Inefficacy of Repetitive Transcranial Magnetic Stimulation in Parkinson’s Disease Patients with Levodopa-Induced Dyskinesias: Results from a Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Inclusion Criteria
2.3. Procedure
2.4. Transcranial Magnetic Stimulation
2.5. Clinical Evaluation
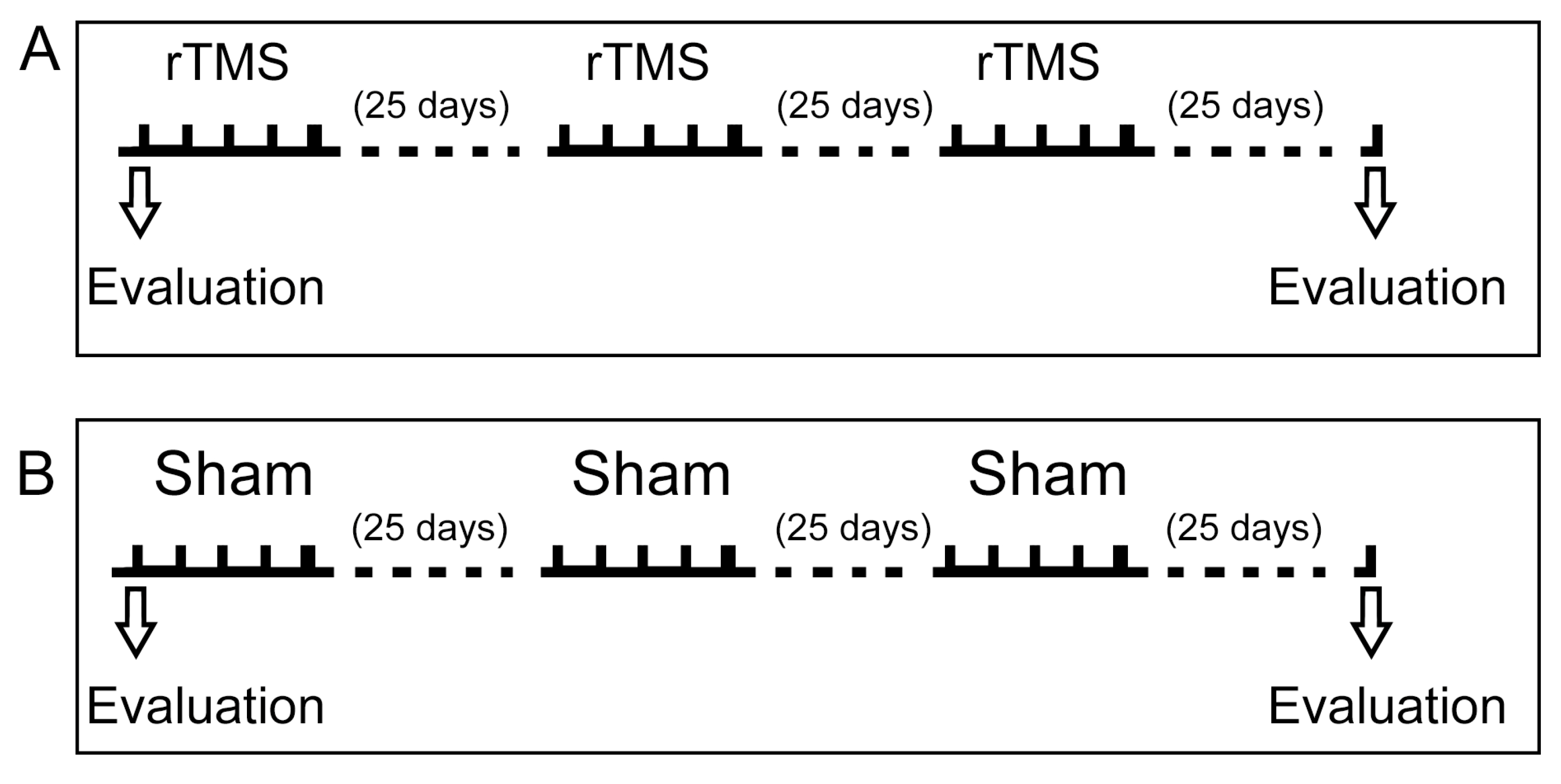
2.6. Study Design
2.7. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Clinical Evaluation Showed No Differences Between Groups Before and After Intervention
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martinez-Ramirez, D.; Rodriguez-Violante, M.; Velazquez-Avila, E.S.; Cervantes-Arriaga, A.; Gonzalez-Cantu, A.; Corona, T.; Velasquez-Perez, L. Incidence and geographic distribution of Parkinson’s disease in Mexico. Salud Publica Mex. 2020, 62, 873–875. [Google Scholar] [CrossRef] [PubMed]
- Kulisevsky, J. Pharmacological management of Parkinson’s disease motor symptoms: Update and recommendations from an expert. Rev. Neurol. 2022, 75, S1–S10. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-Arriaga, A.; Rodríguez-Violante, M.; López-Ruiz, M.; Estrada-Bellmann, I.; Zuñiga-Ramírez, C.; Otero-Cerdeira, E.; Camacho-Ordoñez, A.; González-Latapi, P.; Morales-Briceño, H.; Martínez-Ramírez, D. Profile characterization of Parkinson’s disease in Mexico: ReMePARK study. Gac. Med. Mex. 2013, 149, 497–501. [Google Scholar] [PubMed]
- Guridi, J.; Gonzalez-Redondo, R.; Obeso, J.A. Clinical features, pathophysiology, and treatment of levodopa-induced dyskinesias in Parkinson’s disease. Parkinsons Dis. 2012, 2012, 943159. [Google Scholar] [CrossRef]
- Wu, J.; Lim, E.C.; Nadkarni, N.V.; Tan, E.K.; Kumar, P.M. The impact of levodopa therapy-induced complications on quality of life in Parkinson’s disease patients in Singapore. Sci. Rep. 2019, 9, 9248. [Google Scholar] [CrossRef]
- Hansen, C.A.; Miller, D.R.; Annarumma, S.; Rusch, C.T.; Ramirez-Zamora, A.; Khoshbouei, H. Levodopa-induced dyskinesia: A historical review of Parkinson’s disease, dopamine, and modern advancements in research and treatment. J. Neurol. 2022, 269, 2892–2909. [Google Scholar] [CrossRef]
- Deuschl, G.; Schade-Brittinger, C.; Krack, P.; Volkmann, J.; Schafer, H.; Botzel, K.; Daniels, C.; Deutschlander, A.; Dillmann, U.; Eisner, W.; et al. A randomized trial of deep-brain stimulation for Parkinson’s disease. N. Engl. J. Med. 2006, 355, 896–908. [Google Scholar] [CrossRef]
- Espay, A.J.; Morgante, F.; Merola, A.; Fasano, A.; Marsili, L.; Fox, S.H.; Bezard, E.; Picconi, B.; Calabresi, P.; Lang, A.E. Levodopa-induced dyskinesia in Parkinson disease: Current and evolving concepts. Ann. Neurol. 2018, 84, 797–811. [Google Scholar] [CrossRef]
- Ramirez-Zamora, A.; Ostrem, J.L. Globus Pallidus Interna or Subthalamic Nucleus Deep Brain Stimulation for Parkinson Disease: A Review. JAMA Neurol. 2018, 75, 367–372. [Google Scholar] [CrossRef]
- Fan, S.Y.; Wang, K.L.; Hu, W.; Eisinger, R.S.; Han, A.; Han, C.L.; Wang, Q.; Michitomo, S.; Zhang, J.G.; Wang, F.; et al. Pallidal versus subthalamic nucleus deep brain stimulation for levodopa-induced dyskinesia. Ann. Clin. Transl. Neurol. 2020, 7, 59–68. [Google Scholar] [CrossRef]
- Fitzgerald, P.B.; Fountain, S.; Daskalakis, Z.J. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin. Neurophysiol. 2006, 117, 2584–2596. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, J.P.; Andre-Obadia, N.; Antal, A.; Ayache, S.S.; Baeken, C.; Benninger, D.H.; Cantello, R.M.; Cincotta, M.; de Carvalho, M.; De Ridder, D.; et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin. Neurophysiol. 2014, 125, 2150–2206. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, J.P. Transcranial magnetic stimulation. Handb. Clin. Neurol. 2019, 160, 559–580. [Google Scholar] [CrossRef]
- Kricheldorff, J.; Goke, K.; Kiebs, M.; Kasten, F.H.; Herrmann, C.S.; Witt, K.; Hurlemann, R. Evidence of Neuroplastic Changes after Transcranial Magnetic, Electric, and Deep Brain Stimulation. Brain Sci. 2022, 12, 929. [Google Scholar] [CrossRef]
- Hallett, M. Transcranial magnetic stimulation: A primer. Neuron 2007, 55, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, J.C.; Hallett, M.; Berardelli, A.; Eisen, A.; Rossini, P.; Paulus, W. Magnetic stimulation: Motor evoked potentials. The International Federation of Clinical Neurophysiology. Electroencephalogr. Clin. Neurophysiol. Suppl. 1999, 52, 97–103. [Google Scholar]
- Pascual-Leone, A.; Valls-Sole, J.; Wassermann, E.M.; Hallett, M. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain 1994, 117 Pt 4, 847–858. [Google Scholar] [CrossRef] [PubMed]
- Ziemann, U. TMS induced plasticity in human cortex. Rev. Neurosci. 2004, 15, 253–266. [Google Scholar] [CrossRef]
- Ziemann, U.; Paulus, W.; Nitsche, M.A.; Pascual-Leone, A.; Byblow, W.D.; Berardelli, A.; Siebner, H.R.; Classen, J.; Cohen, L.G.; Rothwell, J.C. Consensus: Motor cortex plasticity protocols. Brain Stimul. 2008, 1, 164–182. [Google Scholar] [CrossRef]
- Siebner, H.R.; Bergmann, T.O.; Bestmann, S.; Massimini, M.; Johansen-Berg, H.; Mochizuki, H.; Bohning, D.E.; Boorman, E.D.; Groppa, S.; Miniussi, C.; et al. Consensus paper: Combining transcranial stimulation with neuroimaging. Brain Stimul. 2009, 2, 58–80. [Google Scholar] [CrossRef]
- Eldaief, M.C.; Halko, M.A.; Buckner, R.L.; Pascual-Leone, A. Transcranial magnetic stimulation modulates the brain’s intrinsic activity in a frequency-dependent manner. Proc. Natl. Acad. Sci. USA 2011, 108, 21229–21234. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.X.; Rogers, L.M.; Gross, E.Z.; Ryals, A.J.; Dokucu, M.E.; Brandstatt, K.L.; Hermiller, M.S.; Voss, J.L. Targeted enhancement of cortical-hippocampal brain networks and associative memory. Science 2014, 345, 1054–1057. [Google Scholar] [CrossRef] [PubMed]
- Raij, T.; Nummenmaa, A.; Marin, M.F.; Porter, D.; Furtak, S.; Setsompop, K.; Milad, M.R. Prefrontal Cortex Stimulation Enhances Fear Extinction Memory in Humans. Biol. Psychiatry 2018, 84, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Ziemann, U.; Hallett, M.; Cohen, L.G. Mechanisms of deafferentation-induced plasticity in human motor cortex. J. Neurosci. 1998, 18, 7000–7007. [Google Scholar] [CrossRef]
- Cirillo, G.; Di Pino, G.; Capone, F.; Ranieri, F.; Florio, L.; Todisco, V.; Tedeschi, G.; Funke, K.; Di Lazzaro, V. Neurobiological after-effects of non-invasive brain stimulation. Brain Stimul. 2017, 10, 1–18. [Google Scholar] [CrossRef]
- Touge, T.; Gerschlager, W.; Brown, P.; Rothwell, J.C. Are the after-effects of low-frequency rTMS on motor cortex excitability due to changes in the efficacy of cortical synapses? Clin. Neurophysiol. 2001, 112, 2138–2145. [Google Scholar] [CrossRef]
- Peinemann, A.; Reimer, B.; Loer, C.; Quartarone, A.; Munchau, A.; Conrad, B.; Siebner, H.R. Long-lasting increase in corticospinal excitability after 1800 pulses of subthreshold 5 Hz repetitive TMS to the primary motor cortex. Clin. Neurophysiol. 2004, 115, 1519–1526. [Google Scholar] [CrossRef]
- Rothkegel, H.; Sommer, M.; Paulus, W. Breaks during 5Hz rTMS are essential for facilitatory after effects. Clin. Neurophysiol. 2010, 121, 426–430. [Google Scholar] [CrossRef]
- Lefaucheur, J.P.; Aleman, A.; Baeken, C.; Benninger, D.H.; Brunelin, J.; Di Lazzaro, V.; Filipovic, S.R.; Grefkes, C.; Hasan, A.; Hummel, F.C.; et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014–2018). Clin. Neurophysiol. 2020, 131, 474–528. [Google Scholar] [CrossRef]
- Gonzalez-Garcia, N.; Armony, J.L.; Soto, J.; Trejo, D.; Alegria, M.A.; Drucker-Colin, R. Effects of rTMS on Parkinson’s disease: A longitudinal fMRI study. J. Neurol. 2011, 258, 1268–1280. [Google Scholar] [CrossRef]
- Chou, Y.H.; Hickey, P.T.; Sundman, M.; Song, A.W.; Chen, N.K. Effects of repetitive transcranial magnetic stimulation on motor symptoms in Parkinson disease: A systematic review and meta-analysis. JAMA Neurol. 2015, 72, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Chang, W.H.; Cho, J.W.; Youn, J.; Kim, Y.K.; Kim, S.W.; Kim, Y.H. Efficacy of cumulative high-frequency rTMS on freezing of gait in Parkinson’s disease. Restor. Neurol. Neurosci. 2015, 33, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Zanjani, A.; Zakzanis, K.K.; Daskalakis, Z.J.; Chen, R. Repetitive transcranial magnetic stimulation of the primary motor cortex in the treatment of motor signs in Parkinson’s disease: A quantitative review of the literature. Mov. Disord. 2015, 30, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Brys, M.; Fox, M.D.; Agarwal, S.; Biagioni, M.; Dacpano, G.; Kumar, P.; Pirraglia, E.; Chen, R.; Wu, A.; Fernandez, H.; et al. Multifocal repetitive TMS for motor and mood symptoms of Parkinson disease: A randomized trial. Neurology 2016, 87, 1907–1915. [Google Scholar] [CrossRef]
- Makkos, A.; Pal, E.; Aschermann, Z.; Janszky, J.; Balazs, E.; Takacs, K.; Karadi, K.; Komoly, S.; Kovacs, N. High-Frequency Repetitive Transcranial Magnetic Stimulation Can Improve Depression in Parkinson’s Disease: A Randomized, Double-Blind, Placebo-Controlled Study. Neuropsychobiology 2016, 73, 169–177. [Google Scholar] [CrossRef]
- Yang, C.; Guo, Z.; Peng, H.; Xing, G.; Chen, H.; McClure, M.A.; He, B.; He, L.; Du, F.; Xiong, L.; et al. Repetitive transcranial magnetic stimulation therapy for motor recovery in Parkinson’s disease: A Meta-analysis. Brain Behav. 2018, 8, e01132. [Google Scholar] [CrossRef]
- Khedr, E.M.; Farweez, H.M.; Islam, H. Therapeutic effect of repetitive transcranial magnetic stimulation on motor function in Parkinson’s disease patients. Eur. J. Neurol. 2003, 10, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Khedr, E.M.; Rothwell, J.C.; Shawky, O.A.; Ahmed, M.A.; Hamdy, A. Effect of daily repetitive transcranial magnetic stimulation on motor performance in Parkinson’s disease. Mov. Disord. 2006, 21, 2201–2205. [Google Scholar] [CrossRef]
- Maruo, T.; Hosomi, K.; Shimokawa, T.; Kishima, H.; Oshino, S.; Morris, S.; Kageyama, Y.; Yokoe, M.; Yoshimine, T.; Saitoh, Y. High-frequency repetitive transcranial magnetic stimulation over the primary foot motor area in Parkinson’s disease. Brain Stimul. 2013, 6, 884–891. [Google Scholar] [CrossRef]
- Koch, G.; Brusa, L.; Caltagirone, C.; Peppe, A.; Oliveri, M.; Stanzione, P.; Centonze, D. rTMS of supplementary motor area modulates therapy-induced dyskinesias in Parkinson disease. Neurology 2005, 65, 623–625. [Google Scholar] [CrossRef]
- Brusa, L.; Versace, V.; Koch, G.; Iani, C.; Stanzione, P.; Bernardi, G.; Centonze, D. Low frequency rTMS of the SMA transiently ameliorates peak-dose LID in Parkinson’s disease. Clin. Neurophysiol. 2006, 117, 1917–1921. [Google Scholar] [CrossRef]
- Rektorova, I.; Sedlackova, S.; Telecka, S.; Hlubocky, A.; Rektor, I. Dorsolateral prefrontal cortex: A possible target for modulating dyskinesias in Parkinson’s disease by repetitive transcranial magnetic stimulation. Int. J. Biomed. Imaging 2008, 2008, 372125. [Google Scholar] [CrossRef] [PubMed]
- Cerasa, A.; Koch, G.; Donzuso, G.; Mangone, G.; Morelli, M.; Brusa, L.; Stampanoni Bassi, M.; Ponzo, V.; Picazio, S.; Passamonti, L.; et al. A network centred on the inferior frontal cortex is critically involved in levodopa-induced dyskinesias. Brain 2015, 138, 414–427. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, Y.; Cao, X.B.; Zeng, W.Q.; Zhai, H.; Zhang, X.Q.; Yang, X.M.; Cheng, C.; Wang, J.L.; Yang, X.M.; Xu, Y. Transcranial Magnetic Stimulation Alleviates Levodopa-Induced Dyskinesia in Parkinson’s Disease and the Related Mechanisms: A Mini-Review. Front. Neurol. 2021, 12, 758345. [Google Scholar] [CrossRef] [PubMed]
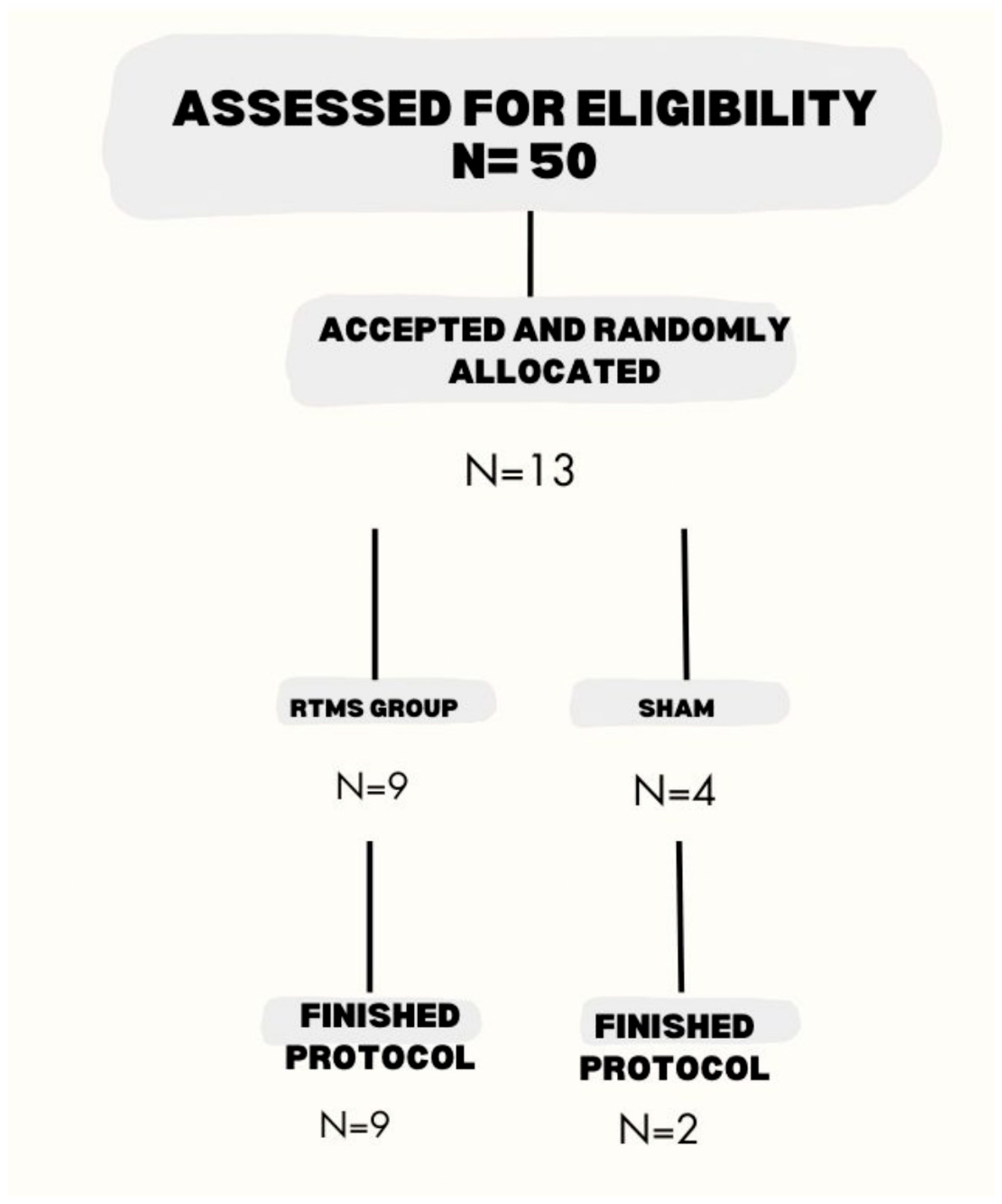
| Population Characteristics | rTMS | Sham | p | |
| Number of patients | 9 | 2 | ||
| Age (years; median; 25–75 centile) | 73(65.5–77) | 53.5 (53–54) | 0.098 | |
| Sex distribution (%) | Female | 3 (33.33%) | 1 (50%) | 0.658 |
| Male | 6 (66.66%) | 1 (50%) | ||
| Laterality (%) | Right | 9 (100%) | 2 (100%) | - |
| Left | 0 | 0 | ||
| Scholarity (years; median; 25–75 centile) | 6(4.5–9) | 8 (7–9) | 0.329 | |
| Age at diagnosis of PD (years; median; 25–75 centile) | 62(52.5–68.5) | 43 (39–47) | 0.098 | |
| Years with PD (years; median; 25–75 centile) | 10 (5.5–15) | 10.5 (7–14) | 0.813 | |
| Initial affected site (%) | Right | 3 (33.33%) | 0 | 0.887 |
| Left | 6 (66.66%) | 2 (100%) | ||
| Years with levodopa treatment (years; median; 25–75 centile) | 7(4.5–13) | 5 (0) | 0.469 | |
| Actual levodopa dose (mg/day; median; 25–75 centile) | 800 (762.5–1212.5) | 875 (700–1050) | 0.477 | |
| Actual dopa agonist treatment (%) | Yes | 7 (77.77%) | 2 (100%) | 0.461 |
| No | 2 (22.22%) | 0 | ||
| Actual amantadine treatment (%) | Yes | 3 (33.33%) | 1 (50%) | 0.658 |
| No | 6 (66.66%) | 1 (50%) | ||
| Clinical Evaluation | rTMS (n = 9) | p1 | Sham (n = 2) | p2 | p3 | p4 | |||
|---|---|---|---|---|---|---|---|---|---|
| First Evaluation (Centile 25–75) | Last Evaluation (Centile 25–75) | First Evaluation (Range) | Last Evaluation (Range) | ||||||
| MDS-UPDRS | Part 1 (median) | 16(6.5–20) | 14(8.5–17) | 0.859 | 18 (16–20) | 13.5 (5–22) | 0.655 | 0.55 | 0.813 |
| Part 2 (median) | 19 (8.5–24.5) | 14 (11.5–23) | 0.888 | 21(8–34 | 19 (11–28) | 0.655 | 0.723 | 0.906 | |
| Part 3 (median) | 34 (27.5–37) | 40 (33–44.5) | 0.021 | 55(43–67) | 42 (30–55) | 0.18 | 0.059 | 0.813 | |
| Part 4 (median) | 4 (1.5–6.5) | 7 (3–8.5) | 0.153 | 6.5(1–12) | 7 (1–13) | 0.317 | 0.812 | 1 | |
| H&Y | 2 (total; %) | 5 (55.55%) | 6 (66.66%) | 0.317 | 1 (50%) | 1 (50%) | 0.317 | 0.509 | 0.673 |
| 3 (total; %) | 4 (44.44%) | 3 (33.33) | 0 | 1 (50%) | |||||
| 4 (total, %) | 0 | 0 | 1 (50%) | 0 | |||||
| NMS | Cardiovascular (median) | 1 (0–3) | 0 (0- 0.5) | 0.48 | 5 (0–10) | 8 | 0.317 | 0.62 | 0.294 |
| Sleep/fatigue (median) | 8 (3.5–21) | 5 (2–14) | 0.179 | 27.5 (27–28) | 17(5–29) | 0.655 | 0.099 | 0.406 | |
| Mood/cognition (median) | 0 (0–7.5) | 1 (0–11) | 0.066 | 10.5 (9–12) | 9 (0–18) | 0.655 | 0.23 | 0.805 | |
| Perceptual problems/hallucinations (median) | 0 (0–4) | 0 (0–2.5) | 0.891 | 1 (0–2) | 0 (0) | 0.317 | 0.898 | 0.275 | |
| Attention/memory(median) | 3 (0–13) | 3 (0–8.5) | 1 | 1.5(1–2) | 0.5 (0–1) | 0.157 | 0.72 | 0.227 | |
| Gastrointestinal tract (median) | 6 (5–13) | 8(5.5–19.5) | 0.066 | 5 (1–9) | 6 (0–12) | 0.655 | 0.72 | 0.476 | |
| Urinary (median) | 18 (3–20.5) | 12 (4.5–32) | 0.44 | 12 (7–17) | 16 (0–32) | 0.655 | 0.637 | 0.721 | |
| Sexual function (median) | 1 (0–15) | 0 (0–6.5) | 0.109 | 4.5 (0–9) | 0 (0) | 0.317 | 0.805 | 0.368 | |
| Miscellaneous (median) | 13 (5.5–16.5) | 4 (0–4) | 0.018 | 13 (12–14) | 4 (0–8) | 0.18 | 1 | 0.612 | |
| Total score (median) | 79 (31.5–91.5) | 52 (14.5–102) | 0.26 | 80 (72–88) | 60.5 (5–116) | 0.655 | 0.722 | 0.814 | |
| UDysRS | Historical (median) | 14 (3.23.5) | 15 (12.5–26) | 0.312 | 15.5 (7–24) | 24 (10–38) | 0.18 | 0.55 | 0.814 |
| Objective (median) | 7(3–8.5) | 9 (8–16.5) | 0.074 | 6 (5–7) | 12.5 (7–18) | 0.18 | 1 | 0.905 | |
| Total (median) | 21(7–31.5) | 25 (20.5–42) | 0.213 | 21.5 (12–31) | 36.5 (17–56) | 0.18 | 0.637 | 0.814 | |
| Clinical Evaluation | rTMS (n = 9) | ||||
|---|---|---|---|---|---|
| First Evaluation (Centile 25–75) | Last Evaluation (Centile 25–75) | p | |||
| MDS-UPDRS | Part 3 (median) | General (median; centile 25–72) | 34 (27.5–37) | 40 (33–44.5) | 0.021 |
| Right-hand movement | 2 (1–2) | 2 (2–2) | 0.083 | ||
| Left-hand movement | 2 (1–2) | 3 (2–3) | 0.053 | ||
| Right pronation/supination | 1 (1–1) | 1 (1–1.5) | 0.083 | ||
| Right leg agility | 1 (0.5–2) | 1 (1–2) | 0.083 | ||
| Posture | 1 (1–2) | 2 (1.5–2) | 0.059 | ||
| Left-hand postural tremor | 0 (0) | 0 (0–1) | 0.083 | ||
| Left-hand kinetic tremor | 0 (0–0.5) | 1 (1–1) | 0.02 | ||
| NMS | Mood/cognition (median) | General (median; centile 25–72) | 0 (0–7.5) | 1 (0–11) | 0.066 |
| Sad or depressed | 0 (0–3) | 0 (0–5) | 0.066 | ||
| Gastrointestinal tract (median) | 6 (5–13) | 8 (5.5–19.5) | 0.066 | ||
| Miscellaneous (median) a | General (median; centile 25–72) | 13 (5.5–16.5) | 4 (0–4) | 0.018 | |
| Recent change in weight | 2(0–4) | 0(0) | 0.038 | ||
| Excessive sweating | 4 (0–12) | 0 (0–2.5) | 0.041 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medrano-Hernández, A.; Neri-Nani, G.; Rodríguez-Violante, M.; Drucker-Colín, R.; Chavarría, A. Inefficacy of Repetitive Transcranial Magnetic Stimulation in Parkinson’s Disease Patients with Levodopa-Induced Dyskinesias: Results from a Pilot Study. Biomedicines 2025, 13, 1663. https://doi.org/10.3390/biomedicines13071663
Medrano-Hernández A, Neri-Nani G, Rodríguez-Violante M, Drucker-Colín R, Chavarría A. Inefficacy of Repetitive Transcranial Magnetic Stimulation in Parkinson’s Disease Patients with Levodopa-Induced Dyskinesias: Results from a Pilot Study. Biomedicines. 2025; 13(7):1663. https://doi.org/10.3390/biomedicines13071663
Chicago/Turabian StyleMedrano-Hernández, Alma, Gabriel Neri-Nani, Mayela Rodríguez-Violante, René Drucker-Colín, and Anahí Chavarría. 2025. "Inefficacy of Repetitive Transcranial Magnetic Stimulation in Parkinson’s Disease Patients with Levodopa-Induced Dyskinesias: Results from a Pilot Study" Biomedicines 13, no. 7: 1663. https://doi.org/10.3390/biomedicines13071663
APA StyleMedrano-Hernández, A., Neri-Nani, G., Rodríguez-Violante, M., Drucker-Colín, R., & Chavarría, A. (2025). Inefficacy of Repetitive Transcranial Magnetic Stimulation in Parkinson’s Disease Patients with Levodopa-Induced Dyskinesias: Results from a Pilot Study. Biomedicines, 13(7), 1663. https://doi.org/10.3390/biomedicines13071663





