Pulmonary Hemorrhage in Premature Infants: Pathophysiology, Risk Factors and Clinical Management
Abstract
1. Introduction
2. Epidemiology
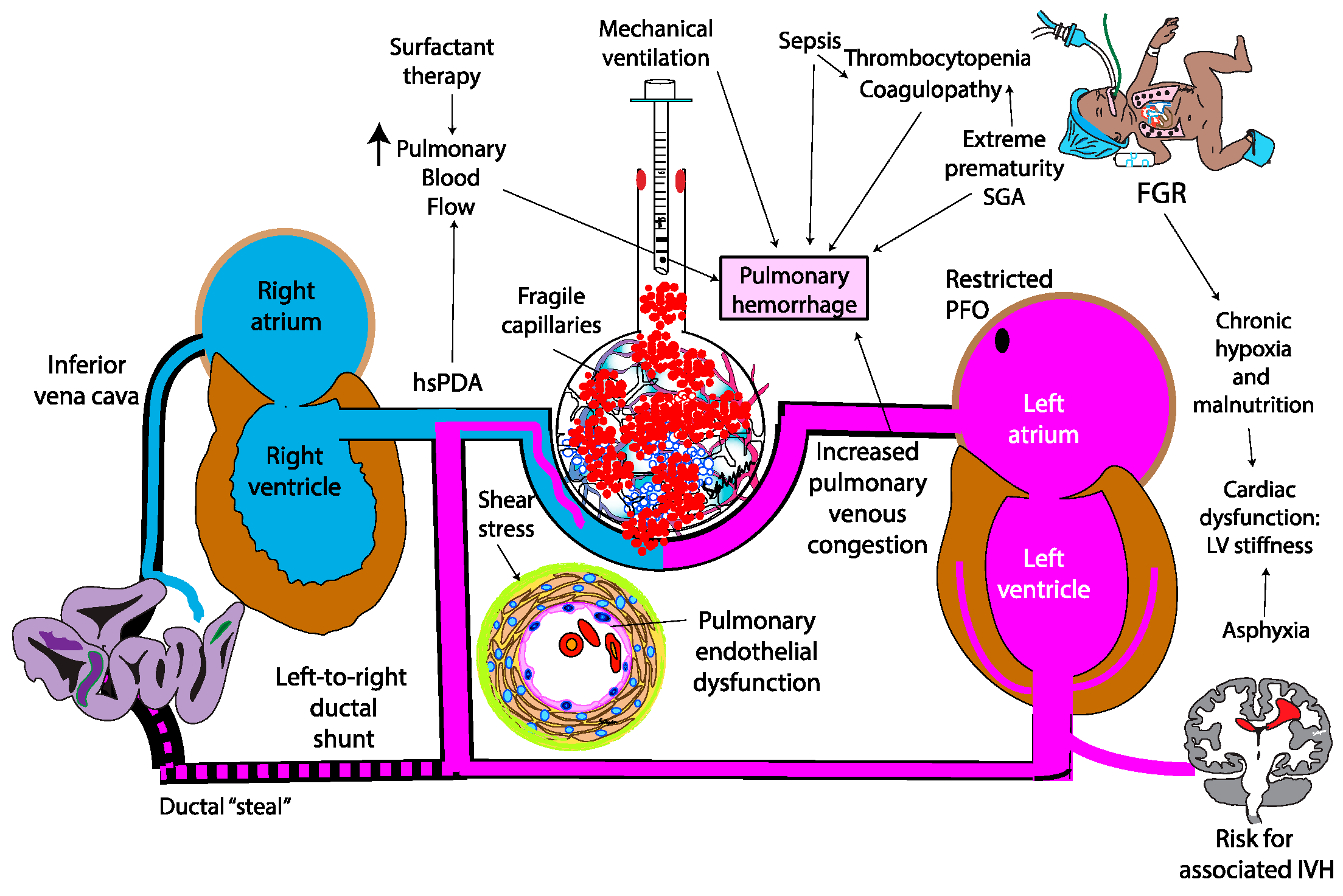
3. Pathogenesis
4. Factors Contributing to Pulmonary Hemorrhage
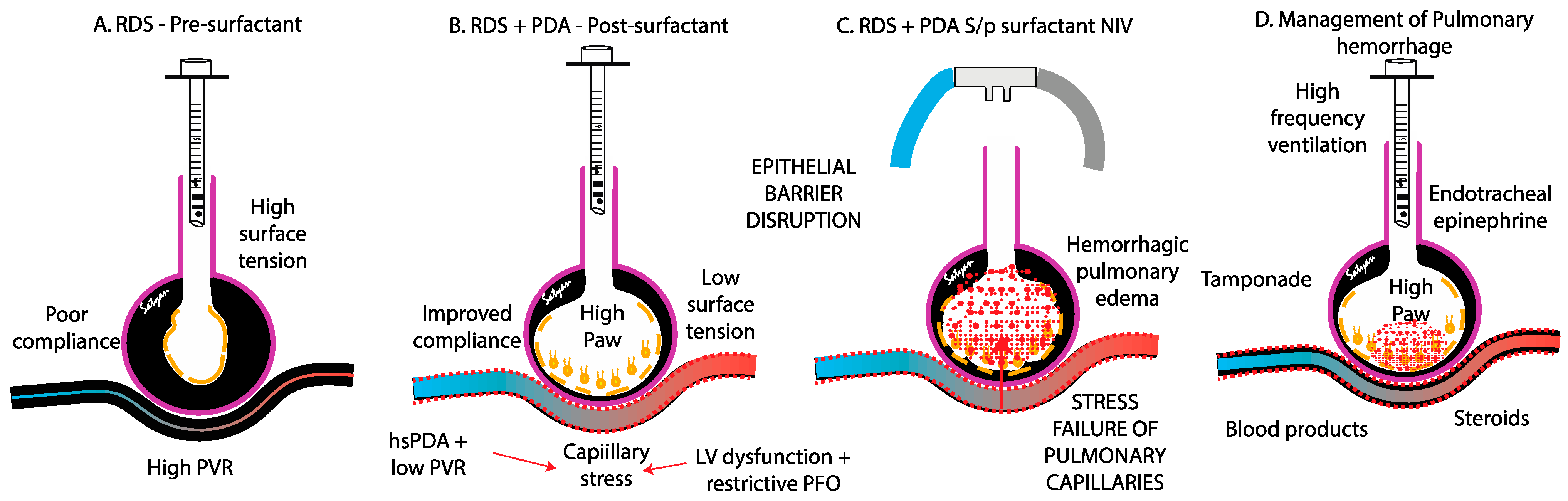
4.1. Respiratory Distress Syndrome (RDS)
4.2. Patent Ductus Arteriosus (PDA) and Pulmonary over Circulation
4.3. Stress Failure of Pulmonary Capillaries
4.4. Left Ventricular Stiffness and Diastolic Properties
4.5. Genetic Factors
4.6. Infection and Sepsis
4.7. Fetal Growth Restriction (FGR)
4.8. Other Conditions Possibly Associated with PH
5. Protective Factors
5.1. Antenatal Glucocorticoids
5.2. Prophylactic Indomethacin
6. Clinical Diagnosis
6.1. Clinical Presentation
6.2. Laboratory Diagnosis
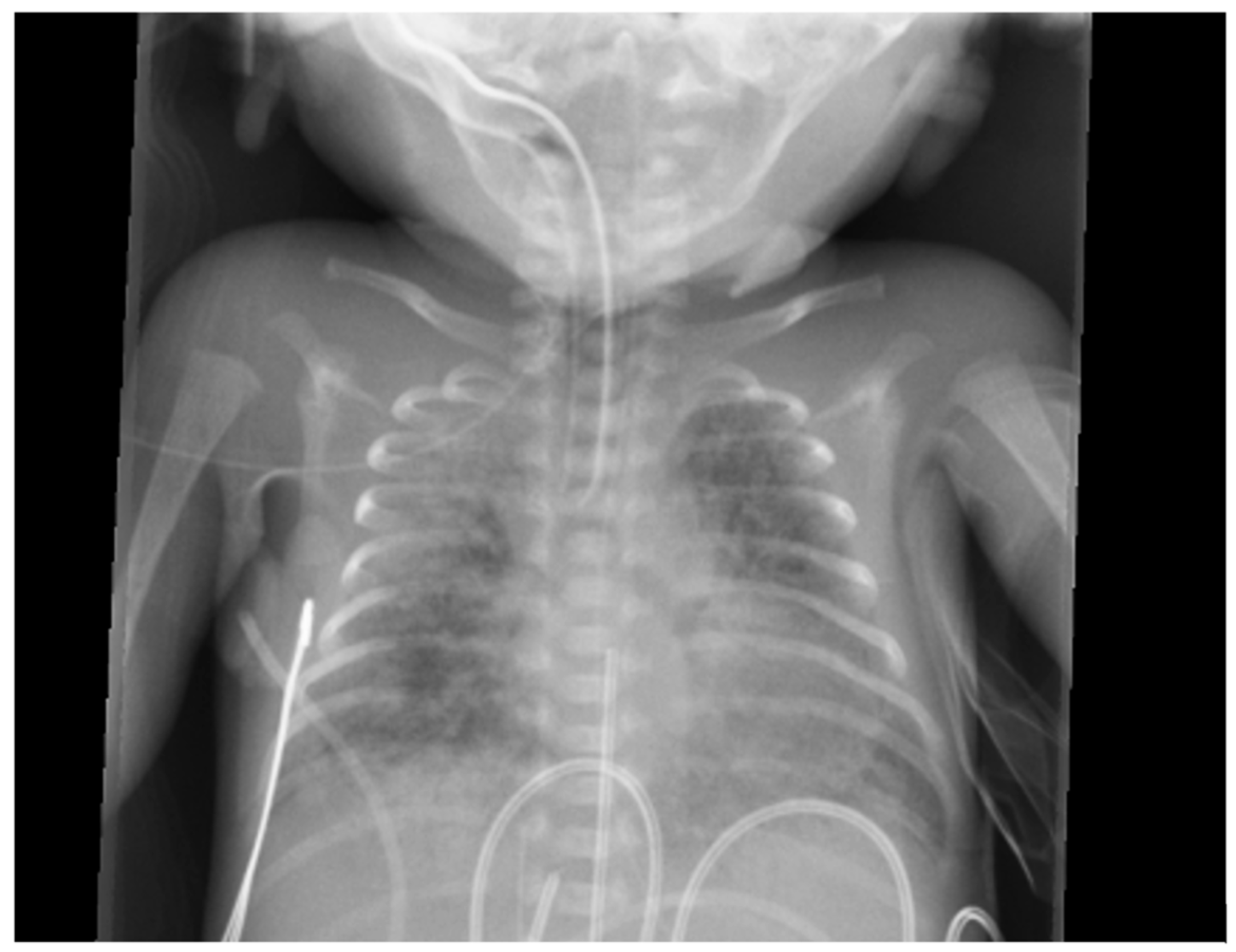
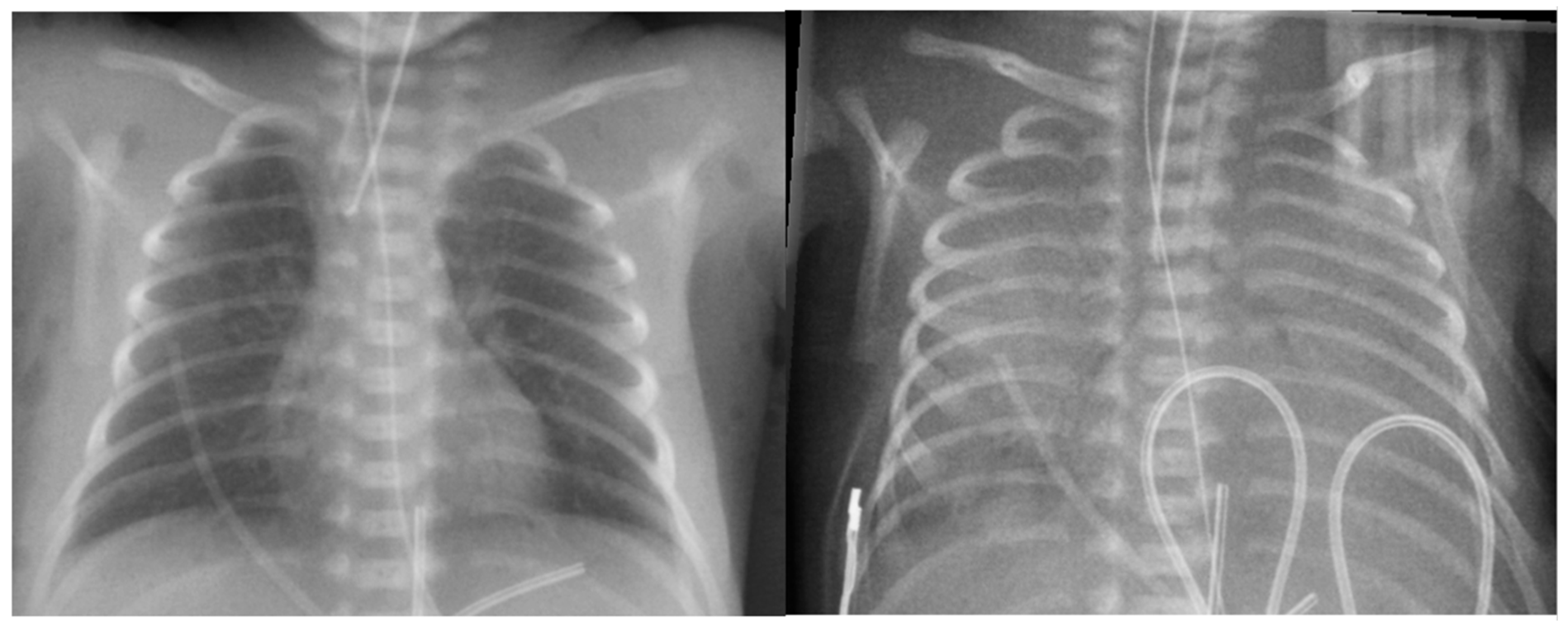
6.3. Radiological Diagnosis
6.4. Lung Ultrasound in the Evaluation of PH
6.5. Echocardiography Assessment
6.5.1. Patent Ductus Arteriosus
6.5.2. Pulmonary Hypertension
6.5.3. Systemic Hypotension
- The assessment of biventricular systolic function (the impact of hemorrhage on contractility).
- The evaluation of preload and afterload (volume status and vascular resistance).
- The characterization of intra- and extracardiac shunts (PDA and atrial shunt), influencing pulmonary and systemic flow.
- Chamber morphology (LV and RV size and hypertrophy, dilation) secondary to volume or pressure overload.
- The exclusion or inclusion of a diagnosis of congenital heart disease, which may present with hemodynamic instability and contribute to PH [75].
7. Management
7.1. Resuscitation and Stabilization
7.2. Mechanical Ventilation
- High-Frequency Oscillatory Ventilation (HFOV): HFOV is often favored over conventional mechanical ventilation (CMV), although no RCT has confirmed its superiority. Several studies have demonstrated that HFOV can significantly reduce FiO2 requirements and improve the oxygenation index in critically ill neonates with massive PH and respiratory failure [8,76,77]. AlKharfy et al. further reported that HFOV effectively promotes adequate ventilation [78]. Additionally, a study by Duval et al. highlighted the potential life-saving benefits of HFOV in cases of severe PH, showing a rapid improvement in oxygenation [79]. Its sustained high distending pressures, which may tamponade alveolar bleeding, reduce pulmonary blood flow, and limit further capillary rupture, make it a valuable lung-protective strategy.
- Positive End-Expiratory Pressure (PEEP): Trompeter et al. demonstrated that increasing MAP through PEEP optimization alongside acidosis correction, morphine administration, and diuretic (furosemide) can stabilize hemorrhage [5]. Similarly, Bhandari et al. reported benefits from combining increased MAPs with endotracheal epinephrine (1:10,000 at 0.1 mL/kg) and/or 4% cocaine (4 mL/kg) [80]. These strategies aim to improve lung recruitment, stabilize alveolar capillary membranes, and mitigate further hemorrhagic episodes by reducing excessive pulmonary capillary pressure and improving gas exchange.
7.3. Surfactant Therapy
7.4. Blood Product Transfusions and Coagulation Support
- Recombinant Factor VII (rFVIIa): A few case reports have documented the use of intravenous recombinant activated factor VII (rFVIIa) in neonates with life-threatening PH, demonstrating positive outcomes. rFVIIa acts by directly activating the extrinsic coagulation pathway, leading to thrombin generation and fibrin clot formation, which may help control severe bleeding. However, further prospective studies are needed to establish the optimal dosage, timing of administration, and overall efficacy, safety, and tolerability of rFVIIa in both preterm and term neonates [86].
- Hemocoagulase: Some studies have reported the use of hemocoagulase, a snake venom-derived enzyme, as a hemostatic agent in neonatal hemorrhage. Hemocoagulase promotes blood coagulation by activating prothrombin and accelerating fibrin clot formation. While its use in neonates remains limited, preliminary reports suggest its potential efficacy in controlling pulmonary hemorrhage. Further research is needed to evaluate its safety, optimize dosing, and enhance its overall effectiveness in neonatal care [87,88,89].
- Antifibrinolytic Agents: Tranexamic acid (TXA) is an antifibrinolytic agent that prevents fibrin clot degradation by inhibiting plasminogen activation. It has been used intravenously in some cases of neonatal hemorrhage, including PH, to stabilize existing clots and reduce ongoing bleeding [90]. While TXA has shown promise in adult and pediatric populations [91], its use in neonates remains limited, and further research is needed to establish its safety, optimize dosing, and improve overall efficacy in preterm and critically ill infants.
- Ankaferd Blood Stopper (ABS): Two cases from a report described the successful use of ABS in treating massive PH in neonates. The first case involved a term male newborn at 38 3/7 weeks of gestation, while the second case involved a late preterm infant at 33 6/7 weeks. Both neonates experienced severe PH, and ABS was administered directly via the endotracheal tube. In both cases, the hemorrhage ceased immediately following ABS administration, highlighting its potential as an emergency hemostatic intervention in neonatal PH. However, further studies are necessary to evaluate its safety, optimize dosing, and apply it to broader clinical applications, especially in premature infants where there is no reported evidence or experience [92].
7.5. Endotracheal Epinephrine
7.6. Tolazoline
8. Prognosis
9. Future Directions
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barnes, M.E.; Feeney, E.; Duncan, A.; Jassim, S.; MacNamara, H.; O’Hara, J.; Refila, B.; Allen, J.; McCollum, D.; Meehan, J.; et al. Pulmonary haemorrhage in neonates: Systematic review of management. Acta Paediatr. 2022, 111, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewska, M.; Stork, E.; Minich, N.M.; Friedman, H.; Berlin, S.; Hack, M. Pulmonary Hemorrhage: Clinical Course and Outcomes Among Very Low-Birth-Weight Infants. Arch. Pediatr. Adolesc. Med. 1999, 153, 715–721. [Google Scholar] [CrossRef] [PubMed]
- De Carolis, M.P.; Romagnoli, C.; Cafforio, C.; Piersigilli, F.; Papacci, P.; Vento, G.; Tortorolo, G. Pulmonary haemorrhage in infants with gestational age of less than 30 weeks. Eur. J. Pediatr. 1998, 157, 1037–1038. [Google Scholar] [CrossRef] [PubMed]
- Zahr, R.A.; Ashfaq, A.; Marron-Corwin, M. Neonatal Pulmonary Hemorrhage. NeoReviews 2012, 13, e302–e306. [Google Scholar] [CrossRef]
- Trompeter, R.; Yu, V.Y.; Aynsley-Green, A.; Roberton, N.R. Massive pulmonary haemorrhage in the newborn infant. Arch. Dis. Child. 1975, 50, 123–127. [Google Scholar] [CrossRef] [PubMed]
- van Houten, J.; Long, W.; Mullett, M.; Finer, N.; Derleth, D.; McMurray, B.; Peliowski, A.; Walker, D.; Wold, D.; Sankaran, K.; et al. Pulmonary hemorrhage in premature infants after treatment with synthetic surfactant: An autopsy evaluation. The American Exosurf Neonatal Study Group I, and the Canadian Exosurf Neonatal Study Group. J. Pediatr. 1992, 120, S40–S44. [Google Scholar] [CrossRef] [PubMed]
- Pappin, A.; Shenker, N.; Hack, M.; Redline, R.W. Extensive intraalveolar pulmonary hemorrhage in infants dying after surfactant therapy. J. Pediatr. 1994, 124, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.Y.; Chang, Y.S.; Park, W.S. Massive pulmonary hemorrhage in newborn infants successfully treated with high frequency oscillatory ventilation. J. Korean Med. Sci. 1998, 13, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.Y. Massive pulmonary hemorrhage in neonatal infection. Can. Med. Assoc. J. 1976, 114, 135–138. [Google Scholar] [PubMed]
- Finlay, E.R.; Subhedar, N.V. Pulmonary haemorrhage in preterm infants. Eur. J. Pediatr. 2000, 159, 870–871. [Google Scholar] [CrossRef] [PubMed]
- Pandit, P.B.; O’Brien, K.; Asztalos, E.; Colucci, E.; Dunn, M.S. Outcome following pulmonary haemorrhage in very low birthweight neonates treated with surfactant. Arch. Dis. Child. Fetal Neonatal Ed. 1999, 81, F40–F44. [Google Scholar] [CrossRef] [PubMed]
- Sadeh-Vered, T.; Rosenberg, N.; Morag, I.; Berg, A.A.; Kenet, G.; Strauss, T. A Proposed Role of Surfactant in Platelet Function and Treatment of Pulmonary Hemorrhage in Preterm and Term Infants. Acta Haematol. 2018, 140, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Yum, S.K.; Moon, C.J.; Youn, Y.A.; Lee, H.S.; Kim, S.Y.; Sung, I.K. Risk factor profile of massive pulmonary haemorrhage in neonates: The impact on survival studied in a tertiary care centre. J. Matern. Fetal Neonatal Med. 2016, 29, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, K.A.; Bennett, M.M.; Ahmad, S.F.; Clark, R.H.; Tolia, V.N. Morbidity and mortality with early pulmonary haemorrhage in preterm neonates. Arch. Dis. Child.—Fetal Neonatal Ed. 2019, 104, F63. [Google Scholar] [CrossRef] [PubMed]
- Gezmu, A.M.; Tefera, E.; Mochankana, K.; Imran, F.; Joel, D.; Pelaelo, I.; Nakstad, B. Pulmonary hemorrhage and associated risk factors among newborns admitted to a tertiary level neonatal unit in Botswana. Front. Pediatr. 2023, 11, 1171223. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xia, H.; Ye, L.; Li, X.; Zhang, Z. Exploring prediction model and survival strategies for pulmonary hemorrhage in premature infants: A single-center, retrospective study. Transl. Pediatr. 2021, 10, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Wang, H.P.; Lin, S.M.; Chang, J.T.; Hsieh, K.S.; Huang, F.K.; Chiou, Y.H.; Huang, Y.F.; Taiwan Premature Infant Development Collaborative Study, G. Pulmonary hemorrhage in very low-birthweight infants: Risk factors and management. Pediatr. Int. 2012, 54, 743–747. [Google Scholar] [CrossRef] [PubMed]
- Peacock, J.L.; Marston, L.; Marlow, N.; Calvert, S.A.; Greenough, A. Neonatal and infant outcome in boys and girls born very prematurely. Pediatr. Res. 2012, 71, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Berger, T.M.; Allred, E.N.; Van Marter, L.J. Antecedents of clinically significant pulmonary hemorrhage among newborn infants. J. Perinatol. 2000, 20, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Gludovacz, K.; Vlasselaer, J.; Mesens, T.; Van Holsbeke, C.; Van Robays, J.; Gyselaers, W. Early neonatal complications from pulmonary arteriovenous malformations in hereditary hemorrhagic telangiectasia: Case report and review of the literature. J. Matern. Fetal Neonatal Med. 2012, 25, 1494–1498. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.H.; Carmona, F.; Martinez, F.E. Prevalence, risk factors and outcomes associated with pulmonary hemorrhage in newborns. J. Pediatr. 2014, 90, 316–322. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, T.T.; Zhou, M.; Hu, X.F.; Liu, J.Q. Perinatal risk factors for pulmonary hemorrhage in extremely low-birth-weight infants. World J. Pediatr. 2020, 16, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Saxonhouse, M.A.; Sola, M.C. Platelet function in term and preterm neonates. Clin. Perinatol. 2004, 31, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Welde, M.A.; Sanford, C.B.; Mangum, M.; Paschal, C.; Jnah, A.J. Pulmonary Hemorrhage in the Neonate. Neonatal Netw. 2021, 40, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Johnson, D.L.; Stewart, L.; Waite, K.; Elliott, D.; Wilson, J.M. Rab14 regulation of claudin-2 trafficking modulates epithelial permeability and lumen morphogenesis. Mol. Biol. Cell 2014, 25, 1744–1754. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.W.; Su, B.H.; Lin, H.C.; Hu, P.S.; Peng, C.T.; Tsai, C.H.; Liang, W.M. Risk factors of pulmonary hemorrhage in very-low-birth-weight infants: A two-year retrospective study. Acta Paediatr. Taiwan. 2000, 41, 255–258. [Google Scholar] [PubMed]
- Bendapudi, P.; Narasimhan, R.; Papworth, S. Causes and management of pulmonary haemorrhage in the neonate. Paediatr. Child Health 2012, 22, 528–531. [Google Scholar] [CrossRef]
- Raju, T.N.; Langenberg, P. Pulmonary hemorrhage and exogenous surfactant therapy: A metaanalysis. J. Pediatr. 1993, 123, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.L.; Siu, K.L. Pulmonary Complications in Premature Infants Using a Beractant or Poractant for Respiratory Distress Syndrome: A Retrospective Cohort Study. Am. J. Perinatol. 2024, 41, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Speer, C.P.; Gefeller, O.; Groneck, P.; Laufkotter, E.; Roll, C.; Hanssler, L.; Harms, K.; Herting, E.; Boenisch, H.; Windeler, J.; et al. Randomised clinical trial of two treatment regimens of natural surfactant preparations in neonatal respiratory distress syndrome. Arch. Dis. Child. Fetal Neonatal Ed. 1995, 72, F8–F13. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, S.B.; Beresford, M.W.; Milligan, D.W.; Shaw, N.J.; Matthews, J.N.; Fenton, A.C.; Ward Platt, M.P. Pumactant and poractant alfa for treatment of respiratory distress syndrome in neonates born at 25–29 weeks’ gestation: A randomised trial. Lancet 2000, 355, 1387–1392. [Google Scholar] [CrossRef] [PubMed]
- Strauss, T.; Rozenzweig, N.; Rosenberg, N.; Shenkman, B.; Livnat, T.; Morag, I.; Fruchtman, Y.; Martinowitz, U.; Kenet, G. Surfactant impairs coagulation in-vitro: A risk factor for pulmonary hemorrhage? Thromb. Res. 2013, 132, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.J.; Kohn, A. Pulmonary Hemorrhage, Transient Tachypnea and Neonatal Pneumonia. In Neonatology: A Practical Approach to Neonatal Diseases; Buonocore, G., Bracci, R., Weindling, M., Eds.; Springer: Milano, Italy, 2012; pp. 455–459. [Google Scholar]
- Buvaneswarran, S.; Wong, Y.L.; Liang, S.; Quek, S.C.; Lee, J. Active Treatment vs Expectant Management of Patent Ductus Arteriosus in Preterm Infants: A Meta-Analysis. JAMA Pediatr. 2025. ahead-of-print. [Google Scholar] [CrossRef]
- Spenard, S.; Backes, C.; Fitzgerald, D.A.; Sant’Anna, G.; Altit, G. Current approaches to the patent ductus arteriosus: Implications for pulmonary morbidities. Paediatr. Respir. Rev. 2025. ahead-of-print. [Google Scholar] [CrossRef]
- Ambalavanan, N.; Aucott, S.W.; Salavitabar, A.; Levy, V.Y.; Committee on Fetus and Newborn; Section on Cardiology and Cardiac Surgery. Patent Ductus Arteriosus in Preterm Infants. Pediatrics 2025, 155, e2025071425. [Google Scholar] [CrossRef] [PubMed]
- Kluckow, M.; Jeffery, M.; Gill, A.; Evans, N. A randomised placebo-controlled trial of early treatment of the patent ductus arteriosus. Arch. Dis. Child. Fetal Neonatal Ed. 2014, 99, F99–F104. [Google Scholar] [CrossRef] [PubMed]
- Gournay, V.; Roze, J.C.; Kuster, A.; Daoud, P.; Cambonie, G.; Hascoet, J.M.; Chamboux, C.; Blanc, T.; Fichtner, C.; Savagner, C.; et al. Prophylactic ibuprofen versus placebo in very premature infants: A randomised, double-blind, placebo-controlled trial. Lancet 2004, 364, 1939–1944. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Han, D.; Wang, X.; Huang, X.; Huang, Z.; Liu, Y.; Zhong, J.; Walther, F.J.; Yang, C.; Wagenaar, G.T.M. Vascular and pulmonary effects of ibuprofen on neonatal lung development. Respir. Res. 2023, 24, 39. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.; Davis, P.; Moddemann, D.; Ohlsson, A.; Roberts, R.S.; Saigal, S.; Solimano, A.; Vincer, M.; Wright, L.L. Long-term effects of indomethacin prophylaxis in extremely-low-birth-weight infants. N. Engl. J. Med. 2001, 344, 1966–1972. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho Nunes, G.; Wutthigate, P.; Simoneau, J.; Beltempo, M.; Sant’Anna, G.M.; Altit, G. Natural evolution of the patent ductus arteriosus in the extremely premature newborn and respiratory outcomes. J. Perinatol. 2022, 42, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Hundscheid, T.; Onland, W.; Kooi, E.M.W.; Vijlbrief, D.C.; de Vries, W.B.; Dijkman, K.P.; van Kaam, A.H.; Villamor, E.; Kroon, A.A.; Visser, R.; et al. Expectant Management or Early Ibuprofen for Patent Ductus Arteriosus. N. Engl. J. Med. 2023, 388, 980–990. [Google Scholar] [CrossRef] [PubMed]
- West, J.B.; Mathieu-Costello, O. Stress failure of pulmonary capillaries: Role in lung and heart disease. Lancet 1992, 340, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Toyoshima, K.; Kawataki, M.; Ohyama, M.; Shibasaki, J.; Yamaguchi, N.; Hoshino, R.; Itani, Y.; Nakazawa, M. Tailor-made circulatory management based on the stress-velocity relationship in preterm infants. J. Formos. Med. Assoc. 2013, 112, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Noori, S.; McCoy, M.; Anderson, M.P.; Ramji, F.; Seri, I. Changes in cardiac function and cerebral blood flow in relation to peri/intraventricular hemorrhage in extremely preterm infants. J. Pediatr. 2014, 164, e261–e263. [Google Scholar] [CrossRef] [PubMed]
- Änghagen, O.; Engvall, J.; Gottvall, T.; Nelson, N.; Nylander, E.; Bang, P. Developmental Differences in Left Ventricular Strain in IUGR vs. Control Children the First Three Months of Life. Pediatr. Cardiol. 2022, 43, 1286–1297. [Google Scholar] [CrossRef] [PubMed]
- Zaharie, G.C.; Hasmasanu, M.; Blaga, L.; Matyas, M.; Muresan, D.; Bolboaca, S.D. Cardiac left heart morphology and function in newborns with intrauterine growth restriction: Relevance for long-term assessment. Med. Ultrason. 2019, 21, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Dearborn, D.G. Pulmonary hemorrhage in infants and children. Curr. Opin. Pediatr. 1997, 9, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Pace, L.M.; Lee, A.Y.; Nath, S.; Alviedo, N.B. Pulmonary Hemorrhage: An Unusual Life-Threatening Presentation of Factor IX Deficiency in a Monochorionic-Diamniotic Twin Neonate. Cureus 2021, 13, e20352. [Google Scholar] [CrossRef] [PubMed]
- Orbach, R.; Mandel, D.; Lubetzky, R.; Ovental, A.; Haham, A.; Halutz, O.; Grisaru-Soen, G. Pulmonary hemorrhage due to Coxsackievirus B infection-A call to raise suspicion of this important complication as an end-stage of enterovirus sepsis in preterm twin neonates. J. Clin. Virol. 2016, 82, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Sollinger, C.; Steiner, L. Case 2: Respiratory Failure, Myocardial Dysfunction, and Pulmonary Hemorrhage in a Full-Term Newborn. NeoReviews 2016, 17, e282–e284. [Google Scholar] [CrossRef]
- Pallotto, E.K.; Kilbride, H.W. Perinatal Outcome and Later Implications of Intrauterine Growth Restriction. Clin. Obstet. Gynecol. 2006, 49, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Nishida, H.; Arai, T.; Kaneda, Y. Abnormal cardiac histology in severe intrauterine growth retardation infants. Acta Paediatr. Jpn. 1995, 37, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Healy, F.; Hanna, B.D.; Zinman, R. Pulmonary complications of congenital heart disease. Paediatr. Respir. Rev. 2012, 13, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Welsh, S.K.; Casey, A.M.; Fishman, M.P. Pulmonary hemorrhage in infancy: A 10-year single-center experience. Pediatr. Pulmonol. 2018, 53, 1559–1564. [Google Scholar] [CrossRef] [PubMed]
- Tran, G.T.M.; Nguyen, N.P.M.; Long, N.P.; Nguyen, D.N.; Nguyen, T.-T. Risk Factors and Outcomes of Pulmonary Hemorrhage in Preterm Infants born before 32 weeks. medRxiv 2024. [Google Scholar] [CrossRef]
- Polglase, G.R.; Ong, T.; Hillman, N.H. Cardiovascular Alterations and Multiorgan Dysfunction After Birth Asphyxia. Clin. Perinatol. 2016, 43, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Morishita, S. Hypercoagulability and DIC in high-risk infants. Semin. Thromb. Hemost. 1998, 24, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Cohen, I.J. Room temperature ADP-induced first-stage hyperaggregation of human platelets: The cause of rewarming deaths by thrombocytopenia in neonatal cold injury. Pediatr. Hematol. Oncol. 1991, 8, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Curley, A.; Stanworth, S.J. Interpretation of clotting tests in the neonate. Arch. Dis. Child. Fetal Neonatal Ed. 2015, 100, F270–F274. [Google Scholar] [CrossRef] [PubMed]
- Cole, V.A.; Normand, I.C.; Reynolds, E.O.; Rivers, R.P. Pathogenesis of hemorrhagic pulmonary edema and massive pulmonary hemorrhage in the newborn. Pediatrics 1973, 51, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Alfaleh, K.; Smyth, J.A.; Roberts, R.S.; Solimano, A.; Asztalos, E.V.; Schmidt, B.; Trial of Indomethacin Prophylaxis in Preterms, I. Prevention and 18-month outcomes of serious pulmonary hemorrhage in extremely low birth weight infants: Results from the trial of indomethacin prophylaxis in preterms. Pediatrics 2008, 121, e233–e238. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, F.; Yousef, N.; Migliaro, F.; Capasso, L.; De Luca, D. Point-of-care lung ultrasound in neonatology: Classification into descriptive and functional applications. Pediatr. Res. 2021, 90, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Ruoss, J.L.; Bazacliu, C.; Cacho, N.; De Luca, D. Lung Ultrasound in the Neonatal Intensive Care Unit: Does It Impact Clinical Care? Children 2021, 8, 1098. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.L.; Fu, W.; Liu, J.; Liu, Y.; Xia, R.M. Lung ultrasonography to diagnose pulmonary hemorrhage of the newborn. J. Matern. Fetal Neonatal Med. 2017, 30, 2601–2606. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chi, J.H.; Lu, Z.L.; Fu, W. The specific signs of lung ultrasound to diagnose pulmonary hemorrhage of the newborns: Evidence from a multicenter retrospective case-control study. Front. Pediatr. 2023, 11, 1090332. [Google Scholar] [CrossRef] [PubMed]
- Kluckow, M.; Evans, N. Ductal shunting, high pulmonary blood flow, and pulmonary hemorrhage. J. Pediatr. 2000, 137, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Barrington, K.J.; Finer, N.; Pennaforte, T.; Altit, G. Nitric oxide for respiratory failure in infants born at or near term. Cochrane Database Syst. Rev. 2017, 1, CD000399. [Google Scholar] [CrossRef] [PubMed]
- Barrington, K.J.; Finer, N.; Pennaforte, T. Inhaled nitric oxide for respiratory failure in preterm infants. Cochrane Database Syst. Rev. 2017, 1, CD000509. [Google Scholar] [CrossRef] [PubMed]
- Chock, V.Y.; Van Meurs, K.P.; Hintz, S.R.; Ehrenkranz, R.A.; Lemons, J.A.; Kendrick, D.E.; Stevenson, D.K. Inhaled nitric oxide for preterm premature rupture of membranes, oligohydramnios, and pulmonary hypoplasia. Am. J. Perinatol. 2009, 26, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Kettle, R.; Subhedar, N.V. Nitric Oxide in Pulmonary Hypoplasia: Results from the European iNO Registry. Neonatology 2019, 116, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Dani, C.; Corsini, I.; Cangemi, J.; Vangi, V.; Pratesi, S. Nitric oxide for the treatment of preterm infants with severe RDS and pulmonary hypertension. Pediatr. Pulmonol. 2017, 52, 1461–1468. [Google Scholar] [CrossRef] [PubMed]
- McMullan, D.M.; Bekker, J.M.; Johengen, M.J.; Hendricks-Munoz, K.; Gerrets, R.; Black, S.M.; Fineman, J.R. Inhaled nitric oxide-induced rebound pulmonary hypertension: Role for endothelin-1. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H777–H785. [Google Scholar] [CrossRef] [PubMed]
- Vijlbrief, D.C.; Benders, M.J.; Kemperman, H.; van Bel, F.; de Vries, W.B. B-type natriuretic peptide and rebound during treatment for persistent pulmonary hypertension. J. Pediatr. 2012, 160, 111–115.e111. [Google Scholar] [CrossRef] [PubMed]
- McNamara, P.J.; Jain, A.; El-Khuffash, A.; Giesinger, R.; Weisz, D.; Freud, L.; Levy, P.T.; Bhombal, S.; de Boode, W.; Leone, T.; et al. Guidelines and Recommendations for Targeted Neonatal Echocardiography and Cardiac Point-of-Care Ultrasound in the Neonatal Intensive Care Unit: An Update from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2024, 37, 171–215. [Google Scholar] [CrossRef] [PubMed]
- Pappas, M.D.; Sarnaik, A.P.; Meert, K.L.; Hasan, R.A.; Lieh-Lai, M.W. Idiopathic pulmonary hemorrhage in infancy. Clinical features and management with high frequency ventilation. Chest 1996, 110, 553–555. [Google Scholar] [CrossRef] [PubMed]
- Yen, T.A.; Wang, C.C.; Hsieh, W.S.; Chou, H.C.; Chen, C.Y.; Tsao, P.N. Short-term outcome of pulmonary hemorrhage in very-low-birth-weight preterm infants. Pediatr. Neonatol. 2013, 54, 330–334. [Google Scholar] [CrossRef] [PubMed]
- AlKharfy, T.M. High-frequency ventilation in the management of very-low-birth-weight infants with pulmonary hemorrhage. Am. J. Perinatol. 2004, 21, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Duval, E.L.I.M.; Markhorst, D.G.; Ramet, J.; van Vught, A.J. High-frequency oscillatory ventilation in severe lung haemorrhage: A case study of three centres. Respir. Med. CME 2009, 2, 92–98. [Google Scholar] [CrossRef]
- Bhandari, V.; Gagnon, C.; Rosenkrantz, T.; Hussain, N. Pulmonary hemorrhage in neonates of early and late gestation. J. Perinat. Med. 1999, 27, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.; Ohlsson, A. Surfactant for pulmonary haemorrhage in neonates. Cochrane Database Syst. Rev. 2020, 2, CD005254. [Google Scholar] [CrossRef] [PubMed]
- Pandit, P.B.; Dunn, M.S.; Colucci, E.A. Surfactant therapy in neonates with respiratory deterioration due to pulmonary hemorrhage. Pediatrics 1995, 95, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Amizuka, T.; Shimizu, H.; Niida, Y.; Ogawa, Y. Surfactant therapy in neonates with respiratory failure due to haemorrhagic pulmonary oedema. Eur. J. Pediatr. 2003, 162, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Christensen, R.D.; Bahr, T.M.; Davenport, P.; Sola-Visner, M.C.; Ohls, R.K.; Ilstrup, S.J.; Kelley, W.E. Implementing evidence-based restrictive neonatal intensive care unit platelet transfusion guidelines. J. Perinatol. 2024, 44, 1394–1401. [Google Scholar] [CrossRef] [PubMed]
- New, H.V.; Berryman, J.; Bolton-Maggs, P.H.; Cantwell, C.; Chalmers, E.A.; Davies, T.; Gottstein, R.; Kelleher, A.; Kumar, S.; Morley, S.L.; et al. Guidelines on transfusion for fetuses, neonates and older children. Br. J. Haematol. 2016, 175, 784–828. [Google Scholar] [CrossRef] [PubMed]
- Poralla, C.; Hertfelder, H.-J.; Oldenburg, J.; Müller, A.; Bartmann, P.; Heep, A. Treatment of acute pulmonary haemorrhage in extremely preterm infants with recombinant activated factor VII. Acta Paediatr. 2010, 99, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Tang, S.; Li, H.; Zhao, J.; Pan, F. New Treatment of Neonatal Pulmonary Hemorrhage with Hemocoagulase in Addition to Mechanical Ventilation. Biol. Neonate 2005, 88, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Lodha, A.; Kamaluddeen, M.; Akierman, A.; Amin, H. Role of Hemocoagulase in Pulmonary Hemorrhage in Preterm Infants: A Systematic Review. Indian J. Pediatr. 2011, 78, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhao, J.; Tang, S.; Pan, F.; Liu, L.; Tian, Z.; Li, H. Effect of hemocoagulase for prevention of pulmonary hemorrhage in critical newborns on mechanical ventilation: A randomized controlled trial. Indian Pediatr. 2008, 45, 199–202. [Google Scholar] [PubMed]
- Can, E.; Hamilçıkan, Ş. Efficacy of Tranexamic Acid in Severe Pulmonary Hemorrhage in a Asphyctic Neonate. Iran. J. Ped. Hematol. Oncol. 2018, 8, 139–141. [Google Scholar]
- Singleton, L.; Kennedy, C.; Philip, B.; Navaei, A.; Bhar, S.; Ankola, A.; Doane, K.; Ontaneda, A. Use of Inhaled Tranexamic Acid for Pulmonary Hemorrhage in Pediatric Patients on Extracorporeal Membrane Oxygenation Support. ASAIO J. 2025, 36, 1. [Google Scholar] [CrossRef] [PubMed]
- Tufekci, S.; Halis, H. Treatment of Acute Pulmonary Hemorrhage in Neonates with Endotracheal Ankaferd Blood Stopper Use. Ulutas Med. J. 2021, 7, 248. [Google Scholar] [CrossRef]
- Markestad, T.; Finne, P.H. Effect of tolazoline in pulmonary hemorrhage in the newborn. Acta Paediatr. Scand. 1980, 69, 425–426. [Google Scholar] [CrossRef] [PubMed]


| Risk Factors | Protective Factors |
|---|---|
|
|
| Initial Assessment and Stabilization |
|
| Mechanical Ventilation |
|
| Surfactant Therapy |
|
| Blood Product Transfusions |
|
| Coagulation Support |
|
| Endotracheal Epinephrine |
|
| Inotropic Drug or Vasopressor |
|
| Steroids |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahussarungsi, S.; Lapointe, A.; Villeneuve, A.; Hebert, A.; Nouraeyan, N.; Lakshminrusimha, S.; Singh, Y.; Sabapathy, C.; Cavallé-Garrido, T.; Sant’Anna, G.; et al. Pulmonary Hemorrhage in Premature Infants: Pathophysiology, Risk Factors and Clinical Management. Biomedicines 2025, 13, 1744. https://doi.org/10.3390/biomedicines13071744
Sahussarungsi S, Lapointe A, Villeneuve A, Hebert A, Nouraeyan N, Lakshminrusimha S, Singh Y, Sabapathy C, Cavallé-Garrido T, Sant’Anna G, et al. Pulmonary Hemorrhage in Premature Infants: Pathophysiology, Risk Factors and Clinical Management. Biomedicines. 2025; 13(7):1744. https://doi.org/10.3390/biomedicines13071744
Chicago/Turabian StyleSahussarungsi, Sariya, Anie Lapointe, Andréanne Villeneuve, Audrey Hebert, Nina Nouraeyan, Satyan Lakshminrusimha, Yogen Singh, Christine Sabapathy, Tiscar Cavallé-Garrido, Guilherme Sant’Anna, and et al. 2025. "Pulmonary Hemorrhage in Premature Infants: Pathophysiology, Risk Factors and Clinical Management" Biomedicines 13, no. 7: 1744. https://doi.org/10.3390/biomedicines13071744
APA StyleSahussarungsi, S., Lapointe, A., Villeneuve, A., Hebert, A., Nouraeyan, N., Lakshminrusimha, S., Singh, Y., Sabapathy, C., Cavallé-Garrido, T., Sant’Anna, G., & Altit, G. (2025). Pulmonary Hemorrhage in Premature Infants: Pathophysiology, Risk Factors and Clinical Management. Biomedicines, 13(7), 1744. https://doi.org/10.3390/biomedicines13071744





