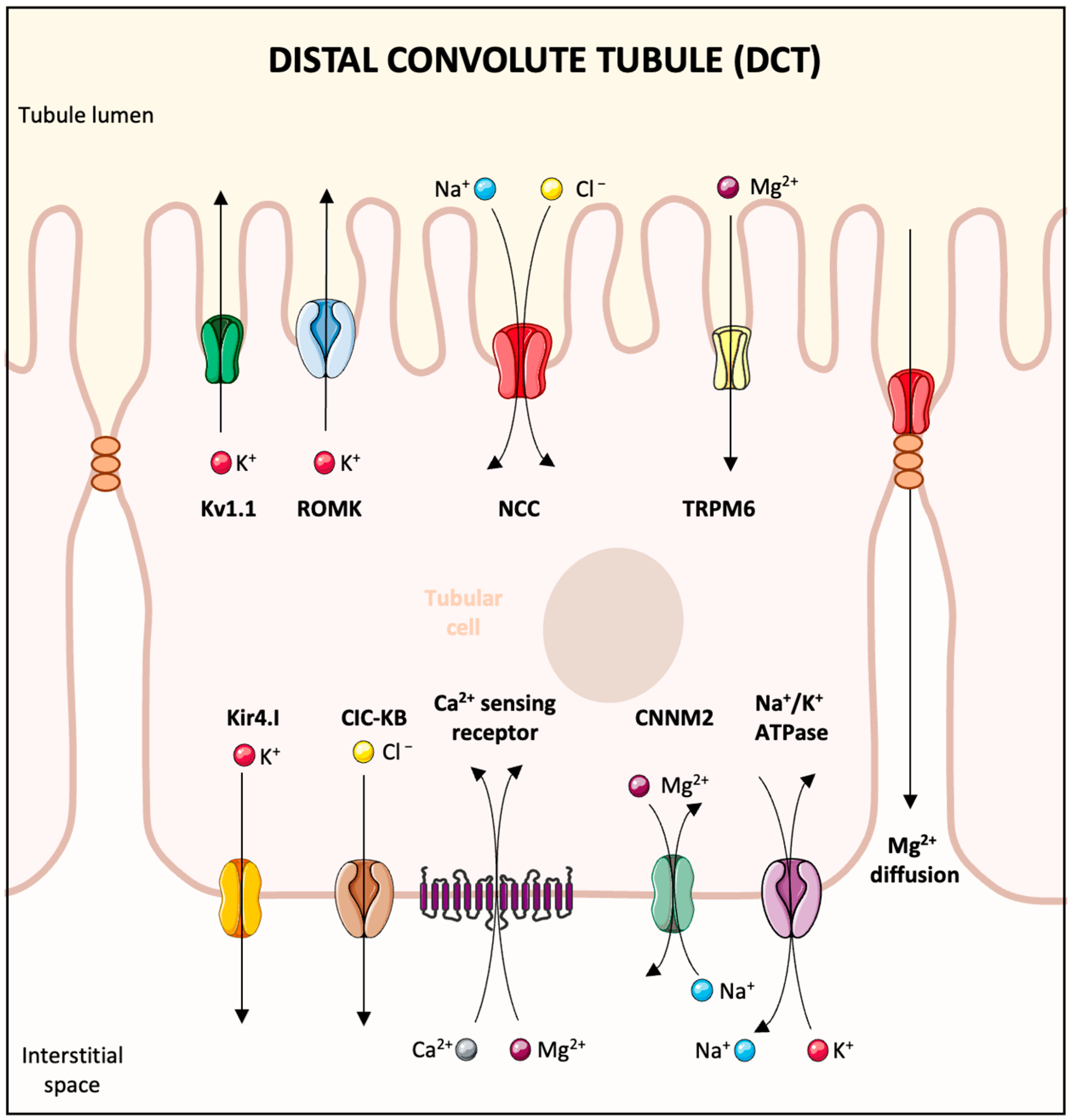
2. Overview of Magnesium Absorption and Renal Handling
3. The Magnesium Role in Physiological Processes
4. Causes of Hypomagnesemia
4.1. Decreased Magnesium Intake
4.2. Increased Digestive Losses
4.3. Increased Urinary Loss
5. Medications
5.1. Diuretics
5.2. Antibiotics
5.2.1. Aminoglycosides
5.2.2. Amphotericin B
5.2.3. Pentamidine
5.3. Calcineurin Inhibitors
5.4. Antineoplastic Drugs
5.4.1. Platinum-Based Compounds
5.4.2. EGF Receptor Antagonist
5.4.3. Other Antineoplastic Drugs
6. Miscellaneous Etiologies
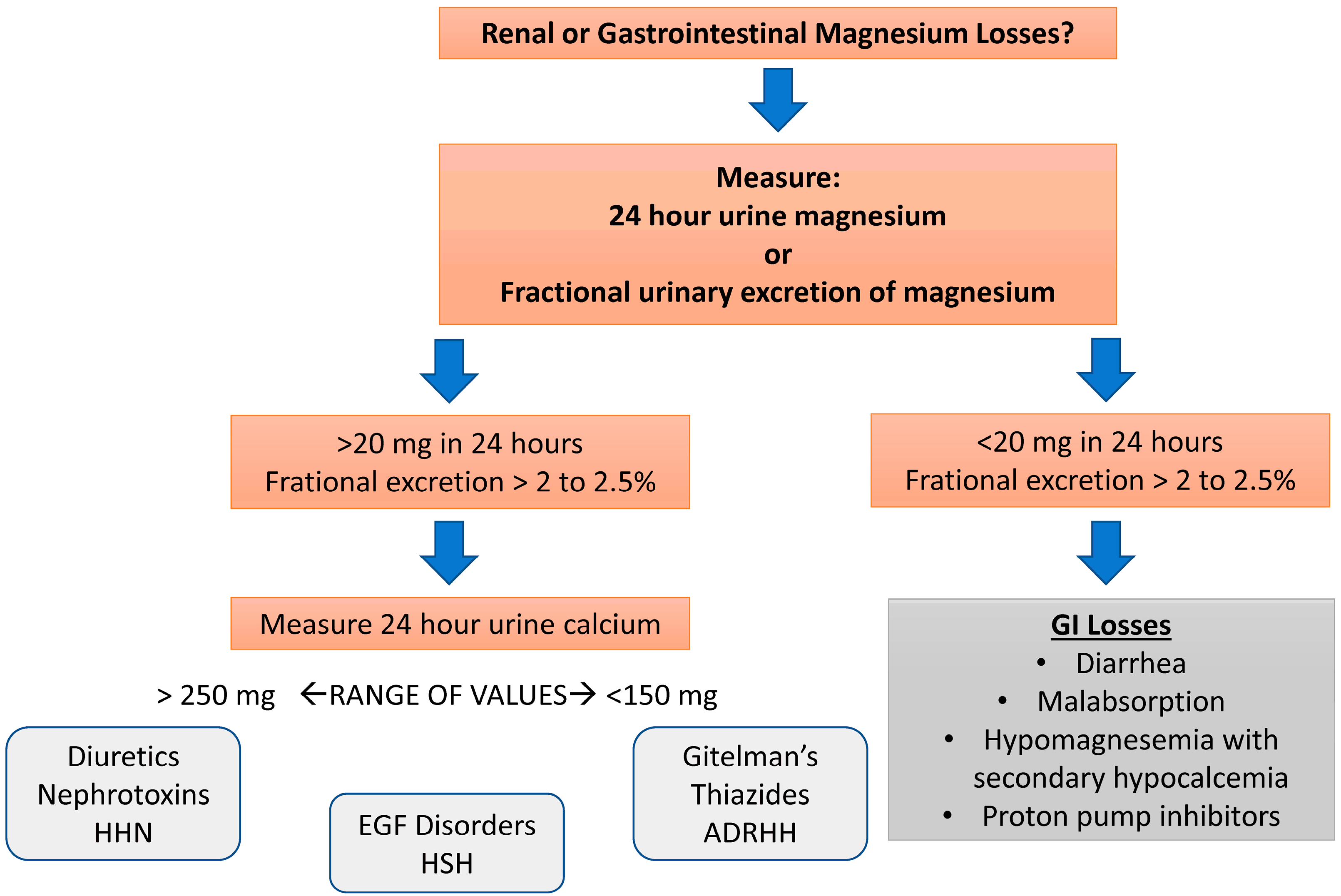
7. Clinical Manifestations and Diagnosis of Magnesium Depletion
Diagnostic Testing for Magnesium Disorders
8. Treatment of Hypomagnesemia (Figure 5)

8.1. Severe Hypomagnesemia
8.2. Mild Hypomagnesemia
8.3. Drugs to Limit Kidney Magnesium Wasting
8.3.1. Amiloride
8.3.2. Sodium-Glucose Cotransporter 2 Inhibitors (SGLT2i)
8.4. Nutritional Management of Hypomagnesemia
9. Research Agenda
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liamis, G.; Rodenburg, E.M.; Hofman, A.; Zietse, R.; Stricker, B.H.; Hoorn, E.J. Electrolyte disorders in community subjects: Prevalence and risk factors. Am. J. Med. 2013, 126, 256–263. [Google Scholar] [CrossRef]
- Santosh Raju, K.; BhaskaraRao, J.V.; Naidu, B.T.K.; Sunil Kumar, N. A Study of Hypomagnesemia in Patients Admitted to the ICU. Cureus 2023, 15, e41949. [Google Scholar] [CrossRef]
- de Baaij, J.H.; Hoenderop, J.G.; Bindels, R.J. Magnesium in man: Implications for health and disease. Physiol. Rev. 2015, 95, 1–46. [Google Scholar] [CrossRef]
- Liamis, G.; Hoorn, E.J.; Florentin, M.; Milionis, H. An overview of diagnosis and management of drug-induced hypomagnesemia. Pharmacol. Res. Perspect. 2021, 9, e00829. [Google Scholar] [CrossRef]
- Rosner, M.H.; Ha, N.; Palmer, B.F.; Perazella, M.A. Acquired Disorders of Hypomagnesemia. Mayo Clin. Proc. 2023, 98, 581–596. [Google Scholar] [CrossRef] [PubMed]
- Tangvoraphonkchai, K.; Davenport, A. Magnesium and Cardiovascular Disease. Adv. Chronic Kidney Dis. 2018, 25, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; He, T.; Wang, M.; Nie, L.; Zhang, Y.; Wang, Y.; Huang, Y.; Feng, B.; Zhang, J.; Zhao, J. Serum magnesium, mortality, and cardiovascular disease in chronic kidney disease and end-stage renal disease patients: A systematic review and meta-analysis. J. Nephrol. 2019, 32, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Arpaci, D.; Tocoglu, A.G.; Ergenc, H.; Korkmaz, S.; Ucar, A.; Tamer, A. Associations of serum Magnesium levels with diabetes mellitus and diabetic complications. Hippokratia 2015, 19, 153–157. [Google Scholar]
- Nielsen, F.H. Guidance for the determination of status indicators and dietary requirements for magnesium. Magnes. Res. 2016, 29, 154–160. [Google Scholar] [CrossRef]
- Schwalfenberg, G.K.; Genuis, S.J. The Importance of Magnesium in Clinical Healthcare. Scientifica 2017, 2017, 4179326. [Google Scholar] [CrossRef]
- Jahnen-Dechent, W.; Ketteler, M. Magnesium basics. Clin. Kidney J. 2012, 5, i3–i14. [Google Scholar] [CrossRef]
- Konrad, M.; Schlingmann, K.P.; Gudermann, T. Insights into the molecular nature of magnesium homeostasis. Am. J. Physiol. Renal Physiol. 2004, 286, F599–F605. [Google Scholar] [CrossRef]
- Curry, J.N.; Yu, A.S.L. Magnesium Handling in the Kidney. Adv. Chronic Kidney Dis. 2018, 25, 236–243. [Google Scholar] [CrossRef]
- Nielsen, F.H.; Lukaski, H.C. Update on the relationship between magnesium and exercise. Magnes. Res. 2006, 19, 180–189. [Google Scholar]
- Houillier, P.; Lievre, L.; Hureaux, M.; Prot-Bertoye, C. Mechanisms of paracellular transport of magnesium in intestinal and renal epithelia. Ann. N. Y. Acad. Sci. 2023, 1521, 14–31. [Google Scholar] [CrossRef]
- Groenestege, W.M.; Hoenderop, J.G.; van den Heuvel, L.; Knoers, N.; Bindels, R.J. The epithelial Mg2+ channel transient receptor potential melastatin 6 is regulated by dietary Mg2+ content and estrogens. J. Am. Soc. Nephrol. 2006, 17, 1035–1043. [Google Scholar] [CrossRef]
- Voets, T.; Nilius, B.; Hoefs, S.; van der Kemp, A.W.; Droogmans, G.; Bindels, R.J.; Hoenderop, J.G. TRPM6 forms the Mg2+ influx channel involved in intestinal and renal Mg2+ absorption. J. Biol. Chem. 2004, 279, 19–25. [Google Scholar] [CrossRef]
- Yamazaki, D.; Funato, Y.; Miura, J.; Sato, S.; Toyosawa, S.; Furutani, K.; Kurachi, Y.; Omori, Y.; Furukawa, T.; Tsuda, T.; et al. Basolateral Mg2+ extrusion via CNNM4 mediates transcellular Mg2+ transport across epithelia: A mouse model. PLoS Genet. 2013, 9, e1003983. [Google Scholar] [CrossRef]
- Schuchardt, J.P.; Hahn, A. Intestinal Absorption and Factors Influencing Bioavailability of Magnesium—An Update. Curr. Nutr. Food Sci. 2017, 13, 260–278. [Google Scholar] [CrossRef]
- Fox, C.; Ramsoomair, D.; Carter, C. Magnesium: Its proven and potential clinical significance. South. Med. J. 2001, 94, 1195–1201. [Google Scholar] [CrossRef]
- Ellison, D.H.; Maeoka, Y.; McCormick, J.A. Molecular Mechanisms of Renal Magnesium Reabsorption. J. Am. Soc. Nephrol. 2021, 32, 2125–2136. [Google Scholar] [CrossRef]
- de Baaij, J.H.F. Magnesium reabsorption in the kidney. Am. J. Physiol. Renal Physiol. 2023, 324, F227–F244. [Google Scholar] [CrossRef]
- Lameris, A.L.; Nevalainen, P.I.; Reijnen, D.; Simons, E.; Eygensteyn, J.; Monnens, L.; Bindels, R.J.; Hoenderop, J.G. Segmental transport of Ca2+ and Mg2+ along the gastrointestinal tract. Am. J. Physiol. Gastrointestnal Liver Physiol. 2015, 308, G206–G216. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; van der Wijst, J.; van der Kemp, A.; van Zeeland, F.; Bindels, R.J.; Hoenderop, J.G. Regulation of the epithelial Mg2+ channel TRPM6 by estrogen and the associated repressor protein of estrogen receptor activity (REA). J. Biol. Chem. 2009, 284, 14788–14795. [Google Scholar] [CrossRef] [PubMed]
- Bertinato, J.; Wu Xiao, C.; Ratnayake, W.M.; Fernandez, L.; Lavergne, C.; Wood, C.; Swist, E. Lower serum magnesium concentration is associated with diabetes, insulin resistance, and obesity in South Asian and white Canadian women but not men. Food Nutr. Res. 2015, 59, 25974. [Google Scholar] [CrossRef]
- Grober, U.; Schmidt, J.; Kisters, K. Magnesium in Prevention and Therapy. Nutrients 2015, 7, 8199–8226. [Google Scholar] [CrossRef]
- Mutnuri, S.; Fernandez, I.; Kochar, T. Suppression of Parathyroid Hormone in a Patient with Severe Magnesium Depletion. Case Rep. Nephrol. 2016, 2016, 2608538. [Google Scholar] [CrossRef]
- Killilea, D.W.; Killilea, A.N. Mineral requirements for mitochondrial function: A connection to redox balance and cellular differentiation. Free Radic. Biol. Med. 2022, 182, 182–191. [Google Scholar] [CrossRef]
- Chaigne-Delalande, B.; Lenardo, M.J. Divalent cation signaling in immune cells. Trends Immunol. 2014, 35, 332–344. [Google Scholar] [CrossRef]
- Guth-Metzler, R.; Mohamed, A.M.; Cowan, E.T.; Henning, A.; Ito, C.; Frenkel-Pinter, M.; Wartell, R.M.; Glass, J.B.; Williams, L.D. Goldilocks and RNA: Where Mg2+ concentration is just right. Nucleic Acids Res. 2023, 51, 3529–3539. [Google Scholar] [CrossRef]
- Mubagwa, K.; Gwanyanya, A.; Zakharov, S.; Macianskiene, R. Regulation of cation channels in cardiac and smooth muscle cells by intracellular magnesium. Arch. Biochem. Biophys. 2007, 458, 73–89. [Google Scholar] [CrossRef]
- Ricketts, S.N.; Khanal, P.; Rust, M.J.; Das, M.; Ross, J.L.; Robertson-Anderson, R.M. Triggering Cation-Induced Contraction of Cytoskeleton Networks via Microfluidics. Front. Phys. 2020, 8, 596699. [Google Scholar] [CrossRef]
- Souza, A.C.R.; Vasconcelos, A.R.; Dias, D.D.; Komoni, G.; Name, J.J. The Integral Role of Magnesium in Muscle Integrity and Aging: A Comprehensive Review. Nutrients 2023, 15, 5127. [Google Scholar] [CrossRef]
- Al Alawi, A.M.; Majoni, S.W.; Falhammar, H. Magnesium and Human Health: Perspectives and Research Directions. Int. J. Endocrinol. 2018, 2018, 9041694. [Google Scholar] [CrossRef]
- Jeong, E.M.; Lee, K.B.; Kim, G.E.; Kim, C.M.; Lee, J.H.; Kim, H.J.; Shin, J.W.; Kwon, M.A.; Park, H.H.; Kim, I.G. Competitive Binding of Magnesium to Calcium Binding Sites Reciprocally Regulates Transamidase and GTP Hydrolysis Activity of Transglutaminase 2. Int. J. Mol. Sci. 2020, 21, 791. [Google Scholar] [CrossRef]
- Liu, M.; Dudley, S.C., Jr. Magnesium, Oxidative Stress, Inflammation, and Cardiovascular Disease. Antioxidants 2020, 9, 907. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Food Labeling: Revision of the Nutrition and Supplement Facts Labels. 2016. Available online: https://www.federalregister.gov/documents/2016/05/27/2016-11867/food-labeling-revision-of-the-nutrition-and-supplement-facts-labels (accessed on 31 May 2025).
- EFSA Panel on Dietetic Products; Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for magnesium. EFSA J. 2015, 13, 4186. [Google Scholar] [CrossRef]
- Jackson, S.E.; Smith, L.; Grabovac, I.; Haider, S.; Demurtas, J.; Lopez-Sanchez, G.F.; Soysal, P.; Redsell, S.; Isik, A.T.; Yang, L. Ethnic Differences in Magnesium Intake in U.S. Older Adults: Findings from NHANES 2005–2016. Nutrients 2018, 10, 1901. [Google Scholar] [CrossRef] [PubMed]
- Shariat Moharari, R.; Motalebi, M.; Najafi, A.; Zamani, M.M.; Imani, F.; Etezadi, F.; Pourfakhr, P.; Khajavi, M.R. Magnesium Can Decrease Postoperative Physiological Ileus and Postoperative Pain in Major non Laparoscopic Gastrointestinal Surgeries: A Randomized Controlled Trial. Anesth. Pain Med. 2014, 4, e12750. [Google Scholar] [CrossRef]
- Workeneh, B.T.; Uppal, N.N.; Jhaveri, K.D.; Rondon-Berrios, H. Hypomagnesemia in the Cancer Patient. Kidney360 2021, 2, 154–166. [Google Scholar] [CrossRef]
- Barbagallo, M.; Dominguez, L.J. Magnesium and type 2 diabetes. World J. Diabetes 2015, 6, 1152–1157. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, L.; Veronese, N.; Barbagallo, M. Magnesium and Hypertension in Old Age. Nutrients 2020, 13, 139. [Google Scholar] [CrossRef] [PubMed]
- Aziz, D.A.; Sajjad, M.A.; Iftikhar, H. Clinical outcomes of children with acute asthma managed with intravenous magnesium sulfate outside intensive care setting. Monaldi Arch. Chest Dis. 2024, 94, 2664. [Google Scholar] [CrossRef]
- Akbarali, H.I.; Muchhala, K.H.; Jessup, D.K.; Cheatham, S. Chemotherapy induced gastrointestinal toxicities. Adv. Cancer Res. 2022, 155, 131–166. [Google Scholar] [CrossRef]
- Salinas, M.; Lopez-Garrigos, M.; Flores, E.; Leiva-Salinas, C. Improving diagnosis and treatment of hypomagnesemia. Clin. Chem. Lab. Med. 2024, 62, 234–248. [Google Scholar] [CrossRef]
- Mori, H.; Tack, J.; Suzuki, H. Magnesium Oxide in Constipation. Nutrients 2021, 13, 421. [Google Scholar] [CrossRef]
- Ramirez, J.; Guarner, F.; Bustos Fernandez, L.; Maruy, A.; Sdepanian, V.L.; Cohen, H. Antibiotics as Major Disruptors of Gut Microbiota. Front. Cell. Infect. Microbiol. 2020, 10, 572912. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Z. Progress in the management of acute colchicine poisoning in adults. Intern. Emerg. Med. 2022, 17, 2069–2081. [Google Scholar] [CrossRef]
- Bushinsky, D.A.; Spiegel, D.M.; Yuan, J.; Warren, S.; Fogli, J.; Pergola, P.E. Effects of the Potassium-Binding Polymer Patiromer on Markers of Mineral Metabolism. Clin. J. Am. Soc. Nephrol. 2019, 14, 103–110. [Google Scholar] [CrossRef]
- Srinutta, T.; Chewcharat, A.; Takkavatakarn, K.; Praditpornsilpa, K.; Eiam-Ong, S.; Jaber, B.L.; Susantitaphong, P. Proton pump inhibitors and hypomagnesemia: A meta-analysis of observational studies. Medicine 2019, 98, e17788. [Google Scholar] [CrossRef] [PubMed]
- Epstein, M.; McGrath, S.; Law, F. Proton-pump inhibitors and hypomagnesemic hypoparathyroidism. N. Engl. J. Med. 2006, 355, 1834–1836. [Google Scholar] [CrossRef]
- Hess, M.W.; Hoenderop, J.G.; Bindels, R.J.; Drenth, J.P. Systematic review: Hypomagnesaemia induced by proton pump inhibition. Aliment. Pharmacol. Ther. 2012, 36, 405–413. [Google Scholar] [CrossRef]
- Gommers, L.M.M.; Ederveen, T.H.A.; van der Wijst, J.; Overmars-Bos, C.; Kortman, G.A.M.; Boekhorst, J.; Bindels, R.J.M.; de Baaij, J.H.F.; Hoenderop, J.G.J. Low gut microbiota diversity and dietary magnesium intake are associated with the development of PPI-induced hypomagnesemia. FASEB J. 2019, 33, 11235–11246. [Google Scholar] [CrossRef] [PubMed]
- Gommers, L.M.M.; Hoenderop, J.G.J.; de Baaij, J.H.F. Mechanisms of proton pump inhibitor-induced hypomagnesemia. Acta Physiol. 2022, 235, e13846. [Google Scholar] [CrossRef] [PubMed]
- William, J.H.; Danziger, J. Proton-pump inhibitor-induced hypomagnesemia: Current research and proposed mechanisms. World J. Nephrol. 2016, 5, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Reed, L.G.; Mok, J.Y. Proton Pump Inhibitor-Associated Hypomagnesemia: A Retrospective Case-Control Study. Fed. Pract. 2016, 33, 18–21. [Google Scholar]
- FDA Drug Safety Communication: Low Magnesium Levels Can Be Associated with Long-Term Use of Proton Pump Inhibitor Drugs (PPIs). 2011. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-low-magnesium-levels-can-be-associated-long-term-use-proton-pump (accessed on 31 May 2025).
- Alexander, R.T.; Dimke, H. Effect of diuretics on renal tubular transport of calcium and magnesium. Am. J. Physiol. Renal Physiol. 2017, 312, F998–F1015. [Google Scholar] [CrossRef]
- Katopodis, P.; Karteris, E.; Katopodis, K.P. Pathophysiology of Drug-Induced Hypomagnesaemia. Drug Saf. 2020, 43, 867–880. [Google Scholar] [CrossRef]
- Rondon, L.J.; Groenestege, W.M.; Rayssiguier, Y.; Mazur, A. Relationship between low magnesium status and TRPM6 expression in the kidney and large intestine. Am. J. Physiol. Regul Integr. Comp. Physiol. 2008, 294, R2001–R2007. [Google Scholar] [CrossRef]
- Toto, R.D.; Goldenberg, R.; Chertow, G.M.; Cain, V.; Stefansson, B.V.; Sjostrom, C.D.; Sartipy, P. Correction of hypomagnesemia by dapagliflozin in patients with type 2 diabetes: A post hoc analysis of 10 randomized, placebo-controlled trials. J. Diabetes Complicat. 2019, 33, 107402. [Google Scholar] [CrossRef]
- Maeoka, Y.; McCormick, J.A. NaCl cotransporter activity and Mg2+ handling by the distal convoluted tubule. Am. J. Physiol. Renal Physiol. 2020, 319, F1043–F1053. [Google Scholar] [CrossRef]
- Kieboom, B.C.T.; Zietse, R.; Ikram, M.A.; Hoorn, E.J.; Stricker, B.H. Thiazide but not loop diuretics is associated with hypomagnesaemia in the general population. Pharmacoepidemiol. Drug Saf. 2018, 27, 1166–1173. [Google Scholar] [CrossRef]
- Alkharfy, K.M.; Al-Rasheed, M.; Al-Otaibi, B.; Al-Mitwazy, A.; Al-Mutairi, M. Effect of aminoglycoside dosing on magnesium levels in hospitalised patients. Int. J. Antimicrob. Agents 2007, 30, 189–190. [Google Scholar] [CrossRef]
- Singh, J.; Patel, M.L.; Gupta, K.K.; Pandey, S.; Dinkar, A. Acquired Bartter syndrome following gentamicin therapy. Indian J. Nephrol. 2016, 26, 461–463. [Google Scholar] [CrossRef]
- Bosman, W.; Hoenderop, J.G.J.; de Baaij, J.H.F. Genetic and drug-induced hypomagnesemia: Different cause, same mechanism. Proc. Nutr. Soc. 2021, 80, 327–338. [Google Scholar] [CrossRef]
- Dutta, P.; Hakimi, S.; Layton, A.T. How the kidney regulates magnesium: A modelling study. R. Soc. Open Sci. 2024, 11, 231484. [Google Scholar] [CrossRef]
- Kim, Y.W. Antimicrobial-induced Electrolyte and Acid-Base Disturbances. Electrolytes Blood Press. 2007, 5, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Wade, R.L.; Chaudhari, P.; Natoli, J.L.; Taylor, R.J.; Nathanson, B.H.; Horn, D. Comparison of adverse events and hospital length of stay associated with various amphotericin B formulations: Sequential conventional amphotericin b/lipid versus lipid-only therapy for the treatment of invasive fungal infections in hospitalized patients. P&T 2013, 38, 278–287. [Google Scholar]
- Le, T.; Kinh, N.V.; Cuc, N.T.K.; Tung, N.L.N.; Lam, N.T.; Thuy, P.T.T.; Cuong, D.D.; Phuc, P.T.H.; Vinh, V.H.; Hanh, D.T.H.; et al. A Trial of Itraconazole or Amphotericin B for HIV-Associated Talaromycosis. N. Engl. J. Med. 2017, 376, 2329–2340. [Google Scholar] [CrossRef]
- Sabra, R.; Branch, R.A. Amphotericin B nephrotoxicity. Drug Saf. 1990, 5, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Kleyman, T.R.; Roberts, C.; Ling, B.N. A mechanism for pentamidine-induced hyperkalemia: Inhibition of distal nephron sodium transport. Ann. Intern. Med. 1995, 122, 103–106. [Google Scholar] [CrossRef]
- Gratreak, B.D.K.; Swanson, E.A.; Lazelle, R.A.; Jelen, S.K.; Hoenderop, J.; Bindels, R.J.; Yang, C.L.; Ellison, D.H. Tacrolimus-induced hypomagnesemia and hypercalciuria requires FKBP12 suggesting a role for calcineurin. Physiol. Rep. 2020, 8, e14316. [Google Scholar] [CrossRef]
- Stefanelli, L.F.; Alessi, M.; Bertoldi, G.; Rossato, V.; Di Vico, V.; Nalesso, F.; Calo, L.A. Calcineurin-Inhibitor-Induced Hypomagnesemia in Kidney Transplant Patients: A Monocentric Comparative Study between Sucrosomial Magnesium and Magnesium Pidolate Supplementation. J. Clin. Med. 2023, 12, 752. [Google Scholar] [CrossRef]
- Navaneethan, S.D.; Sankarasubbaiyan, S.; Gross, M.D.; Jeevanantham, V.; Monk, R.D. Tacrolimus-associated hypomagnesemia in renal transplant recipients. Transplant. Proc. 2006, 38, 1320–1322. [Google Scholar] [CrossRef]
- Al-Rasheed, A.K.; Blaser, S.I.; Minassian, B.A.; Benson, L.; Weiss, S.K. Cyclosporine A neurotoxicity in a patient with idiopathic renal magnesium wasting. Pediatr. Neurol. 2000, 23, 353–356. [Google Scholar] [CrossRef]
- Van Laecke, S.; Van Biesen, W. Hypomagnesaemia in kidney transplantation. Transplant. Rev. 2015, 29, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Holzmacher, R.; Kendziorski, C.; Michael Hofman, R.; Jaffery, J.; Becker, B.; Djamali, A. Low serum magnesium is associated with decreased graft survival in patients with chronic cyclosporin nephrotoxicity. Nephrol. Dial. Transplant. 2005, 20, 1456–1462. [Google Scholar] [CrossRef]
- Isakov, O.; Patibandla, B.K.; Christopher, K.B.; Chandraker, A.; Hod, T. Impact of Post-Transplantation Hypomagnesemia on Long-Term Graft and Patient Survival after Transplantation. Kidney Blood Press. Res. 2022, 47, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Oronsky, B.; Caroen, S.; Oronsky, A.; Dobalian, V.E.; Oronsky, N.; Lybeck, M.; Reid, T.R.; Carter, C.A. Electrolyte disorders with platinum-based chemotherapy: Mechanisms, manifestations and management. Cancer Chemother. Pharmacol. 2017, 80, 895–907. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.P.; Tadagavadi, R.K.; Ramesh, G.; Reeves, W.B. Mechanisms of Cisplatin nephrotoxicity. Toxins 2010, 2, 2490–2518. [Google Scholar] [CrossRef]
- Ledeganck, K.J.; Boulet, G.A.; Bogers, J.J.; Verpooten, G.A.; De Winter, B.Y. The TRPM6/EGF pathway is downregulated in a rat model of cisplatin nephrotoxicity. PLoS ONE 2013, 8, e57016. [Google Scholar] [CrossRef]
- Stohr, W.; Paulides, M.; Bielack, S.; Jurgens, H.; Koscielniak, E.; Rossi, R.; Langer, T.; Beck, J.D. Nephrotoxicity of cisplatin and carboplatin in sarcoma patients: A report from the late effects surveillance system. Pediatr. Blood Cancer 2007, 48, 140–147. [Google Scholar] [CrossRef]
- Borghaei, H.; Langer, C.J.; Millenson, M.; Ruth, K.J.; Litwin, S.; Tuttle, H.; Seldomridge, J.S.; Rovito, M.; Mintzer, D.; Cohen, R.; et al. Phase II study of paclitaxel, carboplatin, and cetuximab as first line treatment, for patients with advanced non-small cell lung cancer (NSCLC): Results of OPN-017. J. Thorac. Oncol. 2008, 3, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Konner, J.; Schilder, R.J.; DeRosa, F.A.; Gerst, S.R.; Tew, W.P.; Sabbatini, P.J.; Hensley, M.L.; Spriggs, D.R.; Aghajanian, C.A. A phase II study of cetuximab/paclitaxel/carboplatin for the initial treatment of advanced-stage ovarian, primary peritoneal, or fallopian tube cancer. Gynecol. Oncol. 2008, 110, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Sikking, C.; Niggebrugge-Mentink, K.L.; van der Sman, A.S.E.; Smit, R.H.P.; Bouman-Wammes, E.W.; Beex-Oosterhuis, M.M.; van Kesteren, C. Hydration Methods for Cisplatin Containing Chemotherapy: A Systematic Review. Oncologist 2024, 29, e173–e186. [Google Scholar] [CrossRef] [PubMed]
- Tejpar, S.; Piessevaux, H.; Claes, K.; Piront, P.; Hoenderop, J.G.; Verslype, C.; Van Cutsem, E. Magnesium wasting associated with epidermal-growth-factor receptor-targeting antibodies in colorectal cancer: A prospective study. Lancet Oncol. 2007, 8, 387–394. [Google Scholar] [CrossRef]
- Wang, Q.; Qi, Y.; Zhang, D.; Gong, C.; Yao, A.; Xiao, Y.; Yang, J.; Zhou, F.; Zhou, Y. Electrolyte disorders assessment in solid tumor patients treated with anti-EGFR monoclonal antibodies: A pooled analysis of 25 randomized clinical trials. Tumour Biol. 2015, 36, 3471–3482. [Google Scholar] [CrossRef]
- Garcia-Foncillas, J.; Sunakawa, Y.; Aderka, D.; Wainberg, Z.; Ronga, P.; Witzler, P.; Stintzing, S. Distinguishing Features of Cetuximab and Panitumumab in Colorectal Cancer and Other Solid Tumors. Front. Oncol. 2019, 9, 849. [Google Scholar] [CrossRef]
- Saloura, V.; Cohen, E.E.; Licitra, L.; Billan, S.; Dinis, J.; Lisby, S.; Gauler, T.C. An open-label single-arm, phase II trial of zalutumumab, a human monoclonal anti-EGFR antibody, in patients with platinum-refractory squamous cell carcinoma of the head and neck. Cancer Chemother. Pharmacol. 2014, 73, 1227–1239. [Google Scholar] [CrossRef]
- Dimke, H.; van der Wijst, J.; Alexander, T.R.; Meijer, I.M.; Mulder, G.M.; van Goor, H.; Tejpar, S.; Hoenderop, J.G.; Bindels, R.J. Effects of the EGFR Inhibitor Erlotinib on Magnesium Handling. J. Am. Soc. Nephrol. 2010, 21, 1309–1316. [Google Scholar] [CrossRef]
- Maliakal, P.; Ledford, A. Electrolyte and protein imbalance following anti-EGFR therapy in cancer patients: A comparative study. Exp. Ther. Med. 2010, 1, 307–311. [Google Scholar] [CrossRef]
- Fakih, M.G.; Wilding, G.; Lombardo, J. Cetuximab-induced hypomagnesemia in patients with colorectal cancer. Clin. Colorectal Cancer 2006, 6, 152–156. [Google Scholar] [CrossRef]
- Hsieh, M.C.; Wu, C.F.; Chen, C.W.; Shi, C.S.; Huang, W.S.; Kuan, F.C. Hypomagnesemia and clinical benefits of anti-EGFR monoclonal antibodies in wild-type KRAS metastatic colorectal cancer: A systematic review and meta-analysis. Sci. Rep. 2018, 8, 2047. [Google Scholar] [CrossRef] [PubMed]
- Florakis, D.; Karakozis, S.; Tseleni-Balafouta, S.; Makras, P. Lessons learned from the management of Hungry Bone Syndrome following the removal of an Atypical Parathyroid Adenoma. J. Musculoskelet. Neuronal Interact. 2019, 19, 379–384. [Google Scholar]
- Khositseth, S.; Sudjaritjan, N.; Tananchai, P.; Ong-ajyuth, S.; Sitprija, V.; Thongboonkerd, V. Renal magnesium wasting and tubular dysfunction in leptospirosis. Nephrol. Dial. Transplant. 2008, 23, 952–958. [Google Scholar] [CrossRef]
- Pitt, B.; Bakris, G.L. New potassium binders for the treatment of hyperkalemia: Current data and opportunities for the future. Hypertension 2015, 66, 731–738. [Google Scholar] [CrossRef]
- Megapanou, E.; Florentin, M.; Milionis, H.; Elisaf, M.; Liamis, G. Drug-Induced Hypophosphatemia: Current Insights. Drug Saf. 2020, 43, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Huycke, M.M.; Naguib, M.T.; Stroemmel, M.M.; Blick, K.; Monti, K.; Martin-Munley, S.; Kaufman, C. A double-blind placebo-controlled crossover trial of intravenous magnesium sulfate for foscarnet-induced ionized hypocalcemia and hypomagnesemia in patients with AIDS and cytomegalovirus infection. Antimicrob. Agents Chemother. 2000, 44, 2143–2148. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Begin, M.J.; Ste-Marie, L.G.; Coupal, L.; Ethier, J.; Rakel, A. Hypomagnesemia During Teriparatide Treatment in Osteoporosis: Incidence and Determinants. J. Bone Miner. Res. 2018, 33, 1444–1449. [Google Scholar] [CrossRef]
- Marlow, C.F.; Sharma, S.; Babar, F.; Lin, J. Severe Hypocalcemia and Hypomagnesemia with Denosumab in Advanced Chronic Kidney Disease: Case Report and Literature Review. Case Rep. Oncol. Med. 2018, 2018, 2059364. [Google Scholar] [CrossRef]
- Filippatos, T.; Tzavella, E.; Rizos, C.; Elisaf, M.; Liamis, G. Acid-base and electrolyte disorders associated with the use of antidiabetic drugs. Expert Opin. Drug Saf. 2017, 16, 1121–1132. [Google Scholar] [CrossRef]
- Vatsalya, V.; Gala, K.S.; Mishra, M.; Schwandt, M.L.; Umhau, J.; Cave, M.C.; Parajuli, D.; Ramchandani, V.A.; McClain, C.J. Lower Serum Magnesium Concentrations are associated With Specific Heavy Drinking Markers, Pro-Inflammatory Response and Early-Stage Alcohol-associated Liver Injury section sign. Alcohol Alcohol. 2020, 55, 164–170. [Google Scholar] [CrossRef]
- Vanoni, F.O.; Milani, G.P.; Agostoni, C.; Treglia, G.; Fare, P.B.; Camozzi, P.; Lava, S.A.G.; Bianchetti, M.G.; Janett, S. Magnesium Metabolism in Chronic Alcohol-Use Disorder: Meta-Analysis and Systematic Review. Nutrients 2021, 13, 1959. [Google Scholar] [CrossRef]
- Kirkland, A.E.; Sarlo, G.L.; Holton, K.F. The Role of Magnesium in Neurological Disorders. Nutrients 2018, 10, 730. [Google Scholar] [CrossRef]
- Tinawi, M. Disorders of Calcium Metabolism: Hypocalcemia and Hypercalcemia. Cureus 2021, 13, e12420. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; O’Keefe, J.H.; Wilson, W. Subclinical magnesium deficiency: A principal driver of cardiovascular disease and a public health crisis. Open Heart 2018, 5, e000668. [Google Scholar] [CrossRef]
- Izzedine, H.; Benalia, H.; Arzouk, N.; Jeunemaitre, X.; Hacini, S.; Bourry, E.; Barrou, B. Nephrolithiasis with hypomagnesemia: What is the cause? Am. J. Kidney Dis. 2007, 49, 862–864. [Google Scholar] [CrossRef]
- Alkazemi, D.; Alsouri, N.; Zafar, T.; Kubow, S. Hypomagnesemia and the Metabolic Syndrome among Apparently Healthy Kuwaiti Adults: A Cross-Sectional Study. Nutrients 2022, 14, 5257. [Google Scholar] [CrossRef] [PubMed]
- Kieboom, B.C.; Stricker, B.H. Low serum magnesium is associated with hypertension. J. Pediatr. 2016, 174, 279–280. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.W.; Famure, O.; Li, Y.; Kim, S.J. Hypomagnesemia and the Risk of New-Onset Diabetes Mellitus after Kidney Transplantation. J. Am. Soc. Nephrol. 2016, 27, 1793–1800. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, L.J.; Veronese, N.; Ciriminna, S.; Perez-Albela, J.L.; Vasquez-Lopez, V.F.; Rodas-Regalado, S.; Di Bella, G.; Parisi, A.; Tagliaferri, F.; Barbagallo, M. Association between Serum Magnesium and Fractures: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients 2023, 15, 1304. [Google Scholar] [CrossRef]
- Aal-Hamad, A.H.; Al-Alawi, A.M.; Kashoub, M.S.; Falhammar, H. Hypermagnesemia in Clinical Practice. Medicina 2023, 59, 1190. [Google Scholar] [CrossRef] [PubMed]
- Dent, A.; Selvaratnam, R. Measuring magnesium—Physiological, clinical and analytical perspectives. Clin. Biochem. 2022, 105–106, 1–15. [Google Scholar] [CrossRef]
- Zakharchenko, M.; Leden, P.; Rulisek, J.; Los, F.; Brodska, H.; Balik, M. Ionized Magnesium and Regional Citrate Anticoagulation for Continuous Renal Replacement Therapy. Blood Purif. 2016, 41, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Lowenstein, F.W.; Stanton, M.F. Serum magnesium levels in the United States, 1971–1974. J. Am. Coll. Nutr. 1986, 5, 399–414. [Google Scholar] [CrossRef]
- Costello, R.B.; Elin, R.J.; Rosanoff, A.; Wallace, T.C.; Guerrero-Romero, F.; Hruby, A.; Lutsey, P.L.; Nielsen, F.H.; Rodriguez-Moran, M.; Song, Y.; et al. Perspective: The Case for an Evidence-Based Reference Interval for Serum Magnesium: The Time Has Come. Adv. Nutr. 2016, 7, 977–993. [Google Scholar] [CrossRef]
- Elin, R.J. Assessment of magnesium status for diagnosis and therapy. Magnes. Res. 2010, 23, S194–S198. [Google Scholar] [CrossRef]
- Touyz, R.M. Magnesium in clinical medicine. Front. Biosci. 2004, 9, 1278–1293. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.L.; Kuo, E. Mechanism of hypokalemia in magnesium deficiency. J. Am. Soc. Nephrol. 2007, 18, 2649–2652. [Google Scholar] [CrossRef]
- Yamamoto, M.; Yamaguchi, T.; Yamauchi, M.; Yano, S.; Sugimoto, T. Acute-onset hypomagnesemia-induced hypocalcemia caused by the refractoriness of bones and renal tubules to parathyroid hormone. J. Bone Miner. Metab. 2011, 29, 752–755. [Google Scholar] [CrossRef]
- Koebnick, C.; Leitzmann, R.; Garcia, A.L.; Heins, U.A.; Heuer, T.; Golf, S.; Katz, N.; Hoffmann, I.; Leitzmann, C. Long-term effect of a plant-based diet on magnesium status during pregnancy. Eur. J. Clin. Nutr. 2005, 59, 219–225. [Google Scholar] [CrossRef]
- Dalton, L.M.; Ni Fhloinn, D.M.; Gaydadzhieva, G.T.; Mazurkiewicz, O.M.; Leeson, H.; Wright, C.P. Magnesium in pregnancy. Nutr. Rev. 2016, 74, 549–557. [Google Scholar] [CrossRef]
- Moller, A.S.; Bressendorff, I.; Nordholm, A.; Egstrand, S.; Jorgensen, N.R.; Klausen, T.W.; Olgaard, K.; Hansen, D. Diurnal variation of magnesium and the mineral metabolism in patients with chronic kidney disease. Bone Rep. 2021, 15, 101130. [Google Scholar] [CrossRef]
- Lysakowski, C.; Von Elm, E.; Dumont, L.; Junod, J.D.; Tassonyi, E.; Kayser, B.; Tramer, M.R. Effect of magnesium, high altitude and acute mountain sickness on blood flow velocity in the middle cerebral artery. Clin. Sci. 2004, 106, 279–285. [Google Scholar] [CrossRef]
- Lima-Oliveira, G.; Salvagno, G.L.; Danese, E.; Brocco, G.; Guidi, G.C.; Lippi, G. Contamination of lithium heparin blood by K2-ethylenediaminetetraacetic acid (EDTA): An experimental evaluation. Biochem. Med. 2014, 24, 359–367. [Google Scholar] [CrossRef]
- Lippi, G.; Salvagno, G.L.; Montagnana, M.; Brocco, G.; Guidi, G.C. Influence of hemolysis on routine clinical chemistry testing. Clin. Chem. Lab. Med. 2006, 44, 311–316. [Google Scholar] [CrossRef]
- Makowsky, M.J.; Bell, P.; Gramlich, L. Subcutaneous Magnesium Sulfate to Correct High-Output Ileostomy-Induced Hypomagnesemia. Case Rep. Gastroenterol. 2019, 13, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Bundy, J.T.; Connito, D.; Mahoney, M.D.; Pontier, P.J. Treatment of idiopathic renal magnesium wasting with amiloride. Am. J. Nephrol. 1995, 15, 75–77. [Google Scholar] [CrossRef] [PubMed]
- Hamm, L.L.; Feng, Z.; Hering-Smith, K.S. Regulation of sodium transport by ENaC in the kidney. Curr. Opin. Nephrol. Hypertens. 2010, 19, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, R.E.; Mende, C.; Vijapurkar, U.; Sha, S.; Davies, M.J.; Desai, M. Effects of Canagliflozin on Serum Magnesium in Patients With Type 2 Diabetes Mellitus: A Post Hoc Analysis of Randomized Controlled Trials. Diabetes Ther. 2017, 8, 451–458. [Google Scholar] [CrossRef]
- Tang, H.; Zhang, X.; Zhang, J.; Li, Y.; Del Gobbo, L.C.; Zhai, S.; Song, Y. Elevated serum magnesium associated with SGLT2 inhibitor use in type 2 diabetes patients: A meta-analysis of randomised controlled trials. Diabetologia 2016, 59, 2546–2551. [Google Scholar] [CrossRef] [PubMed]
- Mende, C.W. Diabetes and kidney disease: The role of sodium-glucose cotransporter-2 (SGLT-2) and SGLT-2 inhibitors in modifying disease outcomes. Curr. Med. Res. Opin. 2017, 33, 541–551. [Google Scholar] [CrossRef]
- Ferrannini, E.; Muscelli, E.; Frascerra, S.; Baldi, S.; Mari, A.; Heise, T.; Broedl, U.C.; Woerle, H.J. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J. Clin. Investig. 2014, 124, 499–508. [Google Scholar] [CrossRef]
- Blau, J.E.; Bauman, V.; Conway, E.M.; Piaggi, P.; Walter, M.F.; Wright, E.C.; Bernstein, S.; Courville, A.B.; Collins, M.T.; Rother, K.I.; et al. Canagliflozin triggers the FGF23/1,25-dihydroxyvitamin D/PTH axis in healthy volunteers in a randomized crossover study. JCI Insight 2018, 3, e99123. [Google Scholar] [CrossRef] [PubMed]
- Verschuren, E.H.J.; Hoenderop, J.G.J.; Peters, D.J.M.; Arjona, F.J.; Bindels, R.J.M. Tubular flow activates magnesium transport in the distal convoluted tubule. FASEB J. 2019, 33, 5034–5044. [Google Scholar] [CrossRef] [PubMed]
| Mechanisms of Drug-Induced Hypomagnesemia |
|---|
| Intracellular shift of magnesium |
| Insulin therapy |
| Beta-agonists: Epinephrine, Salbutamol, Terbutaline, Rimiterol |
| Xanthines: Theophylline |
| Correction of metabolic acidosis with alkali therapy |
| Metformin |
| Gastrointestinal loss of Magnesium |
| Laxative abuse |
| Antibiotics, antineoplastic agents |
| Proton pump inhibitors |
| Colchicine |
| Patiromer |
| Chemotherapeutic agents causing intestinal mucosal injury |
| Increased urinary Magnesium excretion |
| Antineoplastics: Carboplatin, Cisplatin |
| Monoclonal antibody EGFR inhibitors: Cetuximab, Panitumumab |
| mTOR inhibitors |
| Calcineurin inhibitors: Cyclosporine, Tacrolimus |
| Aminoglycosides |
| Amphotericin B |
| Diuretics: Thiazides, Furosemide |
| Digoxin |
| Miscellaneous |
| Alcohol |
| Massive transfusions |
| Teriparatide |
| Bisphosphonates |
| Denosumab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Floris, M.; Angioi, A.; Lepori, N.; Piras, D.; Cabiddu, G.; Pani, A.; Rosner, M.H. The Clinical Spectrum of Acquired Hypomagnesemia: From Etiology to Therapeutic Approaches. Biomedicines 2025, 13, 1862. https://doi.org/10.3390/biomedicines13081862
Floris M, Angioi A, Lepori N, Piras D, Cabiddu G, Pani A, Rosner MH. The Clinical Spectrum of Acquired Hypomagnesemia: From Etiology to Therapeutic Approaches. Biomedicines. 2025; 13(8):1862. https://doi.org/10.3390/biomedicines13081862
Chicago/Turabian StyleFloris, Matteo, Andrea Angioi, Nicola Lepori, Doloretta Piras, Gianfranca Cabiddu, Antonello Pani, and Mitchell H. Rosner. 2025. "The Clinical Spectrum of Acquired Hypomagnesemia: From Etiology to Therapeutic Approaches" Biomedicines 13, no. 8: 1862. https://doi.org/10.3390/biomedicines13081862
APA StyleFloris, M., Angioi, A., Lepori, N., Piras, D., Cabiddu, G., Pani, A., & Rosner, M. H. (2025). The Clinical Spectrum of Acquired Hypomagnesemia: From Etiology to Therapeutic Approaches. Biomedicines, 13(8), 1862. https://doi.org/10.3390/biomedicines13081862






