3.2. Expression Analysis of NRP1 and ACE2 in Lung Tissues
Table 3 presents transcriptomic expression data (TPM) for NRP1 and ACE2 in lung tissue samples from COVID-19 patients and healthy controls, based on publicly available RNA-Seq data (GSE150316). A total of 11 samples were included: 5 control lung tissues (3 males, 2 females; average age ~55 years) and 6 post-mortem COVID-19 lung tissues (4 males, 2 females; average age ~58 years). All COVID-19 cases corresponded to patients who died from severe disease, according to dataset annotations.
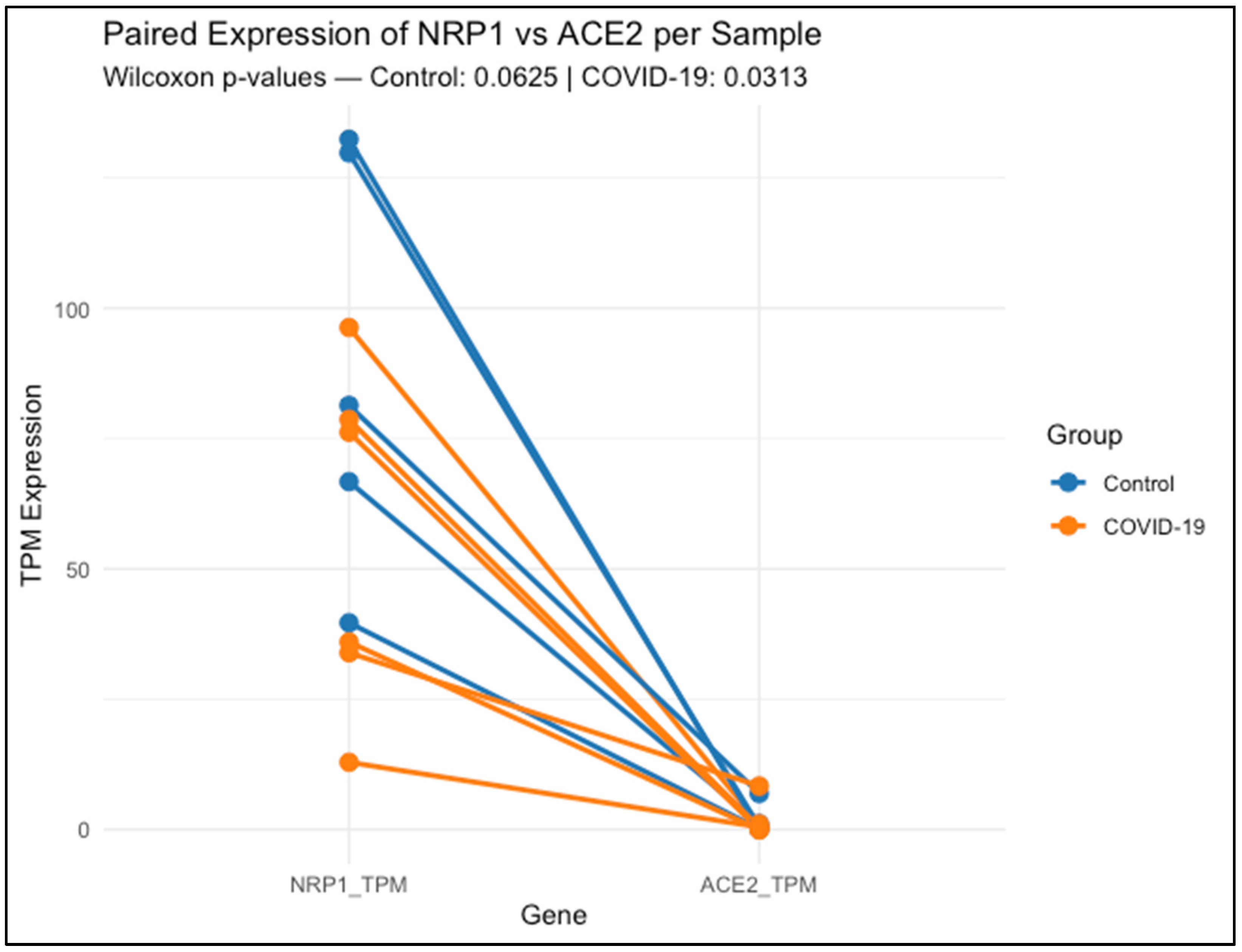
Figure 1 shows that each line connects the expression levels of NRP1 and ACE2 within the same lung tissue sample, either from healthy controls (blue) or COVID-19 patients (orange).
Data represent transcript abundance in transcripts per million (TPM), as obtained from publicly available RNA-Seq data (GSE150316). Across nearly all samples, NRP1 expression was higher than ACE2, regardless of clinical group. Statistical comparison using the Wilcoxon signed-rank test revealed a non-significant trend in the control group (p = 0.0625), and a significant difference in the COVID-19 group (p = 0.03125), indicating that NRP1 expression remains elevated relative to ACE2 even during infection.
NRP1 expression was clearly higher and more consistent among control lung samples, ranging from 39.67 to 132.40 TPM. COVID-19 samples exhibited greater variability (range: 12.91 to 96.30 TPM), suggesting inter-individual differences in NRP1 regulation during infection. A paired Wilcoxon test comparing NRP1 and ACE2 expression within each sample showed a non-significant trend in controls (p = 0.0625), while a statistically significant difference was observed in COVID-19 patients (p = 0.03125), with NRP1 consistently exceeding ACE2 levels.
By contrast, ACE2 expression was low overall, with a heterogeneous and sporadic distribution across both groups. Several samples—both controls and COVID-19—displayed undetectable levels (0.000 TPM), while isolated cases reached moderate to high values (e.g., 6.927 TPM in one control and 8.355 TPM in a COVID-19 sample). This distribution highlights the limited and variable expression of ACE2 in lung tissue compared to the broader and more stable profile of NRP1.
3.3. Design and Cross-Species Conservation of siRNAs
Table 4 summarizes the coordinates of the coding region sequences (CDS) for multiple human NRP1 mRNA variants and the corresponding region in
Rattus norvegicus, based on NCBI reference transcripts. In human transcripts, most variants (1–5 and 7) share a common translation start site at position 286, with coding region endpoints ranging between 2105 and 3057, depending on transcript length. Variant 6 was identified as non-coding, and was excluded from siRNA targeting. The rat NRP1 transcript exhibits a coding region extending from position 511 to 3295, slightly shifted relative to the human variants.
This comparative mapping confirmed that the 5′ region of the CDS is highly conserved across human variants and rat, supporting its use as a reliable target site for siRNA design. The siRNA target sequences were selected within this conserved region to ensure cross-species efficacy.
Figure 2 illustrates the sequence alignment of the target site for the first designed siRNA, highlighting its position within the coding region of NRP1 mRNA across multiple human transcript variants and the rat (
Rattus norvegicus) reference transcript (NM_145098.2).
The siRNA-binding site, shown in yellow, corresponds to the 19-nucleotide sequence GAGACTGCAAGTATGACTA, and is located within a highly conserved region present in all human coding variants assessed (NM_001024628.3, NM_001024629.3, NM_003873.3, NM_001244972.2, and NM_001244973.2), with exact sequence identity. Asterisks below the alignment indicate positions that are conserved across all sequences shown.
Table 5 presents the hybridization coordinates of the first designed siRNA (GAGACTGCAAGTATGACTA) within the mRNA transcripts of
Rattus norvegicus and multiple human NRP1 variants. The siRNA aligns with a fully conserved region in both species, highlighting its suitability for cross-species silencing applications.
In all human variants analyzed (1–5 and 7), the siRNA-binding site is located between nucleotides 524 and 542, showing perfect sequence identity. In the rat transcript (NM_145098.2), the target region maps to positions 777–797, also exhibiting complete conservation of the 19-nt sequence.
These conserved coordinates confirm that the designed siRNA hybridizes within the coding region of NRP1 in all relevant isoforms and across species, providing strong support for its broad applicability in preclinical models and its potential translational relevance in therapeutic silencing strategies targeting NRP1.
Figure 3 shows the multiple sequence alignment of the binding site for the second siRNA candidate, highlighting its position across human and rat NRP1 mRNA transcript variants. The target sequence CATTCTGACCAGATCAC (highlighted in yellow) aligns perfectly across all human coding variants (including NM_001024628.3, NM_001024629.3, NM_003873.3, NM_001244972.2, and NM_001244973.2), as well as the rat reference transcript (NM_145098.2). Asterisks below the alignment indicate positions that are conserved across all sequences shown.
Table 6 provides the hybridization coordinates of the second siRNA designed against NRP1, targeting the 17-nucleotide sequence CATTCTGACCAGATCAC. This sequence was selected for its high conservation across species and was confirmed to be present without mismatches in all analyzed human NRP1 variants (1–5, 7), as well as in the rat transcript NM_145098.2.
In human transcripts, the siRNA target site consistently maps to positions 1144–1160 nt, while in Rattus norvegicus, it aligns between 1399–1415 nt, reflecting species-specific differences in untranslated regions but full conservation of the coding region itself.
These findings confirm that both siRNA-1 and siRNA-2 bind to structurally favorable, exposed regions of the transcript, supporting effective and reproducible gene silencing across species. This structural validation complements the sequence conservation data and reinforces the rationale for selecting these sites in the design of robust siRNA-based therapies targeting NRP1.
Table 7 summarizes the physicochemical characteristics of the siRNA-1 duplex used in this study. The duplex consists of a 21-nucleotide sense strand (5′-GAG ACT GCA AGT ATG ACT A-3′) and its complementary antisense strand (5′-TAG TCA TAC TTG CAG TCT CTT-3′), designed to target a conserved region of the NRP1 coding sequence.
The duplex displays a GC content of 38.1%, which favors moderate thermodynamic stability while minimizing off-target interactions. The melting temperature (Tm) was calculated at 61.3 °C, indicating a stable duplex formation under physiological conditions. The molecular weight of the duplex is 6362.2 g/mol, with an extinction coefficient of 193,600 L/(mol·cm), enabling precise quantification by spectrophotometry.
For optical density measurements at 260 nm, the siRNA has a conversion factor of 5.17 nmol per OD260 and 32.86 μg per OD260, which facilitates accurate dosing in experimental applications.
Details the physicochemical parameters of the second siRNA duplex designed to target NRP1. The duplex consists of a 19-nucleotide sense strand (5′-CATTCTGACCAGATCAC-3′) and a complementary antisense strand (5′-GTGATCTGGTCAGAATGTT-3′). Its shorter length compared to siRNA-1 contributes to enhanced target specificity and potentially reduced off-target effects (
Table 8).
The GC content of 42.1% provides balanced thermodynamic stability, while the melting temperature (Tm) of 55.4 °C supports effective hybridization under physiological conditions. The calculated molecular weight is 5873.9 g/mol, and the extinction coefficient is 186,500 L/(mol·cm), enabling precise quantification by UV absorbance. For OD260-based quantification, this duplex yields 5.36 nmol and 31.5 µg per unit, which facilitates accurate preparation for experimental dosing.
3.4. Evaluation of Relative NRP1 mRNA Expression in Lung Tissue
The relative expression of NRP1 mRNA in lung tissue was evaluated by RT-PCR in rats, aiming to confirm its pulmonary expression. Primers were designed using the Roche ProbeFinder tool based on the reference sequence NM_145098.2 (Rattus norvegicus neuropilin-1 mRNA), and were synthesized by the Oligo T4 laboratory.
Two primer sets were used targeting distinct regions of NRP1, both optimized for real-time PCR and designed using the Universal ProbeLibrary system (Roche). Primers designed for detection of NRP1 in
Rattus norvegicus are shown in
Table 9.
This table lists the primer sequences used for the quantification of NRP1 mRNA expression by RT-qPCR, along with two reference genes, β-actin (ACTB) and hypoxanthine-guanine phosphoribosyltransferase (HPRT), selected for normalization purposes.
Importantly, the use of two independent housekeeping genes—β-actin and HPRT-1—for normalization enhances the robustness and reliability of the findings. This dual-normalization approach minimizes potential reference gene bias and supports the reproducibility and consistency of the observed NRP1 downregulation.
3.5. Physiological Parameters Following siRNA Administration
Table 10,
Table 11 and
Table 12 summarize the systolic arterial pressure (SAP), diastolic arterial pressure (DAP), and heart rate (HR) measured at baseline (time zero) and three days after siRNA administration across all experimental groups: Control, Sham, Control + Vehicle, and siRNA + Vehicle.
At baseline, all groups showed comparable SAP, DAP, and HR values. Notably, the siRNA + Vehicle group displayed physiological parameters within normal ranges, similar to those of the control groups, indicating that systemic administration of the siRNA formulation did not induce any acute cardiovascular alterations.
Three days post-injection, all hemodynamic parameters remained stable, with no significant differences among the groups. This reinforces the hemodynamic safety profile of the siRNA compound, even after systemic exposure and potential intracellular uptake.
In addition,
Table 12 presents the body weight measurements recorded at time zero and three days post-treatment. All groups showed a slight increase in weight, proportional to normal growth patterns expected during the experimental period. No statistically significant differences in weight gain were observed, further supporting the absence of systemic toxicity or adverse metabolic effects associated with the siRNA treatment.
3.6. Evaluation of the Effect of siRNA Administration in a Melittin-Induced Lung Injury Model
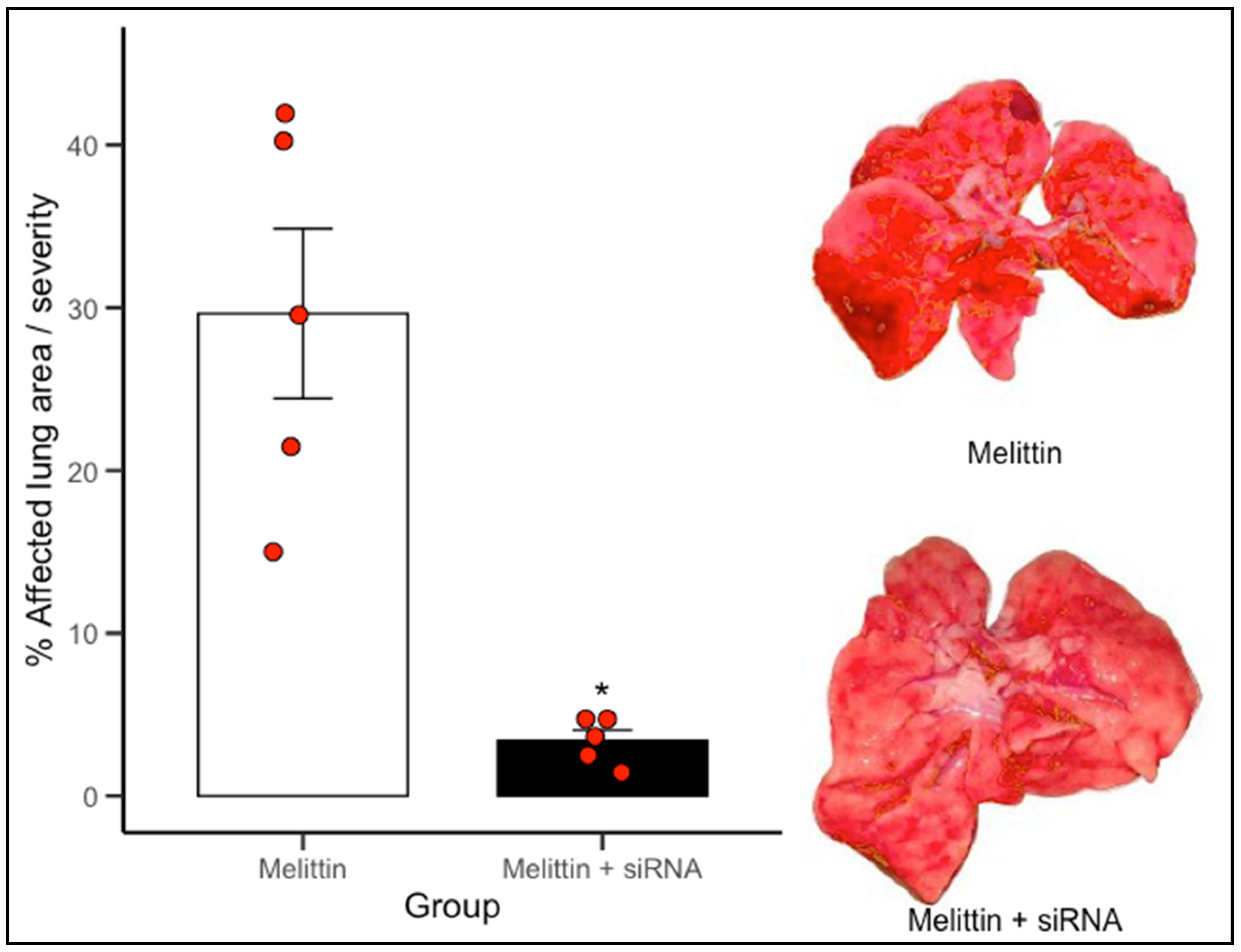
To determine the effect of the designed siRNAs, lung damage was assessed in a melittin-induced injury model in both the control group (vehicle) and the siRNA-treated group. ImageJ software was used to quantify the total lung area, lesion area, and percentage of affected (injured) tissue.
The protective effect observed in the Melittin + siRNA group was achieved by administering 50 µL of siRNA formulation intravenously via the jugular vein, 30 min prior to melittin exposure. This timing was selected to allow for sufficient intracellular uptake before the onset of inflammatory injury.
The administered siRNAs exerted a protective effect on lung tissue, as the extent of injury following melittin administration was significantly lower in the siRNA-treated groups compared to the control group.
The
Figure 4 illustrates the extent of macroscopic lung damage following melittin-induced inflammation, quantified as the percentage of visibly affected pulmonary surface. Lungs from animals treated only with melittin displayed extensive tissue injury, with mean lesion areas approximating 30%, characteristic of acute inflammatory insult. In contrast, animals pretreated with siRNA 1–2 directed against NRP1 showed a notable reduction in pulmonary damage, with lesion coverage falling to below 10% on average. This difference was statistically significant (
p < 0.05). The visual comparison between groups, supported by representative lung images, underscores the protective effect of NRP1 knockdown. * Indicate statistically significant differences (
p < 0.05) versus Melittin.