Involvement of Microglia in Retinal Ganglion Cell Injury Induced by IOP Elevation in a Rat Ex Vivo Acute Glaucoma Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Rat Ex Vivo Eyecup Preparation
2.2. Whole Mounted Retinas and Immunostaining
2.3. Microglial Sholl Analysis
2.4. Microglial Soma Area Measurement
2.5. Iba-1 and CD68 Double Immunofluorescence on Cryosections
2.6. Apoptosis
2.7. Preparation of Materials for Light Microscopy
2.8. Data Analysis of Morphometrics
2.9. Western Blot Analysis
2.10. Image Processing
2.11. Statistical Analysis
3. Results
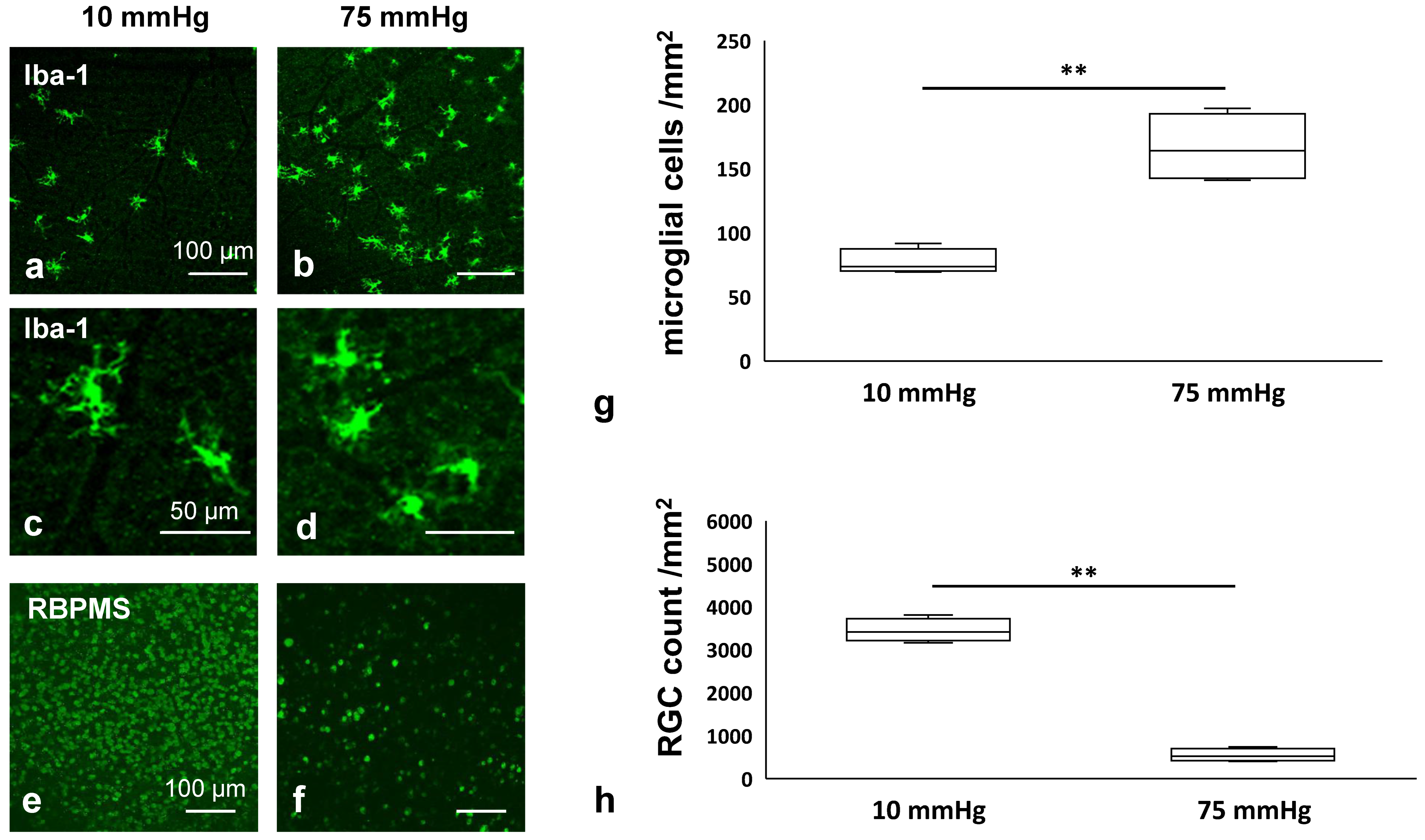
3.1. Pressure-Mediated Microglial Proliferation and Retinal Degeneration in an Ex Vivo Model
3.2. Changes in Markers Associated with NLRP3 Inflammasome Pathway
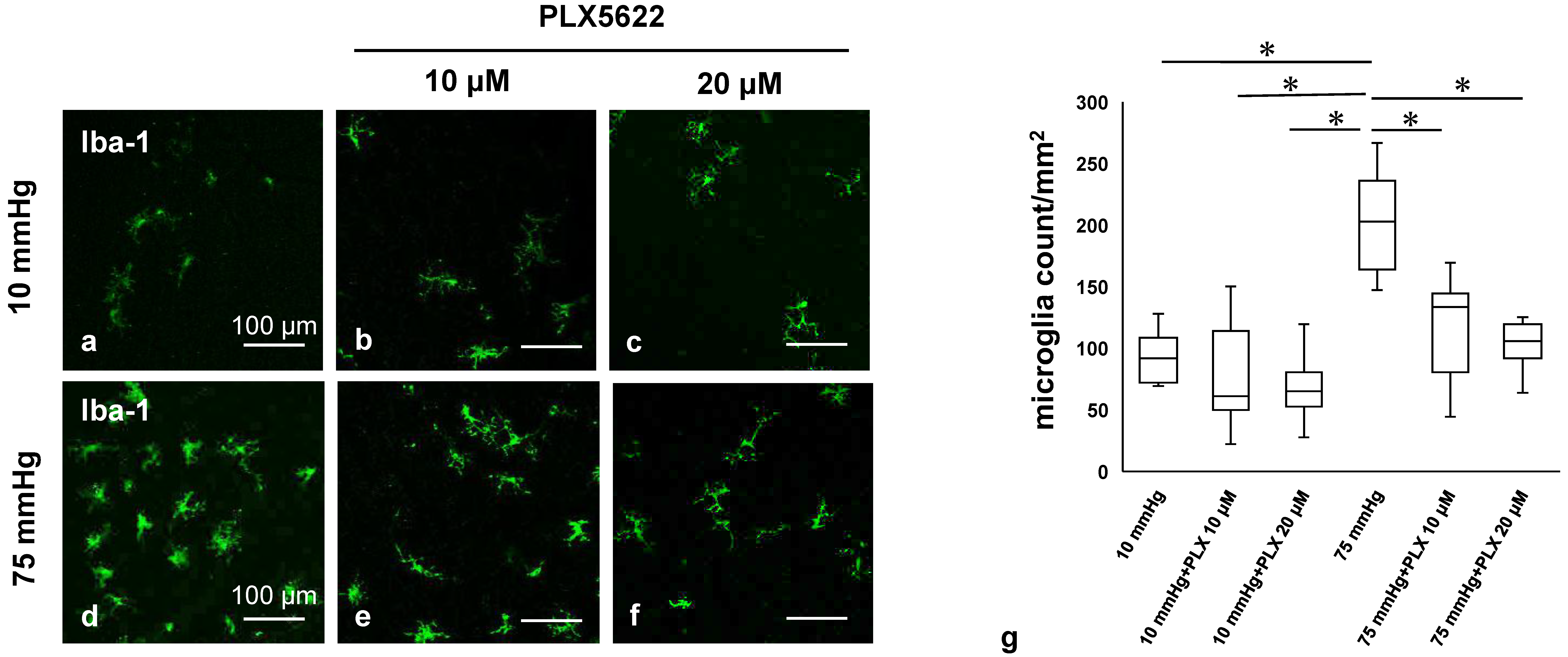
3.3. PLX5622 Prevents Pressure-Induced Microglial Proliferation
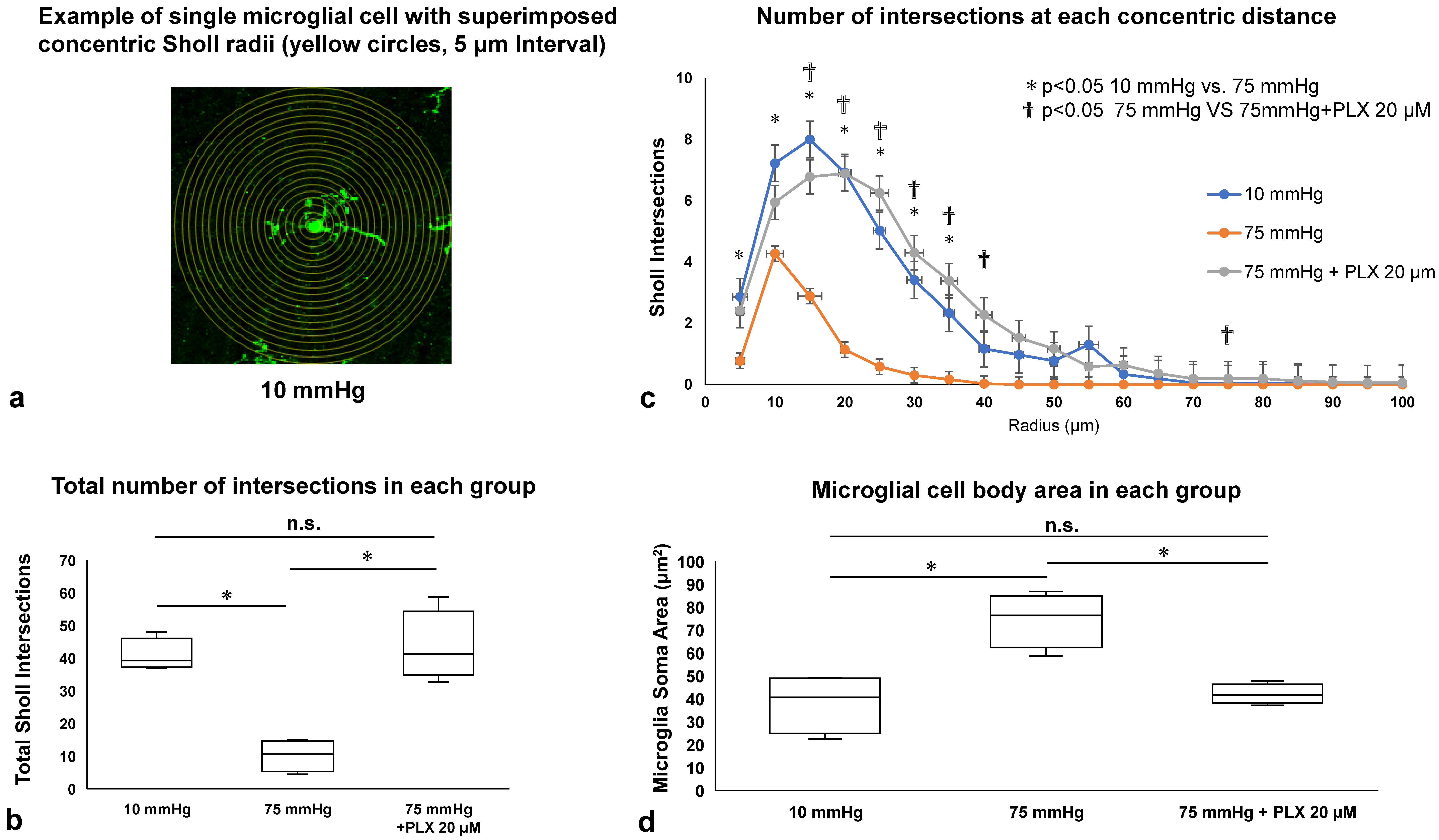
3.4. Microglial Morphology Assessed by Sholl Analysis and Soma Area Measurement
3.5. CD68 Expression Among Iba-1-Positive Microglia
3.6. PLX5622 Preserves the RNA-Binding Protein (RBPMS) Under High Pressure
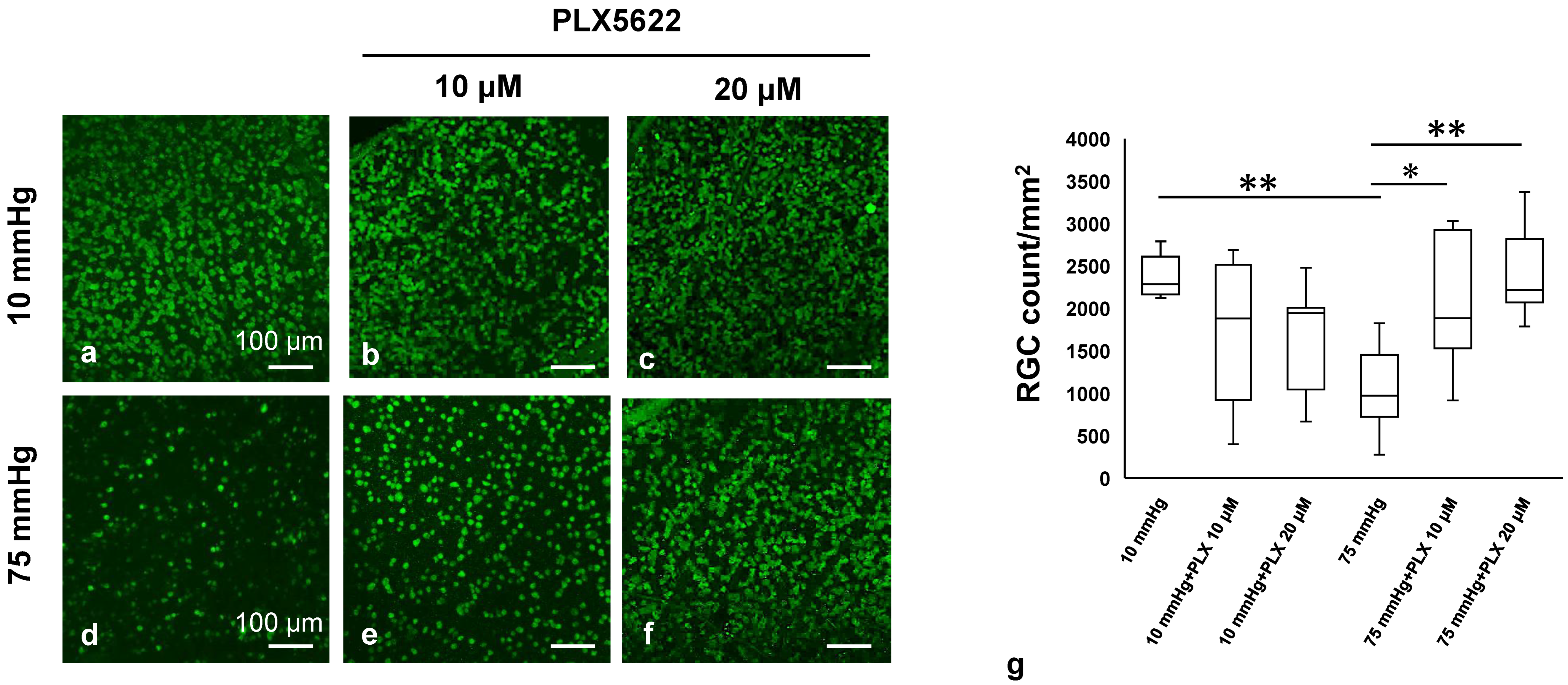
3.7. PLX5622 Prevents Pressure-Induced Apoptosis and Retinal Degeneration
3.8. Effects of PLX5622 on NLRP3 Inflammasome Pathway Markers
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RGC | Retinal ganglion cells |
| AAC | Acute angle-closure attack |
| IOP | Intraocular pressure |
| IL | Interleukin |
| NLRP3 | Nucleotide-binding and oligomerization domain, leucine-rich repeat-, and pyrin domain-containing protein 3 |
| NLR | NOD-like receptor |
| PRR | Pattern recognition receptor |
| TLR | Toll-like receptor |
| I/R | Ischemia/reperfusion |
| CSF-1R | Colony stimulating factor 1 receptor |
| aCSF | Artificial cerebrospinal fluid |
| SD rat | Sprague Dawley rat |
| RBPMS | mRNA processing factor |
| Iba-1 | Ionizing calcium-binding adaptor molecule 1 |
| NFLT | Nerve fiber layer thickness |
| NDS | Neuronal damage score |
| INL | Inner nuclear layer |
| IPL | Inner plexiform layer |
| GCL | Ganglion cell layer |
References
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Quigley, H.A. Neuronal Death in Glaucoma. Prog. Retin. Eye Res. 1999, 18, 39–57. [Google Scholar] [CrossRef]
- Aung, T.; Ang, L.P.; Chan, S.P.; Chew, P.T. Acute primary angle-closure: Long-term intraocular pressure outcome in Asian eyes. Am. J. Ophthalmol. 2001, 131, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Ritch, R. Chapter 4. Glaucoma. In Vision Impairment and Vision Rehabilitation; Silverstone, B., Lang, M.A., Rosenthal, B.P., Faye, E.E., Eds.; Oxford University Press Inc.: New York, NY, USA, 2000; pp. 53–81. [Google Scholar]
- Lowe, R.F. Primary angle-closure glaucoma: A review 5 years after bilateral surgery. Br. J. Ophthalmol. 1973, 57, 457–463. [Google Scholar] [CrossRef][Green Version]
- Lowe, R.F. Acute angle-closure glaucoma the second eye: An analysis of 200 cases. Br. J. Ophthalmol. 1962, 46, 641–650. [Google Scholar] [CrossRef]
- Jiang, N.; Li, Z.; Li, Z.; Zhang, Y.; Yu, Z.; Wan, P.; Zhu, Y.; Li, Y.; Su, W.; Zhuo, Y. Laquinimod exerts anti-inflammatory and antiapoptotic effects in retinal ischemia/reperfusion injury. Int. Immunopharmacol. 2020, 8, 106989. [Google Scholar] [CrossRef]
- Labzin, L.I.; Heneka, M.T.; Latz, E. Innate immunity and neurodegeneration. Annu. Rev. Med. 2018, 69, 437–449. [Google Scholar] [CrossRef]
- Chi, W.; Li, F.; Chen, H.; Wang, Y.; Zhu, Y.; Yang, X.; Zhu, J.; Wu, F.; Ouyang, H.; Ge, J.; et al. Caspase-8 promotes NLRP1/NLRP3 inflammasome activation and IL-1β production in acute glaucoma. Proc. Natl. Acad. Sci. USA 2014, 111, 11181–11186. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.L.; Shi, J.M. The role of microglia in the progression of glaucomatous neurodegeneration. A review. Int. J. Ophthalmol. 2018, 11, 143–149. [Google Scholar] [CrossRef]
- Paschalis, E.I.; Lei, F.; Zhou, C.; Kapoulea, V.; Thanos, A.; Dana, R.; Vavvas, D.G.; Chodosh, J.; Dohlman, C.H. The Role of Microglia and Peripheral Monocytes in Retinal Damage after Corneal Chemical Injury. Am. J. Pathol. 2018, 188, 1580–1596. [Google Scholar] [CrossRef]
- Wei, X.; Cho, K.S.; Thee, E.F.; Jager, M.J.; Chen, D.F. Neuroinflammation and microglia in glaucoma: Time for a paradigm shift. J. Neurosci. Res. 2019, 97, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Wooff, Y.; Man, S.M.; Aggio-Bruce, R.; Natoli, R.; Fernando, N. IL-1 family members mediate cell death, inflammation and angiogenesis in retinal degenerative diseases. Front. Immunol. 2019, 10, 1618. [Google Scholar] [CrossRef]
- Mookherjee, S.; Banerjee, D.; Chakraborty, S.; Banerjee, A.; Mukhopadhyay, I.; Sen, A.; Ray, K. Association of IL1A and IL1B loci with primary open angle glaucoma. BMC Med. Genet. 2010, 11, 99. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Fu, X.X.; Duan, R.; Wei, B.; Cao, H.M.; Yan, E.; Chen, S.Y.; Zhang, Y.D.; Jiang, T. The Alzheimer’s disease-associated gene TREML2 modulates inflammation by regulating microglia polarization and NLRP3 inflammasome activation. Neural Regen. Res. 2023, 18, 434–438. [Google Scholar] [CrossRef]
- Jin, C.; Flavell, R.A. Molecular mechanism of NLRP3 inflammasome activation. J. Clin. Immunol. 2010, 30, 628–631. [Google Scholar] [CrossRef]
- Hanamsagar, R.; Hanke, M.L.; Kielian, T. Toll-like receptor (TLR) and inflammasome actions in the central nervous system. Trends Immunol. 2012, 33, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Lehnardt, S. Innate immunity and neuroinflammation in the CNS: The role of microglia in Toll-like receptor-mediated neuronal injury. Glia 2010, 58, 253–263. [Google Scholar] [CrossRef]
- Saijo, K.; Crotti, A.; Glass, C.K. Regulation of microglia activation and deactivation by nuclear receptors. Glia 2013, 61, 104–111. [Google Scholar] [CrossRef]
- Ganbold, T.; Bao, Q.; Xiao, H.; Zurgaanjin, D.; Liu, C.; Han, S.; Hasi, A.; Baigude, H. Peptidomimetic Lipid-Nanoparticle-Mediated Knockdown of TLR4 in CNS Protects against Cerebral Ischemia/Reperfusion Injury in Mice. Nanomaterials 2022, 12, 2072. [Google Scholar] [CrossRef]
- Shen, Y.; Gong, Z.; Zhang, S.; Cao, J.; Mao, W.; Yao, Y.; Zhao, J.; Li, Q.; Liu, K.; Liu, B.; et al. Besides TLR2 and TLR4, NLRP3 is also involved in regulating Escherichia coli infection-induced inflammatory responses in mice. Int. Immunopharmacol. 2023, 121, 110556. [Google Scholar] [CrossRef]
- Hyakkoku, K.; Hamanaka, J.; Tsuruma, K.; Shimazawa, M.; Tanaka, H.; Uematsu, S.; Akira, S.; Inagaki, N.; Nagai, H.; Hara, H. Toll-like receptor 4 (TLR4), but not TLR3 or TLR9, knock-out mice have neuroprotective effects against focal cerebral ischemia. Neuroscience 2010, 171, 258–267. [Google Scholar] [CrossRef]
- Han, J.; Zhu, K.; Zhang, X.M.; Harris, R.A. Enforced microglial depletion and repopulation as a promising strategy for the treatment of neurological disorders. Glia 2019, 67, 217–231. [Google Scholar] [CrossRef]
- Spiteri, A.G.; Ni, D.; Ling, Z.L.; Macia, L.; Campbell, I.L.; Hofer, M.J.; King, N.J.C. PLX5622 reduces disease severity in lethal CNS infection by off-target inhibition of peripheral inflammatory monocyte production. Front. Immunol. 2022, 13, 851556. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Takaseki, S.; Yoshitomi, T.; Covey, D.F.; Zorumski, C.F.; Izumi, Y. The neurosteroid allopregnanolone protects retinal neurons by effects on autophagy and GABRs/GABAA receptors in rat glaucoma models. Autophagy 2021, 17, 743–760. [Google Scholar] [CrossRef] [PubMed]
- Kwong, J.M.; Caprioli, J.; Piri, N. RNA binding protein with multiple splicing: A new marker for retinal ganglion cells. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1052–1058. [Google Scholar] [CrossRef] [PubMed]
- Sholl, D.A. Dendritic organization in the neurons of the visual and motor cortices of the cat. J. Anat. 1953, 87 Pt 4, 387–406. [Google Scholar]
- Holness, C.L.; Simmons, D.L. Molecular cloning of CD68, a human macrophage marker related to lysosomal glycoproteins. Blood 1993, 6, 1607–1613. [Google Scholar] [CrossRef]
- Yu, C.; Chen, P.; Xu, J.; Liu, Y.; Li, H.; Wang, L.; Di, G. hADSCs derived extracellular vesicles inhibit NLRP3inflammasome activation and dry eye. Sci. Rep. 2020, 10, 14521. [Google Scholar] [CrossRef]
- Teng, J.F.; Mei, Q.B.; Zhou, X.G.; Tang, Y.; Xiong, R.; Qiu, W.Q.; Pan, R.; Law, B.Y.; Wong, V.K.; Yu, C.L.; et al. Polyphyllin VI induces caspase-1-mediated pyroptosis via the induction of ROS/NF-κB/NLRP3/GSDMD signal axis in non-small cell lung cancer. Cancers 2020, 12, 193. [Google Scholar] [CrossRef]
- Baptiste, D.C.; Powell, K.J.; Jollimore, C.A.; Hamilton, C.; LeVatte, T.L.; Archibald, M.L.; Chauhan, B.C.; Robertson, G.S.; Kelly, M.E. Effects of minocycline and tetracycline on retinal ganglion cell survival after axotomy. Neuroscience 2005, 134, 575–582. [Google Scholar] [CrossRef]
- Levkovitch-Verbin, H.; Kalev-Landoy, M.; Habot-Wilner, Z.; Melamed, S. Minocycline delays death of retinal ganglion cells in experimental glaucoma and after optic nerve transection. Arch. Ophthalmol. 2006, 124, 520–526. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kumar, S.; Akopian, A.; Bloomfield, S.A. Neuroprotection of Retinal Ganglion Cells Suppresses Microglia Activation in a Mouse Model of Glaucoma. Investig. Ophthalmol. Vis. Sci. 2023, 64, 24. [Google Scholar] [CrossRef]
- Nakazawa, T.; Nakazawa, C.; Matsubara, A.; Noda, K.; Hisatomi, T.; She, H.; Michaud, N.; Hafezi-Moghadam, A.; Miller, J.W.; Benowitz, L.I. Tumor necrosis factor-alpha mediates oligodendrocyte death and delayed retinal ganglion cell loss in a mouse model of glaucoma. J. Neurosci. 2006, 26, 12633–12641. [Google Scholar] [CrossRef] [PubMed]
- Bosco, A.; Inman, D.M.; Steele, M.R.; Wu, G.; Soto, I.; Marsh-Armstrong, N.; Hubbard, W.C.; Calkins, D.J.; Horner, P.J.; Vetter, M.L. Reduced retina microglial activation and improved optic nerve integrity with minocycline treatment in the DBA/2J mouse model of glaucoma. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1437–1446. [Google Scholar] [CrossRef]
- Patel, S.; Player, M.R. Colony-stimulating factor-1 receptor inhibitors for the treatment of cancer and inflammatory disease. Curr. Top. Med. Chem. 2009, 9, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Chitu, V.; Gokhan, Ş.; Nandi, S.; Mehler, M.F.; Stanley, E.R. Emerging Roles for CSF-1 Receptor and its Ligands in the Nervous System. Trends Neurosci. 2016, 39, 378–393. [Google Scholar] [CrossRef]
- Ruytinx, P.; Proost, P.; Van Damme, J.; Struyf, S. Chemokine-Induced Macrophage Polarization in Inflammatory Conditions. Front. Immunol. 2018, 9, 1930. [Google Scholar] [CrossRef]
- Spangenberg, E.; Severson, P.L.; Hohsfield, L.A.; Crapser, J.; Zhang, J.; Burton, E.A.; Zhang, Y.; Spevak, W.; Lin, J.; Phan, N.Y.; et al. Sustained microglial depletion with CSF1R inhibitor impairs parenchymal plaque development in an Alzheimer’s disease model. Nat. Commun. 2019, 10, 3758. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, L.; Wang, X.; Ma, W.; Lazere, A.; Qian, H.H.; Zhang, J.; Abu-Asab, M.; Fariss, R.N.; Roger, J.E.; et al. Repopulating retinal microglia restore endogenous organization and function under CX3CL1-CX3CR1 regulation. Sci. Adv. 2018, 4, eaap8492. [Google Scholar] [CrossRef]
- Mou, Q.; Yao, K.; Ye, M.; Zhao, B.; Hu, Y.; Lou, X.; Li, H.; Zhang, H.; Zhao, Y. Modulation of Sirt1-mTORC1 Pathway in Microglia Attenuates Retinal Ganglion Cell Loss After Optic Nerve Injury. J. Inflamm. Res. 2021, 14, 6857–6869. [Google Scholar] [CrossRef]
- Takeda, A.; Shinozaki, Y.; Kashiwagi, K.; Ohno, N.; Eto, K.; Wake, H.; Nabekura, J.; Koizumi, S. Microglia mediate non-cell-autonomous cell death of retinal ganglion cells. Glia 2018, 66, 2366–2384. [Google Scholar] [CrossRef]
- Tan, Z.; Guo, Y.; Shrestha, M.; Sun, D.; Gregory-Ksander, M.; Jakobs, T.C. Microglia depletion exacerbates retinal ganglion cell loss in a mouse model of glaucoma. Exp. Eye Res. 2022, 225, 109273. [Google Scholar] [CrossRef]
- Varvel, N.H.; Grathwohl, S.A.; Baumann, F.; Liebig, C.; Bosch, A.; Brawek, B.; Thal, D.R.; Charo, I.F.; Heppner, F.L.; Aguzzi, A.; et al. Microglial repopulation model reveals a robust homeostatic process for replacing CNS myeloid cells. Proc. Natl. Acad. Sci. USA 2012, 109, 18150–18155. [Google Scholar] [CrossRef] [PubMed]
- Bruttger, J.; Karram, K.; Wörtge, S.; Regen, T.; Marini, F.; Hoppmann, N.; Klein, M.; Blank, T.; Yona, S.; Wolf, Y.; et al. Genetic cell ablation reveals clusters of local self-renewing microglia in the mammalian central nervous system. Immunity 2015, 43, 92–106. [Google Scholar] [CrossRef]
- Lund, H.; Pieber, M.; Parsa, R.; Han, J.; Grommisch, D.; Ewing, E.K.; Kular, L.; Needhamsen, M.; Alexander Espinosa, A.; Nilsson, E.; et al. Competitive repopulation of an empty microglial niche yields functionally distinct subsets of microglia-like cells. Nat. Commun. 2018, 9, 4845. [Google Scholar] [CrossRef] [PubMed]
- Margeta, M.A.; Lad, E.M.; Proia, A.D. CD163+ macrophages infiltrate axon bundles of postmortem optic nerves with glaucoma. Graefes Arch. Clin. Exp. Ophthalmol. 2018, 256, 2449–2456. [Google Scholar]
- Lee, N.Y.; Park, H.Y.; Park, C.K.; Ahn, M.D. Analysis of systemic endothelin-1, matrix metalloproteinase-9, macrophage chemoattractant protein-1, and high-sensitivity C-reactive protein in normal-tension glaucoma. Curr. Eye Res. 2012, 37, 1121–1126. [Google Scholar] [CrossRef]
- Gschwandtner, M.; Derler, R.; Midwood, K.S. More than just attractive: How CCL2 influences myeloid cell behavior beyond chemotaxis. Front. Immunol. 2019, 10, 2759. [Google Scholar] [CrossRef]
- Fernandez-Lizarbe, S.; Montesinos, J.; Guerri, C. Ethanol induces TLR4/TLR2 association, triggering an inflammatory response in microglial cells. J. Neurochem. 2013, 126, 261–273. [Google Scholar] [CrossRef]
- Takano, Y.; Shi, D.; Shimizu, A.; Funayama, T.; Mashima, Y.; Yasuda, N.; Fukuchi, T.; Abe, H.; Ideta, H.; Zheng, X.; et al. Association of toll-like receptor 4 gene polymorphisms in Japanese subjects with primary open-angle, normal-tension, and exfoliation glaucoma. Am. J. Ophthalmol. 2012, 154, 825–832. [Google Scholar] [CrossRef]
- Iannucci, A.; Caneparo, V.; Raviola, S.; Debernardi, I.; Colangelo, D.; Miggiano, R.; Griffante, G.; Landolfo, S.; Gariglio, M.; Andrea, M.D. Toll-like receptor 4-mediated inflammation triggered by extracellular IFI16 is enhanced by lipopolysaccharide binding. PLoS Pathog. 2020, 16, e1008811. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F.; Lerner, A. The second phase of brain trauma can be controlled by nutraceuticals that suppress DAMP-mediated microglial activation. Expert. Rev. Neurother. 2021, 21, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Halle, A.; Hornung, V.; Petzold, G.C.; Stewart, C.R.; Monk, S.; Reinheckel, T.; Fitzgerald, K.A.; Latz, E.; Moore, K.J.; Golenbock, D.T. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat. Immunol. 2008, 9, 857–865. [Google Scholar] [CrossRef]
- Fernández-Albarral, J.A.; Salazar, J.J.; de Hoz, R.; Marco, E.M.; Martín-Sánchez, B.; Flores-Salguero, E.; Salobrar-García, E.; López-Cuenca, I.; Barrios-Sabador, V.; Avilés-Trigueros, M.; et al. Retinal molecular changes are associated with neuroinflammation and loss of RGCs in an experimental model of glaucoma. Int. J. Mol. Sci. 2021, 22, 2066. [Google Scholar] [CrossRef]
- Nakano, Y.; Shimazawa, M.; Ojino, K.; Izawa, H.; Takeuchi, H.; Inoue, Y.; Tsuruma, K.; Hara, H. Toll-like receptor 4 inhibitor protects against retinal ganglion cell damage induced by optic nerve crush in mice. J. Pharmacol. Sci. 2017, 133, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Abcouwer, S.F.; Shanmugam, S.; Muthusamy, A.; Lin, C.M.; Kong, D.; Hager, H.; Liu, X.; Antonetti, D.A. Inflammatory resolution and vascular barrier restoration after retinal ischemia/reperfusion injury. J. Neuroinflam. 2021, 18, 186. [Google Scholar] [CrossRef]
- Li, X.; Ye, Z.; Pei, S.; Zheng, D.; Zhu, L. Neuroprotective effect of minocycline on rat retinal ischemia-reperfusion injury. Mol. Vis. 2021, 27, 438–456. [Google Scholar]
- Halder, S.K.; Matsunaga, H.; Ishii, K.J.; Ueda, H. Prothymosin-alpha preconditioning activates TLR4-TRIF signaling to induce protection of ischemic retina. J. Neurochem. 2015, 135, 1161–1177. [Google Scholar] [CrossRef]
- Levkovitch-Verbin, H.; Waserzoog, Y.; Vander, S.; Makarovsky, D.; Piven, I. Minocycline upregulates pro-survival genes and downregulates pro-apoptotic genes in experimental glaucoma. Graefe’s Arch. Clin. Exp. Ophthalmol. 2014, 252, 761–772. [Google Scholar] [CrossRef]
- Ishikawa, M.; Yoshitomi, T.; Zorumski, C.F.; Izumi, Y. Downregulation of glutamine synthetase via GLAST suppression induces retinal axonal swelling in a rat ex vivo hydrostatic pressure model. Investig. Ophthalmol. Vis. Sci. 2011, 52, 6604–6616. [Google Scholar] [CrossRef]
- King, A.E.; Dickson, T.C.; Blizzard, C.A.; Foster, S.S.; Chung, R.S.; Chuah, M.I.; Vickers, J.C. Excitotoxicity mediated by non-NMDA receptors causes distal axonopathy in long-term cultured spinal motor neurons. Eur. J. Neurosci. 2007, 26, 2151–2159. [Google Scholar] [CrossRef] [PubMed]
- Lambuk, L.; Iezhitsa, I.; Agarwal, R.; Bakar, N.S.; Agarwal, P.; Ismail, N.M. Antiapoptotic effect of taurine against NMDA-induced retinal excitotoxicity in rats. Neurotoxicology 2019, 70, 62–71. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, T.; Ishikawa, M.; Izumi, Y.; Shibata, N.; Sato, K.; Ohno-Oishi, M.; Tawarayama, H.; Kunikata, H.; Zorumski, C.F.; Nakazawa, T. Involvement of Microglia in Retinal Ganglion Cell Injury Induced by IOP Elevation in a Rat Ex Vivo Acute Glaucoma Model. Biomedicines 2025, 13, 1670. https://doi.org/10.3390/biomedicines13071670
Sato T, Ishikawa M, Izumi Y, Shibata N, Sato K, Ohno-Oishi M, Tawarayama H, Kunikata H, Zorumski CF, Nakazawa T. Involvement of Microglia in Retinal Ganglion Cell Injury Induced by IOP Elevation in a Rat Ex Vivo Acute Glaucoma Model. Biomedicines. 2025; 13(7):1670. https://doi.org/10.3390/biomedicines13071670
Chicago/Turabian StyleSato, Taimu, Makoto Ishikawa, Yukitoshi Izumi, Naoya Shibata, Kota Sato, Michiko Ohno-Oishi, Hiroshi Tawarayama, Hiroshi Kunikata, Charles F. Zorumski, and Toru Nakazawa. 2025. "Involvement of Microglia in Retinal Ganglion Cell Injury Induced by IOP Elevation in a Rat Ex Vivo Acute Glaucoma Model" Biomedicines 13, no. 7: 1670. https://doi.org/10.3390/biomedicines13071670
APA StyleSato, T., Ishikawa, M., Izumi, Y., Shibata, N., Sato, K., Ohno-Oishi, M., Tawarayama, H., Kunikata, H., Zorumski, C. F., & Nakazawa, T. (2025). Involvement of Microglia in Retinal Ganglion Cell Injury Induced by IOP Elevation in a Rat Ex Vivo Acute Glaucoma Model. Biomedicines, 13(7), 1670. https://doi.org/10.3390/biomedicines13071670





