Neurophysiological Effects of Virtual Reality Multitask Training in Cardiac Surgery Patients: A Study with Standardized Low-Resolution Electromagnetic Tomography (sLORETA)
Abstract
1. Introduction
2. Materials and Methods
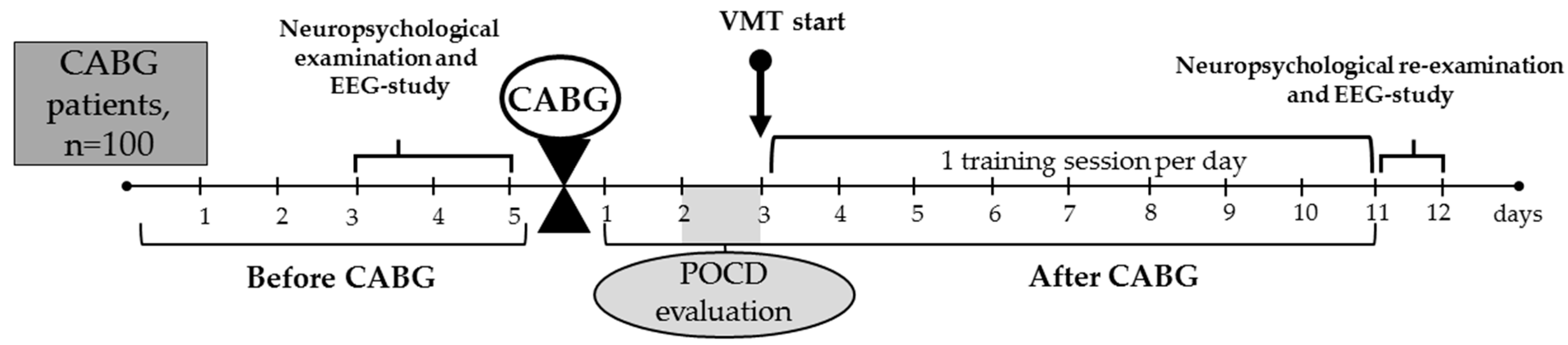
2.1. Data Collection and Sampling
2.2. Neuropsychological Examination
2.3. Virtual Reality-Based Multitask Training Paradigm
2.4. EEG Data Recording and Processing
2.5. Statistical Analysis
3. Results
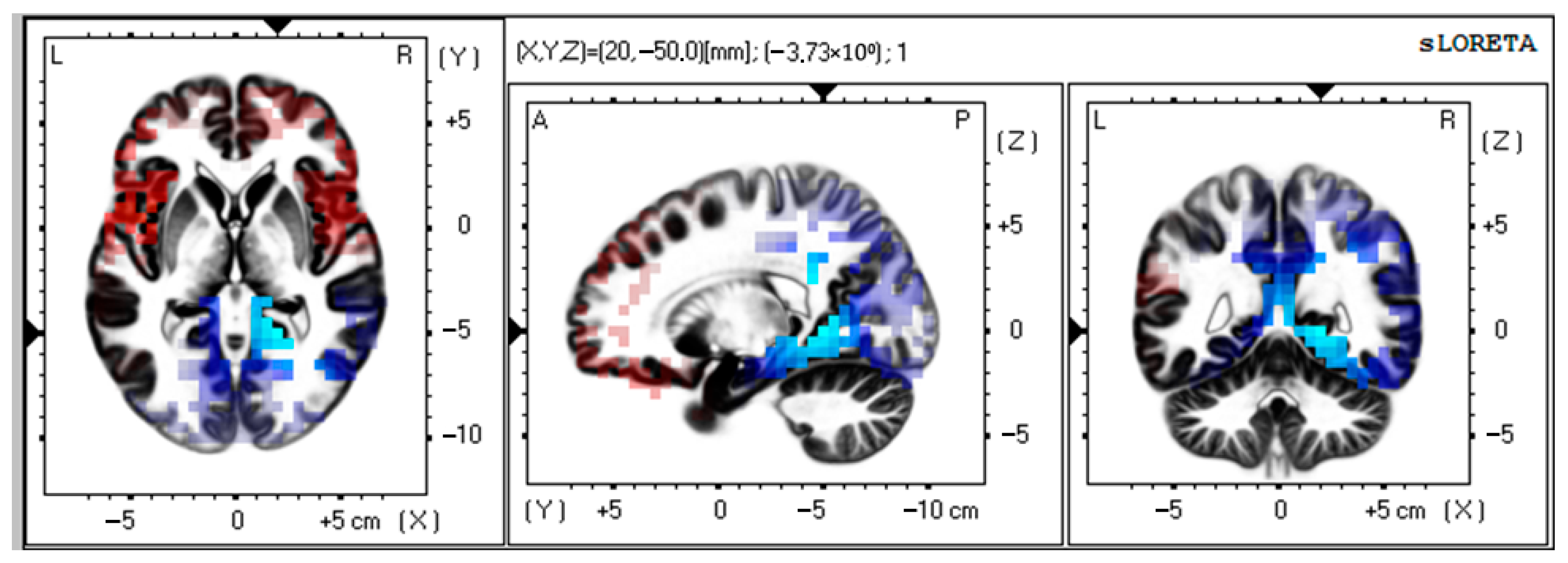
3.1. Source Estimation Analysis in VMT Group
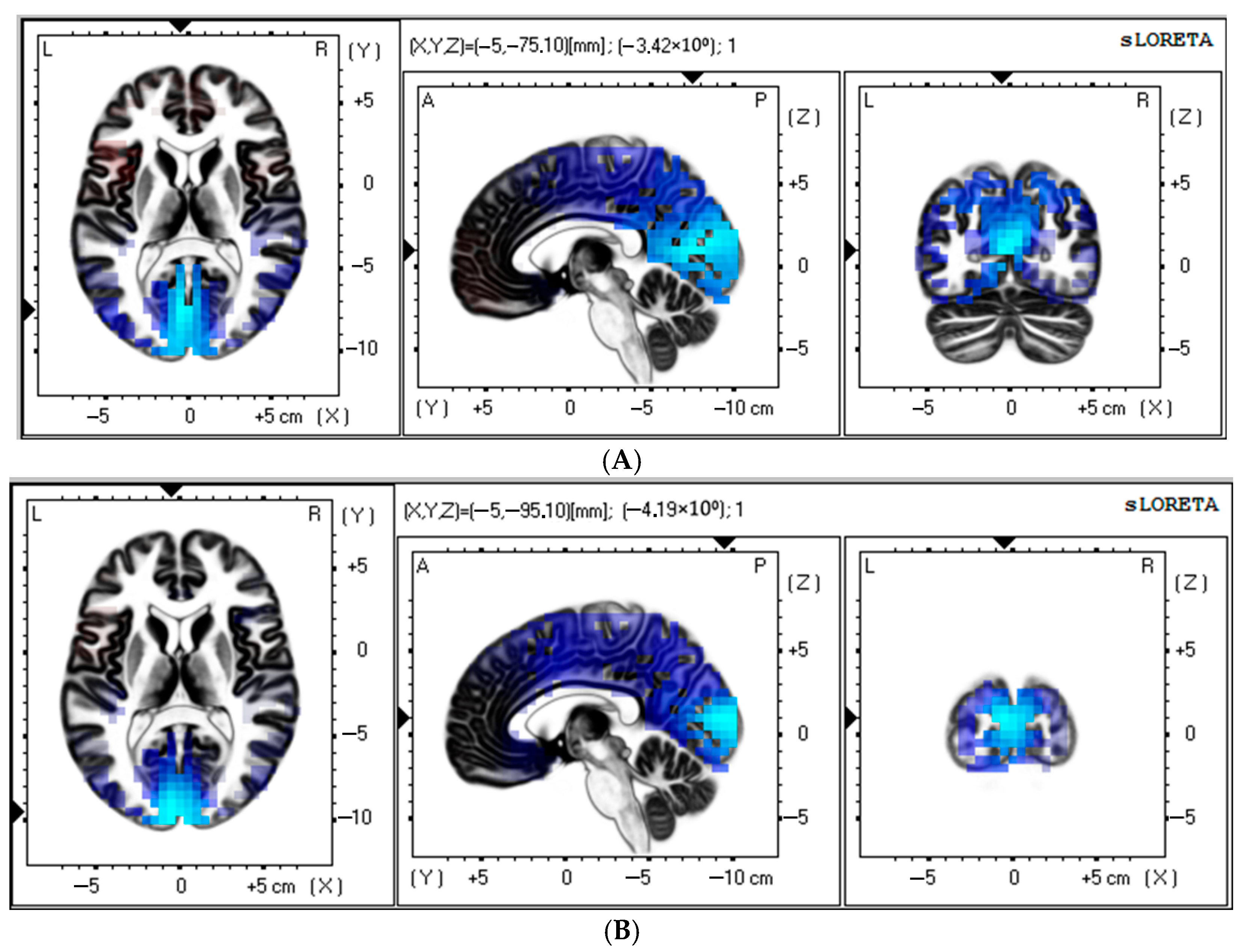
3.2. Source Estimation Analysis in Control Group
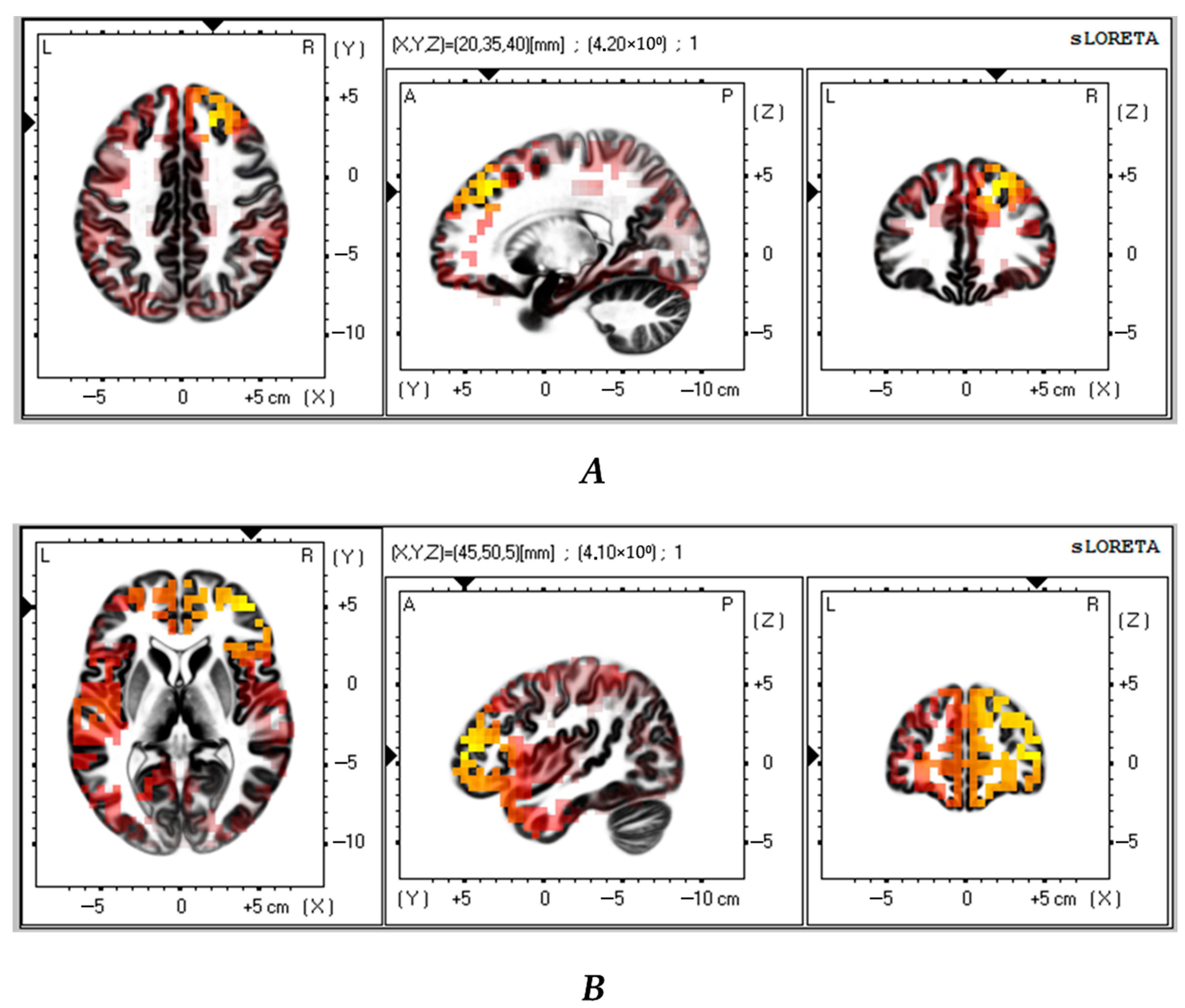
3.3. Contrast Between VMT and Control Groups
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Colita, E.; Mateescu, V.O.; Olaru, D.G.; Popa-Wagner, A. Cognitive decline in ageing and disease: Risk factors, genetics and treatments. Curr. Health Sci. J. 2024, 50, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.L.; Grandpre, J.; Katz, D.L.; Shenson, D. Cognitive impairment and cardiovascular disease: A comparison of risk factors, disability, quality of life, and access to health care. Public Health Rep. 2020, 135, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Lindsay, J.; Monsein, L.H.; Hill, P.C.; Corso, P.J. Silent brain injury after cardiac surgery: A review: Cognitive dysfunction and magnetic resonance imaging diffusion-weighted imaging findings. J. Am. Coll. Cardiol. 2012, 60, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Indja, B.; Seco, M.; Seamark, R.; Kaplan, J.; Bannon, P.G.; Grieve, S.M.; Vallely, M.P. Neurocognitive and psychiatric issues post cardiac surgery. Heart Lung Circ. 2017, 26, 779–785. [Google Scholar] [CrossRef]
- Butz, M.; Gerriets, T.; Sammer, G.; El-Shazly, J.; Braun, T.; Sünner, L.; Meyer, R.; Tschernatsch, M.; Schramm, P.; Gerner, S.T.; et al. The impact of white matter lesions and subclinical cerebral ischemia on postoperative cognitive training outcomes after heart valve surgery: A randomized clinical trial. J. Neurol. Sci. 2025, 15, 123370. [Google Scholar] [CrossRef]
- de Tournay-Jetté, E.; Dupuis, G.; Denault, A.; Cartier, R.; Bherer, L. The benefits of cognitive training after a coronary artery bypass graft surgery. J. Behav. Med. 2012, 35, 557–568. [Google Scholar] [CrossRef]
- Zhang, R.; Zhu, C.; Chen, S.; Tian, F.; Huang, P.; Chen, Y. Effects of cognitive training on cognitive function in patients after cardiac surgery: A systematic review and meta-analysis of randomized controlled trials. Medicine 2024, 103, e40324. [Google Scholar] [CrossRef]
- Gkintoni, E.; Vassilopoulos, S.P.; Nikolaou, G.; Vantarakis, A. Neurotechnological approaches to cognitive rehabilitation in mild cognitive impairment: A systematic review of neuromodulation, EEG, virtual reality, and emerging AI applications. Brain Sci. 2025, 15, 582. [Google Scholar] [CrossRef]
- Alashram, A.R.; Padua, E.; Annino, G. Virtual reality for balance and mobility rehabilitation following traumatic brain injury: A systematic review of randomized controlled trials. J. Clin. Neurosci. 2022, 105, 115–121. [Google Scholar] [CrossRef]
- Maddox, T.; Oldstone, L.; Linde-Zwirble, W.; Bonakdar, R.; Maddox, R.; Sackman, J.; Adair, T.; Ffrench, K.; Sparks, C.; Darnall, B.D. Differential treatment response to virtual reality in high-impact chronic pain: Secondary analysis of a randomized trial. Sci. Rep. 2025, 15, 14430. [Google Scholar] [CrossRef]
- Baldimtsi, E.; Mouzakidis, C.; Karathanasi, E.M.; Verykouki, E.; Hassandra, M.; Galanis, E.; Hatzigeorgiadis, A.; Goudas, M.; Zikas, P.; Evangelou, G.; et al. Effects of virtual reality physical and cognitive training intervention on cognitive abilities of elders with mild cognitive impairment. J. Alzheimers Dis. Rep. 2023, 7, 1475–1490. [Google Scholar] [CrossRef]
- Chatterjee, R.; Moussavi, Z. Evaluation of a cognition-sensitive spatial virtual reality game for Alzheimer’s disease. Med. Biol. Eng. Comput. 2025, 63, 1355–1365. [Google Scholar] [CrossRef]
- Hassandra, M.; Galanis, E.; Hatzigeorgiadis, A.; Goudas, M.; Mouzakidis, C.; Karathanasi, E.M.; Petridou, N.; Tsolaki, M.; Zikas, P.; Evangelou, G.; et al. A Virtual Reality App for Physical and Cognitive Training of Older People With Mild Cognitive Impairment: Mixed Methods Feasibility Study. JMIR Serious Games 2021, 9, e24170. [Google Scholar] [CrossRef]
- De Luca, R.; Maggio, M.G.; Maresca, G.; Latella, D.; Cannavò, A.; Sciarrone, F.; Lo Voi, E.; Accorinti, M.; Bramanti, P.; Calabrò, R.S. Improving cognitive function after traumatic brain injury: A clinical trial on the potential use of the semi-immersive virtual reality. Behav. Neurol. 2019, 2019, 9268179. [Google Scholar] [CrossRef] [PubMed]
- Bouraghi, H.; Mohammadpour, A.; Khodaveisi, T.; Ghazisaeedi, M.; Saeedi, S.; Familgarosian, S. Virtual reality and cardiac diseases: A systematic review of applications and effects. J. Healthc. Eng. 2023, 2023, 8171057. [Google Scholar] [CrossRef] [PubMed]
- Hogue, C.W.; Gottesman, R.F.; Stearns, J. Mechanisms of cerebral injury from cardiac surgery. Crit. Care Clin. 2008, 24, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Blue, M.E.; Wilson, M.A.; Beaty, C.A.; George, T.J.; Arnaoutakis, G.J.; Haggerty, K.A.; Jones, M.; Brawn, J.; Manmohan, S.; Lange, M.S.; et al. Brain injury in canine models of cardiac surgery. Neuropathol. Exp. Neurol. 2014, 73, 1134–1143. [Google Scholar] [CrossRef][Green Version]
- Tarasova, I.; Trubnikova, O.; Kupriyanova, D.S.; Maleva, O.; Syrova, I.; Kukhareva, I.; Sosnina, A.; Tarasov, R.; Barbarash, O. Cognitive functions and patterns of brain activity in patients after simultaneous coronary and carotid artery revascularization. Front. Hum. Neurosci. 2023, 17, 996359. [Google Scholar] [CrossRef]
- Tan, W.; Xu, Y.; Liu, P.; Liu, C.; Li, Y.; Du, Y.; Chen, C.; Wang, Y.; Zhang, Y. A method of VR-EEG scene cognitive rehabilitation training. Health Inf. Sci. Syst. 2020, 9, 4. [Google Scholar] [CrossRef]
- Sadeghi, M.; Bristow, T.; Fakorede, S.; Liao, K.; Palmer, J.A.; Lyons, K.E.; Pahwa, R.; Huang, C.K.; Akinwuntan, A.; Devos, H. The effect of sensory reweighting on postural control and cortical activity in Parkinson’s disease: A pilot study. Arch. Rehabil. Res. Clin. Transl. 2024, 6, 100368. [Google Scholar] [CrossRef]
- Annaka, H.; Hiraoka, T.; Nomura, T. Effects of a virtual reality game with leap motion controller on brain activity related to attentional function in healthy adults—A pilot EEG study. Cureus 2024, 16, e71838. [Google Scholar] [CrossRef]
- Pappalettera, C.; Miraglia, F.; Cacciotti, A.; Nucci, L.; Tufo, G.; Rossini, P.M.; Vecchio, F. The impact of virtual reality and distractors on attentional processes: Insights from EEG. Pflugers Arch. 2024, 476, 1727–1742. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Marqui, R.D. Standardized low-resolution brain electromagnetic tomography (sLORETA): Technical details. Methods Find. Exp. Clin. Pharmacol. 2002, 24 (Suppl. D), 5–12. [Google Scholar] [PubMed]
- Sadat-Nejad, Y.; Beheshti, S. Efficient high resolution sLORETA in brain source localization. J. Neural Eng. 2021, 18, 016013. [Google Scholar] [CrossRef] [PubMed]
- Tarasova, I.; Trubnikova, O.; Kukhareva, I.; Syrova, I.; Sosnina, A.; Kupriyanova, D.; Barbarash, O.A. Comparison of two multi-tasking approaches to cognitive training in cardiac surgery patients. Biomedicines 2023, 11, 2823. [Google Scholar] [CrossRef]
- Tarasova, I.; Trubnikova, O.; Kukhareva, I.; Kupriyanova, D.; Sosnina, A. The neurophysiological effects of virtual reality application and perspectives of using for multitasking training in cardiac surgery patients: Pilot study. Appl. Sci. 2024, 14, 10893. [Google Scholar] [CrossRef]
- Privodnova, E.Y.; Volf, N.V. Association of the brain-derived neurotrophic factor gene (BDNF) Val66Met polymorphism with individual alpha peak frequency and alpha power in adults. Hum. Physiol. 2023, 49, 357–363. [Google Scholar] [CrossRef]
- Babiloni, C.; Arakaki, X.; Azami, H.; Bennys, K.; Blinowska, K.; Bonanni, L.; Bujan, A.; Carrillo, M.C.; Cichocki, A.; de Frutos-Lucas, J.; et al. Measures of resting state EEG rhythms for clinical trials in Alzheimer’s disease: Recommendations of an expert panel. Alzheimers Dement. 2021, 17, 1528–1553. [Google Scholar] [CrossRef]
- Smailovic, U.; Koenig, T.; Savitcheva, I.; Chiotis, K.; Nordberg, A.; Blennow, K.; Winblad, B.; Jelic, V. Regional disconnection in Alzheimer dementia and amyloid-positive mild cognitive impairment: Association between EEG functional connectivity and brain glucose metabolism. Brain Connect. 2020, 10, 555–565. [Google Scholar] [CrossRef]
- van Vliet, M.; Liljeström, M.; Aro, S.; Salmelin, R.; Kujala, J. Analysis of functional connectivity and oscillatory power using DICS: From raw MEG data to group-level statistics in Python. Front. Neurosci. 2018, 12, 586. [Google Scholar] [CrossRef]
- Soltani Zangbar, H.; Ghadiri, T.; Seyedi Vafaee, M.; Ebrahimi Kalan, A.; Fallahi, S.; Ghorbani, M.; Shahabi, P. Theta oscillations through hippocampal/prefrontal pathway: Importance in cognitive performances. Brain Connect. 2020, 10, 157–169. [Google Scholar] [CrossRef]
- Sandri Heidner, G.; O’Connell, C.; Domire, Z.J.; Rider, P.; Mizelle, C.; Murray, N.P. Concussed neural signature is substantially different than fatigue neural signature in non-concussed controls. J. Mot. Behav. 2023, 55, 302–312. [Google Scholar] [CrossRef]
- Vanneste, S.; Song, J.J.; De Ridder, D. Thalamocortical dysrhythmia detected by machine learning. Nat. Commun. 2018, 9, 1103. [Google Scholar] [CrossRef]
- Daulatzai, M.A. Cerebral hypoperfusion and glucose hypometabolism: Key pathophysiological modulators promote neurodegeneration, cognitive impairment, and Alzheimer’s disease. J. Neurosci. Res. 2017, 95, 943–972. [Google Scholar] [CrossRef]
- McDonald, A.J.; Mott, D.D. Functional neuroanatomy of amygdalohippocampal interconnections and their role in learning and memory. J. Neurosci. Res. 2017, 95, 797–820. [Google Scholar] [CrossRef]
- Crivelli, D.; Balconi, M. Electrophysiological signature of interoceptive attention: A spectral and source localization analysis. Cogn. Affect. Behav. Neurosci. 2025, 25, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Frank, S.M.; Epstein, R.A.; Tse, P.U. The parahippocampal place area and hippocampus encode the spatial significance of landmark objects. NeuroImage 2021, 236, 118081. [Google Scholar] [CrossRef] [PubMed]
- Clancy, K.J.; Andrzejewski, J.A.; Simon, J.; Ding, M.; Schmidt, N.B.; Li, W. Posttraumatic stress disorder is associated with α dysrhythmia across the visual cortex and the default mode network. eNeuro 2020, 7, ENEURO.0053-20.2020. [Google Scholar] [CrossRef] [PubMed]
- Puma, S.; Matton, N.; Paubel, P.V.; Raufaste, É.; El-Yagoubi, R. Using theta and alpha band power to assess cognitive workload in multitasking environments. Int. J. Psychophysiol. 2018, 123, 111–120. [Google Scholar] [CrossRef]
- Wascher, E.; Arnau, S.; Gutberlet, I.; Karthaus, M.; Getzmann, S. Evaluating pro- and re-active driving behavior by means of the EEG. Front. Hum. Neurosci. 2018, 12, 205. [Google Scholar] [CrossRef]
- Callan, D.E.; Fukada, T.; Dehais, F.; Ishii, S. The role of brain-localized gamma and alpha oscillations in inattentional deafness: Implications for understanding human attention. Front. Hum. Neurosci. 2023, 17, 1168108. [Google Scholar] [CrossRef]
- Dong, Y.; Ma, M.; Li, Y.; Shao, Y.; Shi, G. Association between enhanced effective connectivity from the cuneus to the middle frontal gyrus and impaired alertness after total sleep deprivation. J. Integr. Neurosci. 2024, 23, 174. [Google Scholar] [CrossRef]
- Misselhorn, J.; Friese, U.; Engel, A.K. Frontal and parietal alpha oscillations reflect attentional modulation of cross-modal matching. Sci. Rep. 2019, 9, 5030. [Google Scholar] [CrossRef] [PubMed]
- Xia, R.; Chen, X.; Engel, T.A.; Moore, T. Common and distinct neural mechanisms of attention. Trends Cogn. Sci. 2024, 28, 554–567. [Google Scholar] [CrossRef] [PubMed]
- Sadaghiani, S.; Dombert, P.L.; Løvstad, M.; Funderud, I.; Meling, T.R.; Endestad, T.; Knight, R.T.; Solbakk, A.K.; D’Esposito, M. Lesions to the fronto-parietal network impact alpha-band phase synchrony and cognitive control. Cereb. Cortex 2019, 29, 4143–4153. [Google Scholar] [CrossRef] [PubMed]
- Tarasova, I.V.; Trubnikova, O.A.; Syrova, I.D.; Barbarash, O.L. Long-term neurophysiological outcomes in patients undergoing coronary artery bypass grafting. Braz. J. Cardiovasc. Surg. 2021, 36, 629–638. [Google Scholar] [CrossRef]
| Variable | VMT (n = 50) | Control (n = 50) | p-Value |
|---|---|---|---|
| Age, years, mean (SD) | 62.5 (7.69) | 62.9 (7.61) | 0.77 * |
| Educational attainment, years, mean (SD) | 12.9 (2.75) | 13.1 (2.93) | 0.54 * |
| MoCA, scores, mean (SD) | 26.7 (2.13) | 26.2 (2.29) | 0.32 * |
| BDI-II, scores, mean (SD) | 3.3 (3.1) | 2.1 (2.04) | 0.13 * |
| Smoking, n (%) | 22 (44) | 25 (50) | 0.67 # |
| Functional class NYHA, n (%) | 0.55 # | ||
| I–II | 43 (86) | 44 (88) | |
| III | 7 (14) | 9 (18) | |
| History of myocardial infarction, n (%) | 22 (44) | 29 (58) | 0.09 # |
| Fraction of left ventricle ejection, %, mean (SD) | 61.6 (7.64) | 58.4 (9.68) | 0.31 * |
| Arterial hypertension, n (%) | 42 (84) | 43 (86) | 0.55 # |
| Type 2 diabetes mellitus, n (%) | 11 (22) | 13 (26) | 0.61 # |
| CA stenosis < 50%, n (%) | 17 (34) | 14 (28) | 0.22 # |
| Cognitive Indicators | Patients with Successful VMT | Patients with Unsuccessful VMT | p |
|---|---|---|---|
| Psychomotor and executive functions | |||
| Complex visual–motor reaction | |||
| Reaction time, ms | 4.53 | 7.62 | 0.32 |
| Errors, n | 23.62 | −14.81 | 0.18 |
| Level of functional mobility of nervous processes: responses to feedback | |||
| Reaction time, ms | 0.61 | −6.07 | 0.01 |
| Errors, n | −13.15 | −9.38 | 0.68 |
| Missed signals, n | 5.10 | 2.64 | 0.76 |
| Attention | |||
| Bourdon’s test | |||
| Processed letters per min, n | −14.83 | −10.79 | 0.79 |
| Processed letters per 4 min, n | −16.90 | −74.78 | 0.36 |
| Attention ratio, scores | −34.93 | 3.83 | 0.002 |
| Attention span test, scores | −21.10 | 14.61 | 0.001 |
| Short-term memory | |||
| 10-word memorizing test, n | −28.36 | −18.10 | 0.03 |
| 10-number memorizing test, n | −23.26 | 1.79 | 0.63 |
| Figurative memory, n | 3.20 | −4.26 | 0.44 |
| Rank | t-Value | MNI Coordinate | Brodmann Area | Brain Structure | p-Value | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| 1 | −3.83 | 20 | −50 | 0 | 30 | Limbic Lobe, Parahippocampal Gyrus | <0.05 |
| 2 | −3.74 | 15 | −45 | 0 | 30 | Limbic Lobe, Parahippocampal Gyrus | <0.05 |
| 3 | −3.73 | 10 | −45 | 5 | 29 | Limbic Lobe, Posterior Cingulate | <0.05 |
| 4 | −3.69 | 20 | −45 | −5 | 19 | Limbic Lobe, Parahippocampal Gyrus | <0.05 |
| 5 | −3.68 | 25 | −55 | 0 | 30 | Limbic Lobe, Posterior Cingulate | <0.05 |
| Rank | t-Value | MNI Coordinate | Brodmann Area | Brain Structure | p-Value | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Theta-frequency band (3–5 Hz) | |||||||
| 1 | −3.42 | −5 | −75 | 10 | 23 | Occipital Lobe, Cuneus | <0.05 |
| 2 | −3.41 | −5 | −85 | 15 | 18 | Occipital Lobe, Cuneus | <0.05 |
| 3 | −3.40 | 0 | −75 | 15 | 18 | Occipital Lobe, Cuneus | <0.05 |
| 4 | −3.38 | −5 | −80 | 10 | 17 | Occipital Lobe, Cuneus | <0.05 |
| 5 | −3.38 | 0 | −80 | 15 | 18 | Occipital Lobe, Cuneus | <0.05 |
| Alpha-frequency band (7–9 Hz) | |||||||
| 1 | −4.19 | −5 | −95 | 10 | 18 | Occipital Lobe, Cuneus | <0.05 |
| 2 | −4.17 | −5 | −100 | 10 | 18 | Occipital Lobe, Middle Occipital Gyrus | <0.05 |
| 3 | −4.15 | −5 | −90 | 10 | 18 | Occipital Lobe, Cuneus | <0.05 |
| 4 | −4.12 | −5 | −100 | 5 | 18 | Occipital Lobe, Cuneus | <0.05 |
| 5 | −4.10 | 0 | −100 | 5 | 18 | Occipital Lobe, Cuneus | <0.05 |
| Rank | t-Value | MNI Coordinate | Brodmann Area | Brain Structure | p-Value | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Theta-frequency band (3–5 Hz) | |||||||
| 1 | 3.89 | 20 | 30 | 45 | 8 | Frontal Lobe, Middle Frontal Gyrus | <0.05 |
| 2 | 3.85 | 20 | 35 | 45 | 8 | Frontal Lobe, Superior Frontal Gyrus | <0.05 |
| 3 | 3.83 | −20 | −65 | 15 | 31 | Limbic Lobe, Posterior Cingulate | <0.05 |
| 4 | 3.80 | 25 | 30 | 45 | 8 | Frontal Lobe, Middle Frontal Gyrus | <0.05 |
| 5 | 3.76 | 25 | 35 | 50 | 8 | Frontal Lobe, Superior Frontal Gyrus | <0.05 |
| Alpha-frequency band (7–9 Hz) | |||||||
| 1 | 3.62 | 10 | 55 | 35 | 9 | Frontal Lobe, Superior Frontal Gyrus | <0.05 |
| 2 | 3.62 | 30 | 40 | 40 | 9 | Frontal Lobe, Middle Frontal Gyrus | <0.05 |
| 3 | 3.61 | 10 | 50 | 40 | 9 | Frontal Lobe, Medial Frontal Gyrus | <0.05 |
| 4 | 3.60 | 15 | 50 | 45 | 8 | Frontal Lobe, Superior Frontal Gyrus | <0.05 |
| 5 | 3.60 | 35 | 40 | 15 | 10 | Frontal Lobe, Middle Frontal Gyrus | <0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarasova, I.; Trubnikova, O.; Kupriyanova, D.; Kukhareva, I.; Sosnina, A. Neurophysiological Effects of Virtual Reality Multitask Training in Cardiac Surgery Patients: A Study with Standardized Low-Resolution Electromagnetic Tomography (sLORETA). Biomedicines 2025, 13, 1755. https://doi.org/10.3390/biomedicines13071755
Tarasova I, Trubnikova O, Kupriyanova D, Kukhareva I, Sosnina A. Neurophysiological Effects of Virtual Reality Multitask Training in Cardiac Surgery Patients: A Study with Standardized Low-Resolution Electromagnetic Tomography (sLORETA). Biomedicines. 2025; 13(7):1755. https://doi.org/10.3390/biomedicines13071755
Chicago/Turabian StyleTarasova, Irina, Olga Trubnikova, Darya Kupriyanova, Irina Kukhareva, and Anastasia Sosnina. 2025. "Neurophysiological Effects of Virtual Reality Multitask Training in Cardiac Surgery Patients: A Study with Standardized Low-Resolution Electromagnetic Tomography (sLORETA)" Biomedicines 13, no. 7: 1755. https://doi.org/10.3390/biomedicines13071755
APA StyleTarasova, I., Trubnikova, O., Kupriyanova, D., Kukhareva, I., & Sosnina, A. (2025). Neurophysiological Effects of Virtual Reality Multitask Training in Cardiac Surgery Patients: A Study with Standardized Low-Resolution Electromagnetic Tomography (sLORETA). Biomedicines, 13(7), 1755. https://doi.org/10.3390/biomedicines13071755





