Targeting Resistance Pathways in Breast Cancer Through Precision Oncology: Nanotechnology and Immune Modulation Approaches
Abstract
1. Introduction
2. Advances in Molecular Targeted Therapy for BC
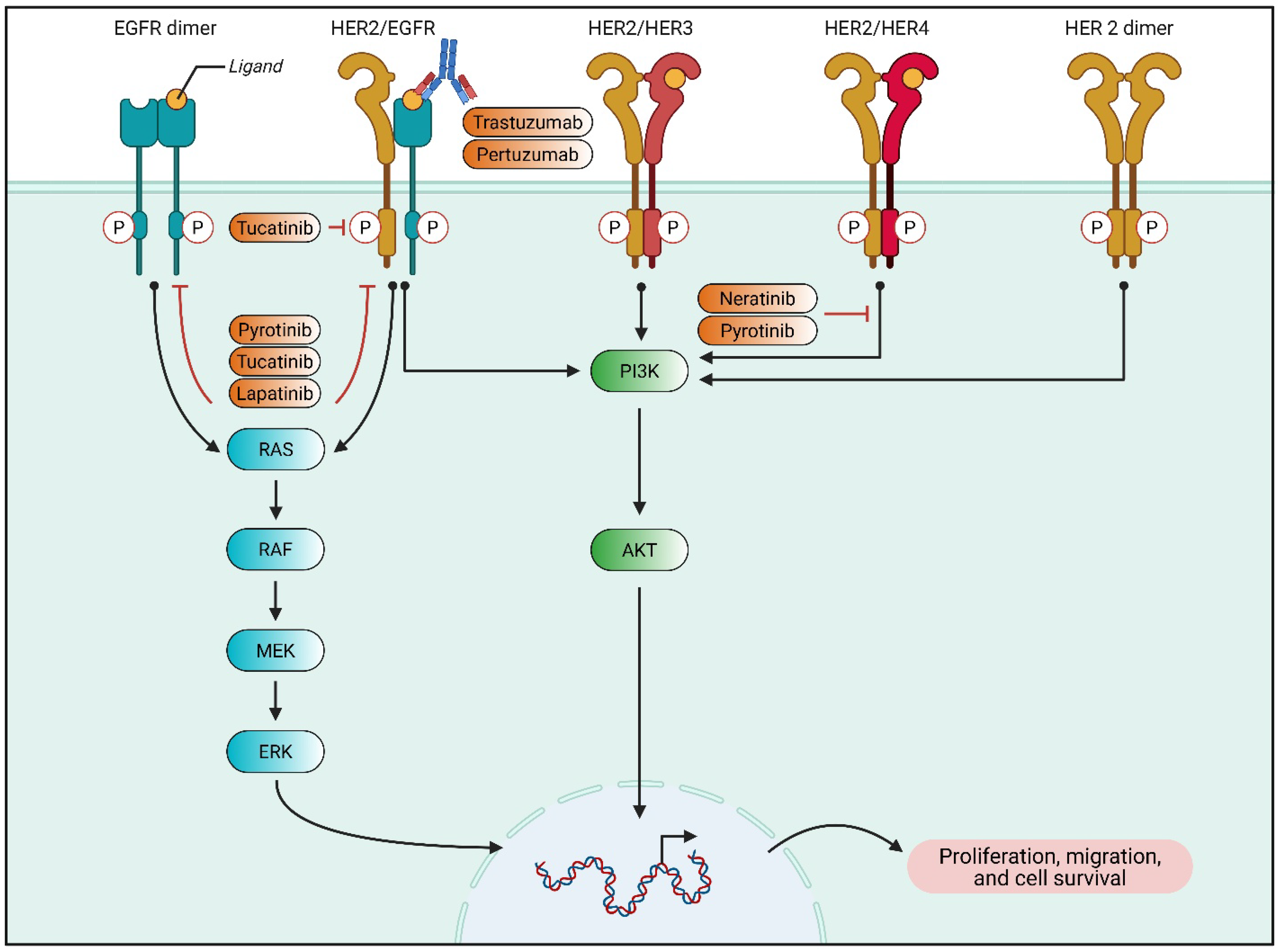
2.1. Molecular Profiling and Targeted Therapy in BC
2.2. HR+/HER2− BC and the Challenge of Resistance
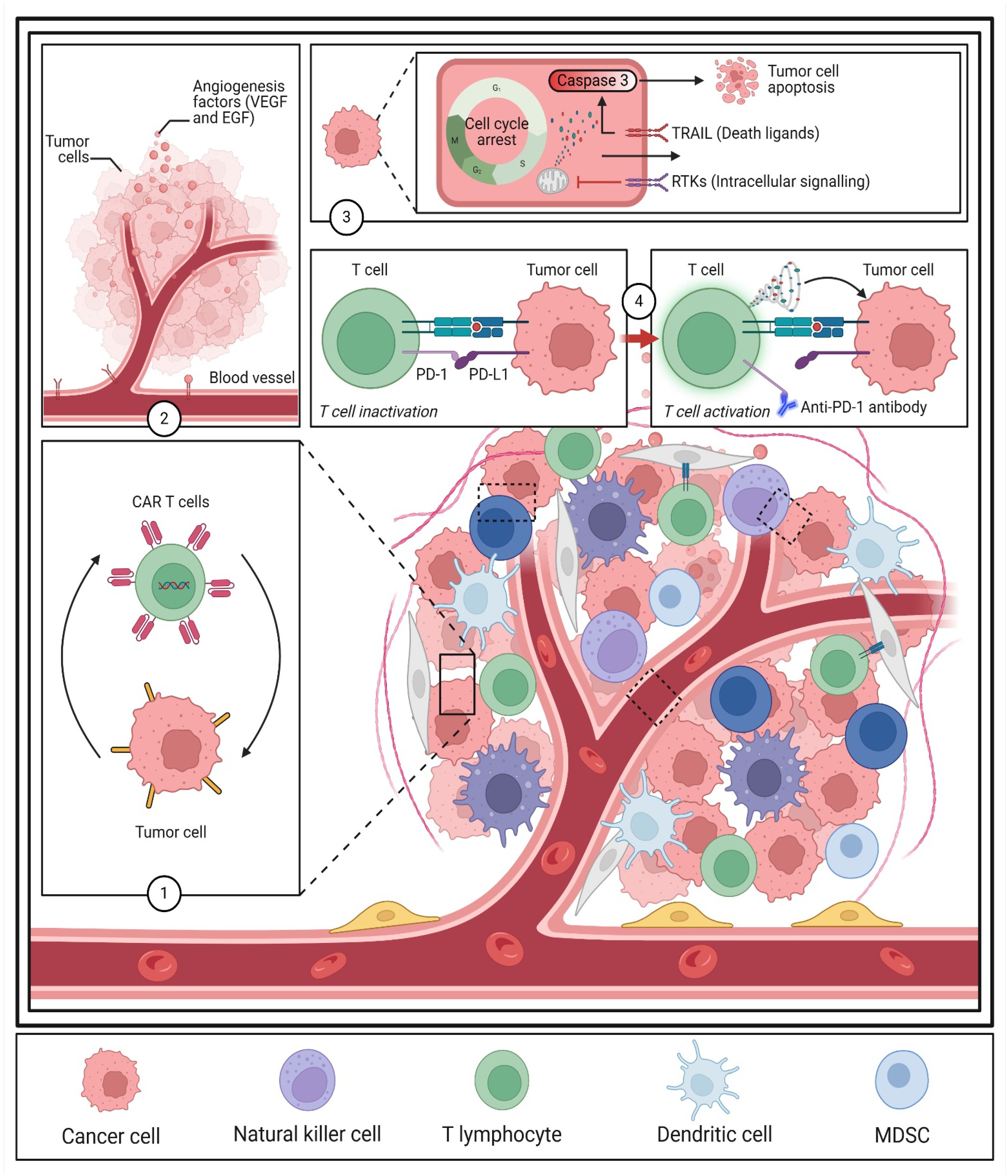
2.3. TME and Immune Modulation in Targeted Therapy
2.4. Emerging Strategies for Drug Delivery
3. Advances in Targeted Therapy for BC
3.1. ADCs: Precision in Targeted Therapy
3.2. Nanotechnology in Drug Delivery
3.3. Immunotherapy and TME Modulation
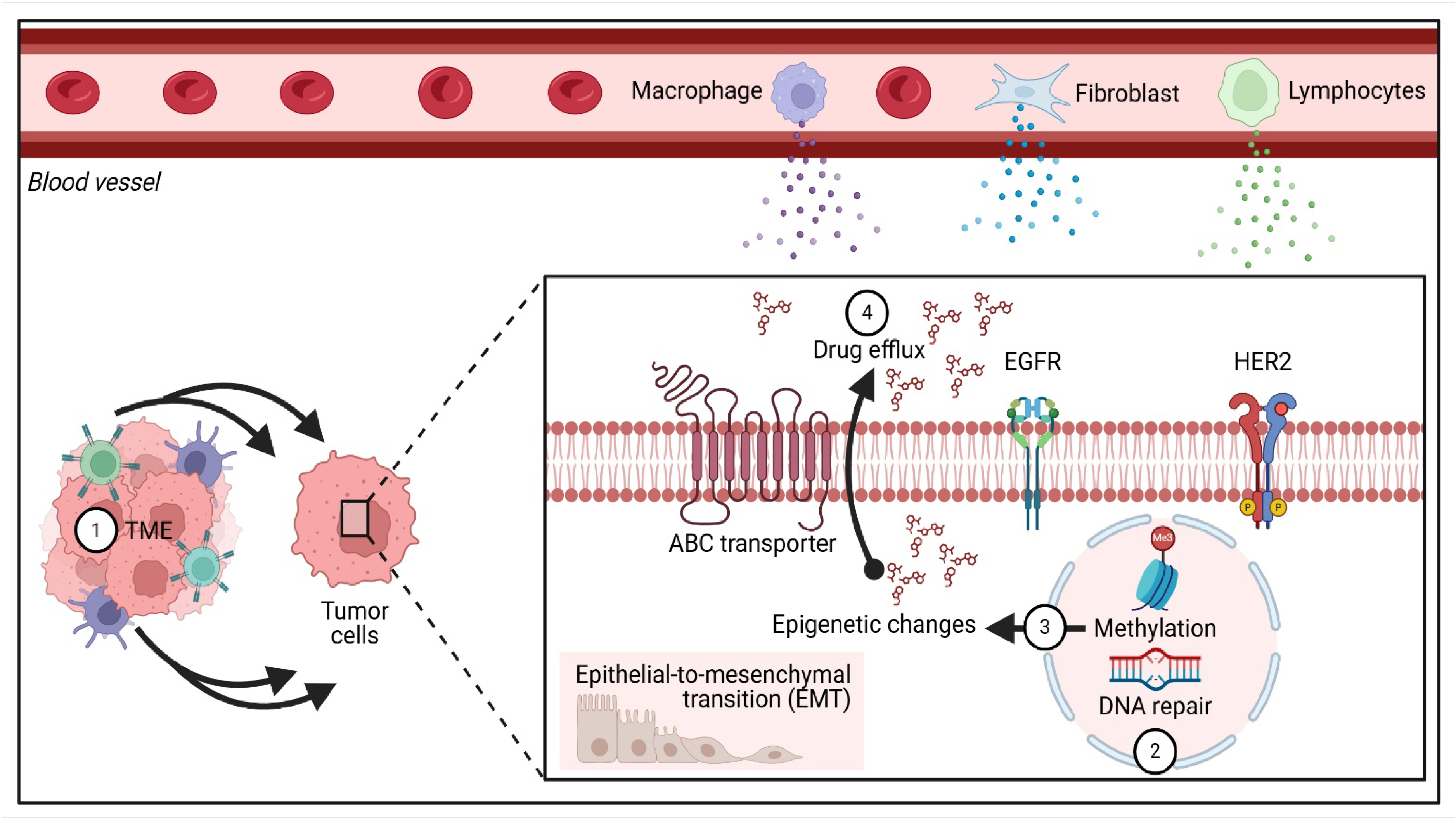
3.4. Addressing Drug Resistance in Targeted Therapy
4. Overcome Drug Resistance in BC
4.1. Drug Resistance in BC
4.1.1. Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL) Resistance
4.1.2. Role of Autophagy in Chemoresistance
4.1.3. Iron Modulation and Cancer Stem-like Phenotypes
4.1.4. Cancer Stem Cell (CSC)-Mediated Resistance
4.1.5. Wnt/β-Catenin Signaling and OTULIN Phosphorylation
4.1.6. Fibroblast-Mediated Resistance in HER2+ BC
4.1.7. Single-Cell Transcriptomics and Drug Resistance Acquisition
4.2. Strategies to Overcome Drug Resistance
4.2.1. Nanotechnology-Based Drug Delivery
4.2.2. Targeting Cancer Stem Cells (CSCs)
4.2.3. Targeting Apoptotic and Survival Pathways
4.2.4. Overcoming Metabolic Adaptations
5. Therapeutic Strategies
5.1. Targeted Approaches for Overcoming Drug Resistance
5.2. Emerging Therapeutic Strategies
5.2.1. Nanotechnology-Based Drug Delivery
5.2.2. Photoimmunotherapy and Macrophage Reprogramming
5.2.3. Targeting Exosomal miRNAs
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Brantley, K.D.; Rosenberg, S.M.; Collins, L.C.; Ruddy, K.J.; Tamimi, R.M.; Schapira, L.; Borges, V.F.; Warner, E.; Come, S.E.; Zheng, Y.; et al. Second Primary Breast Cancer in Young Breast Cancer Survivors. JAMA Oncol. 2024, 10, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Giaquinto, A.N.; Sung, H.; Newman, L.A.; Freedman, R.A.; Smith, R.A.; Star, J.; Jemal, A.; Siegel, R.L. Breast cancer statistics 2024. CA A Cancer J. Clin. 2024, 74, 477–495. [Google Scholar] [CrossRef] [PubMed]
- Del Vecchio, V.; Rehman, A.; Panda, S.K.; Torsiello, M.; Marigliano, M.; Nicoletti, M.M.; Ferraro, G.A.; De Falco, V.; Lappano, R.; Lieto, E.; et al. Mitochondrial transfer from Adipose stem cells to breast cancer cells drives multi-drug resistance. J. Exp. Clin. Cancer Res. 2024, 43, 166. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Cao, S.; Long, X.; Xiao, L.; Yang, L.; Zhang, P.; Li, L.; Chen, F.; Lei, T.; Gao, H.; et al. DNAJC12 causes breast cancer chemotherapy resistance by repressing doxorubicin-induced ferroptosis and apoptosis via activation of AKT. Redox Biol. 2024, 70, 103035. [Google Scholar] [CrossRef]
- O’Connell, B.C.; Hubbard, C.; Zizlsperger, N.; Fitzgerald, D.; Kutok, J.L.; Varner, J.; Ilaria, R., Jr.; Cobleigh, M.A.; Juric, D.; Tkaczuk, K.H.R.; et al. Eganelisib combined with immune checkpoint inhibitor therapy and chemotherapy in frontline metastatic triple-negative breast cancer triggers macrophage reprogramming, immune activation and extracellular matrix reorganization in the tumor microenvironment. J. Immunother. Cancer 2024, 12, e009160. [Google Scholar] [CrossRef]
- Tran, H.C.M.; Mbemba, E.; Mourot, N.; Faltas, B.; Rousseau, A.; Lefkou, E.; Sabbah, M.; van Dreden, P.; Gerotziafas, G. The procoagulant signature of cancer cells drives fibrin network formation in tumor microenvironment and impacts its quality. Implications in cancer cell migration and the resistance to anticancer agents. Thromb. Res. 2024, 238, 172–183. [Google Scholar] [CrossRef]
- Abbasi, A.B.; Wu, V.; Lang, J.E.; Esserman, L.J. Precision Oncology in Breast Cancer Surgery. Surg. Oncol. Clin. N. Am. 2024, 33, 293–310. [Google Scholar] [CrossRef]
- Pensabene, M.; Calabrese, A.; von Arx, C.; Caputo, R.; De Laurentiis, M. Cancer genetic counselling for hereditary breast cancer in the era of precision oncology. Cancer Treat. Rev. 2024, 125, 102702. [Google Scholar] [CrossRef]
- Pu, H.; Huang, J.; Gui, B.; Chen, Y.; Guo, Y.; Lian, Y.; Pan, J.; Hu, Y.; Jiang, N.; Deng, Q.; et al. Ultrasound-Responsive Nanobubbles for Breast Cancer: Synergistic Sonodynamic, Chemotherapy, and Immune Activation through the cGAS-STING Pathway. ACS Appl. Mater. Interfaces 2025, 17, 19317–19334. [Google Scholar] [CrossRef]
- Qian, X.; Shi, R.; Chen, J.; Wang, Y.; Han, X.; Sun, Y.; Ling, C.; Wang, G.; Xu, A.W.; Pan, Y. The single-atom iron nanozyme mimicking peroxidase remodels energy metabolism and tumor immune landscape for synergistic chemodynamic therapy and photothermal therapy of triple-negative breast cancer. Front. Bioeng. Biotechnol. 2022, 10, 1026761. [Google Scholar] [CrossRef]
- Ming, H.; Li, B.; Tian, H.; Zhou, L.; Jiang, J.; Zhang, T.; Qiao, L.; Wu, P.; Nice, E.C.; Zhang, W.; et al. A minimalist and robust chemo-photothermal nanoplatform capable of augmenting autophagy-modulated immune response against breast cancer. Mater. Today Bio 2022, 15, 100289. [Google Scholar] [CrossRef] [PubMed]
- Mimansa; Zafar, M.A.; Verma, D.K.; Das, R.; Agrewala, J.N.; Shanavas, A. Shielding against breast tumor relapse with an autologous chemo-photo-immune active Nano-Micro-Sera based fibrin implant. Nanoscale 2024, 16, 14006–14019. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhao, M.; Wu, Z.; Chen, C.; Zhang, Y.; Wang, L.; Guo, Q.; Wang, Q.; Liang, S.; Hu, S.; et al. Nano-ultrasonic Contrast Agent for Chemoimmunotherapy of Breast Cancer by Immune Metabolism Reprogramming and Tumor Autophagy. ACS Nano 2022, 16, 3417–3431. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Yang, X.; Li, R.; Shao, L.; Zhao, W.; Hu, X.; Fang, K.; Chai, K.; Shi, S.; Dong, C. Immunomodulator-Mediated Suppressive Tumor Immune Microenvironment Remodeling Nanoplatform for Enhanced Immuno/Chemo/Photothermal Combination Therapy of Triple Negative Breast Cancer. ACS Appl. Mater. Interfaces 2023, 15, 53318–53332. [Google Scholar] [CrossRef]
- Falcone, R.; Lombardi, P.; Filetti, M.; Fabi, A.; Altamura, V.; Scambia, G.; Daniele, G. Molecular Profile and Matched Targeted Therapy for Advanced Breast Cancer Patients. Curr. Oncol. 2023, 30, 2501–2509. [Google Scholar] [CrossRef]
- Jiao, X.D.; Qin, B.D.; Wang, Z.; Liu, K.; Wu, Y.; Ling, Y.; Qin, W.X.; Wang, M.M.; Yuan, L.Y.; Barreto, S.G.; et al. Targeted therapy for intractable cancer on the basis of molecular profiles: An open-label, phase II basket trial (Long March Pathway). Front. Oncol. 2023, 13, 860711. [Google Scholar] [CrossRef]
- Yi, Z.; Rong, G.; Guan, Y.; Li, J.; Chang, L.; Li, H.; Liu, B.; Wang, W.; Guan, X.; Ouyang, Q.; et al. Molecular landscape and efficacy of HER2-targeted therapy in patients with HER2-mutated metastatic breast cancer. NPJ Breast Cancer 2020, 6, 59. [Google Scholar] [CrossRef]
- Liu, B.; Yi, Z.; Guan, Y.; Ouyang, Q.; Li, C.; Guan, X.; Lv, D.; Li, L.; Zhai, J.; Qian, H.; et al. Molecular landscape of TP53 mutations in breast cancer and their utility for predicting the response to HER-targeted therapy in HER2 amplification-positive and HER2 mutation-positive amplification-negative patients. Cancer Med. 2022, 11, 2767–2778. [Google Scholar] [CrossRef]
- Nakano, S.; Imawari, Y.; Mibu, A.; Kato, S.; Yamaguchi, S.; Otsuka, M.; Sano, M. Molecular Targeted Therapy for Hormone Receptor-Positive, Human Epidermal Growth Factor 2-Negative Metastatic Breast Cancer in Clinical Practice. J. Nippon. Med. Sch. 2022, 89, 88–94. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, Z.; He, D.; Zhu, Y.; Chen, X.; Cao, K. Identification of novel subtypes based on ssGSEA in immune-related prognostic signature for tongue squamous cell carcinoma. Cancer Med. 2021, 10, 8693–8707. [Google Scholar] [CrossRef]
- Dong, X.; Sun, R.; Wang, J.; Yu, S.; Cui, J.; Guo, Z.; Pan, X.; Sun, J.; Yang, J.; Pan, L.L. Corrigendum to “Glutathione S-transferases P1-mediated interleukin-6 in tumor-associated macrophages augments drug-resistance in MCF-7 breast cancer”. Biochem. Pharmacol. 2020, 182, 114289, Erratum in Biochem. Pharmacol. 2021, 185, 114289. [Google Scholar] [CrossRef]
- Hu, Q.; He, C.; Lu, Z.; He, Y.; Xie, H.; Li, J.; Fu, Z.; Guo, B. Engineering of small molecular organic nanoparticles for mitochondria-targeted mild photothermal therapy of malignant breast cancers. Biomater. Sci. 2022, 10, 6013–6023. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, Y.; Xu, J.; Luo, H.; Zhu, Y.; Zeng, X.; Dong, F.; Wei, Z.; Yan, F.; Zheng, H. Ultrasound molecular imaging-guided tumor gene therapy through dual-targeted cationic microbubbles. Biomater. Sci. 2021, 9, 2454–2466. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhang, Z.; Zhang, H.; Guo, H.; Tan, C.; Xu, N.; Tan, Y.; Jiang, Y. Aptamer Proteolysis-Targeting Chimeras (PROTACs): A Novel Strategy to Combat Drug Resistance in Estrogen Receptor α-Positive Breast Cancer. ACS Pharmacol. Transl. Sci. 2024, 7, 3945–3954. [Google Scholar] [CrossRef]
- Jhaveri, K.L.; Lim, E.; Jeselsohn, R.; Ma, C.X.; Hamilton, E.P.; Osborne, C.; Bhave, M.; Kaufman, P.A.; Beck, J.T.; Manso Sanchez, L.; et al. Imlunestrant, an Oral Selective Estrogen Receptor Degrader, as Monotherapy and in Combination With Targeted Therapy in Estrogen Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: Phase Ia/Ib EMBER Study. J. Clin. Oncol. 2024, 42, 4173–4186. [Google Scholar] [CrossRef]
- Montazeri Aliabadi, H. Molecular Targets for Breast Cancer Therapy. Biomolecules 2024, 14, 1219. [Google Scholar] [CrossRef]
- Amjad, M.T.; Chidharla, A.; Kasi, A. Cancer Chemotherapy. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Cui, G.; Deng, S.; Zhang, B.; Wang, M.; Lin, Z.; Lan, X.; Li, Z.; Yao, G.; Yu, M.; Yan, J. Overcoming the Tumor Collagen Barriers: A Multistage Drug Delivery Strategy for DDR1-Mediated Resistant Colorectal Cancer Therapy. Adv. Sci. 2024, 11, e2402107. [Google Scholar] [CrossRef]
- Wang, S.; Li, J.; Xu, S.; Wang, N.; Pan, B.; Yang, B.; Zheng, Y.; Zhang, J.; Peng, F.; Peng, C.; et al. Baohuoside I chemosensitises breast cancer to paclitaxel by suppressing extracellular vesicle/CXCL1 signal released from apoptotic cells. J. Extracell. Vesicles 2024, 13, e12493. [Google Scholar] [CrossRef]
- Yin, X.; Ke, Y.; Liang, Y.; Zhang, S.; Chen, Z.; Yu, L.; Jiang, M.; Liu, Q.; Gu, X. An Immune-Enhancing Injectable Hydrogel Loaded with Esketamine and DDP Promotes Painless Immunochemotherapy to Inhibit Breast Cancer Growth. Adv. Healthc. Mater. 2024, 13, e2401373. [Google Scholar] [CrossRef]
- Li, Y.W.; Dai, L.J.; Wu, X.R.; Zhao, S.; Xu, Y.Z.; Jin, X.; Xiao, Y.; Wang, Y.; Lin, C.J.; Zhou, Y.F.; et al. Molecular Characterization and Classification of HER2-Positive Breast Cancer Inform Tailored Therapeutic Strategies. Cancer Res. 2024, 84, 3669–3683. [Google Scholar] [CrossRef]
- Wang, K.; Zerdes, I.; Johansson, H.J.; Sarhan, D.; Sun, Y.; Kanellis, D.C.; Sifakis, E.G.; Mezheyeuski, A.; Liu, X.; Loman, N.; et al. Longitudinal molecular profiling elucidates immunometabolism dynamics in breast cancer. Nat. Commun. 2024, 15, 3837. [Google Scholar] [CrossRef] [PubMed]
- Duro-Sánchez, S.; Nadal-Serrano, M.; Lalinde-Gutiérrez, M.; Arenas, E.J.; Morales, C.B.; Morancho, B.; Escorihuela, M.; Pérez-Ramos, S.; Escrivá-de-Romaní, S.; Gandullo-Sánchez, L.; et al. Therapy-Induced Senescence Enhances the Efficacy of HER2-Targeted Antibody-Drug Conjugates in Breast Cancer. Cancer Res. 2022, 82, 4670–4679. [Google Scholar] [CrossRef] [PubMed]
- Cecco, S.; Puligheddu, S.; Fusaroli, M.; Gerratana, L.; Yan, M.; Zamagni, C.; De Ponti, F.; Raschi, E. Emerging Toxicities of Antibody-Drug Conjugates for Breast Cancer: Clinical Prioritization of Adverse Events from the FDA Adverse Event Reporting System. Target Oncol. 2024, 19, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Huang, R.; Chen, R.; Pan, F.; Shen, X.; Li, H.; Rong, Q.; An, X.; Xue, C.; Shi, Y. Optimal Sequential Strategies for Antibody-Drug Conjugate in Metastatic Breast Cancer: Evaluating Efficacy and Cross-Resistance. Oncologist 2024, 29, e957–e966. [Google Scholar] [CrossRef]
- Cortés, J.; Hurvitz, S.A.; Im, S.A.; Iwata, H.; Curigliano, G.; Kim, S.B.; Chiu, J.W.Y.; Pedrini, J.L.; Li, W.; Yonemori, K.; et al. Trastuzumab deruxtecan versus trastuzumab emtansine in HER2-positive metastatic breast cancer: Long-term survival analysis of the DESTINY-Breast03 trial. Nat. Med. 2024, 30, 2208–2215. [Google Scholar] [CrossRef]
- Turner, N.; Saura, C.; Aftimos, P.; van den Tweel, E.; Oesterholt, M.; Koper, N.; Colleoni, M.; Kaczmarek, E.; Punie, K.; Song, X.; et al. Trastuzumab Duocarmazine in Pretreated Human Epidermal Growth Factor Receptor 2-Positive Advanced or Metastatic Breast Cancer: An Open-Label, Randomized, Phase III Trial (TULIP). J. Clin. Oncol. 2025, 43, 513–523. [Google Scholar] [CrossRef]
- Klapp, V.; Buqué, A.; Bloy, N.; Sato, A.; Yamazaki, T.; Zhou, X.K.; Formenti, S.C.; Galluzzi, L.; Petroni, G. Cellular senescence in the response of HR(+) breast cancer to radiotherapy and CDK4/6 inhibitors. J. Transl. Med. 2023, 21, 110. [Google Scholar] [CrossRef]
- Luo, Z.; Lu, L.; Xu, W.; Meng, N.; Wu, S.; Zhou, J.; Xu, Q.; Xie, C.; Liu, Y.; Lu, W. In vivo self-assembled drug nanocrystals for metastatic breast cancer all-stage targeted therapy. J. Control Release 2022, 346, 32–42. [Google Scholar] [CrossRef]
- Chang, J.; Mo, L.; Song, J.; Wang, X.; Liu, H.; Meng, C.; Wu, Y. A pH-responsive mesoporous silica nanoparticle-based drug delivery system for targeted breast cancer therapy. J. Mater. Chem. B 2022, 10, 3375–3385. [Google Scholar] [CrossRef]
- Mariadoss, A.V.A.; Saravanakumar, K.; Sathiyaseelan, A.; Venkatachalam, K.; Wang, M.H. Folic acid functionalized starch encapsulated green synthesized copper oxide nanoparticles for targeted drug delivery in breast cancer therapy. Int. J. Biol. Macromol. 2020, 164, 2073–2084. [Google Scholar] [CrossRef]
- Zoghi, M.; Pourmadadi, M.; Yazdian, F.; Nigjeh, M.N.; Rashedi, H.; Sahraeian, R. Synthesis and characterization of chitosan/carbon quantum dots/Fe2O3 nanocomposite comprising curcumin for targeted drug delivery in breast cancer therapy. Int. J. Biol. Macromol. 2023, 249, 125788. [Google Scholar] [CrossRef] [PubMed]
- Gundamaraju, R.; Papadakis, M. Exosomal miRNAs and breast cancer: A complex theranostics interlink with clinical significance. Biomarkers 2023, 28, 502–518. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, M.; Weng, B.; Mao, H.; Zhao, J. Exosome-based delivery nanoplatforms: Next-generation theranostic platforms for breast cancer. Biomater. Sci. 2022, 10, 1607–1625. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhao, D.; Lu, J.; Li, J.; Dao, G.; Chen, B.; Huang, B.; Pan, X. Multiple roles of nanomaterials along with their based nanotechnologies in the elimination and dissemination of antibiotic resistance. Chem. Eng. J. 2022, 455, 140927. [Google Scholar] [CrossRef]
- Davatgaran Taghipour, Y.; Zarebkohan, A.; Salehi, R.; Rahimi, F.; Torchilin, V.P.; Hamblin, M.R.; Seifalian, A.M. An update on dual targeting strategy for cancer treatment. J. Control Release 2022, 349, 67–96. [Google Scholar] [CrossRef]
- Ranjan, P.; Colin, K.; Dutta, R.; Verma, S.K. Challenges and future scope of exosomes in the treatment of cardiovascular diseases. J. Physiol. 2022, 601, 4873–4893. [Google Scholar] [CrossRef]
- Song, I.W.; Vo, H.H.; Chen, Y.S.; Kahle, M.P.; Johnson, A.; Tsimberidou, A.M. Precision Oncology: Evolving Clinical Trials across Tumor Types. Cancers 2023, 15, 1967. [Google Scholar] [CrossRef]
- Si, Y.; Zhang, Y.; Guan, J.S.; Ngo, H.G.; Totoro, A.; Singh, A.P.; Chen, K.; Xu, Y.; Yang, E.S.; Zhou, L.; et al. Anti-CD47 Monoclonal Antibody-Drug Conjugate: A Targeted Therapy to Treat Triple-Negative Breast Cancers. Vaccines 2021, 9, 882. [Google Scholar] [CrossRef]
- Bai, M.; Sun, C. Determination of Breast Metabolic Phenotypes and Their Associations With Immunotherapy and Drug-Targeted Therapy: Analysis of Single-Cell and Bulk Sequences. Front. Cell Dev. Biol. 2022, 10, 829029. [Google Scholar] [CrossRef]
- Xuhong, J.; Wu, N.; Shi, Q.; Tian, H.; Peng, Z.; Jiang, J.; Zhang, J.; Qi, X. Targeted multimodal synergistic therapy of drug-resistant HER2-positive breast cancer by pyrotinib-ICG self-assembled nanoparticles. Am. J. Cancer Res. 2024, 14, 3976–3993. [Google Scholar] [CrossRef]
- Xia, L.; Ni, C.; Sun, H.; Guo, H.; Huang, H.; Cao, X.; Xia, J.; Shi, X.; Guo, R. Dual drug-loaded metal-phenolic networks for targeted magnetic resonance imaging and synergistic chemo-chemodynamic therapy of breast cancer. J. Mater. Chem. B 2024, 12, 6480–6491. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.J.; Han, Y.Q.; Li, Q.; Mo, H.N.; Li, Y.Q.; Guan, X.W.; Chen, Y.M.; Lin, S.Y.; Xu, B.H.; Li, Q.; et al. A real world study on the relationship between drug resistance of targeted therapy and prognosis of HER-2-positive advanced breast cancer. Chin. J. Oncol. 2022, 44, 360–363. [Google Scholar] [CrossRef]
- Raikwar, S.; Jain, A.; Saraf, S.; Bidla, P.D.; Panda, P.K.; Tiwari, A.; Verma, A.; Jain, S.K. Opportunities in combinational chemo-immunotherapy for breast cancer using nanotechnology: An emerging landscape. Expert Opin. Drug Deliv. 2022, 19, 247–268. [Google Scholar] [CrossRef] [PubMed]
- Subhan, M.A.; Parveen, F.; Shah, H.; Yalamarty, S.S.K.; Ataide, J.A.; Torchilin, V.P. Recent Advances with Precision Medicine Treatment for Breast Cancer including Triple-Negative Sub-Type. Cancers 2023, 15, 2204. [Google Scholar] [CrossRef]
- Aljadani, M.A. Nanotechnology-based Therapeutic Strategies for Breast Cancer. Int. J. Pharm. Investig. 2022, 12, 145–150. [Google Scholar] [CrossRef]
- Davies, E. Nanotechnology and Immunomodulators in Cancer. In Immunomodulators and Human Health; Springer: Berlin/Heidelberg, Germany, 2022; pp. 125–186. [Google Scholar]
- Chen, Z.D.; Chen, X.; Liu, G.; Han, K.; Chen, J.; Wang, J. Editorial: The Application of Nanoengineering in Advanced Drug Delivery and Translational Research. Front. Bioeng. Biotechnol. 2022, 10, 886109. [Google Scholar] [CrossRef]
- Cheng, X.; Xie, Q.; Sun, Y. Advances in nanomaterial-based targeted drug delivery systems. Front. Bioeng. Biotechnol. 2023, 11, 1177151. [Google Scholar] [CrossRef]
- Valerio, T.I.; Furrer, C.; Sadeghipour, N.; Patrock, S.J.X.; Tillery, S.A.; Hoover, A.R.; Liu, K.; Chen, W.R. Immune Modulations of the Tumor Microenvironment in Response to Phototherapy. J. Innov. Opt. Health Sci. 2023, 16, 2330007. [Google Scholar] [CrossRef]
- Amen, T.P.J.; Davronova, N. Modulating undruggable targets to overcome cancer therapy resistance. Drug Resist. Updates 2022, 60, 100788. [Google Scholar] [CrossRef]
- Wang, H. Perspectives for Interdisciplinary Methodological Approaches for Technology Research in Healthcare; IGI Global: New York, NY, USA, 2022. [Google Scholar]
- Xu, G.; Chen, S.; Yang, H.; Feng, X.; Li, F.; Zhao, H.; Sun, L.; Yan, P.; Chen, Y.; Guo, G.; et al. An ER stress and mitochondrial apoptosis Co-inducer for enhanced cancer immunotherapy. Cancer Lett. 2025, 612, 217485. [Google Scholar] [CrossRef]
- He, J.; Fortunati, E.; Liu, D.X.; Li, Y. Pleiotropic Roles of ABC Transporters in Breast Cancer. Int. J. Mol. Sci. 2021, 22, 3199. [Google Scholar] [CrossRef] [PubMed]
- Debaugnies, M.; Rodríguez-Acebes, S.; Blondeau, J.; Parent, M.A.; Zocco, M.; Song, Y.; de Maertelaer, V.; Moers, V.; Latil, M.; Dubois, C.; et al. RHOJ controls EMT-associated resistance to chemotherapy. Nature 2023, 616, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R. DPEITC and MDR1 suppression in breast cancer. Oncol. Rep. 2023, 40, 1250–1265. [Google Scholar]
- Zheng, H.C. The molecular mechanisms of chemoresistance in cancers. Oncotarget 2017, 8, 59950–59964. [Google Scholar] [CrossRef]
- Chow, L.M.C.; Chan, T.H. ATP-binding cassette (ABC) transporter proteins, multidrug resistance, and novel flavonoid dimers as potent, nontoxic, and selective inhibitors. Can. J. Chem. 2022, 100, 85–97. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, J.; Ma, L.; Zhao, Y.; Xu, S.; Shi, J.; Qian, K.; Gu, M.; Tan, H.; Xu, L.; et al. Reversing multi-drug resistance by polymeric metformin to enhance antitumor efficacy of chemotherapy. Int. J. Pharm. 2022, 624, 121931. [Google Scholar] [CrossRef]
- Brindisi, M. Cholesterol metabolism in endocrine therapy resistance. Breast Cancer Res. 2020, 22, 130–144. [Google Scholar]
- Henriques Palma, C.; Kaur, P. Lipid metabolism and endocrine resistance in breast cancer. Nat. Commun. 2022, 15, 199–213. [Google Scholar]
- Wang, B.; Wang, Y.; Wang, X.; Gu, J.; Wu, W.; Wu, H.; Wang, Q.; Zhou, D. Extracellular Vesicles Carrying miR-887-3p Promote Breast Cancer Cell Drug Resistance by Targeting BTBD7 and Activating the Notch1/Hes1 Signaling Pathway. Dis. Markers 2022, 2022, 5762686. [Google Scholar] [CrossRef]
- Kaur, S. Nanoemulsions in paclitaxel drug delivery. Adv. Drug Deliv. Rev. 2022, 35, 550–564. [Google Scholar]
- Gómez Tejeda Zañudo, J. FOXO3 downregulation and PI3K resistance. Cell Syst. 2021, 12, 105–118. [Google Scholar]
- Amioka, Y. Wnt5a and CYP enzymes in endocrine resistance. Mol. Oncol. 2021, 38, 1340–1355. [Google Scholar]
- Geng, W.; Cao, M.; Dong, K.; An, J.; Gao, H. SHOC2 mediates the drug-resistance of triple-negative breast cancer cells to everolimus. Cancer Biol. Ther. 2023, 24, 2206362. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wu, X.; Wang, C.; Liu, R.; Wang, J. Resistance to TRAIL-induced apoptosis in breast cancer: Molecular mechanisms and therapeutic strategies. Front. Oncol. 2021, 11, 650428. [Google Scholar]
- Cheng, Y.; Gao, S.; Wang, W.; Ding, L. Tumor microenvironment-targeted nanoplatforms for sensitizing TRAIL therapy in breast cancer. Biomater. Sci. 2022, 10, 784–797. [Google Scholar]
- Li, N.; Gao, D.; Li, C.; Wang, B.; Li, B.; Bao, B.; Wu, M.; Li, M.; Xing, C. Polymer Nanoparticles Overcome Drug Resistance by a Dual-Targeting Apoptotic Signaling Pathway in Breast Cancer. ACS Appl. Mater. Interfaces 2022, 14, 23117–23128. [Google Scholar] [CrossRef]
- Meng, Y.; Zhou, J.; Liu, X.; Zeng, F.; Wen, T.; Xu, H. CXC chemokine receptor type 4 antagonistic gold nanorods induce specific immune responses and long-term immune memory to combat triple-negative breast cancer. ACS Appl. Mater. Interfaces 2023, 15, 18734–18746. [Google Scholar] [CrossRef]
- Li, T.; Zhang, S.; Chen, F.; Hu, J.; Yuan, S.; Li, C.; Wang, X.; Zhang, W.; Tang, R. Formononetin ameliorates the drug resistance of Taxol resistant triple negative breast cancer by inhibiting autophagy. Am. J. Transl. Res. 2021, 13, 497–514. [Google Scholar]
- Pan, Y. A novel autophagy inhibitor Formononetin sensitizes breast cancer cells to paclitaxel by downregulating long non-coding RNA AFAP1-AS1. Int. J. Mol. Sci. 2023, 24, 9719. [Google Scholar]
- Tan, Y. MiR-199a-3p functions as a tumor suppressor in breast cancer by targeting autophagy-related genes. Cancer Cell Int. 2023, 23, 56. [Google Scholar]
- Jiang, Y.; Xie, D.; Wang, H.; Zuo, L.; Huang, S.; Liu, S.; Zhang, W.; Li, T. Formononetin reverses Taxol resistance in triple-negative breast cancer through targeting the miR-199a-3p/mTOR axis. Genes Dis. 2024, 11, 459–471. [Google Scholar] [CrossRef]
- Zhou, Y. Labile iron pool modulates cancer stem cell-like properties and confers resistance to anti-estrogen therapy in ER+ breast cancer. Nat. Commun. 2021, 12, 4567. [Google Scholar]
- Wang, H. Iron metabolism drives breast cancer stemness and metastasis via the epithelial-mesenchymal transition pathway. Theranostics 2022, 12, 3583. [Google Scholar]
- Li, X. Targeting iron metabolism in breast cancer stem-like cells with a lysosomal iron chelator restores drug sensitivity. ACS Nano 2023, 17, 12204. [Google Scholar] [CrossRef]
- Ambrosio, M.R.; Mosca, G.; Migliaccio, T.; Liguoro, D.; Nele, G.; Schonauer, F.; D’Andrea, F.; Liotti, F.; Prevete, N.; Melillo, R.M.; et al. Glucose Enhances Pro-Tumorigenic Functions of Mammary Adipose-Derived Mesenchymal Stromal/Stem Cells on Breast Cancer Cell Lines. Cancers 2022, 14, 5421. [Google Scholar] [CrossRef]
- Buschhaus, J.M.; Rajendran, S.; Humphries, B.A.; Cutter, A.C.; Muñiz, A.J.; Ciavattone, N.G.; Buschhaus, A.M.; Cañeque, T.; Nwosu, Z.C.; Sahoo, D.; et al. Effects of iron modulation on mesenchymal stem cell-induced drug resistance in estrogen receptor-positive breast cancer. Oncogene 2022, 41, 3705–3718. [Google Scholar] [CrossRef]
- Lee, J.; You, J.H.; Kim, M.S.; Roh, J.L. Epigenetic reprogramming of epithelial-mesenchymal transition promotes ferroptosis of head and neck cancer. Redox Biol. 2020, 37, 101697. [Google Scholar] [CrossRef]
- Han, Y.; Ye, L.; Du, F.; Ye, M.; Li, C.; Zhu, X.; Wang, Q.; Jiang, H.; Liu, Z.; Ma, J.; et al. Iron metabolism regulation of epithelial-mesenchymal transition in idiopathic pulmonary fibrosis. Ann. Transl. Med. 2021, 9, 1755. [Google Scholar] [CrossRef]
- Ozer, U. The role of Iron on breast cancer stem-like cells. Cell. Mol. Biol. 2016, 62, 25–30. [Google Scholar]
- Wang, L.; Li, X.; Mu, Y.; Lu, C.; Tang, S.; Lu, K.; Qiu, X.; Wei, A.; Cheng, Y.; Wei, W. The iron chelator desferrioxamine synergizes with chemotherapy for cancer treatment. J. Trace Elem. Med. Biol. 2019, 56, 131–138. [Google Scholar] [CrossRef]
- Wang, Y.; Lv, Z.; Chen, F.; Wang, X.; Gou, S. Conjugates Derived from Lapatinib Derivatives with Cancer Cell Stemness Inhibitors Effectively Reversed Drug Resistance in Triple-Negative Breast Cancer. J. Med. Chem. 2021, 64, 12877–12892. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Wu, H.; Du, X.; Li, C.; Zeng, W.; Qu, L.; Cang, C. Inhibition of lysosomal TRPML1 channel eliminates breast cancer stem cells by triggering ferroptosis. Cell Death Discov. 2024, 10, 256. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, X.; Yu, Y.; Liu, Z.; Zhang, C. OTULIN phosphorylation regulates β-catenin signaling and promotes breast cancer chemoresistance. Cell Death Dis. 2022, 13, 245. [Google Scholar]
- Chen, Y.; Gao, X.; Sun, W. OTULIN inhibition sensitizes breast cancer to chemotherapy by destabilizing β-catenin. Mol. Cancer Ther. 2023, 22, 89–101. [Google Scholar]
- Zhou, Q.; Liu, C.; Li, J.; Zhang, X. Targeting Wnt/β-catenin signaling to overcome chemoresistance in breast cancer. Front. Oncol. 2021, 11, 648402. [Google Scholar]
- Park, J.H.; Lee, S.H.; Park, S. Wnt signaling as a therapeutic target in triple-negative breast cancer: Current status and challenges. Int. J. Mol. Sci. 2022, 23, 11159. [Google Scholar] [CrossRef]
- Nguyen, T.; Tran, L.; Doan, M. Inhibition of β-catenin signaling reduces tumor stemness and improves chemosensitivity in breast cancer. Breast Cancer Res. Treat. 2023, 200, 101–113. [Google Scholar]
- Zhang, Y.; Liu, T.; Chen, H. Fibroblast-driven PLK1 signaling mediates HER2 inhibitor resistance in breast cancer. Cell Rep. 2023, 42, 112345. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Kim, J.S.; Park, S. Targeting PAI-1 reverses stromal-induced lapatinib resistance in HER2+ breast cancer. Nat. Commun. 2022, 13, 1789. [Google Scholar]
- Wang, F.; Zhao, L.; Yu, X. Tumor-associated fibroblasts enhance chemoresistance via PLK1 upregulation. Mol. Cancer 2021, 20, 98. [Google Scholar]
- Chen, M.; Tan, J.; Liu, Q. HER2+ breast cancer resistance and fibroblast secretome interactions. Oncogene 2024, 43, 113. [Google Scholar]
- Lee, Y.H.; Jung, H.J.; Choi, Y. Dual inhibition of PLK1 and HER2 overcomes fibroblast-induced resistance. Clin. Cancer Res. 2023, 29, 765. [Google Scholar]
- Lengrand, J.; Pastushenko, I.; Vanuytven, S.; Song, Y.; Venet, D.; Sarate, R.M.; Bellina, M.; Moers, V.; Boinet, A.; Sifrim, A.; et al. Pharmacological targeting of netrin-1 inhibits EMT in cancer. Nature 2023, 620, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Sun, L.; Gai, J.; Cao, Y.; Zhang, S. Exploring the resistance mechanism of triple-negative breast cancer to paclitaxel through the scRNA-seq analysis. PLoS ONE 2024, 19, e0297260. [Google Scholar] [CrossRef]
- Iida, K.; Okada, M. Identifying Key Regulatory Genes in Drug Resistance Acquisition: Modeling Pseudotime Trajectories of Breast Cancer Single-Cell Transcriptome. Cancers 2024, 16, 1884. [Google Scholar] [CrossRef]
- Fang, K.; Ohihoin, A.G.; Liu, T.; Choppavarapu, L.; Nosirov, B.; Wang, Q.; Yu, X.-Z.; Kamaraju, S.; Leone, G.; Jin, V.X. Integrative analysis of scRNA-seq and scATAC-seq reveals epigenetic regulation in tamoxifen-resistant breast cancer. Genome Med. 2024, 16, 134. [Google Scholar] [CrossRef]
- Luo, L.; Mastoraki, S.; Rao, X.; Wang, Y.; Kettner, N.M.; Raghavendra, A.S.; Tripathy, D.; Damodaran, S.; Hunt, K.K.; Wang, J.; et al. Single-cell transcriptomic profiling identifies markers of late progression in HR+/HER2- metastatic breast cancer under CDK4/6 inhibitor therapy. Mol. Cancer 2025, 24, 48. [Google Scholar] [CrossRef]
- Wu, P. Apatinib and vinorelbine combination in HER2-negative breast cancer. J. Exp. Oncol. 2024, 48, 300–315. [Google Scholar]
- Talib, W.H. A ketogenic diet combined with melatonin overcomes cisplatin and vincristine drug resistance in breast carcinoma syngraft. Nutrition 2020, 72, 110659. [Google Scholar] [CrossRef]
- Gadwal, R. GALNT14-mediated stemness in drug resistance. Cancer Cell Rep. 2024, 19, 205–217. [Google Scholar]
- Sahoo, S. EMT in therapy-resistant breast cancer. Cancer Ther. Rev. 2021, 17, 810–823. [Google Scholar]
- Liu, C.; Zhao, Z.; Gao, R.; Zhang, X.; Sun, Y.; Wu, J.; Liu, J.; Chen, C. Matrix Metalloproteinase-2-Responsive Surface-Changeable Liposomes Decorated by Multifunctional Peptides to Overcome the Drug Resistance of Triple-Negative Breast Cancer through Enhanced Targeting and Penetrability. ACS Biomater. Sci. Eng. 2022, 8, 2979–2994. [Google Scholar] [CrossRef] [PubMed]
- González-Callejo, P.; García-Astrain, C.; Herrero-Ruiz, A.; Henriksen-Lacey, M.; Seras-Franzoso, J.; Abasolo, I.; Liz-Marzán, L.M. 3D Bioprinted Tumor-Stroma Models of Triple-Negative Breast Cancer Stem Cells for Preclinical Targeted Therapy Evaluation. ACS Appl. Mater. Interfaces 2024, 16, 27151–27163. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.; Han, J.; Choi, J.; Kang, H.W. Embedded Bioprinting of Breast Cancer-Adipose Composite Tissue Model for Patient-Specific Paracrine Interaction Analysis. Adv. Healthc. Mater. 2025, 14, e2401887. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, X.; Mao, J.; Zheng, H.; Ji, R.; Wang, Z.; Guo, M.; Yuan, H.; Anwar, A.; Chen, C.; et al. Reverse anti-breast cancer drug resistance effects by a novel two-step assembled nano-celastrol medicine. Nanoscale 2022, 14, 7856–7863. [Google Scholar] [CrossRef]
- Li, H.; Fan, Y.; Shen, Y.; Xu, H.; Zhang, H.; Chen, F.; Feng, S. Acid-Activated TAT Peptide-Modified Biomimetic Boron Nitride Nanoparticles for Enhanced Targeted Codelivery of Doxorubicin and Indocyanine Green: A Synergistic Cancer Photothermal and Chemotherapeutic Approach. ACS Appl. Mater. Interfaces 2024, 16, 25101–25112. [Google Scholar] [CrossRef]
- Gadwal, A.; Purohit, P.; Khokhar, M.; Vishnoi, J.R.; Pareek, P.; Choudhary, R.; Elhence, P.; Banerjee, M.; Sharma, P. GALNT6, GALNT14, and Gal-3 in association with GDF-15 promotes drug resistance and stemness of breast cancer via β-catenin axis. Growth Factors 2024, 42, 84–100. [Google Scholar] [CrossRef]
- Tan, E.W.; Abdullah, A.D.I.; Ming, L.C.; Poh, C.L.; Goh, B.H.; Lau, T.P.; Tan, K.O. Adenovirus-mediated expression of MOAP-1, Bax and RASSF1A antagonizes chemo-drug resistance of human breast cancer cells expressing cancer stem cell markers. Biomed. Pharmacother. 2024, 176, 116744. [Google Scholar] [CrossRef]
- Yahya, S.M.M.; Nabih, H.K.; Elsayed, G.H.; Mohamed, S.I.A.; Elfiky, A.M.; Salem, S.M. Restoring microRNA-34a overcomes acquired drug resistance and disease progression in human breast cancer cell lines via suppressing the ABCC1 gene. Breast Cancer Res. Treat. 2024, 204, 133–149. [Google Scholar] [CrossRef]
- Delbue, D.; Mendonça, B.S.; Robaina, M.C.; Lemos, L.G.T.; Lucena, P.I.; Viola, J.P.B.; Magalhães, L.M.; Crocamo, S.; Oliveira, C.A.B.; Teixeira, F.R.; et al. Expression of nuclear XIAP associates with cell growth and drug resistance and confers poor prognosis in breast cancer. Biochim. Biophys. Acta BBA-Mol. Cell Res. 2020, 1867, 118761. [Google Scholar] [CrossRef]
- Talib, W. Ketogenic diet and chemotherapy resistance. Cancer Metab. 2020, 10, 215–228. [Google Scholar]
- Brindisi, M.; Fiorillo, M.; Frattaruolo, L.; Sotgia, F.; Lisanti, M.P.; Cappello, A.R. Cholesterol and Mevalonate: Two Metabolites Involved in Breast Cancer Progression and Drug Resistance through the ERRα Pathway. Cells 2020, 9, 1819. [Google Scholar] [CrossRef] [PubMed]
- Kunhoth, S. Computational Methods for Breast Cancer Molecular Profiling through Routine Histopathology: A Review. arXiv 2024, arXiv:2412.10392. [Google Scholar]
- Kim, M. Patient-derived organoids in precision cancer medicine. Medicine 2023, 4, 843–856. [Google Scholar]
- Zhang, X. Targeted therapy and mechanisms of drug resistance in breast cancer. Front. Oncol. 2024, 13, 9954028. [Google Scholar]
- Lee, Y.H. Advanced drug delivery platforms target cancer stem cells. J. Control Release 2023, 350, 1–19. [Google Scholar]
- Wang, F. Emerging agents that target signaling pathways in cancer stem cells. J. Hematol. Oncol. 2023, 13, 94. [Google Scholar] [CrossRef]
- Katuwal, N.B.; Kang, M.S.; Ghosh, M.; Hong, S.D.; Jeong, Y.G.; Park, S.M.; Kim, S.G.; Sohn, J.; Kim, T.H.; Moon, Y.W. Targeting PEG10 as a novel therapeutic approach to overcome CDK4/6 inhibitor resistance in breast cancer. J. Exp. Clin. Cancer Res. 2023, 42, 325. [Google Scholar] [CrossRef]
- Fujihara, M.; Shien, T.; Shien, K.; Suzawa, K.; Takeda, T.; Zhu, Y.; Mamori, T.; Otani, Y.; Yoshioka, R.; Uno, M.; et al. YES1 as a Therapeutic Target for HER2-Positive Breast Cancer after Trastuzumab and Trastuzumab-Emtansine (T-DM1) Resistance Development. Int. J. Mol. Sci. 2021, 22, 12809. [Google Scholar] [CrossRef]
- Ippolitov, D.; Lin, Y.H.; Spence, J.; Glogowska, A.; Thanasupawat, T.; Beiko, J.; Del Bigio, M.R.; Xu, X.; Wang, A.; Calvo, R.; et al. Overcoming brain-derived therapeutic resistance in HER2+ breast cancer brain metastasis. bioRxiv 2024. [Google Scholar] [CrossRef]
- Traphagen, N.A.; Hosford, S.R.; Jiang, A.; Marotti, J.D.; Brauer, B.L.; Demidenko, E.; Miller, T.W. High estrogen receptor alpha activation confers resistance to estrogen deprivation and is required for therapeutic response to estrogen in breast cancer. Oncogene 2021, 40, 3408–3421. [Google Scholar] [CrossRef] [PubMed]
- Dustin, D.; Gu, G.; Beyer, A.R.; Herzog, S.K.; Edwards, D.G.; Lin, H.; Gonzalez, T.L.; Grimm, S.L.; Coarfa, C.; Chan, D.W.; et al. RON signalling promotes therapeutic resistance in ESR1 mutant breast cancer. Br. J. Cancer 2021, 124, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Kim, B.J.; Mitra, A.; Vollert, C.T.; Lei, J.T.; Fandino, D.; Anurag, M.; Holt, M.V.; Gou, X.; Pilcher, J.B.; et al. PKMYT1 Is a Marker of Treatment Response and a Therapeutic Target for CDK4/6 Inhibitor-Resistance in ER+ Breast Cancer. Mol. Cancer Ther. 2024, 23, 1494–1510. [Google Scholar] [CrossRef] [PubMed]
- Egeland, E.V.; Seip, K.; Skourti, E.; Øy, G.F.; Pettersen, S.J.; Pandya, A.D.; Dahle, M.A.; Haugen, M.H.; Kristian, A.; Nakken, S.; et al. The SRC-family serves as a therapeutic target in triple negative breast cancer with acquired resistance to chemotherapy. Br. J. Cancer 2024, 131, 1656–1667. [Google Scholar] [CrossRef]
- Yi, Y.W.; You, K.S.; Han, S.; Ha, I.J.; Park, J.S.; Lee, S.G.; Seong, Y.S. Inhibition of IκB Kinase Is a Potential Therapeutic Strategy to Circumvent Resistance to Epidermal Growth Factor Receptor Inhibition in Triple-Negative Breast Cancer Cells. Cancers 2022, 14, 5215. [Google Scholar] [CrossRef]
- Payton, C.; Pang, L.Y.; Gray, M.; Argyle, D.J. Exosomes Derived from Radioresistant Breast Cancer Cells Promote Therapeutic Resistance in Naïve Recipient Cells. J. Pers. Med. 2021, 11, 1310. [Google Scholar] [CrossRef]
- Lin, B.; Huang, G.; Yuan, Z.; Peng, X.; Yu, C.; Zheng, J.; Li, Z.; Li, J.; Liang, J.; Xu, B. RPS15 Coordinates with CtIP to Facilitate Homologous Recombination and Confer Therapeutic Resistance in Breast Cancer. Radiat. Res. 2024, 202, 775–784. [Google Scholar] [CrossRef]
- Zhang, R.; Jiang, W.J.; Zhao, S.; Kang, L.J.; Wang, Q.S.; Feng, Y.M. FOXF2 expression triggered by endocrine therapy orchestrates therapeutic resistance through reorganization of chromatin architecture in breast cancer. Cancer Lett. 2025, 612, 217463. [Google Scholar] [CrossRef]
- Castillo, A.F.; Orlando, U.D.; Maloberti, P.M.; Prada, J.G.; Dattilo, M.A.; Solano, A.R.; Bigi, M.M.; Ríos Medrano, M.A.; Torres, M.T.; Indo, S.; et al. New inhibitor targeting Acyl-CoA synthetase 4 reduces breast and prostate tumor growth, therapeutic resistance and steroidogenesis. Cell Mol. Life Sci. 2021, 78, 2893–2910. [Google Scholar] [CrossRef]
- Rahem, S.M.; Epsi, N.J.; Coffman, F.D.; Mitrofanova, A. Genome-wide analysis of therapeutic response uncovers molecular pathways governing tamoxifen resistance in ER+ breast cancer. EBioMedicine 2020, 61, 103047. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, M.; Jiang, Z.; Fu, Z.; Ma, J.; Gao, S. Candidate Oligo Therapeutic Target, miR-330-3p, Induces Tamoxifen Resistance in Estrogen Receptor-Positive Breast Cancer Cells via HDAC4. Breast J. 2023, 2023, 2875972. [Google Scholar] [CrossRef] [PubMed]
- Crump, L.S.; Wyatt, G.L.; Rutherford, T.R.; Richer, J.K.; Porter, W.W.; Lyons, T.R. Hormonal Regulation of Semaphorin 7a in ER(+) Breast Cancer Drives Therapeutic Resistance. Cancer Res. 2021, 81, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Aka, Y.; Karakas, B.; Acikbas, U.; Basaga, H.; Gul, O.; Kutuk, O. Kinome-wide RNAi screening for mediators of ABT-199 resistance in breast cancer cells identifies Wee1 as a novel therapeutic target. Int. J. Biochem. Cell Biol. 2021, 137, 106028. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Jimenez, C.; Galan-Moya, E.M.; Corrales-Sanchez, V.; Noblejas-Lopez, M.D.M.; Burgos, M.; Domingo, B.; Montero, J.C.; Gomez-Juarez, M.; Picazo-Martinez, M.G.; Esparis-Ogando, A.; et al. Inhibition of the mitotic kinase PLK1 overcomes therapeutic resistance to BET inhibitors in triple negative breast cancer. Cancer Lett. 2020, 491, 50–59. [Google Scholar] [CrossRef]
- Zhu, J.; Wu, W.; Togashi, Y.; Taira Nihira, N.; Johmura, Y.; Zhu, D.; Nakanishi, M.; Miyoshi, Y.; Ohta, T. Alteration of Trop-2 expression in breast cancer cells by clinically used therapeutic agents and acquired tamoxifen resistance. Breast Cancer 2022, 29, 1076–1087. [Google Scholar] [CrossRef]
- Radomska, D.; Czarnomysy, R.; Marciniec, K.; Nowakowska, J.; Domínguez-Álvarez, E.; Bielawski, K. Short Communication: Novel Di- and Triselenoesters as Effective Therapeutic Agents Inhibiting Multidrug Resistance Proteins in Breast Cancer Cells. Int. J. Mol. Sci. 2024, 25, 9732. [Google Scholar] [CrossRef]
- Jin, X.; Wang, J.; Wang, Z.; Pang, W.; Chen, Y.; Yang, L. Chromatin-modifying protein 4C (CHMP4C) affects breast cancer cell growth and doxorubicin resistance as a potential breast cancer therapeutic target. J. Antibiot. 2024, 77, 93–101. [Google Scholar] [CrossRef]
- Chen, H. Nanoparticle-based drug delivery in cancer therapy. Adv. Drug Deliv. Rev. 2023, 182, 114124. [Google Scholar]
- Yang, K.; Xiao, Q.; Niu, M.; Pan, X.; Zhu, X. Exosomes in atherosclerosis: Convergence on macrophages. Int. J. Biol. Sci. 2022, 18, 3266–3281. [Google Scholar] [CrossRef]
- Wang, S. Lipid nanoparticles for targeted drug delivery. Biomater. Sci. 2021, 9, 178–195. [Google Scholar]
- Cui, Y.; Zhao, J.; Li, H. Chromogenic Mechanisms of Colorimetric Sensors Based on Gold Nanoparticles. Biosensors 2023, 13, 801. [Google Scholar] [CrossRef] [PubMed]
- Carbone, C.; Rigogliuso, S.; Brucato, V.M.B.; Cusimano, A.; Labbozzetta, M.; La Carrubba, V.; Poma, P.; Notarbartolo, M.; Carfì Pavia, F. PLLA Porous Scaffold as a 3D Breast Cancer Model to Investigate Drug Resistance. J. Biomed. Mater. Res. A 2025, 113, e37836. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, M.; Sohail, M.; Minhas, M.U.; Iqbal, J.; Mahmood, A.; Shaikh, A.J. Folic acid-functionalized nanoparticles-laden biomaterials for the improved oral delivery of hydrophobic drug in colorectal cancer. J. Drug Deliv. Sci. Technol. 2022, 71, 103287. [Google Scholar] [CrossRef]
- Shang, J.; Zhou, C.; Jiang, C.; Huang, X.; Liu, Z.; Zhang, H.; Zhao, J.; Liang, W.; Zeng, B. Recent developments in nanomaterials for upgrading treatment of orthopedics diseases. Front. Bioeng. Biotechnol. 2023, 11, 1221365. [Google Scholar] [CrossRef]
- Kharlamov, A.N.; Feinstein, J.A.; Cramer, J.A.; Boothroyd, J.A.; Shishkina, E.V.; Shur, V. Plasmonic photothermal therapy of atherosclerosis with nanoparticles: Long-term outcomes and safety in NANOM-FIM trial. Future Cardiol. 2017, 13, 345–363. [Google Scholar] [CrossRef]
- Fernandez Alarcon, J.; Soliman, M.; Lüdtke, T.U.; Clemente, E.; Dobricic, M.; Violatto, M.B.; Corbelli, A.; Fiordaliso, F.; Cordiglieri, C.; Talamini, L.; et al. Long-term retention of gold nanoparticles in the liver is not affected by their physicochemical characteristics. Nanoscale 2023, 15, 8740–8753. [Google Scholar] [CrossRef]
- Huang, Y. Challenges and opportunities in exosome-based therapy. Nat. Rev. Drug Discov. 2023, 22, 355–372. [Google Scholar]
- Emami, F.; Pathak, S.; Nguyen, T.T.; Shrestha, P.; Maharjan, S.; Kim, J.O.; Jeong, J.H.; Yook, S. Photoimmunotherapy with cetuximab-conjugated gold nanorods reduces drug resistance in triple negative breast cancer spheroids with enhanced infiltration of tumor-associated macrophages. J. Control Release 2021, 329, 645–664. [Google Scholar] [CrossRef]
- Wang, Z.; Zhong, H.; Liang, X.; Ni, S. Targeting tumor-associated macrophages for the immunotherapy of glioblastoma: Navigating the clinical and translational landscape. Front. Immunol. 2022, 13, 1024921. [Google Scholar] [CrossRef]
- Liu, L.; Li, S.; Qu, Y.; Bai, H.; Pan, X.; Wang, J.; Wang, Z.; Duan, J.; Zhong, J.; Wan, R.; et al. Ablation of ERO1A induces lethal endoplasmic reticulum stress responses and immunogenic cell death to activate anti-tumor immunity. Cell Rep. Med. 2023, 4, 101206. [Google Scholar] [CrossRef]
- Zhu, M.Y.; Wang, T.; Wang, H.D.; Wang, H.Z.; Chen, H.Y.; Zhang, S.; Guo, Y.J.; Li, H.; Hui, H. LW-213 induces immunogenic tumor cell death via ER stress mediated by lysosomal TRPML1. Cancer Lett. 2023, 577, 216435. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Miao, K.; Sun, H.; Deng, C.X. Tumor heterogeneity reshapes the tumor microenvironment to influence drug resistance. Int. J. Biol. Sci. 2022, 18, 3019–3033. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Yao, Z.; Chen, Z.; Ge, X.; Su, L.; Wang, S.; Wu, Y.; Song, J. Ultrasound-Activated NIR Chemiluminescence for Deep Tissue and Tumor Foci Imaging. Anal. Chem. 2023, 95, 11219–11226. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Jike, Y.; Liu, K.; Gan, F.; Zhang, K.; Xie, M.; Zhang, J.; Chen, C.; Zou, X.; Jiang, X.; et al. Exosome-mediated miR-144-3p promotes ferroptosis to inhibit osteosarcoma proliferation, migration, and invasion through regulating ZEB1. Mol. Cancer 2023, 22, 113. [Google Scholar] [CrossRef]
- Xian, X.; Cai, L.L.; Li, Y.; Wang, R.C.; Xu, Y.H.; Chen, Y.J.; Xie, Y.H.; Zhu, X.L.; Li, Y.F. Neuron secrete exosomes containing miR-9-5p to promote polarization of M1 microglia in depression. J. Nanobiotechnol. 2022, 20, 122. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, S.; Tang, W.; Huang, Q.; Mei, Y.; Yang, H. Exosomes from tamoxifen-resistant breast cancer cells transmit drug resistance partly by delivering miR-9-5p. Cancer Cell Int. 2021, 21, 55. [Google Scholar] [CrossRef]
- Kim, J.Y.; Jung, E.J.; Kim, J.M.; Son, Y.; Lee, H.S.; Kwag, S.J.; Park, J.H.; Cho, J.K.; Kim, H.G.; Park, T.; et al. MiR-221 and miR-222 regulate cell cycle progression and affect chemosensitivity in breast cancer by targeting ANXA3. Exp. Ther. Med. 2023, 25, 127. [Google Scholar] [CrossRef]
- Zhang, L. Synthetic anti-miRNAs as novel cancer therapeutics. J. Mol. Med. 2022, 100, 345–362. [Google Scholar]
- Peng, Y.; Zhao, M.; Hu, Y.; Guo, H.; Zhang, Y.; Huang, Y.; Zhao, L.; Chai, Y.; Wang, Z. Blockade of exosome generation by GW4869 inhibits the education of M2 macrophages in prostate cancer. BMC Immunol. 2022, 23, 37. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, Y.; Shi, Y.; Zhang, Y.; Liu, K.; Liang, R.; Sun, P.; Chang, X.; Tang, W.; Zhang, Y.; et al. Expression of miRNA-29 in Pancreatic β Cells Promotes Inflammation and Diabetes via TRAF3. Cell Rep. 2021, 34, 108576. [Google Scholar] [CrossRef]
- Huang, C.; Zhou, Y.; Feng, X.; Wang, J.; Li, Y.; Yao, X. Delivery of Engineered Primary Tumor-Derived Exosomes Effectively Suppressed the Colorectal Cancer Chemoresistance and Liver Metastasis. ACS Nano 2023, 17, 10313–10326. [Google Scholar] [CrossRef]

| Therapeutic Agent/Approach | Mechanism/Regimen Type | Primary Outcome/Effect |
|---|---|---|
| Ultrasound-responsive nanobubbles co-loaded with chlorin-e6 and paclitaxel | Chemotherapy, sonodynamic therapy, and immune activation via cGAS-STING pathway | Suppressing tumor progression and enhancing CD8+ T cell infiltration in TNBC models |
| Single-atom iron nanozymes (Fe-N-C SAzymes) | Mimicking peroxidase activity to remodel tumor metabolism and immune landscape | Potentiating chemodynamic therapy (CDT) and photothermal therapy (PTT), eliminating immunosuppressive myeloid-derived suppressor cells |
| Gold nanorods and CXCR4 antagonist peptide E5 (AuNRs-E5) | Disrupting tumor proliferation, initiating endoplasmic reticulum stress, promoting dendritic cell maturation | Long-term immune memory and suppression of TNBC recurrence |
| IC/IR820 nanoparticles (combination of chemotherapy with photothermal and immune stimulation) | Inducing robust autophagy-dependent immunogenic cell death | Inducing robust autophagy-dependent immunogenic cell death |
| Nano–Micro-Sera-based fibrin implant (photothermal agents, chemotherapeutics, and immune agonists) | Multi-agent, locally administered system to prevent TNBC relapse post-surgery | Enhanced immune infiltration at the tumor site, prevented local recurrence, and achieved remarkable tumor suppression |
| Pt(IV)/CQ/PFH constructs (nanoparticles) | Inhibiting protective autophagy while reprogramming innate immune metabolism | Boosting maturation of dendritic cells and polarization of macrophages to pro-inflammatory phenotypes |
| Multi-modal nanoplatforms (immunogenic cell death inducers, e.g., mitoxantrone, immune adjuvants, e.g., TLR7/8 agonists, and photothermal agents) | Repolarizing tumor-associated macrophages, enhancing dendritic cell activation, converting cold tumors into immunologically hot ones | Enhanced clinical efficacy in BC, particularly TNBC |
| CDK4/6 inhibitors (palbociclib, abemaciclib) | Molecularly targeted therapy for HR+/HER2− metastatic BC | Median progression-free survival (PFS) of 5.3 months in heavily pretreated patients |
| mTOR inhibitors (everolimus) | Molecularly targeted therapy for HR+/HER2− metastatic BC | Median progression-free survival (PFS) of 5.3 months in heavily pretreated patients |
| Selective Estrogen Receptor Degraders (SERDs) | Addressing ESR1 mutations in endocrine therapy resistance | More effective inhibition of ESR1 mutations |
| Mitochondria-targeted organic nanoparticles | Mild photothermal therapy | Improved cancer cell apoptosis, reduced tumor metastasis |
| Dual-targeted cationic microbubbles | Enhanced gene therapy delivery | Improved tumor suppression |
| Aptamer-PROTAC approach targeting ERα | Degrading mutant ERα variants that drive resistance to tamoxifen | Overcoming endocrine resistance in ERα-positive BC |
| Trastuzumab emtansine (T-DM1) | ADC: Lysosomal processing to release cytotoxic payloads | Widely adopted for HER2-positive BC |
| Trastuzumab duocarmazine (SYD985) | Next-generation ADC: Incorporates protease-cleavable linkers and membrane-permeable drugs, enabling a bystander effect | Targeting antigen-negative neighboring cells |
| Self-assembled cabazitaxel nanocrystals (PC/CNC) | Protein corona-bridged natural targeting | Enhancing drug accumulation in primary tumors, circulating tumor cells, and metastatic lesions |
| Mesoporous silica nanoparticle-based systems | pH-responsive targeted drug release | Improving doxorubicin efficacy against BC cells |
| Folic acid-functionalized starch-encapsulated copper oxide nanoparticles | Enhancing tumor penetration and inducing apoptosis via reactive oxygen species generation | Promising in TNBC treatment |
| Chitosan/carbon quantum dots/Fe2O3 nanocomposites loaded with curcumin | Targeted drug release in tumor microenvironments | Targeted drug release in tumor microenvironments |
| Anti-CD47 monoclonal ADCs | Targeting immune checkpoint molecule CD47; facilitating macrophage-mediated phagocytosis and promoting natural killer cell activation | Enhanced cytotoxicity in TNBC models |
| Photothermal therapy (PTT) and Photodynamic therapy (PDT) with tyrosine kinase inhibitors (e.g., pyrotinib) | Enhancing oxidative stress-induced cell death | Promoting ferroptosis and improving treatment efficacy in HER2-positive BC |
| Dual drug-loaded metal–phenolic networks | Simultaneous chemodynamic therapy and MRI-guided treatment; amplifying oxidative stress through glutathione depletion; enhancing chemotherapeutic efficacy via mitochondrial inhibition | Facilitate tumor-targeted drug release, amplify oxidative stress, and enhance chemotherapeutic efficacy |
| Oxovanadium (IV) complexes | Inhibiting ABC transporters (P-gp/ABCB1 and BCRP/ABCG2) | Restoring drug sensitivity in BC cells, improving therapeutic efficacy of existing chemotherapeutics |
| 2,2-diphenethyl isothiocyanate (DPEITC) | Suppressing MDR1 expression | Enhancing topoisomerase inhibitor-induced cell death |
| Acetyl plumbagin | Cholesterol depletion | Sensitized tamoxifen-resistant BC cells |
| Nanoemulsions incorporating paclitaxel and erucin in frankincense oil | Enhanced tumor penetration and sustained drug release | Overcoming resistance |
| Combination therapies with BH3 mimetics (for PI3Kα inhibitor resistance) | Targeting FOXO3 downregulation | Enhancing efficacy against resistance to PI3K inhibitors |
| Near-infrared (NIR) light-activated conjugated polymer nanoparticles (CPNs) with W-7 | Inducing endoplasmic reticulum (ER) stress, upregulating heat shock protein 70 (HSP70), enhancing TRAIL gene expression and apoptotic signaling, potentiating caspase-8 activation | Enhancing TRAIL sensitivity and inducing immunogenic apoptosis in resistant BC cells, particularly in TNBC |
| Formononetin (FMNT) | Downregulating miR-199a-3p, restoring mTOR activity, inhibiting BC cell proliferation, invasion, and migration | Reduction in autophagic processes, sensitization of TNBC cells to Taxol, potential for reversing Taxol resistance |
| Lysosomal iron chelators (e.g., deferoxamine) and novel nanoformulated agents | Depleting labile iron pools within CSCs | Re-sensitizing CSCs to endocrine therapy, suppressing CSC proliferation, enhancing efficacy of ROS-generating chemotherapeutics |
| Lapatinib derivatives with CSC inhibitors (conjugates) | Concurrently inhibiting AKT/ERK and Wnt/β-catenin signaling pathways | Reversing resistance in TNBC by overcoming CSC-mediated resistance |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabit, H.; Rashwan, S.; Albrahim, Y.; Wadan, A.-H.S.; Radwan, F.; Alqosaibi, A.I.; Abdel-Ghany, S.; Arneth, B. Targeting Resistance Pathways in Breast Cancer Through Precision Oncology: Nanotechnology and Immune Modulation Approaches. Biomedicines 2025, 13, 1691. https://doi.org/10.3390/biomedicines13071691
Sabit H, Rashwan S, Albrahim Y, Wadan A-HS, Radwan F, Alqosaibi AI, Abdel-Ghany S, Arneth B. Targeting Resistance Pathways in Breast Cancer Through Precision Oncology: Nanotechnology and Immune Modulation Approaches. Biomedicines. 2025; 13(7):1691. https://doi.org/10.3390/biomedicines13071691
Chicago/Turabian StyleSabit, Hussein, Sanaa Rashwan, Yasser Albrahim, Al-Hassan Soliman Wadan, Faisal Radwan, Amany I. Alqosaibi, Shaimaa Abdel-Ghany, and Borros Arneth. 2025. "Targeting Resistance Pathways in Breast Cancer Through Precision Oncology: Nanotechnology and Immune Modulation Approaches" Biomedicines 13, no. 7: 1691. https://doi.org/10.3390/biomedicines13071691
APA StyleSabit, H., Rashwan, S., Albrahim, Y., Wadan, A.-H. S., Radwan, F., Alqosaibi, A. I., Abdel-Ghany, S., & Arneth, B. (2025). Targeting Resistance Pathways in Breast Cancer Through Precision Oncology: Nanotechnology and Immune Modulation Approaches. Biomedicines, 13(7), 1691. https://doi.org/10.3390/biomedicines13071691








