Experimental Study of Two-Bite Test Parameters for Effective Drug Release from Chewing Gum Using a Novel Bio-Engineered Testbed
Abstract
1. Introduction
- (1)
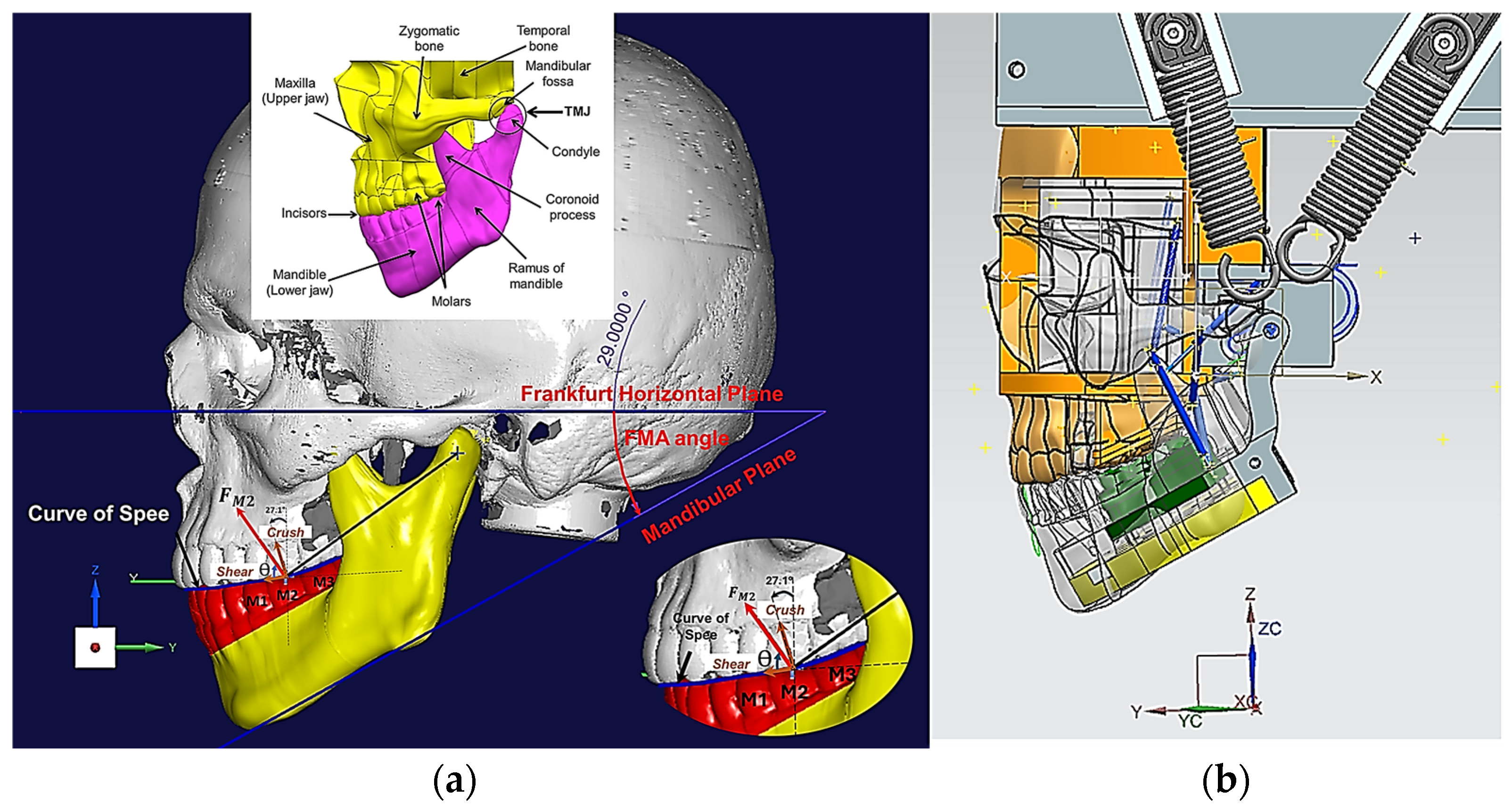
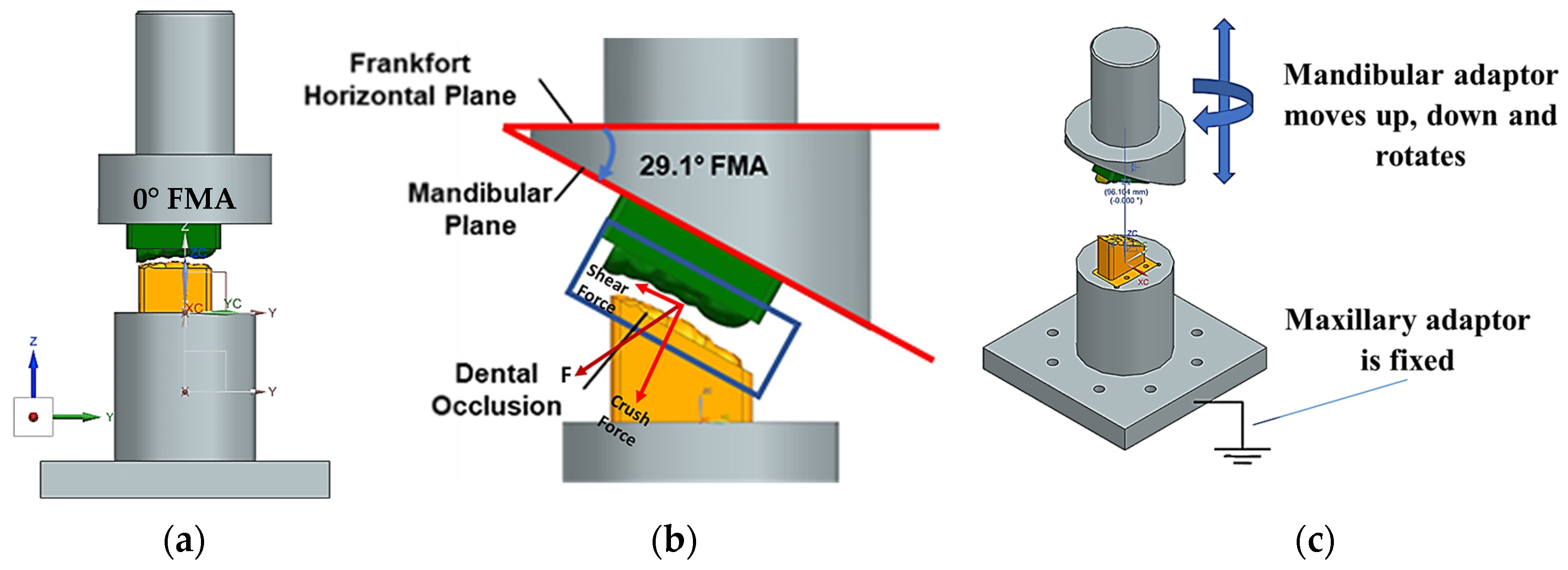
- The inherent technical and scientific challenges in realising in vitro apparatus to test drug release prior to human studies. These challenges arise from the need to replicate the intricate jaw physiology and human oral environment with masticatory motions and forces, and the flow of saliva over the artificial gum surface necessary for the release of the API from the MCG [10]. The Frankfort-mandibular plane angle (FMA) and Bennett angle (BA) are important cephalometric measurements that reflect the interplay between jaw structure, dental morphology and overall facial form–function. The FMA indicates the vertical jaw relationship and the BA is related to mandibular movement during lateral excursions, which reflects the temporomandibular joint’s (TMJ) influence on jaw function. The FMA and BA are crucial in understanding chewing because they influence jaw movement and the way teeth come together during mastication. The FMA impacts on bite force and tooth arrangement. The BA is vital for proper occlusal relationships and the design of prosthetic restorations. The FMA and the BA are critical parameters in understanding the biomechanics of chewing (i.e., crushing, shearing and grinding), impacting both the natural occlusion and the design of prosthetic restorations.
- (2)
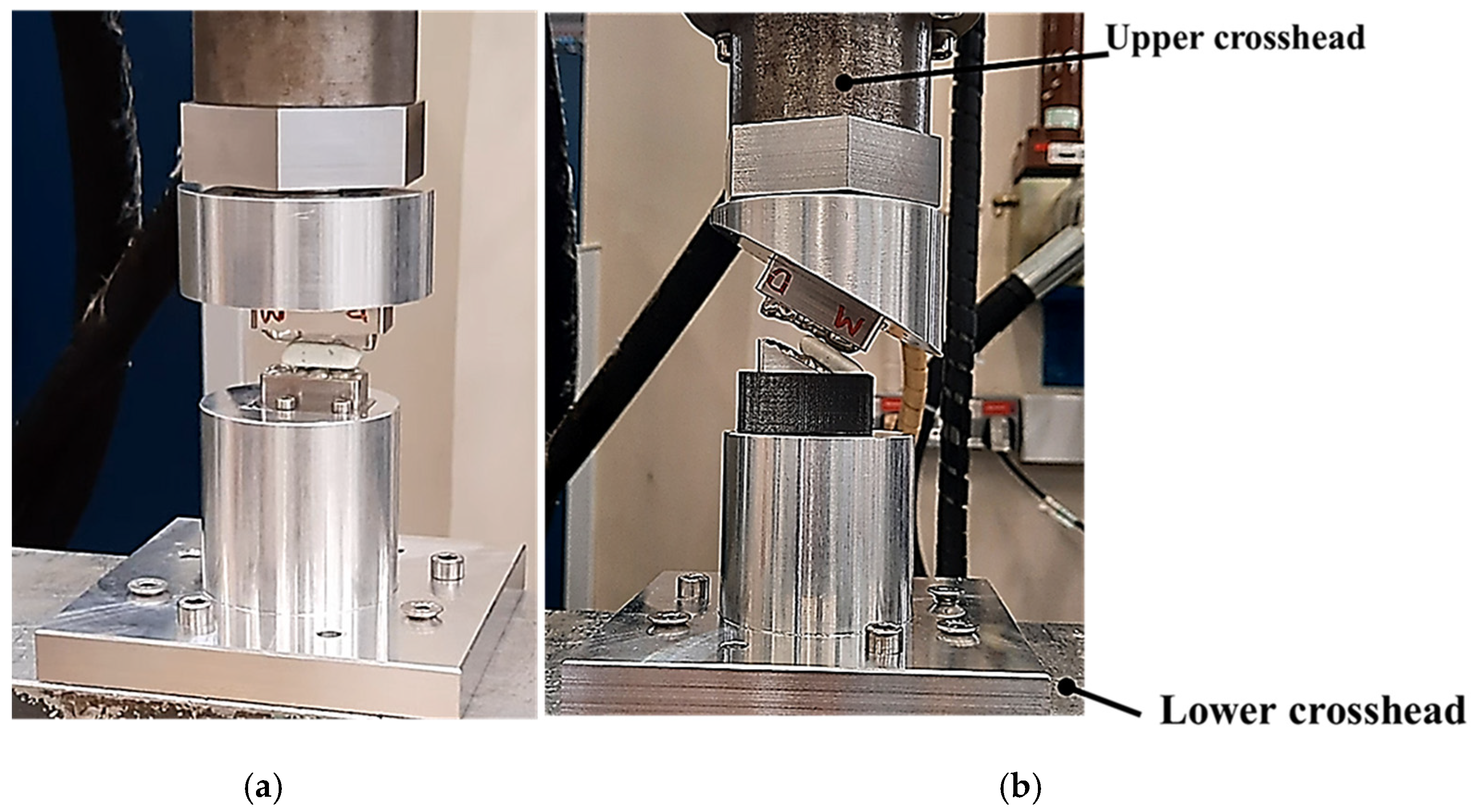
2. Materials and Methods: The Novel Two-Bite Bio-Engineered Testbed
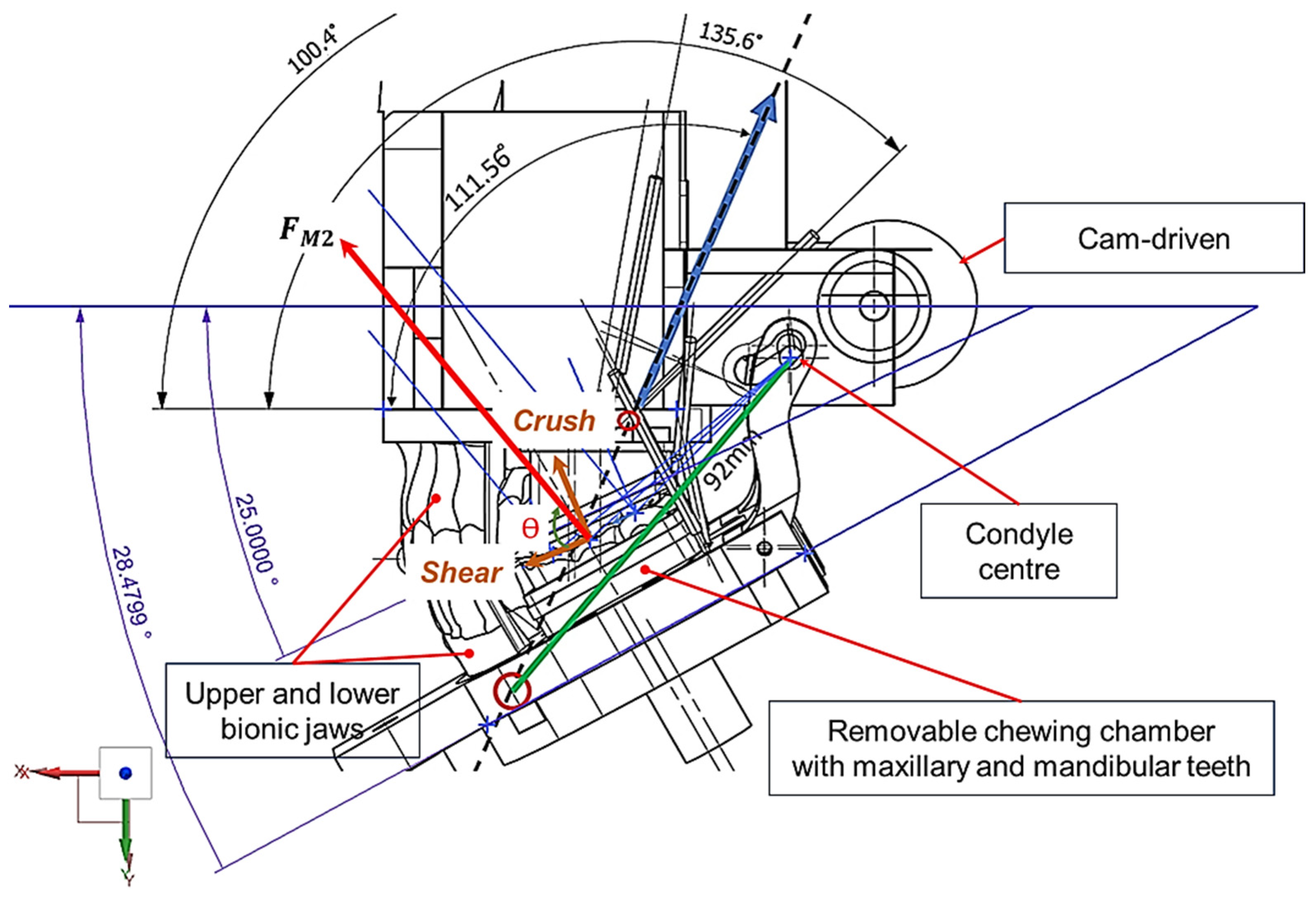
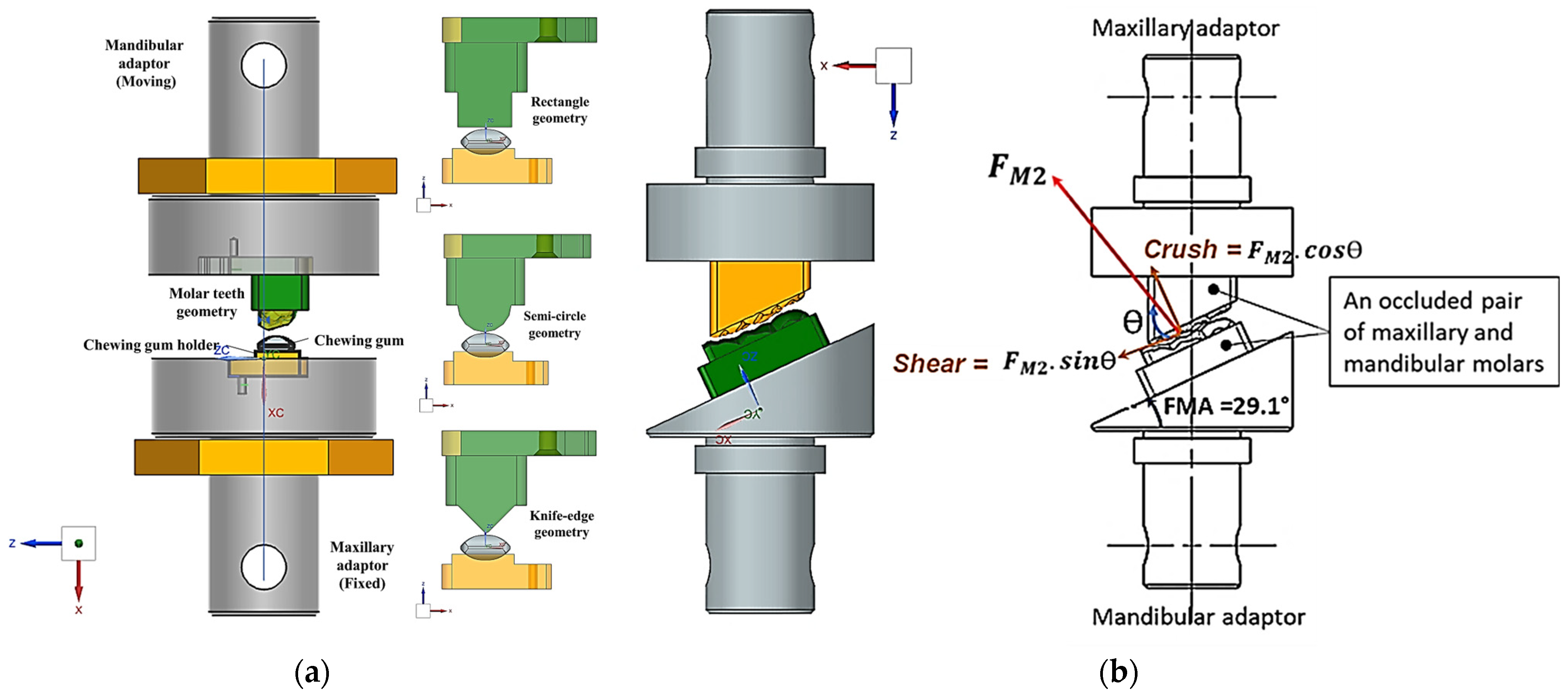
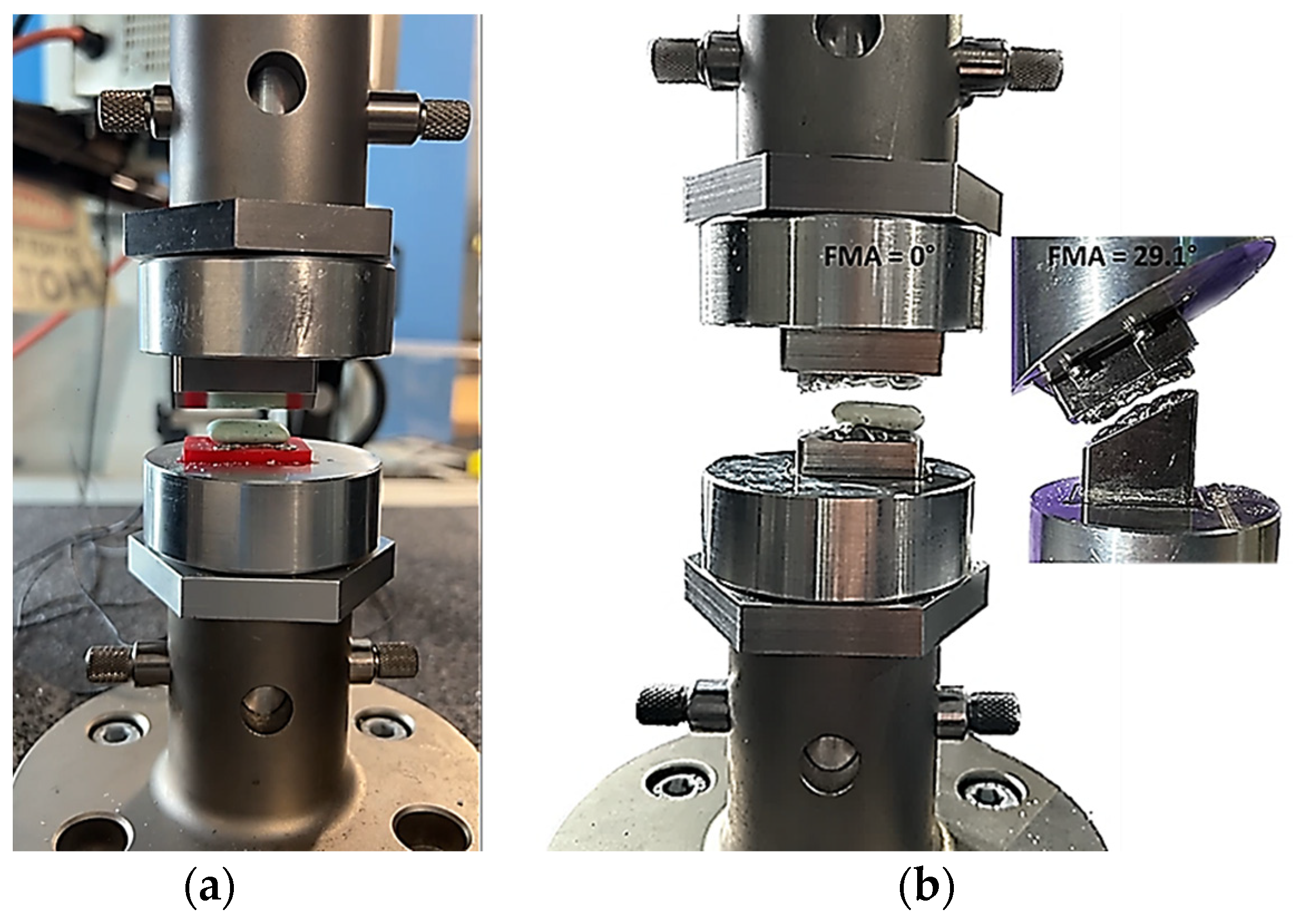
2.1. Design and Modelling of Maxillary–Mandibular–Bio Testbed
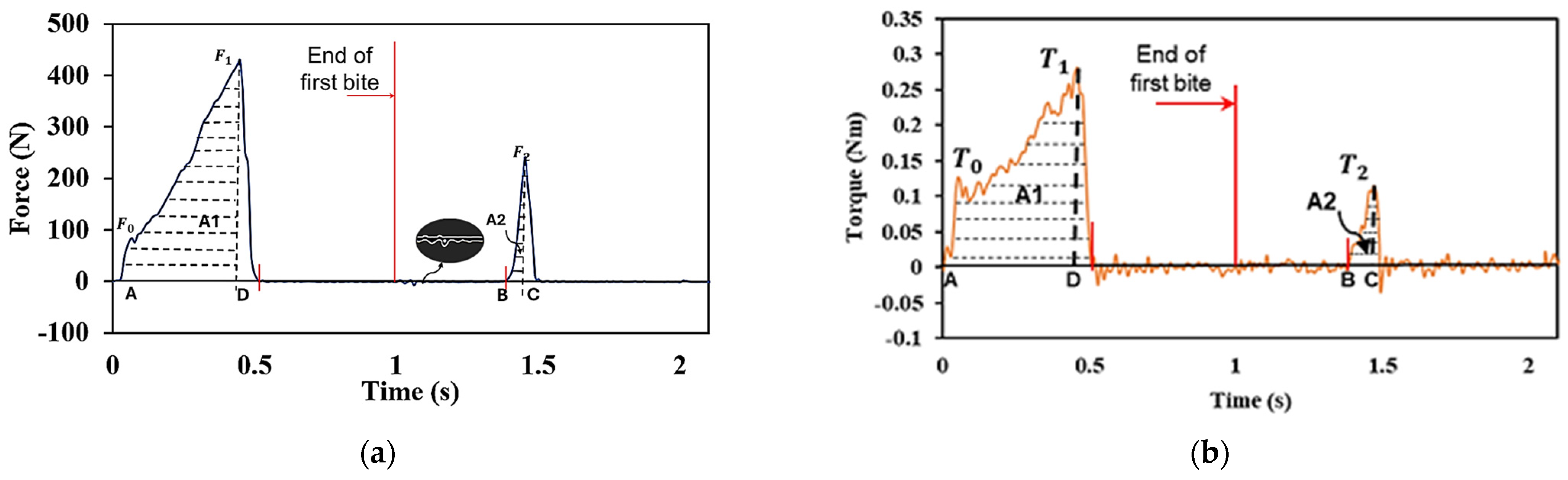
2.2. Two-Bite Test–Texture Profile Analysis (TPA)
3. Results
3.1. Set-Up A: Instron Using 10 mm/s Compression and FMA = 0 to Evaluate Tooth Morphologies (Including an Occluded Pair of Molars)
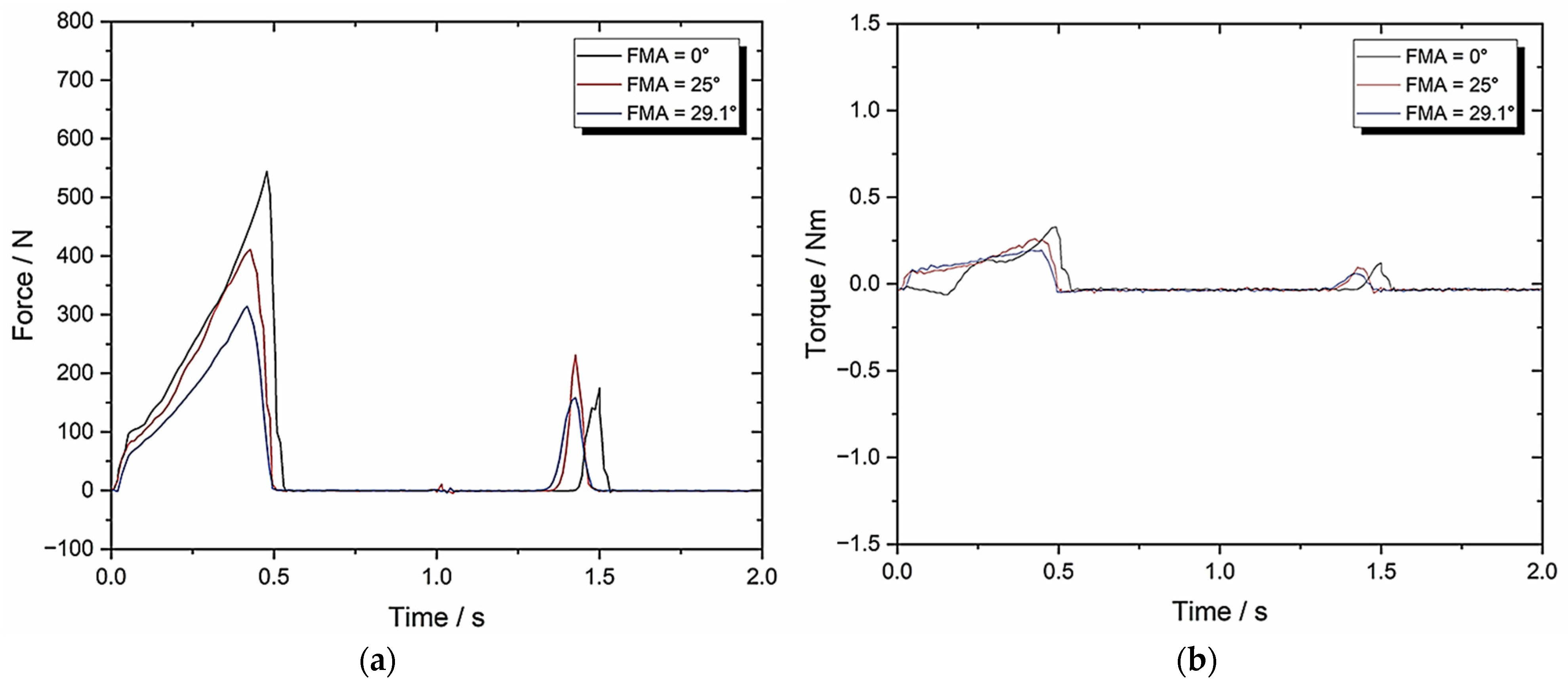
3.2. Set-Up B: Zwick–Roell Using 10 mm/s Compression and BA = 0 Whilst Varying FMA
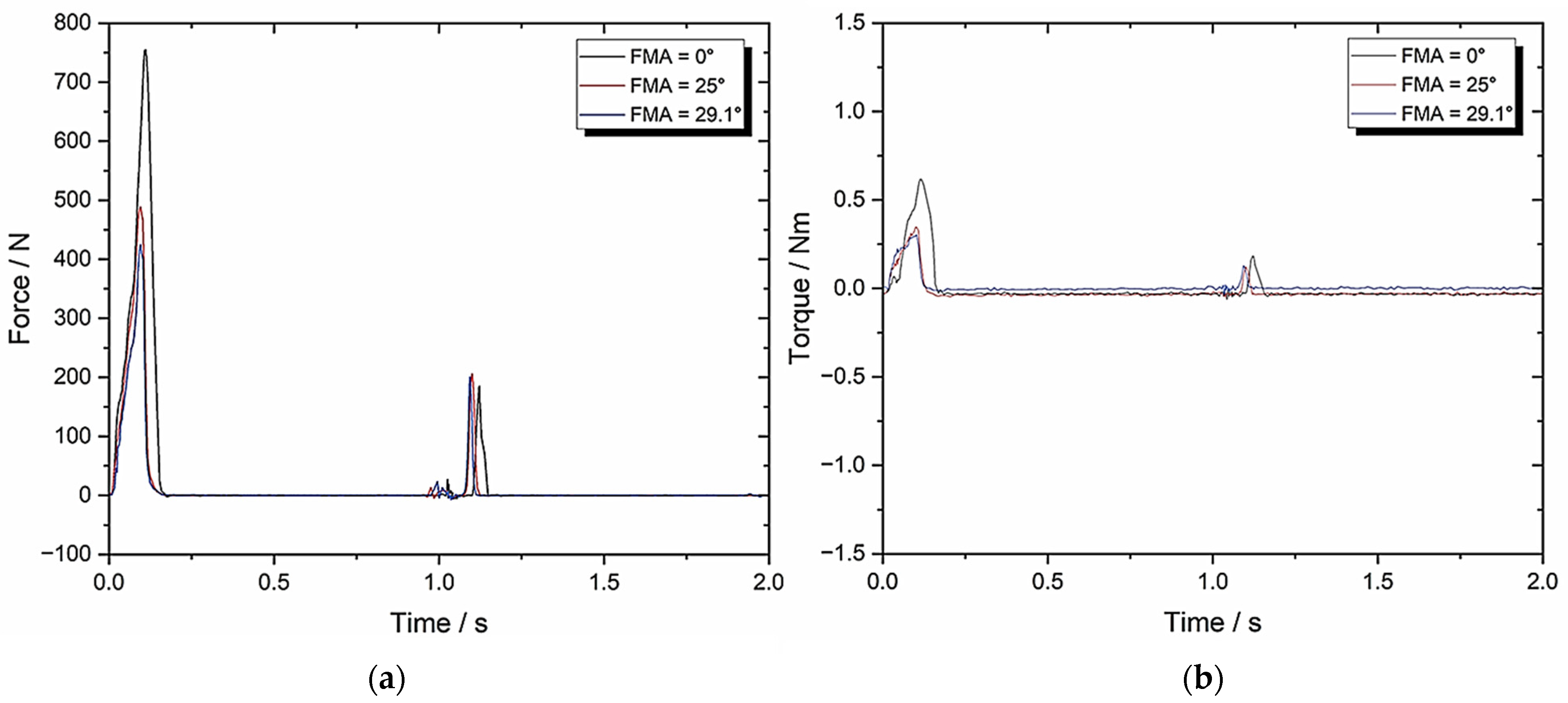
3.3. Set-Up C: Zwick–Roell Using 49.5 mm/s Compression and BA = 0 Whilst Varying FMA (To Emulate Human Chewing Speed)
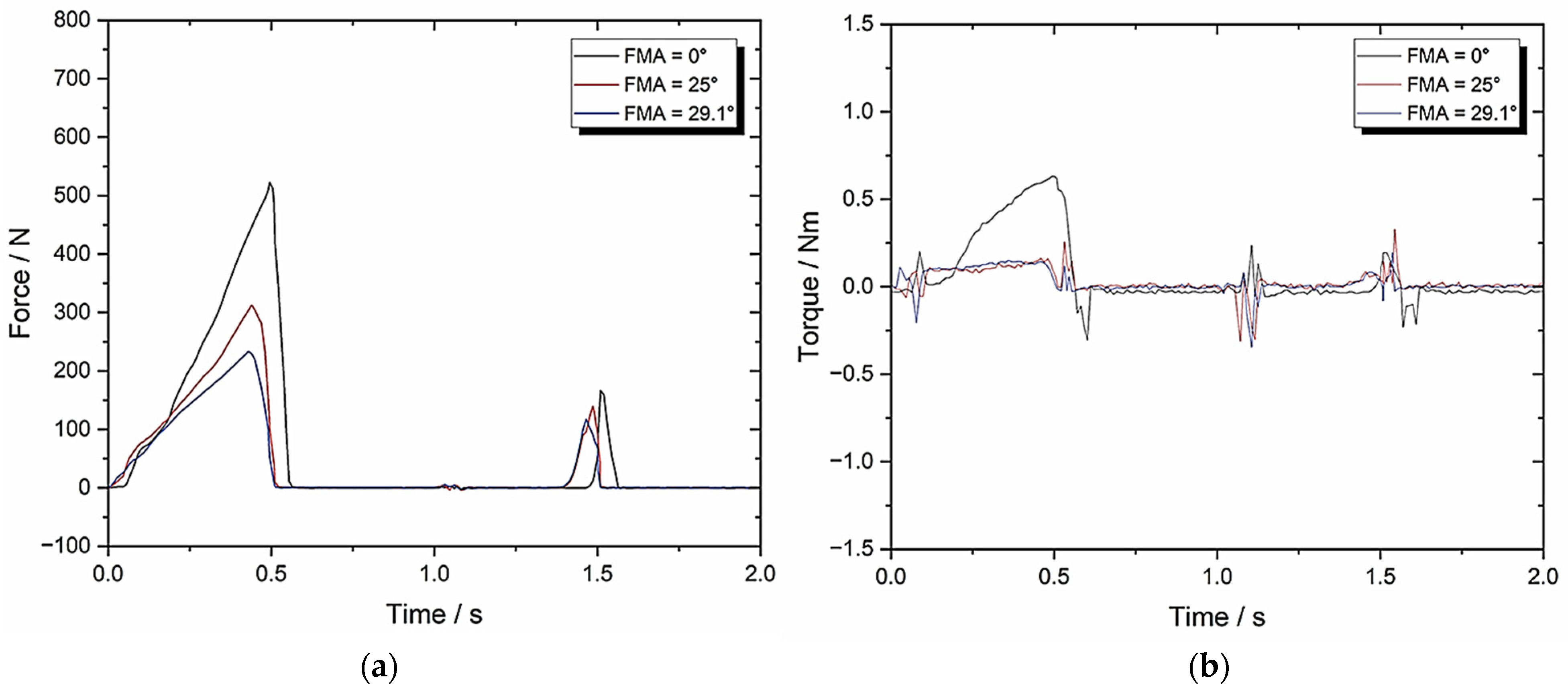
3.4. Set-Up D: Zwick–Roell Using 10 mm/s Compression and BA = 8 Whilst Varying FMA (To Evaluate the Change in BA)
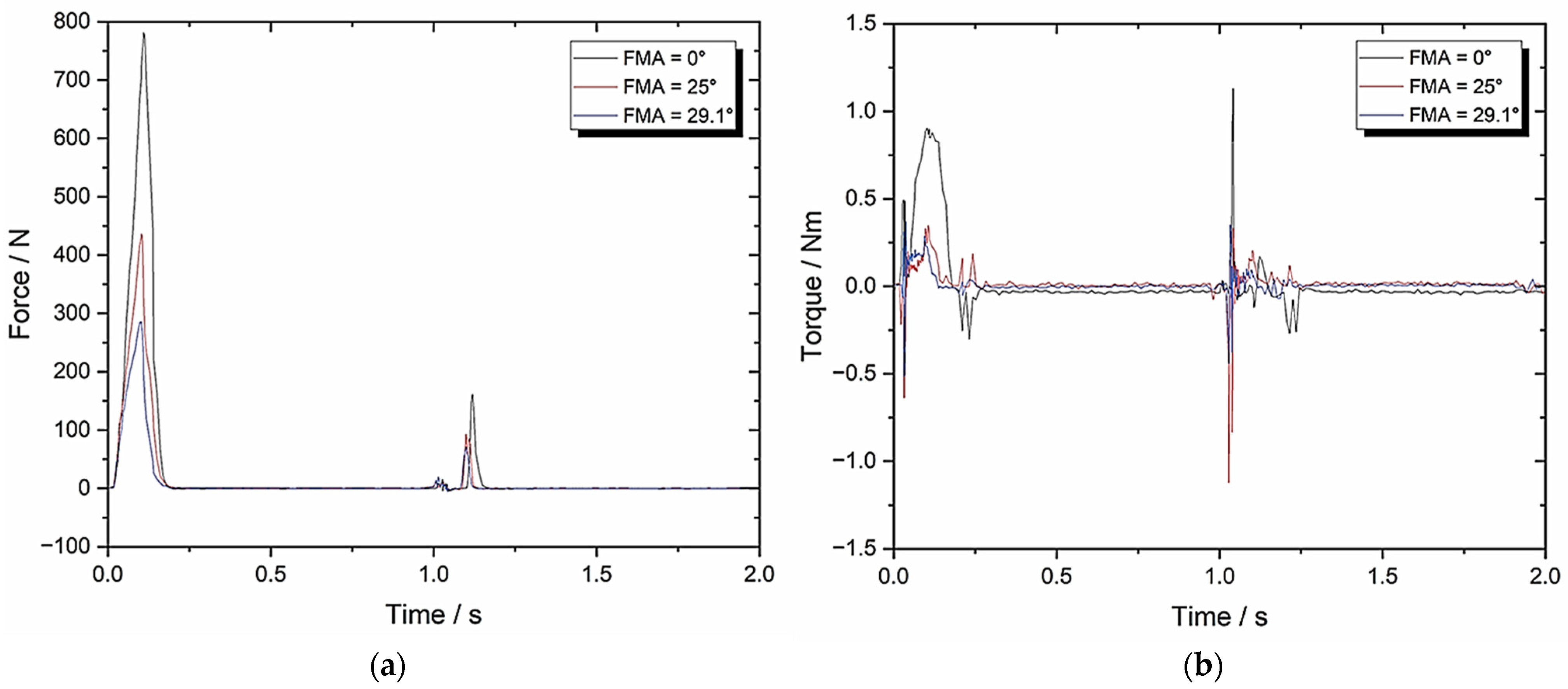
3.5. Set-Up E: Zwick–Roell Using 49.5 mm/s Compression and BA = 8 Whilst Varying FMA (To Emulate Human Chewing Speed)
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MCG | Medicated chewing gum |
| PLM | Product lifecycle management |
| API | Active pharmaceutical ingredient |
| BA | Bennett angle of mandible |
| FMA | Frankfort-mandibular plane angle |
| TMJ | Temporomandibular Joint |
| CUR | Curcumin |
| TPA | Texture profile analysis |
| CeA | Cephalometric analysis |
| FHP | Frankfurt Horizontal plane |
| MP | Mandibular Plane |
| ART | Artificial Resynthesis Technology |
| CNS | Central Nervous System |
| POC | Proof of Concept |
| CPP-ACP | Casein Phosphopeptide-Amorphous Calcium Phosphate |
References
- Alemzadeh, K.; Raabe, D. Prototyping artificial jaws for the robotic dental testing simulator. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2008, 222, 1209–1220. [Google Scholar] [CrossRef]
- Grau, A.; Stawarczyk, B.; Roos, M.; Theelke, B.; Hampe, R. Reliability of wear measurements of CAD-CAM restorative materials after artificial aging in a mastication simulator. J. Mech. Behav. Biomed. Mater. 2018, 86, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Steiner, M.; Mitsias, M.E.; Ludwig, K.; Kern, M. In vitro evaluation of a mechanical testing chewing simulator. Dent. Mater. 2009, 25, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Morell, P.; Hernando, I.; Fiszman, S.M. Understanding the relevance of in-mouth food processing. A review of in vitro techniques. Trends Food Sci. Technol. 2014, 35, 18–31. [Google Scholar] [CrossRef]
- Peyron, M.-A.; Woda, A. An update about artificial mastication. Curr. Opin. Food Sci. 2016, 9, 21–28. [Google Scholar] [CrossRef]
- Salles, C.; Tarrega, A.; Mielle, P.; Maratray, J.; Gorria, P.; Liaboeuf, J.; Liodenot, J.-J. Development of a chewing simulator for food breakdown and the analysis of in vitro flavor compound release in a mouth environment. J. Food Eng. 2007, 82, 189–198. [Google Scholar] [CrossRef]
- Woda, A.; Mishellany-Dutour, A.; Batier, L.; François, O.; Meunier, J.P.; Reynaud, B.; Alric, M.; Peyron, M.A. Development and validation of a mastication simulator. J. Biomech. 2010, 43, 1667–1673. [Google Scholar] [CrossRef]
- Christrup, L.L.; Moeller, N. Chewing gum as a drug delivery system. Arch. Pharm. Chem. Sci. Ed. 1986, 14, 30–36. [Google Scholar]
- Kvist, C.; Andersson, S.B.; Fors, S.; Wennergren, B.; Berglund, J. Apparatus for studying in vitro drug release from medicated chewing gums. Int. J. Pharm. 1999, 189, 57–65. [Google Scholar] [CrossRef]
- Alemzadeh, K.; Jones, S.B.; Davies, M.; West, N. Development of a Chewing Robot With Built-in Humanoid Jaws to Simulate Mastication to Quantify Robotic Agents Release From Chewing Gums Compared to Human Participants. IEEE Trans. Biomed. Eng. 2020, 68, 492–504. [Google Scholar] [CrossRef]
- Vincent, J. Biomimetics with Trade-Offs. Biomimetics 2023, 8, 265. [Google Scholar] [CrossRef]
- Swiderski, D.L.; Zelditch, M.L. Complex adaptive landscape for a “Simple” structure: The role of trade-offs in the evolutionary dynamics of mandibular shape in ground squirrels. Evolution 2022, 76, 946–965. [Google Scholar] [CrossRef] [PubMed]
- Heintze, S.D.; Reichl, F.-X.; Hickel, R. Wear of dental materials: Clinical significance and laboratory wear sim-ulation methods—A review. Dent. Mater. J. 2019, 38, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Krueger, K.L.; Chwa, E.; Peterson, A.S.; Willman, J.C.; Fok, A.; van Heel, B.; Heo, Y.; Weston, M.; DeLong, R. Technical note: Artificial Resynthesis Technology for the experimental formation of dental microwear textures. Am. J. Phys. Anthr. 2021, 176, 703–712. [Google Scholar] [CrossRef]
- Daegling, D.J.; Hua, L.-C.; Ungar, P.S. The role of food stiffness in dental microwear feature formation. Arch. Oral Biol. 2016, 71, 16–23. [Google Scholar] [CrossRef]
- Pu, D.; Shan, Y.; Wang, J.; Sun, B.; Xu, Y.; Zhang, W.; Zhang, Y. Recent trends in aroma release and perception during food oral processing: A review. Crit. Rev. Food Sci. Nutr. 2022, 64, 3441–3457. [Google Scholar] [CrossRef]
- Krause, A.J.; Henson, L.S.; Reineccius, G.A. Use of a chewing device to perform a mass balance on chewing gum components. Flavour Fragr. J. 2010, 26, 47–54. [Google Scholar] [CrossRef]
- Peyron, M.-A.; Santé-Lhoutellier, V.; Dardevet, D.; Hennequin, M.; Rémond, D.; François, O.; Woda, A. Addressing various challenges related to food bolus and nutrition with the AM2 mastication simulator. Food Hydrocoll. 2019, 97. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, Q.; Li, T.; Mao, Q. Masticatory simulators based on oral physiology in food research: A systematic review. J. Texture Stud. 2024, 55, e12864. [Google Scholar] [CrossRef]
- Walker, A.E.; Guy, F.; Salles, C.; Thiery, G.; Lazzari, V. Assessment of comminution capacity related to molar intercuspation in catarrhines using a chewing simulator. BMSAP 2022, 34, 10052. [Google Scholar] [CrossRef]
- Lucas, P.W.; Corlett, R.T.; Luke, D.A. Postcanine tooth size and diet in anthropoid primates. Z. Für Morphol. Und Anthropol. 1986, 76, 253–276. [Google Scholar] [CrossRef]
- Lucas, P.W. Dental Functional Morphology: How Teeth Work; Cambridge University Press: Cambridge, UK, 2004; pp. 1–372. [Google Scholar]
- Berthaume, M.A.; Dumont, E.R.; Godfrey, L.R.; Grosse, I.R. The effects of relative food item size on optimal tooth cusp sharpness during brittle food item processing. J. R. Soc. Interface 2014, 11, 20140965. [Google Scholar] [CrossRef] [PubMed]
- Jeannin, C.; Gritsch, K.; Liodénot, J.J.; Grosgogeat, B. MARIO: The first chewing bench used for ageing and analysis the released compounds of dental materials. Comput. Methods Biomech. Biomed. Eng. 2019, 22, S62–S64. [Google Scholar] [CrossRef]
- Chen, B.; Dhupia, J.S.; Morgenstern, M.P.; Bronlund, J.E.; Xu, W. Development of a Biomimetic Masticating Robot for Food Texture Analysis. J. Mech. Robot. 2021, 14, 1–28. [Google Scholar] [CrossRef]
- Rathbone, M.J.; Şenel, S.; Pather, I. Design and development of systemic oral mucosal drug delivery systems. Oral Mucosal Drug Deliv. Ther. 2015, 149–167. [Google Scholar] [CrossRef]
- Wanasathop, A.; Li, S.K. Iontophoretic Drug Delivery in the Oral Cavity. Pharmaceutics 2018, 10, 121. [Google Scholar] [CrossRef]
- Al Hagbani, T.; Nazzal, S. Medicated Chewing Gums (MCGs): Composition, Production, and Mechanical Testing. Aaps Pharmscitech 2018, 19, 2908–2920. [Google Scholar] [CrossRef]
- Rassing, M.R. Chewing gum as a drug delivery system. Adv. Drug Deliv. Rev. 1994, 13, 89–121. [Google Scholar] [CrossRef]
- Ferrazzano, G.F.; Cantile, T.; Coda, M.; Alcidi, B.; Sangianantoni, G.; Ingenito, A.; Di Stasio, M.; Volpe, M.G. In Vivo Release Kinetics and Antibacterial Activity of Novel Polyphenols-Enriched Chewing Gums. Molecules 2016, 21, 1008. [Google Scholar] [CrossRef]
- Giacaman, R.A.; Maturana, C.A.; Molina, J.; Volgenant, C.; Fernández, C.E. Effect of casein phosphopep-tide-amorphous calcium phosphate added to milk, chewing gum, and candy on dental caries: A systematic review. Caries Res. 2023, 57, 106–118. [Google Scholar] [CrossRef]
- Kassebaum, N.J.; Bernabé, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.L.; Marcenes, W. Global burden of untreated caries: A systematic review and metaregression. J. Dent. Res. 2015, 94, 650–658. [Google Scholar] [CrossRef] [PubMed]
- GBD 2017 Oral Disorders Collaborators; Bernabe, E.; Marcenes, W.; Hernandez, C.R.; Bailey, J.; Abreu, L.G.; Alipour, V.; Amini, S.; Arabloo, J.; Arefi, Z.; et al. Global, Regional, and National Levels and Trends in Burden of Oral Conditions from 1990 to 2017: A Systematic Analysis for the Global Burden of Disease 2017 Study. J. Dent. Res. 2020, 99, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Aspirin Market Size, Share, Value and Forecast 2030. Available online: https://www.zionmarketresearch.com (accessed on 31 May 2025).
- Original Source: Zion Market Research, Found on Globenewswire, 4 September 2018. Available online: http://www.globenewswire.com (accessed on 31 May 2025).
- Al Hagbani, T.; Nazzal, S. Curcumin complexation with cyclodextrins by the autoclave process: Method development and characterization of complex formation. Int. J. Pharm. 2017, 520, 173–180. [Google Scholar] [CrossRef]
- Al Hagbani, T.; Altomare, C.; Kamal, M.M.; Nazzal, S. Mechanical Characterization and Dissolution of Chewing Gum Tablets (CGTs) Containing Co-compressed Health in Gum® and Curcumin/Cyclodextrin Inclusion Complex. Aaps Pharmscitech 2018, 19, 3742–3750. [Google Scholar] [CrossRef] [PubMed]
- Daniell, H.; Nair, S.K.; Esmaeili, N.; Wakade, G.; Shahid, N.; Ganesan, P.K.; Islam, R.; Shepley-McTaggart, A.; Feng, S.; Gary, E.N.; et al. Debulking SARS-CoV-2 in saliva using angiotensin converting enzyme 2 in chewing gum to decrease oral virus transmission and infection. Mol. Ther. 2022, 30, 1966–1978. [Google Scholar] [CrossRef]
- Imfeld, T. Chewing Gum—Facts and Fiction: A Review of Gum-Chewing and Oral Health. Crit. Rev. Oral Biol. Med. 1999, 10, 405–419. [Google Scholar] [CrossRef]
- Konar, N.; Palabiyik, I.; Toker, O.S.; Sagdic, O. Chewing gum: Production, quality parameters and opportunities for delivering bioactive compounds. Trends Food Sci. Technol. 2016, 55, 29–38. [Google Scholar] [CrossRef]
- Al Hagbani, T.; Nazzal, S. Development of postcompressional textural tests to evaluate the mechanical properties of medicated chewing gum tablets with high drug loadings. J. Texture Stud. 2017, 49, 30–37. [Google Scholar] [CrossRef]
- Maslii, Y.; Kolisnyk, T.; Ruban, O.; Yevtifieieva, O.; Gureyeva, S.; Goy, A.; Kasparaviciene, G.; Kalveniene, Z.; Bernatoniene, J. Impact of Compression Force on Mechanical, Textural, Release and Chewing Perception Properties of Compressible Medicated Chewing Gums. Pharmaceutics 2021, 13, 1808. [Google Scholar] [CrossRef]
- Monograph 2.9.25 (2012). In Ph.Eur.9.0. Available online: http://uspbpep.com/ep60/2.9.25.%20dissolution%20test%20for%20medicated%20chewing%20gums%2020925e.pdf (accessed on 31 May 2025).
- Stomberg, C.; Kanikanti, V.-R.; Hamann, H.-J.; Kleinebudde, P. Development of a New Dissolution Test Method for Soft Chewable Dosage Forms. Aaps Pharmscitech 2017, 18, 2446–2453. [Google Scholar] [CrossRef]
- Zieschang, L.; Klein, M.; Krämer, J.; Windbergs, M. In Vitro Performance Testing of Medicated Chewing Gums. Dissolution Technol. 2018, 25, 64–69. [Google Scholar] [CrossRef]
- Externbrink, A.; Sharan, S.; Sun, D.; Jiang, W.; Keire, D.; Xu, X. An in vitro approach for evaluating the oral abuse deterrence of solid oral extended-release opioids with properties intended to deter abuse via chewing. Int. J. Pharm. 2019, 561, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Cai, F.; Shen, P.; Walker, G.D.; Reynolds, C.; Yuan, Y.; Reynolds, E.C. Remineralization of enamel subsurface lesions by chewing gum with added calcium. J. Dent. 2009, 37, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Alemzadeh, K. Innovative Bionics Product Life-Cycle Management Methodology Framework with Built-In Reverse Biomimetics: From Inception to Clinical Validation. Biomimetics 2025, 10, 158. [Google Scholar] [CrossRef]
- Schreuders, F.K.; Schlangen, M.; Kyriakopoulou, K.; Boom, R.M.; van der Goot, A.J. Texture methods for evaluating meat and meat analogue structures: A review. Food Control. 2021, 127, 108103. [Google Scholar] [CrossRef]
- Al Hagbani, T.; Altomare, C.; Salawi, A.; Nazzal, S. D-optimal mixture design: Formulation development, mechanical characterization, and optimization of curcumin chewing gums using oppanol® B 12 elastomer as a gum-base. Int. J. Pharm. 2018, 553, 210–219. [Google Scholar] [CrossRef]
- Bogdan, C.; Hales, D.; Cornilă, A.; Casian, T.; Iovanov, R.; Tomuță, I.; Iurian, S. Texture analysis—A versatile tool for pharmaceutical evaluation of solid oral dosage forms. Int. J. Pharm. 2023, 638, 122916. [Google Scholar] [CrossRef]
- Peleg, M. The instrumental texture profile analysis revisited. J. Texture Stud. 2019, 50, 362–368. [Google Scholar] [CrossRef]
- Benazzi, S.; Grosse, I.R.; Gruppioni, G.; Weber, G.W.; Kullmer, O. Comparison of occlusal loading conditions in a lower second premolar using three-dimensional finite element analysis. Clin. Oral Investig. 2013, 18, 369–375. [Google Scholar] [CrossRef]
- Katona, T.R.; Eckert, G.J. The mechanics of dental occlusion and disclusion. Clin. Biomech. 2017, 50, 84–91. [Google Scholar] [CrossRef]
- Katona, T.R. An engineering analysis of dental occlusion principles. Am. J. Orthod. Dentofacial Orthop. 2009, 135, 696–697. [Google Scholar] [CrossRef]
- Katona, T.R. Engineering analyses of the link between occlusion and temporomandibular joint disorders. Int. J. Stomatol. occlusion Med. 2012, 6, 16–21. [Google Scholar] [CrossRef]
- Katona, T.R.; Isikbay, S.C.; Chen, J. An analytical approach to 3D orthodontic load systems. Angle Orthod. 2014, 84, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Bourdiol, P.; Mioche, L. Correlations between functional and occlusal tooth-surface areas and food texture during natural chewing sequences in humans. Arch. Oral Biol. 2000, 45, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Ungar, P.S. Mammalian dental function and wear: A review. Biosurface Biotribology 2015, 1, 25–41. [Google Scholar] [CrossRef]
- Osborn, J.W. Relationship between the mandibular condyle and the occlusal plane during hominid evolution: Some of its effects on jaw mechanics. Am. J. Phys. Anthr. 1987, 73, 193–207. [Google Scholar] [CrossRef]
- Osborn, J.W. Orientation of the masseter muscle and the curve of Spee in relation to crushing forces on the molar teeth of primates. Am. J. Phys. Anthr. 1993, 92, 99–106. [Google Scholar] [CrossRef]
- Fukoe, H.; Basili, C.; Slavicek, R.; Sato, S.; Akimoto, S. Three-dimensional analyses of the mandible and the oc-clusal architecture of mandibular dentition. J. Stomat. Occ. Med. 2012, 5, 119–129. [Google Scholar] [CrossRef]
- Cimić, S.; Simunković, S.K.; Catić, A. The relationship between Angle type of occlusion and recorded Bennett angle values. J. Prosthet. Dent. 2016, 115, 729–735. [Google Scholar] [CrossRef]
- Andrews, L.F. The six keys to normal occlusion. Am. J. Orthod. 1972, 62, 296–309. [Google Scholar] [CrossRef]
- Nam, S.-E.; Park, Y.-S.; Lee, W.; Ahn, S.-J.; Lee, S.-P. Making three-dimensional Monson’s sphere using virtual dental models. J. Dent. 2013, 41, 336–344. [Google Scholar] [CrossRef]
- Spee, F.G.; Biedenbach, M.A.; Hotz, M.; Hitchcock, H.P. The Gliding Path of the Mandible along the Skull. J. Am. Dent. Assoc. 1980, 100, 670–675. [Google Scholar] [CrossRef]
- Marshall, S.D.; Caspersen, M.; Hardinger, R.R.; Franciscus, R.G.; Aquilino, S.A.; Southard, T.E. Development of the curve of Spee. Am. J. Orthod. Dentofacial Orthop. 2008, 134, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Lynch, C.D.; McConnell, R.J. Prosthodontic management of the curve of Spee: Use of the Broadrick flag. J. Prosthet. Dent. 2002, 87, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Monson, G.S. Applied mechanics to the theory of mandibular movements. Dent. Cosmos. 1932, 74, 1039–1053. [Google Scholar]
- Artificial Human Skull (Separates into 3 Parts). Available online: https://www.adam-rouilly.co.uk/product/po10-artificial-human-skull-separates-into-3-parts (accessed on 31 May 2025).
- Johnson, M. Overview of Texture Profile Analysis, Modified October 2023. Available online: https://texturetechnologies.com/resources/texture-profile-analysis (accessed on 31 May 2025).
- Bourne, M.C. Texture measurement of individual cooked dry beans by the puncture test. J. Food Sci. 1972, 37, 751–753. [Google Scholar] [CrossRef]
- Rosenthal, A.J. Texture profile analysis–how important are the parameters? J. Texture Stud. 2010, 41, 672–684. [Google Scholar] [CrossRef]
- Madieta, E.; Symoneaux, R.; Mehinagic, E. Textural properties of fruit affected by experimental conditions in TPA tests: An RSM approach. Int. J. Food Sci. Technol. 2011, 46, 1044–1052. [Google Scholar] [CrossRef]
- Rahman, M.S.; Al-Attabi, Z.H.; Al-Habsi, N.; Al-Khusaibi, M. Measurement of instrumental texture profile analysis 804 (TPA) of foods. In Techniques to Measure Food Safety and Quality: Microbial, Chemical, and Sensory; Springer: Berlin/Heidelberg, Germany, 2021; pp. 427–465. [Google Scholar]
- Nishinari, K.; Fang, Y. Perception and measurement of food texture: Solid foods. J. Texture Stud. 2018, 49, 160–201. [Google Scholar] [CrossRef]
- Paredes, J.; Cortizo-Lacalle, D.; Imaz, A.M.; Aldazabal, J.; Vila, M. Application of texture analysis methods for the characterization of cultured meat. Sci. Rep. 2022, 12, 3898. [Google Scholar] [CrossRef]
- Salejda, A.M.; Janiewicz, U.; Korzeniowska, M.; Kolniak-Ostek, J.; Krasnowska, G. Effect of walnut green husk addition on some quality properties of cooked sausages. LWT 2016, 65, 751–757. [Google Scholar] [CrossRef]
- Florek, M.; Junkuszew, A.; Bojar, W.; Skałecki, P.; Greguła-Kania, M.; Litwińczuk, A.; Gruszecki, T.M. Effect of Vacuum Ageing on Instrumental and Sensory Textural Properties of Meat from Uhruska Lambs. Ann. Anim. Sci. 2016, 16, 601–609. [Google Scholar] [CrossRef]
- Rapisarda, M.; Valenti, G.; Carbone, D.C.; Rizzarelli, P.; Recca, G.; La Carta, S.; Paradisi, R.; Fincchiaro, S. Strength, fracture and compression properties of gelatins by a new 3D printed tool. J. Food Eng. 2018, 220, 38–48. [Google Scholar] [CrossRef]
- Nitschke, I.; Moede, C.; Hopfenmüller, W.; Sobotta, B.A.J.; Koenig, A.; Jockusch, J. Validation of a New Measuring Instrument for the Assessment of Bite Force. Diagnostics 2023, 13, 3498. [Google Scholar] [CrossRef]
- Saberi, F.; Azmoon, E.; Nouri, M. Effect of thermal processing and mixing time on textural and sensory properties of stick chewing gum. Food Struct. 2019, 22, 100129. [Google Scholar] [CrossRef]
- Mehta, F.; Trivedi, P. Formulation and Characterization of Biodegradable Medicated Chewing Gum Delivery System for Motion Sickness using Corn Zein as Gum Former. Trop. J. Pharm. Res. 2015, 14, 753. [Google Scholar] [CrossRef]
- Palabiyik, I.; Güleri, T.; Gunes, R.; Öner, B.; Toker, O.S.; Konar, N. A fundamental optimization study on chewing gum textural and sensorial properties: The effect of ingredients. Food Struct. 2020, 26, 100155. [Google Scholar] [CrossRef]
- Jonkers, N.; van Dommelen, J.A.W.; Geers, M.G.D. Intrinsic mechanical properties of food in relation to texture parameters. Mech. Time-Dependent Mater. 2021, 26, 323–346. [Google Scholar] [CrossRef]
- Nishinari, K.; Fang, Y.; Rosenthal, A. Human oral processing and texture profile analysis parameters: Bridging the gap between the sensory evaluation and the instrumental measurements. J. Texture Stud. 2019, 50, 369–380. [Google Scholar] [CrossRef]
- Foster, K.D.; Woda, A.; Peyron, M.A. Effect of Texture of Plastic and Elastic Model Foods on the Parameters of Mastication. J. Neurophysiol. 2006, 95, 3469–3479. [Google Scholar] [CrossRef]
- Adeleke, O.A.; Abedin, S. Characterization of Prototype Gummy Formulations Provides Insight into Setting Quality Standards. Aaps Pharmscitech 2024, 25, 155. [Google Scholar] [CrossRef]
- Lira-Morales, D.; Montoya-Rojo, M.B.; Varela-Bojórquez, N.; González-Ayón, M.; Vélez-De La Rocha, R.; Verdu-go-Perales, M.; Sañudo-Barajas, J.A. Dietary fiber and lycopene from tomato processing. In Plant Food by-Products: Industrial Relevance for Food Additives and Nutraceuticals; Apple Academic Press: Palm Bay, FL, USA, 2018; pp. 256–281. [Google Scholar]
- Noren, N.E.; Scanlon, M.G.; Arntfield, S.D. Differentiating between tackiness and stickiness and their induction in foods. Trends Food Sci. Technol. 2019, 88, 290–301. [Google Scholar] [CrossRef]
- Salahi, M.R.; Mohebbi, M.; Razavi, S.M.A. Analyzing the effects of aroma and texture interactions on oral processing behavior and dynamic sensory perception: A case study on cold-set emulsion-filled gels containing limonene and menthol. Food Hydrocoll. 2024, 154, 110128. [Google Scholar] [CrossRef]
- Simpson, G.G. Paleobiology of Jurassic mammals; Paleobiologica, Emil Haim & Co.: Vienna, Austria, 1933; Volume 5, pp. 127–158. [Google Scholar]
- Fiorenza, L.; Huynh Nguyen, N.; Benazzi, S. Stress Distribution and Molar Macrowear in Pongo pygmaeus: A New Approach through Finite Element and Occlusal Fingerprint Analyses. Hum. Evol. 2015, 30, 215–226. [Google Scholar]
- Kay, R.F.; Hiiemae, K.M. Jaw movement and tooth use in recent and fossil primates. Am. J. Phys. Anthr. 1974, 40, 227–256. [Google Scholar] [CrossRef] [PubMed]
- DiPietro, G.J.; Moergeli, J.R., Jr. Significance of the Frankfort-mandibular plane angle to prosthodontics. J. Prosthet. Dent. 1976, 36, 624–635. [Google Scholar] [CrossRef]
- Kaushik, P.; Mittal, V.; Kaushik, D. Unleashing the Potential of β–cyclodextrin Inclusion Complexes in Bitter Taste Abatement: Development, Optimization and Evaluation of Taste Masked Anti-emetic Chewing Gum of Promethazine Hydrochloride. AAPS PharmSciTech 2024, 25, 169. [Google Scholar] [CrossRef]
- Xu, Y.; Lv, B.; Wu, P.; Chen, X.D. Creating similar food boluses as that in vivo using a novel in vitro bio-inspired oral mastication simulator (iBOMS-III): The cases with cooked rice and roasted peanuts. Food Res. Int. 2024, 190, 114630. [Google Scholar] [CrossRef]
- Market Segmentation by Product, News, Technavio, Functional Chewing Gum Market Size to Increase by USD 3.15 Billion Between 2023 to 2028. Available online: https://www.prnewswire.com/news-releases/functional-chewing-gum-market-size-to-increase-by-usd-3-15-billion-between-2023-to-2028--market-segmentation-by-product-distribution-channel-geography-technavio-302353616.html (accessed on 31 May 2025).
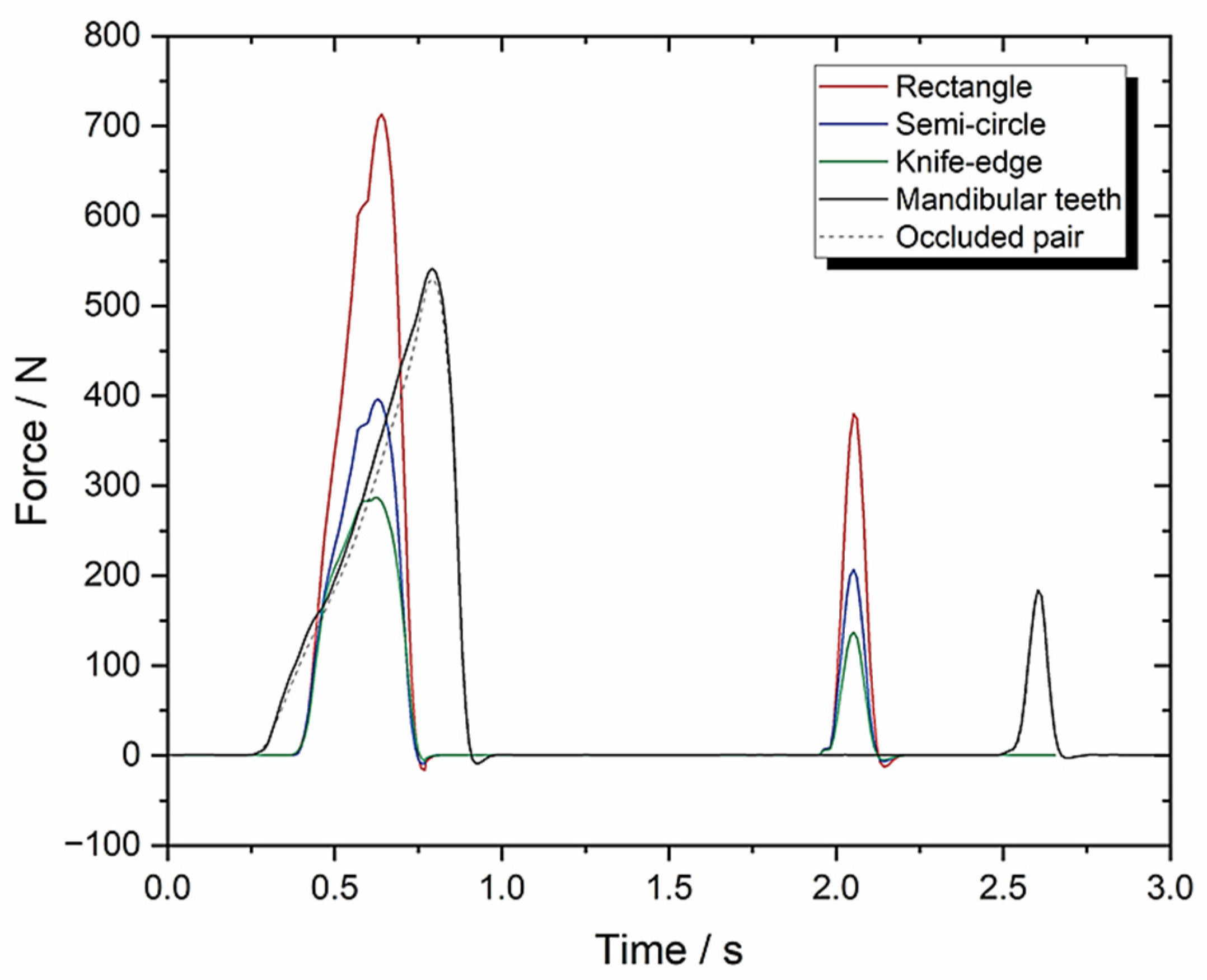
| TPA Parameters | Knife-Edge | Rectangle or Flat | Semi-Circle | Mandibular Teeth | Occluded Pair of Molars Teeth |
|---|---|---|---|---|---|
| Hardness (N) | 287.20 ± 14.36 | 713.51 ± 35.68 | 396.32 ± 19.82 | 546.5 ± 27.33 | 541.7 ± 27.09 |
| Cohesiveness | 0.16 ± 0.008 | 0.205 ± 0.010 | 0.18 ± 0.009 | 0.09 ± 0.005 | 0.06 ± 0.003 |
| Springiness | 0.29 ± 0.015 | 0.33 ± 0.02 | 0.28 ± 0.014 | 0.242 ± 0.012 | 0.225 ± 0.011 |
| Chewiness | 13.33 ± 0.67 | 48.27 ± 2.41 | 19.97 ± 1 | 11.9 ± 0.6 | 7.31 ± 0.37 |
| Compressibility | 0.44 ± 0.022 | 0.52 ± 0.03 | 0.44 ± 0.022 | 0.75 ± 0.038 | 0.827 ± 0.041 |
| Work Done (J) | 40.74 ± 2.04 | 91.07 ± 4.55 | 54.17 ± 2.71 | 1480.5 ± 74.03 | 1448.67 ± 72.43 |
| TPA Parameters | 0° FMA | 25° FMA | 29.1° FMA |
|---|---|---|---|
| Hardness (N) | 558.01 ± 27.90 | 419.88 ± 21 | 318.87 ± 15.94 |
| Cohesiveness | 0.05 ± 0.003 | 0.10 ± 0.005 | 0.12 ± 0.006 |
| Springiness | 0.10 ± 0.005 | 0.17 ± 0.009 | 0.20 ± 0.01 |
| Chewiness | 2.79 ± 0.14 | 7.14 ± 0.36 | 7.65 ± 0.38 |
| Compressibility | 0.82 ± 0.041 | 0.87 ± 0.044 | 0.89 ± 0.045 |
| Max Torque (Nm) | 0.31 ± 0.016 | 0.24 ± 0.012 | 0.17 ± 0.009 |
| Work Done (J) | 1295.05 ± 64.75 | 958.67 ± 47.93 | 709.18 ± 35.46 |
| Crush to Shear Ratio | ∞ | 4.22 ± 0.21 | 3.41 ± 0.17 |
| TPA Parameters | 0° FMA | 25° FMA | 29.1° FMA |
|---|---|---|---|
| Hardness (N) | 760.12 ± 38.01 | 496.54 ± 24.83 | 430.85 ± 21.54 |
| Cohesiveness | 0.09 ± 0.005 | 0.17 ± 0.009 | 0.19 ± 0.01 |
| Springiness | 0.29 ± 0.015 | 0.21 ± 0.011 | 0.22 ± 0.011 |
| Chewiness | 19.84 ± 1.0 | 17.73 ± 0.887 | 18.01 ± 0.9 |
| Compressibility | 0.92 ± 0.046 | 0.83 ± 0.042 | 0.86 ± 0.043 |
| Max Torque (Nm) | 0.59 ± 0.03 | 0.33 ± 0.017 | 0.3 ± 0.015 |
| Work Done (J) | 1301.71 ± 65.09 | 1524.69 ± 76.23 | 1421.68 ± 71.08 |
| Crush to Shear Ratio | ∞ | 4.22 ± 0.21 | 3.41 ± 0.17 |
| TPA Parameters | 0° FMA | 25° FMA | 29.1° FMA |
|---|---|---|---|
| Hardness (N) | 581.66 ± 29.08 | 347.69 ± 17.38 | 232.59 ± 11.63 |
| Cohesiveness | 0.05 ± 0.003 | 0.09 ± 0.005 | 0.10 ± 0.005 |
| Springiness | 0.11 ± 0.005 | 0.27 ± 0.014 | 0.23 ± 0.012 |
| Chewiness | 3.2 ± 0.16 | 8.45 ± 0.42 | 5.35 ± 0.27 |
| Compressibility | 0.83 ± 0.042 | 0.95 ± 0.048 | 0.91 ± 0.046 |
| Max Torque (Nm) | 0.65 ± 0.033 | 0.19 ± 0.01 | 0.19 ± 0.01 |
| Work Done (J) | 1292.67 ± 64.63 | 805.15 ± 40.26 | 590.48 ± 29.52 |
| Crush to Shear Ratio | 26157 ± 1307.85 | 4.22 ± 0.21 | 3.41 ± 0.17 |
| TPA Parameters | 0° FMA | 25° FMA | 29.1° FMA |
|---|---|---|---|
| Hardness (N) | 793.47 ± 39.67 | 446.75 ± 22.34 | 294.36 ± 14.72 |
| Cohesiveness | 0.06 ± 0.003 | 0.11 ± 0.006 | 0.13 ± 0.007 |
| Springiness | 0.19 ± 0.01 | 0.23 ± 0.012 | 0.23 ± 0.012 |
| Chewiness | 9.04 ± 0.45 | 11.3 ± 0.57 | 8.8 ± 0.44 |
| Compressibility | 0.84 ± 0.042 | 0.84 ± 0.042 | 0.85 ± 0.043 |
| Max Torque (Nm) | 0.96 ± 0.048 | 0.45 ± 0.023 | 0.38 ± 0.019 |
| Work Done (J) | 1971.80 ± 98.59 | 1123.21 ± 56.16 | 791.09 ± 39.55 |
| Crush to Shear Ratio | 19634 ± 981.7 | 4.22 ± 0.21 | 3.41 ± 0.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alemzadeh, K.; Alemzadeh, J. Experimental Study of Two-Bite Test Parameters for Effective Drug Release from Chewing Gum Using a Novel Bio-Engineered Testbed. Biomedicines 2025, 13, 1811. https://doi.org/10.3390/biomedicines13081811
Alemzadeh K, Alemzadeh J. Experimental Study of Two-Bite Test Parameters for Effective Drug Release from Chewing Gum Using a Novel Bio-Engineered Testbed. Biomedicines. 2025; 13(8):1811. https://doi.org/10.3390/biomedicines13081811
Chicago/Turabian StyleAlemzadeh, Kazem, and Joseph Alemzadeh. 2025. "Experimental Study of Two-Bite Test Parameters for Effective Drug Release from Chewing Gum Using a Novel Bio-Engineered Testbed" Biomedicines 13, no. 8: 1811. https://doi.org/10.3390/biomedicines13081811
APA StyleAlemzadeh, K., & Alemzadeh, J. (2025). Experimental Study of Two-Bite Test Parameters for Effective Drug Release from Chewing Gum Using a Novel Bio-Engineered Testbed. Biomedicines, 13(8), 1811. https://doi.org/10.3390/biomedicines13081811






