Neuromodulation of the Cardiac Autonomic Nervous System for Arrhythmia Treatment
Abstract
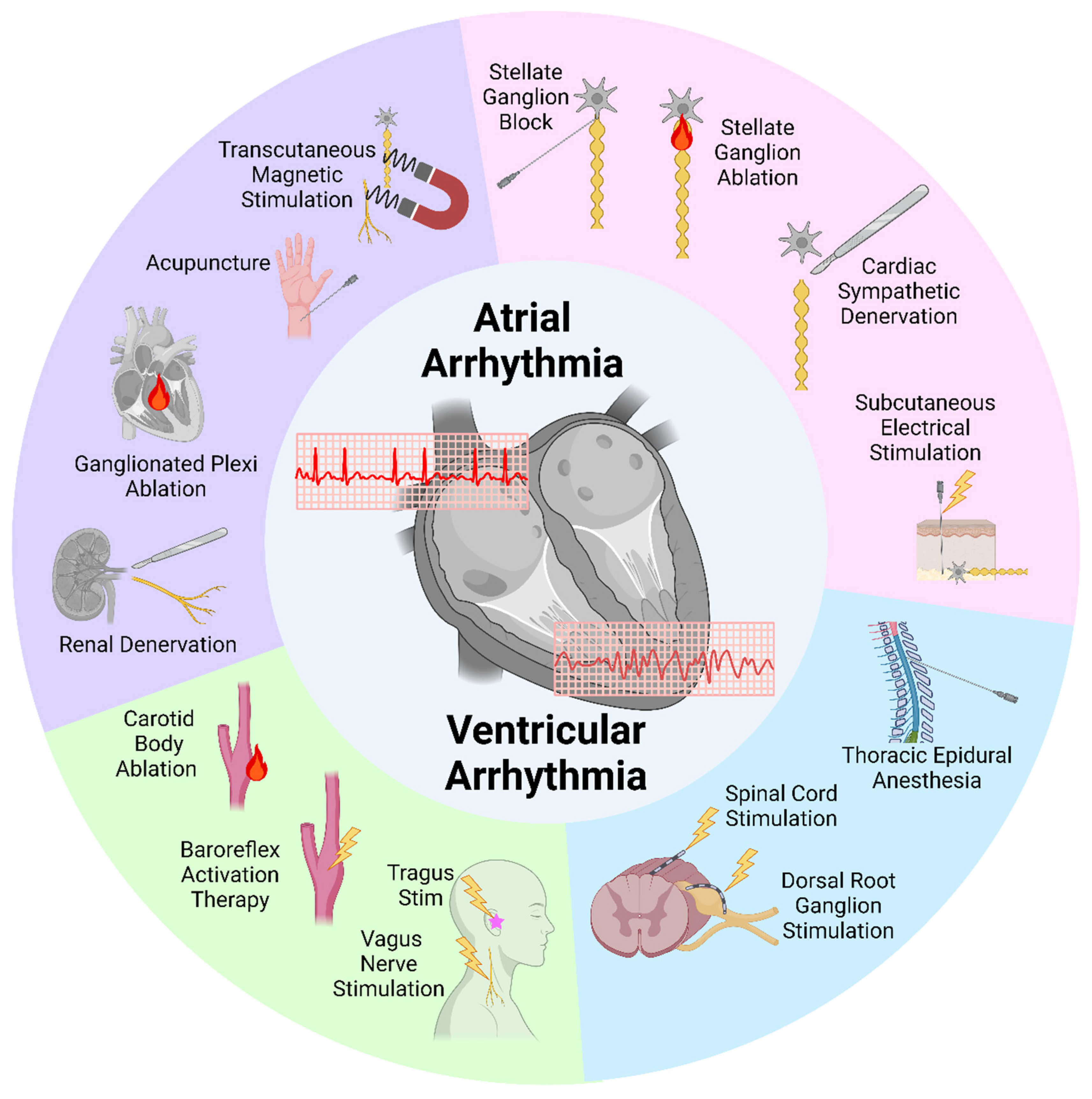
1. Introduction
2. Targeting the Stellate Ganglion
2.1. Stellate Ganglion Block
2.2. Stellate Ganglion Ablation
2.3. Cardiac Sympathetic Denervation
2.4. Subcutaneous Nerve Stimulation
3. Targeting the Spinal Cord and Dorsal Root Ganglion
3.1. Thoracic Epidural Anesthesia
3.2. Spinal Cord Stimulation
3.3. Dorsal Root Ganglion Stimulation
4. Targeting Parasympathetic Systems
4.1. Vagus Nerve Stimulation
4.2. Baroreflex Activation Therapy
4.3. Carotid Body Resection or Ablation
5. Other Potential Neuromodulation Techniques
5.1. Renal Denervation
5.2. Ganglionated Plexus Ablation
5.3. Acupuncture
5.4. Transcutaneous Magnetic Stimulation
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CANS | Cardiac Autonomic Nervous System |
| SG | Stellate Ganglion |
| SGB | Stellate Ganglion Block |
| BoNT-A | Botulinum Toxin A |
| VA | Ventricular Arrhythmia |
| VF | Ventricular Fibrillation |
| AA | Atrial Arrhythmia |
| AF | Atrial Fibrillation |
| MI | Myocardial Infarction |
| CSD | Cardiac Sympathetic Denervation |
| QTc | Corrected QT |
| LCSD | Left Cardiac Sympathetic Denervation |
| BCSD | Bilateral Cardiac Sympathetic Denervation |
| ICD | Implantable Cardioverter-Defibrillator |
| VT | Ventricular Tachycardia |
| TEA | Thoracic Epidural Anesthesia |
| SCS | Spinal Cord Stimulation |
| IPG | Implantable Programmable Pulse Generator |
| DRG | Dorsal Root Ganglion |
| DRGS | Dorsal Root Ganglion Stimulation |
| VNS | Vagus Nerve Stimulation |
| GP | Ganglionated Plexi |
| CB | Carotid Body |
| RDN | Renal Denervation |
| PV | Pulmonary Vein |
| AV | Atrioventricular |
| AFACT | AF Ablation and Autonomic Modulation via Thoracoscopic Surgery |
| TcMS | Transcutaneous Magnetic Stimulation |
References
- Tang, D.H.; Gilligan, A.M.; Romero, K. Economic Burden and Disparities in Healthcare Resource Use Among Adult Patients with Cardiac Arrhythmia. Appl. Health Econ. Health Policy 2014, 12, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Sussman, M.; Menzin, J.; Lin, I.; Kwong, W.J.; Munsell, M.; Friedman, M.; Selim, M. Impact of atrial fibrillation on stroke-related healthcare costs. J. Am. Heart Assoc. 2013, 2, e000479. [Google Scholar] [CrossRef] [PubMed]
- Chugh, S.S.; Reinier, K.; Teodorescu, C.; Evanado, A.; Kehr, E.; Al Samara, M.; Mariani, R.; Gunson, K.; Jui, J. Epidemiology of sudden cardiac death: Clinical and research implications. Prog. Cardiovasc. Dis. 2008, 51, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Ko, D.; Chung, M.K.; Evans, P.T.; Benjamin, E.J.; Helm, R. Atrial Fibrillation: A Review. JAMA 2025, 333, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Samuel, M.; Healey, J.S.; Nault, I.; Sterns, L.D.; Essebag, V.; Gray, C.; Hruczkowski, T.; Gardner, M.; Parkash, R.; Sapp, J.L. Ventricular Tachycardia and ICD Therapy Burden With Catheter Ablation Versus Escalated Antiarrhythmic Drug Therapy. JACC Clin. Electrophysiol. 2023, 9, 808–821. [Google Scholar] [CrossRef] [PubMed]
- Herring, N.; Kalla, M.; Paterson, D.J. The autonomic nervous system and cardiac arrhythmias: Current concepts and emerging therapies. Nat. Rev. Cardiol. 2019, 16, 707–726. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Kanazawa, H.; Aizawa, Y.; Ardell, J.L.; Shivkumar, K. Cardiac innervation and sudden cardiac death. Circ. Res. 2015, 116, 2005–2019. [Google Scholar] [CrossRef] [PubMed]
- Giannino, G.; Braia, V.; Brookles, C.G.; Giacobbe, F.; D’ascenzo, F.; Angelini, F.; Saglietto, A.; De Ferrari, G.M.; Dusi, V. The Intrinsic Cardiac Nervous System: From Pathophysiology to Therapeutic Implications. Biology 2024, 13, 105. [Google Scholar] [CrossRef] [PubMed]
- Shivkumar, K.; Ardell, J.L. Cardiac autonomic control in health and disease. J. Physiol. 2016, 594, 3851. [Google Scholar] [CrossRef] [PubMed]
- Ajijola, O.A.; Aksu, T.; Arora, R.; Biaggioni, I.; Chen, P.; De Ferrari, G.; Dusi, V.; Fudim, M.; Goldberger, J.J.; Green, A.L.; et al. Clinical neurocardiology: Defining the value of neuroscience-based cardiovascular therapeutics—2024 update. J. Physiol. 2025, 603, 1781–1839. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.J.; Zipes, D.P. Role of the autonomic nervous system in modulating cardiac arrhythmias. Circ. Res. 2014, 114, 1004–1021. [Google Scholar] [CrossRef] [PubMed]
- Salavatian, S.; Beaumont, E.; Gibbons, D.; Hammer, M.; Hoover, D.B.; Armour, J.A.; Ardell, J.L. Thoracic spinal cord and cervical vagosympathetic neuromodulation obtund nodose sensory transduction of myocardial ischemia. Auton. Neurosci. 2017, 208, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Salavatian, S.; Ardell, S.M.; Hammer, M.; Gibbons, D.; Armour, J.A.; Ardell, J.L. Thoracic spinal cord neuromodulation obtunds dorsal root ganglion afferent neuronal transduction of the ischemic ventricle. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H1134–H1141. [Google Scholar] [CrossRef] [PubMed]
- Salavatian, S.; Kuwabara, Y.; Wong, B.; Fritz, J.R.; Howard-Quijano, K.; Foreman, R.D.; Armour, J.A.; Ardell, J.L.; Mahajan, A. Spinal neuromodulation mitigates myocardial ischemia-induced sympathoexcitation by suppressing the intermediolateral nucleus hyperactivity and spinal neural synchrony. Front. Neurosci. 2023, 17, 1180294. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, Y.; Matsumoto, S.; Fujinaka, W.; Takatori, M.; Nishioka, K.; Namera, A. Left Stellate Ganglion Blockade for Refractory Ventricular Arrhythmias With Aconitine Poisoning: A Case Report. A A Pract. 2023, 17, e01666. [Google Scholar] [CrossRef] [PubMed]
- Wink, J.; Veering, B.T.; Aarts, L.P.H.J.; Wouters, P.F. Effects of Thoracic Epidural Anesthesia on Neuronal Cardiac Regulation and Cardiac Function. Anesthesiology 2019, 130, 472–491. [Google Scholar] [CrossRef] [PubMed]
- Mahfoud, F.; E Kandzari, D.; Kario, K.; Townsend, R.R.; A Weber, M.; E Schmieder, R.; Tsioufis, K.; Pocock, S.; Dimitriadis, K.; Choi, J.W.; et al. Long-term efficacy and safety of renal denervation in the presence of antihypertensive drugs (SPYRAL HTN-ON MED): A randomised, sham-controlled trial. Lancet 2022, 399, 1401–1410. [Google Scholar] [CrossRef] [PubMed]
- Bradfield, J.S.; Ajijola, O.A.; Vaseghi, M.; Shivkumar, K. Mechanisms and management of refractory ventricular arrhythmias in the age of autonomic modulation. Heart Rhythm 2018, 15, 1252–1260. [Google Scholar] [CrossRef] [PubMed]
- Lemery, R. Cardiac Neuromodulation and Neurocardiology. J. Cardiovasc. Electrophysiol. 2025, 36, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Markman, T.M.; Gugger, D.; Arkles, J.; Riley, M.P.; Dixit, S.; Guandalini, G.S.; Frankel, D.S.; Epstein, A.E.; Callans, D.J.; Singhal, S.; et al. Neuromodulation for the Treatment of Refractory Ventricular Arrhythmias. JACC Clin. Electrophysiol. 2023, 9, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Consoli, L.N.; Cetinel, E.; Lajczak, P.; Koziakas, I.G.; Majeed, M.W.; Wijaya, P.; Salha, I.; Samanidis, G. Surgical neuromodulation therapies to prevent postoperative atrial fibrillation: A meta-analysis, meta-regression, and trial sequential analysis of randomized controlled trials. Heart Rhythm, 2025; in press. [Google Scholar] [CrossRef] [PubMed]
- Cha, Y.; Li, X.; Yang, M.; Han, J.; Wu, G.; Kapa, S.C.; McLeod, C.J.; Noseworthy, P.A.; Mulpuru, S.K.; Asirvatham, S.J.; et al. Stellate ganglion block and cardiac sympathetic denervation in patients with inappropriate sinus tachycardia. J. Cardiovasc. Electrophysiol. 2019, 30, 2920–2928. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, A.; Qadri, Y.J.; Boortz-Marx, R.L.; Al-Khatib, S.M.; Harpole, D.H.; Katz, J.N.; Koontz, J.I.; Mathew, J.P.; Ray, N.D.; Sun, A.Y.; et al. Stellate Ganglion Blockade: An Intervention for the Management of Ventricular Arrhythmias. Curr. Hypertens. Rep. 2020, 22, 100. [Google Scholar] [CrossRef] [PubMed]
- Dusi, V.; Angelini, F.; Gravinese, C.; Frea, S.; De Ferrari, G.M. The role of antiarrhythmic drugs and stellate ganglion block in the acute management of electrical storm. Eur. Heart J. Suppl. 2025, 27 (Suppl. S1), i154–i161. [Google Scholar] [CrossRef] [PubMed]
- Yeo, Y.H.; Thong, J.Y.; Tan, M.-C.; Lee, J.Z.; Tan, J.L.; Markman, T.; Rattanawong, P. Stellate ganglion block for refractory ventricular arrhythmias: An updated systematic review. J. Interv. Card. Electrophysiol. 2025, 68, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Savastano, S.; Baldi, E.; Compagnoni, S.; Rordorf, R.; Sanzo, A.; Gentile, F.R.; Dusi, V.; Frea, S.; Gravinese, C.; Cauti, F.M.; et al. Electrical storm treatment by percutaneous stellate ganglion block: The STAR study. Eur. Heart J. 2024, 45, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Chouairi, F.; Rajkumar, K.; Benak, A.; Qadri, Y.; Piccini, J.P.; Mathew, J.; Ray, N.D.; Toman, J.; Kautzner, J.; Ganesh, A.; et al. A Multicenter Study of Stellate Ganglion Block as a Temporizing Treatment for Refractory Ventricular Arrhythmias. JACC Clin. Electrophysiol. 2024, 10, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, M.; Jiao, L.; Liu, C.; Chen, H.; Zhou, L.; Wang, Y.; Wang, Y.; Liu, Z.; Liu, Z.; et al. Ultrasound-guided injection of botulinum toxin type A blocks cardiac sympathetic ganglion to improve cardiac remodeling in a large animal model of chronic myocardial infarction. Heart Rhythm 2022, 19, 2095–2104. [Google Scholar] [CrossRef] [PubMed]
- Reinertsen, E.; Sabayon, M.; Riso, M.; Lloyd, M.; Spektor, B. Stellate ganglion blockade for treating refractory electrical storm: A historical cohort study. Can. J. Anaesth. 2021, 68, 1683–1689. [Google Scholar] [CrossRef] [PubMed]
- Sanghai, S.; Abbott, N.J.; Dewland, T.A.; Henrikson, C.A.; Elman, M.R.; Wollenberg, M.; Ivie, R.; Gonzalez-Sotomayor, J.; Nazer, B. Stellate Ganglion Blockade With Continuous Infusion Versus Single Injection for Treatment of Ventricular Arrhythmia Storm. JACC Clin. Electrophysiol. 2021, 7, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Pak, A.T.; Üstün, I.; Sengul, Y. Botulinum toxin type A wear-off phenomenon in chronic migraine patients: How long does the maximum efficiency last? Arq. Neuropsiquiatr. 2021, 79, 886–890. [Google Scholar] [CrossRef] [PubMed]
- Kamanli, A.; Kaya, A.; Ardicoglu, O.; Ozgocmen, S.; Zengin, F.O.; Bayık, Y. Comparison of lidocaine injection, botulinum toxin injection, and dry needling to trigger points in myofascial pain syndrome. Rheumatol. Int. 2005, 25, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.-H.; Zheng, L.-H.; Yao, Y. Ultrasound-guided stellate ganglion blockade: An appealing tactic for cardiac electrical storm. J. Geriatr. Cardiol. 2023, 20, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Bhattaram, S.; Shinde, V.; Hm, A. Star in the storm: Percutaneous stellate ganglion blockade for drug-refractory electrical storm in the emergency department. Clin. Exp. Emerg. Med. 2023, 10, 122–124. [Google Scholar] [CrossRef] [PubMed]
- Wittwer, E.D.; Radosevich, M.A.; Ritter, M.; Cha, Y.-M. Stellate Ganglion Blockade for Refractory Ventricular Arrhythmias: Implications of Ultrasound-Guided Technique and Review of the Evidence. J. Cardiothorac. Vasc. Anesth. 2020, 34, 2245–2252. [Google Scholar] [CrossRef] [PubMed]
- Savastano, S.; Dusi, V.; Baldi, E.; Rordorf, R.; Sanzo, A.; Camporotondo, R.; Fracchia, R.; Compagnoni, S.; Frigerio, L.; Visconti, L.O.; et al. Anatomical-based percutaneous left stellate ganglion block in patients with drug-refractory electrical storm and structural heart disease: A single-centre case series. Europace 2021, 23, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Motazedian, P.; Quinn, N.; Wells, G.A.; Beauregard, N.; Lam, E.; Mathieu, M.-E.; Knoll, W.; Prosperi-Porta, G.; Ly, V.; Parlow, S.; et al. Efficacy of stellate ganglion block in treatment of electrical storm: A systematic review and meta-analysis. Sci. Rep. 2024, 14, 24719. [Google Scholar] [CrossRef] [PubMed]
- Gulcu-Bulut, N.; Gonca, E.; Kocoglu, H.; Bozdoğan, Ö.; Karaaslan, K. Pretreatment with stellate ganglion blockade before ischemia reduces infarct size in rat hearts. Saudi Med. J. 2010, 31, 148–152. [Google Scholar] [PubMed]
- Gu, Y.; Wang, L.; Wang, X.; Tang, Y.; Cao, F.; Fang, Y. Assessment of ventricular electrophysiological characteristics at periinfarct zone of postmyocardial infarction in rabbits following stellate ganglion block. J. Cardiovasc. Electrophysiol. 2012, 23 (Suppl. S1), S29–S35. [Google Scholar] [CrossRef] [PubMed]
- Callipari, C.; Stone, M.; John, D.; Keceli, M.; Giles, R.A. Intra-Cardiac Arrest Use of Stellate Ganglion Block for Refractory Ventricular Tachycardia. J. Emerg. Med. 2023, 64, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Fudim, M.; Qadri, Y.J.; Waldron, N.H.; Boortz-Marx, R.L.; Ganesh, A.; Patel, C.B.; Podgoreanu, M.V.; Sun, A.Y.; Milano, C.A.; Tong, B.C.; et al. Stellate Ganglion Blockade for the Treatment of Refractory Ventricular Arrhythmias. JACC Clin. Electrophysiol. 2020, 6, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, L.; Liu, H.; Shao, Y.; Zhang, S. Cardiac Sympathetic Denervation Suppresses Atrial Fibrillation and Blood Pressure in a Chronic Intermittent Hypoxia Rat Model of Obstructive Sleep Apnea. J. Am. Heart Assoc. 2019, 8, e010254. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Tseng, C.H.; Shivkumar, K.; Ajijola, O. Efficacy of Stellate Ganglion Blockade in Managing Electrical Storm: A Systematic Review. JACC Clin. Electrophysiol. 2017, 3, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Baldi, E.; Dusi, V.; Rordorf, R.; Currao, A.; Compagnoni, S.; Sanzo, A.; Gentile, F.R.; Frea, S.; Gravinese, C.; Angelini, F.; et al. Efficacy of early use of percutaneous stellate ganglion block for electrical storms. Eur. Heart J. Acute Cardiovasc. Care 2024, 13, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Du, Y.; Yuan, C.; Han, L.; Zhao, Y.; Xie, Y.; Peng, W. Effect of ultrasound-guided stellate ganglion block on cerebral oxygen metabolism and S100B protein during carotid endarterectomy. Am. J. Transl. Res. 2024, 16, 1018. [Google Scholar] [CrossRef] [PubMed]
- Leftheriotis, D.; Flevari, P.; Kossyvakis, C.; Katsaras, D.; Batistaki, C.; Arvaniti, C.; Giannopoulos, G.; Deftereos, S.; Kostopanagiotou, G.; Lekakis, J. Acute effects of unilateral temporary stellate ganglion block on human atrial electrophysiological properties and atrial fibrillation inducibility. Heart Rhythm 2016, 13, 2111–2117. [Google Scholar] [CrossRef] [PubMed]
- Groenendyk, J.; Mandler, A.; Luan, D.; Goljo, E.; Tedore, T.; Cheung, J.W.; Markowitz, S.M. Management of Rapid Atrial Fibrillation Using Stellate Ganglion Blockade. JACC Case Rep. 2024, 29, 102530. [Google Scholar] [CrossRef] [PubMed]
- Rao, B.H.; Lokre, A.; Patnala, N.; Padmanabhan, T.N.C. Stellate ganglion ablation by conventional radiofrequency in patients with electrical storm. Europace 2023, 25, euad290. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yu, X.; Luo, D.; Qin, Z.; Wang, X.; He, W.; Ma, R.; Hu, H.; Xie, J.; He, B.; et al. Ablation of the Ligament of Marshall and Left Stellate Ganglion Similarly Reduces Ventricular Arrhythmias During Acute Myocardial Infarction. Circ. Arrhythm. Electrophysiol. 2018, 11, e005945. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, S.; Xiong, X.; Liu, J.; Xie, B.; Yao, Y.; Yin, J.; Zi, L.; Wang, X.; Tang, Y.; et al. Left Stellate Ganglion Ablation Inhibits Ventricular Arrhythmias through Macrophage Regulation in Canines with Acute Ischemic Stroke. Int. J. Med. Sci. 2021, 18, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Shelton, R.S.; Ogawa, M.; Lin, H.; Shen, C.; Wong, J.; Lin, S.-F.; Chen, P.-S.; Everett, T.H. Effects of Stellate Ganglion Cryoablation on Subcutaneous Nerve Activity and Atrial Tachyarrhythmias in a Canine Model of Pacing-Induced Heart Failure. JACC Clin. Electrophysiol. 2018, 4, 686–695. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.J.; Wittbrodt, M.T.; Bremner, J.D.; Vaccarino, V. Cardiovascular pathophysiology from the cardioneural perspective and its clinical applications. Trends Cardiovasc. Med. 2022, 32, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Buckley, U.; Yamakawa, K.; Takamiya, T.; Armour, J.A.; Shivkumar, K.; Ardell, J.L. Targeted stellate decentralization: Implications for sympathetic control of ventricular electrophysiology. Heart Rhythm. 2016, 13, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Irie, T.; Yamakawa, K.; Hamon, D.; Nakamura, K.; Shivkumar, K.; Vaseghi, M. Cardiac sympathetic innervation via middle cervical and stellate ganglia and antiarrhythmic mechanism of bilateral stellectomy. Am. J. Physiol.-Heart Circ. Physiol. 2017, 312, H392–H405. [Google Scholar] [CrossRef] [PubMed]
- Dhanse, S.; Rao, M.S.; Ramachandran, P.; Devasia, T.; Ashwal, A.J.; Paramasivam, G.; Prabhu, M. Effectiveness of ultrasonography-guided cardiac sympathetic denervation in acute control of electrical storm: A retrospective case series. J. Anaesthesiol. Clin. Pharmacol. 2022, 38, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Melinosky, K.; Leng, A.; Johnson, C.R.; Verdi, K.G.; Etchill, E.W.; Tandri, H.; Brock, M.V.; Ha, J.S. Outcomes Comparison of Robot-Assisted and Video-Assisted Thoracoscopic Cardiac Sympathetic Denervation. Innovations 2023, 18, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Dusi, V.; Sorg, J.M.; Gornbein, J.; Gima, J.; Yanagawa, J.; Lee, J.M.; Vecerek, N.; Vaseghi, M.; Bradfield, J.S.; De Ferrari, G.M.; et al. Prognostic impact of atrial rhythm and dimension in patients with structural heart disease undergoing cardiac sympathetic denervation for ventricular arrhythmias. Heart Rhythm 2020, 17 Pt A, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Moss, A.J.; McDonald, J. Unilateral cervicothoracic sympathetic ganglionectomy for the treatment of long QT interval syndrome. New Engl. J. Med. 1971, 285, 903–904. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, P.J.; Snebold, N.G.; Brown, A.M. Effects of unilateral cardiac sympathetic denervation on the ventricular fibrillation threshold. Am. J. Cardiol. 1976, 37, 1034–1040. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, P.J.; Priori, S.G.; Cerrone, M.; Spazzolini, C.; Odero, A.; Napolitano, C.; Bloise, R.; De Ferrari, G.M.; Klersy, C.; Moss, A.J.; et al. Left cardiac sympathetic denervation in the management of high-risk patients affected by the long-QT syndrome. Circulation 2004, 109, 1826–1833. [Google Scholar] [CrossRef] [PubMed]
- Surman, T.L.; Stuklis, R.G.; Chan, J.C. Thoracoscopic Sympathectomy for Long QT Syndrome. Literature Review and Case Study. Heart Lung Circ. 2019, 28, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Ertugrul, I.; Donmez, Y.N.; Aydın, A.; Aykan, H.H.; Sel, K.; Uysal, S.; Yilmaz, M.; Karagoz, T. Bilateral thoracoscopic sympathectomy for cardiac denervation in pediatric population: Does Kuntz nerve cauterization have an impact on success? J. Card. Surg. 2021, 36, 2705–2713. [Google Scholar] [CrossRef] [PubMed]
- Bourke, T.; Vaseghi, M.; Michowitz, Y.; Sankhla, V.; Shah, M.; Swapna, N.; Boyle, N.G.; Mahajan, A.; Narasimhan, C.; Lokhandwala, Y.; et al. Neuraxial modulation for refractory ventricular arrhythmias: Value of thoracic epidural anesthesia and surgical left cardiac sympathetic denervation. Circulation 2010, 121, 2255–2262. [Google Scholar] [CrossRef] [PubMed]
- Hanna, D.B.; Karimianpour, A.; Mamprejew, N.; Fiechter, C.; Verghese, D.; Navas, V.; Sharma, D. The role of cardiac sympathetic denervation for ventricular arrhythmias: An updated systematic review and meta-analysis. J. Interv. Card. Electrophysiol. 2025, 68, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Assis, F.R.; Sharma, A.; Shah, R.; Akhtar, T.; Adari, S.; Calkins, H.; Ha, J.S.; Mandal, K.; Tandri, H. Long-Term Outcomes of Bilateral Cardiac Sympathetic Denervation for Refractory Ventricular Tachycardia. JACC Clin. Electrophysiol. 2021, 7, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Charate, R.; Bawa, D.; Ghazal, R.; Garg, J.; Pothineni, N.V.K.; Kabra, R.; Della Rocca, D.G.; Atkins, D.; Lakkireddy, P.; et al. Bilateral Cardiac Sympathetic Denervation for Refractory Multifocal Premature Ventricular Contractions in Patients With Nonischemic Cardiomyopathy. JACC Clin. Electrophysiol. 2024, 10, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Vaseghi, M.; Barwad, P.; Corrales, F.J.M.; Tandri, H.; Mathuria, N.; Shah, R.; Sorg, J.M.; Gima, J.; Mandal, K.; Morales, L.C.S.; et al. Cardiac Sympathetic Denervation for Refractory Ventricular Arrhythmias. J. Am. Coll. Cardiol. 2017, 69, 3070–3080. [Google Scholar] [CrossRef] [PubMed]
- Hofferberth, S.C.; Cecchin, F.; Loberman, D.; Fynn-Thompson, F. Left thoracoscopic sympathectomy for cardiac denervation in patients with life-threatening ventricular arrhythmias. J. Thorac. Cardiovasc. Surg. 2014, 147, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Dai, M.; Tian, Y.; Zhang, P.; Wittwer, E.D.; Rho, R.H.; Kapa, S.; McLeod, C.J.; Mulpuru, S.K.; Lee, H.; et al. Electrophysiologic effects and outcomes of sympatholysis in patients with recurrent ventricular arrhythmia and structural heart disease. J. Cardiovasc. Electrophysiol. 2019, 30, 1499–1507. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Assis, F.; Alugubelli, N.; Okada, D.R.; Cardoso, R.; Shivkumar, K.; Tandri, H. Cardiac sympathetic denervation for refractory ventricular arrhythmias in patients with structural heart disease: A systematic review. Heart Rhythm 2019, 16, 1499–1505. [Google Scholar] [CrossRef] [PubMed]
- Barwad, P.; Sinkar, K.; Bachani, N.; Shah, R.; Shah, V.; Kumar, B.; Bhoskar, S.; Desai, N.; Lokhandwala, Y. Long-term clinical outcomes of cardiac sympathetic denervation in patients with refractory ventricular arrhythmias. J. Cardiovasc. Electrophysiol. 2021, 32, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Yalin, K.; Liosis, S.; Palade, E.; Fink, T.; Schierholz, S.; Sawan, N.; Eitel, C.; Heeger, C.H.; Sciacca, V.; Sano, M.; et al. Cardiac sympathetic denervation in patients with nonischemic cardiomyopathy and refractory ventricular arrhythmias: A single-center experience. Clin. Res. Cardiol. 2021, 110, 21–28. [Google Scholar] [CrossRef] [PubMed]
- König, S.; Schröter, T.; A Borger, M.; Bertagnolli, L.; Nedios, S.; Darma, A.; Hindricks, G.; Arya, A.; Dinov, B. Outcomes following cardiac sympathetic denervation in patients with structural heart disease and refractory ventricular arrhythmia. Europace 2022, 24, 1800–1808. [Google Scholar] [CrossRef] [PubMed]
- Brady, S.; Matthia, E.; Antoine, S.; Aranda, J.; Miles, W.; Vilaro, J.; Al-Ani, M.; Oduntan, O.; Guo, Y.; Li, Y.; et al. Outcome of Patients With Systolic Heart Failure Who Underwent Sympathectomy for Ventricular Arrhythmia. Am. J. Cardiol. 2024, 225, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Robinson, E.A.; Rhee, K.; Doytchinova, A.; Kumar, M.; Shelton, R.; Jiang, Z.; Kamp, N.J.; Adams, D.; Wagner, D.; Shen, C.; et al. Estimating sympathetic tone by recording subcutaneous nerve activity in ambulatory dogs. J. Cardiovasc. Electrophysiol. 2015, 26, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Doytchinova, A.; Patel, J.; Zhou, S.; Chen, L.S.; Lin, H.; Shen, C.; Everett, T.H.; Lin, S.-F.; Chen, P.-S. Subcutaneous nerve activity and spontaneous ventricular arrhythmias in ambulatory dogs. Heart Rhythm 2015, 12, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Zhao, Y.; Doytchinova, A.; Kamp, N.J.; Tsai, W.-C.; Yuan, Y.; Adams, D.; Wagner, D.; Shen, C.; Chen, L.S.; et al. Using skin sympathetic nerve activity to estimate stellate ganglion nerve activity in dogs. Heart Rhythm 2015, 12, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Jiang, Z.; Zhao, Y.; Tsai, W.-C.; Patel, J.; Chen, L.S.; Shen, C.; Lin, S.-F.; Chen, H.-S.V.; Everett, T.H.; et al. Long-term intermittent high-amplitude subcutaneous nerve stimulation reduces sympathetic tone in ambulatory dogs. Heart Rhythm 2018, 15, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Kusayama, T.; Wan, J.; Yuan, Y.; Liu, X.; Li, X.; Shen, C.; Fishbein, M.C.; Everett, T.H.; Chen, P.-S. Effects of subcutaneous nerve stimulation with blindly inserted electrodes on ventricular rate control in a canine model of persistent atrial fibrillation. Heart Rhythm 2021, 18, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhao, Y.; Wong, J.; Tsai, W.-C.; Jiang, Z.; Kabir, R.A.; Han, S.; Shen, C.; Fishbein, M.C.; Chen, L.S.; et al. Subcutaneous nerve stimulation reduces sympathetic nerve activity in ambulatory dogs with myocardial infarction. Heart Rhythm 2020, 17, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Boezaart, A.P.; Smith, C.R.; Chembrovich, S.; Zasimovich, Y.; Server, A.; Morgan, G.; Theron, A.; Booysen, K.; A Reina, M. Visceral versus somatic pain: An educational review of anatomy and clinical implications. Reg. Anesth. Pain. Med. 2021, 46, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Hoang, J.D.; van Weperen, V.Y.; Kang, K.-W.; Jani, N.R.; Swid, M.A.; Chan, C.A.; Lokhandwala, Z.A.; Lux, R.L.; Vaseghi, M. Antiarrhythmic Mechanisms of Epidural Blockade After Myocardial Infarction. Circ. Res. 2024, 135, e57–e75. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.W. Successful neural modulation of bedside modified thoracic epidural anesthesia for ventricular tachycardia electrical storm. Acute Crit. Care 2022, 39, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Howard-Quijano, K.; Takamiya, T.; Dale, E.A.; Yamakawa, K.; Zhou, W.; Buckley, U.; Mahajan, A. Effect of Thoracic Epidural Anesthesia on Ventricular Excitability in a Porcine Model. Anesthesiology 2017, 126, 1096–1106. [Google Scholar] [CrossRef] [PubMed]
- Do, D.H.; Bradfield, J.; Ajijola, O.A.; Vaseghi, M.; Le, J.; Rahman, S.; Mahajan, A.; Nogami, A.; Boyle, N.G.; Shivkumar, K. Thoracic Epidural Anesthesia Can Be Effective for the Short-Term Management of Ventricular Tachycardia Storm. J. Am. Heart Assoc. 2017, 6, e007080. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Horner, F.; Aly, M.; Nair, G.S.; Lin, C. Why do thoracic epidurals fail? A literature review on thoracic epidural failure and catheter confirmation. World J. Crit. Care Med. 2024, 13, 94157. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.-M.; Lee, H.J.; Oh, Y.-J.; Cho, A.R.; Kim, H.J.; Lee, D.-W.; Do, W.-S.; Kwon, J.-Y.; Kim, H. Observations on significant hemodynamic changes caused by a high concentration of epidurally administered ropivacaine: Correlation and prediction study of stroke volume variation and central venous pressure in thoracic epidural anesthesia. BMC Anesthesiol. 2017, 17, 153. [Google Scholar] [CrossRef] [PubMed]
- Hoang, J.D.; van Weperen, V.Y.; Kang, K.W.; Jani, N.R.; Swid, M.A.; Chan, C.A.; Lokhandwala, Z.A.; Lux, R.L.; Vaseghi, M. Thoracic epidural blockade after myocardial infarction benefits from anti-arrhythmic pathways mediated in part by parasympathetic modulation. bioRxiv 2024. bioRxiv:2024.03.14.585127. [Google Scholar]
- Smith, D.I.; Kralovic, S.A.; Hegazy, R.A.; Tran, H. Continuous Thoracic Paravertebral Block to Treat Electrical Storm. Tex. Heart Inst. J. 2022, 49, e176433. [Google Scholar] [CrossRef] [PubMed]
- Shinde, S.R.; Basantwani, S.; Tendolkar, B. Management of ventricular storm with thoracic epidural anesthesia. Ann. Card. Anaesth. 2019, 22, 439–441. [Google Scholar] [CrossRef] [PubMed]
- Ardell, J.L.; Nier, H.; Hammer, M.; Southerland, E.M.; Ardell, C.L.; Beaumont, E.; KenKnight, B.H.; Armour, J.A. Defining the neural fulcrum for chronic vagus nerve stimulation: Implications for integrated cardiac control. J. Physiol. 2017, 595, 6887–6903. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.-Y.; Liu, Y.; Zuo, M.; Zhang, Y.; Yue, W.; Au, K.-W.; Lai, W.-H.; Wu, Y.; Shuto, C.; Chen, P.; et al. Remodelling of cardiac sympathetic re-innervation with thoracic spinal cord stimulation improves left ventricular function in a porcine model of heart failure. Europace 2015, 17, 1875–1883. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhou, X.; Huang, B.; Wang, Z.; Zhou, L.; Chen, M.; Yu, L.; Jiang, H. Spinal cord stimulation suppresses atrial fibrillation by inhibiting autonomic remodeling. Heart Rhythm 2016, 13, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Caylor, J.; Reddy, R.; Yin, S.; Cui, C.; Huang, M.; Huang, C.; Rao, R.; Baker, D.G.; Simmons, A.; Souza, D.; et al. Spinal cord stimulation in chronic pain: Evidence and theory for mechanisms of action. Bioelectron. Med. 2019, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Sdrulla, A.D.; Guan, Y.; Raja, S.N. Spinal Cord Stimulation: Clinical Efficacy and Potential Mechanisms. Pain. Pract. 2018, 18, 1048–1067. [Google Scholar] [CrossRef] [PubMed]
- Kapural, L.; Yu, C.; Doust, M.W.; Gliner, B.E.; Vallejo, R.; Sitzman, B.T.; Amirdelfan, K.; Morgan, D.M.; Brown, L.L.; Yearwood, T.L.; et al. Novel 10-kHz High-frequency Therapy (HF10 Therapy) Is Superior to Traditional Low-frequency Spinal Cord Stimulation for the Treatment of Chronic Back and Leg Pain. Anesthesiology 2015, 123, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Conic, R.R.Z.; Caylor, J.; Cui, C.L.; Reyes, Z.; Nelson, E.; Yin, S.; Lerman, I. Sex-specific differences in the efficacy of traditional low frequency versus high frequency spinal cord stimulation for chronic pain. Bioelectron. Med. 2022, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Hoelzer, B.C.; Edgar, D.; Lu, S.-P.; Taylor, R.S. Indirect Comparison of 10 kHz Spinal Cord Stimulation (SCS) versus Traditional Low-Frequency SCS for the Treatment of Painful Diabetic Neuropathy: A Systematic Review of Randomized Controlled Trials. Biomedicines 2022, 10, 2630. [Google Scholar] [CrossRef] [PubMed]
- Sagalajev, B.; Zhang, T.; Abdollahi, N.; Yousefpour, N.; Medlock, L.; Al-Basha, D.; Ribeiro-Da-Silva, A.; Esteller, R.; Ratté, S.; Prescott, S.A. Absence of paresthesia during high-rate spinal cord stimulation reveals importance of synchrony for sensations evoked by electrical stimulation. Neuron 2024, 112, 404–420.e6. [Google Scholar] [CrossRef] [PubMed]
- Salavatian, S.; Wong, B.; Kuwabara, Y.; Fritz, J.R.; Varghese, C.G.; Howard-Quijano, K.; Armour, J.A.; Foreman, R.D.; Ardell, J.L.; Mahajan, A. Comparing the Memory Effects of 50-Hz Low-Frequency and 10-kHz High-Frequency Thoracic Spinal Cord Stimulation on Spinal Neural Network in a Myocardial Infarction Porcine Model. Neuromodulation 2024, 27, 1177–1186. [Google Scholar] [CrossRef] [PubMed]
- Solanes, C.; Durá, J.L.; Canós, M.Á.; De Andrés, J.; Martí-Bonmatí, L.; Saiz, J. 3D patient-specific spinal cord computational model for SCS management: Potential clinical applications. J. Neural Eng. 2021, 18, 036017. [Google Scholar] [CrossRef] [PubMed]
- Rogers, E.R.; Mirzakhalili, E.; Lempka, S.F. Model-based analysis of subthreshold mechanisms of spinal cord stimulation for pain. J. Neural Eng. 2023, 20, 066003. [Google Scholar] [CrossRef] [PubMed]
- Howard-Quijano, K.; Takamiya, T.; Dale, E.A.; Kipke, J.; Kubo, Y.; Grogan, T.; Afyouni, A.; Shivkumar, K.; Mahajan, A. Spinal cord stimulation reduces ventricular arrhythmias during acute ischemia by attenuation of regional myocardial excitability. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H421–H431. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Howard-Quijano, K.; Yamaguchi, T.; Gao, F.; Kuwabara, Y.; Puig, S.; Lundquist, E.; Salavatian, S.; Taylor, B.; Mahajan, A. Spinal Cord Stimulation Reduces Ventricular Arrhythmias by Attenuating Reactive Gliosis and Activation of Spinal Interneurons. JACC Clin. Electrophysiol. 2021, 7, 1211–1225. [Google Scholar] [CrossRef] [PubMed]
- Howard-Quijano, K.; Kuwabara, Y.; Yamaguchi, T.; Roman, K.; Salavatian, S.; Taylor, B.; Mahajan, A. GABAergic signaling during spinal cord stimulation reduces cardiac arrhythmias in a porcine model. Anesthesiology 2023, 138, 372–387. [Google Scholar] [CrossRef] [PubMed]
- Ardell, J.L.; Foreman, R.D.; Armour, J.A.; Shivkumar, K. Cardiac sympathectomy and spinal cord stimulation attenuate reflex-mediated norepinephrine release during ischemia preventing ventricular fibrillation. JCI Insight 2019, 4, 131648. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhou, X.; Huang, B.; Wang, Z.; Liao, K.; Saren, G.; Lu, Z.; Chen, M.; Yu, L.; Jiang, H. Spinal cord stimulation protects against ventricular arrhythmias by suppressing left stellate ganglion neural activity in an acute myocardial infarction canine model. Heart Rhythm 2015, 12, 1628–1635. [Google Scholar] [CrossRef] [PubMed]
- Walega, D.; Rosenow, J.M. Spinal cord stimulation for electrical storm refractory to conventional medical treatment: An emerging indication? Neuromodulation Technol. Neural Interface 2015, 18, 194–196. [Google Scholar] [CrossRef] [PubMed]
- Saraste, A.; Ukkonen, H.; Varis, A.; Vasankari, T.; Tunturi, S.; Taittonen, M.; Rautakorpi, P.; Luotolahti, M.; Airaksinen, K.J.; Knuuti, J. Effect of spinal cord stimulation on myocardial perfusion reserve in patients with refractory angina pectoris. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Bao, H.; Si, Y.; Xu, C.; Chen, H.; Gao, X.; Xie, X.; Xu, Y.; Sun, F.; Zeng, L. Spinal Cord Stimulation for Refractory Angina Pectoris: A Systematic Review and Meta-analysis. Clin. J. Pain. 2017, 33, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, S.A.; Wong, B.; Vasquez, C.; Rosenberg, S.P.; Rooke, R.; Kuznekoff, L.M.; Lader, J.M.; Mahoney, V.M.; Budylin, T.; Älvstrand, M.; et al. Spinal cord stimulation protects against atrial fibrillation induced by tachypacing. Heart Rhythm 2012, 9, 1426–1433.e3. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Chang, Y.; Song, J. Advances in preclinical surgical therapy of cardiovascular diseases. Int. J. Surg. 2024, 110, 4965–4975. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Huang, B.; He, W.; Wang, S.; Liao, K.; Zhou, X.; He, B.; Lu, Z.; Jiang, H. Spinal cord stimulation suppresses focal rapid firing–induced atrial fibrillation by inhibiting atrial ganglionated plexus activity. J. Cardiovasc. Pharmacol. 2014, 64, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Romanov, A.; Lomivorotov, V.; Chernyavskiy, A.; Murtazin, V.; Kliver, E.; Ponomarev, D.; Mikheenko, I.; Yakovlev, A.; Yakovleva, M.; Steinberg, J.S. Temporary spinal cord stimulation to prevent postcardiac surgery atrial fibrillation: 30-day safety and efficacy outcomes. J. Am. Coll. Cardiol. 2022, 79, 754–756. [Google Scholar] [CrossRef] [PubMed]
- Ardell, J.L.; Andresen, M.C.; Armour, J.A.; Billman, G.E.; Chen, P.-S.; Foreman, R.D.; Herring, N.; O’Leary, D.S.; Sabbah, H.N.; Schultz, H.; et al. Translational neurocardiology: Preclinical models and cardioneural integrative aspects. J. Physiol. 2016, 594, 3877–3909. [Google Scholar] [CrossRef] [PubMed]
- Krames, E.S. The dorsal root ganglion in chronic pain and as a target for neuromodulation: A review. Neuromodulation Technol. Neural Interface 2015, 18, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Sverrisdottir, Y.B.; Martin, S.C.; Hadjipavlou, G.; Kent, A.R.; Paterson, D.J.; FitzGerald, J.J.; Green, A.L. Human Dorsal Root Ganglion Stimulation Reduces Sympathetic Outflow and Long-Term Blood Pressure. JACC Basic. Transl. Sci. 2020, 5, 973–985. [Google Scholar] [CrossRef] [PubMed]
- Deer, T.R.; Levy, R.M.; Kramer, J.; Poree, L.; Amirdelfan, K.; Grigsby, E.; Staats, P.; Burton, A.W.; Burgher, A.H.; Obray, J.; et al. Dorsal root ganglion stimulation yielded higher treatment success rate for CRPS and causalgia at 3 and 12 months: Randomized comparative trial. Pain 2017, 158, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Deer, T.R.; Hunter, C.W.; Mehta, P.; Sayed, D.; Grider, J.S.; Lamer, T.J.; E Pope, J.; Falowski, S.; A Provenzano, D.; Esposito, M.F.; et al. A systematic literature review of dorsal root ganglion neurostimulation for the treatment of pain. Pain Med. 2020, 21, 1581–1589. [Google Scholar] [CrossRef] [PubMed]
- D’sOuza, R.S.; Kubrova, E.; Her, Y.F.; Barman, R.A.; Smith, B.J.; Alvarez, G.M.; West, T.E.; Abd-Elsayed, A. Dorsal root ganglion stimulation for lower extremity neuropathic pain syndromes: An evidence-based literature review. Adv. Ther. 2022, 39, 4440–4473. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, Y.; Howard-Quijano, K.; Salavatian, S.; Yamaguchi, T.; Saba, S.; Mahajan, A. Thoracic dorsal root ganglion stimulation reduces acute myocardial ischemia induced ventricular arrhythmias. Front. Neurosci. 2023, 17, 1091230. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, Y.; Salavatian, S.; Howard-Quijano, K.; Yamaguchi, T.; Lundquist, E.; Mahajan, A. Neuromodulation With Thoracic Dorsal Root Ganglion Stimulation Reduces Ventricular Arrhythmogenicity. Front. Physiol. 2021, 12, 713717. [Google Scholar] [CrossRef] [PubMed]
- Garamendi-Ruiz, I.; Gomez-Esteban, J.C. Cardiovascular autonomic effects of vagus nerve stimulation. Clin. Auton. Res. 2019, 29, 183–194. [Google Scholar] [CrossRef] [PubMed]
- De Ferrari, G.M.; Crijns, H.J.; Borggrefe, M.; Milasinovic, G.; Smid, J.; Zabel, M.; Gavazzi, A.; Sanzo, A.; Dennert, R.; Kuschyk, J.; et al. Chronic vagus nerve stimulation: A new and promising therapeutic approach for chronic heart failure. Eur. Heart J. 2011, 32, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Ardell, J.L.; Rajendran, P.S.; Nier, H.A.; KenKnight, B.H.; Armour, J.A. Central-peripheral neural network interactions evoked by vagus nerve stimulation: Functional consequences on control of cardiac function. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1740–H1752. [Google Scholar] [CrossRef] [PubMed]
- Yoo, P.B.; Liu, H.; Hincapie, J.G.; Ruble, S.B.; Hamann, J.J.; Grill, W.M. Modulation of heart rate by temporally patterned vagus nerve stimulation in the anesthetized dog. Physiol. Rep. 2016, 4, e12689. [Google Scholar] [CrossRef] [PubMed]
- Stavrakis, S.; Stoner, J.A.; Humphrey, M.B.; Morris, L.; Filiberti, A.; Reynolds, J.C.; Elkholey, K.; Javed, I.; Twidale, N.; Riha, P.; et al. TREAT AF (Transcutaneous Electrical Vagus Nerve Stimulation to Suppress Atrial Fibrillation): A Randomized Clinical Trial. JACC Clin. Electrophysiol. 2020, 6, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Antonino, D.; Teixeira, A.L.; Maia-Lopes, P.M.; Souza, M.C.; Sabino-Carvalho, J.L.; Murray, A.R.; Deuchars, J.; Vianna, L.C. Non-invasive vagus nerve stimulation acutely improves spontaneous cardiac baroreflex sensitivity in healthy young men: A randomized placebo-controlled trial. Brain Stimul. 2017, 10, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Katsunuma, R.; Takamura, T.; Yamada, M.; Sekiguchi, A. Proof of mechanism investigation of Transcutaneous auricular vagus nerve stimulation through simultaneous measurement of autonomic functions: A randomized controlled trial protocol. Biopsychosoc. Med. 2024, 18, 15. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; Scherlag, B.J.; Yu, L.; Li, S.; Ali, R.; Zhang, Y.; Fu, G.; Nakagawa, H.; Jackman, W.M.; Lazzara, R.; et al. Prevention and reversal of atrial fibrillation inducibility and autonomic remodeling by low-level vagosympathetic nerve stimulation. J. Am. Coll. Cardiol. 2011, 57, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Scherlag, B.J.; Li, S.; Sheng, X.; Lu, Z.; Nakagawa, H.; Zhang, Y.; Jackman, W.M.; Lazzara, R.; Jiang, H.; et al. Low-level vagosympathetic nerve stimulation inhibits atrial fibrillation inducibility: Direct evidence by neural recordings from intrinsic cardiac ganglia. J. Cardiovasc. Electrophysiol. 2011, 22, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Salavatian, S.; Beaumont, E.; Longpré, J.-P.; Armour, J.A.; Vinet, A.; Jacquemet, V.; Shivkumar, K.; Ardell, J.L. Vagal stimulation targets select populations of intrinsic cardiac neurons to control neurally induced atrial fibrillation. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H1311–H1320. [Google Scholar] [CrossRef] [PubMed]
- Hanna, P.; Buch, E.; Stavrakis, S.; Meyer, C.; Tompkins, J.D.; Ardell, J.L.; Shivkumar, K. Neuroscientific therapies for atrial fibrillation. Cardiovasc. Res. 2021, 117, 1732–1745. [Google Scholar] [CrossRef] [PubMed]
- Kharbanda, R.K.; van der Does, W.F.; van Staveren, L.N.; Taverne, Y.J.; Bogers, A.J.; de Groot, N.M. Vagus Nerve Stimulation and Atrial Fibrillation: Revealing the Paradox. Neuromodulation Technol. Neural Interface 2022, 25, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mazgalev, T.N. Arrhythmias and vagus nerve stimulation. Heart Fail. Rev. 2011, 16, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Machetanz, K.; Berelidze, L.; Guggenberger, R.; Gharabaghi, A. Transcutaneous auricular vagus nerve stimulation and heart rate variability: Analysis of parameters and targets. Auton. Neurosci. 2021, 236, 102894. [Google Scholar] [CrossRef] [PubMed]
- Stavrakis, S.; Humphrey, M.B.; Scherlag, B.; Iftikhar, O.; Parwani, P.; Abbas, M.; Filiberti, A.; Fleming, C.; Hu, Y.; Garabelli, P.; et al. Low-Level Vagus Nerve Stimulation Suppresses Post-Operative Atrial Fibrillation and Inflammation: A Randomized Study. JACC Clin. Electrophysiol. 2017, 3, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Stavrakis, S.; Humphrey, M.B.; Scherlag, B.J.; Hu, Y.; Jackman, W.M.; Nakagawa, H.; Lockwood, D.; Lazzara, R.; Po, S.S. Low-level transcutaneous electrical vagus nerve stimulation suppresses atrial fibrillation. J. Am. Coll. Cardiol. 2015, 65, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Nasi-Er, B.; Wenhui, Z.; HuaXin, S.; Xianhui, Z.; Yaodong, L.; Yanmei, L.; Hongli, W.; TuEr-Hong, Z.; Qina, Z.; BaoPeng, T. Vagus nerve stimulation reduces ventricular arrhythmias and increases ventricular electrical stability. Pacing Clin. Electrophysiol. 2019, 42, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Stavrakis, S.; Kulkarni, K.; Singh, J.P.; Katritsis, D.G.; Armoundas, A.A. Autonomic Modulation of Cardiac Arrhythmias: Methods to Assess Treatment and Outcomes. JACC Clin. Electrophysiol. 2020, 6, 467–483. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lu, Y.; Sun, J.; Zhou, X.; Tang, B. Subthreshold vagal stimulation suppresses ventricular arrhythmia and inflammatory response in a canine model of acute cardiac ischaemia and reperfusion. Exp. Physiol. 2016, 101, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Wang, Y.; Zang, M.; Li, G.; Wang, G.; Hu, D.; Zheng, L.; Yao, Y.; Pu, J. Remote-controlled vagal nerve stimulation attenuates ventricular arrhythmias and prevents heart failure progression in a rat model of acute myocardial infarction. Auton. Neurosci. 2025, 260, 103279. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Wang, S.; Zhou, X.; Wang, Z.; Huang, B.; Liao, K.; Saren, G.; Chen, M.; Po, S.S.; Jiang, H. Chronic Intermittent Low-Level Stimulation of Tragus Reduces Cardiac Autonomic Remodeling and Ventricular Arrhythmia Inducibility in a Post-Infarction Canine Model. JACC Clin. Electrophysiol. 2016, 2, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.; Kerndt, C.C.; Moore, R.A. Physiology, Baroreceptors. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Abraham, W.T.; Zile, M.R.; Weaver, F.A.; Butter, C.; Ducharme, A.; Halbach, M.; Klug, D.; Lovett, E.G.; Müller-Ehmsen, J.; Schafer, J.E.; et al. Baroreflex Activation Therapy for the Treatment of Heart Failure With a Reduced Ejection Fraction. JACC Heart Fail. 2015, 3, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Linz, D.; Mahfoud, F.; Schotten, U.; Ukena, C.; Neuberger, H.; Wirth, K.; Böhm, M. Effects of electrical stimulation of carotid baroreflex and renal denervation on atrial electrophysiology. J. Cardiovasc. Electrophysiol. 2013, 24, 1028–1033. [Google Scholar] [CrossRef] [PubMed]
- Liao, K.; Yu, L.; Zhou, X.; Saren, G.; Wang, S.; Wang, Z.; Huang, B.; Yang, K.; Jiang, H. Low-level baroreceptor stimulation suppresses atrial fibrillation by inhibiting ganglionated plexus activity. Can. J. Cardiol. 2015, 31, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Bao, M.; Zhang, Y.; Yu, L.; Cao, Q.; Tang, Y.; Huang, H.; Wang, X.; Hu, D.; Huang, C. Low-level carotid baroreflex stimulation suppresses atrial fibrillation by inhibiting left stellate ganglion activity in an acute canine model. Heart Rhythm 2016, 13, 2203–2212. [Google Scholar] [CrossRef] [PubMed]
- Liao, K.; Yu, L.; Yang, K.; Saren, G.; Wang, S.; Huang, B.; Jiang, H.; Resstel, L.B.M. Low-level carotid baroreceptor stimulation suppresses ventricular arrhythmias during acute ischemia. PLoS ONE 2014, 9, e109313. [Google Scholar] [CrossRef] [PubMed]
- Iturriaga, R.; Alcayaga, J.; Chapleau, M.W.; Somers, V.K. Carotid body chemoreceptors: Physiology, pathology, and implications for health and disease. Physiol. Rev. 2021, 101, 1177–1235. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Li, Y.; Schultz, H.D. Role of blood flow in carotid body chemoreflex function in heart failure. J. Physiol. 2011, 589, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Prabhakhar, N.R.; Joyner, M.J. Tasting arterial blood: What do the carotid chemoreceptors sense? Front. Physiol. 2015, 5, 524. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P. Systemic effects resulting from carotid body stimulation–invited article. Arter. Chemorecept. 2009, 223–233. [Google Scholar] [CrossRef]
- Whayne, T.F., Jr. Cardiovascular medicine at high altitude. Angiology 2014, 65, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Bilo, G.; Villafuerte, F.C.; Faini, A.; Anza-Ramírez, C.; Revera, M.; Giuliano, A.; Caravita, S.; Gregorini, F.; Lombardi, C.; Salvioni, E.; et al. Ambulatory Blood Pressure in Untreated and Treated Hypertensive Patients at High Altitude: The High Altitude Cardiovascular Research–Andes Study. Hypertension 2015, 65, 1266–1272. [Google Scholar] [CrossRef] [PubMed]
- Marcus, N.J.; Del Rio, R.; Schultz, H.D. Central role of carotid body chemoreceptors in disordered breathing and cardiorenal dysfunction in chronic heart failure. Front. Physiol. 2014, 5, 438. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, R.; Andrade, D.C.; Lucero, C.; Arias, P.; Iturriaga, R. Carotid Body Ablation Abrogates Hypertension and Autonomic Alterations Induced by Intermittent Hypoxia in Rats. Hypertension 2016, 68, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, R.; Marcus, N.J.; Schultz, H.D. Carotid chemoreceptor ablation improves survival in heart failure: Rescuing autonomic control of cardiorespiratory function. J. Am. Coll. Cardiol. 2013, 62, 2422–2430. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Patel, K.P. Integration of renal sensory afferents at the level of the paraventricular nucleus dictating sympathetic outflow. Auton. Neurosci. 2017, 204, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Sata, Y.; Head, G.A.; Denton, K.; May, C.N.; Schlaich, M.P. Role of the Sympathetic Nervous System and Its Modulation in Renal Hypertension. Front. Med. 2018, 5, 82. [Google Scholar] [CrossRef] [PubMed]
- Kario, K.; Yokoi, Y.; Okamura, K.; Fujihara, M.; Ogoyama, Y.; Yamamoto, E.; Urata, H.; Cho, J.-M.; Kim, C.-J.; Choi, S.-H.; et al. Catheter-based ultrasound renal denervation in patients with resistant hypertension: The randomized, controlled REQUIRE trial. Hypertens. Res. 2022, 45, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Mahfoud, F.; Weber, M.; Schmieder, R.E.; Lobo, M.D.; Blankestijn, P.J.; Persu, A.; Fischell, T.A.; Parise, H.; Pathak, A.; Kandzari, D.E. Catheter-based alcohol-mediated renal denervation for the treatment of uncontrolled hypertension: Design of two sham-controlled, randomized, blinded trials in the absence (TARGET BP OFF-MED) and presence (TARGET BP I) of antihypertensive medications. Am. Heart J. 2021, 239, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Jain, H.; Verma, A.; Jain, J.; Shamim, U.; Kanagala, S.G.; Motwani, J.; Dey, R.C.; Chunawala, Z.; Sohail, A.H.; et al. The role of renal denervation in cardiology and beyond: An updated comprehensive review and future directives. Curr. Probl. Cardiol. 2024, 49, 102196. [Google Scholar] [CrossRef] [PubMed]
- Jackson, N.; Gizurarson, S.; Azam, M.A.; King, B.; Ramadeen, A.; Zamiri, N.; Porta-Sánchez, A.; Al-Hesayen, A.; Graham, J.; Kusha, M.; et al. Effects of Renal Artery Denervation on Ventricular Arrhythmias in a Postinfarct Model. Circ. Cardiovasc. Interv. 2017, 10, e004172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhou, Q.; Lu, Y.; Li, Y.; Zhang, L.; Zhang, J.; Xing, Q.; Lv, W.; Cheng, X.; Zhang, G.; et al. Renal Denervation Reduced Ventricular Arrhythmia After Myocardial Infarction by Inhibiting Sympathetic Activity and Remodeling. J. Am. Heart Assoc. 2018, 7, e009938. [Google Scholar] [CrossRef] [PubMed]
- Ukena, C.; Bauer, A.; Mahfoud, F.; Schreieck, J.; Neuberger, H.-R.; Eick, C.; Sobotka, P.A.; Gawaz, M.; Böhm, M. Renal sympathetic denervation for treatment of electrical storm: First-in-man experience. Clin. Res. Cardiol. 2012, 101, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Evranos, B.; Canpolat, U.; Kocyigit, D.; Coteli, C.; Yorgun, H.; Aytemir, K. Role of adjuvant renal sympathetic denervation in the treatment of ventricular arrhythmias. Am. J. Cardiol. 2016, 118, 1207–1210. [Google Scholar] [CrossRef] [PubMed]
- Bradfield, J.S.; Hayase, J.; Liu, K.; Moriarty, J.; Kee, S.T.; Do, D.; Ajijola, O.A.; Vaseghi, M.; Gima, J.; Sorg, J.; et al. Renal denervation as adjunctive therapy to cardiac sympathetic denervation for ablation refractory ventricular tachycardia. Heart Rhythm 2020, 17, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Nammas, W.; Airaksinen, J.K.; Paana, T.; Karjalainen, P.P. Renal sympathetic denervation for treatment of patients with atrial fibrillation: Reappraisal of the available evidence. Heart Rhythm 2016, 13, 2388–2394. [Google Scholar] [CrossRef] [PubMed]
- Feyz, L.; Theuns, D.A.; Bhagwandien, R.; Strachinaru, M.; Kardys, I.; Van Mieghem, N.M.; Daemen, J. Atrial fibrillation reduction by renal sympathetic denervation: 12 months’ results of the AFFORD study. Clin. Res. Cardiol. 2019, 108, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Mark, D.B.; Anstrom, K.J.; Sheng, S.; Piccini, J.P.; Baloch, K.N.; Monahan, K.H.; Daniels, M.R.; Bahnson, T.D.; Poole, J.E.; Rosenberg, Y.; et al. Effect of catheter ablation vs. medical therapy on quality of life among patients with atrial fibrillation: The CABANA randomized clinical trial. JAMA 2019, 321, 1275–1285. [Google Scholar] [CrossRef] [PubMed]
- Calkins, H.; Hindricks, G.; Cappato, R.; Kim, Y.H.; Saad, E.B.; Aguinaga, L.; Akar, J.G.; Badhwar, V.; Brugada, J.; Camm, J.; et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Ep Europace 2018, 20, e1–e160. [Google Scholar] [CrossRef] [PubMed]
- Packer, D.L.; Mark, D.B.; Robb, R.A.; Monahan, K.H.; Bahnson, T.D.; Poole, J.E.; Noseworthy, P.A.; Rosenberg, Y.D.; Jeffries, N.; Mitchell, L.B.; et al. Effect of catheter ablation vs. antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: The CABANA randomized clinical trial. JAMA 2019, 321, 1261–1274. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, J.S.; Shabanov, V.; Ponomarev, D.; Losik, D.; Ivanickiy, E.; Kropotkin, E.; Polyakov, K.; Ptaszynski, P.; Keweloh, B.; Yao, C.J.; et al. Effect of Renal Denervation and Catheter Ablation vs. Catheter Ablation Alone on Atrial Fibrillation Recurrence Among Patients With Paroxysmal Atrial Fibrillation and Hypertension: The ERADICATE-AF Randomized Clinical Trial. JAMA 2020, 323, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Stavrakis, S.; Po, S. Ganglionated plexi ablation: Physiology and clinical applications. Arrhythmia Electrophysiol. Rev. 2017, 6, 186. [Google Scholar] [CrossRef] [PubMed]
- Aksu, T.; Yalin, K.; Bozyel, S.; Gopinathannair, R.; Gupta, D. The anatomical basis behind the neuromodulation effects associated with pulmonary vein isolation. J. Cardiovasc. Electrophysiol. 2021, 32, 1733–1736. [Google Scholar] [CrossRef] [PubMed]
- Saburkina, I.; Pauziene, N.; Solomon, O.I.; Rysevaite-Kyguoliene, K.; Pauza, D.H. Comparative gross anatomy of epicardiac ganglionated nerve plexi on the human and sheep cardiac ventricles. Anat. Rec. 2023, 306, 2302–2312. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, X.; Xie, X.; Wang, Z.; Liu, X.; Guan, J.; Wang, W.; Li, Z.; Wang, J.; Gao, M.; et al. Long-Term Effects of Atrial Ganglionated Plexi Ablation on Function and Structure of Sinoatrial and Atrioventricular Node in Canine. Pacing Clin. Electrophysiol. 2015, 38, 1181–1191. [Google Scholar] [CrossRef] [PubMed]
- Malcolme-Lawes, L.C.; Lim, P.B.; Wright, I.; Kojodjojo, P.; Koa-Wing, M.; Jamil-Copley, S.; Dehbi, H.-M.; Francis, D.P.; Davies, D.W.; Peters, N.S.; et al. Characterization of the left atrial neural network and its impact on autonomic modification procedures. Circ. Arrhythmia Electrophysiol. 2013, 6, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, Y.; Li, S.; Ma, J.; Liu, J.; Ruan, Y.; Zhang, J. Comparative study of the therapeutic effects of radiofrequency ablation of ganglionated plexi guided by high-frequency stimulation and anatomical localization methods in the treatment of vagal syncope in young people. Cardiology 2024, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Yin, X.; Zhang, Y.; Yan, Q.; Dong, J.; Ma, C.; Liu, X. Ablation of epicardial ganglionated plexi increases atrial vulnerability to arrhythmias in dogs. Circ. Arrhythm. Electrophysiol. 2014, 7, 711–717. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Lu, Z.; He, W.; Wu, L.; Cui, B.; Hu, X.; Yu, L.; Huang, C.; Jiang, H. Effects of ganglionated plexi ablation on ventricular electrophysiological properties in normal hearts and after acute myocardial ischemia. Int. J. Cardiol. 2013, 168, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.-K.; Chen, P.-S. Is the Atrial Neural Plexis a Therapeutic Target in Atrial Fibrillation? Methodist. Debakey Cardiovasc. J. 2015, 11, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Hertel, J.N.; Nissen, S.D.; Lindberg, L.A.; Schneider, M.; Niskala, A.; Isaksen, J.L.; Jerltorp, K.; Ye, C.; Hermans, B.J.M.; Aerts, L.; et al. Targeted epicardial pulsed field ablation of atrial ganglionated plexi: Electrophysiological and histological analysis in pigs. Heart Rhythm, 2025; in press. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Jiang, Z.; Tsai, W.-C.; Yuan, Y.; Chinda, K.; Choi, E.-K.; Fishbein, M.C.; Lin, S.-F.; Chen, P.-S.; Everett, T.H. Ganglionated plexi and ligament of Marshall ablation reduces atrial vulnerability and causes stellate ganglion remodeling in ambulatory dogs. Heart Rhythm 2016, 13, 2083–2090. [Google Scholar] [CrossRef] [PubMed]
- Berger, W.R.; Neefs, J.; van den Berg, N.W.; Krul, S.P.; van Praag, E.M.; Piersma, F.R.; de Jong, J.S.S.G.; van Boven, W.-J.P.; Driessen, A.H.G.; de Groot, J.R. Additional Ganglion Plexus Ablation During Thoracoscopic Surgical Ablation of Advanced Atrial Fibrillation: Intermediate Follow-Up of the AFACT Study. JACC Clin. Electrophysiol. 2019, 5, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Kampaktsis, P.N.; Oikonomou, E.K.; Choi, D.Y.; Cheung, J.W. Efficacy of ganglionated plexi ablation in addition to pulmonary vein isolation for paroxysmal versus persistent atrial fibrillation: A meta-analysis of randomized controlled clinical trials. J. Interv. Card. Electrophysiol. 2017, 50, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Katritsis, D.G.; Pokushalov, E.; Romanov, A.; Giazitzoglou, E.; Siontis, G.C.; Po, S.S.; Camm, A.J.; Ioannidis, J.P.A. Autonomic denervation added to pulmonary vein isolation for paroxysmal atrial fibrillation: A randomized clinical trial. J. Am. Coll. Cardiol. 2013, 62, 2318–2325. [Google Scholar] [CrossRef] [PubMed]
- Avazzadeh, S.; McBride, S.; O’brien, B.; Coffey, K.; Elahi, A.; O’halloran, M.; Soo, A.; Quinlan, L.R. Ganglionated Plexi Ablation for the Treatment of Atrial Fibrillation. J. Clin. Med. 2020, 9, 3081. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.; Fu, Y.; Xue, F.; Xu, M.; Ling, L.; Jiang, T. Which ablation strategy is the most effective for treating persistent atrial fibrillation? A systematic review and Bayesian network meta-analysis of randomized controlled trials. Heart Rhythm 2025, 22, e60–e73. [Google Scholar] [CrossRef] [PubMed]
- Aksu, T.; Skeete, J.R.; Huang, H.H. Ganglionic Plexus Ablation: A Step-by-step Guide for Electrophysiologists and Review of Modalities for Neuromodulation for the Management of Atrial Fibrillation. Arrhythmia Electrophysiol. Rev. 2023, 12, 2023. [Google Scholar] [CrossRef] [PubMed]
- Benabou, L.; Ascione, C.; Soré, B.; Cherbi, M.; Labrousse, R.; Tixier, R.; Bouyer, B.; Arnaud, M.; Buliard, S.; Pambrun, T.; et al. A computed tomography–based evaluation and comparison of ganglionated plexus targeting techniques for cardioneuroablation. Heart Rhythm, 2025; in press. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Fang, R.; Huang, L.; Zhou, L.; Liu, H.; Cai, M.; Sha’aBan, A.; Yu, C.; Akkaif, M.A. Acupuncture in Traditional Chinese Medicine: A Complementary Approach for Cardiovascular Health. J. Multidiscip. Healthc. 2024, 17, 3459–3473. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jung, H.J.; Kim, T.H.; Lee, S.; Kim, J.E.; Kang, K.W.; Jung, S.Y.; Kim, A.R.; Park, H.J.; Shin, M.S.; et al. Auricular acupuncture for prehypertension and stage 1 hypertension: Study protocol for a pilot multicentre randomised controlled trial. Trials 2013, 14, 303. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Ko, Y.; Benharash, P.; Yamakawa, K.; Patel, S.; Ajijola, O.A.; Mahajan, A. Cardioprotection of electroacupuncture against myocardial ischemia-reperfusion injury by modulation of cardiac norepinephrine release. Am. J. Physiol.-Heart Circ. Physiol. 2012, 302, H1818–H1825. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Cui, B.; Shao, Y.; Ni, B.; Zhang, W.; Luo, Y.; Zhang, S. Electroacupuncture improves cardiac function and remodeling by inhibition of sympathoexcitation in chronic heart failure rats. Am. J. Physiol.-Heart Circ. Physiol. 2014, 306, H1464–H1471. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Lu, S.-F.; Hu, C.-J.; Fu, S.-P.; Shen, W.-X.; Liu, W.-X.; Li, Q.; Wang, N.; He, S.-Y.; Liang, F.-R.; et al. Electro-acupuncture at Neiguan pretreatment alters genome-wide gene expressions and protects rat myocardium against ischemia-reperfusion. Molecules 2014, 19, 16158–16178. [Google Scholar] [CrossRef] [PubMed]
- Longhurst, J. Acupuncture’s cardiovascular actions: A mechanistic perspective. Med. Acupunct. 2013, 25, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, D.; Zheng, H.; Chang, X.; Cui, J.; Wang, R.; Shi, J.; Fan, H.; Li, Y.; Sun, X.; et al. Acupuncture as Adjunctive Therapy for Chronic Stable Angina: A Randomized Clinical Trial. JAMA Intern. Med. 2019, 179, 1388–1397. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wang, X.; Li, X.-B.; Wu, B.-Q.; Zhang, Z.-H.; Guo, W.-H.; Wu, C.-C.; Chen, X.; Chen, M.-L.; Dai, Z.; et al. Acupuncture for persistent atrial fibrillation after catheter ablation: Study protocol for a pilot randomized controlled trial. Trials 2021, 22, 35. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Kitagawa, Y.; Tajima, F. Effects of a Single Session of Acupuncture Treatment on Blood Pressure and Heart Rate Variability in Patients with Mild Hypertension. J. Altern. Complement. Med. 2021, 27, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liang, D.; Wang, F.; Li, W.; Han, Y.; Zhang, W.; Xie, Y.; Xin, W.; Zhou, B.; Sun, D.; et al. Efficacy of electroacupuncture pretreatment for myocardial injury in patients undergoing percutaneous coronary intervention: A randomized clinical trial with a 2-year follow-up. Int. J. Cardiol. 2015, 194, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.; Xie, Y.; Wang, Q.; Zhong, H.; Chen, M.; Wang, F.; Xiong, L. Cardioprotective effect of transcutaneous electric acupoint stimulation in the pediatric cardiac patients: A randomized controlled clinical trial. Paediatr. Anaesth. 2012, 22, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Abdi, H.; Tayefi, M.; Moallem, S.R.; Zhao, B.; Fayaz, M.; Ardabili, H.M.; Razavi, A.-A.; Darbandi, M.; Darbandi, S.; Abbasi, P.; et al. Abdominal and auricular acupuncture reduces blood pressure in hypertensive patients. Complement. Ther. Med. 2017, 31, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Chunling, G.; Kai, C.; Shouqin, Y.; Shaofeng, R.; Hengyap, T.; Xiaofeng, G.; Dandan, P. Efficacy on rabbits with arrhythmia: Needling acupoint of Neiguan (PC6) at shallow or deep depth, and retaining needles for 10, 20, or 30 min. J. Tradit. Chin. Med. 2021, 41, 968–973. [Google Scholar]
- Sun, Q.; Cheng, K.; Dai, X.; Yang, Z.; Wu, X.; Xu, C.; Qiu, X.; Gao, X.; Liu, D.; Yang, Q. Effect of electroacupuncture at Neiguan (PC6) at different time points on myocardial ischemia reperfusion arrhythmia in rats. J. Tradit. Chin. Med. 2024, 44, 113–121. [Google Scholar]
- Zuo, H.; Cui, S.; Wang, K.; Wu, X.; Zhou, J.; Qu, Q.; Tong, Y.; Wu, S.; Zhou, M. Electroacupuncture Ameliorates Acute Myocardial Ischemic Injury and Long QT Interval in Mice through the α1A-Adrenergic Receptor: Electrophysiological, Morphological, and Molecular Evidence. Oxidative Med. Cell. Longev. 2022, 2022, 1984706. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Yang, M.; Yu, S.; Fu, H.; Huang, H.; Yang, B.; Liu, Y.; He, B.; Bao, M.; Wu, G.; et al. Effect of acupuncture at Neiguan point combined with amiodarone therapy on early recurrence after pulmonary vein electrical isolation in patients with persistent atrial fibrillation. J. Cardiovasc. Electrophysiol. 2019, 30, 910–917. [Google Scholar] [CrossRef] [PubMed]
- Lomuscio, A.; Belletti, S.; Battezzati, P.M.; Lombardi, F. Efficacy of acupuncture in preventing atrial fibrillation recurrences after electrical cardioversion. J. Cardiovasc. Electrophysiol. 2011, 22, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Markman, T.M.; Pothineni, N.V.K.; Zghaib, T.; Smietana, J.; McBride, D.; Amankwah, N.A.; Linn, K.A.; Kumareswaran, R.; Hyman, N.; Arkles, J.; et al. Effect of transcutaneous magnetic stimulation in patients with ventricular tachycardia storm: A randomized clinical trial. JAMA Cardiol. 2022, 7, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Norise, C.; Hamilton, R.H. Non-invasive Brain Stimulation in the Treatment of Post-stroke and Neurodegenerative Aphasia: Parallels, Differences, and Lessons Learned. Front. Hum. Neurosci. 2016, 10, 675. [Google Scholar] [CrossRef] [PubMed]
- Sheen, J.Z.; Miron, J.-P.; Mansouri, F.; Dunlop, K.; Russell, T.; Zhou, R.; Hyde, M.; Fox, L.; Voetterl, H.; Daskalakis, Z.J.; et al. Cardiovascular biomarkers of response to accelerated low frequency repetitive transcranial magnetic stimulation in major depression. J. Affect. Disord. 2022, 318, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Gulli, G.; Tarperi, C.; Cevese, A.; Acler, M.; Bongiovanni, G.; Manganotti, P. Effects of prefrontal repetitive transcranial magnetic stimulation on the autonomic regulation of cardiovascular function. Exp. Brain Res. 2013, 226, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Rast, J.; Sohinki, D.; Warner, A. Non-invasive Neuromodulation of Arrhythmias. J. Innov. Card. Rhythm. Manag. 2024, 15, 5757–5766. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhou, X.; Wang, Z.; Huang, B.; Zhou, L.; Chen, M.; Yu, L.; Jiang, H. Magnetic fields in noninvasive heart stimulation: A novel approach for anti-atrial fibrillation. Int. J. Cardiol. 2015, 190, 54–55. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Dyer, J.W.; Scherlag, B.J.; Stavrakis, S.; Sha, Y.; Sheng, X.; Garabelli, P.; Jacobson, J.; Po, S.S. The use of low-level electromagnetic fields to suppress atrial fibrillation. Heart Rhythm 2015, 12, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Sohinki, D.; Thomas, J.; Scherlag, B.; Stavrakis, S.; Yousif, A.; Po, S.; Dasari, T. Impact of low-level electromagnetic fields on the inducibility of atrial fibrillation in the electrophysiology laboratory. Heart Rhythm O2 2021, 2, 239–246. [Google Scholar] [CrossRef] [PubMed]
| Study (Year) | Population (n) | Age (Years) | Cardiac Pathology | CSD Approach | Primary Outcomes |
|---|---|---|---|---|---|
| Hofferberth et al. [68] (2014) | 24 | 5 weeks to 27 years | Long QT syndrome, catecholaminergic polymorphic VT, idiopathic VT | Left | 73% arrhythmia reduction; 55% arrhythmia-free |
| Vaseghi et al. [67] (2017) | 121 | 55 ± 13 | Structural heart disease, refractory VT | Left or bilateral | 1-year freedom from VT/ICD shock: 58%; ICD shocks reduced |
| Cai et al. [69] (2019) | 19 | 60.3 ± 14.6 | Structural heart disease, recurrent VA | Left (14), bilateral (5) | VA/ICD therapies reduced; 3-year heart transplant/death-free: 52.6% |
| Shah et al. [70] (2019) | 173 | 54.6 ± 2 | Structural heart disease, refractory VA | 82% bilateral | Event-free: 58–100%; 28% complication rate (mostly minor) |
| Barwad et al. [71] (2021) | 65 | 50 ± 18 | Structural heart disease, refractory VT | Surgical (mostly bilateral) | 92% reduction in defibrillation shocks; 2-year ICD shock/death-free: 51.5% |
| Ertugrul et al. [62] (2021) | 14 | 8–19 years | Long QT syndrome, catecholaminergic polymorphic VT, other | Bilateral + Kuntz ablation | Arrhythmia reduction; no major complications |
| Yalin et al. [72] (2021) | 10 | 61.6 ± 19.6 | Nonischemic cardiomyopathy, r efractory VA | Left (6), bilateral (4) | VA/ICD shocks reduced; 2 deaths (not CSD-related) |
| König et al. [73] (2022) | 21 | 63.7 ± 14.4 | Structural heart disease, refractory VA | 90.5% bilateral | 77% ICD shock-free at 9 mo; 9.5% major complications |
| Brady et al. [74] (2024) | 32 | 62 ± 11.6 | Systolic heart failure, refractory VA | Bilateral (27), unilateral (5) | 1-year survival: 61.4% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, B.; Kuwabara, Y.; Salavatian, S. Neuromodulation of the Cardiac Autonomic Nervous System for Arrhythmia Treatment. Biomedicines 2025, 13, 1776. https://doi.org/10.3390/biomedicines13071776
Wong B, Kuwabara Y, Salavatian S. Neuromodulation of the Cardiac Autonomic Nervous System for Arrhythmia Treatment. Biomedicines. 2025; 13(7):1776. https://doi.org/10.3390/biomedicines13071776
Chicago/Turabian StyleWong, Benjamin, Yuki Kuwabara, and Siamak Salavatian. 2025. "Neuromodulation of the Cardiac Autonomic Nervous System for Arrhythmia Treatment" Biomedicines 13, no. 7: 1776. https://doi.org/10.3390/biomedicines13071776
APA StyleWong, B., Kuwabara, Y., & Salavatian, S. (2025). Neuromodulation of the Cardiac Autonomic Nervous System for Arrhythmia Treatment. Biomedicines, 13(7), 1776. https://doi.org/10.3390/biomedicines13071776






