Systemic Inflammation Index (SII) as a Predictor of Mortality in Intensive Care Units
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vincent, J.-L.; Marshall, J.C.; A Ñamendys-Silva, S.A.; François, B.; Martin-Loeches, I.; Lipman, J.; Reinhart, K.; Antonelli, M.; Pickkers, P.; Njimi, H.; et al. Assessment of the worldwide burden of critical illness: The Intensive Care Over Nations (ICON) audit. Lancet Respir. Med. 2014, 2, 380–386. [Google Scholar] [CrossRef]
- Wang, R.-H.; Wen, W.-X.; Jiang, Z.-P.; Du, Z.-P.; Ma, Z.-H.; Lu, A.-L.; Li, H.-P.; Yuan, F.; Wu, S.-B.; Guo, J.-W.; et al. The clinical value of neutrophil-to-lymphocyte ratio (NLR), systemic immune-inflammation index (SII), platelet-to-lymphocyte ratio (PLR) and systemic inflammation response index (SIRI) for predicting the occurrence and severity of pneumonia in patients with intracerebral hemorrhage. Front. Immunol. 2023, 14, 1115031. [Google Scholar] [CrossRef]
- Wang, J.; Sun, Y.; Teng, S.; Li, K. Prediction of sepsis mortality using metabolite biomarkers in the blood: A meta-analysis of death-related pathways and prospective validation. BMC Med. 2020, 18, 83. [Google Scholar] [CrossRef]
- Fois, A.G.; Paliogiannis, P.; Scano, V.; Cau, S.; Babudieri, S.; Perra, R.; Ruzzittu, G.; Zinellu, E.; Pirina, P.; Carru, C.; et al. The Systemic Inflammation Index on Admission Predicts In-Hospital Mortality in COVID-19 Patients. Molecules 2020, 25, 5725. [Google Scholar] [CrossRef]
- Jia, L.; Li, C.; Bi, X.; Wei, F.; Meng, J.; Sun, G.; Yu, H.; Dong, H.; Li, B.; Cao, Y.; et al. Prognostic Value of Systemic Immune-Inflammation Index among Critically Ill Patients with Acute Kidney Injury: A Retrospective Cohort Study. J. Clin. Med. 2022, 11, 3978. [Google Scholar] [CrossRef]
- Jiang, D.; Bian, T.; Shen, Y.; Huang, Z. Association between admission systemic immune-inflammation index and mortality in critically ill patients with sepsis: A retrospective cohort study based on MIMIC-IV database. Clin. Exp. Med. 2023, 23, 3641–3650. [Google Scholar] [CrossRef]
- Mangalesh, S.; Dudani, S.; Malik, A. The systemic immune-inflammation index in predicting sepsis mortality. Postgrad. Med. 2023, 135, 345–351. [Google Scholar] [CrossRef]
- Sun, J.; Qi, Y.; Wang, W.; Meng, P.; Han, C.; Chen, B. Systemic Immune-Inflammation Index (SII) as a Predictor of Short-Term Mortality Risk in Sepsis-Associated Acute Kidney Injury: A Retrospective Cohort Study. Med. Sci. Monit. 2024, 30, e943414–1. [Google Scholar] [CrossRef]
- Zhao, G.; Gu, Y.; Wang, Z.; Chen, Y.; Xia, X. The clinical value of inflammation index in predicting ICU mortality of critically ill patients with intracerebral hemorrhage. Front. Public Health 2024, 12, 1373585. [Google Scholar] [CrossRef]
- Parmana, I.M.A.; Boom, C.E.; Poernomo, H.; Gani, C.; Nugroho, B.; Cintyandy, R.; Sanjaya, L.; Hadinata, Y.; Parna, D.R.; Hanafy, D.A. Systemic Immune-Inflammation Index Predicts Prolonged Mechanical Ventilation and Intensive Care Unit Stay After off-Pump Coronary Artery Bypass Graft Surgery: A Single-Center Retrospective Study. Vasc. Health Risk Manag. 2023, 19, 353–361. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, S.; Zhang, Y. High level of systemic immune inflammation index elevates delirium risk among patients in intensive care unit. Sci. Rep. 2024, 14, 30265. [Google Scholar] [CrossRef]
- Nøst, T.H.; Alcala, K.; Urbarova, I.; Byrne, K.S.; Guida, F.; Sandanger, T.M.; Johansson, M. Systemic inflammation markers and cancer incidence in the UK Biobank. Eur. J. Epidemiol. 2021, 36, 841. [Google Scholar] [CrossRef]
- Takahashi, H.; Tsuda, Y.; Kobayashi, M.; Herndon, D.N.; Suzuki, F. CCL2 as a trigger of manifestations of compensatory anti-inflammatory response syndrome in mice with severe systemic inflammatory response syndrome. J. Leukoc. Biol. 2006, 79, 789–796. [Google Scholar] [CrossRef][Green Version]
- Arslan, K.; Sahin, A.S. Prognostic value of systemic immune-inflammation index, neutrophil-lymphocyte ratio, and thrombocyte-lymphocyte ratio in critically ill patients with moderate to severe traumatic brain injury. Medicine 2024, 103, e39007. [Google Scholar] [CrossRef]
- Fever and Antipyretic in Critically ill patients Evaluation (FACE) Study Group; Lee, B.H.; Inui, D.; Suh, G.Y.; Kim, J.Y.; Kwon, J.Y.; Park, J.; Tada, K.; Tanaka, K.; Ietsugu, K.; et al. Association of body temperature and antipyretic treatments with mortality of critically ill patients with and without sepsis: Multi-centered prospective observational study. Crit. Care 2012, 16, R33. [Google Scholar] [CrossRef]
- Weissman, G.E.M.; Hubbard, R.A.; Ungar, L.H.; Harhay, M.O.; Greene, C.S.; Himes, B.E.; Halpern, S.D. Inclusion of Unstructured Clinical Text Improves Early Prediction of Death or Prolonged ICU Stay. Crit. Care Med. 2018, 46, 1125–1132. [Google Scholar] [CrossRef]
- Emgin, O.; Rollas, K.; Saritas, A.; Ersan, G.; Senoglu, N. Dexamethasone in critically ill patients admitted to intensive care unit with COVID-19 pneumonia. Egypt. J. Crit. Care Med. 2023, 10, 35–40. [Google Scholar]
- Gourd, N.M.; Nikitas, N. Multiple Organ Dysfunction Syndrome. J. Intensive Care Med. 2019, 35, 1564–1575. [Google Scholar] [CrossRef]
- Yu, Z.; Ashrafi, N.; Li, H.; Alaei, K.; Pishgar, M. Prediction of 30-day mortality for ICU patients with Sepsis-3. BMC Med. Inform. Decis. Mak. 2024, 24, 223. [Google Scholar] [CrossRef]
- Juneja, D.; Jain, N.; Singh, O.; Goel, A.; Arora, S. Comparison between presepsin, procalcitonin, and CRP as biomarkers to diagnose sepsis in critically ill patients. J. Anaesthesiol. Clin. Pharmacol. 2023, 39, 458. [Google Scholar] [CrossRef]
- Zhan, Y.-F.; Li, F.; Wu, L.-C.; Li, J.-M.; Zhu, C.-Y.; Han, M.-S.; Sheng, Y. Role of Charlson comorbidity index in predicting the ICU admission in patients with thoracic aortic aneurysm undergoing surgery. J. Orthop. Surg. Res. 2023, 18, 1–10. [Google Scholar] [CrossRef]
- Fang, X.; Li, S.; Yu, H.; Wang, P.; Zhang, Y.; Chen, Z.; Li, Y.; Cheng, L.; Li, W.; Jia, H.; et al. Epidemiological, comorbidity factors with severity and prognosis of COVID-19: A systematic review and meta-analysis. Aging 2020, 12, 12493–12503. [Google Scholar] [CrossRef]
- Duarte, P.A.; Costa, J.B.; Duarte, S.T.; Taba, S.; Lordani, C.R.F.; Osaku, E.F.; Costa, C.R.L.M.; Miglioranza, D.C.; Gund, D.P.; Jorge, A.C. Characteristics and Outcomes of Intensive Care Unit Survivors: Experience of a Multidisciplinary Outpatient Clinic in a Teaching Hospital. Clinics 2017, 72, 764. [Google Scholar] [CrossRef]
- Gyawali, B.; Ramakrishna, K.; Dhamoon, A.S. Sepsis: The evolution in definition, pathophysiology, and management. SAGE Open Med. 2019, 7, 205031211983504. [Google Scholar] [CrossRef]
- Wunsch, H.; Linde-Zwirble, W.T.; Angus, D.C.; Hartman, M.E.; Milbrandt, E.B.; Kahn, J.M. The epidemiology of mechanical ventilation use in the United States. Crit. Care Med. 2010, 38, 1947–1953. [Google Scholar] [CrossRef]
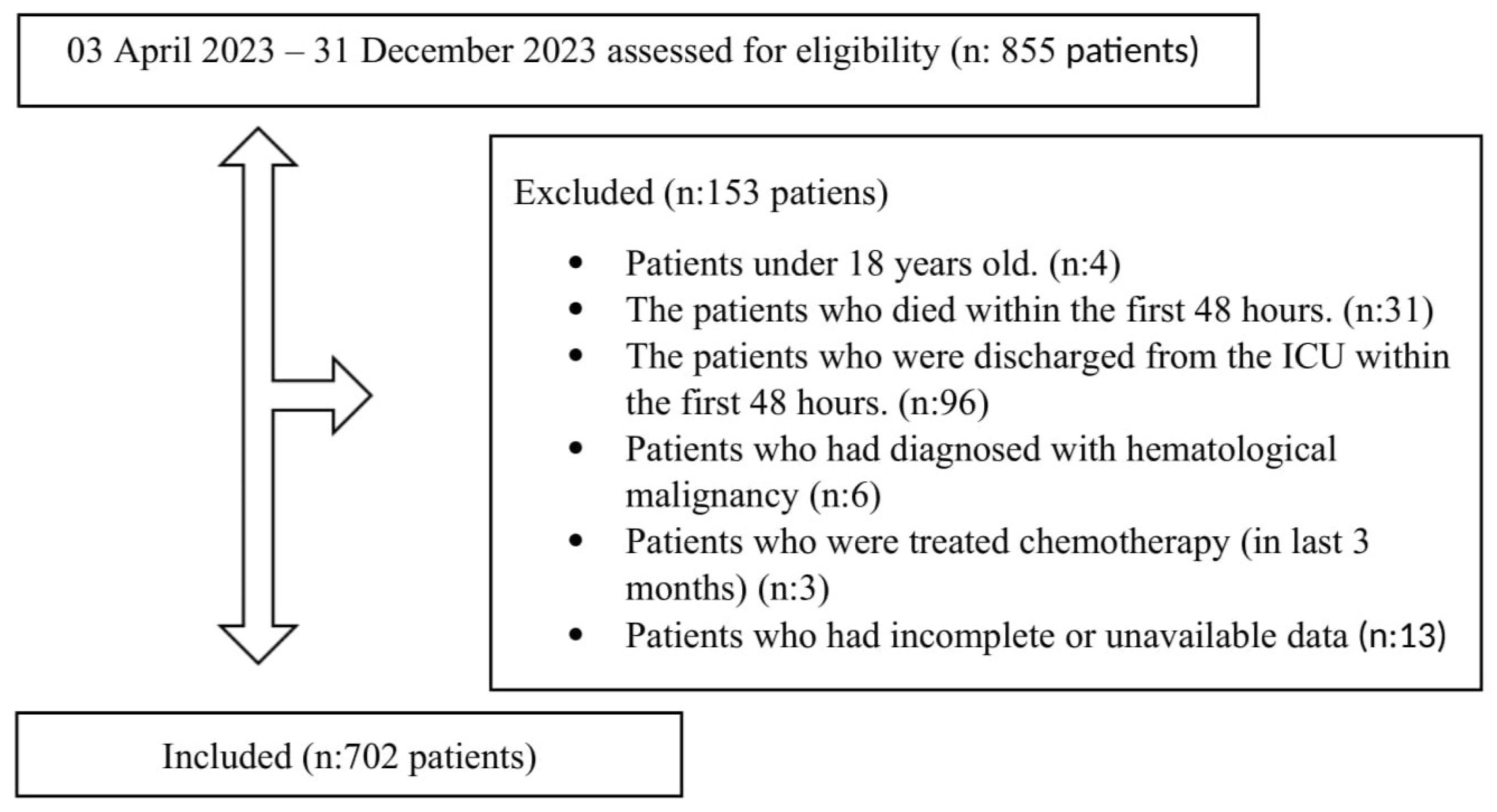
| All Patients n:702 (100%) | Survivors n:443 (63.1%) | Non-Survivors n:259 (36.9%) | p-Value | |
|---|---|---|---|---|
| Age (median years) | 70 (57–80) | 69 (55–79) | 73 (61–81) | 0.001 |
| Gender (n (%)) | ||||
| Female | 292 (41.6) | 194 (43.8) | 98 (37.8) | 0.112 |
| Male | 410 (58.4) | 249 (56.2) | 161 (62.2) | |
| Comorbidities (n (%)) | ||||
| Hypertension | 338 (48.1) | 199 (44.9) | 139 (53.7) | 0.025 |
| Diabetes Mellitus | 219 (31.2) | 127 (28.7) | 92 (35.5) | 0.059 |
| Congestive Heart Disease | 200 (28.5) | 106 (23.9) | 94 (36.3) | <0.001 |
| Malignancy | 165 (23.5) | 88 (19.9) | 77 (29.7) | 0.003 |
| Coronary Artery Disease | 136 (19.4) | 66 (14.9) | 70 (27) | <0.001 |
| COPD | 112 (16.0) | 54 (12.2) | 58 (22.4) | <0.001 |
| Cerebrovascular Disease | 108 (15.4) | 69 (15.6) | 39 (15.1) | 0.854 |
| Chronic Kidney Disease | 73 (10.4) | 32 (7.2) | 41 (15.8) | <0.001 |
| Liver Disease | 6 (0.9) | 2 (0.5) | 4 (1.5) | 0.129 |
| Causes of ICU Admission (n (%)) | ||||
| Sepsis | 182 (25.9) | 77 (17.4) | 105 (40.5) | <0.001 |
| Respiratory causes | 81 (11.5) | 47 (10.6) | 34 (13.1) | 0.314 |
| Neurological causes | 82 (11.7) | 53 (12.0) | 29 (11.2) | 0.760 |
| Trauma | 63 (9.0) | 46 (10.4) | 17 (6.6) | 0.088 |
| Postoperative patients | 247 (35.2) | 192(43.3) | 55 (21.2) | <0.001 |
| The other causes | 39 (5.6) | 23 (5.2) | 16 (6.2) | 0.582 |
| First day diagnosis/treatment (n (%)) | ||||
| Acute Kidney Injury | 100 (14.2) | 44 (9.9) | 56 (21.6) | <0.001 |
| Hemodyalysis | 58 (8.3) | 24 (5.4) | 34 (13.1) | <0.001 |
| Severity Scores | ||||
| APACHE-II scores | 19.97 ± 8.30 | 17.11 ± 7.53 | 24.86 ± 7.21 | <0.001 |
| SOFA scores | 5 (3–8) | 4 (2–7) | 8 (5–10) | <0.001 |
| CCI | 5 (2–9) | 4 (1–8) | 7 (4–10) | <0.001 |
| IMV treatment | 355 (50.6) | 178 (40.2) | 177 (68.3) | <0.001 |
| Duration of IMV (days) | 10 (4–24) | 16 (3–39) | 8 (5–15) | <0.001 |
| Length of stay in ICUs (days) | 8 (5–18) | 7 (4–23) | 9 (5–18) | 0.263 |
| Length of stay in hospital (days) | 15 (8–25) | 15 (10–33) | 14 (7–22) | <0.001 |
| 90-day mortality | 332 (47.3) |
| All Patients n:702 (100%) | Survivors n:443 (63.1%) | Non-Survivors n:259 (36.9%) | p-Value | |
|---|---|---|---|---|
| Hemoglobin (g/dL) | 10.90 (9.40–12.60) | 11.10 (9.70–13.00) | 10.50 (9.00–12.10) | <0.001 |
| Leukocyte (103/μL) | 12.51 (8.83–17.31) | 11.94 (8.70–16.52) | 13.42 (9.22–18.12) | 0.009 |
| Neutrophil (103/μL) | 10.69 (7.02–16.33) | 9.94 (6.71–14.26) | 11.91(7.92–16.73) | <0.001 |
| Lymphocyte (103/μL) | 0.90(0.56–1.40) | 0.95 (0.65–1.48) | 0.79 (0.42–1.22) | <0.001 |
| Platelet (103/μL) | 228 (166–304) | 234 (180–305) | 205 (139–300) | 0.001 |
| INR | 1.15 (1.06–1.30) | 1.12(1.04–1.25) | 1.24 (1.10–1.47) | <0.001 |
| aPTT (second) | 29.55 (26.40–34.70) | 28.80 (26.02–32.80) | 32.05 (27.17–38.80) | <0.001 |
| Glucose (mg/dL) | 148 (117–194) | 146 (117–187) | 151 (120–208) | 0.216 |
| Creatinine (mg/dL) | 0.99 (0.69–1.62) | 0.87 (0.65–1.23) | 1.23 (0.83–2.27) | <0.001 |
| Sodium (mmol/L) | 139 (136–142) | 139 (136–142) | 138 (135–143) | 0.200 |
| Potassium (mmol/L) | 4.20 (3.70–4.80) | 4.20 (3.80–4.60) | 4.20 (3.60–5.00) | 0.409 |
| Chlorine (mmol/L) | 103 (99–107) | 103 (100–107) | 102 (98–108) | 0.047 |
| a-c Calcium (mg/dL) | 9.52 (8.94–10.28) | 9.44 (8.92–10.10) | 9.74 (8.96–10.44) | 0.001 |
| Magnesium (mg/dL) | 1.93 (1.71–2.20) | 1.90 (1.68–2.14) | 2.01 (1.79–2.29) | 0.001 |
| Phosphorus (mg/dL) | 3.60 (2.90–4.50) | 3.50 (2.90–4.20) | 3.90 (3.10–5.30) | <0.001 |
| AST (U/L) | 33 (21–63) | 30 (20–55) | 39 (23–87) | <0.001 |
| ALT (U/L) | 20 (12–39) | 19 (12–36) | 22 (13–46) | 0.034 |
| Total bilirubin (mg/dL) | 0.57 (0.36–0.94) | 0.57 (0.37–0.86) | 0.57 (0.36–1.15) | 0.266 |
| Direct bilirubin (mg/dL) | 0.27 (0.16–0.49) | 0.25 (0.15–0.40) | 0.30 (0.19–0.74) | <0.001 |
| Total Protein (g/dL) | 5.66 (4.98–6.39) | 5.77 (5.08–6.46) | 5.46 (4.81–6.24) | 0.001 |
| Albumin (g/dL) | 3.10 (2.50–3.60) | 3.20 (2.70–3.70) | 2.80 (2.30–3.30) | <0.001 |
| CRP (mg/L) | 65.00 (16.57–158.11 | 50.70 (10.21–143.00) | 92.01 (24.30–179.06) | <0.001 |
| Procalcitonin (µg/L) | 0.42 (0.13–1.80) | 0.26 (0.009–0.89) | 0.86 (0.27–3.9) | <0.001 |
| pH | 7.39 (7.31–7.45) | 7.40 (7.34–7.45) | 7.36 (7.27–7.44) | <0.001 |
| Lactate (mmol/L) | 1.71 (1.22–2.57) | 1.55 (1.13–2.22) | 2.03 (1.44–3.04) | <0.001 |
| Inflammatory Parameters | All Patients n:702 (100%) | Survivors n:443(63.1) | Non-Survivors n:259 (36.9%) | p-Value |
|---|---|---|---|---|
| SII | 2573.19 (1257.06–4805.89) | 2461.73 (1193.89–4295.11) | 2890.33 (1392.34–6645.25) | 0.010 |
| NLR | 11.55 (6.18–20.89) | 10.42 (5.62–17.11) | 15.86 (6.96–28.59) | <0.001 |
| PLR | 243.74 (145.94–412.28) | 243.10 (146.60–397.56) | 259.09 (147.05–444.23) | 0.326 |
| Risk Factors | OR (95% CI) | p Value |
|---|---|---|
| APACHE-II score | 1.065 (1.028–1.102) | <0.001 |
| SOFA | 1.202 (1.107–1.304) | <0.001 |
| CCI | 1.111 (1.064–1.160) | <0.001 |
| CRP (mg/L) | 1.002 (1.000–1.004) | 0.051 |
| Lactate (mmol/liter) | 1.156 (1.053–1.269) | 0.002 |
| Creatinine (mg/dL) | 1.097 (0.937–1.283) | 0.251 |
| SII/1000 | 1.029 (1.001–1.057) | 0.042 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emgin, Ö.; Kılıç, E.R.; Taşkıran, İ.; Haftacı, E.; Ata, A.; Yılmaz, M. Systemic Inflammation Index (SII) as a Predictor of Mortality in Intensive Care Units. Biomedicines 2025, 13, 1669. https://doi.org/10.3390/biomedicines13071669
Emgin Ö, Kılıç ER, Taşkıran İ, Haftacı E, Ata A, Yılmaz M. Systemic Inflammation Index (SII) as a Predictor of Mortality in Intensive Care Units. Biomedicines. 2025; 13(7):1669. https://doi.org/10.3390/biomedicines13071669
Chicago/Turabian StyleEmgin, Ömer, Elif Rana Kılıç, İmren Taşkıran, Engin Haftacı, Adnan Ata, and Mehmet Yılmaz. 2025. "Systemic Inflammation Index (SII) as a Predictor of Mortality in Intensive Care Units" Biomedicines 13, no. 7: 1669. https://doi.org/10.3390/biomedicines13071669
APA StyleEmgin, Ö., Kılıç, E. R., Taşkıran, İ., Haftacı, E., Ata, A., & Yılmaz, M. (2025). Systemic Inflammation Index (SII) as a Predictor of Mortality in Intensive Care Units. Biomedicines, 13(7), 1669. https://doi.org/10.3390/biomedicines13071669






