Comparative Effects of Pulsed Field and Radiofrequency Ablation on Blood Cell Parameters During Pulmonary Vein Isolation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Design of the Study
2.2. Study Endpoints
2.3. Procedural Description and Periprocedural Management
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Study Endpoints
3.2.1. Primary Endpoints
3.2.2. Secondary Endpoints
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACT | Activated Clotting Time |
| AF | Atrial Fibrillation |
| BMI | Body Mass Index |
| LDH | Lactate Dehydrogenase |
| LVEF | Left Ventricular Ejection Fraction |
| PFA | Pulsed Field Ablation |
| PVI | Pulmonary Vein Isolation |
| RBC | Red Blood Cell |
| RF | Radiofrequency |
References
- Popa, M.A.; Venier, S.; Menè, R.; Della Rocca, D.G.; Sacher, F.; Derval, N.; Hocini, M.; Dulucq, S.; Caluori, G.; Combes, S.; et al. Characterization and clinical significance of hemolysis after pulsed field ablation for atrial fibrillation: Results of a multicenter analysis. Circ. Arrhythmia Electrophysiol. Rev. 2024, 17, e012732. [Google Scholar] [CrossRef] [PubMed]
- Osmancik, P.; Bacova, B.; Herman, D.; Hozman, M.; Fiserova, I.; Hassouna, S.; Melenovsky, V.; Karch, J.; Vesela, J.; Benesova, K.; et al. Periprocedural intravascular hemolysis during atrial fibrillation ablation: A comparison of pulsed field with radiofrequency ablation. JACC Clin. Electrophysiol. 2024, 10, 1660–1671. [Google Scholar] [CrossRef] [PubMed]
- Ekanem, E.; Neuzil, P.; Reichlin, T.; Kautzner, J.; van der Voort, P.; Jais, P.; Chierchia, G.-B.; Bulava, A.; Blaauw, Y.; Skala, T.; et al. Safety of pulsed field ablation in more than 17,000 patients with atrial fibrillation in the MANIFEST-17K study. Nat. Med. 2024, 30, 2020–2029. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.; Casella, M.; Compagnucci, P.; Torlapati, P.G.; Della Rocca, D.G.; La Fazia, V.M.; Gianni, C.; Chierchia, G.-B.; MacDonald, B.; Mayedo, A.; et al. Acute Kidney Injury Resulting From Hemoglobinuria After Pulsed-Field Ablation in Atrial Fibrillation: Is it Preventable? JACC Clin. Electrophysiol. 2024, 10, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Mariani, M.V.; Matteucci, A.; Pierucci, N.; Palombi, M.; Compagnucci, P.; Bruti, R.; Cipollone, P.; Vinciullo, S.; Trivigno, S.; Piro, A.; et al. Pentaspline Pulsed Field Ablation Versus High-Power Short-Duration/Very High-Power Short-Duration Radiofrequency Ablation in Atrial Fibrillation: A Meta-Analysis. J. Cardiovasc. Electrophysiol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Stojadinović, P.; Ventrella, N.; Alfredová, H.; Wichterle, D.; Peichl, P.; Čihák, R.; Ing, V.F.; Borišincová, E.; Štiavnický, P.; Hašková, J.; et al. Prediction of major intravascular hemolysis during pulsed electric field ablation of atrial fibrillation using a pentaspline catheter. J. Cardiovasc. Electrophysiol. 2024, 35, 2405–2410. [Google Scholar] [CrossRef] [PubMed]
- Osmancik, P.; Bacova, B.; Hozman, M.; Pistkova, J.; Kunstatova, V.; Sochorova, V.; Waldauf, P.; Hassouna, S.; Karch, J.; Vesela, J.; et al. Myocardial Damage, Inflammation, Coagulation, and Platelet Activity During Catheter Ablation Using Radiofrequency and Pulsed-Field Energy. JACC Clin. Electrophysiol. 2024, 10, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Auf der Heiden, C.A.; Bejinariu, A.; Kelm, M.; Spieker, M.; Rana, O. Hemolysis after pulsed field ablation in pulmonary vein isolation for atrial fibrillation: A prospective controlled trial. Heart Rhythm 2024, in press. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Gulburak, T.K.; Lu, Y.; Zhang, J.; TuErhong, Z.; Tang, B.; Zhou, X. Hemolysis after pulsed-field ablation of atrial fibrillation. Heart Rhythm 2025, 22, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Fiserova, I.; Fiser, O.; Novak, M.; Trnka, J.; Gibalova, A.; Kvapil, D.; Bacova, B.; Hozman, M.; Herman, D.; Benesova, K.; et al. Significant hemolysis is present during irreversible electroporation of cardiomyocytes in vitro. Heart Rhythm 2025, 22, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Lakkireddy, D.; Katapadi, A.; Garg, J.; Herink, E.; Klotz, M.; Ganta, J.; Kabra, A.; Kabra, R.; Pothineni, N.V.; Darden, D.; et al. NEMESIS-PFA Study: LDH release and biochemical impact of PFA correlate with application count. Present. HRS 2025. [Google Scholar] [CrossRef]
- Venier, S.; Vaxelaire, N.; Jacon, P.; Carabelli, A.; Desbiolles, A.; Garban, F.; Defaye, P. Severe acute kidney injury related to haemolysis after pulsed field ablation. Europace 2024, 26, euad371. [Google Scholar] [CrossRef] [PubMed]
| PFA (n = 121) | RF (n = 128) | p-Value | |
|---|---|---|---|
| Males (%) | 60.33% [51.4–68.6] | 64.06% [55.5-71.9] | 0.634 |
| Age | 71 ± 11.9 [69.37–73.61] | 70 ± 10.2 [67.97–71.50] | 0.478 |
| BMI | 26.86 ± 4.08 [26.13–27.59] | 27.01 ± 3.65 [26.38–27.25] | 0.761 |
| Current Smoker (%) | 18.18% [12.3–26.0] | 20.31% [14.3–28.1] | 0.791 |
| Arterial Hypertension (%) | 52.89% [44.0–61.6] | 53.91% [45.3–62.3] | 0.974 |
| Diabetes (%) | 10.74% [6.4–17.5] | 10.16% [6.0–16.6] | 1.000 |
| LVEF (%) | 57 ± 2.82 [56.66–57.66] | 57.66 ± 3.32 [57.09–58.24] | 0.092 |
| ACE/ARBs/ARNI (%) | 48.76% [40.0–57.6] | 53.13% [44.5–61.6] | 0.574 |
| Diuretics (%) | 2.48% [0.8–7.0] | 7.03% [3.7–12.8] | 0.138 |
| β-Blockers (%) | 51.24% [42.4–60.0] | 57.81% [49.2–66.0] | 0.361 |
| Statins (%) | 13.22% [8.3–20.4] | 17.97% [12.3–25.5] | 0.392 |
| Antiarrhythmic Drug Class I/III/IV (%) | 64.46% [55.6–72.4] | 68.75% [60.3–76.1] | 0.560 |
| Oral Anticoagulation (%) | 89.26% [82.5–93.6] | 89.84% [83.4–94.0] | 1.000 |
| Antiplatelet (%) | 0 | 1.56% [0.4–5.5] | 0.498 |
| Liquid infusion (ml) | 731 ± 111 [711.59–751.14] | 757 ± 112 [737.74–776.46] | 0.067 |
| Procedural Time | 58.34 ± 7.64 [56.96–59.72] | 77.23 ± 11.47 [75.22–79.24] | <0.001 |
| PFA (n = 121) | RF (n = 128) | p-Value | |
|---|---|---|---|
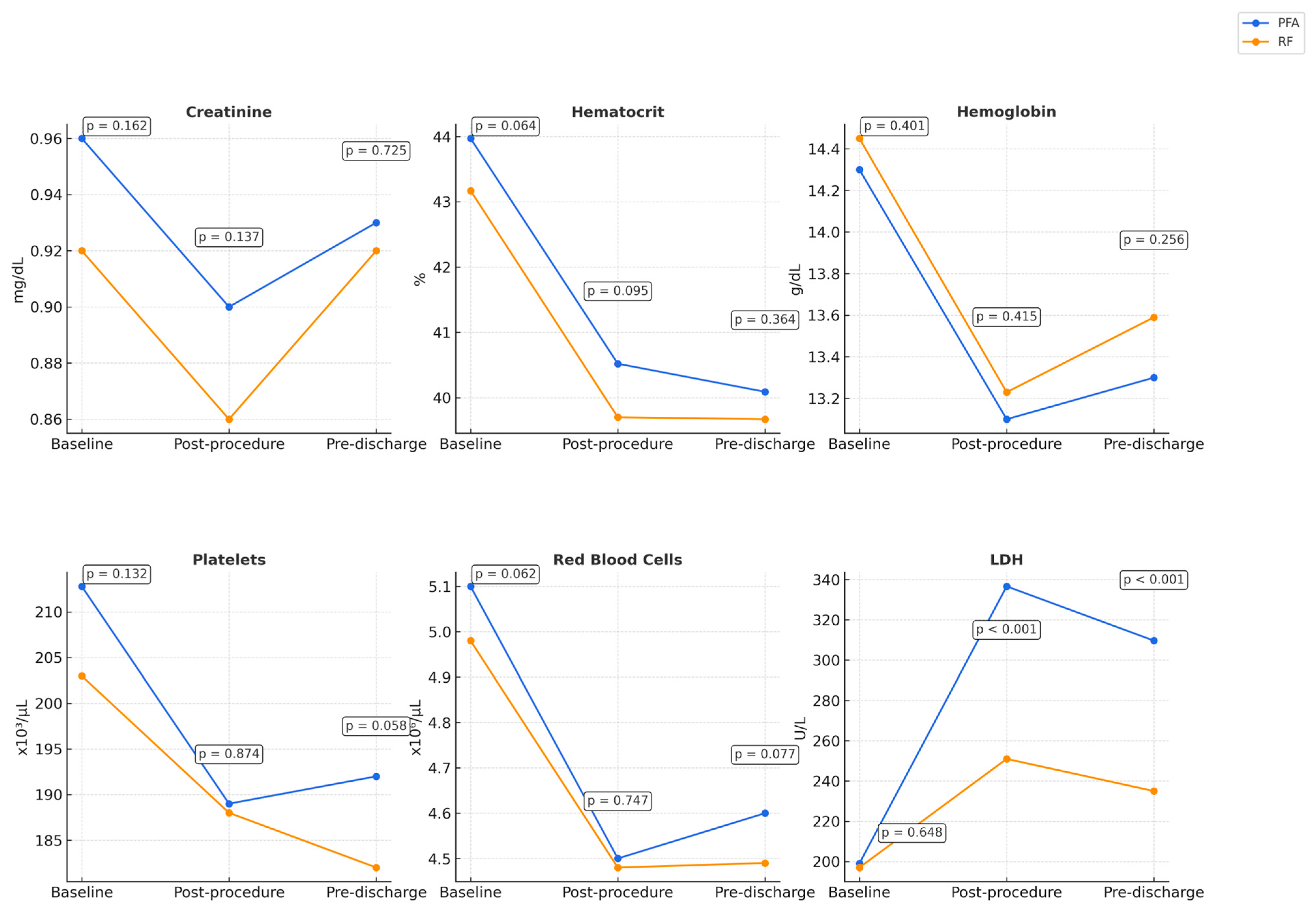
| Creatinine (mg/dL) | 0.96 ± 0.26 [0.91–1.0] | 0.92 ± 0.18 [0.89–0.95] | 0.162 |
| Hematocrit (%) | 43.97 ± 3.51 [43.37–44.61] | 43.17 ± 3.27 [42.61–43.74] | 0.064 |
| Hemoglobin (g/dL) | 14.3 ± 1.46 [14.03–14.55] | 14.45 ± 1.35 [14.21–14.68] | 0.401 |
| Platelets (×109/L) | 212.8 ± 42.27 [205.05–220.21] | 203 ± 59 [192.31–212.96] | 0.132 |
| RBC (×1012/L) | 5.1 ± 0.51 [4.97–5.16] | 4.98 ± 0.50 [4.9–5.07] | 0.062 |
| LDH (U/L) | 199 ± 35 [193.06–205.72] | 197 ± 34 [190.55–202.52] | 0.648 |
| PFA (n = 121) | RF (n = 128) | p-Value | |
|---|---|---|---|
| Creatinine post-procedure (mg/dL) | 0.90 ± 0.24 [0.86–0.94] | 0.86 ± 0.18 [0.83–0.90] | 0.137 |
| Creatinine pre-discharge (mg/dL) | 0.93 ± 0.24 [0.89–0.97] | 0.92 ± 0.21 [0.88–0.96] | 0.725 |
| Hematocrit post- procedure (%) | 40.52 ± 4.17 [39.80–41.28] | 39.70 ± 3.57 [39.08–40.32] | 0.095 |
| Hematocrit pre-discharge (%) | 40.09 ± 3.30 [39.53–40.71] | 39.67 ± 4.06 [38.96–40.38] | 0.364 |
| Hemoglobin post- procedure (g/dL) | 13.1 ± 1.17 [12.89–13.30] | 13.23 ± 1.37 [13.00–13.47] | 0.415 |
| Hemoglobin pre-discharge (g/dL) | 13.3 ± 1.18 [13.06–13.48] | 13.59 ± 2.67 [13.12–14.05] | 0.256 |
| Platelets post- procedure (×109/L) | 189 ± 42 [181.78–196.83] | 188 ± 58 [177.79–197.83] | 0.874 |
| Platelets pre-discharge (×109/L) | 192 ± 33 [186.26–198.17] | 182 ± 50 [173.75–191.13] | 0.058 |
| RBC post- procedure (×1012/L) | 4.5 ± 0.48 [4.44–4.61] | 4.48 ± 0.51 [4.39–4.57] | 0.747 |
| RBC pre-discharge (×1012/L) | 4.6 ± 0.45 [4.56–4.72] | 4.49 ± 0.54 [4.40–4.59] | 0.077 |
| LDH post- procedure (U/L) | 336.6 ± 129.47 [312.72–358.86] | 251 ± 50 [241.80–259.28] | <0.001 |
| LDH pre-discharge (U/L) | 309.6 ± 113.74 [289.89–330.39] | 235 ± 53 [226.14–244.51] | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Addeo, L.; Di Feo, F.; Vaccariello, M.; Varriale, A.; Brescia, B.; Bonadies, D.; Nardi, S.; Argenziano, L.; Marino, V.; Abbate, V.; et al. Comparative Effects of Pulsed Field and Radiofrequency Ablation on Blood Cell Parameters During Pulmonary Vein Isolation. Biomedicines 2025, 13, 1828. https://doi.org/10.3390/biomedicines13081828
Addeo L, Di Feo F, Vaccariello M, Varriale A, Brescia B, Bonadies D, Nardi S, Argenziano L, Marino V, Abbate V, et al. Comparative Effects of Pulsed Field and Radiofrequency Ablation on Blood Cell Parameters During Pulmonary Vein Isolation. Biomedicines. 2025; 13(8):1828. https://doi.org/10.3390/biomedicines13081828
Chicago/Turabian StyleAddeo, Lucio, Federica Di Feo, Mario Vaccariello, Alfonso Varriale, Benedetta Brescia, Davide Bonadies, Stefano Nardi, Luigi Argenziano, Vittoria Marino, Vincenza Abbate, and et al. 2025. "Comparative Effects of Pulsed Field and Radiofrequency Ablation on Blood Cell Parameters During Pulmonary Vein Isolation" Biomedicines 13, no. 8: 1828. https://doi.org/10.3390/biomedicines13081828
APA StyleAddeo, L., Di Feo, F., Vaccariello, M., Varriale, A., Brescia, B., Bonadies, D., Nardi, S., Argenziano, L., Marino, V., Abbate, V., Cocchiara, L., Guarini, P., Dalla Vecchia, L. A., & Donatelli, F. (2025). Comparative Effects of Pulsed Field and Radiofrequency Ablation on Blood Cell Parameters During Pulmonary Vein Isolation. Biomedicines, 13(8), 1828. https://doi.org/10.3390/biomedicines13081828







