Investigation into the Effects of Tramadol, Citalopram, Tianeptine, and Their Combinations on Rat Brain Tissue
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Chemicals
2.3. Experimental Groups
2.4. Experimental Procedure
2.5. Biochemical Analysis
2.5.1. Preparation of Samples
2.5.2. Determination of MDA, GSH, SOD, and CAT in Brain Tissue
2.6. Histopathological Examinations
2.7. Statistical Analyses
3. Results
3.1. Behavioral Test Results
3.2. Biochemical Findings
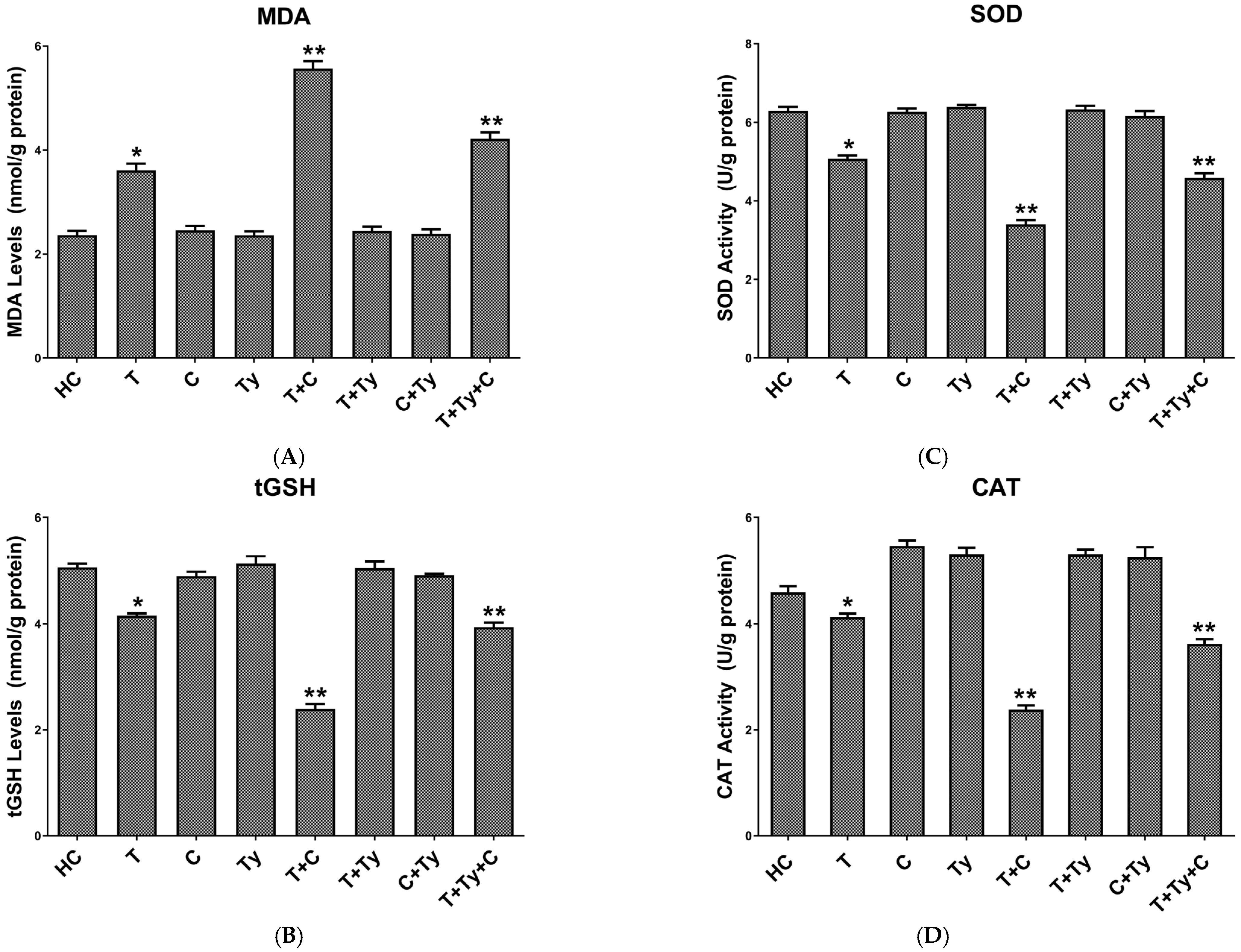
3.2.1. MDA Analysis Results of Brain Tissue
3.2.2. Brain Tissue tGSH Analysis Results
3.2.3. Brain Tissue SOD Analysis Results
3.2.4. Brain Tissue CAT Analysis Results
3.3. Histopathological Findings of Brain Tissue
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FDA | Food and Drug Administration |
| MDA | malondialdehyde |
| GSH | glutathione |
| SOD | superoxide dismutase |
| CAT | catalase |
| SSRI | selective serotonin reuptake inhibitor |
| HC | healthy control |
| T | tramadol alone |
| C | citalopram alone |
| Ty | tianeptine alone |
| T + C | tramadol + citalopram |
| T + Ty | tramadol + tianeptine |
| C + Ty | citalopram + tianeptine |
| T + Ty + C | tramadol + tianeptine + citalopram |
| x ± SEM | mean value ± standard error of mean |
| ANOVA | analysis of variance |
| LPO | lipid peroxidation |
| ROS | reactive oxygen species |
References
- Dhesi, M.; Maldonado, K.A.; Patel, P.; Maani, C.V. Tramadol. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Kaneko, K.; Umehara, M.; Homan, T.; Okamoto, K.; Oka, M.; Oyama, T. The analgesic effect of tramadol in animal models of neuropathic pain and fibromyalgia. Neurosci. Lett. 2014, 562, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Kader, G.A.; Ibrahim, M.A.; Khalifa, A.M.; Mirza, U.; Rashwan, E.K.; Abdel-Hady, Z. Evaluation of vitamin C protective effect on the cerebrocortical antioxidant defense, histopathological, pro-apoptotic p53 and anti-apoptotic Bcl2 expressions against tramadol neurotoxicity in rats. J. Chem. Neuroanat. 2021, 112, 101893. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.A.; Abdeshafy, M.M.; Abdelkawy, K.; Elsabaa, R.M.; Elbarbry, F. Clinical and Laboratory Factors Related to Seizure and Serotonin Toxicity in Tramadol Intoxication: An Egyptian Study. Clin. Drug Investig. 2023, 43, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Hassamal, S.; Miotto, K.; Dale, W.; Danovitch, I. Tramadol: Understanding the Risk of Serotonin Syndrome and Seizures. Am. J. Med. 2018, 131, 1382.e1–1382.e6. [Google Scholar] [CrossRef] [PubMed]
- Shakoor, M.; Ayub, S.; Ahad, A.; Ayub, Z. Transient serotonin syndrome caused by concurrent use of tramadol and selective serotonin reuptake inhibitor. Am. J. Case Rep. 2014, 15, 562–564. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gollapudy, S.; Cronin, D.C.; Pagel, P.S.; Boettcher, B.T. Serotonin Syndrome Resulting from Acute Decompensation of Nonalcoholic Steatohepatitis Cirrhosis in a Patient Chronically Treated with Citalopram and Tramadol. J. Cardiothorac. Vasc. Anesthesia 2017, 31, 1385–1388. [Google Scholar] [CrossRef] [PubMed]
- Perananthan, V.; Buckley, N. Opioids and antidepressants: Which combinations to avoid. Aust. Prescr. 2021, 44, 41–44. [Google Scholar] [CrossRef]
- Mahlberg, R.; Kunz, D.; Sasse, J.; Kirchheiner, J. Serotonin syndrome with tramadol and citalopram. Am. J. Psychiatry 2004, 161, 1129. [Google Scholar] [CrossRef] [PubMed]
- Shoar, N.S.; Fariba, K.A.; Padhy, R.K. Citalopram. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Badea, A.; Ghosh, R.; Lynch, K.L.; Wu, A.H.B. Lack of Cross-Reactivity of Tianeptine with Tricyclic Antidepressant Immunoassays. J. Anal. Toxicol. 2021, 45, e8–e9. [Google Scholar] [CrossRef] [PubMed]
- Nishio, Y.; Lindsley, C.W.; Bender, A.M. Classics in Chemical Neuroscience: Tianeptine. ACS Chem. Neurosci. 2024, 15, 3863–3873. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brink, C.B.; Harvey, B.H.; Brand, L. Tianeptine: A novel atypical antidepressant that may provide new insights into the biomolecular basis of depression. Recent Pat. CNS Drug Discov. 2006, 1, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Edinoff, A.N.; Sall, S.; Beckman, S.P.; Koepnick, A.D.; Gold, L.C.; Jackson, E.D.; Wenger, D.M.; Cornett, E.M.; Murnane, K.S.; Kaye, A.M.; et al. Tianeptine, an Antidepressant with Opioid Agonist Effects: Pharmacology and Abuse Potential, a Narrative Review. Pain Ther. 2023, 12, 1121–1134. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Preskorn, S.H. Tianeptine: A facilitator of the reuptake of serotonin and norepinephrine as an antidepressant? J. Psychiatr. Pract. 2004, 10, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Alamo, C.; García-Garcia, P.; Lopez-Muñoz, F.; Zaragozá, C. Tianeptine, an atypical pharmacological approach to depression. Rev. Psiquiatr. Salud Ment. 2019, 12, 170–186. [Google Scholar] [CrossRef] [PubMed]
- De Simoni, M.G.; De Luigi, A.; Clavenna, A.; Manfridi, A. In vivo studies on the enhancement of serotonin reuptake by tianeptine. Brain Res. 1992, 574, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Mehranpour, M.; Moghaddam, M.H.; Abdollahifar, M.A.; Salehi, M.; Aliaghaei, A. Tramadol induces apoptosis, inflammation, and oxidative stress in rat choroid plexus. Metab. Brain Dis. 2023, 38, 2679–2690. [Google Scholar] [CrossRef] [PubMed]
- Overstreet, D.H.; Keeney, A.; Hogg, S. Antidepressant effects of citalopram and CRF receptor antagonist CP-154,526 in a rat model of depression. Eur. J. Pharmacol. 2004, 492, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, B.K.; Suleyman, Z.; Suleyman, B.; Mammadov, R.; Bulut, S.; Altuner, D.; Alptekin, O.; Coban, T.A.; Suleyman, H. The hormonal mechanism of the effects of meperidine, sertraline, tianeptine, and their combinations on reproductive functions in female rats. Biomed. Pharmacother. 2024, 178, 117160. [Google Scholar] [CrossRef] [PubMed]
- Góth, L. A simple method for determination of serum catalase activity and revision of reference range. Clin. Chim. Acta 1991, 196, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Badar, A. Serotonin syndrome: An often-neglected medical emergency. J. Fam. Community Med. 2024, 31, 1–8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Romero, F.J.; Bosch-Morell, F.; Romero, M.J.; Jareño, E.J.; Romero, B.; Marín, N.; Romá, J. Lipid peroxidation products and antioxidants in human disease. Environ. Health Perspect. 1998, 106 (Suppl. S5), 1229–1234. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Singh, Z.; Karthigesu, I.P.; Singh, P.; Kaur, R. Use of Malondialdehyde as a Biomarker for Assessing Oxidative Stress in Different Disease Pathologies: A Review. Iran. J. Public Health 2014, 43 (Suppl. S3), 7–16. [Google Scholar]
- Ozdemir, E.; Cetinkaya, S.; Ersan, S.; Kucukosman, S.; Ersan, E.E. Serum selenium and plasma malondialdehyde levels and antioxidant enzyme activities in patients with obsessive-compulsive disorder. Prog Neuropsychopharmacol. Biol. Psychiatry 2009, 33, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, S.; Lal, N.; Mahdi, A.A.; Mittal, M.; Singh, B.; Pandey, S. Evaluation of antioxidant enzymes activity and malondialdehyde levels in patients with chronic periodontitis and diabetes mellitus. J. Periodontol. 2014, 85, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Salam, O.M.; Youness, E.R.; Mohammed, N.A.; Abd El-Moneim, O.M.; Shaffie, N. Citicoline protects against tramadol-induced oxidative stress and organ damage. React. Oxyg. Species 2019, 7, 106–120. [Google Scholar] [CrossRef]
- Hussein, S.A.; Ismail, H.K.; Abdel Aal, S.A. Effect of tramadol drug on some biochemical and immunological parameters in albino male rats; evaluation of possible reversal following its withdrawal. Benha Vet. Med. J. 2017, 33, 418–429. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several lines of antioxidant defense against oxidative stress: Antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ali, H.A.; Afifi, M.; Saber, T.M.; Makki, A.A.; Keshta, A.T.; Baeshen, M.; Al-Farga, A. Neurotoxic, Hepatotoxic and Nephrotoxic Effects of Tramadol Administration in Rats. J. Mol. Neurosci. 2020, 70, 1934–1942. [Google Scholar] [CrossRef] [PubMed]
- Mohammadnejad, L.; Soltaninejad, K. Tramadol-Induced Organ Toxicity via Oxidative Stress: A Review Study. Int. J. Med. Toxicol. Forensic Med. 2022, 12, 35430. [Google Scholar] [CrossRef]
- Mikkelsen, N.; Damkier, P.; Pedersen, S.A. Serotonin syndrome-A focused review. Basic Clin. Pharmacol. Toxicol. 2023, 133, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Scotton, W.J.; Hill, L.J.; Williams, A.C.; Barnes, N.M. Serotonin Syndrome: Pathophysiology, Clinical Features, Management, and Potential Future Directions. Int. J. Tryptophan. Res. 2019, 12, 1178646919873925. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Volpi-Abadie, J.; Kaye, A.M.; Kaye, A.D. Serotonin syndrome. Ochsner J. 2013, 13, 533–540. [Google Scholar] [PubMed] [PubMed Central]
- Mason, B.J.; Blackburn, K.H. Possible serotonin syndrome associated with tramadol and sertraline coadministration. Ann. Pharmacother. 1997, 31, 175–177. [Google Scholar] [CrossRef] [PubMed]
- Wrona, M.Z.; Dryhurst, G. Oxidation of serotonin by superoxide radical: Implications to neurodegenerative brain disorders. Chem. Res. Toxicol. 1998, 11, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Della, F.P.; Abelaira, H.M.; Réus, G.Z.; Antunes, A.R.; Dos Santos, M.A.; Zappelinni, G.; Steckert, A.V.; Vuolo, F.; Galant, L.S.; Dal-Pizzol, F.; et al. Tianeptine exerts neuroprotective effects in the brain tissue of rats exposed to the chronic stress model. Pharmacol. Biochem. Behav. 2012, 103, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Slettedal, J.K.; Nilssen, D.O.; Magelssen, M.; Løberg, E.M.; Maehlen, J. Brain pathology in fatal serotonin syndrome: Presentation of two cases. Neuropathology 2011, 31, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.W.; Bhatt, A.; Tien, L.T.; Zheng, B.; Simpson, K.L.; Lin, R.C.; Cai, Z.; Kumar, P.; Pang, Y. Exposure to serotonin adversely affects oligodendrocyte development and myelination in vitro. J. Neurochem. 2015, 133, 532–543. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Then, C.K.; Liu, K.H.; Liao, M.H.; Chung, K.H.; Wang, J.Y.; Shen, S.C. Antidepressants, sertraline and paroxetine, increase calcium influx and induce mitochondrial damage-mediated apoptosis of astrocytes. Oncotarget 2017, 8, 115490–115502. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
 : neuron (A–D,G–I), degenerated neuron (E,F); ⇒: astrocyte (A–D,G–I), swollen astrocyte (E,F); ➢: oligodendrocyte (A,C,D,G,H), slightly edematous oligodendrocyte (B), pericellular edema oligodendrocyte (E,F), moderately edematous oligodendrocyte (I);
: neuron (A–D,G–I), degenerated neuron (E,F); ⇒: astrocyte (A–D,G–I), swollen astrocyte (E,F); ➢: oligodendrocyte (A,C,D,G,H), slightly edematous oligodendrocyte (B), pericellular edema oligodendrocyte (E,F), moderately edematous oligodendrocyte (I);  : microglia (A–D,G–I), increased microglia (E,F); ★: blood vessel (A,C,D,G,H), moderately dilated and congested blood vessel (B,I), severely dilated and congested blood vessel (E); ✹: apoptotic body (F).
: microglia (A–D,G–I), increased microglia (E,F); ★: blood vessel (A,C,D,G,H), moderately dilated and congested blood vessel (B,I), severely dilated and congested blood vessel (E); ✹: apoptotic body (F).
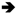 : neuron (A–D,G–I), degenerated neuron (E,F); ⇒: astrocyte (A–D,G–I), swollen astrocyte (E,F); ➢: oligodendrocyte (A,C,D,G,H), slightly edematous oligodendrocyte (B), pericellular edema oligodendrocyte (E,F), moderately edematous oligodendrocyte (I);
: neuron (A–D,G–I), degenerated neuron (E,F); ⇒: astrocyte (A–D,G–I), swollen astrocyte (E,F); ➢: oligodendrocyte (A,C,D,G,H), slightly edematous oligodendrocyte (B), pericellular edema oligodendrocyte (E,F), moderately edematous oligodendrocyte (I);  : microglia (A–D,G–I), increased microglia (E,F); ★: blood vessel (A,C,D,G,H), moderately dilated and congested blood vessel (B,I), severely dilated and congested blood vessel (E); ✹: apoptotic body (F).
: microglia (A–D,G–I), increased microglia (E,F); ★: blood vessel (A,C,D,G,H), moderately dilated and congested blood vessel (B,I), severely dilated and congested blood vessel (E); ✹: apoptotic body (F).
| Groups | Astrocytes Median (Min-Max) | Oligodendrocytes Median (Min-Max) | Microglia Median (Min-Max) | Vascular Dilatation Median (Min-Max) | Congestion Median (Min-Max) | Apoptotic Bodies Median (Min-Max) |
|---|---|---|---|---|---|---|
| HC | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| T | 0 (0–1) | 1 (0–2) | 0 (0–0) | 2 (1–3) | 2 (2–2) * | 0 (0–0) |
| C | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| Ty | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| T + C | 3 (2–3) * | 3 (2–3) ** | 3 (1–3) * | 3 (3–3) * | 3 (2–3) * | 1.5 (1–2) ** |
| T + Ty | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| C + Ty | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| T + Ty + C | 0 (0–0) | 2 (1–3) ** | 0 (0–0) | 2 (1–3) * | 2 (1–2) | 0 (0–0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ates, I.; Isik, B.; Gozen, F.; Yazici, G.N.; Gulaboglu, M.; Mammadov, R.; Huseynova, G.; Altuner, D.; Suleyman, H. Investigation into the Effects of Tramadol, Citalopram, Tianeptine, and Their Combinations on Rat Brain Tissue. Biomedicines 2025, 13, 1690. https://doi.org/10.3390/biomedicines13071690
Ates I, Isik B, Gozen F, Yazici GN, Gulaboglu M, Mammadov R, Huseynova G, Altuner D, Suleyman H. Investigation into the Effects of Tramadol, Citalopram, Tianeptine, and Their Combinations on Rat Brain Tissue. Biomedicines. 2025; 13(7):1690. https://doi.org/10.3390/biomedicines13071690
Chicago/Turabian StyleAtes, Irem, Bahar Isik, Fusun Gozen, Gulce Naz Yazici, Mine Gulaboglu, Renad Mammadov, Gulbeniz Huseynova, Durdu Altuner, and Halis Suleyman. 2025. "Investigation into the Effects of Tramadol, Citalopram, Tianeptine, and Their Combinations on Rat Brain Tissue" Biomedicines 13, no. 7: 1690. https://doi.org/10.3390/biomedicines13071690
APA StyleAtes, I., Isik, B., Gozen, F., Yazici, G. N., Gulaboglu, M., Mammadov, R., Huseynova, G., Altuner, D., & Suleyman, H. (2025). Investigation into the Effects of Tramadol, Citalopram, Tianeptine, and Their Combinations on Rat Brain Tissue. Biomedicines, 13(7), 1690. https://doi.org/10.3390/biomedicines13071690






