Integrating Regenerative Medicine in Chronic Wound Management: A Single-Center Experience
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
- Graft-assisted group: Patients treated with PRP/fat grafting plus a skin graft;
- Regenerative-only group: Patients treated with PRP/fat without grafting.
- (1)
- Chronic wound duration of >6 weeks;
- (2)
- Absence of systemic infection;
- (3)
- ASuitability for surgical debridement.
- (1)
- Signs of acute infection with systemic involvement;
- (2)
- Coagulation disorders;
- (3)
- Contraindications for autologous blood or fat harvesting.
2.2. Data Collection and Clinical Evaluation
2.3. Statistical Analysis
- Student’s T-test for normally distributed quantitative variables;
- Mann–Whitney U test for non-parametric data;
- Fisher’s exact test for categorical variables.
3. Results
3.1. Patient Demographics and Clinical Characteristics
3.2. Wound Etiology, Localization, and Duration
3.3. Association Between Clinical Variables and Use of Skin Grafts
3.4. Treatment Modalities and Therapeutic Interventions
3.5. Healing Outcomes and Wound Surface Reduction
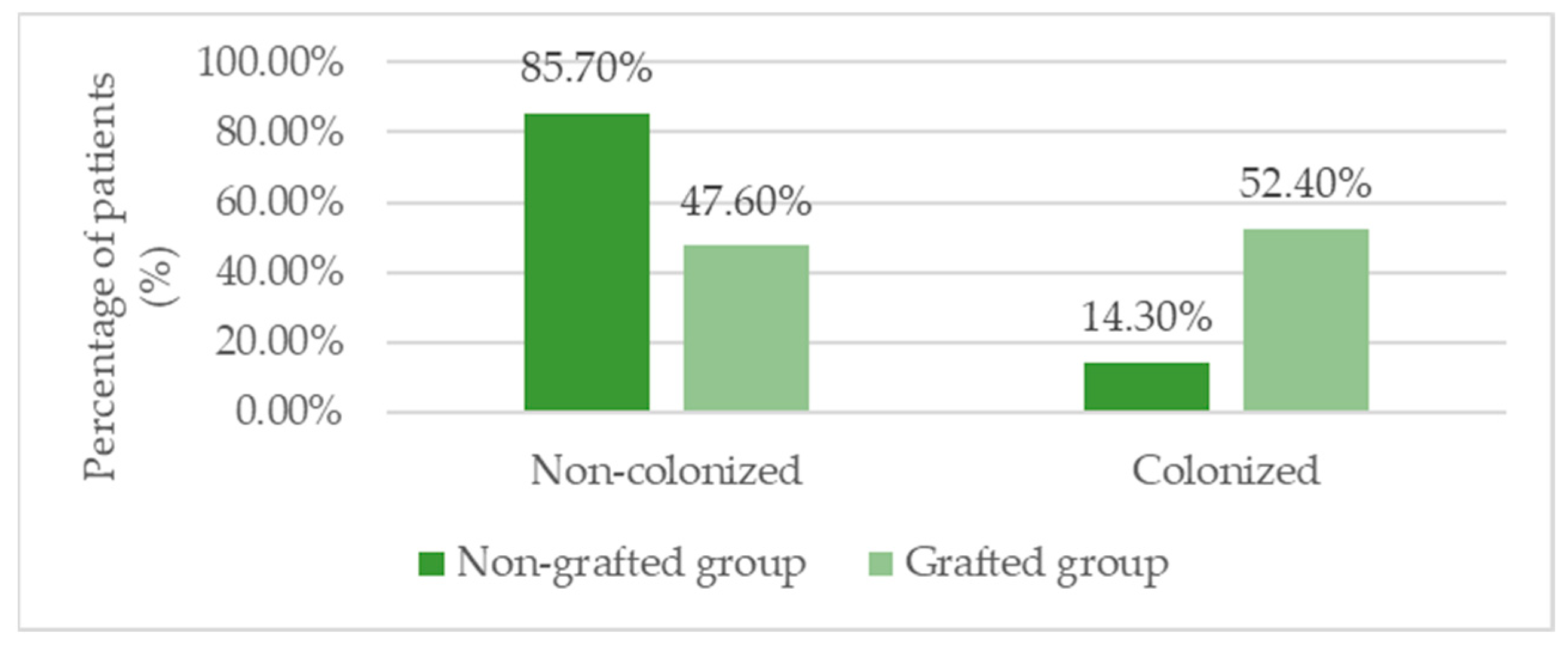
3.6. Infection Profile and Microbiological Findings
3.7. Inflammatory Markers and Laboratory Parameters
3.8. Use of Antibiotic Therapy and Duration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADSCs | Adipose tissue-derived stem cells |
| PRP | Platelet-rich plasma |
| VEGF | Vascular Endothelial Growth Factor |
| HGF | Hepatocyte Growth Factor |
| EGF | Epidermal Growth Factor |
| IGF | Insulin-like Growth Factor (IGF) |
| PDGF | Platelet-Derived Growth Factor |
| FGF | Fibroblast Growth Factor |
| TGF-β | Transforming Growth Factor Beta (TGF-β) |
| NLR | Neutrophil-to-Lymphocyte Ratio |
| NPWT | Negative pressure wound therapy |
References
- Carter, M.J. Economic evaluations of guideline-based or strategic interventions for the prevention or treatment of chronic wounds. Appl. Health Econ. Health Policy 2014, 12, 373–389. [Google Scholar] [CrossRef]
- Bowers, S.; Franco, E. Chronic wounds: Evaluation and management. Am. Fam. Physician 2020, 101, 159–166. [Google Scholar] [PubMed]
- Zhu, X.; Olsson, M.M.; Bajpai, R.; Järbrink, K.; Tang, W.E.; Car, J. Health-related quality of life and chronic wound characteristics among patients with chronic wounds treated in primary care: A cross-sectional study in Singapore. Int. Wound J. 2022, 19, 1121–1132. [Google Scholar] [CrossRef]
- Sen, C.K. Human wound and its burden: Updated 2022 compendium of estimates. Adv. Wound Care 2023, 12, 657–670. [Google Scholar] [CrossRef]
- Riedel, K.; Ryssel, H.; Koellensperger, E.; Germann, G.; Kremer, T. Pathophysiologie der chronischen Wunde. Chirurg 2008, 79, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Mostow, E.N. Diagnosis and classification of chronic wounds. Clin. Dermatol. 1994, 12, 3–9. [Google Scholar] [CrossRef]
- Eriksson, E.; Liu, P.Y.; Schultz, G.S.; Martins-Green, M.M.; Tanaka, R.; Weir, D.; Gould, L.J.; Armstrong, D.G.; Gibbons, G.W.; Wolcott, R.; et al. Chronic wounds: Treatment consensus. Wound Repair Regen. 2022, 30, 156–171, Erratum in: Wound Repair Regen. 2022, 30, 536. [Google Scholar] [CrossRef] [PubMed]
- Izadi, K.; Ganchi, P. Chronic wounds. Clin. Plast. Surg. 2005, 32, 209–222. [Google Scholar] [CrossRef]
- Hopf, H.W.; Hunt, T.K.; West, J.M.; Blomquist, P.; Goodson, W.H.; Jensen, J.A.; Jonsson, K.; Paty, P.B.; Rabkin, J.M.; Upton, R.A.; et al. Wound tissue oxygen tension predicts the risk of wound infection in surgical patients. Arch. Surg. 1997, 132, 997–1005. [Google Scholar] [CrossRef]
- Kurz, A.; Sessler, D.I.; Lenhardt, R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of Wound Infection and Temperature Group. N. Engl. J. Med. 1996, 334, 1209–1215. [Google Scholar] [CrossRef]
- Mustoe, T. Understanding chronic wounds: A unifying hypothesis on their pathogenesis and implications for therapy. Am. J. Surg. 2004, 187, S65–S70. [Google Scholar] [CrossRef]
- James, G.A.; Swogger, E.; Wolcott, R.; deLancey Pulcini, E.; Secor, P.; Sestrich, J.; Costerton, J.W.; Stewart, P.S. Biofilms in chronic wounds. Wound Repair Regen. 2008, 16, 37–44. [Google Scholar] [CrossRef]
- Peña, O.A.; Martin, P. Cellular and molecular mechanisms of skin wound healing. Nat. Rev. Mol. Cell Biol. 2024, 25, 599–616. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Guarino, M.; Hernández-Bule, M.L.; Bacci, S. Cellular and Molecular Processes in Wound Healing. Biomedicines 2023, 13, 2526. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Al Mamun, A.; Shao, C.; Geng, P.; Wang, S.; Xiao, J. Recent advances in molecular mechanisms of skin wound healing and its treatments. Front Immunol. 2024, 21, 1395479. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Samaniego-Ruiz, M.-J.; Llatas, F.P.; Jiménez, O.S. Valoración de las heridas crónicas en el adulto: Una revisión integrativa. Rev. Esc. Enferm. USP 2018, 52, e03315. [Google Scholar] [CrossRef]
- Guo, S.; DiPietro, L. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Frykberg, R.G.; Banks, J. Challenges in the treatment of chronic wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef]
- Altalbawy, F.M.; Mukhlif, B.A.M.; Hussen, A.; Mohammed, J.S.; S, R.J.; Singh, A.; Mishra, S.B.; Chauhan, A.S.; Astaneh, M.E.; Fereydouni, N. Regenerative potential of PRP-based scaffolds in chronic wound healing: Mechanisms, advances, and therapeutic insights. Regen Ther. 2025, 30, 278–298. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Z.; Feng, C.; Chang, G.; Liu, H.; Li, S. The use of platelet-rich plasma in wound healing and vitiligo: A systematic review and meta-analysis. Skin Res. Technol. 2023, 29, e13444. [Google Scholar] [CrossRef] [PubMed]
- Blair, P.; Flaumenhaft, R. Platelet alpha-granules: Basic biology and clinical correlates. Blood Rev. 2009, 23, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Pattani, N.; Sanghera, J.; Langridge, B.J.; Frommer, M.L.; Abu-Hanna, J.; Butler, P.; Liu, L.-P. Exploring the mechanisms behind autologous lipotransfer for radiation-induced fibrosis: A systematic review. PLoS ONE 2024, 19, e0292013. [Google Scholar] [CrossRef]
- Irger, M.; Willinger, L.; Lacheta, L.; Pogorzelski, J.; Imhoff, A.B.; Feucht, M.J. Proximal Hamstring Tendon Avulsion Injuries Occur Predominately in Middle-Aged Patients with Distinct Gender Differences: Epidemiologic Analysis of 263 Surgically Treated Cases. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 1221–1229. [Google Scholar] [CrossRef]
- Queruel, V.; Kabore, R.; Guillaume, A.; Moreau, K.; Leffondre, K.; Merville, P.; Bernhard, J.C. Is Recipient’s Body Mass Index a Determinant of Short Term Complications in Early Renal Transplantation? Prog. Urol. 2020, 30, 663–674. [Google Scholar] [CrossRef]
- Varra, F.N.; Varras, M.; Varra, V.K.; Theodosis-Nobelos, P. Molecular and Pathophysiological Relationship between Obesity and Chronic Inflammation in the Manifestation of Metabolic Dysfunctions and Their Inflammation Mediating Treatment Options. Mol. Med. Rep. 2024, 29, 95. [Google Scholar] [CrossRef]
- Chen, F.; Wang, X.; Pan, Y.; Ni, B.; Wu, J. The Paradox of Obesity in Pressure Ulcers of Critically Ill Patients. Int. Wound J. 2023, 20, 2753–2763. [Google Scholar] [CrossRef]
- Mazini, L.; Rochette, L.; Admou, B.; Amal, S.; Malka, G. Hopes and Limits of Adipose-Derived Stem Cells (ADSCs) and Mesenchymal Stem Cells (MSCs) in Wound Healing. Int. J. Mol. Sci. 2020, 21, 1306. [Google Scholar] [CrossRef] [PubMed]
- Ukaegbu, K.; Allen, E.; Svoboda, K.K. Reactive Oxygen Species and Antioxidants in Wound Healing: Mechanisms and Therapeutic Potential. Int. Wound J. 2025, 22, e70330. [Google Scholar] [CrossRef]
- Leśków, N.; Karp, Z.; Banaszewski, M.; Popielska, K.; Grześkowiak, M.; Mikołajski, J.; Kranc, W. Characteristics and Cellular Mechanism of the Wound Healing Process in the Oral Mucosa. Med. J. Cell Biol. 2023, 11, 1–12. [Google Scholar] [CrossRef]
- Fan Chiang, Y.H.; Lee, Y.W.; Lam, F.; Liao, C.C.; Chang, C.C.; Lin, C.S. Smoking Increases the Risk of Postoperative Wound Complications: A Propensity Score-Matched Cohort Study. Int. Wound J. 2023, 20, 391–402. [Google Scholar] [CrossRef]
- Fong, M.; Kaner, E.; Rowland, M.; Graham, H.E.; McEvoy, L.; Hallsworth, K.; Madigan, C.D. The Effect of Preoperative Behaviour Change Interventions on Pre- and Post-Surgery Health Behaviours, Health Outcomes, and Health Inequalities in Adults: A Systematic Review and Meta-Analyses. PLoS ONE 2023, 18, e0286757. [Google Scholar] [CrossRef]
- Li, C.W.; Yu, K.; Shyh-Chang, N.; Jiang, Z.; Liu, T.; Ma, S.; Liu, G.S. Pathogenesis of Sarcopenia and the Relationship with Fat Mass: Descriptive Review. J. Cachexia Sarcopenia Muscle 2022, 13, 781–794. [Google Scholar] [CrossRef]
- Raffetto, J.D.; Ligi, D.; Maniscalco, R.; Khalil, R.A.; Mannello, F. Why Venous Leg Ulcers Have Difficulty Healing: Overview on Pathophysiology, Clinical Consequences, and Treatment. J. Clin. Med. 2020, 10, 29. [Google Scholar] [CrossRef]
- Isidori, M.; Corbee, R.J.; Trabalza-Marinucci, M. Nonpharmacological Treatment Strategies for the Management of Canine Chronic Inflammatory Enteropathy—A Narrative Review. Vet. Sci. 2022, 9, 37. [Google Scholar] [CrossRef]
- Gwilym, B.L.; Mazumdar, E.; Naik, G.; Tolley, T.; Harding, K.; Bosanquet, D.C. Initial Reduction in Ulcer Size as a Prognostic Indicator for Complete Wound Healing: A Systematic Review of Diabetic Foot and Venous Leg Ulcers. Adv. Wound Care 2023, 12, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Raja, S.S.; Bryant, R.V.; Costello, S.P.; Barnett, M.; Schubert, J.; Rayner, C.K. Systematic Review of Therapies for Refractory Ulcerative Proctitis. J. Gastroenterol. Hepatol. 2023, 38, 496–509. [Google Scholar] [CrossRef] [PubMed]
- Falanga, V.; Isseroff, R.R.; Soulika, A.M.; Romanelli, M.; Margolis, D.; Kapp, S.; Harding, K. Chronic Wounds. Nat. Rev. Dis. Primers 2022, 8, 50. [Google Scholar] [CrossRef] [PubMed]
- Kanta, J.; Zavadakova, A.; Sticova, E.; Dubsky, M. Fibronectin in Hyperglycaemia and Its Potential Use in the Treatment of Diabetic Foot Ulcers: A Review. Int. Wound J. 2023, 20, 1750–1761. [Google Scholar] [CrossRef]
- Liu, L.; Hao, X.; Zhang, J.; Li, S.; Han, S.; Qian, P.; Chai, W. The Wound Healing of Deep Partial-Thickness Burn in Bama Miniature Pigs Is Accelerated by a Higher Dose of hUCMSCs. Stem Cell Res. Ther. 2024, 15, 437. [Google Scholar] [CrossRef]
- Galarraga-Vinueza, M.E.; Barootchi, S.; Nevins, M.L.; Nevins, M.; Miron, R.J.; Tavelli, L. Twenty-Five Years of Recombinant Human Growth Factors rhPDGF-BB and rhBMP-2 in Oral Hard and Soft Tissue Regeneration. Periodontology 2000 2024, 94, 483–509. [Google Scholar] [CrossRef]
- Naderi, N.; Griffin, M.F.; Mosahebi, A.; Butler, P.E.; Seifalian, A.M. Adipose Derived Stem Cells and Platelet Rich Plasma Improve the Tissue Integration and Angiogenesis of Biodegradable Scaffolds for Soft Tissue Regeneration. Mol. Biol. Rep. 2020, 47, 2005–2013. [Google Scholar] [CrossRef]
- Elsaid, A.; El-Said, M.; Emile, S.; Youssef, M.; Khafagy, W.; Elshobaky, A. Randomized Controlled Trial on Autologous Platelet-Rich Plasma versus Saline Dressing in Treatment of Non-Healing Diabetic Foot Ulcers. World J. Surg. 2020, 44, 1294–1301. [Google Scholar] [CrossRef]
- Yu, F.; Witman, N.; Yan, D.; Zhang, S.; Zhou, M.; Yan, Y.; Fu, Y. Human Adipose-Derived Stem Cells Enriched with VEGF-Modified mRNA Promote Angiogenesis and Long-Term Graft Survival in a Fat Graft Transplantation Model. Stem Cell Res. Ther. 2020, 11, 490. [Google Scholar] [CrossRef] [PubMed]
- Hasiba-Pappas, S.K.; Tuca, A.C.; Luze, H.; Nischwitz, S.P.; Zrim, R.; Geissler, J.C.; Winter, R. Platelet-Rich Plasma in Plastic Surgery: A Systematic Review. Transfus. Med. Hemother. 2022, 49, 129–142. [Google Scholar] [CrossRef]
- Lutchminarian, K.; Clarke, D.L. The Microbiology of Ulcerative Skin Cancers: Does the Presence of Pathogenic Bacteria Increase the Risk of Postoperative Complications? S. Afr. J. Surg. 2021, 59, 25a–25e. [Google Scholar] [CrossRef] [PubMed]
- Goswami, A.G.; Basu, S.; Banerjee, T.; Shukla, V.K. Biofilm and Wound Healing: From Bench to Bedside. Eur. J. Med. Res. 2023, 28, 157. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Roy, S.; Mathew-Steiner, S.S.; Gordillo, G.M. Biofilm Management in Wound Care. Plast. Reconstr. Surg. 2021, 148, 275e–288e. [Google Scholar] [CrossRef]
- Cavallo, I.; Sivori, F.; Mastrofrancesco, A.; Abril, E.; Pontone, M.; Di Domenico, E.G.; Pimpinelli, F. Bacterial Biofilm in Chronic Wounds and Possible Therapeutic Approaches. Biology 2024, 13, 109. [Google Scholar] [CrossRef]
- Li, S.; Renick, P.; Senkowsky, J.; Nair, A.; Tang, L. Diagnostics for Wound Infections. Adv. Wound Care 2021, 10, 317–327. [Google Scholar] [CrossRef]
- Chin, J.D.; Zhao, L.; Mayberry, T.G.; Cowan, B.C.; Wakefield, M.R.; Fang, Y. Photodynamic Therapy, Probiotics, Acetic Acid, and Essential Oil in the Treatment of Chronic Wounds Infected with Pseudomonas aeruginosa. Pharmaceutics 2023, 15, 1721. [Google Scholar] [CrossRef]
- Ding, X.; Tang, Q.; Xu, Z.; Xu, Y.; Zhang, H.; Zheng, D.; Chen, J. Challenges and Innovations in Treating Chronic and Acute Wound Infections: From Basic Science to Clinical Practice. Burns Trauma 2022, 10, tkac014. [Google Scholar] [CrossRef]
- Senneville, É.; Albalawi, Z.; Van Asten, S.A.; Abbas, Z.G.; Allison, G.; Aragón-Sánchez, J.; Peters, E.J. IWGDF/IDSA Guidelines on the Diagnosis and Treatment of Diabetes-Related Foot Infections (IWGDF/IDSA 2023). Diabetes Metab. Res. Rev. 2024, 40, e3687. [Google Scholar] [CrossRef]
- Gangat, N.; Karrar, O.; Al-Kali, A.; Begna, K.H.; Elliott, M.A.; Wolanskyj-Spinner, A.P.; Tefferi, A. One Thousand Patients with Essential Thrombocythemia: The Mayo Clinic Experience. Blood Cancer J. 2024, 14, 11. [Google Scholar] [CrossRef] [PubMed]
- Sayed, D.; Grider, J.; Strand, N.; Hagedorn, J.M.; Falowski, S.; Lam, C.M.; Deer, T. The American Society of Pain and Neuroscience (ASPN) Evidence-Based Clinical Guideline of Interventional Treatments for Low Back Pain. J. Pain Res. 2022, 15, 3729–3832. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhu, P.; Liao, B.; You, H.; Cai, Y. Effects and Action Mechanisms of Individual Cytokines Contained in PRP on Osteoarthritis. J. Orthop. Surg. Res. 2023, 18, 713. [Google Scholar] [CrossRef] [PubMed]
- Cecerska-Heryć, E.; Goszka, M.; Serwin, N.; Roszak, M.; Grygorcewicz, B.; Heryć, R.; Dołęgowska, B. Applications of the Regenerative Capacity of Platelets in Modern Medicine. Cytokine Growth Factor Rev. 2022, 64, 84–94. [Google Scholar] [CrossRef]
- Sun, K.; Li, Y.Y.; Jin, J. A Double-Edged Sword of Immuno-Microenvironment in Cardiac Homeostasis and Injury Repair. Signal Transduct. Target. Ther. 2021, 6, 79. [Google Scholar] [CrossRef]
- Gandolfi, S.; Sanouj, A.; Chaput, B.; Coste, A.; Sallerin, B.; Varin, A. The Role of Adipose Tissue-Derived Stromal Cells, Macrophages and Bioscaffolds in Cutaneous Wound Repair. Biol. Direct 2024, 19, 85. [Google Scholar] [CrossRef]
- Vanderstichele, S.; Vranckx, J.J. Anti-Fibrotic Effect of Adipose-Derived Stem Cells on Fibrotic Scars. World J. Stem Cells 2022, 14, 200. [Google Scholar] [CrossRef]
- Sun, T.; Zhou, C.; Lu, F.; Dong, Z.; Gao, J.; Li, B. Adipose-Derived Stem Cells in Immune-Related Skin Disease: A Review of Current Research and Underlying Mechanisms. Stem Cell Res. Ther. 2024, 15, 37. [Google Scholar] [CrossRef]
- Chaisrisawadisuk, S.; Tangjatuporn, W.; Chuangsuwanich, A. The Effect of Gauze-Based, Negative-Pressure Wound Dressing on Skin Graft Survival: A Comparative Study. Bangkok Med. J. 2020, 16, 153. [Google Scholar] [CrossRef]
- Kourbeti, I.; Kamiliou, A.; Samarkos, M. Antibiotic Stewardship in Surgical Departments. Antibiotics 2024, 13, 329. [Google Scholar] [CrossRef]
- Karageorgos, S.; Hibberd, O.; Mullally, P.J.W.; Segura-Retana, R.; Soyer, S.; Hall, D. On Behalf of the Don’t Forget the Bubbles. Antibiotic Use for Common Infections in Pediatric Emergency Departments: A Narrative Review. Antibiotics 2023, 12, 1092. [Google Scholar]
- Sapijaszko, M.; Samadi, S.; Chow, E.Y. Optimizing Surgical Site Infection Prevention in Dermatologic Surgery. J. Cutan. Med. Surg. 2025, 29, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Sartelli, M.; Boermeester, M.A.; Cainzos, M.; Coccolini, F.; de Jonge, S.W.; Rasa, K.; Barie, P.S. Six Long-Standing Questions about Antibiotic Prophylaxis in Surgery. Antibiotics 2023, 12, 908. [Google Scholar] [CrossRef] [PubMed]


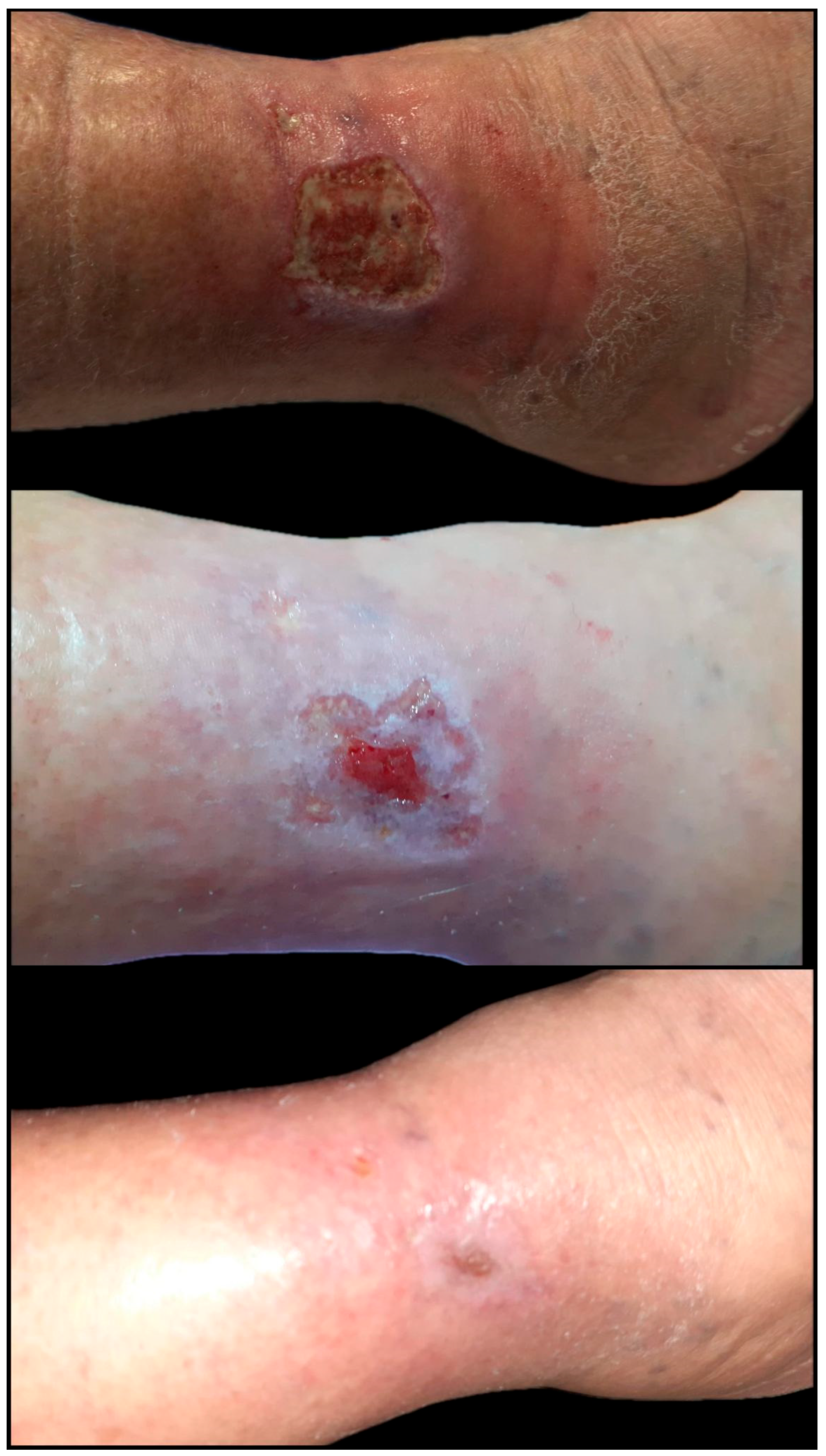
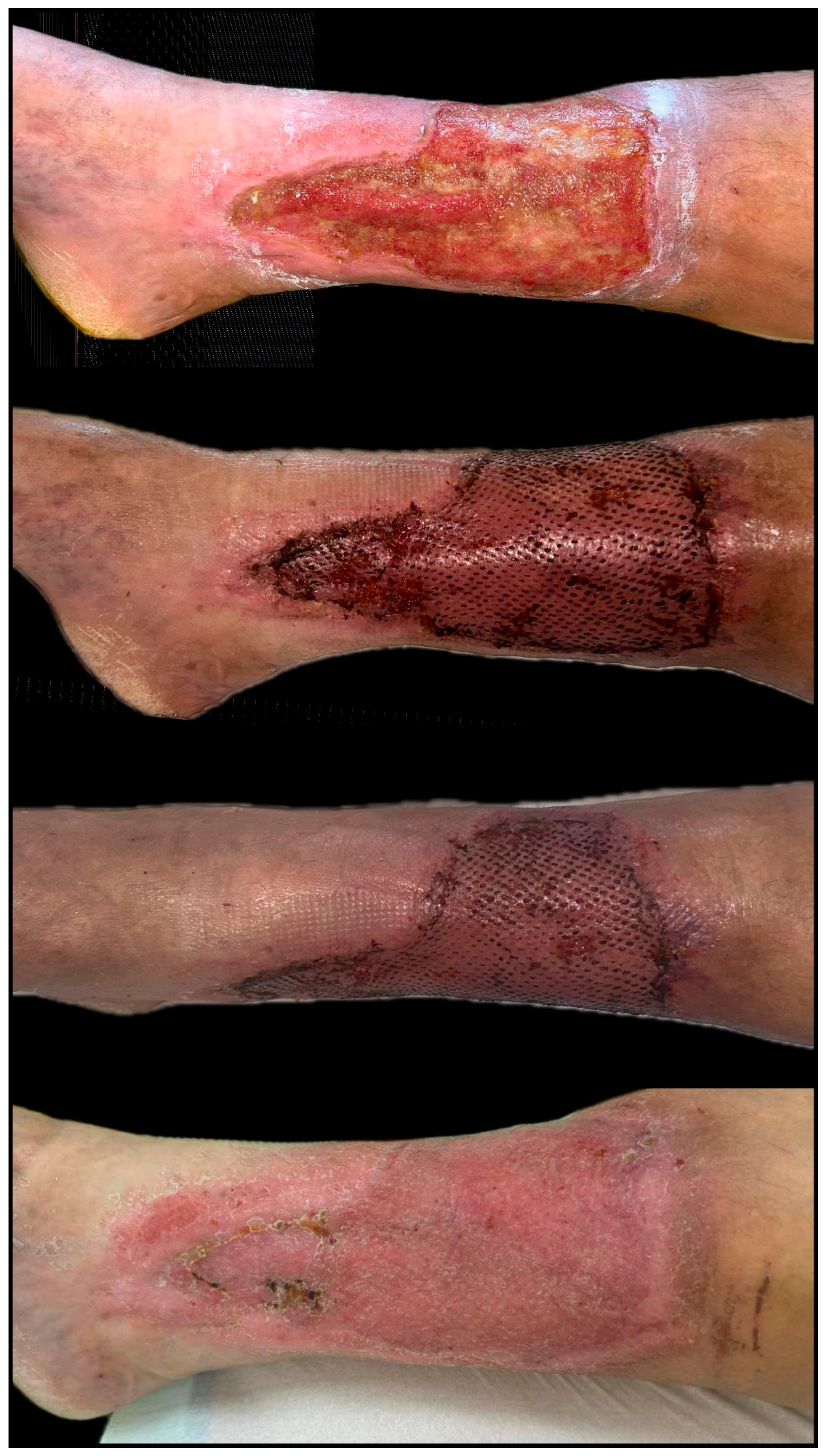
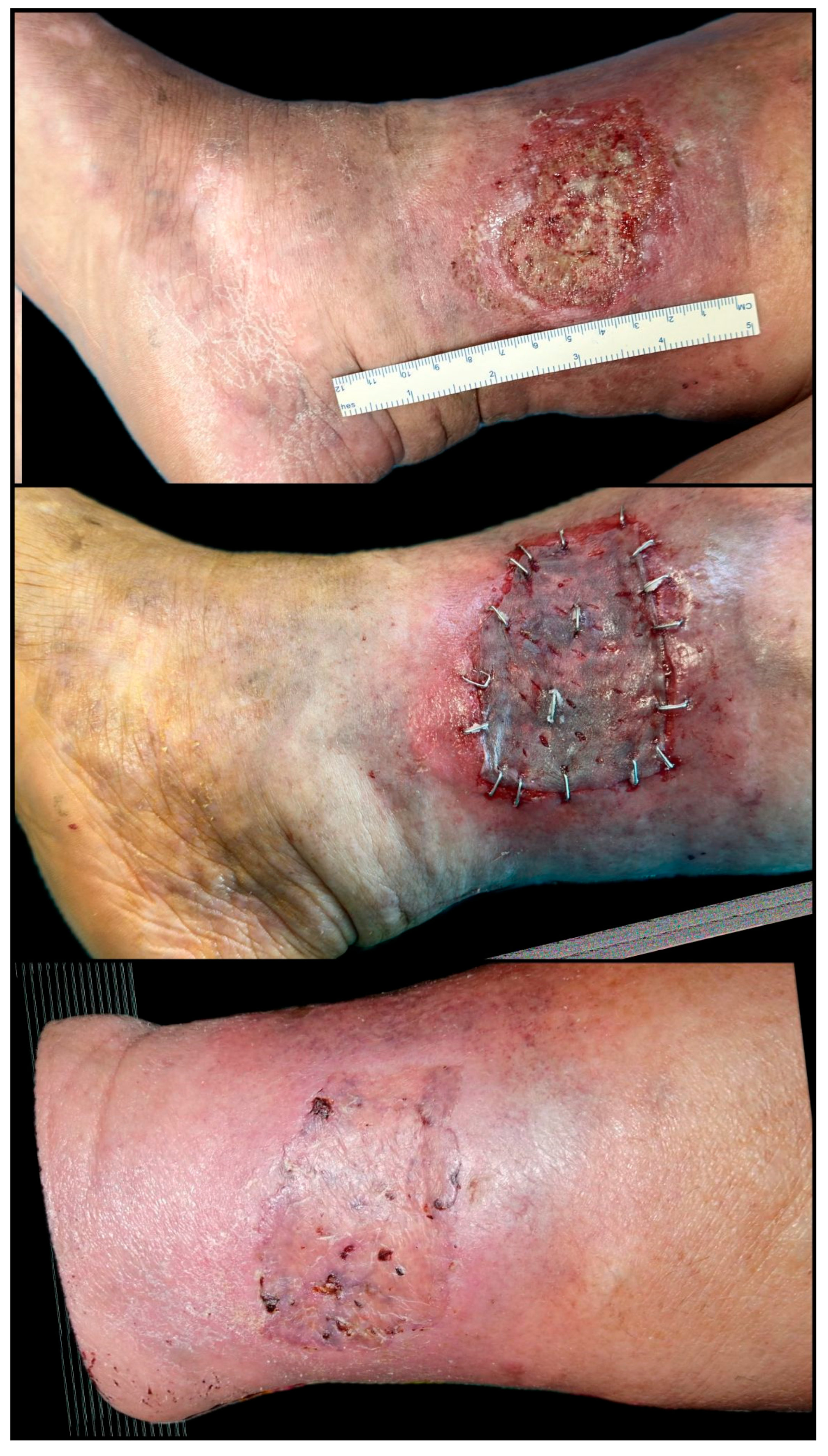
| Parameter | Total (n = 35) | Regenerative-Only Group (n = 14) | Graft-Assisted Group (n = 21) | p-Value |
|---|---|---|---|---|
| Age (mean ± SD) | 59.11 ± 13.64 | 58.14 ± 14.20 | 59.76 ± 13.58 | 0.737 * |
| Gender (male, n/%) | 19 (54.3%) | 9 (64.3%) | 10 (47.6%) | 0.491 ** |
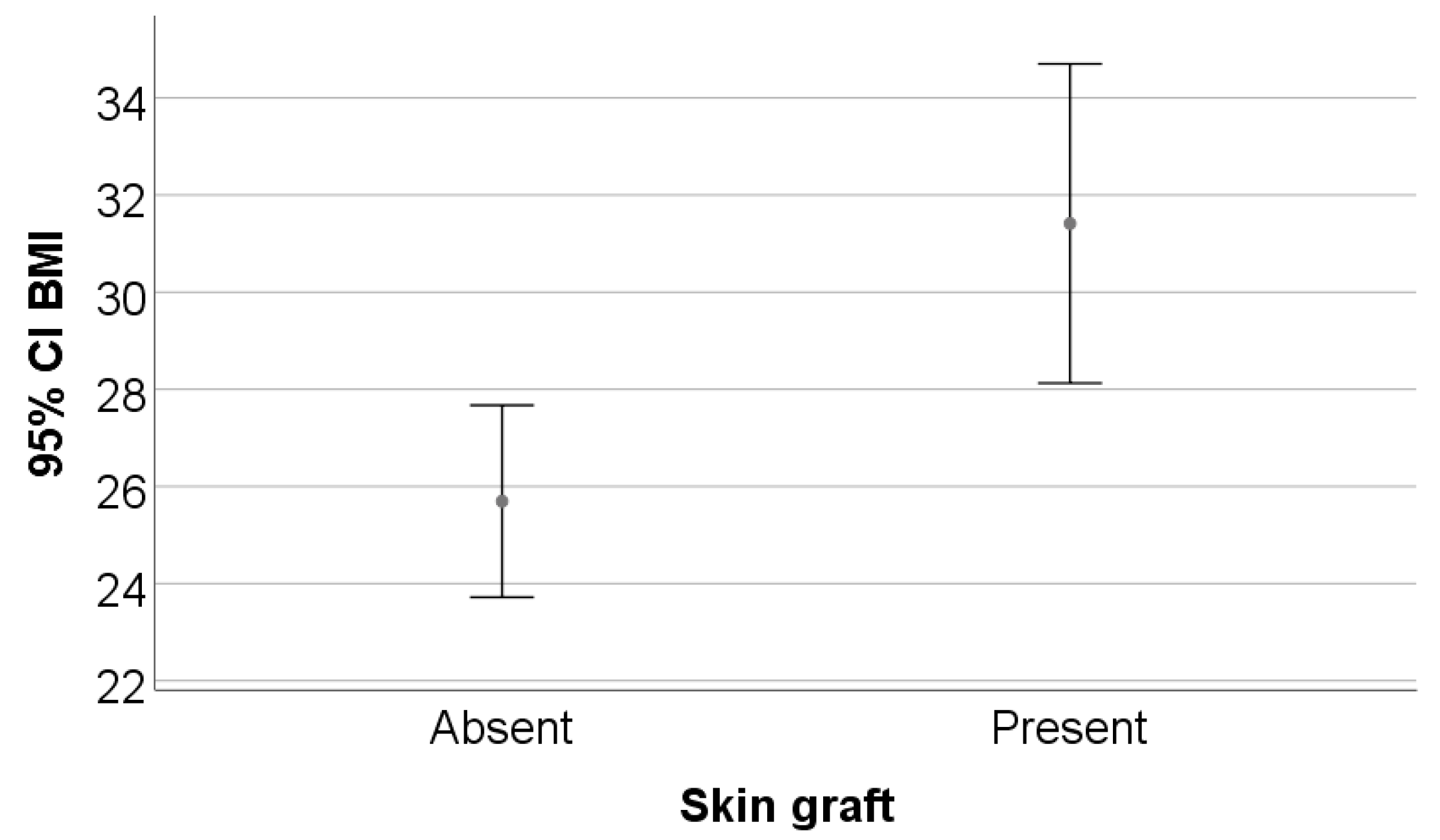
| BMI (mean ± SD) | 29.12 ± 6.57 | 25.69 ± 3.42 | 31.41 ± 7.22 | 0.009 * |
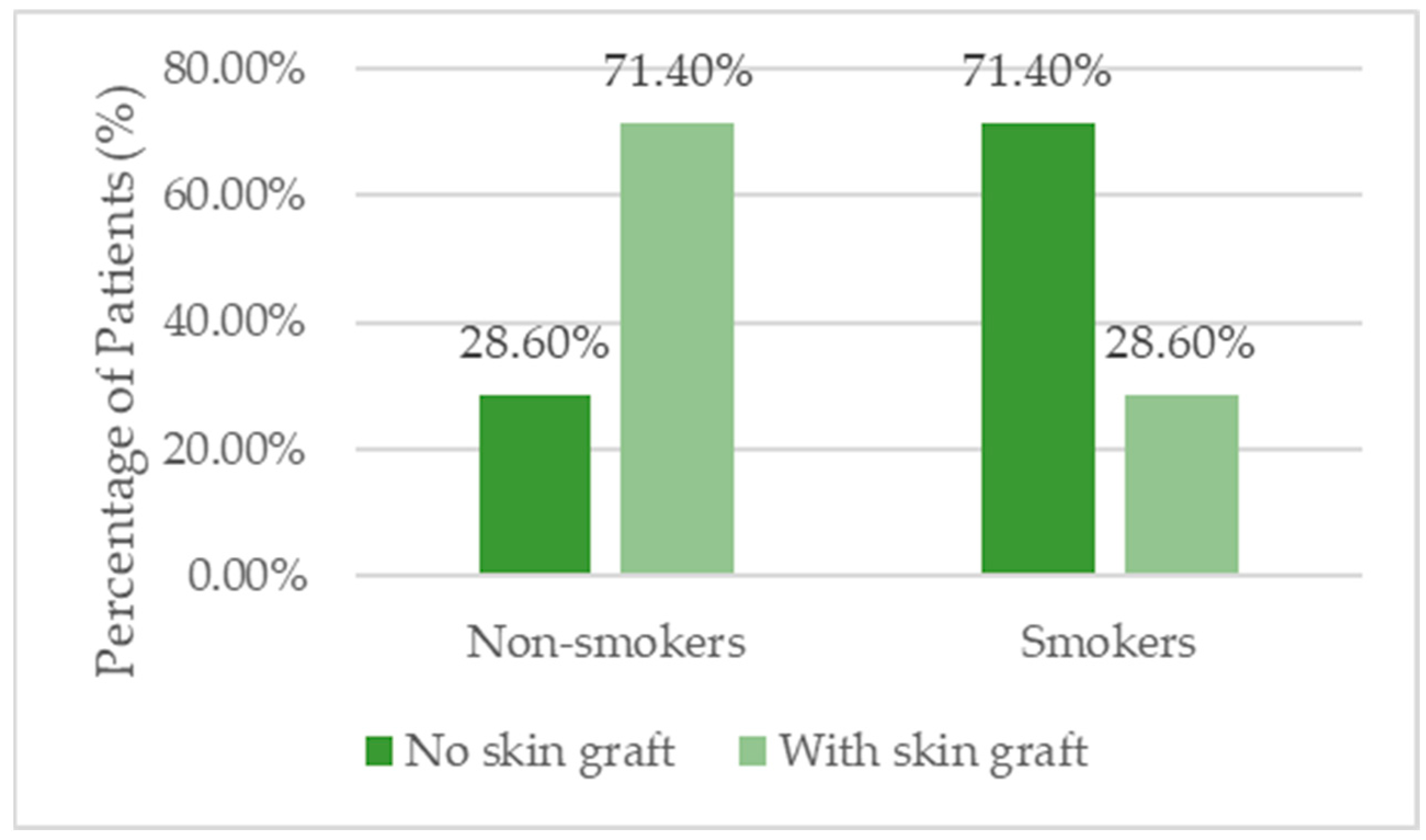
| Smoking (n/%) | 16 (45.7%) | 10 (71.4%) | 6 (28.6%) | 0.018 ** |
| Parameter | Total | Regenerative-Only Group | Graft-Assisted Group | p-Value |
|---|---|---|---|---|
| Venous ulcers (n/%) | 14 (40%) | 3 (21.4%) | 11 (52.4%) | 0.194 ** |
| Arterial ulcers (n/%) | 4 (11.4%) | 3 (21.4%) | 1 (4.8%) | |
| Diabetic ulcers (n/%) | 10 (28.6%) | 5 (35.7%) | 5 (23.8%) | |
| Post-traumatic ulcers (n/%) | 7 (20%) | 3 (21.4%) | 4 (19%) | |
| Localization—lower extremity (n/%) | 32 (91.4%) | 13 (92.9%) | 19 (90.5%) | 1.000 ** |
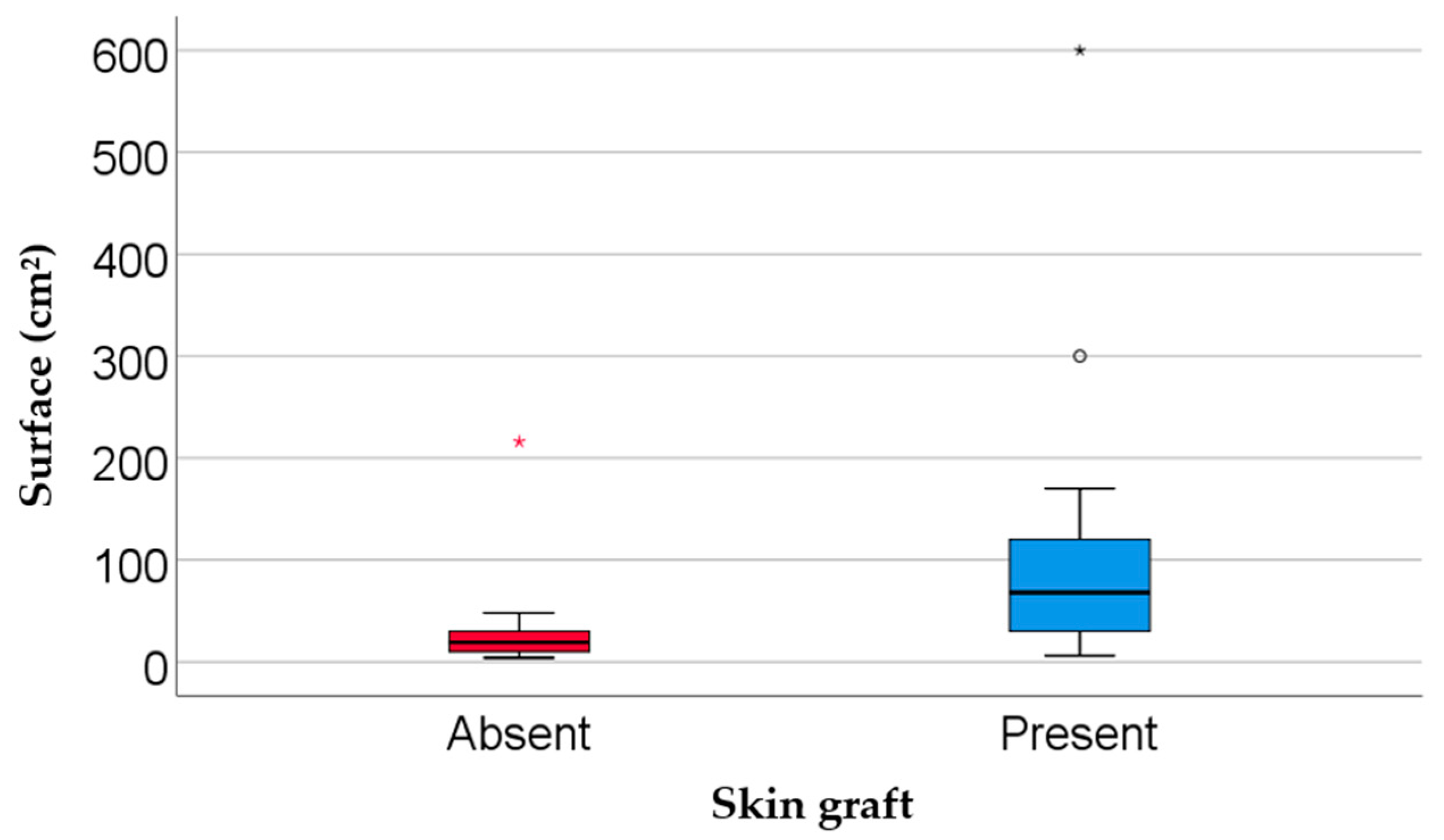
| Surface area (cm2, median IQR) | 45 (18–110) | 19 (9.75–34.5) | 68 (27.5–123) | 0.002 *** |
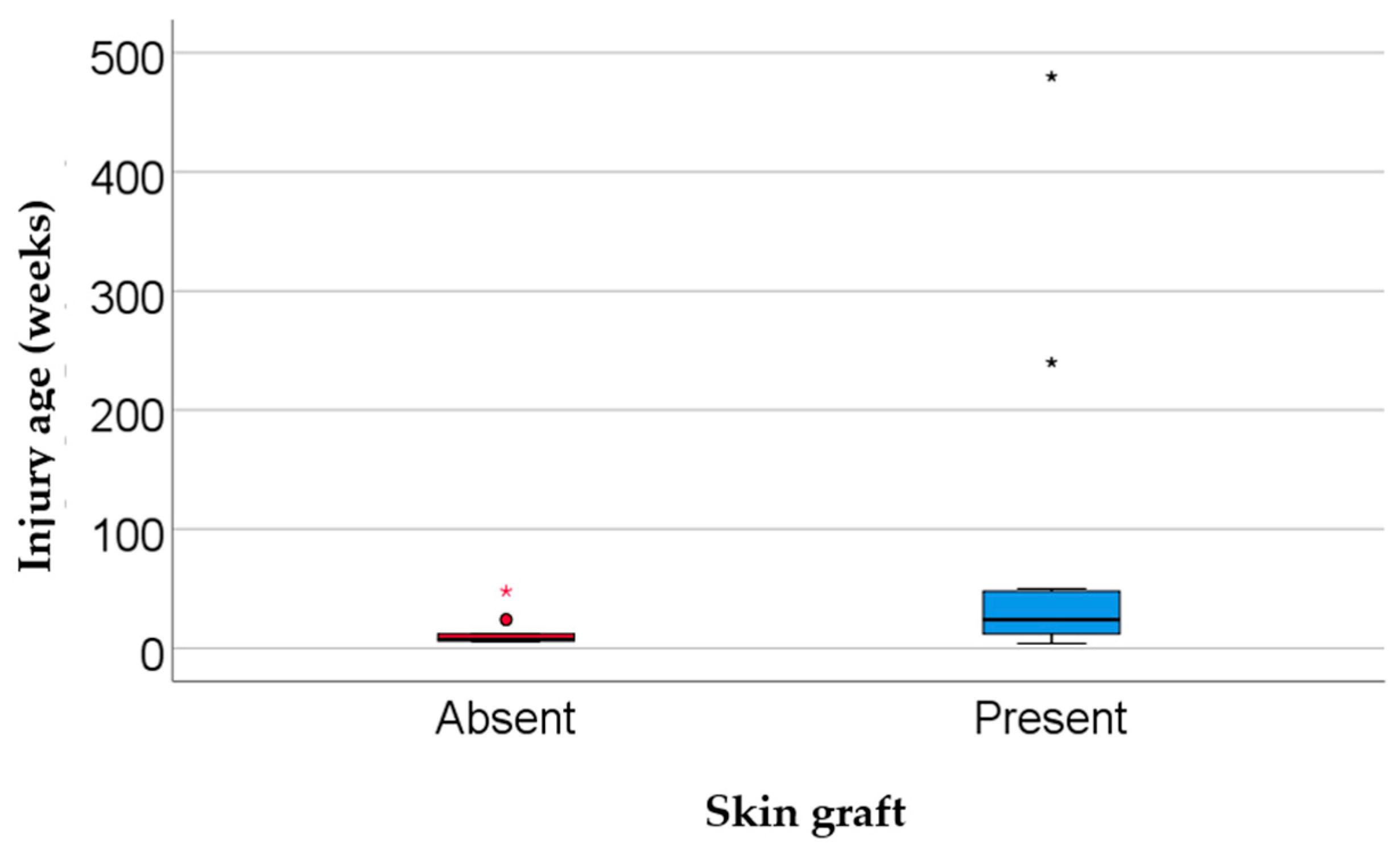
| Injury age (weeks, median IQR) | 12 (6–48) | 7.5 (6–15) | 24 (9.5–49) | 0.024 *** |
| Parameter | Total | Regenerative-Only Group | Graft-Assisted Group | p-Value |
|---|---|---|---|---|
| Diabetes (n/%) | 14 (40%) | 5 (35.7%) | 9 (42.9%) | 0.737 ** |
| Chronic venous insufficiency (n/%) | 17 (48.6%) | 4 (28.6%) | 13 (61.9%) | 0.086 ** |
| Peripheral artery disease (n/%) | 7 (20%) | 4 (28.6%) | 3 (14.3%) | 0.401 ** |
| Autoimmune/vasculitis (n/%) | 7 (20%) | 1 (7.1%) | 6 (28.6%) | 0.203 ** |
| Parameter | Total | Regenerative-Only Group | Graft-Assisted Group | p-Value |
|---|---|---|---|---|
| NPWT (n/%) | 8 (22.9%) | 1 (7.1%) | 7 (33.3%) | 0.108 ** |
| PRP-1 session (n/%) | 29 (82.9%) | 13 (92.9%) | 16 (76.2%) | 0.366 ** |
| PRP-≥2 sessions (n/%) | 6 (17.1%) | 1 (7.1%) | 5 (23.8%) | |
| Fat tissue intervention (n/%) | 6 (17.1%) | 2 (14.3%) | 4 (19%) | 1.000 ** |
| Treatment duration (days, IQR) | 35 (25–70) | 39 (17–67.75) | 34 (28–165) | 0.752 *** |
| Parameter | Total | Regenerative-Only Group | Graft-Assisted Group | p-Value |
|---|---|---|---|---|
| Healing progress (%) (IQR) | 90 (10–100) | 87.5 (0–100) | 90 (40–100) | 0.606 *** |
| No improvement (n/%) | 12 (34.3%) | 6 (42.9%) | 6 (28.6%) | 0.737 ** |
| Satisfactory results (n/%) | 7 (20%) | 2 (14.3%) | 5 (23.8%) | |
| Complete healing (n/%) | 16 (45.7%) | 6 (42.9%) | 10 (47.6%) |
| Parameter | Total | Regenerative-Only Group | Graft-Assisted Group | p-Value |
|---|---|---|---|---|
| P. aeruginosa (n/%) | 13 (37.1%) | 2 (14.3%) | 11 (52.4%) | 0.034 ** |
| S. aureus (n/%) | 6 (17.1%) | 1 (7.1%) | 5 (23.8%) | 0.366 ** |
| E. coli (n/%) | 7 (20%) | 3 (21.4%) | 4 (19%) | 1.000 ** |
| Other bacteria (n/%) | 8 (22.9%) | 1 (7.1%) | 7 (33.3%) | 0.108 ** |
| MDR bacteria (n/%) | 12 (34.3%) | 3 (21.4%) | 9 (42.9%) | 0.282 ** |
| Parameter | Total | Regenerative-Only Group | Graft-Assisted Group | p-Value |
|---|---|---|---|---|
| NLR (median, IQR) | 2 (2–4) | 2 (1.75–4.25) | 3 (2–4) | 0.778 *** |
| Fibrinogen (median, IQR) | 405 (346–478) | 398 (345–484) | 405 (344–473) | 0.829 *** |
| Parameter | Total (n = 35) | Regenerative-Only Group (n = 14) | Graft-Assisted Group (n = 21) | p-Value |
|---|---|---|---|---|
| Antibiotherapy administration rate (n/%) | 26 (74.3%) | 6 (42.9%) | 20 (95.2%) | 0.001 |
| Duration of antibiotherapy (days, IQR) | 7 (7–7.5) | 7 (7–8.75) | 7 (7–8.5) | 1.000 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riza, S.-M.; Porosnicu, A.-L.; Cepi, P.-A.; Parasca, S.V.; Sinescu, R.-D. Integrating Regenerative Medicine in Chronic Wound Management: A Single-Center Experience. Biomedicines 2025, 13, 1827. https://doi.org/10.3390/biomedicines13081827
Riza S-M, Porosnicu A-L, Cepi P-A, Parasca SV, Sinescu R-D. Integrating Regenerative Medicine in Chronic Wound Management: A Single-Center Experience. Biomedicines. 2025; 13(8):1827. https://doi.org/10.3390/biomedicines13081827
Chicago/Turabian StyleRiza, Stefania-Mihaela, Andrei-Ludovic Porosnicu, Patricia-Alina Cepi, Sorin Viorel Parasca, and Ruxandra-Diana Sinescu. 2025. "Integrating Regenerative Medicine in Chronic Wound Management: A Single-Center Experience" Biomedicines 13, no. 8: 1827. https://doi.org/10.3390/biomedicines13081827
APA StyleRiza, S.-M., Porosnicu, A.-L., Cepi, P.-A., Parasca, S. V., & Sinescu, R.-D. (2025). Integrating Regenerative Medicine in Chronic Wound Management: A Single-Center Experience. Biomedicines, 13(8), 1827. https://doi.org/10.3390/biomedicines13081827






