1. Introduction
Herpes zoster (HZ), or shingles, is caused by the reactivation of the varicella-zoster virus (VZV), which remains latent in nerve ganglia after primary infection with chickenpox [
1]. HZ has a global distribution with an age-dependent incidence, ranging from 1.2 to 3.4 per 1000 persons annually in younger adults to 3.9–11.8 per 1000 in those aged 65 years and older [
2,
3]. Risk factors include advancing age, immunosuppression, stress, diabetes, and comorbidities like megaloblastic anemia [
4,
5].
HZ is characterized by a painful vesicular rash that follows a dermatomal distribution [
6]. The disease can lead to severe complications, including postherpetic neuralgia (PHN), vision loss, and, less frequently, hearing impairment [
7,
8,
9]. However, its impact extends beyond the skin, particularly when it involves the central nervous system and its cranial nerves [
10]. HZ involving the ophthalmic branch of the trigeminal nerve, known as herpes zoster ophthalmicus (HZO), presents unique challenges [
9,
11].
Emerging evidence suggests that HZ may also have vascular implications, potentially triggering inflammation in blood vessels and leading to disruptions in microcirculation [
12,
13,
14]. Vasculopathies caused by VZV are believed to result from the trans-axonal spread of VZV to adjacent blood vessels, the viral infection of cerebral blood vessels, vascular remodeling, and thrombosis [
15]. Vascular disturbances in this so-called VZV vasculopathy may contribute to long-term complications such as ischemic tissue damage or an increased risk of cerebrovascular events [
13,
16]. In fact, the risk of stroke is increased by 30% within one year after zoster [
17,
18]. Zoster in the area of the first branch of the trigeminal nerve is associated with a 4.3-fold risk of stroke [
17].
Optical coherence tomography angiography (OCTA) is a non-invasive imaging modality that enables the detailed visualization and quantitative analysis of the retinal and choroidal microvasculature without the need for an intravenous dye injection. Its high reproducibility and ease of use have made it an increasingly valuable tool in both clinical and research settings. One of the primary quantitative metrics derived from OCTA is vessel density (VD), which reflects the proportion of the scanned area occupied by blood vessels and serves as an indicator of microvascular perfusion.
OCTA has been widely applied to evaluate retinal perfusion in systemic inflammatory diseases such as rheumatoid arthritis and systemic sclerosis, where significant alterations in the retinal microvasculature have been documented [
19,
20]. These findings highlight the sensitivity of retinal vessels to systemic inflammatory processes.
Based on these insights, we hypothesized that herpes zoster, as an infection associated with local and systemic inflammation, may also affect retinal perfusion measurable by OCTA VD. To date, no studies have systematically examined the effects of herpes zoster on ocular perfusion using OCTA. Our study is the first to employ this non-invasive imaging modality to quantitatively assess microvascular changes in the retina, optic nerve head, and choriocapillaris in patients with trigeminal herpes zoster. By providing detailed VD measurements, we aim to fill a critical knowledge gap regarding early vascular alterations associated with VZV infection in the eye.
2. Methods
This study included 48 eyes of 24 patients with newly diagnosed trigeminal HZ who were treated as inpatients in the department of dermatology and 48 eyes of 24 healthy controls matched in terms of gender and age from March 2022 to June 2023. In accordance with national guidelines, the diagnosis was made according to the typical clinical signs: the patients exhibited a moderate to severe unilateral skin rash with typical grouped small blisters on reddened skin in the region of the forehead, temples, eyes, and nose bridge, precisely within the innervation territory of the nervus ophthalmicus (V1) and nervus maxillaris (V2), branches of the trigeminal nerve.
In accordance with existing guidelines [
21], the diagnosis of trigeminal herpes zoster was made clinically based on the presence of a characteristic unilateral, dermatomal distribution of painful vesicular lesions, typically preceded by localized neuropathic prodromal pain.
Patients were referred to the department of dermatology by community ophthalmologists, dermatologists, or general practitioners for intravenous antiviral therapy following the acute onset of herpes zoster affecting the V1 and/or V2 dermatomes. Every patient was evaluated clinically by our tertiary university hospital. Hospital-based antiviral treatment was initiated at the time of vesicular eruption, which commonly appeared 24–72 h after the onset of prodromal pain.
The study was approved by the Ethics Committee of the University of Muenster and adhered to the principles of the Declaration of Helsinki (2022-285-f-S). Written informed consent was obtained from all participants after a detailed explanation of the study procedures.
Exclusion criteria included the presence of media opacities affecting imaging quality, vitreoretinal diseases, prior retinal surgery, macular edema, glaucoma, or neurological disorders. A comprehensive ophthalmological assessment was performed for all participants, comprising anterior segment examination, binocular fundus examination, and optical coherence tomography angiography (OCTA). Only patients without signs of intraocular involvement were eligible for inclusion. Individuals presenting with concomitant uveitis, such as anterior chamber inflammation (cells, flare) or retinopathy detected via retinoscopy, were excluded from the study.
Participants provided additional information regarding height, weight, smoking status, and cardiovascular diseases. Patients with diabetes mellitus were excluded due to the known impact of diabetes on ocular perfusion. Individuals with well-controlled arterial hypertension were eligible for inclusion. In cases where patients reported active smoking (n = 3), matched control subjects with similar smoking status were included to eliminate smoking as a potential confounding factor. These parameters were collected solely for the purpose of minimizing confounding influences on ocular perfusion and were not part of the primary outcome analysis.
Detailed information on the pre-existing medical conditions of the included patients can be found in
Supplementary Table S1.
2.1. Optical Coherence Tomography Angiography
OCTA imaging was performed using the AngioVue™ Imaging System (RTVue XR Avanti with AngioVue; Optovue Inc., Fremont, CA, USA). The OCTA data were generated using the split-spectrum amplitude-decorrelation angiography (SSADA) algorithm, as described in previous publications on OCTA technology [
22].
The macula was imaged using a 3 × 3 mm OCTA scan; the optic nerve head OCT-angiogram was obtained using the 4.5 × 4.5 mm scan. The device uses a light source centered at 840 nm and operates at a speed of 70,000 A-scans per second. Each 3 × 3 mm scan consisted of 304 × 304 A-scans. The axial and lateral resolution of the system are approximately 5 µm and 15 µm, respectively.
Only OCTA images with a quality index of ≥6 were included in the study, while images with motion artifacts, signal gaps, or poor signal strength were excluded.
The en face OCT angiogram of the superficial capillary plexus (OCTA-SCP) was segmented with an inner boundary set 3 μm below the internal limiting membrane (ILM) and an outer boundary set 15 μm below the inner plexiform layer (IPL). The deep capillary plexus (DCP) en face OCT-A image was segmented with an inner boundary set 15 μm below the IPL and an outer boundary set 70 μm below the IPL. Choriocapillaris (CC) segmentation was defined from 10 μm above to 30 μm below Bruch’s membrane.
After imaging, the VD of the OCTA-SCP, OCTA-DCP, OCTA-CC, the radial peripapillary capillary density (RPC), and the foveal avascular zone (FAZ) area were analyzed using the integrated AngioAnalytics software Version 2018.1.0.43 provided by the device.
All OCTA images were acquired and reviewed by the same three experienced examiners using a standardized protocol to ensure consistency in data collection and analysis. All OCTA examinations were performed during regular outpatient clinic hours, between 8:00 a.m. and 5:00 p.m., to reduce the potential influence of diurnal fluctuations in ocular blood flow [
23,
24]. The OCTA examinations were performed on the day of admission, ensuring a consistent interval of approximately 1 to 3 days after the clinical onset of herpes zoster across all included patients. This standardized timing enabled uniform data acquisition during the early inflammatory phase of the disease.
Prior to data analysis, an expert reader (EE) reviewed and confirmed the accuracy of the automated segmentation.
2.2. Statistics
Data management was conducted using Microsoft Excel 2016, and statistical analyses were performed using IBM SPSS® Statistics 29 for Windows (IBM Corporation, Somers, NY, USA). The normality of the data distribution was assessed using the Shapiro–Wilk test, which indicated a deviation from normality. Consequently, Spearman’s correlation coefficient (rSp) was applied to assess the degree of association between two variables, while the Mann–Whitney U test was utilized for non-normally distributed variables to compare the two groups. Continuous variables were reported as medians with interquartile ranges (25th percentile; 75th percentile). It is important to note that all inferential statistical analyses were exploratory in nature and intended for hypothesis generation rather than confirmatory testing. The significance level was set at p < 0.05.
3. Results
This prospective study included a total of 48 eyes of 24 patients with newly diagnosed trigeminal herpes zoster (HZ) and 48 eyes of 24 age- and gender-matched healthy controls. There was no significant difference in age between the HZ patient and the healthy control group (
p = 0.60). The demographic characteristics of patients and healthy controls are shown in
Table 1.
OCTA Findings
The OCTA analyses of the superficial (SCP) and deep capillary plexus (DCP) revealed significant reductions in the VD in all measured areas including the whole en face image (SCP whole en face: patients: 44.85 [42.68; 47.08], healthy controls: 47.35 [45.40; 48.38];
p < 0.001; DCP whole en face: patients: 48.85 [44.80; 52.53]; healthy controls: 52.10 [49.40, 53.87];
p < 0.001). See
Figure 1, which shows boxplots illustrating differences in VD between the HZ group and the healthy control HC group in the SCP.
A significant reduction in the VD was also observed in the optic nerve head (ONH), specifically in the inside disk area (patients: 46.20 [42.70, 52.00]; healthy controls: 49.80 [45.63, 52.68]; p = 0.047).
Moreover, statistical analysis revealed significant differences between the VD of the patients and controls in the choriocapillaris (CC) whole image (patients: 71.05 [68.03; 72.70]; healthy controls 72.40 [69.30; 74.78];
p = 0.024). OCTA data are shown in
Table 2.
4. Discussion
In this study, we identified a significant reduction in the VD across all analyzed areas of the superficial and deep macular OCT angiogram, as well as in the radial peripapillary capillaries (inside disk) of the ONH in patients with trigeminal HZ compared with healthy controls. This finding expands on the limited existing research on the vascular implications of HZ, which has primarily focused on the effects of HZ on the vasculature of the central nervous system, with no previous studies specifically evaluating ocular microvasculature in HZ patients using OCTA [
17,
25,
26,
27,
28,
29,
30,
31,
32].
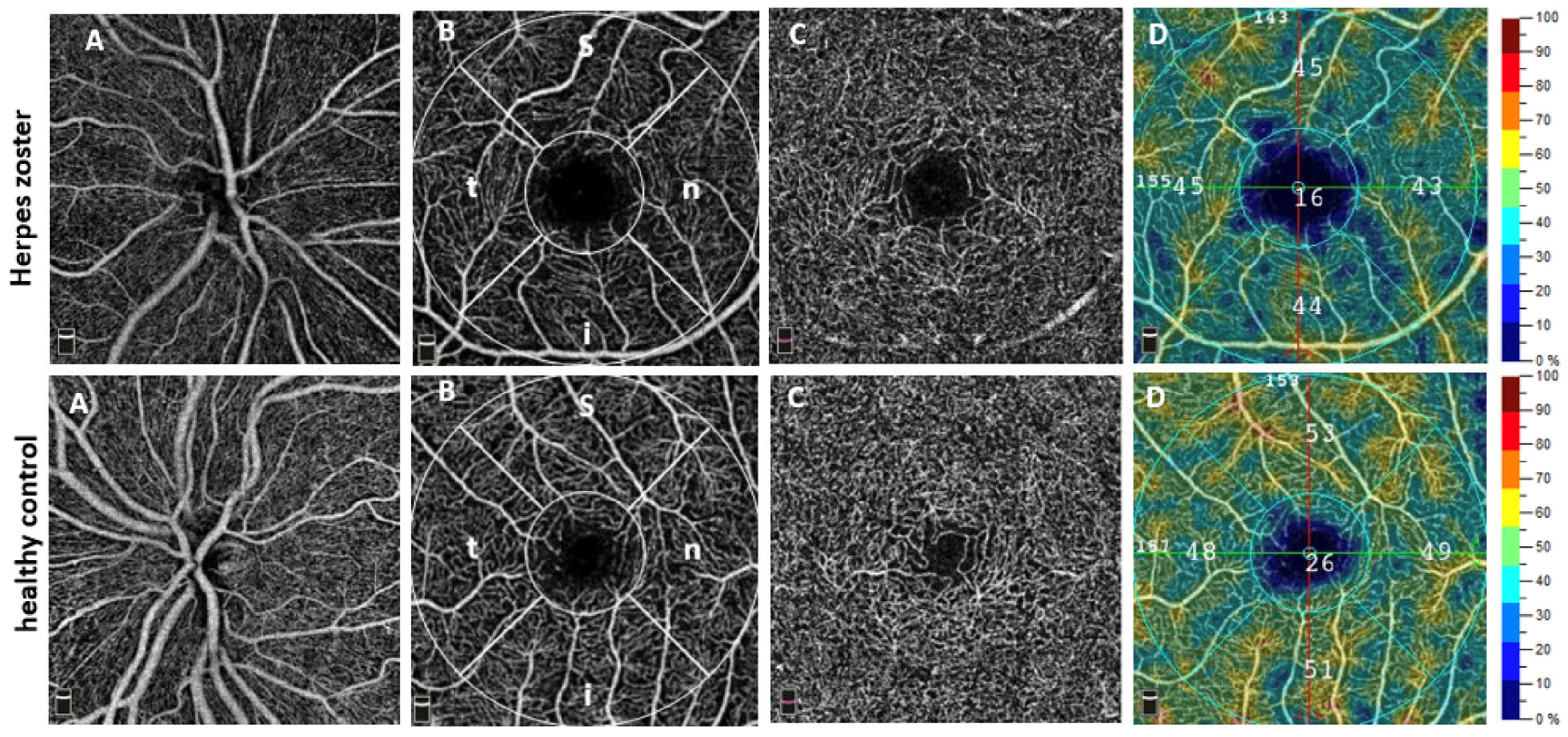
Our results now demonstrate significant alterations of the ocular microvasculature in HZ and could provide indications for VZV-induced microangiopathy. Particularly, we found a reduced VD not only in the SCP but also in the DCP, CC, and the ONH. Moreover, OCT images demonstrated localized non-perfusion areas (
Figure 2D).
In HZ patients with involvement of the V1 (nervus ophthalmicus) and V2 (nervus maxillaris) branches of the trigeminal nerve, OCTA might therefore help identify ischemic changes that correlate with reduced blood flow, potentially aiding in early intervention. Minassian et al. and Yawn et al. provided evidence for a systemic effect of HZ on blood vessels as they not only showed an increased stroke risk but also an increased risk of myocardial infarction following HZ [
29,
32].
Potential mechanisms linking HZ to reduced ocular perfusion may involve both direct viral effects and immune-mediated neurovascular injury. Reactivation of VZV from the trigeminal ganglion may lead to a direct viral invasion of the retinal vessels via trans-axonal spread or through hematogenous dissemination during viremia [
32,
33], resulting in endothelial injury through cytopathic effects.
In parallel, the host’s antiviral immune response triggers a robust inflammatory cascade. Cytokines such as type I interferons (IFN-α/β) play a key role in the innate response to viral infections and have been shown to induce endothelial cell apoptosis and endothelial-to-mesenchymal transition (EndMT), leading to capillary rarefication as described in autoimmune diseases like systemic sclerosis [
34,
35]. Additional pro-inflammatory mediators, including TNF-α, IL-6, and IFN-γ, may further exacerbate endothelial dysfunction and microvascular inflammation [
36,
37].
There are only limited data on retinal microvasculature and infections by herpes viruses. Maier et al. investigated differences in retinal VD using OCT angiography in patients with viral anterior uveitis (VAU) caused by the herpes simplex virus (HSV) and VZV and included 88 eyes of 44 patients, divided into equally sized groups [
38]. They found reduced VD in the superficial vascular plexus of the macula and peripapillary regions in affected eyes compared with non-affected ones.
Similarly to our study, it highlights reduced VD in HSV- and VZV-affected eyes, reflecting microvascular dysfunction. Both conditions demonstrate that inflammation or direct viral effects may impair retinal microcirculation, potentially contributing to complications like ischemia or neurovascular damage. These findings underscore the diagnostic potential of OCTA for the early detection of perfusion abnormalities, aiding in understanding the vascular impact of viral infections like HZ on ocular health.
Additional studies have shown that chronic systemic inflammatory diseases are associated with retinal microvascular rarefaction detectable by OCTA, supporting the concept that OCTA VD is a sensitive marker for systemic vascular involvement [
39]. This aligns with our hypothesis that VZV-induced systemic inflammation and immune-mediated endothelial injury contribute to the observed ocular microvascular impairments.
A key strength of our study is that, to our knowledge, it is the first to demonstrate a significant reduction in ocular perfusion in patients with trigeminal herpes zoster using OCT angiography. By applying a non-invasive, high-resolution imaging technique, we were able to detect microvascular alterations in the retina, choriocapillaris, and optic nerve head at an early stage of the disease. This novel finding not only contributes to our understanding of local ocular involvement in HZ but may also reflect broader systemic microvascular dysfunction induced by varicella-zoster virus reactivation. These findings suggest a potential role for OCTA as a surrogate marker for systemic vascular risk in affected patients. However, further research with larger cohorts and longitudinal study designs will be necessary to confirm this potential and to better evaluate the prognostic value of OCTA for identifying individuals at increased risk of cerebrovascular or cardiovascular complications.
There are certain limitations to our investigation. We only included patients who were hospitalized due to moderate to severe HZ in the department of dermatology. We were not able to recruit outpatients and thus the variability of our HZ group is limited. Likewise, we only included patients with herpes zoster ophthalmicus and no patients with, for example, thoracic herpes. It is possible that herpes in other areas of the body may also lead to reduced blood flow in the eye due to systemic involvement. Unfortunately, we cannot provide longitudinal data on our patients. Future studies with prospective follow-up are warranted to investigate the potential dynamics of ocular perfusion over time following herpes zoster ophthalmicus.
Future research should give priority to such longitudinal studies, especially addressing the question whether alterations of the retinal microvasculature might provide information regarding the risk of future vascular events like stroke or myocardial infarction. However, to address this question, larger cohorts are needed. Comparisons between VD data in the beginning of the disease and after 3–6 months should be part of these investigations.
In conclusion, the ability of OCTA to detect reduced VD in a non-invasive manner provides a window into the microvascular status of HZ patients. Both the stroke/TIA risk demonstrated in previous HZ studies and the reduced ocular perfusion seen in our OCTA study may share same underlying mechanisms of immune-mediated vasculopathy and direct viral effects on vascular structures. OCTA could serve as an early diagnostic tool for assessing the risk of systemic vascular complications in affected individuals, particularly those with trigeminal HZ.
Author Contributions
E.L.E. received support from the Medical Faculty of the University of Münster as part of a research rotation. Study design: N.M., J.E. and E.L.E.; Acquisition of data: E.L.E., S.B., M.D.L. and S.D.; Interpretation of data: J.E., N.M., N.E. and S.B.; Draft: N.M., J.E., E.L.E. and S.B. All authors have read and agreed to the published version of the manuscript.
Funding
There are no fundings to report for this submission.
Institutional Review Board Statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Ethics committee of the University of Muenster, 2022-285-f-S, 4 July 2022.
Informed Consent Statement
Informed consent was obtained from all individual participants included in the study.
Data Availability Statement
The original contributions presented in the study are included in the article/
Supplementary Material, further inquiries can be directed to the corresponding author.
Conflicts of Interest
Author Nataša Mihailovic was employed by the company Klinikum Bielefeld Gem. GmbH. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Patil, A.; Goldust, M.; Wollina, U. Herpes zoster: A Review of Clinical Manifestations and Management. Viruses 2022, 14, 192. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.A.; Patel, B.C. Herpes Zoster; StatPearls: Tampa, FL, USA, 2024. [Google Scholar]
- van Oorschot, D.; Vroling, H.; Bunge, E.; Diaz-Decaro, J.; Curran, D.; Yawn, B. A systematic literature review of herpes zoster incidence worldwide. Hum. Vaccin. Immunother. 2021, 17, 1714–1732. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.-X.; Yeh, F.-Y.; Shen, Y.-J.; Tai, Y.; Huang, N.; Chang, Y.; Chen, T.; Li, C.; Wu, C. Cigarette smoking and risk of herpes zoster: A population-based cohort study in Taiwan. Clin. Exp. Dermatol. 2021, 46, 1293–1298. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-T.; Lee, C.-Y.; Sung, H.-Y.; Liu, S.-J.; Liang, P.-C.; Tsai, M.-C. Association Between Diabetes Mellitus and the Risk of Herpes Zoster: A Systematic Review and Meta-analysis. J. Clin. Endocrinol. Metab. 2022, 107, 586–597. [Google Scholar] [CrossRef]
- Marin, M.; Leung, J.; Lopez, A.S.; Shepersky, L.; Schmid, D.S.; Gershon, A.A. Communicability of varicella before rash onset: A literature review. Epidemiol. Infect. 2021, 149, e131. [Google Scholar] [CrossRef]
- Wollina, U.; Machetanz, J. Herpes zoster und postzosterische Neuralgie. Hautarzt 2016, 67, 653–665. [Google Scholar] [CrossRef]
- Herlin, L.K.; Hansen, K.S.; Bodilsen, J.; Larsen, L.; Brandt, C.; Andersen, C.Ø.; Hansen, B.R.; Lüttichau, H.R.; Helweg-Larsen, J.; Wiese, L.; et al. Varicella Zoster Virus Encephalitis in Denmark from 2015 to 2019-A Nationwide Prospective Cohort Study. Clin. Infect. Dis. 2021, 72, 1192–1199. [Google Scholar] [CrossRef]
- Cohen, E.J.; Jeng, B.H. Herpes Zoster: A Brief Definitive Review. Cornea 2021, 40, 943–949. [Google Scholar] [CrossRef]
- Xie, Z.; Lai, J.; Ning, C.; Ruan, G.; Liang, H. A case of paraplegia due to asymptomatic varicella-zoster virus infection in AIDS patient unexpectedly diagnosed by CSF metagenomic next-generation sequencing. BMC Infect. Dis. 2021, 21, 963. [Google Scholar] [CrossRef]
- Niederer, R.L.; Meyer, J.J.; Liu, K.; Danesh-Meyer, H.V. Herpes Zoster Ophthalmicus Clinical Presentation and Risk Factors for Loss of Vision. Am. J. Ophthalmol. 2021, 226, 83–89. [Google Scholar] [CrossRef]
- Lin, C.-F.; Hong, C.-T.; Lee, W.-H.; Wu, D.; Hu, C.-J.; Chung, C.-C. Disseminated cutaneous herpes zoster and multiple cerebral infarcts in an adult with diabetes mellitus. J. Neurovirol. 2020, 26, 130–132. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, G.; Cammelli, D. Large-vessel Involvement and Varicella Zoster Virus Vasculopathy in Giant Cell Arteritis-related Stroke: Something to Keep an Eye On. J. Rheumatol. 2017, 44, 1566. [Google Scholar] [CrossRef] [PubMed]
- Nagel, M.A.; Jones, D.; Wyborny, A. Varicella zoster virus vasculopathy: The expanding clinical spectrum and pathogenesis. J. Neuroimmunol. 2017, 308, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Gross, G.E.; Eisert, L.; Doerr, H.W.; Fickenscher, H.; Knuf, M.; Maier, P.; Maschke, M.; Müller, R.; Pleyer, U.; Schäfer, M.; et al. S2k-Leitlinie zur Diagnostik und Therapie des Zoster und der Postzosterneuralgie. GMS Infect. Dis. 2020, 8, Doc01. [Google Scholar] [CrossRef]
- Saito, M.; Kawano, H.; Amano, T.; Hirano, T. Acute Stroke Caused by Progressive Intracranial Artery Stenosis Due to Varicella Zoster Virus Vasculopathy after Chemotherapy for Malignant Lymphoma. Intern. Med. 2021, 60, 1769–1773. [Google Scholar] [CrossRef]
- Kang, J.-H.; Ho, J.-D.; Chen, Y.-H.; Lin, H.-C. Increased risk of stroke after a herpes zoster attack: A population-based follow-up study. Stroke 2009, 40, 3443–3448. [Google Scholar] [CrossRef]
- Gupta, A.S.; Pradeep, T.; Yu, Y.; Orlin, S.E.; VanderBeek, B.L. The association of stroke with herpes zoster ophthalmicus. Eye 2024, 38, 488–493. [Google Scholar] [CrossRef]
- Esser, E.L.; Zimmermann, J.A.; Storp, J.J.; Eter, N.; Mihailovic, N. Retinal microvascular density analysis in patients with rheumatoid arthritis treated with hydroxychloroquine. Graefes Arch. Clin. Exp. Ophthalmol. 2023, 261, 1433–1442. [Google Scholar] [CrossRef]
- Mihailovic, N.; Lahme, L.; Braasch, S.; Rosenberger, F.; Eter, N.; Ehrchen, J.; Alnawaiseh, M. Altered ocular microvasculature in patients with systemic sclerosis and very early disease of systemic sclerosis using optical coherence tomography angiography. Sci. Rep. 2022, 12, 10990. [Google Scholar] [CrossRef]
- Gross, G.E.; Eisert, L.; Doerr, H.W.; Fickenscher, H.; Knuf, M.; Maier, P.; Maschke, M.; Müller, R.; Pleyer, U.; Schäfer, M.; et al. S2k guidelines for the diagnosis and treatment of herpes zoster and postherpetic neuralgia. J. Dtsch. Dermatol. Ges. 2020, 18, 55–78. [Google Scholar] [CrossRef]
- Spaide, R.F.; Curcio, C.A. Evaluation of Segmentation of the Superficial and Deep Vascular Layers of the Retina by Optical Coherence Tomography Angiography Instruments in Normal Eyes. JAMA Ophthalmol. 2017, 135, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Rommel, F.; Rothe, M.; Kurz, M.; Prasuhn, M.; Grisanti, S.; Ranjbar, M. Evaluating diurnal variations in retinal perfusion using optical coherence tomography angiography. Int. J. Retin. Vitr. 2020, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Siegfried, F.; Rommel, F.; Rothe, M.; Brinkmann, M.P.; Sochurek, J.A.M.; Freitag, J.; Grisanti, S.; Ranjbar, M. Evaluating diurnal changes in choroidal sublayer perfusion using optical coherence tomography angiography. Acta Ophthalmol. 2019, 97, e1062–e1068. [Google Scholar] [CrossRef] [PubMed]
- Lian, Y.; Zhu, Y.; Tang, F.; Yang, B.; Duan, R. Herpes zoster and the risk of ischemic and hemorrhagic stroke: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0171182. [Google Scholar] [CrossRef]
- Nagel, M.A.; Gilden, D. The relationship between herpes zoster and stroke. Curr. Neurol. Neurosci. Rep. 2015, 15, 16. [Google Scholar] [CrossRef]
- Breuer, J.; Pacou, M.; Gautier, A.; Brown, M.M. Herpes zoster as a risk factor for stroke and TIA: A retrospective cohort study in the UK. Neurology 2014, 83, e27–e33. [Google Scholar] [CrossRef]
- Langan, S.M.; Minassian, C.; Smeeth, L.; Thomas, S.L. Risk of stroke following herpes zoster: A self-controlled case-series study. Clin. Infect. Dis. 2014, 58, 1497–1503. [Google Scholar] [CrossRef]
- Minassian, C.; Thomas, S.L.; Smeeth, L.; Douglas, I.; Brauer, R.; Langan, S.M. Acute Cardiovascular Events after Herpes Zoster: A Self-Controlled Case Series Analysis in Vaccinated and Unvaccinated Older Residents of the United States. PLoS Med. 2015, 12, e1001919. [Google Scholar] [CrossRef]
- Sreenivasan, N.; Basit, S.; Wohlfahrt, J.; Pasternak, B.; Munch, T.N.; Nielsen, L.P.; Melbye, M.; Dowd, J.B. The short- and long-term risk of stroke after herpes zoster—A nationwide population-based cohort study. PLoS ONE 2013, 8, e69156. [Google Scholar] [CrossRef]
- Sundström, K.; Weibull, C.E.; Söderberg-Löfdal, K.; Bergström, T.; Sparén, P.; Arnheim-Dahlström, L. Incidence of herpes zoster and associated events including stroke—A population-based cohort study. BMC Infect. Dis. 2015, 15, 488. [Google Scholar] [CrossRef]
- Yawn, B.P.; Wollan, P.C.; Nagel, M.A.; Gilden, D. Risk of Stroke and Myocardial Infarction After Herpes Zoster in Older Adults in a US Community Population. Mayo Clin. Proc. 2016, 91, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Nagel, M.A.; Lenggenhager, D.; White, T.; Khmeleva, N.; Heintzman, A.; Boyer, P.J.; Gilden, D. Disseminated VZV infection and asymptomatic VZV vasculopathy after steroid abuse. J. Clin. Virol. 2015, 66, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Distler, O.; Shen, L.; Xu, X.; Yuan, Y.; Li, R.; Liu, B.; Li, Q.; Huang, Q.; Xie, F.; et al. Endothelial Response to Type I Interferon Contributes to Vasculopathy and Fibrosis and Predicts Disease Progression of Systemic Sclerosis. Arthritis Rheumatol. 2024, 76, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Romano, E.; Rosa, I.; Fioretto, B.S.; Manetti, M. Recent Insights into Cellular and Molecular Mechanisms of Defective Angiogenesis in Systemic Sclerosis. Biomedicines 2024, 12, 1331. [Google Scholar] [CrossRef]
- Wang, J.P.; Kurt-Jones, E.A.; Shin, O.S.; Manchak, M.D.; Levin, M.J.; Finberg, R.W. Varicella-zoster virus activates inflammatory cytokines in human monocytes and macrophages via Toll-like receptor 2. J. Virol. 2005, 79, 12658–12666. [Google Scholar] [CrossRef]
- Hao, M.; Wang, X.; Du, J.; Liu, L.; Jiao, Y.; Wu, H.; Zheng, J.; Li, W. Cytokine levels are associated with the severity of varicella infections. J. Infect. Dev. Ctries. 2015, 9, 190–196. [Google Scholar] [CrossRef]
- Maier, A.-K.B.; Mandrossa, D.; Reitemeyer, E.; Winterhalter, S.; Rübsam, A.; Pleyer, U. Viral Anterior Uveitis: Differences in Retinal Vessel Area Density between the Affected and Non-Affected Eye Using Optical Coherence Tomography Angiography. Ocul. Immunol. Inflamm. 2024, 32, 1–9. [Google Scholar] [CrossRef]
- Agarwal, A.; Rübsam, A.; zur Bonsen, L.; Pichi, F.; Neri, P.; Pleyer, U. A Comprehensive Update on Retinal Vasculitis: Etiologies, Manifestations and Treatments. J. Clin. Med. 2022, 11, 2525. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).