Effectiveness and Safety of Endovascular Treatment in Large Vessel Occlusion Stroke with an NIHSS Score of ≤5 Exhibiting Predominant Cortical Signs
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection and Definition of Parameters
2.3. Definitions of Cortical Signs
2.4. Outcome Measures
2.5. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients with Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar] [PubMed]
- Duloquin, G.; Crespy, V.; Jakubina, P.; Giroud, M.; Vergely, C.; Bejot, Y. Large Vessel Occlusion in Patients with Minor Ischemic Stroke in a Population-Based Study. The Dijon Stroke Registry. Front. Neurol. 2021, 12, 796046. [Google Scholar] [CrossRef] [PubMed]
- Heldner, M.R.; Jung, S.; Zubler, C.; Mordasini, P.; Weck, A.; Mono, M.L.; Ozdoba, C.; El-Koussy, M.; Mattle, H.P.; Schroth, G.; et al. Outcome of patients with occlusions of the internal carotid artery or the main stem of the middle cerebral artery with NIHSS score of less than 5: Comparison between thrombolysed and non-thrombolysed patients. J. Neurol. Neurosurg. Psychiatry 2015, 86, 755–760. [Google Scholar] [CrossRef]
- Miteff, F.; Levi, C.R.; Bateman, G.A.; Spratt, N.; McElduff, P.; Parsons, M.W. The independent predictive utility of computed tomography angiographic collateral status in acute ischaemic stroke. Brain 2009, 132, 2231–2238. [Google Scholar] [CrossRef]
- Goyal, M.; Menon, B.K.; van Zwam, W.H.; Dippel, D.W.; Mitchell, P.J.; Demchuk, A.M.; Davalos, A.; Majoie, C.B.; van der Lugt, A.; de Miquel, M.A.; et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet 2016, 387, 1723–1731. [Google Scholar] [CrossRef]
- Bracard, S.; Ducrocq, X.; Mas, J.L.; Soudant, M.; Oppenheim, C.; Moulin, T.; Guillemin, F.; THRACE Investigators. Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): A randomised controlled trial. Lancet Neurol. 2016, 15, 1138–1147. [Google Scholar] [CrossRef]
- Jovin, T.G.; Nogueira, R.G.; Lansberg, M.G.; Demchuk, A.M.; Martins, S.O.; Mocco, J.; Ribo, M.; Jadhav, A.P.; Ortega-Gutierrez, S.; Hill, M.D.; et al. Thrombectomy for anterior circulation stroke beyond 6 h from time last known well (AURORA): A systematic review and individual patient data meta-analysis. Lancet 2022, 399, 249–258. [Google Scholar] [CrossRef]
- Goyal, N.; Tsivgoulis, G.; Malhotra, K.; Ishfaq, M.F.; Pandhi, A.; Frohler, M.T.; Spiotta, A.M.; Anadani, M.; Psychogios, M.; Maus, V.; et al. Medical Management vs Mechanical Thrombectomy for Mild Strokes: An International Multicenter Study and Systematic Review and Meta-analysis. JAMA Neurol. 2020, 77, 16–24. [Google Scholar] [CrossRef]
- Nagel, S.; Bouslama, M.; Krause, L.U.; Kupper, C.; Messer, M.; Petersen, M.; Lowens, S.; Herzberg, M.; Ringleb, P.A.; Mohlenbruch, M.A.; et al. Mechanical Thrombectomy in Patients with Milder Strokes and Large Vessel Occlusions. Stroke 2018, 49, 2391–2397. [Google Scholar] [CrossRef]
- Saito, T.; Itabashi, R.; Yazawa, Y.; Uchida, K.; Yamagami, H.; Sakai, N.; Morimoto, T.; Yoshimura, S. Clinical Outcome of Patients with Large Vessel Occlusion and Low National Institutes of Health Stroke Scale Scores: Subanalysis of the RESCUE-Japan Registry 2. Stroke 2020, 51, 1458–1463. [Google Scholar] [CrossRef]
- Qin, B.; Zhang, Y.; Liang, S.; Liang, H.; Tang, S.; Liang, Z. Endovascular treatment versus medical management for mild stroke with acute anterior circulation large vessel occlusion: A meta-analysis. J. Neurointerv. Surg. 2023, 15, e475–e483. [Google Scholar] [CrossRef] [PubMed]
- Safouris, A.; Palaiodimou, L.; Nardai, S.; Kargiotis, O.; Magoufis, G.; Psychogios, K.; Matusevicius, M.; Feil, K.; Ahmed, N.; Kellert, L.; et al. Medical Management Versus Endovascular Treatment for Large-Vessel Occlusion Anterior Circulation Stroke with Low NIHSS. Stroke 2023, 54, 2265–2275. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Saver, J.L.; Ovbiagele, B.; Tang, S.C.; Lee, M.; Liebeskind, D.S. Effects of endovascular therapy for mild stroke due to proximal or M2 occlusions: Meta-analysis. J. Neurointerv. Surg. 2023, 15, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Haussen, D.C.; Bouslama, M.; Grossberg, J.A.; Anderson, A.; Belagage, S.; Frankel, M.; Bianchi, N.; Rebello, L.C.; Nogueira, R.G. Too good to intervene? Thrombectomy for large vessel occlusion strokes with minimal symptoms: An intention-to-treat analysis. J. Neurointerv. Surg. 2017, 9, 917–921. [Google Scholar] [CrossRef]
- Haussen, D.C.; Lima, F.O.; Bouslama, M.; Grossberg, J.A.; Silva, G.S.; Lev, M.H.; Furie, K.; Koroshetz, W.; Frankel, M.R.; Nogueira, R.G. Thrombectomy versus medical management for large vessel occlusion strokes with minimal symptoms: An analysis from STOPStroke and GESTOR cohorts. J. Neurointerv. Surg. 2018, 10, 325–329. [Google Scholar] [CrossRef]
- Heldner, M.R.; Chaloulos-Iakovidis, P.; Panos, L.; Volbers, B.; Kaesmacher, J.; Dobrocky, T.; Mordasini, P.; El-Koussy, M.; Gralla, J.; Arnold, M.; et al. Outcome of patients with large vessel occlusion in the anterior circulation and low NIHSS score. J. Neurol. 2020, 267, 1651–1662. [Google Scholar] [CrossRef]
- Shang, X.J.; Shi, Z.H.; He, C.F.; Zhang, S.; Bai, Y.J.; Guo, Y.T.; Sun, B.; Li, S.; Wang, H.M.; Zhou, Z.M.; et al. Efficacy and safety of endovascular thrombectomy in mild ischemic stroke: Results from a retrospective study and meta-analysis of previous trials. BMC Neurol. 2019, 19, 150. [Google Scholar] [CrossRef]
- Kim, B.J.; Menon, B.K.; Yoo, J.; Han, J.H.; Kim, B.J.; Kim, C.K.; Kim, J.G.; Kim, J.T.; Park, H.; Baik, S.H.; et al. Effectiveness and safety of EVT in patients with acute LVO and low NIHSS. Front. Neurol. 2022, 13, 955725. [Google Scholar] [CrossRef]
- Beume, L.A.; Hieber, M.; Kaller, C.P.; Nitschke, K.; Bardutzky, J.; Urbach, H.; Weiller, C.; Rijntjes, M. Large Vessel Occlusion in Acute Stroke. Stroke 2018, 49, 2323–2329. [Google Scholar] [CrossRef]
- Menon, B.K.; d’Esterre, C.D.; Qazi, E.M.; Almekhlafi, M.; Hahn, L.; Demchuk, A.M.; Goyal, M. Multiphase CT Angiography: A New Tool for the Imaging Triage of Patients with Acute Ischemic Stroke. Radiology 2015, 275, 510–520. [Google Scholar] [CrossRef]
- van der Hoeven, E.J.; McVerry, F.; Vos, J.A.; Algra, A.; Puetz, V.; Kappelle, L.J.; Schonewille, W.J.; BASICS Registry Investigators. Collateral flow predicts outcome after basilar artery occlusion: The posterior circulation collateral score. Int. J. Stroke 2016, 11, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Park, T.H.; Lee, J.K.; Park, M.S.; Park, S.S.; Hong, K.S.; Ryu, W.S.; Kim, D.E.; Park, M.S.; Choi, K.H.; Kim, J.T.; et al. Neurologic deterioration in patients with acute ischemic stroke or transient ischemic attack. Neurology 2020, 95, e2178–e2191. [Google Scholar] [CrossRef] [PubMed]
- Yaghi, S.; Willey, J.Z.; Cucchiara, B.; Goldstein, J.N.; Gonzales, N.R.; Khatri, P.; Kim, L.J.; Mayer, S.A.; Sheth, K.N.; Schwamm, L.H.; et al. Treatment and Outcome of Hemorrhagic Transformation After Intravenous Alteplase in Acute Ischemic Stroke: A Scientific Statement for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2017, 48, e343–e361. [Google Scholar] [CrossRef] [PubMed]
- Renu, A.; Millan, M.; San Roman, L.; Blasco, J.; Marti-Fabregas, J.; Terceno, M.; Amaro, S.; Serena, J.; Urra, X.; Laredo, C.; et al. Effect of Intra-arterial Alteplase vs Placebo Following Successful Thrombectomy on Functional Outcomes in Patients With Large Vessel Occlusion Acute Ischemic Stroke: The CHOICE Randomized Clinical Trial. JAMA 2022, 327, 826–835. [Google Scholar] [CrossRef]
- Kaesmacher, J.; Ospel, J.M.; Meinel, T.R.; Boulouis, G.; Goyal, M.; Campbell, B.C.V.; Fiehler, J.; Gralla, J.; Fischer, U. Thrombolysis in Cerebral Infarction 2b Reperfusions: To Treat or to Stop? Stroke 2020, 51, 3461–3471. [Google Scholar] [CrossRef]
- Jehkonen, M.; Ahonen, J.P.; Dastidar, P.; Koivisto, A.M.; Laippala, P.; Vilkki, J.; Molnar, G. Visual neglect as a predictor of functional outcome one year after stroke. Acta Neurol. Scand. 2000, 101, 195–201. [Google Scholar] [CrossRef]
- Sand, K.M.; Thomassen, L.; Naess, H.; Rodahl, E.; Hoff, J.M. Diagnosis and rehabilitation of visual field defects in stroke patients: A retrospective audit. Cerebrovasc. Dis. Extra 2012, 2, 17–23. [Google Scholar] [CrossRef]
- Writing Group for the BASILAR Group; Zi, W.; Qiu, Z.; Wu, D.; Li, F.; Liu, H.; Liu, W.; Huang, W.; Shi, Z.; Bai, Y.; et al. Assessment of Endovascular Treatment for Acute Basilar Artery Occlusion via a Nationwide Prospective Registry. JAMA Neurol. 2020, 77, 561–573. [Google Scholar]
- Liu, X.; Dai, Q.; Ye, R.; Zi, W.; Liu, Y.; Wang, H.; Zhu, W.; Ma, M.; Yin, Q.; Li, M.; et al. Endovascular treatment versus standard medical treatment for vertebrobasilar artery occlusion (BEST): An open-label, randomised controlled trial. Lancet Neurol. 2020, 19, 115–122. [Google Scholar] [CrossRef]
- Langezaal, L.C.M.; van der Hoeven, E.; Mont’Alverne, F.J.A.; de Carvalho, J.J.F.; Lima, F.O.; Dippel, D.W.J.; van der Lugt, A.; Lo, R.T.H.; Boiten, J.; Lycklama, A.N.G.J.; et al. Endovascular Therapy for Stroke Due to Basilar-Artery Occlusion. N. Engl. J. Med. 2021, 384, 1910–1920. [Google Scholar] [CrossRef]
- Wang, F.; Wang, J.; He, Q.; Wang, L.; Cao, Y.; Zhang, H.; Xu, Z. Mechanical Thrombectomy for Posterior Circulation Occlusion: A Comparison of Outcomes with the Anterior Circulation Occlusion—A Meta-Analysis. J. Atheroscler. Thromb. 2020, 27, 1325–1339. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, R.G.; Doheim, M.F.; Al-Bayati, A.R.; Lee, J.S.; Haussen, D.C.; Mohammaden, M.; Lang, M.; Starr, M.; Rocha, M.; da Camara, C.P.; et al. Distal Medium Vessel Occlusion Strokes: Understanding the Present and Paving the Way for a Better Future. J. Stroke 2024, 26, 190–202. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, M.; Raz, E.; Nossek, E.; Chancellor, B.; Ishida, K.; Nelson, P.K. Neuroanatomy of the middle cerebral artery: Implications for thrombectomy. J. Neurointerv. Surg. 2020, 12, 768–773. [Google Scholar] [CrossRef] [PubMed]




| BMT (n = 196) | EVT (n = 95) | p-Value | |
|---|---|---|---|
| Age, year (SD) | 68.2 (13.8) | 65.3 (14.1) | 0.60 |
| Male, n (%) | 115 (58.7) | 64 (67.4) | 0.16 |
| NIHSS, (IQR) | 4 (2–5) | 4 (2–5) | 0.70 |
| Onset to arrival, hour (IQR) | 3.3 (2.2–6.3) | 0.7 (0.5–0.9) | <0.001 |
| Stroke mechanism, n (%) | 0.93 | ||
| LAA | 71 (36.2) | 36 (37.9) | |
| CE | 70 (35.7) | 34 (35.8) | |
| others | 55 (28.1) | 25 (26.3) | |
| Prior stroke, n (%) | 38 (19.4) | 16 (16.8) | 0.63 |
| Hypertension, n (%) | 127 (64.8) | 54 (56.8) | 0.20 |
| Diabetes mellitus, n (%) | 66 (33.7) | 28 (29.5) | 0.51 |
| Hyperlipidemina, n (%) | 41 (20.9) | 19 (20.0) | 0.88 |
| Smoking, n (%) | 49 (25.0) | 21 (22.1) | 0.66 |
| Atrial fibrillation, n (%) | 72 (36.7) | 33 (34.7 | 0.80 |
| Prior antithrombotics, n (%) | 70 (35.7) | 33 (34.7) | 0.90 |
| Prior statin, n (%) | 51 (26.0) | 26 (27.4) | 0.89 |
| Lesion location, n (%) | 0.17 | ||
| Anterior | 120 (61.2) | 68 (71.6) | |
| Posterior | 35 (17.9) | 15 (15.8) | |
| Multiple | 41 (20.9) | 12 (12.6) | |
| Collateral status, n (%) | 0.32 | ||
| poor | 6 (3.1) | 2 (2.1) | |
| intermediate | 25 (12.8) | 7 (7.4) | |
| good | 165 (84.2) | 86 (90.5) | |
| Cortical signs, n (%) | 0.29 | ||
| Aphasia | 87 (44.4) | 55 (57.9) | |
| Neglect | 61 (31.1) | 24 (25.3) | |
| GP | 13 (6.6) | 4 (4.2) | |
| VFD | 21 (10.7) | 8 (8.4) | |
| 2 or more | 14 (7.1) | 4 (4.2) | |
| ASPECTS, score (IQR) | 8 (8–9) | 6 (9–10) | <0.001 |
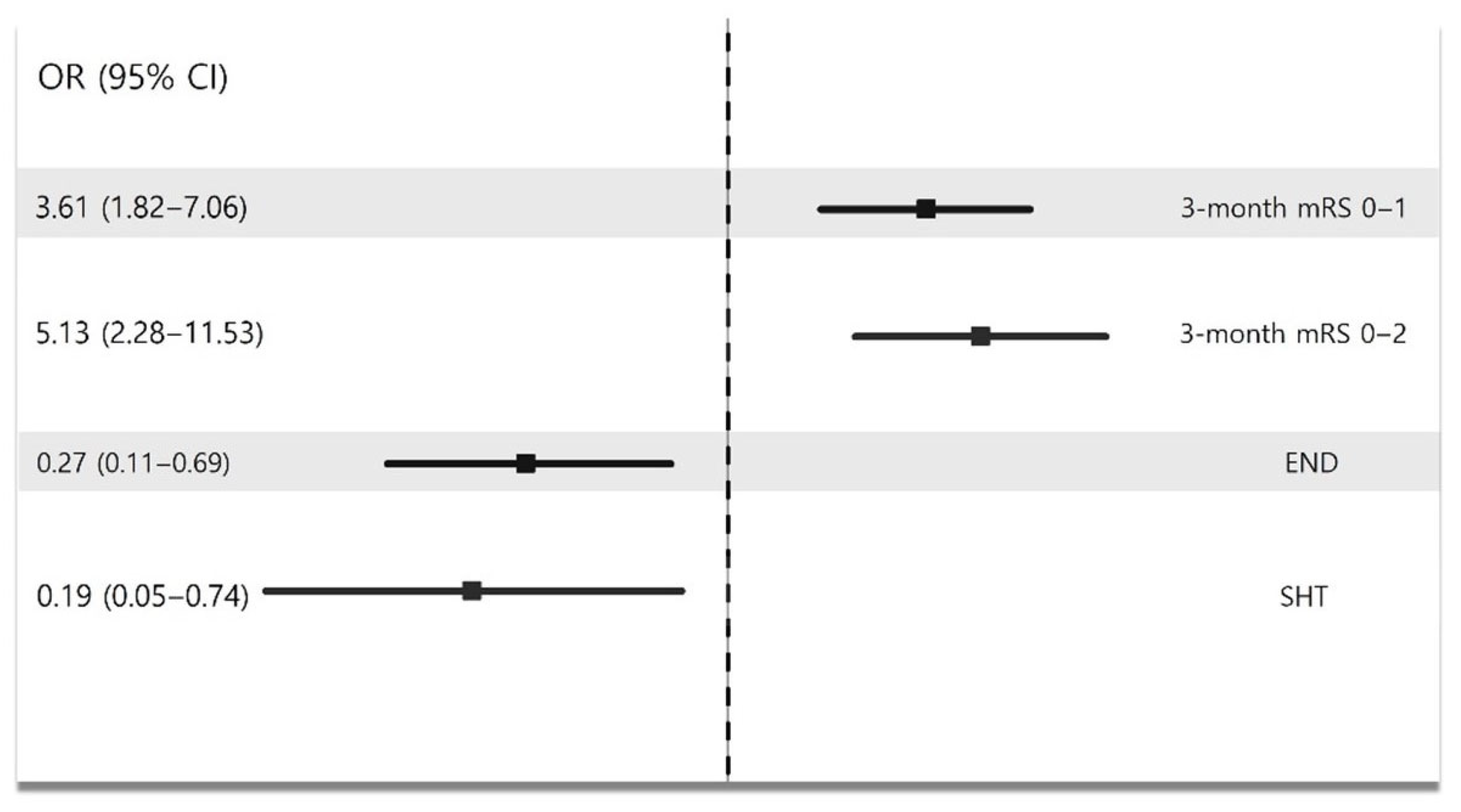
| 3-Month mRS 0–1 | 3-Month mRS 0–2 | END | SHT | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| BMT without IVT | reference | reference | reference | reference | ||||||||
| IVT only | 2.13 | 0.83–5.47 | 0.12 | 1.57 | 0.56–4.35 | 0.39 | 2.81 | 0.91–8.68 | 0.07 | 8.48 | 1.51–47.54 | 0.02 |
| EVT | 7.53 | 2.37–23.99 | 0.001 | 8.13 | 2.14–30.97 | 0.002 | 0.75 | 0.18–3.14 | 0.70 | 1.59 | 0.19–13.56 | 0.67 |
| Age | 0.97 | 0.95–0.99 | 0.004 | 0.97 | 0.94–0.99 | 0.01 | 1.02 | 0.99–1.04 | 0.25 | 1.003 | 0.97–1.04 | 0.84 |
| Male | 0.63 | 0.37–1.09 | 0.10 | 1.42 | 0.78–2.58 | 0.25 | 1.47 | 0.74–2.91 | 0.27 | 0.77 | 0.32–1.56 | 0.57 |
| Stroke mechanism | ||||||||||||
| others | reference | reference | reference | reference | ||||||||
| CE | 0.72 | 0.38–1.37 | 0.32 | 0.63 | 0.33–1.23 | 0.18 | 0.79 | 0.36–1.73 | 0.56 | 0.37 | 0.13–1.05 | 0.06 |
| LAA | 1.25 | 0.65–2.41 | 0.50 | 4.24 | 1.93–9.33 | <0.001 | 0.79 | 0.36–1.74 | 0.55 | 0.43 | 0.16–1.17 | 0.09 |
| Initial NIHSS | 1.11 | 0.96–1.30 | 0.17 | 1.01 | 0.85–1.19 | 0.93 | 1.08 | 0.89–1.31 | 0.45 | 1.09 | 0.85–1.40 | 0.51 |
| Collateral status | ||||||||||||
| poor | reference | reference | reference | refernce | ||||||||
| intermediate | 1.41 | 0.22–9.02 | 0.72 | 0.43 | 0.07–2.64 | 0.36 | 4.40 | 0.43–44.97 | 0.21 | 2.45 | 0.22–27.33 | 0.47 |
| good | 6.38 | 1.4–38.99 | 0.045 | 3.67 | 0.58–23.02 | 0.17 | 1.89 | 0.18–19.87 | 0.60 | 0.86 | 0.07–10.83 | 0.91 |
| ASPECTS | 0.46 | 0.29–0.75 | 0.002 | 0.45 | 0.26–0.77 | 0.004 | 1.29 | 0.72–2.33 | 0.39 | 1.31 | 0.58–2.96 | 0.51 |
| Interval from onset to arrival | 1.01 | 0.86–1.19 | 0.92 | 1.01 | 0.85–1.20 | 0.92 | 1.16 | 0.96–1.39 | 0.12 | 1.29 | 1.00–1.67 | 0.048 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, C.; Oh, S.J.; Lee, J.J.; Sohn, J.-H.; Sung, J.H.; Kim, Y.; Lee, M.; Oh, M.S.; Yu, K.-H.; Mo, H.J.; et al. Effectiveness and Safety of Endovascular Treatment in Large Vessel Occlusion Stroke with an NIHSS Score of ≤5 Exhibiting Predominant Cortical Signs. Biomedicines 2025, 13, 1700. https://doi.org/10.3390/biomedicines13071700
Kim C, Oh SJ, Lee JJ, Sohn J-H, Sung JH, Kim Y, Lee M, Oh MS, Yu K-H, Mo HJ, et al. Effectiveness and Safety of Endovascular Treatment in Large Vessel Occlusion Stroke with an NIHSS Score of ≤5 Exhibiting Predominant Cortical Signs. Biomedicines. 2025; 13(7):1700. https://doi.org/10.3390/biomedicines13071700
Chicago/Turabian StyleKim, Chulho, Seung Joon Oh, Jae Jun Lee, Jong-Hee Sohn, Joo Hye Sung, Yerim Kim, Minwoo Lee, Mi Sun Oh, Kyung-Ho Yu, Hee Jung Mo, and et al. 2025. "Effectiveness and Safety of Endovascular Treatment in Large Vessel Occlusion Stroke with an NIHSS Score of ≤5 Exhibiting Predominant Cortical Signs" Biomedicines 13, no. 7: 1700. https://doi.org/10.3390/biomedicines13071700
APA StyleKim, C., Oh, S. J., Lee, J. J., Sohn, J.-H., Sung, J. H., Kim, Y., Lee, M., Oh, M. S., Yu, K.-H., Mo, H. J., & Lee, S.-H. (2025). Effectiveness and Safety of Endovascular Treatment in Large Vessel Occlusion Stroke with an NIHSS Score of ≤5 Exhibiting Predominant Cortical Signs. Biomedicines, 13(7), 1700. https://doi.org/10.3390/biomedicines13071700






