Protocol Biopsies in Kidney Transplant Recipients: Current Practice After Much Discussion
Abstract
1. Introduction
2. Role and Timing of Protocol Biopsies
3. Advantages and Disadvantages
3.1. Advantages of Kidney Allograft Protocol Biopsies
- I.
- Early Detection of Subclinical Rejection
- II.
- Diagnosis of Rejection:
- Biopsies are considered the ‘gold standard’ for diagnosing acute rejection, crucial for establishing the cause of allograft dysfunction and allowing for accurate histopathological diagnosis [1].
- III.
- Identification of Subclinical Pathologies:
- IV.
- Predictive Value for Graft Function:
- V.
- Assessment of Immunosuppression Efficacy:
- VI.
- Identification of Chronic Pathology:
- Chronic transplant nephropathy and other chronic changes can be detected early through protocol biopsies, facilitating timely interventions that may prevent long-term graft dysfunction [1].
- VII.
- Guidance for Therapeutic Adjustments:
- VIII.
- Monitoring Graft Health:
- IX.
- Standardization of Diagnosis:
- X.
- Research and Data Collection:
- XI.
- Correlation with Long-Term Outcomes:
- XII.
- Improvement in Graft Function Over Time:
- XIII.
- Assessment of Anti-Donor Antibodies:
- Protocol biopsies can help monitor the presence of donor-specific antibodies (DSA), indicating ongoing alloimmune processes [29].
- XIV.
- Patient Stratification:
- Biopsies can help stratify patients based on their risk of graft loss, allowing for tailored follow-up care and management strategies. The histologic findings from protocol biopsies provide prognostic information that is independent of graft function and other clinical parameters. This means they can identify patients at high risk for graft loss who may benefit from targeted therapeutic interventions. Conversely, protocol biopsies can also help identify patients at very low risk for graft loss, who might be candidates for modifications in their immunosuppressive regimens [6,12,31].
- XV.
- Understanding Natural History
- Protocol biopsies contribute to a better understanding of the natural history of transplant rejection, including the prevalence and progression of SCR over time. A study performed by Nankivell et al. reported a prevalence of SCR at various intervals post-transplant (e.g., 60.8% at 1 month, 45.7% at 3 months) [30].
3.2. Disadvantages of Kidney Allograft Protocol Biopsies
- I.
- Invasiveness:
- Biopsies are invasive procedures that carry inherent risks, including bleeding, infection, and potential damage to the allograft. Some non-major safety events that have been reported are transient hematuria, arteriovenous fistula, urinary tract infection and wound infection [1,6,7,9,27,29,30,31,32].
- II.
- Risks Associated with Biopsy Procedures:
- III.
- Interpretation Challenges:
- IV.
- False Sense of Security:
- V.
- Sampling Error:
- There is a possibility that the biopsy may not accurately represent the overall condition of the graft, leading to missed diagnoses [6].
- VI.
- Potential for Overdiagnosis:
- VII.
- Uncertainty Regarding Treatment Necessity:
- VIII.
- Cost and Resource Allocation:
- IX.
- Psychological Impact on Patients:
- X.
- Limited Clinical Relevance:
- XI.
- Need for Standardization:
- Existing classification systems for acute rejection, such as the Banff criteria, require further standardization and development to enhance diagnostic accuracy [1].
- XII.
- Potential for Overtreatment:
- XIII.
- Variability in Histological Interpretation:
- Differences in observer experience and the subjective nature of histological assessment can lead to inconsistent interpretations of biopsy results. There is potential for misinterpretation of biopsy results, leading to false positives or negatives and, therefore, misleading results. This can result in unnecessary anxiety for patients or inadequate management of actual rejection episodes [2,11,27,30,31,32].
- XIV.
- Resource-Intensive:
- XV.
- Unclear Benefit:
- I.
- Procedural Risks and Invasiveness
- -
- -
- -
- II.
- Interpretive and Diagnostic Limitations
- -
- -
- -
- -
- III.
- Resource and Cost-Related Limitations
- -
- -
- -
- -
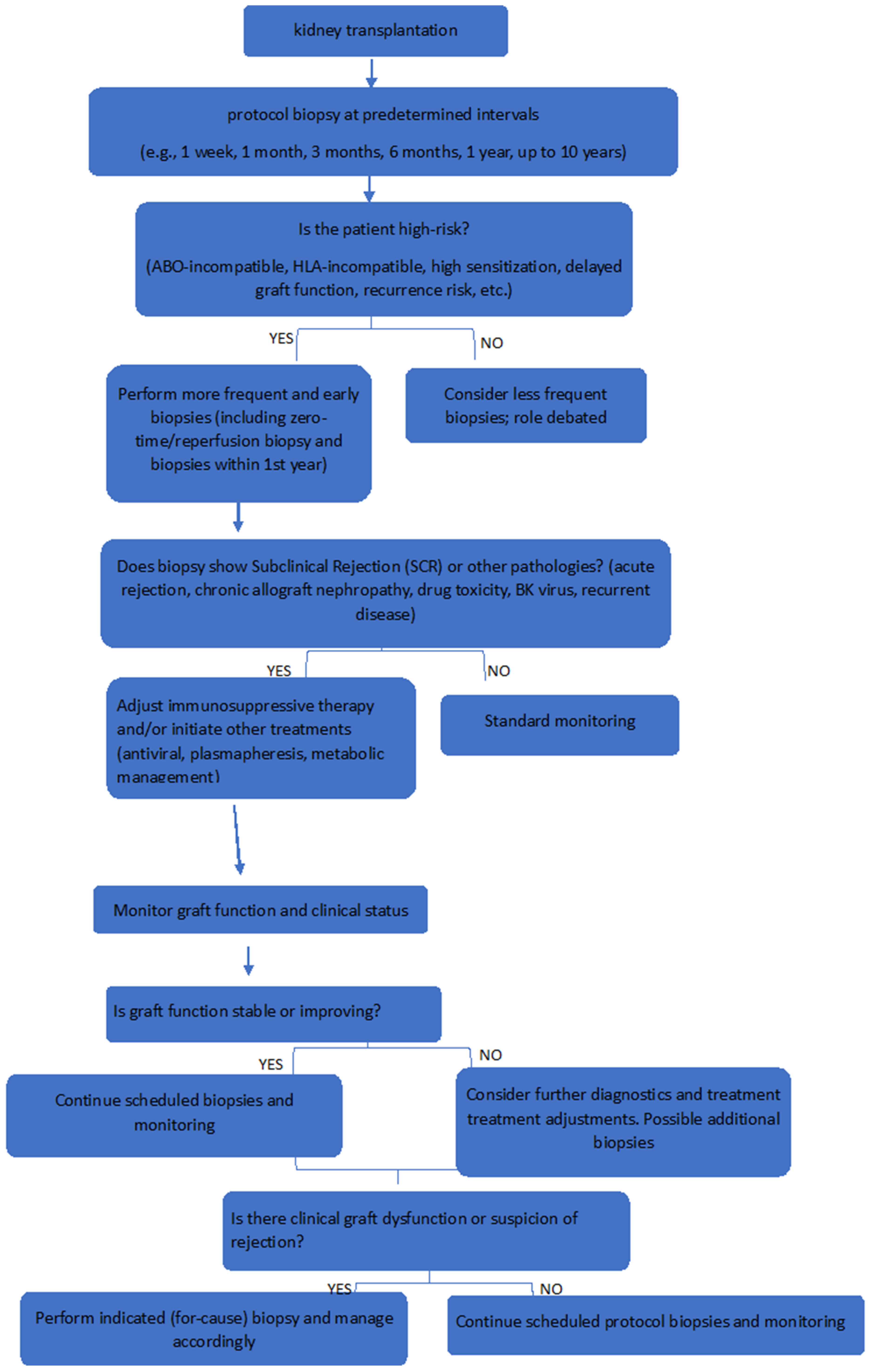
4. The Role of Protocol Biopsies in High-Risk Kidney Transplant Recipients
4.1. Protocol Biopsies in HLA-Incompatible Kidney Transplant Recipients
4.2. Protocol Biopsies in ABO-Incompatible Kidney Transplant Recipients
Psychological Issues Related to Repeated Biopsies
4.3. Protocol Biopsies in Recipients at Increased Risk of Recurrence of Primary Nephropathy
4.3.1. Types of Rejection and Banff Classification
4.3.2. Staining Techniques
- HLA-Incompatible Recipients:
- These recipients face significant immunological challenges, with a predisposition to subclinical rejection (SCR) and antibody-mediated rejection (AMR) that often go undetected clinically.
- Protocol biopsies at 1, 3, 6, and 12 months post-transplant have a high yield in detecting early microcirculation inflammation and SCR, which, if untreated, may progress to chronic damage such as transplant glomerulopathy.
- Early biopsy detection facilitates immunosuppressive regimen adjustments, stabilizing renal function and improving graft survival.
- ABO-Incompatible Recipients:
- Despite advances in desensitization, there remains an increased incidence of subclinical rejection and antibody-mediated injury in ABO-I transplants.
- Protocol biopsies detect these subclinical changes effectively and assist in tailoring immunosuppression.
- Their yield in detecting antibody-mediated rejection despite desensitization is comparable to HLAi recipients.
- Recipients at Risk of Primary Disease Recurrence:
- Recurrence of diseases such as IgA nephropathy, FSGS, membranous nephropathy, and MPGN is a major cause of graft loss and often occurs silently.
- Protocol biopsies reveal recurrence rates much higher than clinical monitoring alone (e.g., up to 60% in IgA nephropathy), enabling earlier and disease-specific interventions such as plasmapheresis in FSGS.
- In primary hyperoxaluria, biopsies detect calcium oxalate deposition early, guiding metabolic management to preserve graft function.
- Impact on Therapeutic Outcomes:
- For example, in HLAi recipients, early identification of microcirculation inflammation allowed for immunosuppressive adjustments that improved graft survival [31].
4.3.3. The Emerging Role of Non-Invasive Biomarkers as Alternatives or Complements to Protocol Biopsies
5. Assessment of Chronic Allograft Nephropathy
6. Post-Acute Rejection Monitoring
Risk–Benefit Ratio in Low-Risk Populations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Rush, D.; Nickerson, P.; Gough, J.; McKenna, R.; Grimm, P.; Cheang, M.; Trpkov, K.; Solez, K.; Jeffery, J. Beneficial effects of treatment of early subclinical rejection: A randomized study. J. Am. Soc. Nephrol. 1998, 9, 2129–2134. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, F.; Gelpi, R.; Helanterä, I.; Melilli, E.; Honkanen, E.; Bestard, O.; Grinyo, J.M.; Cruzado, J.M.; Bagnasco, S.M. Decreased Kidney Graft Survival in Low Immunological Risk Patients Showing Inflammation in Normal Protocol Biopsies. PLoS ONE 2016, 11, e0159717. [Google Scholar] [CrossRef] [PubMed]
- Wohlfahrtova, M.; Tycova, I.; Honsova, E.; Lodererova, A.; Viklicky, O. Molecular Patterns of Subclinical and Clinical Rejection of Kidney Allograft: Quantity Matters. Kidney Blood Press. Res. 2015, 40, 244–257. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Oguchi, H.; Muramatsu, M.; Shishido, S. Protocol graft biopsy in kidney transplantation. Nephrology 2018, 23, 38–44. [Google Scholar] [CrossRef]
- Kalaria, A.L.; Yamada, T.; Klein-Fedyshin, M.; Obata, S.; Cruz-Peralta, M.; Parrish, B.; Rahman, A.Z.; Molinari, M.; Mehta, R.B. Subclinical rejection and allograft survival in kidney transplantation: Protocol for a systematic review and meta-analysis. BMJ Open 2024, 14, e085098. [Google Scholar] [CrossRef]
- Cosio, F.G.; El Ters, M.; Cornell, L.D.; Schinstock, C.A.; Stegall, M.D. Changing Kidney Allograft Histology Early Posttransplant: Prognostic Implications of 1-Year Protocol Biopsies. Am. J. Transplant. 2016, 16, 194–203. [Google Scholar] [CrossRef]
- Rush, D.N.; Jeffery, J.R.; Gough, J. SEQUENTIAL PROTOCOL BIOPSIES IN RENAL TRANSPLANT PATIENTS: Clinico-Pathological Correlations Using the Banff Schema. Transplantation 1995, 59, 511–514. [Google Scholar] [CrossRef]
- Lee, O.; Kim, M.J.; Lee, J.E.; Hwang, N.Y.; Kim, K.; Lee, K.W.; Park, J.B. The Protective Role of Protocol Biopsy for Allograft Kidney Maintenance in Kidney Transplantation. Transplant. Proc. 2023, 55, 756–768. [Google Scholar] [CrossRef]
- Garcia-Lopez, A.; Calderon-Zapata, A.; Gomez-Montero, A.; Lozano-Suarez, N.; Giron-Luque, F. The Value of Protocol Biopsy in Kidney Transplantation on Monitoring Transplant Outcomes: A Systematic Review and Meta-Analysis. Transplant. Proc. 2024, 56, 1231–1240. [Google Scholar] [CrossRef]
- Tanabe, T. The value of long-term protocol biopsies after kidney transplantation. Nephrology 2014, 19, 2–5. [Google Scholar] [CrossRef]
- Miyagi, M.; Ishikawa, Y.; Mizuiri, S.; Aikawa, A.; Ohara, T.; Hasegawa, A. Significance of subclinical rejection in early renal allograft biopsies for chronic allograft dysfunction. Clin. Transplant. 2005, 19, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, R.; Randhawa, P.; Jordan, M.L.; Scantlebury, V.P.; Vivas, C.; Jain, A.; Corry, R.J.; McCauley, J.; Johnston, J.; Donaldson, J.; et al. An Analysis of Early Renal Transplant Protocol Biopsies—The High Incidence of Subclinical Tubulitis. Am. J. Transplant. 2001, 1, 47–50. [Google Scholar] [CrossRef]
- Wilkinson, A. Protocol Transplant Biopsies: Are They Really Needed? Clin. J. Am. Soc. Nephrol. 2006, 1, 130–137. [Google Scholar] [CrossRef]
- Racusen, L.C. Protocol Transplant Biopsies in Kidney Allografts: Why and When Are They Indicated? Clin. J. Am. Soc. Nephrol. 2006, 1, 144–147. [Google Scholar] [CrossRef]
- Bohmig, G.A.; Regele, H.; Horl, W.H. Protocol biopsies after kidney transplantation. Transpl. Int. 2005, 18, 131–139. [Google Scholar] [CrossRef]
- Ho, Q.Y.; Lim, C.C.; Tan, H.Z.; Sultana, R.; Kee, T.; Htay, H. Complications of Percutaneous Kidney Allograft Biopsy: Systematic Review and Meta-analysis. Transplantation 2022, 106, 1497–1506. [Google Scholar] [CrossRef]
- Iwahara, N.; Hotta, K.; Hirose, T.; Okada, H.; Shinohara, N. Protocol biopsy of kidney allograft enables early detection of BK virus nephropathy to preserve kidney allograft function. Transpl. Infect. Dis. 2024, 26, e14338. [Google Scholar] [CrossRef]
- Kurtkoti, J.; Sakhuja, V.; Sud, K.; Minz, M.; Nada, R.; Kohli, H.S.; Gupta, K.L.; Joshi, K.; Jha, V. The Utility of 1-and 3-Month Protocol Biopsies on Renal Allograft Function: A Randomized Controlled Study. Am. J. Transplant. 2008, 8, 317–323. [Google Scholar] [CrossRef]
- Zachariah, M.S.; Dwivedi, A.K.; Yip, C.S.; Chang, S.S.; Gundroo, A.; Venuto, R.C.; Tomaszewski, J.; Patel, S.K.; Sharma, R. Utility of Serial Protocol Biopsies Performed After 1 Year in Predicting Long-Term Kidney Allograft Function According to Histologic Phenotype. Exp. Clin. Transplant. 2018, 16, 391–400. [Google Scholar]
- Naesens, M. Zero-Time Renal Transplant Biopsies: A Comprehensive Review. Transplantation 2016, 100, 1425–1439. [Google Scholar] [CrossRef]
- Mohan, S.; Campenot, E.; Chiles, M.C.; Santoriello, D.; Bland, E.; Crew, R.J.; Rosenstiel, P.; Dube, G.; Batal, I.; Radhakrishnan, J.; et al. Association between Reperfusion Renal Allograft Biopsy Findings and Transplant Outcomes. J. Am. Soc. Nephrol. 2017, 28, 3109–3117. [Google Scholar] [CrossRef] [PubMed]
- Guetta, O.; Osyntsov, A.; Rahamimov, R.; Tobar, A.; Israeli, M.; Masarwa, Y.; Gurevich, M.; Tennak, V.; Mezhybovsky, V.; Gravetz, A.; et al. The Role of Early Sequential Biopsies in Delayed Renal Graft Function of Transplanted Kidney Is Reduced in Modern Immunosuppression Era. Nephron 2023, 147, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.J.; Zhong, W.; Mandelbrot, D.A.; Parajuli, S.M. Histopathological Features and Role of Allograft Kidney Biopsy Among Recipients with Prolonged Delayed Graft Function: A Review. Transplantation 2024, 108, 1911–1921. [Google Scholar] [CrossRef] [PubMed]
- Rush, D.N.; Karpinski, M.E.; Nickerson, P.; Dancea, S.; Birk, P.; Jeffery, J.R. Does subclinical rejection contribute to chronic rejection in renal transplant patients? Clin. Transplant. 1999, 13, 441–446. [Google Scholar] [CrossRef]
- Nankivell, B.J.; Chapman, J.R. The Significance of Subclinical Rejection and the Value of Protocol Biopsies. Am. J. Transplant. 2006, 6, 2006–2012. [Google Scholar] [CrossRef]
- Huang, Y.; Farkash, E. Protocol Biopsies: Utility and Limitations. Adv. Chronic Kidney Dis. 2016, 23, 326–331. [Google Scholar] [CrossRef]
- Nankivell, B.J.; Borrows, R.J.; Fung, C.L.-S.; O’Connell, P.J.; Allen, R.D.M.; Chapman, J.R. Natural History, Risk Factors, and Impact of Subclinical Rejection in Kidney Transplantation. Transplantation 2004, 78, 242–249. [Google Scholar] [CrossRef]
- Cosio, F.G.; Grande, J.P.; Wadei, H.; Larson, T.S.; Griffin, M.D.; Stegall, M.D. Predicting Subsequent Decline in Kidney Allograft Function from Early Surveillance Biopsies. Am. J. Transplant. 2005, 5, 2464–2472. [Google Scholar] [CrossRef]
- Moreso, F.; Ibernon, M.; Gomà, M.; Carrera, M.; Fulladosa, X.; Hueso, M.; Gil-Vernet, S.; Cruzado, J.M.; Torras, J.; Grinyó, J.M.; et al. Subclinical Rejection Associated with Chronic Allograft Nephropathy in Protocol Biopsies as a Risk Factor for Late Graft Loss. Am. J. Transplant. 2006, 6, 747–752. [Google Scholar] [CrossRef]
- Henderson, L.K.; Nankivell, B.J.; Chapman, J.R. Surveillance Protocol Kidney Transplant Biopsies: Their Evolving Role in Clinical Practice. Am. J. Transplant. 2011, 11, 1570–1575. [Google Scholar] [CrossRef]
- Bagnasco, S.M.; Zachary, A.A.; Racusen, L.C.; Arend, L.J.; Carter-Monroe, N.; Alachkar, N.; Nazarian, S.M.; Lonze, B.E.; Montgomery, R.A.; Kraus, E.S. Time Course of Pathologic Changes in Kidney Allografts of Positive Crossmatch HLA-Incompatible Transplant Recipients. Transplantation 2014, 97, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Rennie, T.J.W.; Battle, R.K.; Abel, A.A.; McConnell, S.; McLaren, R.; Phelan, P.J.; Geddes, C.; Padmanabhan, N.; Clancy, M.J.; Little, A.; et al. Comparison of kidney transplant outcomes in HLA compatible and incompatible transplantation: A national cohort study. Nephrology 2022, 27, 962–972. [Google Scholar] [CrossRef] [PubMed]
- Oettl, T.; Halter, J.; Bachmann, A.; Guerke, L.; Infanti, L.; Oertli, D.; Mihatsch, M.; Gratwohl, A.; Steiger, J.; Dickenmann, M. ABO blood group-incompatible living donor kidney transplantation: A prospective, single-centre analysis including serial protocol biopsies. Nephrol. Dial. Transplant. 2008, 24, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Setoguchi, K.; Ishida, H.; Shimmura, H.; Shimizu, T.; Shirakawa, H.; Omoto, K.; Toki, D.; Iida, S.; Setoguchi, S.; Tokumoto, T.; et al. Analysis of Renal Transplant Protocol Biopsies in ABO-Incompatible Kidney Transplantation. Am. J. Transplant. 2007, 8, 86–94. [Google Scholar] [CrossRef]
- Masutani, K.; Nakagawa, K.; Matsukuma, Y.; Ueki, K.; Ataka, E.; Tsuchimoto, A.; Okabe, Y.; Nakamura, M.; Kitazono, T.; Nakano, T. Significance of Perivascular Aggregates in Kidney Allografts: Evaluation of 1-Year Protocol Biopsies Using Recent Banff Classification. Transplant. Proc. 2024, 56, 499–504. [Google Scholar] [CrossRef]
- Dörje, C.; Mjøen, G.; Strøm, E.H.; Holdaas, H.; Jenssen, T.; Øyen, O.; Akkök, Ç.A.; Cvancarova, M.; Midtvedt, K.; Reisæter, A.V. One-year protocol biopsies from ABO-incompatible renal allografts compared with a matched cohort of ABO-compatible allografts. Clin. Transplant. 2015, 29, 268–276. [Google Scholar] [CrossRef]
- Wongsaroj, P.; Kahwaji, J.; Vo, A.; Jordan, S.C. Modern approaches to incompatible kidney transplantation. World J. Nephrol. 2015, 4, 354–362. [Google Scholar] [CrossRef]
- Fu, M.S.; Jalalonmuhali, M.; Ng, K.S.; Lim, S.K.; Ng, K.P. Clinical Significance of Renal Allograft Protocol Biopsies: A Single Tertiary Center Experience in Malaysia. J. Transplant. 2019, 2019, 9153875. [Google Scholar] [CrossRef]
- Cornell, L.D.; Amer, H.; Viehman, J.K.; Mehta, R.A.; Lieske, J.C.; Lorenz, E.C.; Heimbach, J.K.; Stegall, M.D.; Milliner, D.S. Posttransplant recurrence of calcium oxalate crystals in patients with primary hyperoxaluria: Incidence, risk factors, and effect on renal allograft function. Am. J. Transplant. 2021, 22, 85–95. [Google Scholar] [CrossRef]
- Yamamoto, I.; Yamakawa, T.; Katsuma, A.; Kawabe, M.; Katsumata, H.; Hamada, A.M.; Nakada, Y.; Kobayashi, A.; Yamamoto, H.; Yokoo, T. Recurrence of native kidney disease after kidney transplantation. Nephrology 2018, 23, 27–30. [Google Scholar] [CrossRef]
- Scurt, F.G.; Ewert, L.; Mertens, P.R.; Haller, H.; Schmidt, B.M.W.; Chatzikyrkou, C. Clinical outcomes after ABO-incompatible renal transplantation: A systematic review and meta-analysis. Lancet 2019, 393, 2059–2072. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.; Sealfon, R.; Menon, R.; Eadon, M.T.; Lake, B.B.; Steck, B.; Anjani, K.; Parikh, S.; Sigdel, T.K.; Zhang, G.; et al. A reference tissue atlas for the human kidney. Sci. Adv. 2022, 8, eabn4965. [Google Scholar] [CrossRef] [PubMed]
- Rebollo, P.; Ortega, F.; Baltar, J.M.; Díaz-Corte, C.; Navascués, R.A.; Naves, M.; Ureña, A.; Badía, X.; Alvarez-Ude, F.; Alvarez-Grande, J. Health-related quality of life (HRQOL) in end stage renal disease (ESRD) patients over 65 years. Geriatr. Nephrol. Urol. 1998, 8, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Weng, F.L.; Chandwani, S.; Kurtyka, K.M.; Zacker, C.; Chisholm-Burns, M.A.; Demissie, K. Prevalence and correlates of medication nonadherence among kidney transplant recipients more than 6 months post-transplant: A cross-sectional study. BMC Nephrol. 2013, 14, 261. [Google Scholar] [CrossRef]
- Mellon, M.J.; Pillai, U.; Powelson, J.A.; Taber, D.J. Impact of structured emotional support on outcomes in kidney transplant recipients. Clin. Transplant. 2017, 31, e13118. [Google Scholar] [CrossRef]
- Morozumi, K.; Takeda, A.; Otsuka, Y.; Horike, K.; Gotoh, N.; Watarai, Y. Recurrent glomerular disease after kidney transplantation: An update of selected areas and the impact of protocol biopsy. Nephrology 2014, 19, 6–10. [Google Scholar] [CrossRef]
- Crosson, J. Focal Segmental Glomerulosclerosis and Renal Transplantation. Transplant. Proc. 2007, 39, 737–743. [Google Scholar] [CrossRef]
- Heybeli, C.; Alexander, M.P.; Bentall, A.J.; Amer, H.; Buadi, F.K.; Dean, P.G.; Dingli, D.; Dispenzieri, A.; El Ters, M.; Gertz, M.A.; et al. Kidney Transplantation in Patients with Monoclonal Gammopathy of Renal Significance (MGRS)–Associated Lesions: A Case Series. Am. J. Kidney Dis. 2022, 79, 202–216. [Google Scholar] [CrossRef]
- SeróN, D.; Arns, W.; Chapman, J.R. Chronic allograft nephropathy—Clinical guidance for early detection and early intervention strategies. Nephrol. Dial. Transplant. 2008, 23, 2467–2473. [Google Scholar] [CrossRef]
- Bosmans, J.-L.; Ysebaert, D.K.; Verpooten, G.A. Chronic Allograft Nephropathy: What Have We Learned from Protocol Biopsies? Transplantation 2008, 85, S38–S41. [Google Scholar] [CrossRef]
- Nankivell, B.J.; Borrows, R.J.; Fung, C.L.-S.; O’Connell, P.J.; Allen, R.D.; Chapman, J.R. The Natural History of Chronic Allograft Nephropathy. N. Engl. J. Med. 2003, 349, 2326–2333. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.E. Evaluation and Treatment of Acute Rejection in Kidney Allografts. Clin. J. Am. Soc. Nephrol. 2020, 15, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Loupy, A.; Haas, M.; Roufosse, C.; Naesens, M.; Adam, B.; Afrouzian, M.; Akalin, E.; Alachkar, N.; Bagnasco, S.; Becker, J.U.; et al. The Banff 2019 Kidney Meeting Report (I): Updates on and clarification of criteria for T cell– and antibody-mediated rejection. Am. J. Transplant. 2020, 20, 2318–2331. [Google Scholar] [CrossRef]
- Shimizu, T.; Tanabe, T.; Shirakawa, H.; Omoto, K.; Ishida, H.; Tanabe, K. Acute vascular rejection after renal transplantation and isolated v-lesion. Clin. Transplant. 2012, 26, 2–8. [Google Scholar] [CrossRef]
- Sis, B.; Mengel, M.; Haas, M.; Colvin, R.B.; Halloran, P.F.; Racusen, L.C.; Solez, K.; Baldwin, W.; Bracamonte, E.R.; Broecker, V.; et al. Banff 09 Meeting Report: Antibody Mediated Graft Deterioration and Implementation of Banff Working Groups. Am. J. Transplant. 2010, 10, 464–471. [Google Scholar] [CrossRef]
- Agarwal, G.; Diskin, C.D.; Williams, T.A.; Kumar, V. Late Antibody-Mediated Rejection in Kidney Transplant Recipients: Outcomes after Intravenous Immunoglobulin Therapy. Clin. Transpl. 2016, 32, 111–118. [Google Scholar]
- Rafiq, M.A.; De Boccardo, G.; Schröppel, B.; Bromberg, J.S.; Sehgal, V.; Dinavahi, R.; Murphy, B.; Akalin, E. Differential outcomes in 3 types of acute antibody-mediated rejection. Clin. Transplant. 2009, 23, 951–957. [Google Scholar] [CrossRef]
- Colovai, A.I.; Vasilescu, E.R.; Foca-Rodi, A.; Kim-Schulze, S.; Hussaini, N.; DaGati, V.D.; Markowitz, G.S.; Wang, C.; Cohen, D.J.; Hardy, M.A.; et al. Acute and Hyperacute Humoral Rejection in Kidney Allograft Recipients Treated with Anti-Human Thymocyte Antibodies. Hum. Immunol. 2005, 66, 501–512. [Google Scholar] [CrossRef]
- González-Molina, M.; Ruiz-Esteban, P.; Caballero, A.; Burgos, D.; Cabello, M.; Leon, M.; Fuentes, L.; Hernandez, D. Immune response and histology of humoral rejection in kidney transplantation. Nefrología 2016, 36, 354–367. [Google Scholar] [CrossRef]
- Singh, N.; Samant, H.; Hawxby, A.; Samaniego, M.D. Biomarkers of rejection in kidney transplantation. Curr. Opin. Organ Transplant. 2019, 24, 103–110. [Google Scholar] [CrossRef]
- Seemayer, C.A.; Gaspert, A.; Nickeleit, V.; Mihatsch, M.J. C4d staining of renal allograft biopsies: A comparative analysis of different staining techniques. Nephrol. Dial. Transplant. 2006, 22, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, K.; Arai, K.; Aikawa, A.; Miyagi, M.; Ohara, T.; Hasegawa, A.; Muramatsu, M.; Hirayama, N.; Tajima, E.; Kawamura, T.; et al. Reversibility from Delayed Hyperacute Rejection in ABO-Incompatible Renal Transplantation: Histopathological Findings of Renal Allograft Biopsy. Transplant. Proc. 2005, 37, 701–704. [Google Scholar] [CrossRef] [PubMed]
- Biomarkers Definitions Working Group; Atkinson, A.J., Jr.; Colburn, W.A.; DeGruttola, V.G.; DeMets, D.L.; Downing, G.J.; Hoth, D.F.; Oates, J.A.; Peck, C.C.; Spilker, B.A.; et al. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001, 69, 89–95. [Google Scholar] [CrossRef]
- Temerinac-Ott, M.; Forestier, G.; Schmitz, J.; Hermsen, M.; Brasen, J.; Feuerhake, F.; Wemmert, C. Detection of glomeruli in renal pathology by mutual comparison of multiple staining modalities. In Proceedings of the 10th International Symposium on Image and Signal Processing and Analysis (ISPA), Ljubljana, Slovenia, 18–20 September 2017; pp. 19–24. [Google Scholar] [CrossRef]
- Pandey, K.K.; Cr, W.D.S.; Pandey, A.; Agarwal, A. Evaluation of renal biopsies in various kidney diseases with reference to staining. IP Arch. Cytol. Histopathol. Res. 2021, 6, 269–274. [Google Scholar] [CrossRef]
- Walker, P.D.; Cavallo, T.; Bonsib, S.M. Practice guidelines for the renal biopsy. Mod. Pathol. 2004, 17, 1555–1563. [Google Scholar] [CrossRef]
- Messias, N.C.; Walker, P.D.; Larsen, C.P. Paraffin immunofluorescence in the renal pathology laboratory: More than a salvage technique. Mod. Pathol. 2015, 28, 854–860. [Google Scholar] [CrossRef]
- Nasr, S.H.; Fidler, M.E.; Said, S.M. Paraffin Immunofluorescence: A Valuable Ancillary Technique in Renal Pathology. Kidney Int. Rep. 2018, 3, 1260–1266. [Google Scholar] [CrossRef]
- Howell, D.N.; Herrera, G.A. Electron microscopy in renal pathology: Overall applications and guidelines for tissue, collection, preparation, and stains. Ultrastruct. Pathol. 2020, 45, 1–18. [Google Scholar] [CrossRef]
- Voora, S.; Adey, D.B. Management of Kidney Transplant Recipients by General Nephrologists: Core Curriculum 2019. Am. J. Kidney Dis. 2019, 73, 866–879. [Google Scholar] [CrossRef]
- Singh, N.; Nori, U.; Pesavento, T. Kidney transplantation in the elderly. Curr. Opin. Organ Transplant. 2009, 14, 380–385. [Google Scholar] [CrossRef]
- Naesens, M.; Friedewald, J.; Mas, V.; Kaplan, B.; Abecassis, M.M. A Practical Guide to the Clinical Implementation of Biomarkers for Subclinical Rejection Following Kidney Transplantation. Transplantation 2020, 104, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Karuthu, S.; Blumberg, E.A. Common Infections in Kidney Transplant Recipients. Clin. J. Am. Soc. Nephrol. 2012, 7, 2058–2070. [Google Scholar] [CrossRef] [PubMed]
- Kataria, A.; Athreya, A.; Gupta, G. Biomarkers in Kidney Transplantation. Adv. Kidney Dis. Health 2024, 31, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Nickeleit, V.; Mihatsch, M.J. Kidney transplants, antibodies and rejection: Is C4d a magic marker? Nephrol. Dial. Transplant. 2003, 18, 2232–2239. [Google Scholar] [CrossRef]
- Lim, J.-H.; Lee, C.-H.; Kim, K.Y.; Jung, H.-Y.; Choi, J.-Y.; Cho, J.-H.; Park, S.-H.; Kim, Y.-L.; Baek, M.-C.; Park, J.B.; et al. Novel urinary exosomal biomarkers of acute T cell-mediated rejection in kidney transplant recipients: A cross-sectional study. PLoS ONE 2018, 13, e0204204. [Google Scholar] [CrossRef]
- Lai, X.; Zheng, X.; Mathew, J.M.; Gallon, L.; Leventhal, J.R.; Zhang, Z.J. Tackling Chronic Kidney Transplant Rejection: Challenges and Promises. Front. Immunol. 2021, 12, 661643. [Google Scholar] [CrossRef]
- Wu, H.; Malone, A.F.; Donnelly, E.L.; Kirita, Y.; Uchimura, K.; Ramakrishnan, S.M.; Gaut, J.P.; Humphreys, B.D. Single-Cell Transcriptomics of a Human Kidney Allograft Biopsy Specimen Defines a Diverse Inflammatory Response. J. Am. Soc. Nephrol. 2018, 29, 2069–2080. [Google Scholar] [CrossRef]
- Hart, A.; Singh, D.; Brown, S.J.; Wang, J.H.; Kasiske, B.L. Incidence, risk factors, treatment, and consequences of anti-body-mediated kidney transplant rejection: A systematic review. Clin. Transplant. 2021, 35, e14320. [Google Scholar] [CrossRef]
- Vasilescu, E.R.; Ho, E.K.; Colovai, A.I.; Vlad, G.; Foca-Rodi, A.; Markowitz, G.S.; Dagati, V.; Hardy, M.A.; Ratner, L.E.; Suciu-Foca, N. Alloantibodies and the Outcome of Cadaver Kidney Allografts. Hum. Immunol. 2006, 67, 597–604. [Google Scholar] [CrossRef]
- Weir, M.R.; Wali, R.K. Minimizing the Risk of Chronic Allograft Nephropathy. Transplantation 2009, 87, S14–S18. [Google Scholar] [CrossRef]
- Nankivell, B.J.; Chapman, J.R. Chronic Allograft Nephropathy: Current Concepts and Future Directions. Transplantation 2006, 81, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Freese, P.; Svalander, C.T.; Mölne, J.; Nordén, G.; Nyberg, G. Chronic allograft nephropathy—Biopsy findings and outcome. Nephrol. Dial. Transplant. 2001, 16, 2401–2406. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.J. Longitudinal analysis of chronic allograft nephropathy: Clinicopathologic correlations. Kidney Int. 2005, 68, S108–S112. [Google Scholar] [CrossRef] [PubMed]
- Rush, D.N.; Henry, S.F.; Jeffery, J.R.; Schroeder, T.J.; Gough, J. Histological findings in early routine biopsies of stable renal allograft recipients. Transplantation 1994, 57, 208–210. [Google Scholar] [CrossRef]
- El Ters, M.; Grande, J.P.; Keddis, M.T.; Rodrigo, E.; Chopra, B.; Dean, P.G.; Stegall, M.D.; Cosio, F.G.; Stegall, M.D. Kidney Allograft Survival After Acute Rejection, the Value of Follow-Up Biopsies. Am. J. Transplant. 2013, 13, 2334–2341. [Google Scholar] [CrossRef]
- Kee, T.Y.-S.; Chapman, J.R.; OCOnnell, P.J.; Fung, C.L.-S.; Allen, R.D.M.; Kable, K.; Vitalone, M.J.; Nankivell, B.J. Treatment of Subclinical Rejection Diagnosed by Protocol Biopsy of Kidney Transplants. Transplantation 2006, 82, 36–42. [Google Scholar] [CrossRef]
- Uslu, A.; Nart, A. Treatment of First Acute Rejection Episode: Systematic Review of Level I Evidence. Transplant. Proc. 2011, 43, 841–846. [Google Scholar] [CrossRef]
- Moein, M.; Papa, S.; Ortiz, N.; Saidi, R. Protocol biopsy after kidney transplant: Clinical application and efficacy to detect allograft rejection. Cureus 2023, 15, e34505. [Google Scholar] [CrossRef]
- Lim, M.; Park, B.K.; Lee, K.W.; Park, J.B.; Kim, K.D.; Yang, J.; Kwon, J.; Jeong, E.S.; Lee, S. Two-Week Protocol Biopsy in Renal Allograft: Feasibility, Safety, and Outcomes. J. Clin. Med. 2022, 11, 785. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schwarz, A.; Gwinner, W.; Hiss, M.; Radermacher, J.; Mengel, M.; Haller, H. Safety and Adequacy of Renal Transplant Protocol Biopsies. Am. J. Transplant. 2005, 5, 1992–1996. [Google Scholar] [CrossRef]
| Biopsy Time Point | Clinical Recommendations |
|---|---|
| Zero-time/Immediate post-reperfusion |
|
| 1 week post-transplant |
|
| 1 month post-transplant |
|
| 3 months post-transplant |
|
| 6 months post-transplant |
|
| 12 months (1 year) post-transplant |
|
| Beyond 1 year (3, 5, 7, 10 years) |
|
| Early biopsy within 7–10 days (in cases of delayed graft function—DGF) |
|
| Post-acute rejection episodes |
|
| Time Point | Advantages | Disadvantages |
|---|---|---|
| Zero-time/Reperfusion | Baseline assessment of donor kidney quality; detects acute injury; informs management | Invasive; limited evidence for donor discard decisions |
| Early (1 week to 6 months) | Early detection of SCR, drug toxicity, BK virus; guides timely therapy; predicts graft function | Procedural risks; sampling error; psychological impact; resource-intensive |
| Late (1 year and beyond) | Monitors chronic injury; predicts long-term outcomes; detects late rejection | Uncertain clinical relevance of mild findings; possible overtreatment; cost and resource use |
| Post-Acute Rejection | Detects residual SCR; guides therapy adjustments; prognostic value | Interpretation challenges; may not be cost-effective universally |
| Parameter | Data/Findings |
|---|---|
| Subclinical Rejection (SCR) Incidence |
|
| Biopsy-Related Complication Rates | |
| Long-Term Graft Survival and Biopsy Findings |
|
| Impact of Protocol Biopsies on Management |
|
| Risk Group/Patient Type | Biopsy Type Outcomes/Findings | Comments/Notes |
|---|---|---|
| High-Risk Patients (e.g., positive crossmatch, ABOi, HLAi, high sensitization, delayed graft function) | Protocol Biopsy
|
|
For-Cause Biopsy
|
| |
| Low-Risk Patients | Protocol Biopsy
|
|
For-Cause Biopsy
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazarou, C.; Moysidou, E.; Christodoulou, M.; Stai, S.; Lioulios, G.; Kasimatis, E.; Fylaktou, A.; Stangou, M. Protocol Biopsies in Kidney Transplant Recipients: Current Practice After Much Discussion. Biomedicines 2025, 13, 1660. https://doi.org/10.3390/biomedicines13071660
Lazarou C, Moysidou E, Christodoulou M, Stai S, Lioulios G, Kasimatis E, Fylaktou A, Stangou M. Protocol Biopsies in Kidney Transplant Recipients: Current Practice After Much Discussion. Biomedicines. 2025; 13(7):1660. https://doi.org/10.3390/biomedicines13071660
Chicago/Turabian StyleLazarou, Christina, Eleni Moysidou, Michalis Christodoulou, Stamatia Stai, Georgios Lioulios, Efstratios Kasimatis, Asimina Fylaktou, and Maria Stangou. 2025. "Protocol Biopsies in Kidney Transplant Recipients: Current Practice After Much Discussion" Biomedicines 13, no. 7: 1660. https://doi.org/10.3390/biomedicines13071660
APA StyleLazarou, C., Moysidou, E., Christodoulou, M., Stai, S., Lioulios, G., Kasimatis, E., Fylaktou, A., & Stangou, M. (2025). Protocol Biopsies in Kidney Transplant Recipients: Current Practice After Much Discussion. Biomedicines, 13(7), 1660. https://doi.org/10.3390/biomedicines13071660






