Assessment of Tumor Relative Biological Effectiveness in Low-LET Proton Irradiation
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Photon Beam Irradiation
2.3. Proton Beam Irradiation
2.4. Clonogenic Assay
2.5. Image Analysis and Survival Curve Fitting
2.6. Statistical Analyses
3. Results
3.1. Monte Carlo Simulations for the Dose and LET Spectra Along the Beam Direction
3.2. Varied Cell Responses to Photon Irradiation
3.3. Cell Survival and the Calculated RBE Along and Around the Proton SOBP
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LET | Linear energy transfer |
| OAR | Organs at risk |
| RBE | Relative biological effectiveness |
| SOBP | Spread-out Bragg peak |
References
- Vaiserman, A.M. Radiation hormesis: Historical perspective and implications for low-dose cancer risk assessment. Dose Response 2010, 8, 172–191. [Google Scholar] [CrossRef]
- Grassberger, C.; Trofimov, A.; Lomax, A.; Paganetti, H. Variations in linear energy transfer within clinical proton therapy fields and the potential for biological treatment planning. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 1559–1566. [Google Scholar] [CrossRef]
- Bedford, J.S. Sublethal damage, potentially lethal damage, and chromosomal aberrations in mammalian cells exposed to ionizing radiations. Int. J. Radiat. Oncol. Biol. Phys. 1991, 21, 1457–1469. [Google Scholar] [CrossRef]
- Graupner, A.; Eide, D.M.; Instanes, C.; Anderson, J.M.; Brede, D.A.; Dertinger, S.D.; Lind, O.C.; Brandt-Kjelsen, A.; Bjerke, H.; Salbu, B.; et al. Gamma radiation at a human relevant low dose rate is genotoxic in mice. Sci. Rep. 2016, 6, 32977. [Google Scholar] [CrossRef]
- Yang, W.; Reznik, R.; Fraass, B.A.; Nissen, N.; Hendifar, A.; Wachsman, A.; Sandler, H.; Tuli, R. Dosimetric evaluation of simultaneous integrated boost during stereotactic body radiation therapy for pancreatic cancer. Med. Dosim. 2015, 40, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Ohri, N. Radiotherapy dosing for locally advanced non-small cell lung carcinoma: “MTD” or “ALARA”? Front. Oncol. 2017, 7, 205. [Google Scholar] [CrossRef] [PubMed]
- Dauer, L.T.; Yorke, E.; Williamson, M.; Gao, Y.; Dauer, Z.L.; Miller, D.L.; Vañó, E. Radiotherapeutic implications of the updated ICRP thresholds for tissue reactions related to cataracts and circulatory diseases. Ann. ICRP 2018, 47, 196–213. [Google Scholar] [CrossRef] [PubMed]
- Hamada, N.; Fujimichi, Y. Classification of radiation effects for dose limitation purposes: History, current situation and future prospects. J. Radiat. Res. 2014, 55, 629–640. [Google Scholar] [CrossRef]
- Prabhakar, R.; Rath, G.K. A simple plan evaluation index based on the dose to critical structures in radiotherapy. J. Med. Phys. 2011, 36, 192–197. [Google Scholar] [CrossRef]
- Tempero, M.A.; Malafa, M.P.; Al-Hawary, M.; Behrman, S.W.; Benson, A.B.; Cardin, D.B.; Chiorean, E.G.; Chung, V.; Czito, B.; Del Chiaro, M.; et al. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 439–457. [Google Scholar] [CrossRef]
- Pancreatic Adenocarcinoma (Evidence Blocks Version 2.2021). Available online: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic_blocks.pdf (accessed on 1 November 2021).
- Maeda, J.; Froning, C.E.; Brents, C.A.; Rose, B.J.; Thamm, D.H.; Kato, T.A. Intrinsic Radiosensitivity and Cellular Characterization of 27 Canine Cancer Cell Lines. PLoS ONE 2016, 11, e0156689. [Google Scholar] [CrossRef]
- Wang, L.; Han, S.; Zhu, J.; Wang, X.; Li, Y.; Wang, Z.; Lin, E.; Wang, X.; Molkentine, D.P.; Blanchard, P.; et al. Proton versus photon radiation-induced cell death in head and neck cancer cells. Head Neck 2019, 41, 46–55. [Google Scholar] [CrossRef] [PubMed]
- McMahon, S.J.; Paganetti, H.; Prise, K.M. LET-weighted doses effectively reduce biological variability in proton radiotherapy planning. Phys. Med. Biol. 2018, 63, 225009. [Google Scholar] [CrossRef] [PubMed]
- Foote, R.L.; Stafford, S.L.; Petersen, I.A.; Pulido, J.S.; Clarke, M.J.; Schild, S.E. The clinical case for proton beam therapy. Radiat. Oncol. 2012, 7, 174. [Google Scholar] [CrossRef] [PubMed]
- Ilicic, K.; Combs, S.E.; Schmid, T.E. New insight in the relative radiobiological effectiveness of proton irradiation. Radiat. Oncol. 2018, 13, 6. [Google Scholar] [CrossRef]
- Paganetti, H.; Niemierko, A.; Ancukiewicz, M.; Gerweck, L.E.; Goitein, M.; Loeffler, J.S.; Suit, H.D. Relative biological effectiveness (RBE) values for proton beam therapy. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 407–421. [Google Scholar] [CrossRef]
- Guan, F.; Bronk, L.; Titt, U.; Lin, S.H.; Mirkovic, D.; Kerr, M.D.; Zhu, X.R.; Dinh, J.; Sobieski, M.; Stephan, C.; et al. Spatial mapping of the biologic effectiveness of scanned particle beams: Towards biologically optimized particle therapy. Sci. Rep. 2015, 6, 9850. [Google Scholar] [CrossRef]
- Gerweck, L.E.; Kozin, S.V. Relative biological effectiveness of proton beams in clinical therapy. Radiother. Oncol. 1999, 50, 135–142. [Google Scholar] [CrossRef]
- van Leeuwen, C.M.; Oei, A.L.; Crezee, J.; Bel, A.; Franken, N.A.P.; Stalpers, L.J.A.; Kok, H.P. The alfa and beta of tumours: A review of parameters of the linear-quadratic model, derived from clinical radiotherapy studies. Radiat. Oncol. 2018, 13, 96. [Google Scholar] [CrossRef]
- Dahle, T.J.; Rykkelid, A.M.; Stokkevåg, C.H.; Mairani, A.; Görgen, A.; Edin, N.J.; Rørvik, E.; Fjæra, L.F.; Malinen, E.; Ytre-Hauge, K.S. Monte Carlo simulations of a low energy proton beamline for radiobiological experiments. Acta Oncol. 2017, 56, 779–786. [Google Scholar] [CrossRef]
- Gradiz, R.; Silva, H.C.; Carvalho, L.; Botelho, M.F.; Mota-Pinto, A. MIA PaCa-2 and PANC-1-pancreas ductal adenocarcinoma cell lines with neuroendocrine differentiation and somatostatin receptors. Sci. Rep. 2016, 6, 21648. [Google Scholar] [CrossRef]
- Girdhani, S.; Sachs, R.; Hlatky, L. Biological effects of proton radiation: What we know and don’t know. Radiat. Res. 2013, 179, 257–272. [Google Scholar] [CrossRef]
- Sultana, R.; McNeill, D.R.; Abbotts, R.; Mohammed, M.Z.; Zdzienicka, M.Z.; Qutob, H.; Seedhouse, C.; Laughton, C.A.; Fischer, P.M.; Patel, P.M.; et al. Synthetic lethal targeting of DNA double-strand break repair deficient cells by human apurinic/apyrimidinic endonuclease inhibitors. Int. J. Cancer 2012, 131, 2433–2444. [Google Scholar] [CrossRef]
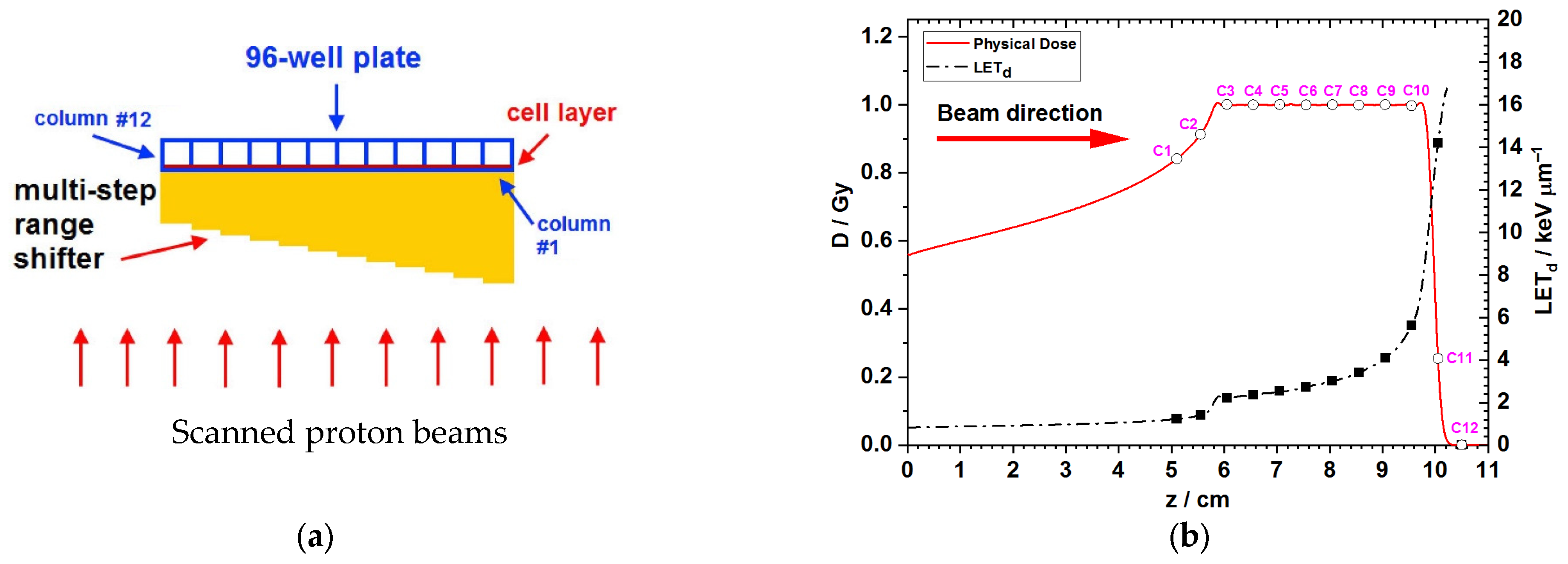
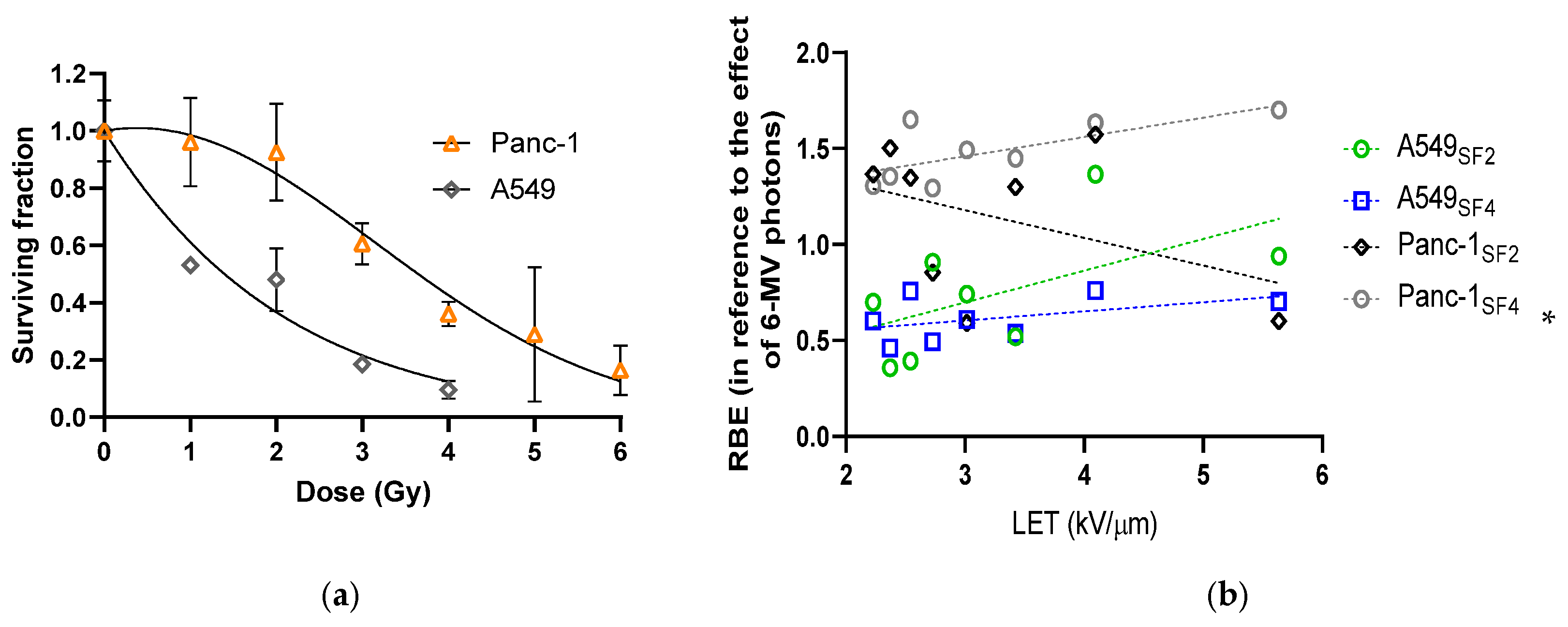
| Column # | Relative Dose Modulation Factor | LET (keV/μm) | Surviving Fractions of A549 Cells | Surviving Fractions of Panc-1 Cells | ||
|---|---|---|---|---|---|---|
| SF2 | SF4 | SF2 | SF4 | |||
| 1 | 0.8415 | 1.2269 | 0.7272 ± 0.3517 | 0.6818 ± 0.4573 | 0.7917 ± 0.2077 | 0.4167 ± 0.1460 |
| 2 | 0.9131 | 1.4134 | 0.9787 ± 0.4724 | 0.6064 ± 0.3943 | 0.7907 ± 0.2886 | 0.5000 ± 0.1990 |
| 3 | 1.0009 | 2.2267 | 0.5000 ± 0.2757 | 0.2963 ± 0.2726 | 0.7027 ± 0.2694 | 0.2162 ± 0.2754 |
| 4 | 1.0004 | 2.3710 | 0.7045 ± 0.3489 | 0.3977 ± 0.4232 | 0.6429 ± 0.2919 | 0.1905 ± 0.2215 |
| 5 | 1.0006 | 2.5418 | 0.6809 ± 0.3975 | 0.2128 ± 0.1712 | 0.7105 ± 0.2190 | 0.0789 ± 0.1878 |
| 6 | 0.9997 | 2.7281 | 0.4043 ± 0.2461 | 0.3723 ± 0.5227 | 1.1111 ± 0.3440 | 0.2222 ± 0.2257 |
| 7 | 1.0002 | 3.0143 | 0.4792 ± 0.2645 | 0.2917 ± 0.2893 | 1.0323 ± 0.4057 | 0.1290 ± 0.2489 |
| 8 | 0.9992 | 3.4235 | 0.6000 ± 0.4695 | 0.3400 ± 0.3974 | 0.7317 ± 0.1987 | 0.1463 ± 0.1807 |
| 9 | 1.0007 | 4.0951 | 0.2500 ± 0.1792 | 0.2115 ± 0.1956 | 0.6111 ± 0.2150 | 0.0833 ± 0.1177 |
| 10 | 0.9978 | 5.6344 | 0.3913 ± 0.2731 | 0.2391 ± 0.2507 | 0.9667 ± 0.3012 | 0.0667 ± 0.1331 |
| 11 | 0.2549 | 14.1959 | 0.5227 ± 0.3763 | 0.3750 ± 0.3141 | 0.7429 ± 0.3620 | 0.5143 ± 0.2590 |
| 12 | 7.94 × 10−4 | 4.15 × 10−6 | 0.7843 ± 0.3894 | 0.9804 ± 0.4558 | 1.1860 ± 0.5081 | 0.8605 ± 0.2584 |
| Average SF over the range of SOBP | 0.9999 ± 0.0010 | 3.2544 ± 1.1389 | 0.5013 ± 0.1127 | 0.2952 ± 0.1185 * | 0.8136 ± 0.1019 | 0.1416 ± 0.0726 * |
| RBE in the range of SOBP | ------ | ------ | 0.7403 ± 0.3324 | 0.6158 ± 0.1158 | 1.0986 ± 0.3984 | 1.4608 ± 0.1620 |
| Cell Lines | A549 | Panc-1 | |
|---|---|---|---|
| Parameter | |||
| α (95% CI) | 0.4822 (0.2930, 0.6831) | −0.05115 (−0.1621, 0.0579) | |
| β (95% CI) | 0.0093 (−0.0602, 0.0886) | 0.0660 (0.0363, 0.1013) | |
| α/β | 51.69 | −0.7747 | |
| R squared | 0.9235 | 0.8629 | |
| Degrees of Freedom | 18 | 26 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-C.; Mo, J.; Lee, Y.-H. Assessment of Tumor Relative Biological Effectiveness in Low-LET Proton Irradiation. Biomedicines 2025, 13, 1823. https://doi.org/10.3390/biomedicines13081823
Lin Y-C, Mo J, Lee Y-H. Assessment of Tumor Relative Biological Effectiveness in Low-LET Proton Irradiation. Biomedicines. 2025; 13(8):1823. https://doi.org/10.3390/biomedicines13081823
Chicago/Turabian StyleLin, Ying-Chun, Jiamin Mo, and Yuan-Hao Lee. 2025. "Assessment of Tumor Relative Biological Effectiveness in Low-LET Proton Irradiation" Biomedicines 13, no. 8: 1823. https://doi.org/10.3390/biomedicines13081823
APA StyleLin, Y.-C., Mo, J., & Lee, Y.-H. (2025). Assessment of Tumor Relative Biological Effectiveness in Low-LET Proton Irradiation. Biomedicines, 13(8), 1823. https://doi.org/10.3390/biomedicines13081823






