Heat-Killed Lactobacillus plantarum beLP1 Attenuates Dexamethasone-Induced Sarcopenia in Rats by Increasing AKT Phosphorylation
Abstract
1. Introduction
2. Materials and Methods
2.1. Postbiotic Lactobacillus plantarum 1 (beLP1)
2.2. Establishment of the Cellular Sarcopenia Model and Cell Viability Assay
2.3. Measurements of Myotube Diameters
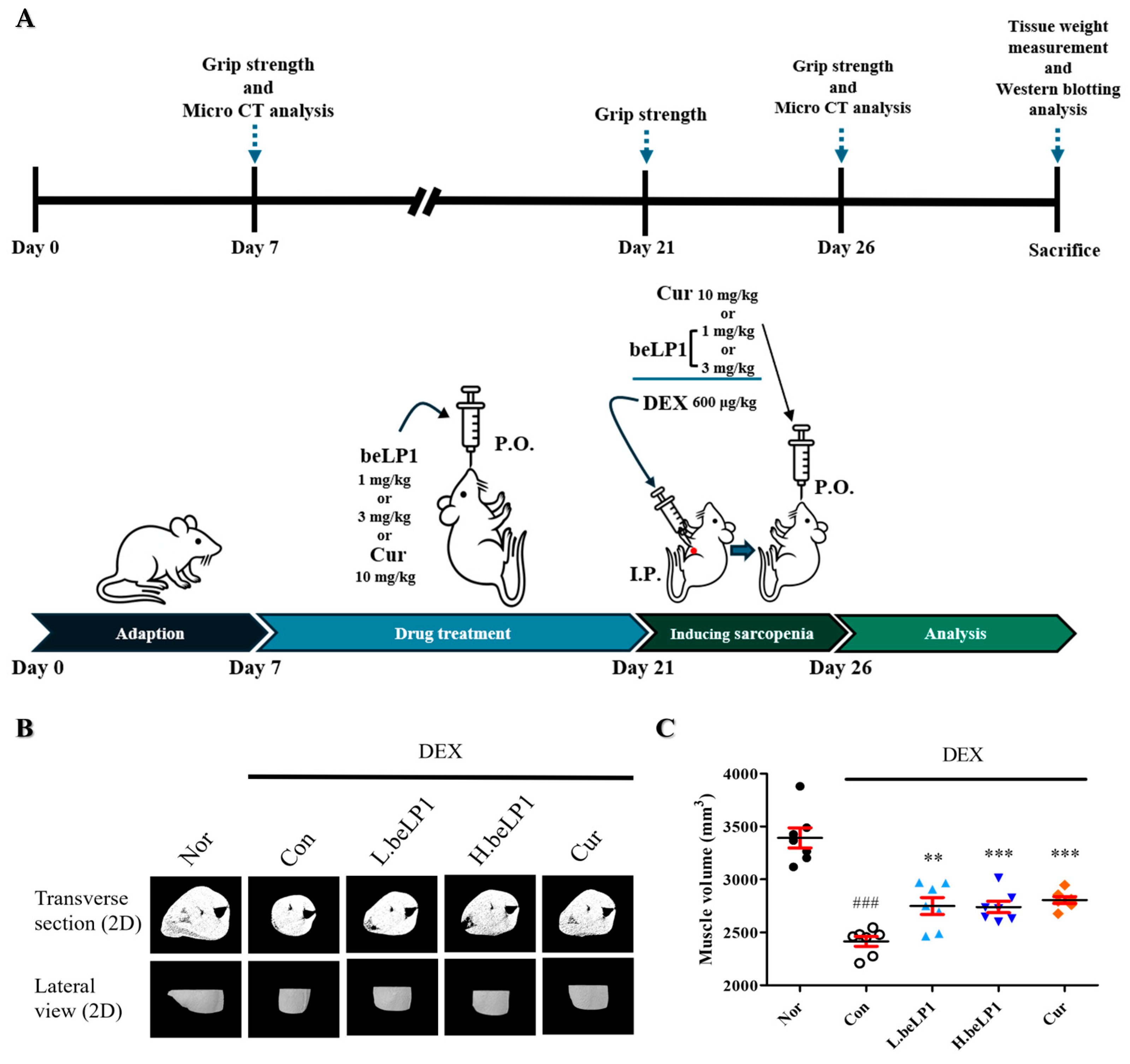
2.4. Animal Experiments
2.5. Grip Strength Test
2.6. Measurement of Muscle Volume Using Micro-Computed Tomography
2.7. Western Blotting Analysis
2.8. Statistical Analysis
3. Results
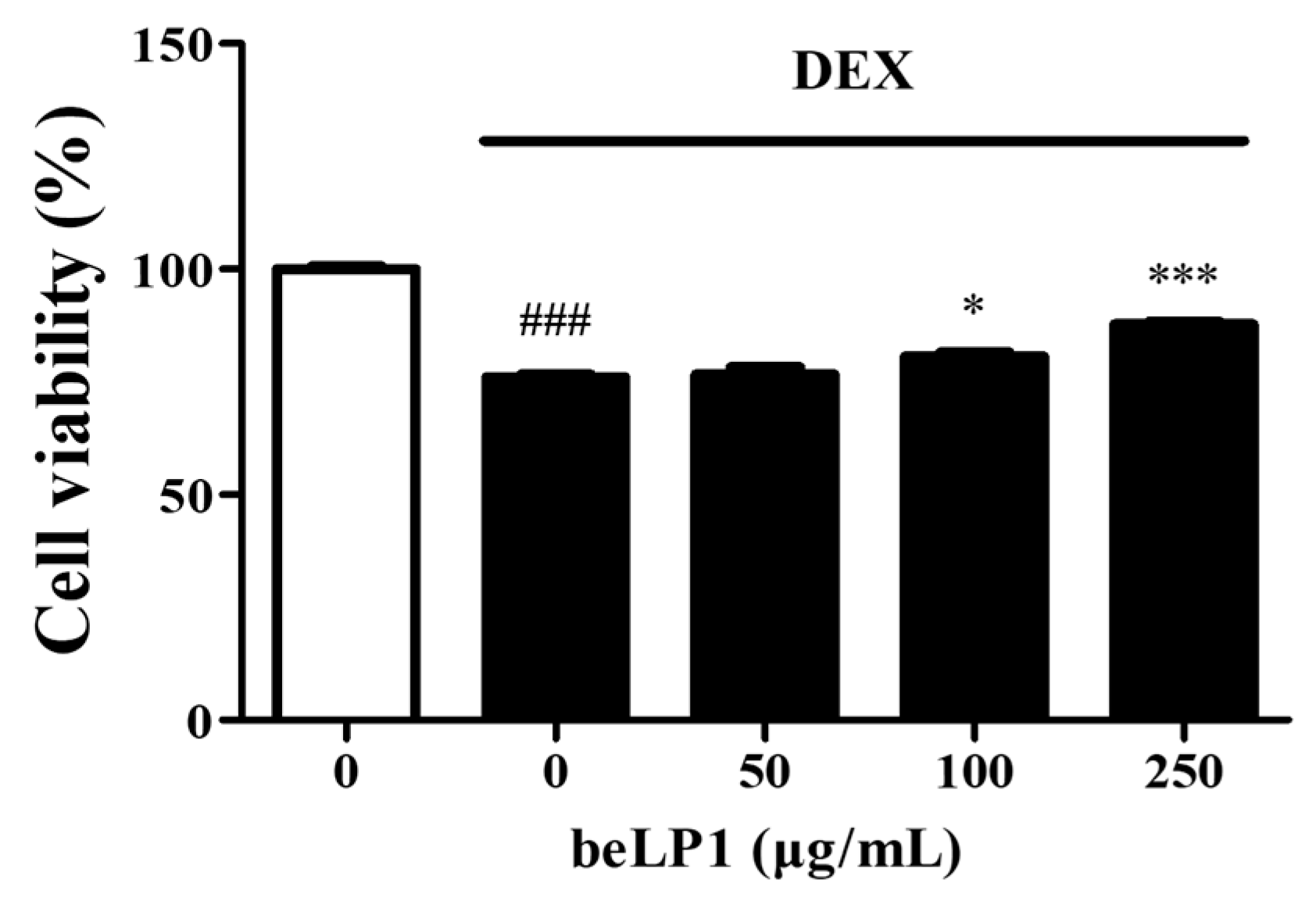
3.1. beLP1 Prevents Dexamethasone-Induced Cell Damage in C2C12 Myotubes
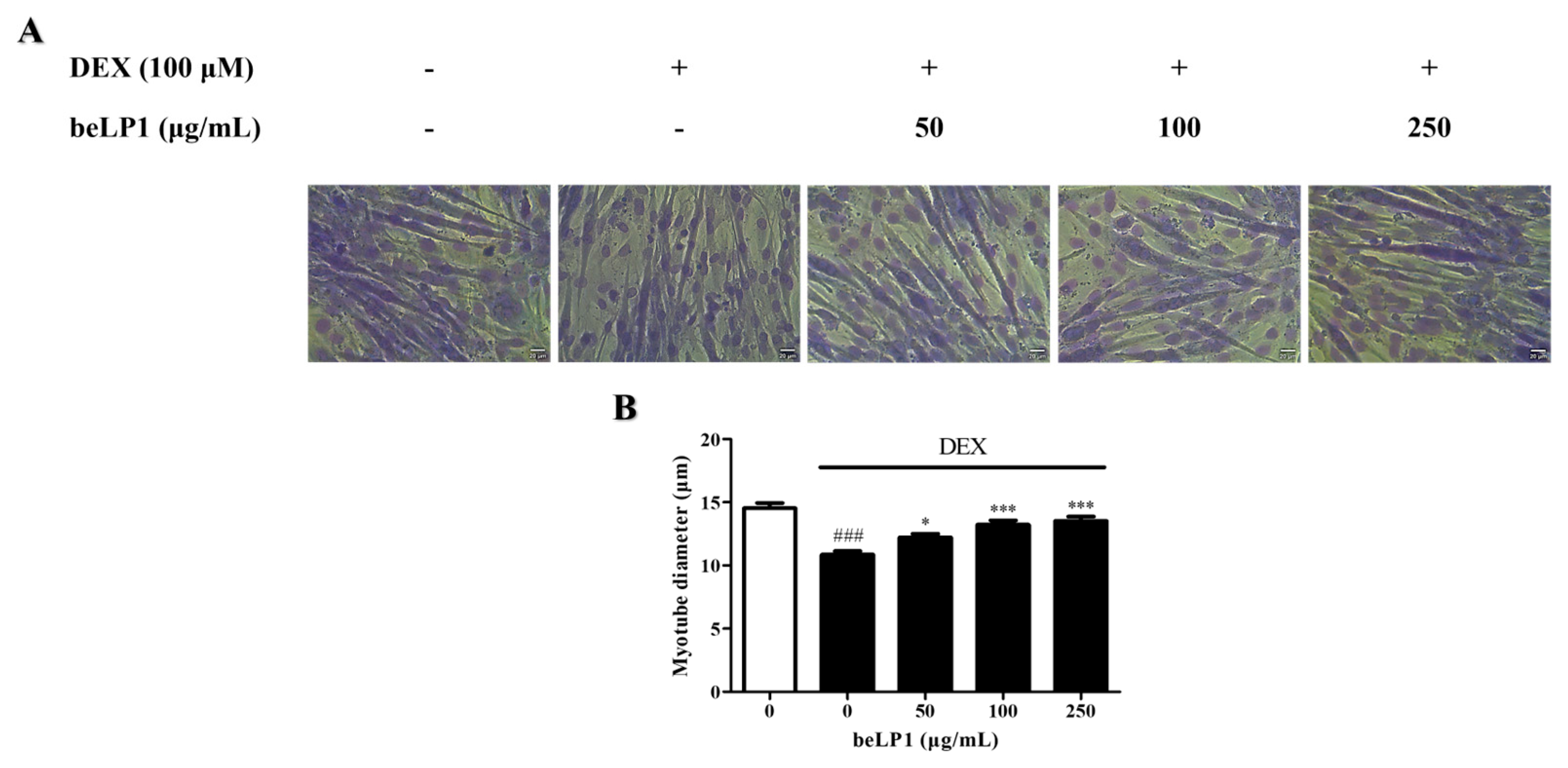
3.2. beLP1 Increases Myotube Diameter Reduced by Dexamethasone in C2C12 Myotubes
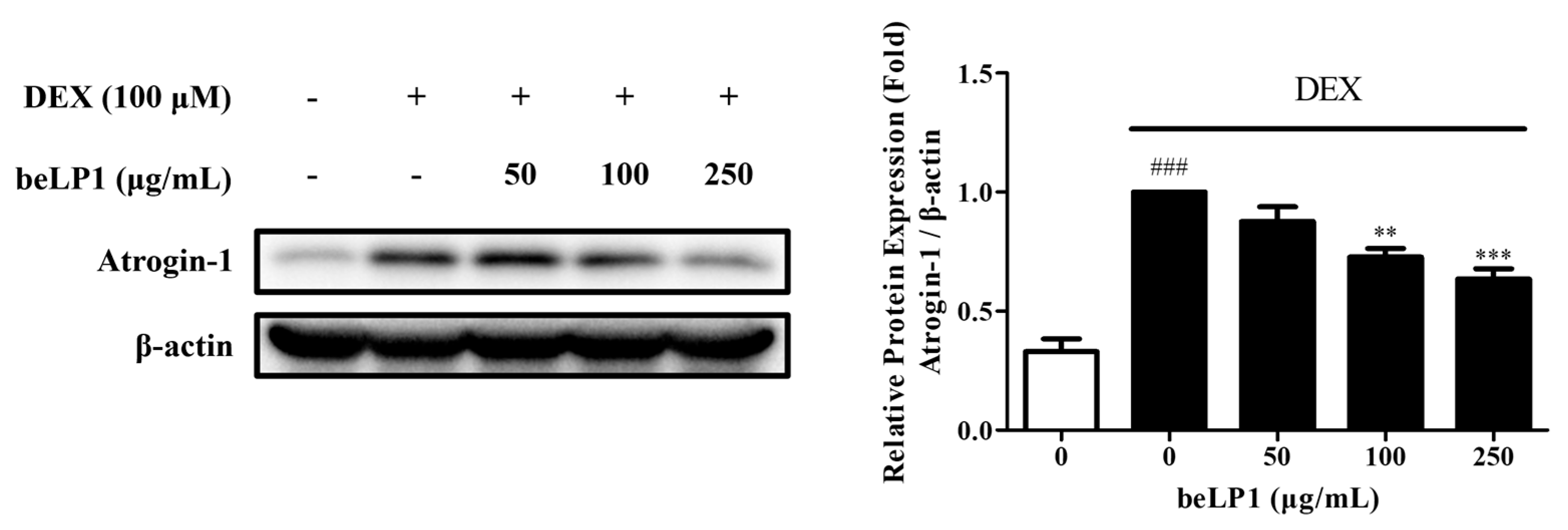
3.3. beLP1 Decreases Atrogin-1 Protein Expression in Dexamethasone-Induced C2C12 Myotube Atrophy Model
3.4. beLP1 Reduces Muscle Volume Loss in Dexamethasone-Induced Sarcopenic Rat Model
3.5. beLP1 Reduces Muscle Weight Loss in Dexamethasone-Induced Sarcopenic Rat Model
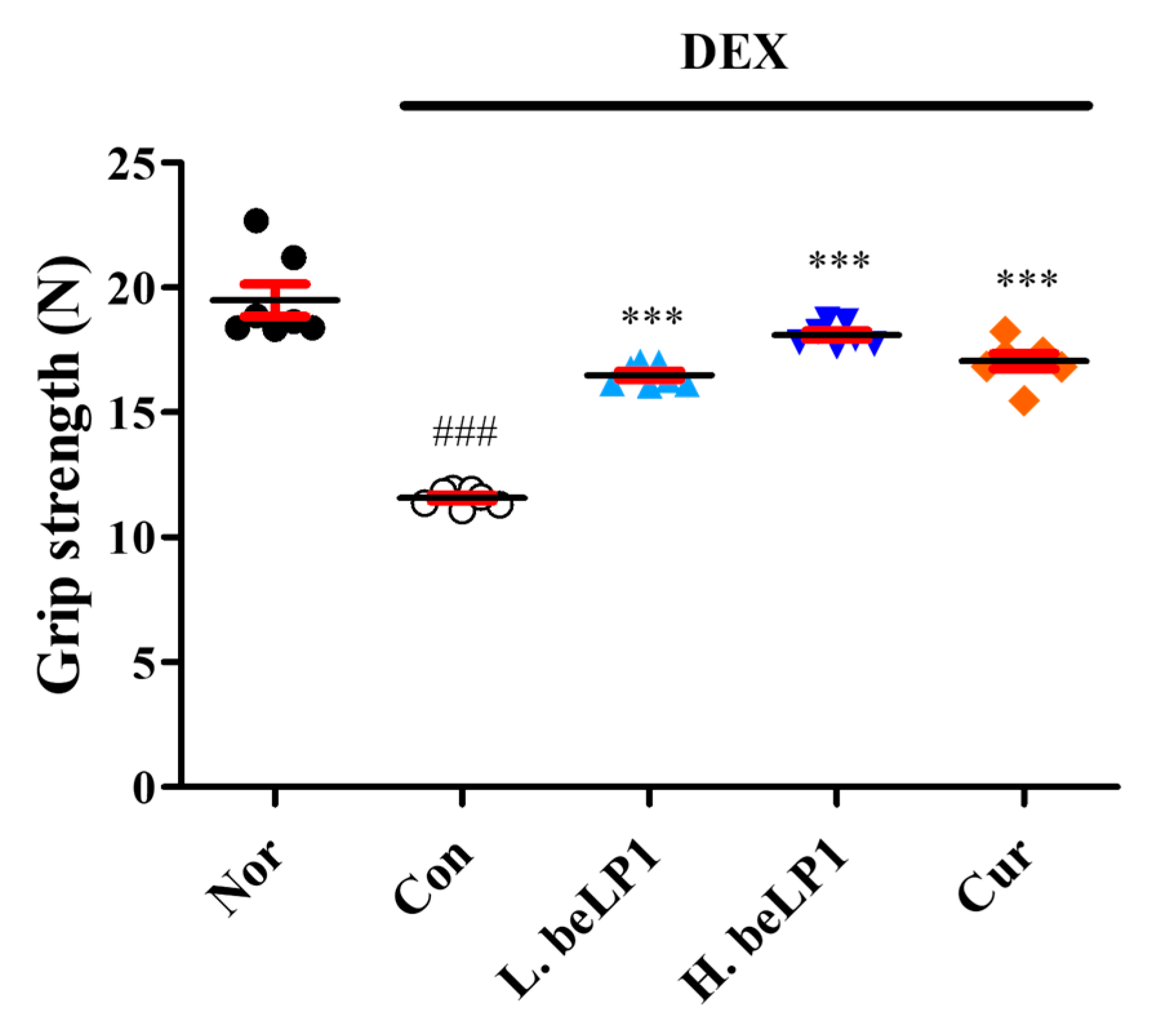
3.6. beLP1 Improves Muscle Strength in Dexamethasone-Induced Sarcopenic Rat Model
3.7. beLP1 Decreases Atrogin-1 and MuRF1 Protein Expression in Dexamethasone-Induced Sarcopenic Rat Model
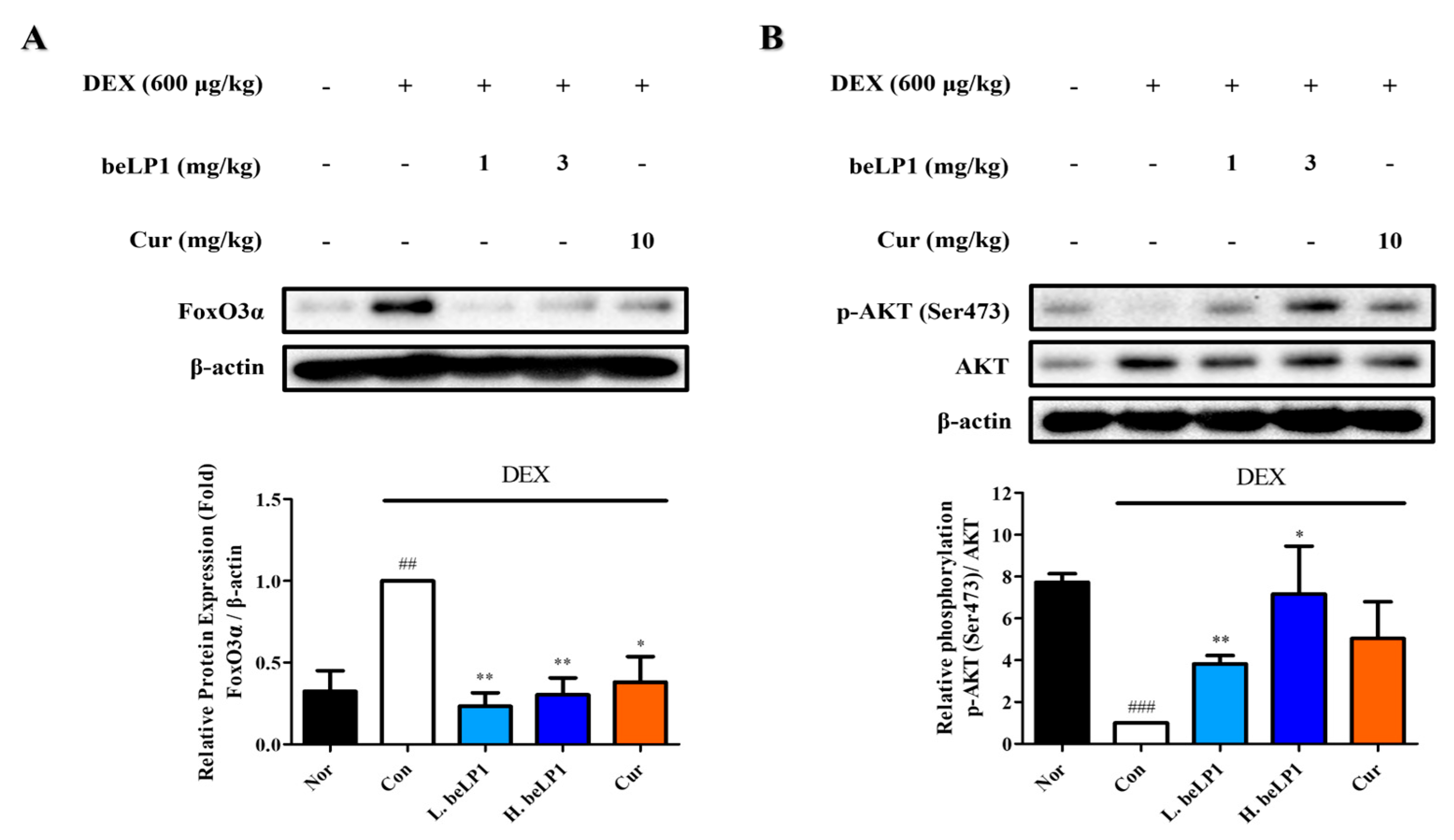
3.8. beLP1 Decreases FoxO3α Protein Expression and Increases AKT Phosphorylation in Dexamethasone-Induced Sarcopenic Rat Model
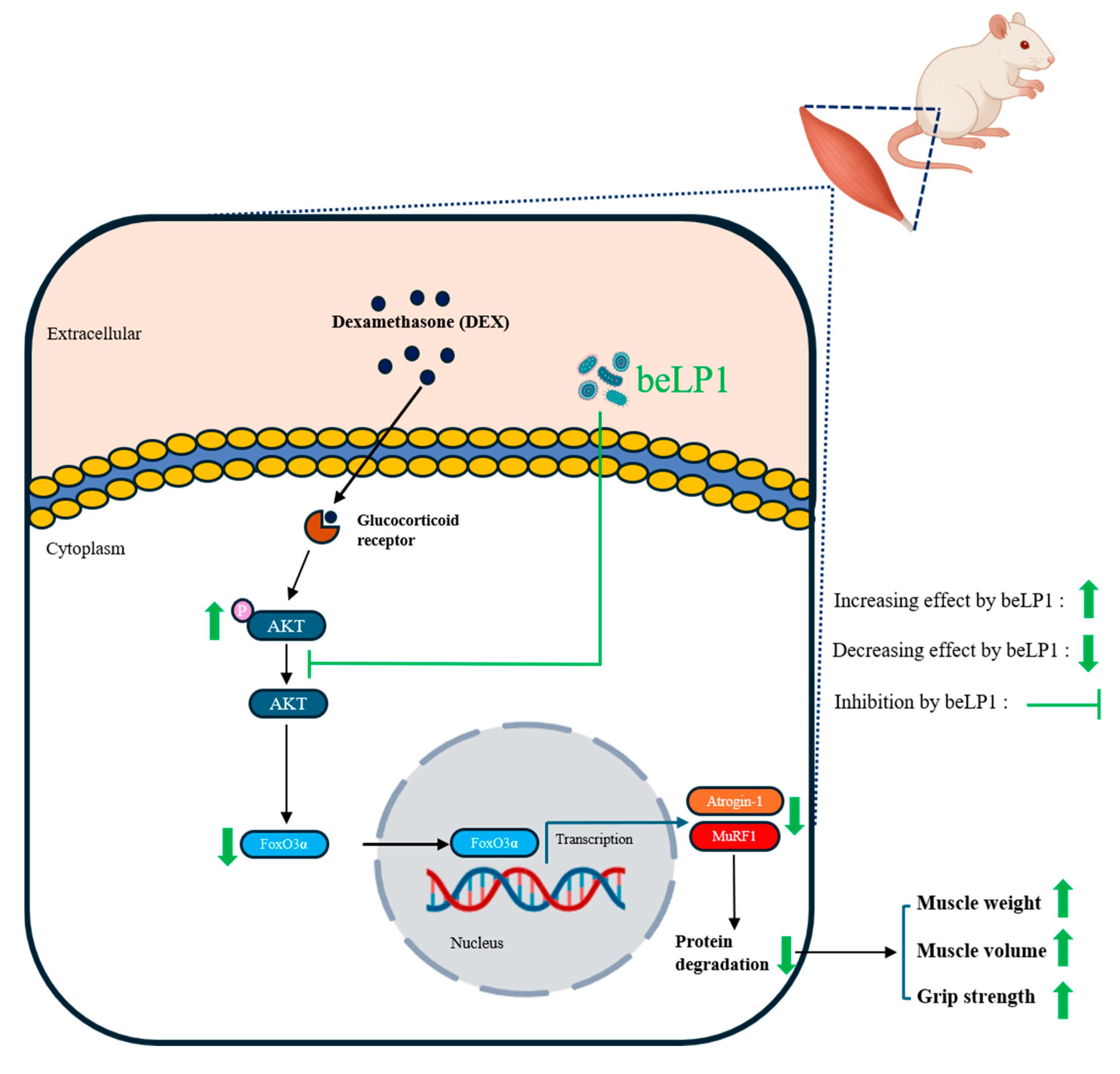
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Nor | Normal group |
| Con | Control group |
| Cur | Curcumin-treated group |
| L.beLP1 | Low-dose beLP1-treated group |
| H.beLP1 | High-dose beLP1-treated group |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| SD | Sprague–Dawley |
| Micro-CT | Micro-computed tomography |
| UPS | Ubiquitin–proteasome system |
References
- Janssen, I.; Heymsfield, S.B.; Ross, R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J. Am. Geriatr. Soc. 2002, 50, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Liperoti, R.; Russo, A.; Giovannini, S.; Tosato, M.; Capoluongo, E.; Bernabei, R.; Onder, G. Sarcopenia as a risk factor for falls in elderly individuals: Results from the ilSIRENTE study. Clin. Nutr. 2012, 31, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; Abbatecola, A.M.; Argiles, J.M.; Baracos, V.; Bauer, J.; Bhasin, S.; Cederholm, T.; Coats, A.J.S.; Cummings, S.R.; Evans, W.J. Sarcopenia with limited mobility: An international consensus. J. Am. Med. Dir. Assoc. 2011, 12, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Morley, J.E.; von Haehling, S. Welcome to the ICD-10 code for sarcopenia. J. Cachexia Sarcopenia Muscle 2016, 7, 512–514. [Google Scholar] [CrossRef] [PubMed]
- Beaudart, C.; Dawson, A.; Shaw, S.; Harvey, N.C.; Kanis, J.; Binkley, N.; Reginster, J.Y.; Chapurlat, R.; Chan, D.; Bruyère, O. Nutrition and physical activity in the prevention and treatment of sarcopenia: Systematic review. Osteoporos. Int. 2017, 28, 1817–1833. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.-R.; Lee, S.; Song, S.-K. A review of sarcopenia pathophysiology, diagnosis, treatment and future direction. J. Korean Med. Sci. 2022, 37, e146. [Google Scholar] [CrossRef] [PubMed]
- Mellen, R.H.; Girotto, O.S.; Marques, E.B.; Laurindo, L.F.; Grippa, P.C.; Mendes, C.G.; Garcia, L.N.H.; Bechara, M.D.; Barbalho, S.M.; Sinatora, R.V. Insights into pathogenesis, nutritional and drug approach in sarcopenia: A systematic review. Biomedicines 2023, 11, 136. [Google Scholar] [CrossRef] [PubMed]
- Bodine, S.C.; Latres, E.; Baumhueter, S.; Lai, V.K.-M.; Nunez, L.; Clarke, B.A.; Poueymirou, W.T.; Panaro, F.J.; Na, E.; Dharmarajan, K. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 2001, 294, 1704–1708. [Google Scholar] [CrossRef] [PubMed]
- Bonaldo, P.; Sandri, M. Cellular and molecular mechanisms of muscle atrophy. Dis. Models Mech. 2013, 6, 25–39. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bodine, S.C.; Stitt, T.N.; Gonzalez, M.; Kline, W.O.; Stover, G.L.; Bauerlein, R.; Zlotchenko, E.; Scrimgeour, A.; Lawrence, J.C.; Glass, D.J. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat. Cell Biol. 2001, 3, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.D.; Lecker, S.H.; Jagoe, R.T.; Navon, A.; Goldberg, A.L. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc. Natl. Acad. Sci. USA 2001, 98, 14440–14445. [Google Scholar] [CrossRef] [PubMed]
- Bilodeau, P.A.; Coyne, E.S.; Wing, S.S. The ubiquitin proteasome system in atrophying skeletal muscle: Roles and regulation. Am. J. Physiol.-Cell Physiol. 2016, 311, C392–C403. [Google Scholar] [CrossRef] [PubMed]
- Sandri, M. Signaling in muscle atrophy and hypertrophy. Physiology 2008, 23, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Altun, M.; Besche, H.C.; Overkleeft, H.S.; Piccirillo, R.; Edelmann, M.J.; Kessler, B.M.; Goldberg, A.L.; Ulfhake, B. Muscle wasting in aged, sarcopenic rats is associated with enhanced activity of the ubiquitin proteasome pathway. J. Biol. Chem. 2010, 285, 39597–39608. [Google Scholar] [CrossRef] [PubMed]
- Glass, D.J. Signalling pathways that mediate skeletal muscle hypertrophy and atrophy. Nat. Cell Biol. 2003, 5, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Chargé, S.B.; Rudnicki, M.A. Cellular and molecular regulation of muscle regeneration. Physiol. Rev. 2004, 84, 209–238. [Google Scholar] [CrossRef] [PubMed]
- Bartke, A. Growth hormone and aging: A challenging controversy. Clin. Interv. Aging 2008, 3, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Verdijk, L.B.; Dirks, M.L.; Snijders, T.; Prompers, J.J.; Beelen, M.; Jonkers, R.; Thijssen, D.; Hopman, M.; Van Loon, L. Reduced satellite cell numbers with spinal cord injury and aging in humans. Med. Sci. Sports Exerc. 2012, 44, 2322–2330. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Jackson, M.J. Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [CrossRef] [PubMed]
- Kozakowska, M.; Pietraszek-Gremplewicz, K.; Jozkowicz, A.; Dulak, J. The role of oxidative stress in skeletal muscle injury and regeneration: Focus on antioxidant enzymes. J. Muscle Res. Cell Motil. 2015, 36, 377–393. [Google Scholar] [CrossRef] [PubMed]
- Shokri-Mashhadi, N.; Navab, F.; Ansari, S.; Rouhani, M.H.; Hajhashemy, Z.; Saraf-Bank, S. A meta-analysis of the effect of probiotic administration on age-related sarcopenia. Food Sci. Nutr. 2023, 11, 4975–4987. [Google Scholar] [CrossRef] [PubMed]
- Saxelin, M.; Tynkkynen, S.; Mattila-Sandholm, T.; de Vos, W.M. Probiotic and other functional microbes: From markets to mechanisms. Curr. Opin. Biotechnol. 2005, 16, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Boyle, R.J.; Robins-Browne, R.M.; Tang, M.L. Probiotic use in clinical practice: What are the risks? Am. J. Clin. Nutr. 2006, 83, 1256–1264. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Toalá, J.; Garcia-Varela, R.; Garcia, H.; Mata-Haro, V.; González-Córdova, A.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Postbiotics: An evolving term within the functional foods field. Trends Food Sci. Technol. 2018, 75, 105–114. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol 2021, 18, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Kim, J.; Park, S.-D.; Shim, J.-J.; Lee, J.-L. Lactobacillus plantarum HY7715 ameliorates sarcopenia by improving skeletal muscle mass and function in aged Balb/c mice. Int. J. Mol. Sci. 2021, 22, 10023. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, A.E.; Chapman, K.E. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol. Cell. Endocrinol. 2011, 335, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Schakman, O.; Kalista, S.; Barbé, C.; Loumaye, A.; Thissen, J.-P. Glucocorticoid-induced skeletal muscle atrophy. Int. J. Biochem. Cell Biol. 2013, 45, 2163–2172. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.Y.-H.; Hsiao, A.W.-T.; Wong, N.; Chen, Y.-F.; Lee, C.-W.; Lee, W.Y.W. Is dexamethasone-induced muscle atrophy an alternative model for naturally aged sarcopenia model? J. Orthop. Transl. 2023, 39, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, K.; Park, J.; Jun, W. Curcuma longa L. Water extract improves dexamethasone-induced sarcopenia by modulating the muscle-related gene and oxidative stress in mice. Antioxidants 2021, 10, 1000. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, K.; Kousaka, M.; Jia, H.; Kato, H. Suppressive effects of turmeric extract on muscle atrophy in dexamethasone-treated mice and myotubes. Nutrients 2022, 14, 3979. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-Y.; Chun, Y.-S.; Kim, J.-K.; Lee, J.-O.; Ku, S.-K.; Shim, S.-M. Curcumin attenuates sarcopenia in chronic forced exercise executed aged mice by regulating muscle degradation and protein synthesis with antioxidant and anti-inflammatory effects. J. Agric. Food Chem. 2021, 69, 6214–6228. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.-J.; Kim, J.-H.; Jung, Y.-J.; Kwak, M.-S.; Sung, M.-H.; Imm, J.-Y. KL-Biome (Postbiotic Formulation of Lactiplantibacillus plantarum KM2) Improves dexamethasone-induced muscle atrophy in mice. Int. J. Mol. Sci. 2024, 25, 7499. [Google Scholar] [CrossRef] [PubMed]
- Jackman, R.W.; Kandarian, S.C. The molecular basis of skeletal muscle atrophy. Am. J. Physiol.-Cell Physiol. 2004, 287, C834–C843. [Google Scholar] [CrossRef] [PubMed]
- Schakman, O.; Gilson, H.; Thissen, J.-P. Mechanisms of glucocorticoid-induced myopathy. J. Endocrinol. 2008, 197, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sandri, M.; Sandri, C.; Gilbert, A.; Skurk, C.; Calabria, E.; Picard, A.; Walsh, K.; Schiaffino, S.; Lecker, S.H.; Goldberg, A.L. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 2004, 117, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Stitt, T.N.; Drujan, D.; Clarke, B.A.; Panaro, F.; Timofeyva, Y.; Kline, W.O.; Gonzalez, M.; Yancopoulos, G.D.; Glass, D.J. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol. Cell 2004, 14, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kang, M.; Yoo, J.; Lee, S.; Kang, M.; Yun, B.; Kim, J.N.; Moon, H.; Chung, Y.; Oh, S. Lactobacillus rhamnosus JY02 ameliorates sarcopenia by anti-atrophic effects in a dexamethasone-induced cellular and murine model. J. Microbiol. Biotechnol. 2023, 33, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Kang, M.; Yoo, J.; Lee, J.; Lee, S.; Yun, B.; Song, M.; Kim, J.-M.; Kim, H.W.; Yang, J. Dietary supplementation with Lacticaseibacillus rhamnosus IDCC3201 alleviates sarcopenia by modulating the gut microbiota and metabolites in dexamethasone-induced models. Food Funct. 2024, 15, 4936–4953. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.-W.; Huang, C.-C.; Hsu, Y.-J.; Chen, C.-J.; Lee, P.-Y.; Huang, Y.-H.; Lee, M.-C.; Chiu, Y.-S.; Tung, Y.-T. Accelerated muscle recovery after in vivo curcumin supplementation. Nat. Prod. Commun. 2020, 15, 1934578X20901898. [Google Scholar] [CrossRef]
- Nishida, K.; Sawada, D.; Kuwano, Y.; Tanaka, H.; Rokutan, K. Health benefits of Lactobacillus gasseri CP2305 tablets in young adults exposed to chronic stress: A randomized, double-blind, placebo-controlled study. Nutrients 2019, 11, 1859. [Google Scholar] [CrossRef] [PubMed]
- Schultz, M.; Linde, H.-J.; Lehn, N.; Zimmermann, K.; Grossmann, J.; Falk, W.; Schölmerich, J. Immunomodulatory consequences of oral administration of Lactobacillus rhamnosus strain GG in healthy volunteers. J. Dairy Res. 2003, 70, 165–173. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.; Jeong, E.; Park, H.; Song, H.-Y.; Moon, J.; Kim, M.-a.; Koo, B.S.; Lee, J.-H.; Hong, J.K.; Han, K.-I.; et al. Heat-Killed Lactobacillus plantarum beLP1 Attenuates Dexamethasone-Induced Sarcopenia in Rats by Increasing AKT Phosphorylation. Biomedicines 2025, 13, 1668. https://doi.org/10.3390/biomedicines13071668
Choi J, Jeong E, Park H, Song H-Y, Moon J, Kim M-a, Koo BS, Lee J-H, Hong JK, Han K-I, et al. Heat-Killed Lactobacillus plantarum beLP1 Attenuates Dexamethasone-Induced Sarcopenia in Rats by Increasing AKT Phosphorylation. Biomedicines. 2025; 13(7):1668. https://doi.org/10.3390/biomedicines13071668
Chicago/Turabian StyleChoi, Jinsu, Eunwoo Jeong, Harang Park, Hye-Yeong Song, Juyeong Moon, Min-ah Kim, Bon Seo Koo, Jin-Ho Lee, Jong Kwang Hong, Kwon-Il Han, and et al. 2025. "Heat-Killed Lactobacillus plantarum beLP1 Attenuates Dexamethasone-Induced Sarcopenia in Rats by Increasing AKT Phosphorylation" Biomedicines 13, no. 7: 1668. https://doi.org/10.3390/biomedicines13071668
APA StyleChoi, J., Jeong, E., Park, H., Song, H.-Y., Moon, J., Kim, M.-a., Koo, B. S., Lee, J.-H., Hong, J. K., Han, K.-I., Kim, D., Kim, H. S., & Kim, T.-J. (2025). Heat-Killed Lactobacillus plantarum beLP1 Attenuates Dexamethasone-Induced Sarcopenia in Rats by Increasing AKT Phosphorylation. Biomedicines, 13(7), 1668. https://doi.org/10.3390/biomedicines13071668






