Narrative Review of Chronic Inflammation in Uterine Myoma: Lack of Specialized Pro-Resolving Lipid Mediators (SPMs) and Vitamin D as a Potential Reason for the Development of Uterine Fibroids
Abstract
1. Introduction
2. Role of Vitamin D
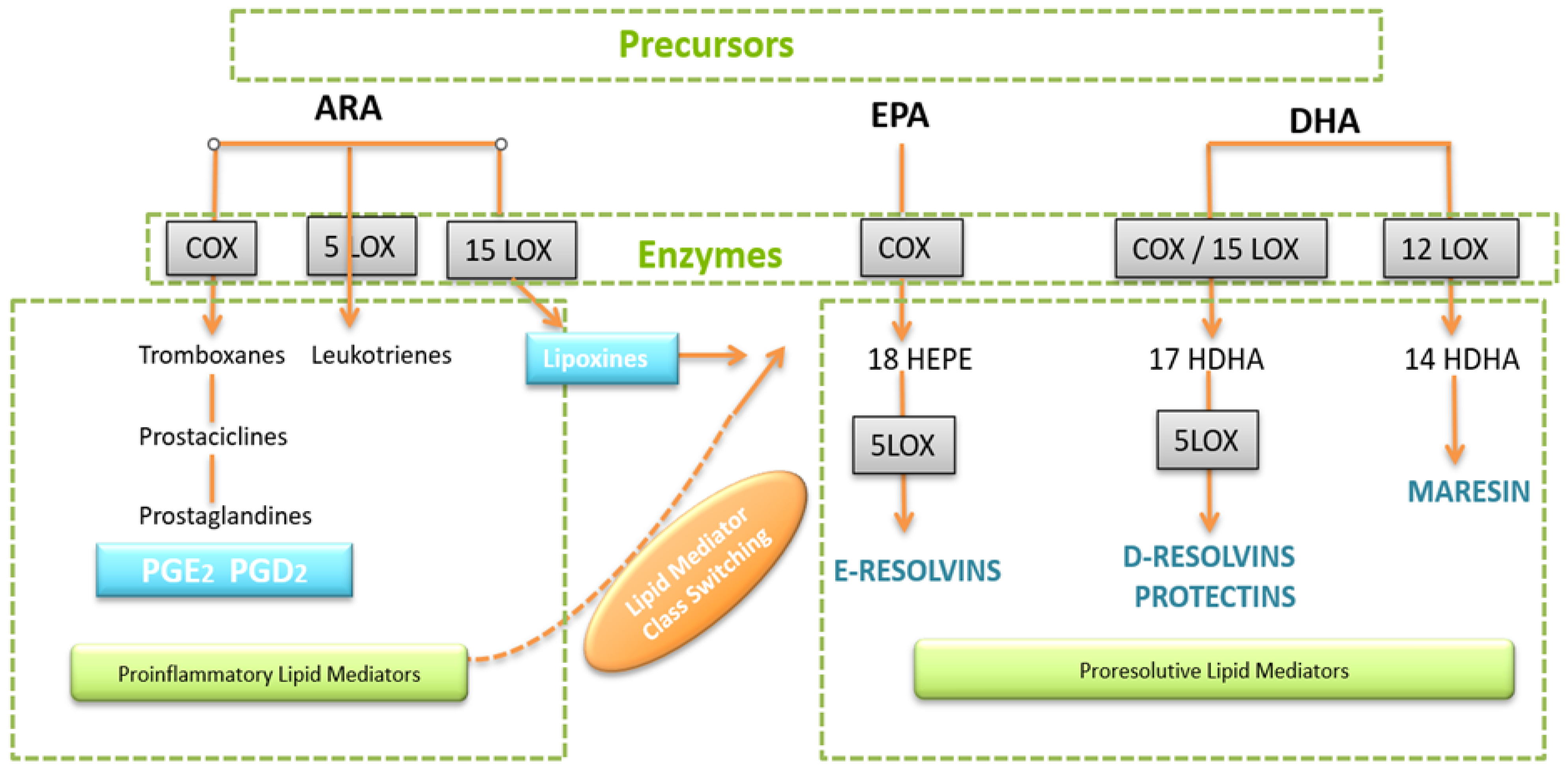
3. Inflammatory Processes and the Role of Lipid Acid-Derived Mediators
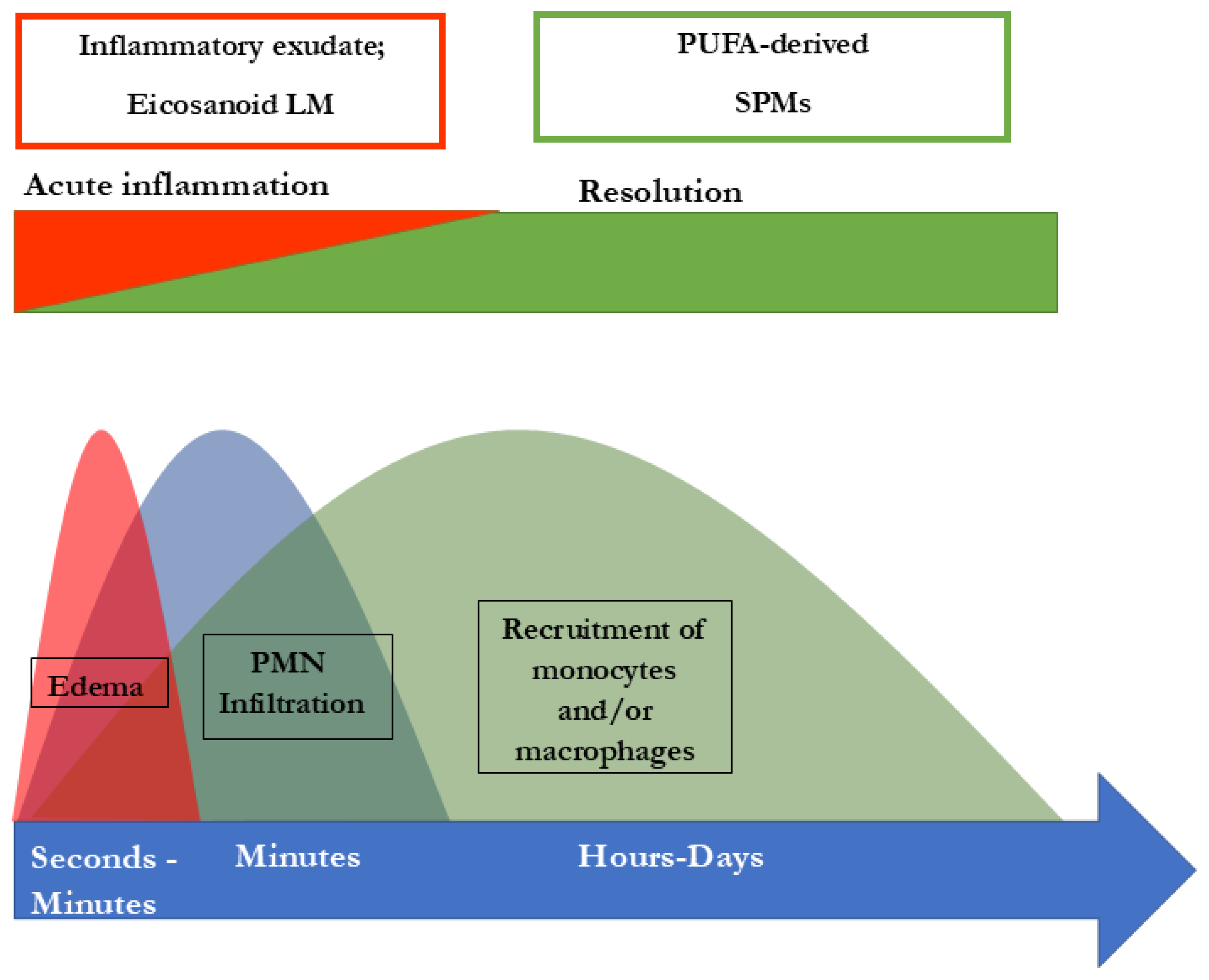
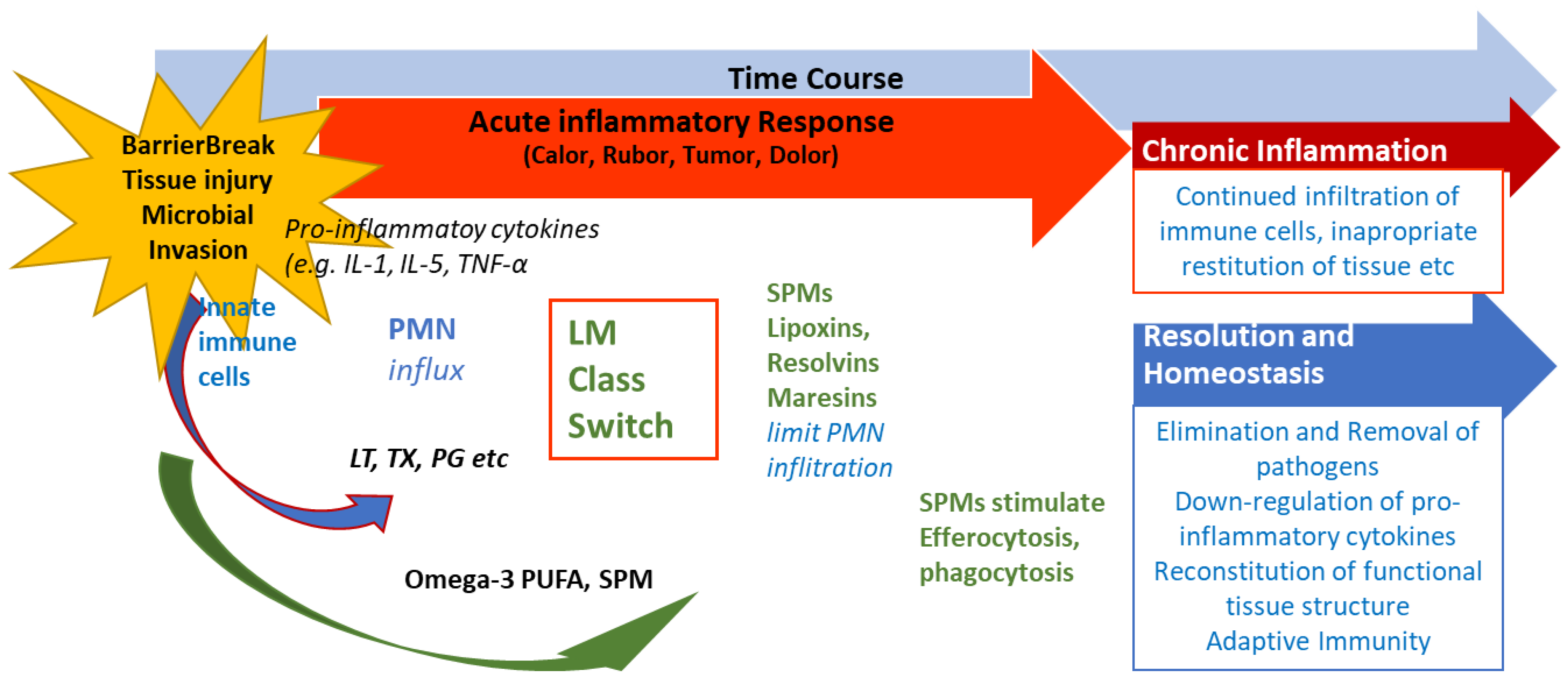
4. SPMs Are Essential for Resolution of Inflammation
5. The Significance of SPMs in Chronic Inflammatory Diseases
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Islam, S.; Ciavattini, A.; Petraglia, F.; Castellucci, M.; Ciarmela, P. Extracellular matrix in uterine leiomyoma pathogenesis: A potential target for future therapeutics. Hum. Reprod. Update 2018, 24, 59–85. [Google Scholar] [CrossRef] [PubMed]
- Buttram, V.C., Jr.; Reiter, R.C. Uterine leiomyomata: Etiology, symptomatology, and management. Fertil. Steril. 1981, 36, 433–445. [Google Scholar] [CrossRef]
- Cramer, S.F.; Patel, A. The frequency of uterine leiomyomas. Am. J. Clin. Pathol. 1990, 94, 435–438. [Google Scholar] [CrossRef]
- Marshall, L.M.; Spiegelman, D.; Barbieri, R.L.; Goldman, M.B.; Manson, J.E.; Colditz, G.A.; Willett, W.C.; Hunter, D.J. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet. Gynecol. 1997, 90, 967–973. [Google Scholar] [CrossRef]
- Baird, D.D.; Dunson, D.B.; Hill, M.C.; Cousins, D.; Schectman, J.M. High cumulative incidence of uterine leiomyoma in black and white women: Ultrasound evidence. Am. J. Obstet. Gynecol. 2003, 188, 100–107. [Google Scholar] [CrossRef]
- Kjerulff, K.H.; Langenberg, P.; Seidman, J.D.; Stolley, P.D.; Guzinski, G.M. Uterine leiomyomas. Racial differences in severity, symptoms and age at diagnosis. J. Reprod. Med. 1996, 41, 483–490. [Google Scholar] [PubMed]
- Szydłowska, I.; Grabowska, M.; Nawrocka-Rutkowska, J.; Kram, A.; Piasecka, M.; Starczewski, A. Markers of Inflammation and Vascular Parameters in Selective Progesterone Receptor Modulator (Ulipristal Acetate)-Treated Uterine Fibroids. J. Clin. Med. 2021, 10, 3721. [Google Scholar] [CrossRef]
- Goad, J.; Rudolph, J.; Wei, J.-J.; Bulun, S.E.; Chakravarti, D.; Rajkovic, A. Single Cell atlas of uterine myometrium and leiomyomas reveals diverse and novel cell types of non-monoclonal origin. bioRxiv 2020. [Google Scholar] [CrossRef]
- Tal, R.; Segars, J.H. The role of angiogenic factors in fibroid pathogenesis: Potential implications for future therapy. Hum. Reprod. Update 2014, 20, 194–216. [Google Scholar] [CrossRef]
- Ciavattini, A.; Di Giuseppe, J.; Stortoni, P.; Montik, N.; Giannubilo, S.R.; Litta, P.; Islam, S.; Tranquilli, A.L.; Reis, F.M.; Ciarmela, P. Uterine fibroids: Pathogenesis and interactions with endometrium and endomyometrial junction. Obstet. Gynecol. Int. 2013, 2013, 173184. [Google Scholar] [CrossRef] [PubMed]
- Galindo, L.J.; Hernández-Beeftink, T.; Salas, A.; Jung, Y.; Reyes, R.; Montes de Oca, F.; Hernández, M.; Almeida, T.A. HMGA2 and MED12 alterations frequently co-occur in uterine leiomyomas. Gynecol. Oncol. 2018, 150, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Grings, A.O.; Lora, V.; Ferreira, G.D.; Brum, I.S.; von Eye Corleta, H.; Capp, E. Protein expression of estrogen receptors alpha and beta and aromatase in myometrium and uterine leiomyoma. Gynecol. Obstet. Investig. 2012, 73, 113–117. [Google Scholar] [CrossRef]
- Ishikawa, H.; Ishi, K.; Serna, V.A.; Kakazu, R.; Bulun, S.E.; Kurita, T. Progesterone is essential for maintenance and growth of uterine leiomyoma. Endocrinology 2010, 151, 2433–2442. [Google Scholar] [CrossRef]
- Kawaguchi, K.; Fujii, S.; Konishi, I.; Nanbu, Y.; Nonogaki, H.; Mori, T. Mitotic activity in uterine leiomyomas during the menstrual cycle. Am. J. Obstet. Gynecol. 1989, 160, 637–641. [Google Scholar] [CrossRef]
- Islam, M.d.S.; Protic, O.; Stortoni, P.; Grechi, G.; Lamanna, P.; Petraglia, F.; Castellucci, M.; Ciarmela, P. Complex networks of multiple factors in the pathogenesis of uterine leiomyoma. Fertil. Steril. 2013, 100, 178–193. [Google Scholar] [CrossRef]
- Modugno, F.; Ness, R.B.; Chen, C.; Weiss, N.S. Inflammation and endometrial cancer: A hypothesis. Cancer Epidemiol. Biomark. Prev. 2005, 14, 2840–2847. [Google Scholar] [CrossRef]
- Orciani, M.; Caffarini, M.; Biagini, A.; Lucarini, G.; Carpini, G.D.; Berretta, A.; Di Primio, R.; Ciavattini, A. Chronic Inflammation May Enhance Leiomyoma Development by the Involvement of Progenitor Cells. Stem Cells Int. 2018, 2018, 1716246. [Google Scholar] [CrossRef]
- Protic, O.; Toti, P.; Islam, M.d.S.; Occhini, R.; Giannubilo, S.R.; Catherino, W.H.; Cinti, S.; Petraglia, F.; Ciavattini, A.; Castellucci, M.; et al. Possible involvement of inflammatory/reparative processes in the development of uterine fibroids. Cell Tissue Res. 2016, 364, 415–427. [Google Scholar] [CrossRef]
- Macdiarmid, F.; Wang, D.; Duncan, L.J.; Purohit, A.; Ghilchick, M.W.; Reed, M.J. Stimulation of aromatase activity in breast fibroblasts by tumor necrosis factor alpha. Mol. Cell Endocrinol. 1994, 106, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Purohit, A.; Newman, S.P.; Reed, M.J. The role of cytokines in regulating estrogen synthesis: Implications for the etiology of breast cancer. Breast Cancer Res. 2002, 4, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Leppert, P.C.; Al-Hendy, A.; Baird, D.D.; Bulun, S.; Catherino, W.; Dixon, D.; Ducharme, M.; Harmon, Q.E.; Jayes, F.L.; Paul, E.; et al. Summary of the Proceedings of the Basic Science of Uterine Fibroids Meeting: New Developments February 28, 2020. F&S Sci. 2021, 2, 88–100. [Google Scholar]
- Ciebiera, M.; Ali, M.; Prince, L.; Jackson-Bey, T.; Atabiekov, I.; Zgliczyński, S.; Al-Hendy, A. The Evolving Role of Natural Compounds in the Medical Treatment of Uterine Fibroids. J. Clin. Med. 2020, 9, 1479. [Google Scholar] [CrossRef]
- Zannotti, A.; Greco, S.; Pellegrino, P.; Giantomassi, F.; Carpini, G.D.; Goteri, G.; Ciavattini, A.; Ciarmela, P. Macrophages and Immune Responses in Uterine Fibroids. Cells 2021, 10, 982. [Google Scholar] [CrossRef]
- AlAshqar, A.; Lulseged, B.; Mason-Otey, A.; Liang, J.; Begum, U.A.M.; Afrin, S.; Borahay, M.A. Oxidative Stress and Antioxidants in Uterine Fibroids: Pathophysiology and Clinical Implications. Antioxidants 2023, 12, 807. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, X.; Asif, H.; Feng, Y.; Kohrn, B.F.; Kennedy, S.R.; Kim, J.J.; Wei, J.J. Myometrial oxidative stress drives MED12 mutations in leiomyoma. Cell Biosci. 2022, 12, 111. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.S.O.; Borges da Silva, B.; Lima de Medeiros, M.; Dos Santos, A.R.; do Nascimento Brazil, E.D.; Eulálio Filho, W.M.N.; Oliveira Ibiapina, J.; Albuquerque Brito, A.G.; Lopes Costa, P.V. Evaluation of vitamin D receptor expression in uterine leiomyoma and nonneoplastic myometrial tissue: A cross-sectional controlled study. Reprod. Biol. Endocrinol. 2021, 19, 67. [Google Scholar] [CrossRef]
- Halder, S.K.; Goodwin, J.S.; Al-Hendy, A. 1,25-Dihydroxyvitamin D3 reduces TGF-beta3-induced fibrosis-related gene expression in human uterine leiomyoma cells. J. Clin. Endocrinol. Metab. 2011, 96, E754–E762. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.K.; Osteen, K.G.; Al-Hendy, A. 1,25-dihydroxyvitamin d3 reduces extracellular matrix-associated protein expression in human uterine fibroid cells. Biol. Reprod. 2013, 89, 150. [Google Scholar] [CrossRef]
- Al-Hendy, A.; Diamond, M.P.; El-Sohemy, A.; Halder, S.K. 1,25-dihydroxyvitamin D3 regulates expression of sex steroid receptors in human uterine fibroid cells. J. Clin. Endocrinol. Metab. 2015, 100, E572–E582. [Google Scholar] [CrossRef]
- Paffoni, A.; Somigliana, E.; Vigano, P.; Benaglia, L.; Cardellicchio, L.; Pagliardini, L.; Papaleo, E.; Candiani, E.M.; Fedele, L. Vitamin D status in women with uterine leiomyomas. J. Clin. Endocrinol. Metab. 2013, 98, E1374–E1378. [Google Scholar] [CrossRef]
- Baird, D.D.; Hill, M.C.; Schectman, J.M.; Hollis, B.W. Vitamin d and the risk of uterine fibroids. Epidemiology 2013, 24, 447–453. [Google Scholar] [CrossRef]
- Sabry, M.; Halder, S.K.; Ait Allah, A.S.; Roshdy, E.; Rajaratnam, V.; Al-Hendy, A. Serum vitamin D3 level inversely correlates with uterine fibroid volume in different ethnic groups: A cross-sectional observational study. Int. J. Womens Health 2013, 5, 93–100. [Google Scholar] [PubMed]
- Wise, L.A.; Ruiz-Narváez, E.A.; Haddad, S.A.; Rosenberg, L.; Julie RPalmer, J.R. Polymorphisms in vitamin D-related genes and risk of uterine leiomyomata. Fertil. Steril. 2014, 102, 503–510. [Google Scholar] [CrossRef]
- Shahbazi, S. Exploring the link between VDR rs2228570 and uterine leiomyoma in Iranian women. Egypt. J. Med. Hum. Genet. 2016, 17, 115–118. [Google Scholar] [CrossRef]
- Yılmaz, G.S.; Gül, T.; Attar, R.; Yıldırım, G.; İşbir, T. Association between fok1 polymorphism of vitamin D receptor gene with uterine leiomyoma in Turkish populations. J. Turk. Ger. Gynecol. Assoc. 2018, 19, 128–131. [Google Scholar] [CrossRef] [PubMed]
- Ciebiera, M.; Wrzosek, M.; Wojtyła, C.; Zaręba, K.; Nowicka, G.; Jakiel, G.; Wlodarczyk, M. Vitamin D receptor gene polymorphisms and uterine fibroid incidence in Caucasian women. Arch. Med. Sci. 2019, 17, 1643–1650. [Google Scholar] [CrossRef]
- Hodge, J.C.; Morton, C.C. Genetic heterogeneity among uterine leiomyomata: Insights into malignant progression. Hum. Mol. Genet. 2007, 16, R7–R13. [Google Scholar] [CrossRef]
- Upadhyay, S.; Dubey, P.K. Gene variants polymorphisms and uterine leiomyoma: An updated review. Front. Genet. 2024, 15, 1330807. [Google Scholar] [CrossRef] [PubMed]
- Chiurchiu, V.; Leuti, A.; Maccarrone, M. Bioactive Lipids and Chronic Inflammation: Managing the Fire Within. Front. Immunol. 2018, 9, 38. [Google Scholar] [CrossRef]
- Flower, R.J. Prostaglandins, bioassay and inflammation. Br. J. Pharmacol. 2006, 147 (Suppl. 1), S182–S192. [Google Scholar] [CrossRef]
- Samuelsson, B. Role of basic science in the development of new medicines: Examples from the eicosanoid field. J. Biol. Chem. 2012, 287, 10070–10080. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Moldawer, L.L.; Opal, S.M.; Reinhart, K.; Turnbull, I.R.; Vincent, J.-L. Sepsis and septic shock. Nat. Rev. Dis. Primers 2016, 2, 16045. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C.; Ding, A. Nonresolving inflammation. Cell 2010, 140, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef]
- Serhan, C.N.; Clish, C.B.; Brannon, J.; Colgan, S.P.; Chiang Gronert, N.K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J. Exp. Med. 2000, 192, 1197–1204. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N.; Dalli, J. The resolution code of acute inflammation: Novel pro-resolving lipid mediators in resolution. Semin. Immunol. 2015, 27, 200–215. [Google Scholar] [CrossRef]
- Serhan, C.N. Treating inflammation and infection in the 21st century: New hints from decoding resolution mediators and mechanisms. FASEB J. 2017, 31, 1273–1288. [Google Scholar] [CrossRef]
- Regidor, P.A.; Eiblwieser, J.; Steeb, T.; Rizo, J.M. Omega-3 long chain fatty acids and their metabolites in pregnancy outcomes for the modulation of maternal inflammatory- associated causes of preterm delivery, chorioamnionitis and preeclampsia. F1000Research 2024, 13, 882. [Google Scholar] [CrossRef]
- Serhan, C.N.; Fredman, G.; Yang, R.; Karamnov, S.; Belayev, L.S.; Bazan, N.G.; Zhu, M.; Winkler, J.W.; Petasis, N.A. Novel proresolving aspirin-triggered DHA pathway. Chem. Biol. 2011, 18, 976–987. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Hong, S.; Gronert, K.; Colgan, S.P.; Devchand, P.R.; Mirick, G.; Moussignac, R.L. Resolvins: A family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J. Exp. Med. 2002, 196, 1025–1037. [Google Scholar] [CrossRef]
- Schett, G.; Neurath, M.F. Resolution of chronic inflammatory disease: Universal and tissue-specific concepts. Nat. Commun. 2018, 9, 3261. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N. Resolution phase lipid mediators of inflammation: Agonists of resolution. Curr. Opin. Pharmacol. 2013, 13, 632–640. [Google Scholar] [CrossRef]
- Serhan, C.N.; Levy, B.D. Resolvins in inflammation: Emergence of the pro-resolving superfamily of mediators. J. Clin. Investig. 2018, 128, 2657–2669. [Google Scholar] [CrossRef]
- Serhan, C.N.; Savill, J. Resolution of inflammation: The beginning programs the end. Nat. Immunol. 2005, 6, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Bandeira-Melo, C.; Serra, M.F.; Diaz, B.L.; Cordeiro, R.S.; Silva, P.M.; Lenzi, H.L.; Bakhle, Y.S.; Serhan, C.N.; Martins, M.A. Cyclooxygenase-2-derived prostaglandin E2 and lipoxin A4 accelerate resolution of allergic edema in Angiostrongylus costaricensis-infected rats: Relationship with concurrent eosinophilia. J. Immunol. 2000, 164, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Levy, B.D.; Clish, C.B.; Schmidt, B.; Gronert, K.; Serhan, C.N. Lipid mediator class switching during acute inflammation: Signals in resolution. Nat. Immunol. 2001, 2, 612–619. [Google Scholar] [CrossRef]
- Barnig, C.; Cernadas, M.; Dutile, S.; Liu, X.; Perrella, M.A.; Kazani, S.; Wechsler, M.E.; Israel, E.; Levy, B.D. Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma. Sci. Transl. Med. 2013, 5, 174ra26. [Google Scholar] [CrossRef] [PubMed]
- Chiurchiù, V.; Leuti, A.; Dalli, J.; Jacobsson, A.; Battistini, L.; Maccarrone, M.; Serhan, C.N. Proresolving lipid mediators resolvin D1, resolvin D2, and maresin 1 are critical in modulating T cell responses. Sci. Transl. Med. 2016, 8, 353ra111. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Jain, A.; Marleau, S.; Clish, C.; Kantarci, A.; Behbehani, B.; Colgan, S.P.; Stahl, G.L.; Merched, A.; Petasis, N.A.; et al. Reduced inflammation and tissue damage in transgenic rabbits overexpressing 15-lipoxygenase and endogenous anti-inflammatory lipid mediators. J. Immunol. 2003, 171, 6856–6865. [Google Scholar] [CrossRef]
- Merched, A.J.; Ko, K.; Gotlinger, K.H.; Serhan, C.N.; Chan, L. Atherosclerosis: Evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. FASEB J. 2008, 22, 3595–3606. [Google Scholar] [CrossRef]
- Merched, A.J.; Serhan, C.N.; Chan, L. Nutrigenetic disruption of inflammation-resolution homeostasis and atherogenesis. J. Nutr. Nutr. 2011, 4, 12–24. [Google Scholar] [CrossRef]
- Lima-Garcia, J.F.; Dutra, R.C.; da Silva, K.A.B.S.; Motta, E.M.; Campos, M.M.; Calixto, J.B. The precursor of resolvin D series and aspirin-triggered resolvin D1 display anti-hyperalgesic properties in adjuvant-induced arthritis in rats. Br. J. Pharmacol. 2011, 164, 278–293. [Google Scholar] [CrossRef]
- Martins, V.; Valença, S.S.; Farias-Filho, F.A.; Molinaro, R.; Simões, R.L.; Ferreira, T.P.T.; e Silva, P.M.R.; Hogaboam, C.M.; Kunkel, S.L.; Fierro, I.M.; et al. ATLa, an aspirin-triggered lipoxin A4 synthetic analog, prevents the inflammatory and fibrotic effects of bleomycin-induced pulmonary fibrosis. J. Immunol. 2009, 182, 5374–5381. [Google Scholar] [CrossRef]
- Börgeson, E.; Docherty, N.G.; Murphy, M.; Rodgers, K.; Ryan, A.; O’Sullivan, T.P.; Guiry, P.J.; Goldschmeding, R.; Higgins, D.F.; Godson, C. Lipoxin A4 and benzo-lipoxin A4 attenuate experimental renal fibrosis. FASEB J. 2011, 25, 2967–2979. [Google Scholar] [CrossRef]
- Qu, X.; Zhang, X.; Yao, J.; Song, J.; Nikolic-Paterson, D.J.; Li, J. Resolvins E1 and D1 inhibit interstitial fibrosis in the obstructed kidney via inhibition of local fibroblast proliferation. J. Pathol. 2012, 228, 506–519. [Google Scholar] [CrossRef]
- Lagana, A.S.; Vitale, S.G.; Ban Frangez, H.; Vrtacnik-Bokal, E.; D’Anna, R. Vitamin D in human reproduction: The more, the better? An evidence-based critical appraisal. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 4243–4251. [Google Scholar] [PubMed]
- Colonese, F.; Laganà, A.S.; Colonese, E.; Sofo, V.; Salmeri, F.M.; Granese, R.; Triolo, O. The pleiotropic Effects of Vitamin D in Gynaecological and Obstetric Diseases: An Overview on a Hot Topic. BioMed Res. Int. 2015, 2015, 986281. [Google Scholar] [CrossRef]
- Vergara, D.; Catherino, W.H.; Trojano, G.; Tinelli, A. Vitamin D: Mechanism of Action and Biological Effects in Uterine Fibroids. Nutrients 2021, 13, 597. [Google Scholar] [CrossRef] [PubMed]
- Harris, H.R.; Eliassen, A.H.; Doody, D.R.; Terry, K.L.; Missmer, S.A. Dietary fat intake, erythrocyte fatty acids, and risk of uterine fibroids. Fertil. Steril. 2020, 114, 837–847. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Regidor, P.-A.; Mayr, M.; Santos, F.G.; Calvo, B.L.; Gutierrez, R.; Rizo, J.M. Narrative Review of Chronic Inflammation in Uterine Myoma: Lack of Specialized Pro-Resolving Lipid Mediators (SPMs) and Vitamin D as a Potential Reason for the Development of Uterine Fibroids. Biomedicines 2025, 13, 1832. https://doi.org/10.3390/biomedicines13081832
Regidor P-A, Mayr M, Santos FG, Calvo BL, Gutierrez R, Rizo JM. Narrative Review of Chronic Inflammation in Uterine Myoma: Lack of Specialized Pro-Resolving Lipid Mediators (SPMs) and Vitamin D as a Potential Reason for the Development of Uterine Fibroids. Biomedicines. 2025; 13(8):1832. https://doi.org/10.3390/biomedicines13081832
Chicago/Turabian StyleRegidor, Pedro-Antonio, Manuela Mayr, Fernando Gonzalez Santos, Beatriz Lazcoz Calvo, Rocio Gutierrez, and Jose Miguel Rizo. 2025. "Narrative Review of Chronic Inflammation in Uterine Myoma: Lack of Specialized Pro-Resolving Lipid Mediators (SPMs) and Vitamin D as a Potential Reason for the Development of Uterine Fibroids" Biomedicines 13, no. 8: 1832. https://doi.org/10.3390/biomedicines13081832
APA StyleRegidor, P.-A., Mayr, M., Santos, F. G., Calvo, B. L., Gutierrez, R., & Rizo, J. M. (2025). Narrative Review of Chronic Inflammation in Uterine Myoma: Lack of Specialized Pro-Resolving Lipid Mediators (SPMs) and Vitamin D as a Potential Reason for the Development of Uterine Fibroids. Biomedicines, 13(8), 1832. https://doi.org/10.3390/biomedicines13081832





