Targeting CEACAM5: Biomarker Characterization and Fluorescent Probe Labeling for Image-Guided Gastric Cancer Surgery
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. Organoid Culture
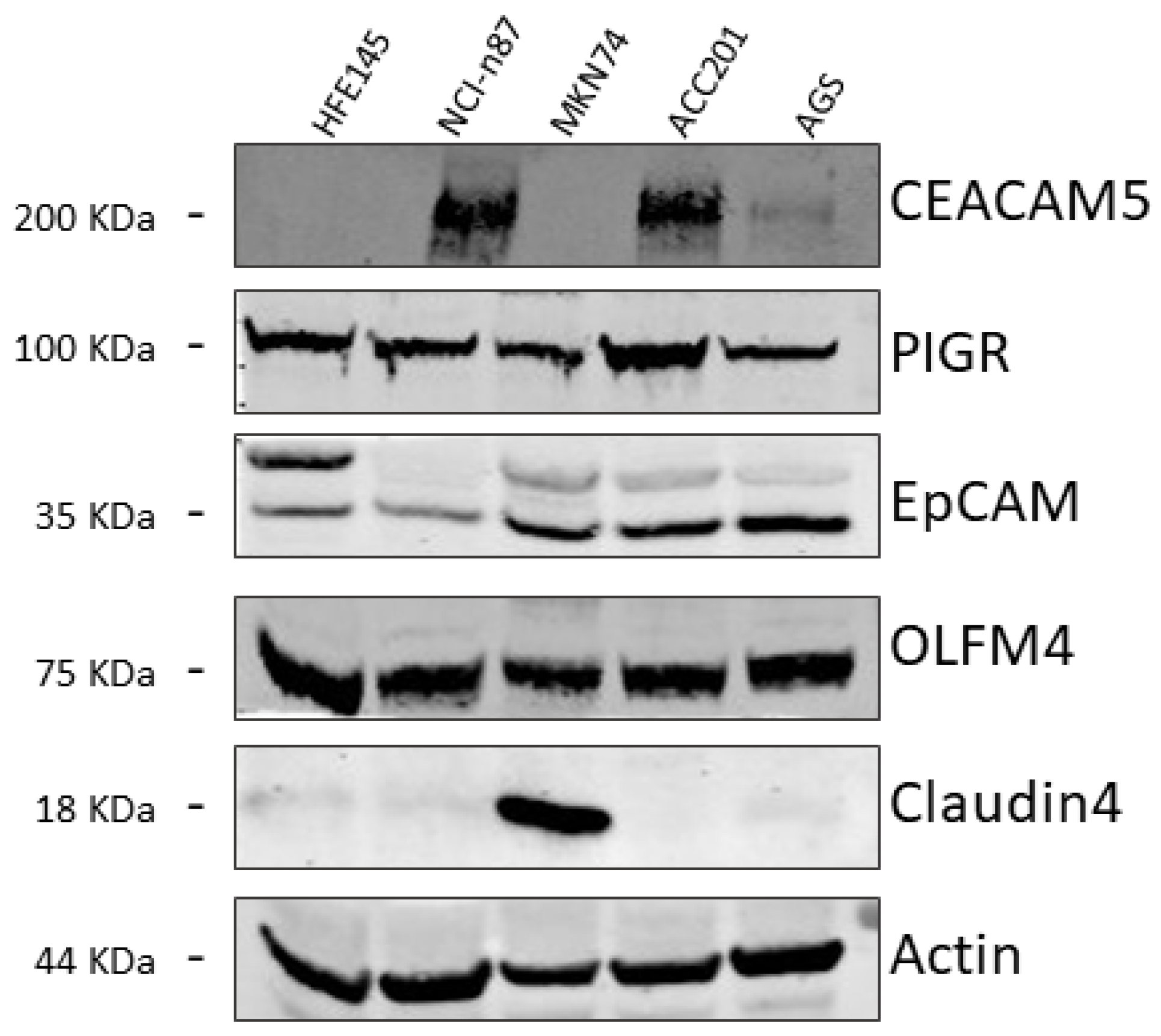
2.3. Western Blotting
2.4. Immunohistochemical Analysis
2.5. Immunofluorescence Staining
2.6. CMG Antibody Generation by CEACAM5 Antibody Conjugation
2.7. In Silico Analysis
2.8. Statistical Analysis
3. Results
3.1. Study Population
3.2. Identification of GC-Associated Surface Biomarkers
3.3. Assessment of CEACAM5 as a Biomarker
3.4. CEACAM5 Antibody Conjugation and Validation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; van Grieken, N.C.; Lordick, F. Gastric cancer. Lancet 2020, 396, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Tringale, K.R.; Pang, J.; Nguyen, Q.T. Image-guided surgery in cancer: A strategy to reduce incidence of positive surgical margins. Wiley Interdiscip. Rev. Syst. Biol. Med. 2018, 10, e1412. [Google Scholar] [CrossRef] [PubMed]
- Ke, B.; Liang, H. Current status of lymph node dissection in gastric cancer. Chin. J. Cancer Res. 2021, 33, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Belia, F.; Biondi, A.; Agnes, A.; Santocchi, P.; Laurino, A.; Lorenzon, L.; Pezzuto, R.; Tirelli, F.; Ferri, L.; D’Ugo, D.; et al. The Use of Indocyanine Green (ICG) and Near-Infrared (NIR) Fluorescence-Guided Imaging in Gastric Cancer Surgery: A Narrative Review. Front. Surg. 2022, 9, 880773. [Google Scholar] [CrossRef] [PubMed]
- DiMaio, S.; Hanuschik, M.; Kreaden, U. The da Vinci Surgical System. In Surgical Robotics: Systems Applications and Visions; Rosen, J., Hannaford, B., Satava, R.M., Eds.; Springer: Boston, MA, USA, 2011; pp. 199–217. [Google Scholar]
- Chen, Q.-Y.; Zhong, Q.; Liu, Z.-Y.; Li, P.; Lin, G.-T.; Zheng, Q.-L.; Wang, J.-B.; Lin, J.-X.; Lu, J.; Cao, L.-L.; et al. Indocyanine green fluorescence imaging-guided versus conventional laparoscopic lymphadenectomy for gastric cancer: Long-term outcomes of a phase 3 randomised clinical trial. Nat. Commun. 2023, 14, 7413. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Berlth, F.; Wang, C.; Wang, S.; Choi, J.-H.; Park, S.-H.; Suh, Y.-S.; Kong, S.-H.; Park, D.J.; Lee, H.-J.; et al. Mapping of the perigastric lymphatic network using indocyanine green fluorescence imaging and tissue marking dye in clinically advanced gastric cancer. Eur. J. Surg. Oncol. 2022, 48, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Mitsumori, N.; Nimura, H.; Takahashi, N.; Kawamura, M.; Aoki, H.; Shida, A.; Omura, N.; Yanaga, K. Sentinel lymph node navigation surgery for early stage gastric cancer. World J. Gastroenterol. 2014, 20, 5685–5693. [Google Scholar] [CrossRef] [PubMed]
- Tajima, Y.; Yamazaki, K.; Masuda, Y.; Kato, M.; Yasuda, D.; Aoki, T.; Kato, T.; Murakami, M.; Miwa, M.; Kusano, M. Sentinel node mapping guided by indocyanine green fluorescence imaging in gastric cancer. Ann. Surg. 2009, 249, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Tummers, Q.R.; Boogerd, L.S.; de Steur, W.O.; Verbeek, F.P.; Boonstra, M.C.; Handgraaf, H.J.; Frangioni, J.V.; van de Velde, C.J.; Hartgrink, H.H.; Vahrmeijer, A.L. Near-infrared fluorescence sentinel lymph node detection in gastric cancer: A pilot study. World J. Gastroenterol. 2016, 22, 3644–3651. [Google Scholar] [CrossRef] [PubMed]
- Baldari, L.; Boni, L.; Cassinotti, E. Lymph node mapping with ICG near-infrared fluorescence imaging: Technique and results. Minim. Invasive Ther. Allied Technol. 2023, 32, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Cianchi, F.; Indennitate, G.; Paoli, B.; Ortolani, M.; Lami, G.; Manetti, N.; Tarantino, O.; Messeri, S.; Foppa, C.; Badii, B.; et al. The Clinical Value of Fluorescent Lymphography with Indocyanine Green During Robotic Surgery for Gastric Cancer: A Matched Cohort Study. J. Gastrointest. Surg. 2020, 24, 2197–2203. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-N.; Su, Y.; Qiu, W.-W.; Liu, C.-H.; Chen, Q.-Y.; Zheng, C.-H.; Li, P.; Wang, J.-B.; Lin, J.-X.; Lu, J.; et al. Assessment of indocyanine green tracer-guided lymphadenectomy in laparoscopic gastrectomy after neoadjuvant chemotherapy for locally advanced gastric cancer: Results from a multicenter analysis based on propensity matching. Gastric Cancer 2021, 24, 1355–1364. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, X.; Zhu, X.; Ai, S.; Guan, W.; Liu, S. Tracers in Gastric Cancer Surgery. Cancers 2022, 14, 5735. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, S.; Fortuna, L.; Coratti, F.; Passagnoli, F.; Amedei, A.; Cianchi, F. Potential Probes for Targeted Intraoperative Fluorescence Imaging in Gastric Cancer. Cancers 2024, 16, 4141. [Google Scholar] [CrossRef] [PubMed]
- Vollmers, H.P.; Stulle, K.; Dämmrich, J.; Pfaff, M.; Papadopoulos, T.; Betz, C.; Saal, K.; Müller-Hermelink, H.K. Characterization of four new gastric cancer cell lines. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1993, 63, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, S.; Amore, F.; Mello, T.; Mannelli, M.; Maggi, M.; Rapizzi, E. Metformin Treatment Induces Different Response in Pheochromocytoma/Paraganglioma Tumour Cells and in Primary Fibroblasts. Cancers 2022, 14, 3471. [Google Scholar] [CrossRef] [PubMed]
- Marroncini, G.; Anceschi, C.; Naldi, L.; Fibbi, B.; Baldanzi, F.; Martinelli, S.; Polvani, S.; Maggi, M.; Peri, A. Low sodium and tolvaptan have opposite effects in human small cell lung cancer cells. Mol. Cell Endocrinol. 2021, 537, 111419. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, S.; Riverso, M.; Mello, T.; Amore, F.; Parri, M.; Simeone, I.; Mannelli, M.; Maggi, M.; Rapizzi, E. SDHB and SDHD silenced pheochromocytoma spheroids respond differently to tumour microenvironment and their aggressiveness is inhibited by impairing stroma metabolism. Mol. Cell Endocrinol. 2022, 547, 111594. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, S.; Cantini, G.; Propato, A.P.; Bani, D.; Guasti, D.; Nardini, P.; Calosi, L.; Mello, T.; Bechmann, N.; Danza, G.; et al. The 3D in vitro Adrenoid cell model recapitulates the complexity of the adrenal gland. Sci. Rep. 2024, 14, 8044. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Kang, B.; Li, C.; Chen, T.; Zhang, Z. GEPIA2: An enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019, 47, W556–W560. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.N.; Wang, H.B.; Zhou, C.C.; Hu, S.Y. CEACAM5 has different expression patterns in gastric non-neoplastic and neoplastic lesions and cytoplasmic staining is a marker for evaluation of tumor progression in gastric adenocarcinoma. Pathol. Res. Pract. 2014, 210, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, T.; Yashiro, M. Biomarkers of gastric cancer: Current topics and future perspective. World J. Gastroenterol. 2018, 24, 2818–2832. [Google Scholar] [CrossRef] [PubMed]
- Toh, J.; Hoppe, M.M.; Thakur, T.; Yang, H.; Tan, K.T.; Pang, B.; Ho, S.; Roy, R.; Ho, K.Y.; Yeoh, K.G.; et al. Profiling of gastric cancer cell-surface markers to achieve tumour-normal discrimination. BMJ Open Gastroenterol. 2020, 7, e000452. [Google Scholar] [CrossRef] [PubMed]
- Fristedt, R.; Gaber, A.; Hedner, C.; Nodin, B.; Uhlén, M.; Eberhard, J.; Jirström, K. Expression and prognostic significance of the polymeric immunoglobulin receptor in esophageal and gastric adenocarcinoma. J. Transl. Med. 2014, 12, 83. [Google Scholar] [CrossRef] [PubMed]
- Chae, J.; Choi, J.; Chung, J. Polymeric immunoglobulin receptor (pIgR) in cancer. J. Cancer Res. Clin. Oncol. 2023, 149, 17683–17690. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Yuan, F.; Fu, C.; Shen, G.; Hu, S. Relationship between epithelial cell adhesion molecule (EpCAM) overexpression and gastric cancer patients: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0175357. [Google Scholar] [CrossRef] [PubMed]
- Guette, C.; Valo, I.; Vétillard, A.; Coqueret, O. Olfactomedin-4 is a candidate biomarker of solid gastric, colorectal, pancreatic, head and neck, and prostate cancers. Proteomics Clin. Appl. 2015, 9, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.L.; He, Z.C.; Yang, C.Q.; Qiao, P.T.; Yin, G.L. Epigenetic silencing of olfactomedin-4 enhances gastric cancer cell invasion via activation of focal adhesion kinase signaling. BMB Rep. 2015, 48, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Li, W.; Zeng, L.; Ding, N.; Li, K.; Yu, H.; Jiang, F.; Yin, H.; Xia, Y.; Deng, C.; et al. OLFM4 promotes the progression of intestinal metaplasia through activation of the MYH9/GSK3β/β-catenin pathway. Mol. Cancer 2024, 23, 124. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, M. The role of claudin-4 in the development of gastric cancer. Scand. J. Gastroenterol. 2020, 55, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Chen, L.; Liu, J. Celastrol inhibits gastric cancer cell proliferation, migration, and invasion via the FOXA1/CLDN4 axis. Toxicol. Res. 2023, 12, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Liu, C.; Cai, Z.; Shen, C.; Yin, Y.; Yin, X.; Zhao, Z.; Mu, M.; Zhang, B. Impact of Surgical Margin Status on Survival in Gastric Cancer: A Systematic Review and Meta-Analysis. Cancer Control 2021, 28, 10732748211043665. [Google Scholar] [CrossRef] [PubMed]
- Hirata, Y.; Agnes, A.; Estrella, J.S.; Blum Murphy, M.; Das, P.; Minsky, B.D.; Ajani, J.A.; Badgwell, B.D.; Mansfield, P.; Ikoma, N. Clinical Impact of Positive Surgical Margins in Gastric Adenocarcinoma in the Era of Preoperative Therapy. Ann. Surg. Oncol. 2023, 30, 4936–4945. [Google Scholar] [CrossRef] [PubMed]
- Meyerholz, D.K.; Beck, A.P. Principles and approaches for reproducible scoring of tissue stains in research. Lab. Investig. 2018, 98, 844–855. [Google Scholar] [CrossRef] [PubMed]
- Houvast, R.D.; van Duijvenvoorde, M.; Thijse, K.; de Steur, W.O.; de Geus-Oei, L.F.; Crobach, A.; Burggraaf, J.; Vahrmeijer, A.L.; Kuppen, P.J.K. Selecting Targets for Molecular Imaging of Gastric Cancer: An Immunohistochemical Evaluation. Mol. Diagn. Ther. 2025, 29, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Seidlitz, T.; Koo, B.-K.; Stange, D.E. Gastric organoids—An in vitro model system for the study of gastric development and road to personalized medicine. Cell Death Differ. 2021, 28, 68–83. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.H.N.; Siu, H.C.; Law, S.; Ho, S.L.; Yue, S.S.K.; Tsui, W.Y.; Chan, D.; Chan, A.S.; Ma, S.; Lam, K.O.; et al. A Comprehensive Human Gastric Cancer Organoid Biobank Captures Tumor Subtype Heterogeneity and Enables Therapeutic Screening. Cell Stem Cell 2018, 23, 882–897.e811. [Google Scholar] [CrossRef] [PubMed]
- Seidlitz, T.; Merker, S.R.; Rothe, A.; Zakrzewski, F.; von Neubeck, C.; Grützmann, K.; Sommer, U.; Schweitzer, C.; Schölch, S.; Uhlemann, H.; et al. Human gastric cancer modelling using organoids. Gut 2019, 68, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Gutowski, M.; Framery, B.; Boonstra, M.C.; Garambois, V.; Quenet, F.; Dumas, K.; Scherninski, F.; Cailler, F.; Vahrmeijer, A.L.; Pèlegrin, A. SGM-101: An innovative near-infrared dye-antibody conjugate that targets CEA for fluorescence-guided surgery. Surg. Oncol. 2017, 26, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Schaap, D.P.; de Valk, K.S.; Deken, M.M.; Meijer, R.P.J.; Burggraaf, J.; Vahrmeijer, A.L.; Kusters, M. Carcinoembryonic antigen-specific, fluorescent image-guided cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for metastatic colorectal cancer. Br. J. Surg. 2020, 107, 334–337. [Google Scholar] [CrossRef] [PubMed]
- Azari, F.; Meijer, R.P.J.; Kennedy, G.T.; Hanna, A.; Chang, A.; Nadeem, B.; Din, A.; Pèlegrin, A.; Framery, B.; Cailler, F.; et al. Carcinoembryonic Antigen-Related Cell Adhesion Molecule Type 5 Receptor-Targeted Fluorescent Intraoperative Molecular Imaging Tracer for Lung Cancer: A Nonrandomized Controlled Trial. JAMA Netw. Open 2023, 6, e2252885. [Google Scholar] [CrossRef] [PubMed]
- Cox, K.E.; Turner, M.A.; Amirfakhri, S.; Lwin, T.M.; Hosseini, M.; Ghosh, P.; Obonyo, M.; Murakami, T.; Hoffman, R.M.; Yazaki, P.J.; et al. Humanized Anti-Carcinoembryonic Antigen Antibodies Brightly Target and Label Gastric Cancer in Orthotopic Mouse Models. J. Surg. Res. 2024, 293, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Cox, K.E.; Turner, M.A.; Lwin, T.M.; Amirfakhri, S.; Kelly, K.J.; Hosseini, M.; Ghosh, P.; Obonyo, M.; Hoffman, R.M.; Yazaki, P.J.; et al. Targeting Patient-Derived Orthotopic Gastric Cancers with a Fluorescent Humanized Anti-CEA Antibody. Ann. Surg. Oncol. 2024, 31, 6291–6299. [Google Scholar] [CrossRef] [PubMed]






| Patients | |
|---|---|
| Number | 30 |
| Age | 74.5 (57–88) |
| Gender | |
| Male | 23 (77%) |
| Female | 7 (23%) |
| Perioperative | |
| chemotherapy | |
| Yes | 18 (60%) |
| No | 12 (40%) |
| TNM stage | |
| I | 14 (47%) |
| II | 9 (30%) |
| III | 7 (23%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinelli, S.; Peri, S.; Anceschi, C.; Laurenzana, A.; Fortuna, L.; Mello, T.; Naldi, L.; Marroncini, G.; Tricomi, J.; Biagioni, A.; et al. Targeting CEACAM5: Biomarker Characterization and Fluorescent Probe Labeling for Image-Guided Gastric Cancer Surgery. Biomedicines 2025, 13, 1812. https://doi.org/10.3390/biomedicines13081812
Martinelli S, Peri S, Anceschi C, Laurenzana A, Fortuna L, Mello T, Naldi L, Marroncini G, Tricomi J, Biagioni A, et al. Targeting CEACAM5: Biomarker Characterization and Fluorescent Probe Labeling for Image-Guided Gastric Cancer Surgery. Biomedicines. 2025; 13(8):1812. https://doi.org/10.3390/biomedicines13081812
Chicago/Turabian StyleMartinelli, Serena, Sara Peri, Cecilia Anceschi, Anna Laurenzana, Laura Fortuna, Tommaso Mello, Laura Naldi, Giada Marroncini, Jacopo Tricomi, Alessio Biagioni, and et al. 2025. "Targeting CEACAM5: Biomarker Characterization and Fluorescent Probe Labeling for Image-Guided Gastric Cancer Surgery" Biomedicines 13, no. 8: 1812. https://doi.org/10.3390/biomedicines13081812
APA StyleMartinelli, S., Peri, S., Anceschi, C., Laurenzana, A., Fortuna, L., Mello, T., Naldi, L., Marroncini, G., Tricomi, J., Biagioni, A., Amedei, A., & Cianchi, F. (2025). Targeting CEACAM5: Biomarker Characterization and Fluorescent Probe Labeling for Image-Guided Gastric Cancer Surgery. Biomedicines, 13(8), 1812. https://doi.org/10.3390/biomedicines13081812











