Advances and Challenges in Targeted Therapy and Its Combination Strategies for Leukemia
Abstract
1. Introduction
2. Targeted Therapy
2.1. Tyrosine Kinase Inhibitors
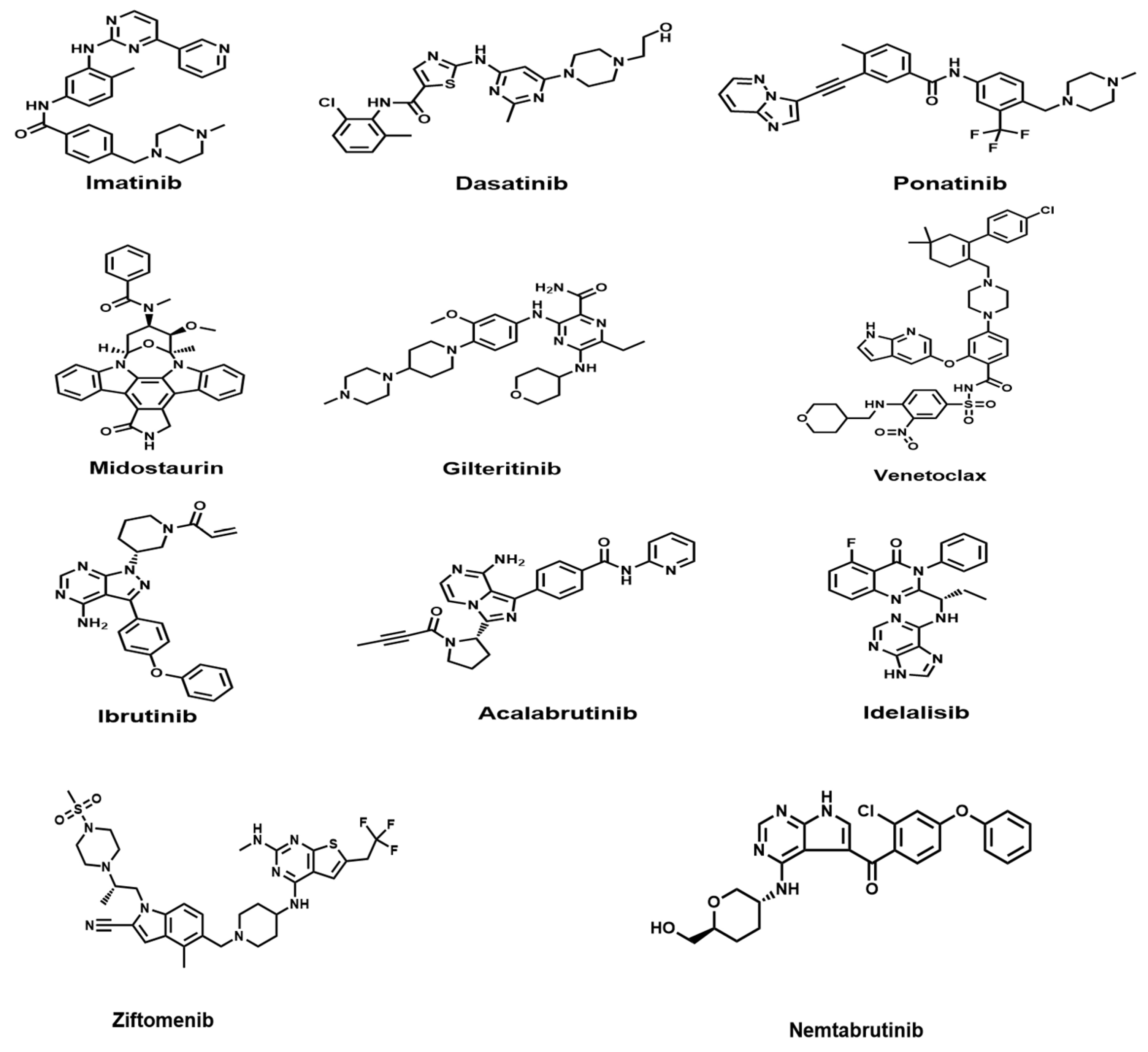
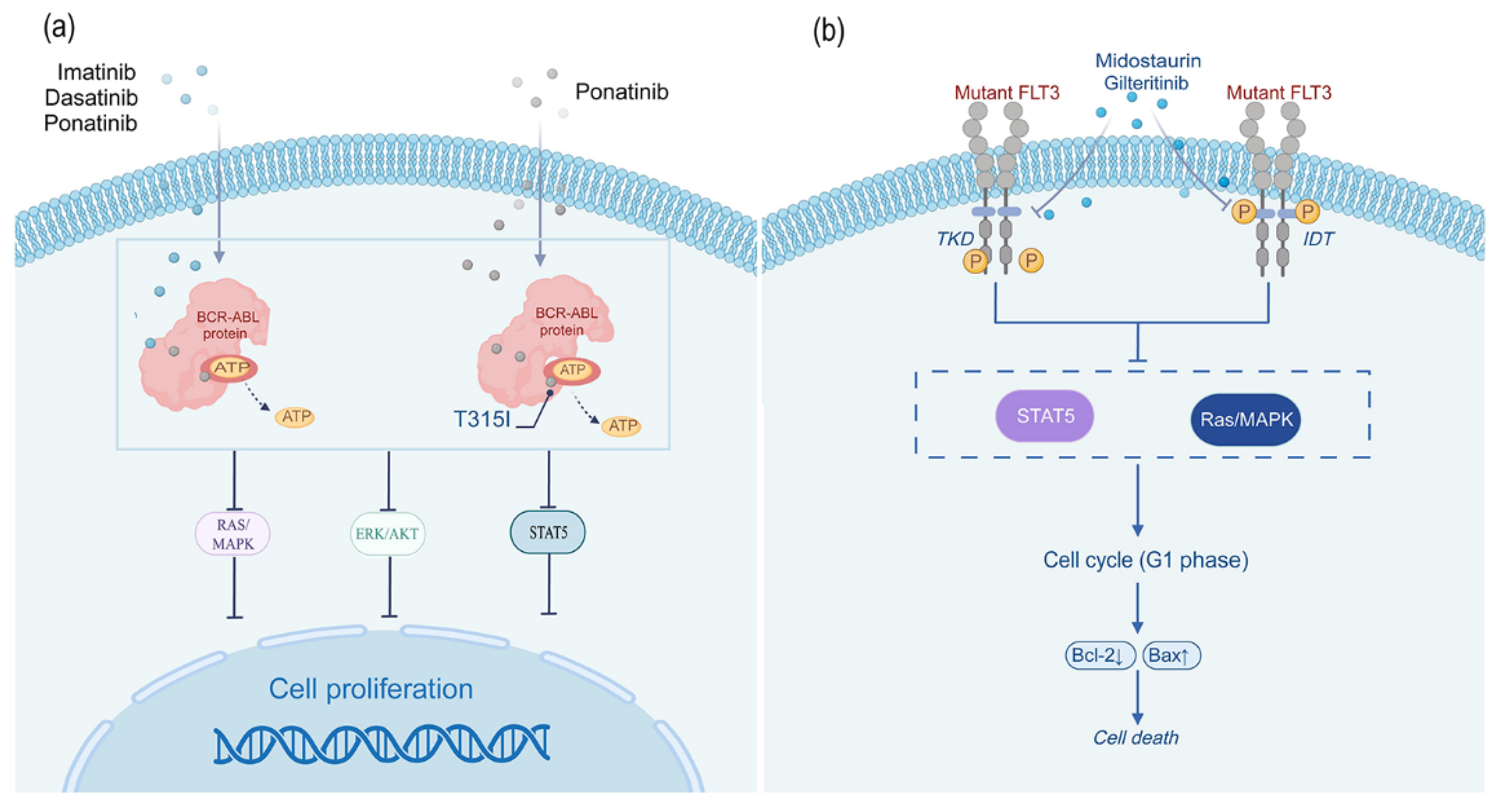
2.1.1. Imatinib
2.1.2. Dasatinib
2.1.3. Ponatinib
2.2. FLT3 Inhibitors
2.2.1. Midostaurin
2.2.2. Gilteritinib
2.3. B-Cell Signaling Pathway Inhibitors
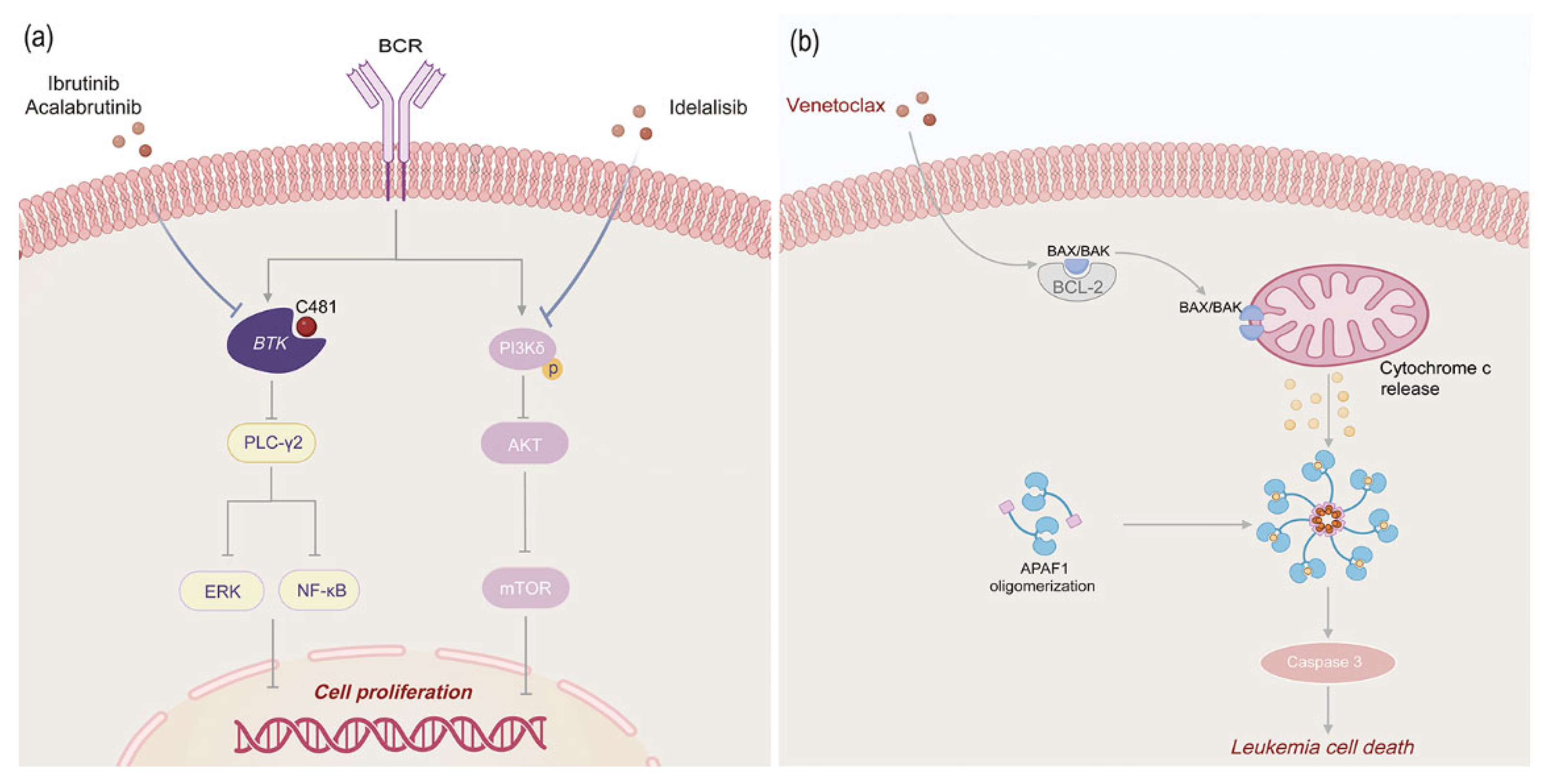
2.3.1. BTK Kinase Inhibitors
Ibrutinib
Acalabrutinib
2.3.2. PI3Kδ Inhibitors
2.4. Anti-Apoptotic Inhibitors
2.5. Immunotherapy Drugs
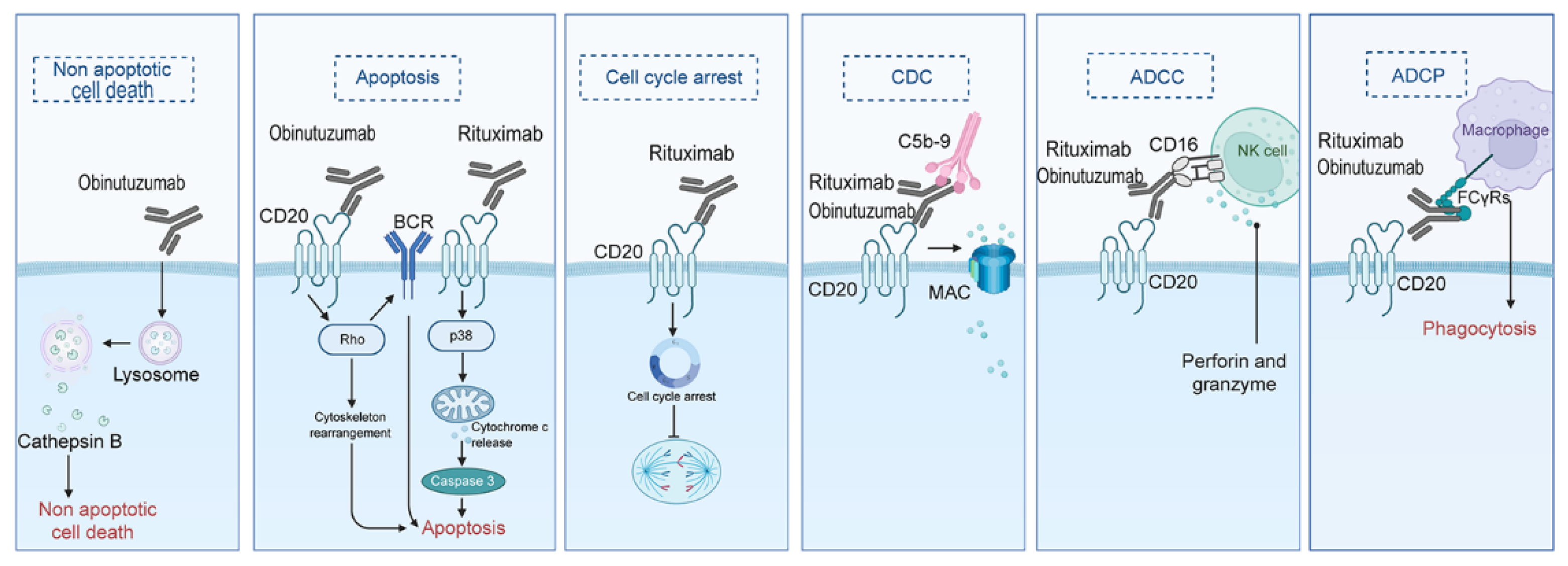
2.5.1. Monoclonal Antibodies
Rituximab
Obinutuzumab
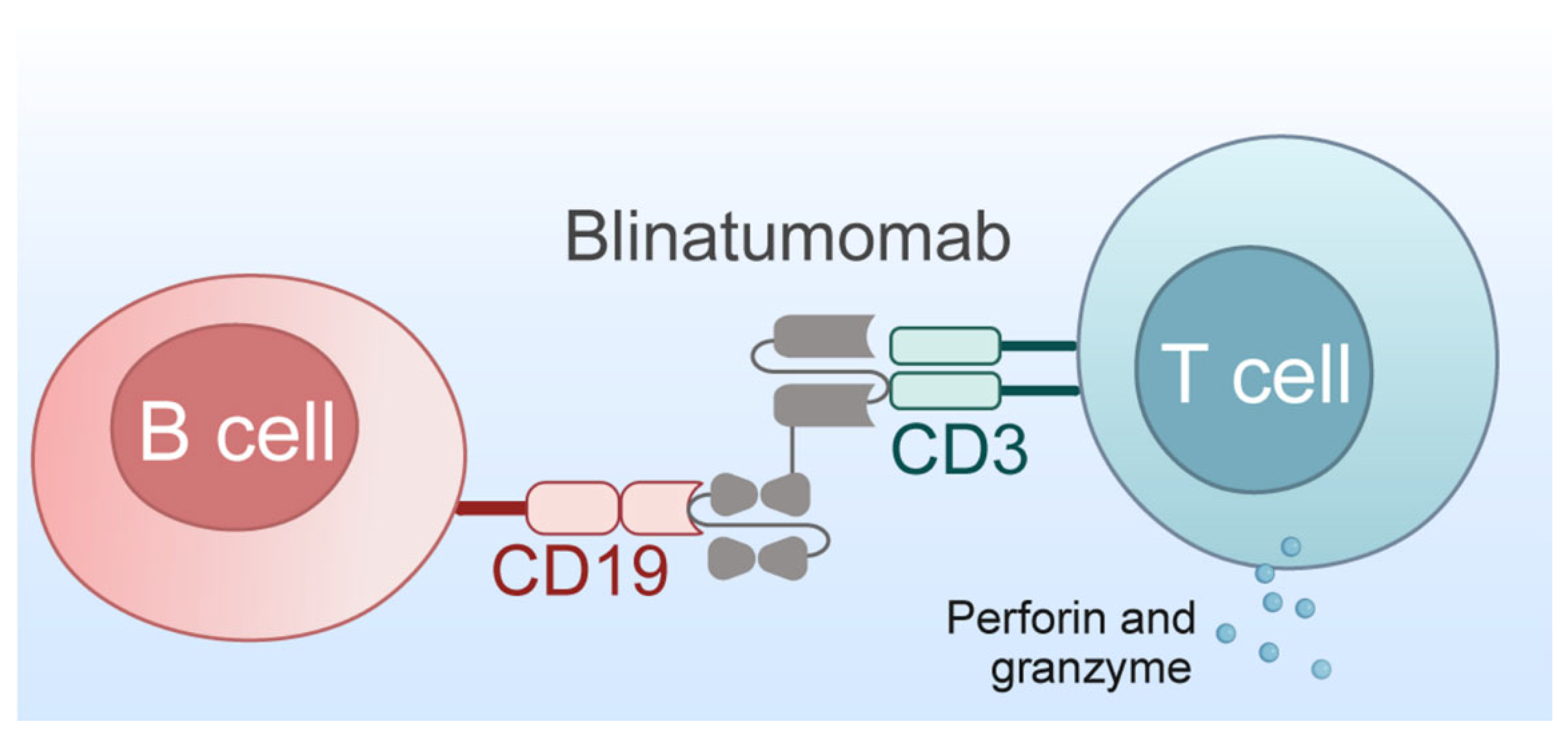
2.5.2. Bispecific T-Cell Engager
2.6. Differentiation Inducers
2.7. Upcoming Targeted Therapies Under Trial
2.8. Limitations of Targeted Therapy
3. Combination Therapy
3.1. Combination of Targeted Drugs and Chemotherapy Drugs
3.2. Internal Combination of Targeted Drugs
3.2.1. Internal Combination of Inhibitors
3.2.2. Combination of Inhibitors and Immunotherapy Drugs
| Combination Therapy Type | Combined Drugs | Indication | Patient Number | Treatment Outcome | Reference |
|---|---|---|---|---|---|
| Inhibitor Internal Combination | Imatinib, Nilotinib | CML | 123 | 5-year OS and PFS rates both 89% | [99] |
| Dasatinib, Venetoclax | CML | 65 | 4-year EFS and OS rates 96% and 100% respectively | [100] | |
| Dasatinib, Selinexor | CML | None | Produces significant inhibitory effects on LAMA84 and K562 cell lines | [101] | |
| Imatinib, Carfilzomib | CML | None | Can significantly reduce proliferation and induce CML stem cell apoptosis | [102] | |
| Ponatinib, Asciminib | CML | None | Effectively overcomes resistance caused by BCR-ABL1 compound mutations | [106] | |
| Imatinib, Venetoclax | CLL, CML | 1 | Achieved DMR | [107] | |
| Acalabrutinib, Venetoclax | CLL | 867 | 36-month OS rate 94.1% | [108] | |
| Idelalisib, Tirabrutinib | CLL | 53 | Objective response rate 93%, CR 7% | [109] | |
| Gilteritinib, MEN1703 | AML | None | Simultaneously inhibits PIM and FLT3, significantly enhances anti-tumor activity in vivo/in vitro | [110] | |
| Gilteritinib, GSK-J4 | AML | None | Combination therapy of Gilteritinib and GSK-J4 can significantly inhibit the growth of MV4-11 and MOLM-13 cells | [111] | |
| Inhibitors in combination with immunologic drugs | Acalabrutinib, Obinutuzumab | CLL | 535 | 72-month OS rate 83.9%, PFS rate 78% | [105] |
| Obinutuzumab, chlorambucil | CLL | 35 | 8-year OS rate 61%, EFS rate 25% | [103] | |
| Obinutuzumab, Venetoclax | CLL | 432 | 6-year OS rate 78.7%, for patients with del(17p) and/or TP53 mutation, 6-year OS rate 60.0% | [104] | |
| Ibrutinib, Ublituximab | CLL | 126 | Median follow-up 41.6 months, ORR 83% | [112] | |
| Rituximab, Idelalisib | CLL | 110 | ORR 83.6% | [45] | |
| Rituximab, Ibrutinib | CLL | 1924 | 4-year OS rate 92.1% | [62] | |
| Rituximab, Venetoclax | CLL | 389 | 2-year OS rate 91.9% | [113] | |
| Dasatinib, Blinatumomab | Ph+ ALL | 63 | Median follow-up 18 months, OS rate 95%, Disease-Free Survival 88% | [114] | |
| Rituximab, Vemurafenib | HCL | 30 | Median follow-up 34 months, Relapse-Free Survival rate 85% | [63] |
3.3. Combination of Targeted Drugs and CAR-T
3.4. Multi-Drug Combination
4. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ALL | acute lymphoblastic leukemia; |
| AML | acute myeloid leukemia; |
| CLL | chronic lymphocytic leukemia; |
| CML | chronic myeloid leukemia; |
| TKIs | Tyrosine kinase inhibitors; |
| CAR | T-Chimeric Antigen Receptor T-Cell; |
| Ph+ ALL | Philadelphia positive acute lymphoblastic leukemia; |
| HSCT | Hematopoietic Stem Cell Transplantation; |
| DMR | Deep Molecular Response; |
| TFR | Treatment-free remission; |
| OS | Overall Survival; |
| MMR | Major Molecular Response; |
| EFS | event-free survival; |
| FLT3-ITD | FLT3 internal tandem duplication mutations; |
| BTK | Bruton’s tyrosine kinase; |
| BCR | B-cell receptor; |
| ORR | Objective Response Rate; |
| PFS | progression-free survival; |
| R/R-CLL | relapsed/refractory chronic lymphocytic leukemia |
| BCL-2 | B-cell lymphoma-2; |
| BiTE | bispecific T-cell engager; |
| HCL | hairy cell leukemia; |
| MRD | minimal residual disease; |
| CR | Complete Remission; |
| scFv | single-chain variable fragments; |
| R/R-ALL | relapsed/refractory acute lymphoblastic leukemia; |
| CML-CP | Chronic Phase CML; |
| LSCs | leukemia stem cells; |
| U-MRD | undetectable Minimal Residual Disease |
| CRi | Complete Remission with Incomplete Blood Count Recovery; |
| ATRA | all-trans retinoic acid; |
| APL | acute promyelocytic leukemia. |
References
- Gale, K.B.; Ford, A.M.; Repp, R.; Borkhardt, A.; Keller, C.; Eden, O.B.; Greaves, M.F. Backtracking leukemia to birth: Identification of clonotypic gene fusion sequences in neonatal blood spots. Proc. Natl. Acad. Sci. USA 1997, 94, 13950–13954. [Google Scholar] [CrossRef]
- Metayer, C.; Zhang, L.; Wiemels, J.L.; Bartley, K.; Schiffman, J.; Ma, X.; Aldrich, M.C.; Chang, J.S.; Selvin, S.; Fu, C.H.; et al. Tobacco Smoke Exposure and the Risk of Childhood Acute Lymphoblastic and Myeloid Leukemias by Cytogenetic Subtype. Cancer Epidemiol. Biomark. Prev. 2013, 22, 1600–1611. [Google Scholar] [CrossRef]
- Deng, W.; Xu, Y.; Yuan, X. Clinical features and prognosis of acute lymphoblastic leukemia in children with Epstein-Barr virus infection. Transl. Pediatr. 2022, 11, 642–650. [Google Scholar] [CrossRef]
- de la Serna, J.; Montesinos, P.; Vellenga, E.; Rayón, C.; Parody, R.; León, A.; Esteve, J.; Bergua, J.M.; Milone, G.; Debén, G.; et al. Causes and prognostic factors of remission induction failure in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and idarubicin. Blood 2008, 111, 3395–3402. [Google Scholar] [CrossRef]
- Vesa, S.; Rusu, R.; Sîrbu, D.; Curşeu, D.; Năsui, B.; Sava, M.; Bojan, A.; Lisencu, C.; Popa, M. Chemotherapy-related infectious complications in patients with Hematologic malignancies. J. Res. Med. Sci. 2018, 23, 68. [Google Scholar] [CrossRef]
- Tada, A.; Kikuchi, Y.; Murase, K.; Takada, K.; Takasawa, A. Drastic Multiorgan Dysfunction Due to Severe Leukostasis: A Case Report. Cureus 2022, 14, e31518. [Google Scholar] [CrossRef]
- Tantiworawit, A.; Power, M.M.; Barnett, M.J.; Hogge, D.E.; Nantel, S.H.; Nevill, T.J.; Shepherd, J.D.; Song, K.W.; Sutherland, H.J.; Toze, C.L.; et al. Long-term follow-up of patients with chronic myeloid leukemia in chronic phase developing sudden blast phase on imatinib therapy. Leuk. Lymphoma 2012, 53, 1321–1326. [Google Scholar] [CrossRef]
- Dong, Y.; Shi, O.; Zeng, Q.; Lu, X.; Wang, W.; Li, Y.; Wang, Q. Leukemia incidence trends at the global, regional, and national level between 1990 and 2017. Exp. Hematol. Oncol. 2020, 9, 14. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Hao, Y.; Zhang, N.; Wei, N.; Yin, H.; Zhang, Y.; Xu, H.; Zhu, C.; Li, D. Matrine induces apoptosis in acute myeloid leukemia cells by inhibiting the PI3K/Akt/mTOR signaling pathway. Oncol. Lett. 2019, 18, 2891–2896. [Google Scholar] [CrossRef]
- Huang, Y.; Qin, Y.; He, Y.; Qiu, D.; Zheng, Y.; Wei, J.; Zhang, L.; Yang, D.; Li, Y. Advances in molecular targeted drugs in combination with CAR-T cell therapy for hematologic malignancies. Drug Resist. Updat. 2024, 74, 101082. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.; Pimentel, L.; Canzian, H.; Oliveira, A.; Junior, F.; Dantas, R.; Hoelz, L.; Marinho, D.; Cunha, A.; Bastos, M.; et al. Hybrids of Imatinib with Quinoline: Synthesis, Antimyeloproliferative Activity Evaluation, and Molecular Docking. Pharmaceuticals 2022, 15, 309. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-E.; Thuy, N.T.T.; Lee, J.; Cho, N.; Yoo, H.M. Platyphylloside Isolated from Betula platyphylla is Antiproliferative and Induces Apoptosis in Colon Cancer and Leukemic Cells. Molecules 2021, 24, 2960. [Google Scholar] [CrossRef] [PubMed]
- Chalandon, Y.; Thomas, X.; Hayette, S.; Cayuela, J.-M.; Abbal, C.; Huguet, F.; Raffoux, E.; Leguay, T.; Rousselot, P.; Lepretre, S.; et al. Randomized study of reduced-intensity chemotherapy combined with imatinib in adults with Ph-positive acute lymphoblastic leukemia. Blood 2015, 125, 3711–3719. [Google Scholar] [CrossRef]
- Hantschel, O.; Rix, U.; Superti-Furga, G. Target spectrum of the BCR-ABL inhibitors imatinib, nilotinib and dasatinib. Leuk. Lymphoma 2008, 49, 615–619. [Google Scholar] [CrossRef]
- Maiti, A.; Cortes, J.E.; Patel, K.P.; Masarova, L.; Borthakur, G.; Ravandi, F.; Verstovsek, S.; Ferrajoli, A.; Estrov, Z.; Garcia-Manero, G.; et al. Long-term results of frontline dasatinib in chronic myeloid leukemia. Cancer 2020, 126, 1502–1511. [Google Scholar] [CrossRef]
- Gong, H.; Jin, X.; Leng, G.; Zhang, M.; Niu, S.; Cao, W.; Zhang, H.; Zeng, Y.; Li, C.; Li, Y.; et al. Licoflavone A Suppresses Gastric Cancer Growth and Metastasis by Blocking the VEGFR-2 Signaling Pathway. J. Oncol. 2022, 2022, 5497991. [Google Scholar] [CrossRef]
- Chang, C.-S.; Yang, Y.-H.; Hsu, C.-N.; Lin, M.-T. Trends in the treatment changes and medication persistence of chronic myeloid leukemia in Taiwan from 1997 to 2007: A longitudinal population database analysis. BMC Health Serv. Res. 2012, 12, 359. [Google Scholar] [CrossRef]
- Vysochinskaya, V.; Dovbysh, O.; Gorshkov, A.; Brodskaia, A.; Dubina, M.; Vasin, A.; Zabrodskaya, Y. Advancements and Future Prospects in Molecular Targeted and siRNA Therapies for Chronic Myeloid Leukemia. Biomolecules 2024, 14, 644. [Google Scholar] [CrossRef]
- Iqbal, N.; Iqbal, N. Imatinib: A Breakthrough of Targeted Therapy in Cancer. Chemother. Res. Pract. 2014, 2014, 357027. [Google Scholar] [CrossRef]
- Mohd Yacob, A.; Muhamad, N.A.; Chang, K.M.; Akmal Hisham, H.; Mat Yusoff, Y.; Ibrahim, L. Hsa-miR-181a-5p, hsa-miR-182-5p, and hsa-miR-26a-5p as potential biomarkers for BCR-ABL1 among adult chronic myeloid leukemia treated with tyrosine kinase inhibitors at the molecular response. BMC Cancer 2022, 22, 332. [Google Scholar] [CrossRef] [PubMed]
- Hochhaus, A.; Larson, R.A.; Guilhot, F.; Radich, J.P.; Branford, S.; Hughes, T.P.; Baccarani, M.; Deininger, M.W.; Cervantes, F.; Fujihara, S.; et al. Long-Term Outcomes of Imatinib Treatment for Chronic Myeloid Leukemia. N. Engl. J. Med. 2017, 376, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Gambacorti-Passerini, C.; Antolini, L.; Mahon, F.-X.; Guilhot, F.; Deininger, M.; Fava, C.; Nagler, A.; Della Casa, C.M.; Morra, E.; Abruzzese, E.; et al. Multicenter Independent Assessment of Outcomes in Chronic Myeloid Leukemia Patients Treated with Imatinib. JNCI J. Natl. Cancer Inst. 2011, 103, 553–561. [Google Scholar] [CrossRef]
- Saußele, S.; Richter, J.; Hochhaus, A.; Mahon, F.-X. The concept of treatment-free remission in chronic myeloid leukemia. Leukemia 2016, 30, 1638–1647. [Google Scholar] [CrossRef]
- Hochhaus, A.; Saglio, G.; Hughes, T.P.; Larson, R.A.; Taningco, L.; Deng, W.; Menssen, H.D.; Kantarjian, H.M. Impact of Treatment with Frontline Nilotinib (NIL) vs Imatinib (IM) on Sustained Deep Molecular Response (MR) in Patients (pts) with Newly Diagnosed Chronic Myeloid Leukemia in Chronic Phase (CML-CP). Blood 2015, 126, 2781. [Google Scholar] [CrossRef]
- Cheng, F.; Yuan, G.; Li, Q.; Cui, Z.; Li, W. Long-term outcomes of frontline imatinib therapy for chronic myeloid leukemia in China. Front. Oncol. 2018, 13, 1172910. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Chen, Q.; Chen, K.; Jiang, Y.; Li, G.; Chen, Q.; Bai, D.; Gao, D.; Deng, M.; Zhang, H.; et al. Simvastatin potentiates the cell-killing activity of imatinib in imatinib-resistant chronic myeloid leukemia cells mainly through PI3K/AKT pathway attenuation and Myc downregulation. Eur. J. Pharmacol. 2021, 913, 174633. [Google Scholar] [CrossRef]
- Vinay, K.; Yanamandra, U.; Dogra, S.; Handa, S.; Suri, V.; Kumari, S.; Khadwal, A.; Prakash, G.; Lad, D.; Varma, S.; et al. Long-term mucocutaneous adverse effects of imatinib in Indian chronic myeloid leukemia patients. Int. J. Dermatol. 2018, 57, 332–338. [Google Scholar] [CrossRef]
- Foà, R.; Vitale, A.; Vignetti, M.; Meloni, G.; Guarini, A.; De Propris, M.S.; Elia, L.; Paoloni, F.; Fazi, P.; Cimino, G.; et al. Dasatinib as first-line treatment for adult patients with Philadelphia chromosome–positive acute lymphoblastic leukemia. Blood 2011, 118, 6521–6528. [Google Scholar] [CrossRef]
- Iurlo, A.; Cattaneo, D.; Malato, A.; Accurso, V.; Annunziata, M.; Gozzini, A.; Scortechini, A.R.; Bucelli, C.; Scalzulli, E.; Attolico, I.; et al. Low-dose ponatinib is a good option in chronic myeloid leukemia patients intolerant to previous TKIs. Am. J. Hematol. 2020, 95, E260–E263. [Google Scholar] [CrossRef]
- Pulte, E.D.; Chen, H.; Price, L.S.L.; Gudi, R.; Li, H.; Okusanya, O.O.; Ma, L.; Rodriguez, L.; Vallejo, J.; Norsworthy, K.J.; et al. FDA Approval Summary: Revised Indication and Dosing Regimen for Ponatinib Based on the Results of the OPTIC Trial. Oncol. 2022, 27, 149–157. [Google Scholar] [CrossRef]
- Haddad, F.G.; Sasaki, K.; Nasr, L.; Short, N.J.; Kadia, T.; Dellasala, S.; Cortes, J.; Nicolini, F.E.; Issa, G.C.; Jabbour, E.; et al. Results of ponatinib as frontline therapy for chronic myeloid leukemia in chronic phase. Cancer 2024, 130, 3344–3352. [Google Scholar] [CrossRef] [PubMed]
- Chan, O.; Talati, C.; Isenalumhe, L.; Shams, S.; Nodzon, L.; Fradley, M.; Sweet, K.; Pinilla-Ibarz, J. Side-effects profile and outcomes of ponatinib in the treatment of chronic myeloid leukemia. Blood Adv. 2020, 4, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.; Gonçalves, A.C.; Rutella, S.; Almeida, A.M.; De Las Rivas, J.; Trougakos, I.P.; Sarmento Ribeiro, A.B. Resistance to Tyrosine Kinase Inhibitors in Chronic Myeloid Leukemia—From Molecular Mechanisms to Clinical Relevance. Cancers 2021, 13, 4820. [Google Scholar] [CrossRef]
- Yuan, S.; Zhou, Y.; Li, Y.; Chen, Z.; Xiao, W.; Jiang, D.; Zhang, P.; Zhang, Y.; Bai, F.; Deng, J.; et al. Mitoxantrone-liposome Sensitizes FLT3-ITD Acute Myeloid Leukemia to Gilteritinib Treatment. J. Cancer 2025, 16, 1905–1917. [Google Scholar] [CrossRef] [PubMed]
- Rong, Q.-Y.; Lu, Y.; Zhang, W.; Rao, G.-W.; Zheng, Q. Targeting FLT3 for treating diseases: FLT3 inhibitors. Drug Discov. Today 2025, 30, 104367. [Google Scholar] [CrossRef]
- Levis, M. Midostaurin approved for FLT3-mutated AML. Blood 2017, 129, 3403–3406. [Google Scholar] [CrossRef]
- Zwaan, C.M.; Söderhäll, S.; Brethon, B.; Luciani, M.; Rizzari, C.; Stam, R.W.; Besse, E.; Dutreix, C.; Fagioli, F.; Ho, P.A.; et al. A phase 1/2, open-label, dose-escalation study of midostaurin in children with relapsed or refractory acute leukaemia. Br. J. Haematol. 2019, 185, 623–627. [Google Scholar] [CrossRef]
- Tzogani, K.; Yu, Y.; Meulendijks, D.; Herberts, C.; Hennik, P.; Verheijen, R.; Wangen, T.; Dahlseng Håkonsen, G.; Kaasboll, T.; Dalhus, M.; et al. European Medicines Agency review of midostaurin (Rydapt) for the treatment of adult patients with acute myeloid leukaemia and systemic mastocytosis. ESMO Open 2019, 4, e000606. [Google Scholar] [CrossRef]
- Perl, A.E.; Hosono, N.; Montesinos, P.; Podoltsev, N.; Martinelli, G.; Panoskaltsis, N.; Recher, C.; Smith, C.C.; Levis, M.J.; Strickland, S.; et al. Clinical outcomes in patients with relapsed/refractory FLT3-mutated acute myeloid leukemia treated with gilteritinib who received prior midostaurin or sorafenib. Blood Cancer J. 2022, 12, 84. [Google Scholar] [CrossRef]
- Perl, A.E.; Larson, R.A.; Podoltsev, N.A.; Strickland, S.; Wang, E.S.; Atallah, E.; Schiller, G.J.; Martinelli, G.; Neubauer, A.; Sierra, J.; et al. Follow-up of patients with R/R FLT3-mutation–positive AML treated with gilteritinib in the phase 3 ADMIRAL trial. Blood 2022, 139, 3366–3375. [Google Scholar] [CrossRef] [PubMed]
- Brinton, L.T.; Zhang, P.; Williams, K.; Canfield, D.; Orwick, S.; Sher, S.; Wasmuth, R.; Beaver, L.; Cempre, C.; Skinner, J.; et al. Synergistic effect of BCL2 and FLT3 co-inhibition in acute myeloid leukemia. J. Hematol. Oncol. 2020, 13, 139. [Google Scholar] [CrossRef]
- Ghia, P.; Pluta, A.; Wach, M.; Lysak, D.; Kozak, T.; Simkovic, M.; Kaplan, P.; Kraychok, I.; Illes, A.; de la Serna, J.; et al. ASCEND: Phase III, Randomized Trial of Acalabrutinib Versus Idelalisib Plus Rituximab or Bendamustine Plus Rituximab in Relapsed or Refractory Chronic Lymphocytic Leukemia. J. Clin. Oncol. 2020, 38, 2849–2861. [Google Scholar] [CrossRef] [PubMed]
- Vetrie, D.; Vořechovský, I.; Sideras, P.; Holland, J.; Davies, A.; Flinter, F.; Hammarström, L.; Kinnon, C.; Levinsky, R.; Bobrow, M.; et al. The gene involved in X-linked agammaglobulinaemia is a member of the src family of protein-tyrosine kinases. Nature 1993, 361, 226–233. [Google Scholar] [CrossRef]
- Sharman, J.P.; Coutre, S.E.; Furman, R.R.; Cheson, B.D.; Pagel, J.M.; Hillmen, P.; Barrientos, J.C.; Zelenetz, A.D.; Kipps, T.J.; Flinn, I.W.; et al. Final Results of a Randomized, Phase III Study of Rituximab With or Without Idelalisib Followed by Open-Label Idelalisib in Patients With Relapsed Chronic Lymphocytic Leukemia. J. Clin. Oncol. 2019, 37, 1391–1402. [Google Scholar] [CrossRef]
- Burger, J.A.; Barr, P.M.; Robak, T.; Owen, C.; Ghia, P.; Tedeschi, A.; Bairey, O.; Hillmen, P.; Coutre, S.E.; Devereux, S.; et al. Long-term efficacy and safety of first-line ibrutinib treatment for patients with CLL/SLL: 5 years of follow-up from the phase 3 RESONATE-2 study. Leukemia 2020, 34, 787–798. [Google Scholar] [CrossRef]
- Mattiello, V.; Barone, A.; Giannarelli, D.; Noto, A.; Cecchi, N.; Rampi, N.; Cassin, R.; Reda, G. Predictors of ibrutinib-associated atrial fibrillation: 5-year follow-up of a prospective study. Hematol. Oncol. 2023, 41, 363–370. [Google Scholar] [CrossRef]
- Woyach, J.A.; Blachly, J.S.; Rogers, K.A.; Bhat, S.A.; Jianfar, M.; Lozanski, G.; Weiss, D.M.; Andersen, B.L.; Gulrajani, M.; Frigault, M.M.; et al. Acalabrutinib plus Obinutuzumab in Treatment-Naïve and Relapsed/Refractory Chronic Lymphocytic Leukemia. Cancer Discov. 2020, 10, 394–405. [Google Scholar] [CrossRef]
- Byrd, J.C.; Hillmen, P.; Ghia, P.; Kater, A.P.; Chanan-Khan, A.; Furman, R.R.; O’Brien, S.; Yenerel, M.N.; Illés, A.; Kay, N.; et al. Acalabrutinib Versus Ibrutinib in Previously Treated Chronic Lymphocytic Leukemia: Results of the First Randomized Phase III Trial. J. Clin. Oncol. 2021, 39, 3441–3452. [Google Scholar] [CrossRef]
- Byrd, J.C.; Wierda, W.G.; Schuh, A.; Devereux, S.; Chaves, J.M.; Brown, J.R.; Hillmen, P.; Martin, P.; Awan, F.T.; Stephens, D.M.; et al. Acalabrutinib monotherapy in patients with relapsed/refractory chronic lymphocytic leukemia: Updated phase 2 results. Blood 2020, 135, 1204–1213. [Google Scholar] [CrossRef]
- Brown, J.R.; Byrd, J.C.; Coutre, S.E.; Benson, D.M.; Flinn, I.W.; Wagner-Johnston, N.D.; Spurgeon, S.E.; Kahl, B.S.; Bello, C.; Webb, H.K.; et al. Idelalisib, an inhibitor of phosphatidylinositol 3-kinase p110δ, for relapsed/refractory chronic lymphocytic leukemia. Blood 2014, 123, 3390–3397. [Google Scholar] [CrossRef] [PubMed]
- Maharaj, D.; Srinivasan, G.; Abreu, M.M.; Ko, M.-W.; Jewett, A.; Gouvea, J. Molecular Remission Using Low-Dose Immunotherapy with Minimal Toxicities for Poor Prognosis IGHV—Unmutated Chronic Lymphocytic Leukemia. Cells 2020, 10, 10. [Google Scholar] [CrossRef]
- Zhu, R.; Li, L.; Nguyen, B.; Seo, J.; Wu, M.; Seale, T.; Levis, M.; Duffield, A.; Hu, Y.; Small, D. FLT3 tyrosine kinase inhibitors synergize with BCL-2 inhibition to eliminate FLT3/ITD acute leukemia cells through BIM activation. Signal Transduct. Target. Ther. 2021, 6, 186. [Google Scholar] [CrossRef]
- Souers, A.J.; Leverson, J.D.; Boghaert, E.R.; Ackler, S.L.; Catron, N.D.; Chen, J.; Dayton, B.D.; Ding, H.; Enschede, S.H.; Fairbrother, W.J.; et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med. 2013, 19, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.A.; Deng, J.; Seymour, J.F.; Tam, C.; Kim, S.Y.; Fein, J.; Yu, L.; Brown, J.R.; Westerman, D.; Si, E.G.; et al. The BCL2 selective inhibitor venetoclax induces rapid onset apoptosis of CLL cells in patients via a TP53-independent mechanism. Blood 2016, 127, 3215–3224. [Google Scholar] [CrossRef]
- Stilgenbauer, S.; Eichhorst, B.; Schetelig, J.; Coutre, S.; Seymour, J.F.; Munir, T.; Puvvada, S.D.; Wendtner, C.-M.; Roberts, A.W.; Jurczak, W.; et al. Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: A multicentre, open-label, phase 2 study. Lancet Oncol. 2016, 17, 768–778. [Google Scholar] [CrossRef]
- Blombery, P.; Lew, T.E.; Dengler, M.A.; Thompson, E.R.; Lin, V.S.; Chen, X.; Nguyen, T.; Panigrahi, A.; Handunnetti, S.M.; Carney, D.A.; et al. Clonal hematopoiesis, myeloid disorders and BAX-mutated myelopoiesis in patients receiving venetoclax for CLL. Blood 2022, 139, 1198–1207. [Google Scholar] [CrossRef]
- Jabbour, E.; O’Brien, S.; Ravandi, F.; Kantarjian, H. Monoclonal antibodies in acute lymphoblastic leukemia. Blood 2015, 125, 4010–4016. [Google Scholar] [CrossRef] [PubMed]
- Hagemeister, F. Rituximab for the Treatment of Non-Hodgkin’s Lymphoma and Chronic Lymphocytic Leukaemia. Drugs 2010, 70, 261–272. [Google Scholar] [CrossRef]
- Ribrag, V.; Koscielny, S.; Bosq, J.; Leguay, T.; Casasnovas, O.; Fornecker, L.-M.; Recher, C.; Ghesquieres, H.; Morschhauser, F.; Girault, S.; et al. Rituximab and dose-dense chemotherapy for adults with Burkitt’s lymphoma: A randomised, controlled, open-label, phase 3 trial. Lancet 2016, 387, 2402–2411. [Google Scholar] [CrossRef]
- Maury, S.; Chevret, S.; Thomas, X.; Heim, D.; Leguay, T.; Huguet, F.; Chevallier, P.; Hunault, M.; Boissel, N.; Escoffre-Barbe, M.; et al. Rituximab in B-Lineage Adult Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2016, 375, 1044–1053. [Google Scholar] [CrossRef] [PubMed]
- Hillmen, P.; Pitchford, A.; Bloor, A.; Broom, A.; Young, M.; Kennedy, B.; Walewska, R.; Furtado, M.; Preston, G.; Neilson, J.R.; et al. Ibrutinib and rituximab versus fludarabine, cyclophosphamide, and rituximab for patients with previously untreated chronic lymphocytic leukaemia (FLAIR): Interim analysis of a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2023, 24, 535–552. [Google Scholar] [CrossRef]
- Tiacci, E.; De Carolis, L.; Simonetti, E.; Capponi, M.; Ambrosetti, A.; Lucia, E.; Antolino, A.; Pulsoni, A.; Ferrari, S.; Zinzani, P.L.; et al. Vemurafenib plus Rituximab in Refractory or Relapsed Hairy-Cell Leukemia. N. Engl. J. Med. 2021, 384, 1810–1823. [Google Scholar] [CrossRef]
- Veeramani, S.; Wang, S.-Y.; Dahle, C.; Blackwell, S.; Jacobus, L.; Knutson, T.; Button, A.; Link, B.K.; Weiner, G.J. Rituximab infusion induces NK activation in lymphoma patients with the high-affinity CD16 polymorphism. Blood 2011, 118, 3347–3349. [Google Scholar] [CrossRef]
- Urlaub, D.; Zhao, S.; Blank, N.; Bergner, R.; Claus, M.; Tretter, T.; Lorenz, H.-M.; Watzl, C.; Merkt, W. Activation of natural killer cells by rituximab in granulomatosis with polyangiitis. Arthritis Res. Ther. 2019, 21, 277. [Google Scholar] [CrossRef]
- Shan, D.; Ledbetter, J.A.; Press, O.W. Apoptosis of Malignant Human B Cells by Ligation of CD20 With Monoclonal Antibodies. Blood 1998, 91, 1644–1652. [Google Scholar] [CrossRef][Green Version]
- Herlyn, D.M.; Koprowski, H. Monoclonal anticolon carcinoma antibodies in complement-dependent cytotoxicity. Int. J. Cancer 1981, 27, 769–774. [Google Scholar] [CrossRef]
- Kamen, L.; Myneni, S.; Langsdorf, C.; Kho, E.; Ordonia, B.; Thakurta, T.; Zheng, K.; Song, A.; Chung, S. A novel method for determining antibody-dependent cellular phagocytosis. J. Immunol. Methods 2019, 468, 55–60. [Google Scholar] [CrossRef]
- Nieva, J.; Bethel, K.; Saven, A. Phase 2 study of rituximab in the treatment of cladribine-failed patients with hairy cell leukemia. Blood 2003, 102, 810–813. [Google Scholar] [CrossRef] [PubMed]
- Amitai, I.; Gafter-Gvili, A.; Shargian-Alon, L.; Raanani, P.; Gurion, R. Obinutuzumab-related adverse events: A systematic review and meta-analysis. Hematol. Oncol. 2021, 39, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Samineni, D.; Gibiansky, L.; Wang, B.; Vadhavkar, S.; Rajwanshi, R.; Tandon, M.; Sinha, A.; Al-Sawaf, O.; Fischer, K.; Hallek, M.; et al. Pharmacokinetics and Exposure-Response Analysis of Venetoclax + Obinutuzumab in Chronic Lymphocytic Leukemia: Phase 1b Study and Phase 3 CLL14 Trial. Adv. Ther. 2022, 39, 3635–3653. [Google Scholar] [CrossRef]
- Cartron, G.; de Guibert, S.; Dilhuydy, M.-S.; Morschhauser, F.; Leblond, V.; Dupuis, J.; Mahe, B.; Bouabdallah, R.; Lei, G.; Wenger, M.; et al. Obinutuzumab (GA101) in relapsed/refractory chronic lymphocytic leukemia: Final data from the phase 1/2 GAUGUIN study. Blood 2014, 124, 2196–2202. [Google Scholar] [CrossRef] [PubMed]
- Brischwein, K.; Parr, L.; Pflanz, S.; Volkland, J.; Lumsden, J.; Klinger, M.; Locher, M.; Hammond, S.A.; Kiener, P.; Kufer, P.; et al. Strictly Target Cell-dependent Activation of T Cells by Bispecific Single-chain Antibody Constructs of the BiTE Class. J. Immunother. 2007, 30, 798–807. [Google Scholar] [CrossRef]
- Yuraszeck, T.; Kasichayanula, S.; Benjamin, J. Translation and Clinical Development of Bispecific T-cell Engaging Antibodies for Cancer Treatment. Clin. Pharmacol. Ther. 2017, 101, 634–645. [Google Scholar] [CrossRef]
- Jabbour, E.; Short, N.J.; Jain, N.; Thompson, P.A.; Kadia, T.M.; Ferrajoli, A.; Huang, X.; Yilmaz, M.; Alvarado, Y.; Patel, K.P.; et al. Hyper-CVAD and sequential blinatumomab for newly diagnosed Philadelphia chromosome-negative B-cell acute lymphocytic leukaemia: A single-arm, single-centre, phase 2 trial. Lancet Haematol. 2022, 9, e878–e885. [Google Scholar] [CrossRef]
- Kantarjian, H.; Stein, A.; Gökbuget, N.; Fielding, A.K.; Schuh, A.C.; Ribera, J.-M.; Wei, A.; Dombret, H.; Foà, R.; Bassan, R.; et al. Blinatumomab versus Chemotherapy for Advanced Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2017, 376, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Gökbuget, N.; Dombret, H.; Bonifacio, M.; Reichle, A.; Graux, C.; Faul, C.; Diedrich, H.; Topp, M.S.; Brüggemann, M.; Horst, H.-A.; et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood 2018, 131, 1522–1531. [Google Scholar] [CrossRef]
- Huang, M.; Ye, Y.; Chen, S.; Chai, J.; Lu, J.; Zhoa, L.; Gu, L.; Wang, Z. Use of all-trans retinoic acid in the treatment of acute promyelocytic leukemia. Blood 1988, 72, 567–572. [Google Scholar] [CrossRef]
- Liang, C.; Qiao, G.; Liu, Y.; Tian, L.; Hui, N.; Li, J.; Ma, Y.; Li, H.; Zhao, Q.; Cao, W.; et al. Overview of all-trans-retinoic acid (ATRA) and its analogues: Structures, activities, and mechanisms in acute promyelocytic leukaemia. Eur. J. Med. Chem. 2021, 220, 113451. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Mu, X.; Li, F.; Wang, Y.; Yang, X.; Guo, Q. Roles of PADI4 in the expression of cytokines involved in inflammation and adhesion in differentiated NB4 cells treated with ATRA. Exp. Ther. Med. 2018, 25, 118. [Google Scholar] [CrossRef]
- Fiskus, W.; Daver, N.; Boettcher, S.; Mill, C.P.; Sasaki, K.; Birdwell, C.E.; Davis, J.A.; Das, K.; Takahashi, K.; Kadia, T.M.; et al. Activity of menin inhibitor ziftomenib (KO-539) as monotherapy or in combinations against AML cells with MLL1 rearrangement or mutant NPM1. Leukemia 2022, 36, 2729–2733. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.S.; Issa, G.C.; Erba, H.P.; Altman, J.K.; Montesinos, P.; DeBotton, S.; Walter, R.B.; Pettit, K.; Savona, M.R.; Shah, M.V.; et al. Ziftomenib in relapsed or refractory acute myeloid leukaemia (KOMET-001): A multicentre, open-label, multi-cohort, phase 1 trial. Lancet Oncol. 2024, 25, 1310–1324. [Google Scholar] [CrossRef] [PubMed]
- Reiff, S.D.; Mantel, R.; Smith, L.L.; Greene, J.T.; Muhowski, E.M.; Fabian, C.A.; Goettl, V.M.; Tran, M.; Harrington, B.K.; Rogers, K.A.; et al. The BTK Inhibitor ARQ 531 Targets Ibrutinib-Resistant CLL and Richter Transformation. Cancer Discov. 2018, 8, 1300–1315. [Google Scholar] [CrossRef]
- Woyach, J.A.; Stephens, D.M.; Flinn, I.W.; Bhat, S.A.; Savage, R.E.; Chai, F.; Eathiraj, S.; Reiff, S.D.; Muhowski, E.M.; Granlund, L.; et al. First-in-Human Study of the Reversible BTK Inhibitor Nemtabrutinib in Patients with Relapsed/Refractory Chronic Lymphocytic Leukemia and B-Cell Non–Hodgkin Lymphoma. Cancer Discov. 2024, 14, 66–75. [Google Scholar] [CrossRef]
- Hochhaus, A.; O’Brien, S.G.; Guilhot, F.; Druker, B.J.; Branford, S.; Foroni, L.; Goldman, J.M.; Müller, M.C.; Radich, J.P.; Rudoltz, M.; et al. Six-year follow-up of patients receiving imatinib for the first-line treatment of chronic myeloid leukemia. Leukemia 2009, 23, 1054–1061. [Google Scholar] [CrossRef] [PubMed]
- DiNardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Döhner, H.; Letai, A.; Fenaux, P.; et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef]
- Jabbour, E.; Kantarjian, H.M.; Aldoss, I.; Montesinos, P.; Leonard, J.T.; Gómez-Almaguer, D.; Baer, M.R.; Gambacorti-Passerini, C.; McCloskey, J.; Minami, Y.; et al. Ponatinib vs Imatinib in Frontline Philadelphia Chromosome–Positive Acute Lymphoblastic Leukemia. JAMA 2024, 331, 1814. [Google Scholar] [CrossRef]
- Kantarjian, H.; Short, N.J.; Jain, N.; Sasaki, K.; Huang, X.; Haddad, F.G.; Khouri, I.; DiNardo, C.D.; Pemmaraju, N.; Wierda, W.; et al. Frontline combination of ponatinib and hyper-CVAD in Philadelphia chromosome-positive acute lymphoblastic leukemia: 80-months follow-up results. Am. J. Hematol. 2023, 98, 493–501. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Lachowiez, C.A.; Takahashi, K.; Loghavi, S.; Kadia, T.; Daver, N.; Xiao, L.; Adeoti, M.; Short, N.J.; Sasaki, K.; et al. Venetoclax combined with FLAG-IDA induction and consolidation in newly diagnosed acute myeloid leukemia. Am. J. Hematol. 2022, 97, 1035–1043. [Google Scholar] [CrossRef]
- Stone, R.M.; Mandrekar, S.J.; Sanford, B.L.; Laumann, K.; Geyer, S.; Bloomfield, C.D.; Thiede, C.; Prior, T.W.; Döhner, K.; Marcucci, G.; et al. Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation. N. Engl. J. Med. 2017, 377, 454–464. [Google Scholar] [CrossRef]
- Sierra, J.; Montesinos, P.; Thomas, X.; Griskevicius, L.; Cluzeau, T.; Caillot, D.; Legrand, O.; Minotti, C.; Luppi, M.; Farkas, F.; et al. Midostaurin plus daunorubicin or idarubicin for young and older adults with FLT3-mutated AML: A phase 3b trial. Blood Adv. 2023, 7, 6441–6450. [Google Scholar] [CrossRef]
- Morita, K.; Kantarjian, H.M.; Sasaki, K.; Issa, G.C.; Jain, N.; Konopleva, M.; Short, N.J.; Takahashi, K.; DiNardo, C.D.; Kadia, T.M.; et al. Outcome of patients with chronic myeloid leukemia in lymphoid blastic phase and Philadelphia chromosome–positive acute lymphoblastic leukemia treated with hyper-CVAD and dasatinib. Cancer 2021, 127, 2641–2647. [Google Scholar] [CrossRef]
- Abaza, Y.; Kantarjian, H.; Alwash, Y.; Borthakur, G.; Champlin, R.; Kadia, T.; Garcia-Manero, G.; Daver, N.; Ravandi, F.; Verstovsek, S.; et al. Phase I/II study of dasatinib in combination with decitabine in patients with accelerated or blast phase chronic myeloid leukemia. Am. J. Hematol. 2020, 95, 1288–1295. [Google Scholar] [CrossRef]
- Marcucci, G.; Geyer, S.; Laumann, K.; Zhao, W.; Bucci, D.; Uy, G.L.; Blum, W.; Eisfeld, A.-K.; Pardee, T.S.; Wang, E.S.; et al. Combination of dasatinib with chemotherapy in previously untreated core binding factor acute myeloid leukemia: CALGB 10801. Blood Adv. 2020, 4, 696–705. [Google Scholar] [CrossRef]
- Cairoli, R.; Gatti, A.; Grillo, G.; Stefanucci, M.R.; Di Camillo, B.; Fumagalli, M.; Krampera, M.; Nadali, G.; Zappasodi, P.; Borlenghi, E.; et al. Efficacy of Midostaurin Combined With Intensive Chemotherapy in Core Binding Factor Leukemia: A Phase II Clinical Trial. Am. J. Hematol. 2025, 100, 346–349. [Google Scholar] [CrossRef]
- Döhner, H.; Weber, D.; Krzykalla, J.; Fiedler, W.; Wulf, G.; Salih, H.; Lübbert, M.; Kühn, M.W.M.; Schroeder, T.; Salwender, H.; et al. Midostaurin plus intensive chemotherapy for younger and older patients with AML and FLT3 internal tandem duplications. Blood Adv. 2022, 6, 5345–5355. [Google Scholar] [CrossRef]
- Pratz, K.W.; Cherry, M.; Altman, J.K.; Cooper, B.W.; Podoltsev, N.A.; Cruz, J.C.; Lin, T.L.; Schiller, G.J.; Jurcic, J.G.; Asch, A.; et al. Gilteritinib in Combination With Induction and Consolidation Chemotherapy and as Maintenance Therapy: A Phase IB Study in Patients With Newly Diagnosed AML. J. Clin. Oncol. 2023, 41, 4236–4246. [Google Scholar] [CrossRef]
- Stilgenbauer, S.; Bosch, F.; Ilhan, O.; Kisro, J.; Mahé, B.; Mikuskova, E.; Osmanov, D.; Reda, G.; Robinson, S.; Tausch, E.; et al. Safety and efficacy of obinutuzumab alone or with chemotherapy in previously untreated or relapsed/refractory chronic lymphocytic leukaemia patients: Final analysis of the Phase IIIb GREEN study. Br. J. Haematol. 2021, 193, 325–338. [Google Scholar] [CrossRef]
- Gugliotta, G.; Castagnetti, F.; Breccia, M.; Gozzini, A.; Usala, E.; Carella, A.M.; Rege-Cambrin, G.; Martino, B.; Abruzzese, E.; Albano, F.; et al. Rotation of nilotinib and imatinib for first-line treatment of chronic phase chronic myeloid leukemia. Am. J. Hematol. 2016, 91, 617–622. [Google Scholar] [CrossRef]
- Jabbour, E.; Haddad, F.G.; Sasaki, K.; Carter, B.Z.; Alvarado, Y.; Nasnas, C.; Nasr, L.; Masarova, L.; Daver, N.; Pemmaraju, N.; et al. Combination of dasatinib and venetoclax in newly diagnosed chronic phase chronic myeloid leukemia. Cancer 2024, 130, 2652–2659. [Google Scholar] [CrossRef] [PubMed]
- Spampinato, M.; Zuppelli, T.; Dulcamare, I.; Longhitano, L.; Sambataro, D.; Santisi, A.; Alanazi, A.M.; Barbagallo, I.A.; Vicario, N.; Parenti, R.; et al. Enhanced Antitumor Activity by the Combination of Dasatinib and Selinexor in Chronic Myeloid Leukemia. Pharmaceuticals 2024, 17, 894. [Google Scholar] [CrossRef] [PubMed]
- Crawford, L.J.; Chan, E.T.; Aujay, M.; Holyoake, T.L.; Melo, J.V.; Jorgensen, H.G.; Suresh, S.; Walker, B.; Irvine, A.E. Synergistic effects of proteasome inhibitor carfilzomib in combination with tyrosine kinase inhibitors in imatinib-sensitive and -resistant chronic myeloid leukemia models. Oncogenesis 2024, 3, e90. [Google Scholar] [CrossRef] [PubMed]
- Goede, V.; Fischer, K.; Robrecht, S.; Giza, A.; Dyer, M.J.; Eckart, M.J.; Müller, L.; Smolej, L.; Kutsch, N.; Fink, A.-M.; et al. Long-term outcomes of chemoimmunotherapy with obinutuzumab/chlorambucil in chronic lymphocytic leukemia. Blood Adv. 2025, 9, 2431–2435. [Google Scholar] [CrossRef]
- Al-Sawaf, O.; Robrecht, S.; Zhang, C.; Olivieri, S.; Chang, Y.M.; Fink, A.M.; Tausch, E.; Schneider, C.; Ritgen, M.; Kreuzer, K.-A.; et al. Venetoclax-obinutuzumab for previously untreated chronic lymphocytic leukemia: 6-year results of the randomized phase 3 CLL14 study. Blood 2024, 144, 1924–1935. [Google Scholar] [CrossRef] [PubMed]
- Sharman, J.P.; Egyed, M.; Jurczak, W.; Skarbnik, A.P.; Patel, K.; Flinn, I.W.; Kamdar, M.; Munir, T.; Walewska, R.; Hughes, M.; et al. Acalabrutinib-Obinutuzumab Improves Survival vs Chemoimmunotherapy in treatment-naive CLL in the 6-year Follow-up of ELEVATE-TN. Blood J. 2025, in press. [Google Scholar] [CrossRef]
- Eide, C.A.; Brewer, D.; Xie, T.; Schultz, A.R.; Savage, S.L.; Muratcioglu, S.; Merz, N.; Press, R.D.; O’Hare, T.; Jacob, T.; et al. Overcoming clinical BCR-ABL1 compound mutant resistance with combined ponatinib and asciminib therapy. Cancer Cell 2024, 42, 1486–1488. [Google Scholar] [CrossRef]
- Przespolewski, E.R.; Baron, J.; Kashef, F.; Fu, K.; Jani Sait, S.N.; Hernandez-Ilizaliturri, F.; Thompson, J. Concomitant Venetoclax and Imatinib for Comanaging Chronic Lymphocytic Leukemia and Chronic Myeloid Leukemia: A Case Report. J. Natl. Compr. Canc. Netw. 2023, 21, 102–107. [Google Scholar] [CrossRef]
- Brown, J.R.; Seymour, J.F.; Jurczak, W.; Aw, A.; Wach, M.; Illes, A.; Tedeschi, A.; Owen, C.; Skarbnik, A.; Lysak, D.; et al. Fixed-Duration Acalabrutinib Combinations in Untreated Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2025, 392, 748–762. [Google Scholar] [CrossRef]
- Danilov, A.V.; Herbaux, C.; Walter, H.S.; Hillmen, P.; Rule, S.A.; Kio, E.A.; Karlin, L.; Dyer, M.J.S.; Mitra, S.S.; Yi, P.C.; et al. Phase Ib Study of Tirabrutinib in Combination with Idelalisib or Entospletinib in Previously Treated Chronic Lymphocytic Leukemia. Clin. Cancer Res. 2020, 26, 2810–2818. [Google Scholar] [CrossRef]
- Zicari, S.; Merlino, G.; Paoli, A.; Fiascarelli, A.; Tunici, P.; Bisignano, D.; Belli, F.; Irrissuto, C.; Talucci, S.; Cirigliano, E.; et al. The Dual PIM/FLT3 Inhibitor MEN1703 Combines Synergistically With Gilteritinib in FLT3-ITD-Mutant Acute Myeloid Leukaemia. J. Cell. Mol. Med. 2024, 28, e70235. [Google Scholar] [CrossRef]
- Zhou, Q.; Guan, Y.; Zhao, P.; Chu, H.; Xi, Y. Combined anti-leukemic effect of gilteritinib and GSK-J4 in FLT3-ITD+ acute myeloid leukemia. Transl. Oncol. 2025, 52, 102271. [Google Scholar] [CrossRef]
- Sharman, J.P.; Brander, D.M.; Mato, A.R.; Ghosh, N.; Schuster, S.J.; Kambhampati, S.; Burke, J.M.; Lansigan, F.; Schreeder, M.T.; Lunin, S.D.; et al. Ublituximab plus ibrutinib versus ibrutinib alone for patients with relapsed or refractory high-risk chronic lymphocytic leukaemia (GENUINE): A phase 3, multicentre, open-label, randomised trial. Lancet Haematol. 2021, 8, e254–e266. [Google Scholar] [CrossRef] [PubMed]
- Seymour, J.F.; Kipps, T.J.; Eichhorst, B.; Hillmen, P.; D’Rozario, J.; Assouline, S.; Owen, C.; Gerecitano, J.; Robak, T.; De la Serna, J.; et al. Venetoclax–Rituximab in Relapsed or Refractory Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2018, 378, 1107–1120. [Google Scholar] [CrossRef]
- Foà, R.; Bassan, R.; Vitale, A.; Elia, L.; Piciocchi, A.; Puzzolo, M.-C.; Canichella, M.; Viero, P.; Ferrara, F.; Lunghi, M.; et al. Dasatinib–Blinatumomab for Ph-Positive Acute Lymphoblastic Leukemia in Adults. N. Engl. J. Med. 2020, 383, 1613–1623. [Google Scholar] [CrossRef]
- Lee, H.H.; Kim, I.; Kim, U.K.; Choi, S.S.; Kim, T.Y.; Lee, D.; Lee, Y.; Lee, J.; Jo, J.; Lee, Y.-T.; et al. Therapeutic effiacy of T cells expressing chimeric antigen receptor derived from a mesothelin-specific scFv in orthotopic human pancreatic cancer animal models. Neoplasia 2022, 24, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Cheng, Q.; Xu, N.; Zhao, C.; Xu, J.; Kang, L.; Lou, X.; Yu, L.; Feng, W. Investigation of CRS-associated cytokines in CAR-T therapy with meta-GNN and pathway crosstalk. BMC Bioinform. 2022, 23, 373. [Google Scholar] [CrossRef] [PubMed]
- Castellote-Borrell, M.; Domingo, M.; Merlina, F.; Lu, H.; Colell, S.; Bachiller, M.; Juan, M.; Guedan, S.; Faraudo, J.; Guasch, J. Lymph-Node Inspired Hydrogels Enhance CAR Expression and Proliferation of CAR T Cells. ACS Appl. Mater. Interfaces 2025, 17, 16548–16560. [Google Scholar] [CrossRef]
- Hill, J.A.; Li, D.; Hay, K.A.; Green, M.L.; Cherian, S.; Chen, X.; Riddell, S.R.; Maloney, D.G.; Boeckh, M.; Turtle, C.J. Infectious complications of CD19-targeted chimeric antigen receptor–modified T-cell immunotherapy. Blood 2018, 131, 121–130. [Google Scholar] [CrossRef]
- Gill, S.; Vides, V.; Frey, N.V.; Hexner, E.O.; Metzger, S.; O’Brien, M.; Hwang, W.-T.; Brogdon, J.L.; Davis, M.M.; Fraietta, J.A.; et al. Anti-CD19 CAR T cells in combination with ibrutinib for the treatment of chronic lymphocytic leukemia. Blood Adv. 2022, 6, 5774–5785. [Google Scholar] [CrossRef]
- Zhang, M.; Fu, S.; Feng, J.; Hong, R.; Wei, G.; Zhao, H.; Zhao, M.; Xu, H.; Cui, J.; Huang, S.; et al. Dasatinib and CAR T-Cell Therapy in Newly Diagnosed Philadelphia Chromosome–Positive Acute Lymphoblastic Leukemia. JAMA Oncol. 2025, 11, 625–629. [Google Scholar] [CrossRef]
- Li, K.; Wu, H.; Pan, W.; Guo, M.; Qiu, D.; He, Y.; Li, Y.; Yang, D.-H.; Huang, Y. A novel approach for relapsed/refractory FLT3mut+ acute myeloid leukaemia: Synergistic effect of the combination of bispecific FLT3scFv/NKG2D-CAR T cells and gilteritinib. Mol. Cancer 2022, 21, 66. [Google Scholar] [CrossRef] [PubMed]
- Funk, C.R.; Wang, S.; Chen, K.Z.; Waller, A.; Sharma, A.; Edgar, C.L.; Gupta, V.A.; Chandrakasan, S.; Zoine, J.T.; Fedanov, A.; et al. PI3Kδ/γ inhibition promotes human CART cell epigenetic and metabolic reprogramming to enhance antitumor cytotoxicity. Blood 2022, 139, 523–537. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Thompson, P.; Burger, J.; Ferrajoli, A.; Takahashi, K.; Estrov, Z.; Borthakur, G.; Bose, P.; Kadia, T.; Pemmaraju, N.; et al. Ibrutinib, fludarabine, cyclophosphamide, and obinutuzumab (iFCG) regimen for chronic lymphocytic leukemia (CLL) with mutated IGHV and without TP53 aberrations. Leukemia 2021, 35, 3421–3429. [Google Scholar] [CrossRef]
- Kutsch, N.; Pallasch, C.; Decker, T.; Hebart, H.; Chow, K.U.; Graeven, U.; Kisro, J.; Kroeber, A.; Tausch, E.; Fischer, K.; et al. Efficacy and Safety of Tirabrutinib and Idelalisib With or Without Obinutuzumab in Relapsed Chronic Lymphocytic Leukemia. HemaSphere 2020, 6, e729. [Google Scholar] [CrossRef] [PubMed]
- Fürstenau, M.; Robrecht, S.; Schneider, C.; Tausch, E.; Giza, A.; Ritgen, M.; Bittenbring, J.; Hebart, H.; Schöttker, B.; Illert, A.L.; et al. MRD-guided zanubrutinib, venetoclax, and obinutuzumab in relapsed CLL: Primary end point analysis from the CLL2-BZAG trial. Blood 2025, 145, 1282–1292. [Google Scholar] [CrossRef]
- Rogers, K.A.; Huang, Y.; Ruppert, A.S.; Abruzzo, L.V.; Andersen, B.L.; Awan, F.T.; Bhat, S.A.; Dean, A.; Lucas, M.; Banks, C.; et al. Phase II Study of Combination Obinutuzumab, Ibrutinib, and Venetoclax in Treatment-Naïve and Relapsed or Refractory Chronic Lymphocytic Leukemia. J. Clin. Oncol. 2020, 38, 3626–3637. [Google Scholar] [CrossRef]
- Short, N.J.; Daver, N.; Dinardo, C.D.; Kadia, T.; Nasr, L.F.; Macaron, W.; Yilmaz, M.; Borthakur, G.; Montalban-Bravo, G.; Garcia-Manero, G.; et al. Azacitidine, Venetoclax, and Gilteritinib in Newly Diagnosed and Relapsed or Refractory FLT3-Mutated AML. J. Clin. Oncol. 2024, 42, 1499–1508. [Google Scholar] [CrossRef]
- Luskin, M.R.; Murakami, M.A.; Keating, J.; Flamand, Y.; Winer, E.S.; Garcia, J.S.; Stahl, M.; Stone, R.M.; Wadleigh, M.; Jaeckle, S.L.; et al. Asciminib plus dasatinib and prednisone for Philadelphia chromosome–positive acute leukemia. Blood 2025, 145, 577–589. [Google Scholar] [CrossRef]
- Advani, A.S.; Moseley, A.; O’Dwyer, K.M.; Wood, B.L.; Park, J.; Wieduwilt, M.; Jeyakumar, D.; Yaghmour, G.; Atallah, E.L.; Gerds, A.T.; et al. Dasatinib/prednisone induction followed by blinatumomab/dasatinib in Ph+ acute lymphoblastic leukemia. Blood Adv. 2023, 7, 1279–1285. [Google Scholar] [CrossRef]
- Short, N.J.; Nguyen, D.; Jabbour, E.; Senapati, J.; Zeng, Z.; Issa, G.C.; Abbas, H.; Nasnas, C.; Qiao, W.; Huang, X.; et al. Decitabine, venetoclax, and ponatinib for advanced phase chronic myeloid leukaemia and Philadelphia chromosome-positive acute myeloid leukaemia: A single-arm, single-centre phase 2 trial. Lancet Haematol. 2024, 11, e839–e849. [Google Scholar] [CrossRef]
- Moore, A.; Soosay Raj, T.; Smith, A. Vincristine sulfate liposomal injection for acute lymphoblastic leukemia. Int. J. Nanomed. 2013, 8, 4361–4369. [Google Scholar] [CrossRef]
- Watson, N.A.; Notley, R.G. Urological Complications of Cyclophosphamide. Br. J. Urol. 1973, 45, 606–609. [Google Scholar] [CrossRef] [PubMed]
- Hanekamp, D.; Denys, B.; Kaspers, G.J.L.; te Marvelde, J.G.; Schuurhuis, G.J.; De Haas, V.; De Moerloose, B.; de Bont, E.S.; Zwaan, C.M.; de Jong, A.; et al. Leukaemic stem cell load at diagnosis predicts the development of relapse in young acute myeloid leukaemia patients. Br. J. Haematol. 2018, 183, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Hyland, K.A.; Nelson, A.M.; Eisel, S.L.; Hoogland, A.I.; Ibarz-Pinilla, J.; Sweet, K.; Jacobsen, P.B.; Knoop, H.; Jim, H.S.L. Fatigue Perpetuating Factors as Mediators of Change in a Cognitive Behavioral Intervention for Targeted Therapy-Related Fatigue in Chronic Myeloid Leukemia: A Pilot Study. Ann. Behav. Med. 2022, 56, 137–145. [Google Scholar] [CrossRef]
- Wang, H.; Chan, K.Y.Y.; Cheng, C.K.; Ng, M.H.L.; Lee, P.Y.; Cheng, F.W.T.; Lam, G.K.S.; Chow, T.W.; Ha, S.Y.; Chiang, A.K.S.; et al. Pharmacogenomic Profiling of Pediatric Acute Myeloid Leukemia to Identify Therapeutic Vulnerabilities and Inform Functional Precision Medicine. Blood Cancer Discov. 2022, 3, 516–535. [Google Scholar] [CrossRef] [PubMed]
- Marrero, R.J.; Wu, H.; Cao, X.; Parcha, P.K.; Elsayed, A.H.; Inaba, H.; Kuo, D.J.; Degar, B.A.; Heym, K.; Taub, J.W.; et al. Pharmacogenomic Score Effectively Personalizes Treatment of Acute Myeloid Leukemia. Clin. Cancer Res. 2024, 30, 4388–4396. [Google Scholar] [CrossRef]
- Luo, Q.; Raulston, E.G.; Prado, M.A.; Wu, X.; Gritsman, K.; Whalen, K.S.; Yan, K.; Booth, C.A.G.; Xu, R.; van Galen, P.; et al. Targetable leukaemia dependency on noncanonical PI3Kγ signalling. Nature 2024, 630, 198–205. [Google Scholar] [CrossRef]
| Combination Therapy Type | Combined Drugs | Indication | Patient Number | Treatment Outcome | Reference |
|---|---|---|---|---|---|
| Tyrosine kinase inhibitors | Imatinib | Ph+ALL, CML | 1106 | 10-year OS rate 83.3% | [22] |
| Dasatinib | 149 | 5-year OS rate 96%, treatment failure-free survival rate 95%. | [16] | ||
| Ponatinib | 51 | 10-year OS rate 90%, 2-year EFS 97% | [32] | ||
| FLT3 Inhibitors | Midostaurin | AML | 22 | overall response rate was 55.5%, OS 3.7 months. | [38] |
| Gilteritinib | 247 | 26 patients survived for 2 years or longer without recurrence | [41] | ||
| B-Cell Signaling Pathway Inhibitors | Ibrutinib | CLL | 269 | ORR 92%, PFS 70%, OS rate 83%. | [46] |
| Acalabrutinib | 134 | 45 months PFS 62% | [50] | ||
| Idelalisib | 54 | 81.5% of patients achieved lymph node response during treatment | [51] | ||
| Anti-apoptotic Inhibitors | Venetoclax | CLL and AML | - | - | - |
| Immunotherapy Drugs | Rituximab | ALL, CLL, HCL | 209 | 2-year EFS 65% | [61] |
| Obinutuzumab | 33 | OS rate 62%, best overall response rate 62% | [72] | ||
| Blinatumomab | 405 | Median OS 7.7 months, CR rate was 34%. | [76] | ||
| Differentiation Inducers | ATRA | APL | - | - | - |
| up coming targeted therapies under trial | Ziftomenib | AML | 83 | 25% achieved complete remission or complete remission with partial hematologic recovery | [82] |
| Nemtabrutinib | CLL | 48 | OS rate in patients with CLL was 75%. | [84] |
| Combination Therapy Type | Combined Drugs | Indication | Patient Number | Treatment Outcome | Reference |
|---|---|---|---|---|---|
| TKI + Chemotherapy Drugs | Imatinib, CVAD (Cyclophosphamide/Vincristine/Doxorubicin/Dexamethasone) | Ph+ ALL | 268 | 5-year OS rate 45.6%, EFS rate 37.1% | [14] |
| Ponatinib, low-intensity chemotherapy regimen | Ph+ ALL | 245 | MRD 34.4% | [87] | |
| Ponatinib, Hyper-CVAD (Cyclophosphamide/Vincristine/Doxorubicin/Dexamethasone) | Ph+ ALL | 86 | 6-year OS rate 75% | [88] | |
| Dasatinib, Hyper-CVAD (Cyclophosphamide/Vincristine/Doxorubicin/Dexamethasone) | CML-LBP, Ph+ ALL | 85 | CML-LBP and Ph+ ALL 5-year OS rates were 59% and 48%, respectively | [92] | |
| Dasatinib, Decitabine | CML | 30 | Median OS of 13.8 months | [93] | |
| Dasatinib, Cytarabine, Daunorubicin | CBF-AML | 61 | 3-year Disease-Free Survival and OS 75% and 77% respectively | [94] | |
| Anti-apoptotic inhibitors + chemotherapeutic agents | Venetoclax, Azacitidine | AML | 431 | Median follow-up 20.5 months, OS 14.7 months | [86] |
| Venetoclax, FLAG-IDA (Fludarabine, Cytarabine, Granulocyte Colony-Stimulating Factor, Idarubicin) | AML | 45 | ORR 98%, MRD 93%, 24-month OS rate 76%, EFS 64% | [89] | |
| B-cell signaling pathway inhibitors + chemotherapeutic agents | Midostaurin, Daunorubicin, Cytarabine | AML | 717 | Median OS 74.7 months | [90] |
| Midostaurin, Daunorubicin or Idarubicin | AML | 301 | CR+CRi 80.7%, of which 65.3% reached CR | [91] | |
| Midostaurin, Cytarabine, Daunorubicin or Idarubicin | Core binding factor leukemia (CBFL) | 34 | Median follow-up 31.5 months, OS rate 73.52% | [95] | |
| Midostaurin, intensive chemotherapy | AML | 440 | 2-year OS rate 55%, EFS rate 41%, CR/CRi 74.9% | [96] | |
| Gilteritinib, Mitoxantrone | AML | none | Significantly inhibited the proliferation of MV4-11 and MOLM13 cells | [35] | |
| Gilteritinib, intensive induction and consolidation chemotherapy | AML | 103 | Median overall survival time 46.1 months | [97] | |
| Immunotherapeutic drugs + chemotherapeutic drugs | Blinatumomab, Hyper-CVAD (Cyclophosphamide/Vincristine/Doxorubicin/Dexamethasone) | B-ALL | 38 | 3-year OS rate 81% | [75] |
| Rituximab, Fludarabine, Cyclophosphamide | CLL | 1924 | 4-year OS rate 93.5% | [62] | |
| Obinutuzumab, Fludarabine, Cyclophosphamide | CLL | 630 | 4-year OS rate in G-FC group reached 90%+ | [98] |
| Combination Therapy Type | Combined Drugs | Indication | Patient Number | Treatment Outcome |
|---|---|---|---|---|
| CAR-T, Ibrutinib | CLL | 20 | At 48 months, OS rate 84%, progression-free survival rate 70% | [119] |
| CAR-T, Dasatinib | Ph+ AML | 28 | 2-year OS and leukemia-free survival rates both 92% | [120] |
| CAR-T, Gilteritinib | AML | None | Significantly enhanced the anti-AML effect of CAR T cells | [121] |
| CAR-T, Duvelisib | CLL | None | Significantly improved the survival rate of CLL mice | [122] |
| Combination Therapy Type | Combined Drugs | Indication | Patient Number | Treatment Outcome |
|---|---|---|---|---|
| Ibrutinib, Fludarabine, Cyclophosphamide, Obinutuzumab | CLL | 45 | 3-year OS rate 98% | [123] |
| Idelalisib, Ttirabrutinib, Obinutuzumab | CLL | 35 | ORR 93.3% | [124] |
| Zanubrutinib, Venetoclax, Obinutuzumab | CLL | 42 | 18-month OS rate 96.8%, PFS rate 96% | [125] |
| Ibrutinib, Venetoclax, Obinutuzumab | CLL | 50 | 2 months after end of treatment, CR 28% | [126] |
| Gilteritinib, Azacitidine, Venetoclax | AML | 52 | 18-month OS rate 72% | [127] |
| Dasatinib, Asciminib, Prednisone | Ph+ ALL | 24 | 2-year OS rate 75% | [128] |
| Dasatinib, Prednisone, Blinatumomab | Ph+ ALL | 24 | 3-year OS rate 87% | [129] |
| Ponatinib, Decitabine, Venetoclax | CML | 20 | 1-year and 2-year OS rates 41% and 34% respectively | [130] |
| Acalabrutinib, Venetoclax, Obinutuzumab | CLL | 867 | 36-month OS rate 87.7% | [108] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, Z.; Yao, R.; Duan, Y.; Ouyang, C.; Du, Z.; Li, L.; Zou, H.; Liu, Y.; Xue, H.; Li, L.; et al. Advances and Challenges in Targeted Therapy and Its Combination Strategies for Leukemia. Biomedicines 2025, 13, 1652. https://doi.org/10.3390/biomedicines13071652
Zhong Z, Yao R, Duan Y, Ouyang C, Du Z, Li L, Zou H, Liu Y, Xue H, Li L, et al. Advances and Challenges in Targeted Therapy and Its Combination Strategies for Leukemia. Biomedicines. 2025; 13(7):1652. https://doi.org/10.3390/biomedicines13071652
Chicago/Turabian StyleZhong, Zhiyuan, Ran Yao, Yifei Duan, Cheng Ouyang, Zefan Du, Lindi Li, Hailin Zou, Yong Liu, Hongman Xue, Liang Li, and et al. 2025. "Advances and Challenges in Targeted Therapy and Its Combination Strategies for Leukemia" Biomedicines 13, no. 7: 1652. https://doi.org/10.3390/biomedicines13071652
APA StyleZhong, Z., Yao, R., Duan, Y., Ouyang, C., Du, Z., Li, L., Zou, H., Liu, Y., Xue, H., Li, L., & Chen, C. (2025). Advances and Challenges in Targeted Therapy and Its Combination Strategies for Leukemia. Biomedicines, 13(7), 1652. https://doi.org/10.3390/biomedicines13071652





