The Role of IL-6 and TNF-α as Early Biomarkers in the Prediction and Diagnosis of Gestational Diabetes Mellitus
Abstract
1. Introduction
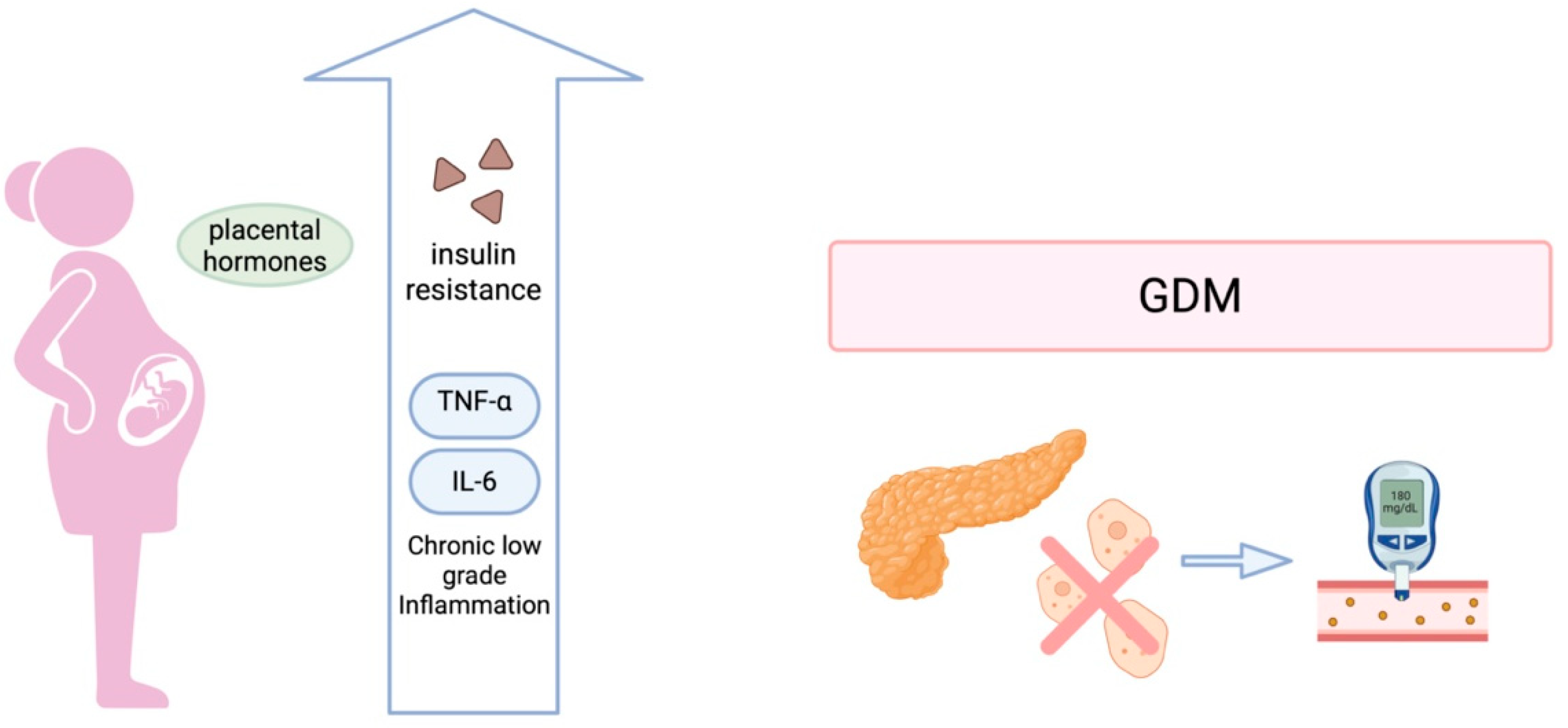
2. Pathophysiology of Gestational Diabetes Mellitus
2.1. Insulin Resistance and β-Cell Dysfunction
2.2. Inflammation and Cytokine-Mediated Insulin Resistance
2.3. Placental and Fetal Contributions
2.4. Non-Cytokine Mediators Involved in Glucose Regulation
3. Biological Functions of Interleukin-6 and TNF-α
4. Predictive Value, Integration into Screening, and Future Perspectives
5. Limitations
6. Future Perspectives
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Plows, J.F.; Stanley, J.L.; Baker, P.N.; Reynolds, C.M.; Vickers, M.H. The Pathophysiology of Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2018, 19, 3342. [Google Scholar] [CrossRef]
- Abel, U.; Deichmann, A.; Nowrouzi, A.; Gabriel, R.; Bartholomae, C.C.; Glimm, H.; von Kalle, C.; Schmidt, M. Analyzing the Number of Common Integration Sites of Viral Vectors—New Methods and Computer Programs. PLoS ONE 2011, 6, e24247. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Singh, S.; Singh, H.; Mahajan, D.; Kolli, P.; Mandadapu, G.; Kumar, B.; Kumar, D.; Kumar, S.; Jena, M.K. Deep Insight of the Pathophysiology of Gestational Diabetes Mellitus. Cells 2022, 11, 2672. [Google Scholar] [CrossRef] [PubMed]
- Hivert, M.-F.; Backman, H.; Benhalima, K.; Catalano, P.; Desoye, G.; Immanuel, J.; McKinlay, C.J.D.; Meek, C.L.; Nolan, C.J.; Ram, U.; et al. Pathophysiology from Preconception, during Pregnancy, and Beyond. Lancet 2024, 404, 158–174. [Google Scholar] [CrossRef]
- Torres-Torres, J.; Monroy-Muñoz, I.E.; Perez-Duran, J.; Solis-Paredes, J.M.; Camacho-Martinez, Z.A.; Baca, D.; Espino-y-Sosa, S.; Martinez-Portilla, R.; Rojas-Zepeda, L.; Borboa-Olivares, H.; et al. Cellular and Molecular Pathophysiology of Gestational Diabetes. Int. J. Mol. Sci. 2024, 25, 11641. [Google Scholar] [CrossRef] [PubMed]
- Harrall, K.K.; Glueck, D.H.; Lange, L.A.; Litkowski, E.M.; Vanderlinden, L.A.; Konigsberg, I.R.; Cree, M.G.; Perng, W.; Dabelea, D. GLP-1R Polymorphisms Modify the Relationship Between Exposure to Gestational Diabetes and Offspring BMI Growth: The EPOCH Study. Diabetes Care 2025, 48, dc250194. [Google Scholar] [CrossRef]
- Chermon, D.; Birk, R. Association of BDNF Polymorphism with Gestational Diabetes Mellitus Risk: A Novel Insight into Genetic Predisposition. J. Perinat. Med. 2024, 52, 611–616. [Google Scholar] [CrossRef]
- Luo, P.; Fan, Y.; Xiong, Y.; Feng, H.; Yang, Z.; Zhang, C.; Mei, B. Genetic Variants of the GLP-1R Gene Affect the Susceptibility and Glucose Metabolism of Gestational Diabetes Mellitus: A Two-Center Nested Case–control Study. Diabetol. Metab. Syndr. 2022, 14, 190. [Google Scholar] [CrossRef]
- Penno, J.R.C.Z.; Santos-Bezerra, D.P.; Cavaleiro, A.M.; Da Silva Sousa, A.M.; Zaccara, T.A.; Da Costa, R.A.; Francisco, R.P.V.; Correa-Giannella, M.L. Variant Rs17619600 in the Gene Encoding Serotonin Receptor 2B (HTR2B) Increases the Risk of Gestational Diabetes Mellitus: A Case–Control Study. Eur. J. Med. Res. 2023, 28, 243. [Google Scholar] [CrossRef]
- Bennet, H.; Mollet, I.G.; Balhuizen, A.; Medina, A.; Nagorny, C.; Bagge, A.; Fadista, J.; Ottosson-Laakso, E.; Vikman, P.; Dekker-Nitert, M.; et al. Serotonin (5-HT) Receptor 2b Activation Augments Glucose-Stimulated Insulin Secretion in Human and Mouse Islets of Langerhans. Diabetologia 2016, 59, 744–754. [Google Scholar] [CrossRef]
- Hussain, M.A.; Akalestou, E.; Song, W. Inter-Organ Communication and Regulation of Beta Cell Function. Diabetologia 2016, 59, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Mittal, R.; Prasad, K.; Lemos, J.R.N.; Arevalo, G.; Hirani, K. Unveiling Gestational Diabetes: An Overview of Pathophysiology and Management. Int. J. Mol. Sci. 2025, 26, 2320. [Google Scholar] [CrossRef] [PubMed]
- Lis-Kuberka, J.; Berghausen-Mazur, M.; Orczyk-Pawiłowicz, M. Evaluation of Selected Pro- and Anti-Inflammatory Adipokines in Colostrum from Mothers with Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2024, 26, 40. [Google Scholar] [CrossRef] [PubMed]
- Ansari-Lari, M.; Kazemipour, N.; Yaghoobi, E.; Masoudian, M. Association between Toxoplasma Gondii and Gestational Diabetes with Regard to Serum Leptin and Tumor Necrosis Factor Alpha, a Preliminary Study. Acta Trop. 2024, 254, 107204. [Google Scholar] [CrossRef]
- Wei, W.; Zhang, X. Expression of ADP and TNF-α in Patients with Gestational Diabetes Mellitus and Its Relationship with Pregnancy Outcomes. Exp. Ther. Med. 2020, 20, 2184–2190. [Google Scholar] [CrossRef]
- Kunicki, M.; Rzewuska, N.; Sopońska, P.; Pawłosek, A.; Sowińska, I.; Kloska, A. Novel Serum Biomarkers for Early Diagnosis of Gestational Diabetes Mellitus—A Review. Gynecol. Endocrinol. 2025, 41, 2455472. [Google Scholar] [CrossRef]
- Ray, G.W.; Zeng, Q.; Kusi, P.; Zhang, H.; Shao, T.; Yang, T.; Wei, Y.; Li, M.; Che, X.; Guo, R. Genetic and Inflammatory Factors Underlying Gestational Diabetes Mellitus: A Review. Front. Endocrinol. 2024, 15, 1399694. [Google Scholar] [CrossRef]
- Brink, H.S.; Van Der Lely, A.J.; Van Der Linden, J. The Potential Role of Biomarkers in Predicting Gestational Diabetes. Endocr. Connect. 2016, 5, R26–R34. [Google Scholar] [CrossRef]
- Atègbo, J.-M.; Grissa, O.; Yessoufou, A.; Hichami, A.; Dramane, K.L.; Moutairou, K.; Miled, A.; Grissa, A.; Jerbi, M.; Tabka, Z.; et al. Modulation of Adipokines and Cytokines in Gestational Diabetes and Macrosomia. J. Clin. Endocrinol. Metab. 2006, 91, 4137–4143. [Google Scholar] [CrossRef]
- Hichami, A.; Grissa, O.; Mrizak, I.; Benammar, C.; Khan, N.A. Role of T-Cell Polarization and Inflammation and Their Modulation by n-3 Fatty Acids in Gestational Diabetes and Macrosomia. J. Nutr. Metab. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Lekva, T.; Norwitz, E.R.; Aukrust, P.; Ueland, T. Impact of Systemic Inflammation on the Progression of Gestational Diabetes Mellitus. Curr. Diab. Rep. 2016, 16, 26. [Google Scholar] [CrossRef]
- Rui, L.; Aguirre, V.; Kim, J.K.; Shulman, G.I.; Lee, A.; Corbould, A.; Dunaif, A.; White, M.F. Insulin/IGF-1 and TNF-α Stimulate Phosphorylation of IRS-1 at Inhibitory Ser307 via Distinct Pathways. J. Clin. Investig. 2001, 107, 181–189. [Google Scholar] [CrossRef]
- Kappes, A.; Löffler, G. Influences of Ionomycin, Dibutyryl-cycloAMP and Tumour Necrosis Factor-Alpha on Intracellular Amount and Secretion of apM1 in Differentiating Primary Human Preadipocytes. Horm. Metab. Res. 2000, 32, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.E.; Ishizuka, T.; Shao, J.; Huston, L.; Highman, T.; Catalano, P. Impaired Glucose Transport and Insulin Receptor Tyrosine Phosphorylation in Skeletal Muscle from Obese Women with Gestational Diabetes. Diabetes 1999, 48, 1807–1814. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Catalano, P.M.; Yamashita, H.; Ruyter, I.; Smith, S.; Youngren, J.; Friedman, J.E. Decreased Insulin Receptor Tyrosine Kinase Activity and Plasma Cell Membrane Glycoprotein-1 Overexpression in Skeletal Muscle from Obese Women with Gestational Diabetes Mellitus (GDM): Evidence for Increased Serine/Threonine Phosphorylation in Pregnancy and GDM. Diabetes 2000, 49, 603–610. [Google Scholar] [CrossRef] [PubMed]
- De Gennaro, G.; Palla, G.; Battini, L.; Simoncini, T.; Del Prato, S.; Bertolotto, A.; Bianchi, C. The Role of Adipokines in the Pathogenesis of Gestational Diabetes Mellitus. Gynecol. Endocrinol. 2019, 35, 737–751. [Google Scholar] [CrossRef]
- Nuamah, M.A.; Yura, S.; Sagawa, N.; Itoh, H.; Mise, H.; Korita, D.; Kakui, K.; Takemura, M.; Ogawa, Y.; Nakao, K.; et al. Significant Increase in Maternal Plasma Leptin Concentration in Induced Delivery: A Possible Contribution of Pro-Inflammatory Cytokines to Placental Leptin Secretion. Endocr. J. 2004, 51, 177–187. [Google Scholar] [CrossRef]
- Catalano, P.; Ehrenberg, H. Review Article: The Short- and Long-term Implications of Maternal Obesity on the Mother and Her Offspring. BJOG 2006, 113, 1126–1133. [Google Scholar] [CrossRef]
- Sun, D.; Li, F.; Zhang, Y.; Xu, X. Associations of the Pre-Pregnancy BMI and Gestational BMI Gain with Pregnancy Outcomes in Chinese Women with Gestational Diabetes Mellitus. Int. J. Clin. Exp. Med. 2014, 7, 5784–5789. [Google Scholar]
- Ellingsgaard, H.; Seelig, E.; Timper, K.; Coslovsky, M.; Soederlund, L.; Lyngbaek, M.P.; Wewer Albrechtsen, N.J.; Schmidt-Trucksäss, A.; Hanssen, H.; Frey, W.O.; et al. GLP-1 Secretion Is Regulated by IL-6 Signalling: A Randomised, Placebo-Controlled Study. Diabetologia 2020, 63, 362–373. [Google Scholar] [CrossRef]
- Zgutka, K.; Tkacz, M.; Tomasiak, P.; Piotrowska, K.; Ustianowski, P.; Pawlik, A.; Tarnowski, M. Gestational Diabetes Mellitus-Induced Inflammation in the Placenta via IL-1β and Toll-like Receptor Pathways. Int. J. Mol. Sci. 2024, 25, 11409. [Google Scholar] [CrossRef]
- Visiedo, F.; Vázquez-Fonseca, L.; Ábalos-Martínez, J.; Broullón-Molanes, J.R.; Quintero-Prado, R.; Mateos, R.M.; Bugatto, F. Maternal Elevated Inflammation Impairs Placental Fatty Acids β-Oxidation in Women with Gestational Diabetes Mellitus. Front. Endocrinol. 2023, 14, 1146574. [Google Scholar] [CrossRef]
- Hosseini, E.; Mokhtari, Z.; Salehi Abargouei, A.; Mishra, G.D.; Amani, R. Maternal Circulating Leptin, Tumor Necrosis Factor-Alpha, and Interleukine-6 in Association with Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis. Gynecol. Endocrinol. 2023, 39, 2183049. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.; Waghdhare, S.; Goel, C.; Panda, M.; Soneja, H.; Sundar, J.; Banerjee, M.; Jha, S.; Dubey, S. Augmentation of IL-6 Production Contributes to Development of Gestational Diabetes Mellitus: An Indian Study. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, C. Comparison of the Expression Levels of TNF-α, IL-6, hsCRP and sICAM-1 Inflammatory Factors, Bone Mineral Density and Nutritional Status between Diabetic and Normal Pregnant Women. Cell Mol. Biol. 2020, 66, 132–137. [Google Scholar] [CrossRef]
- Omazić, J.; Muller, A.; Dumančić, B.; Kadivnik, M.; Aladrović, J.; Pađen, L.; Kralik, K.; Brkić, N.; Dobrošević, B.; Vuković, B.; et al. Metabolic and Immune Parameters in Pregnant Women with Impaired Glucose Metabolism—A Pilot Study. Metabolites 2024, 14, 551. [Google Scholar] [CrossRef] [PubMed]
- Muthuraman, N.; Abraham, P. Serum Tumour Necrosis Factor-α (TNF-α) and Lipid Profile in Gestational Diabetes Mellitus. J. Obstet. Gynaecol. Can. 2024, 46, 102592. [Google Scholar] [CrossRef]
- Tenenbaum-Gavish, K.; Sharabi-Nov, A.; Binyamin, D.; Møller, H.J.; Danon, D.; Rothman, L.; Hadar, E.; Idelson, A.; Vogel, I.; Koren, O.; et al. First Trimester Biomarkers for Prediction of Gestational Diabetes Mellitus. Placenta 2020, 101, 80–89. [Google Scholar] [CrossRef]
- Chiti, H.; Izadi, M.H.; Mazloomzadeh, S. The Association Between Pregnancy Serum TNF-α Level and Postpartum Insulin Resistance in Pregnant Women with Gestational Diabetes Mellitus. ACTA 2020, 150–154. [Google Scholar] [CrossRef]
- Mohammed, A.; Aliyu, I.; Manu, M. Correlation between Circulating Level of Tumor Necrosis Factor-Alpha and Insulin Resistance in Nigerian Women with Gestational Diabetes Mellitus. Ann. Afr. Med. 2018, 17, 168. [Google Scholar] [CrossRef]
- Zhang, J.; Chi, H.; Xiao, H.; Tian, X.; Wang, Y.; Yun, X.; Xu, Y. Interleukin 6 (IL-6) and Tumor Necrosis Factor α (TNF-α) Single Nucleotide Polymorphisms (SNPs), Inflammation and Metabolism in Gestational Diabetes Mellitus in Inner Mongolia. Med. Sci. Monit. 2017, 23, 4149–4157. [Google Scholar] [CrossRef] [PubMed]
- Gueuvoghlanian-Silva, B.Y.; Torloni, M.R.; Mattar, R.; De Oliveira, L.S.; Scomparini, F.B.; Nakamura, M.U.; Daher, S. Profile of Inflammatory Mediators in Gestational Diabetes Mellitus: Phenotype and Genotype. Am. J. Rep. Immunol. 2012, 67, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.P.; Torloni, M.R.; Gueuvoghlanian-Silva, B.Y.; Alexandre, S.M.; Mattar, R.; Daher, S. Cytokine Levels in Gestational Diabetes Mellitus: A Systematic Review of the Literature. Am. J. Rep. Immunol. 2013, 69, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Sengupta, P.; Liew, F.F. Cytokine Landscapes of Pregnancy: Mapping Gestational Immune Phases. GOCM 2024, 4, e000011. [Google Scholar] [CrossRef]
- Zhang, T.; Tian, M.; Zhang, P.; Du, L.; Ma, X.; Zhang, Y.; Tang, Z. Risk of Adverse Pregnancy Outcomes in Pregnant Women with Gestational Diabetes Mellitus by Age: A Multicentric Cohort Study in Hebei, China. Sci. Rep. 2024, 14, 807. [Google Scholar] [CrossRef]
- Singh, T.; Newman, A.B. Inflammatory Markers in Population Studies of Aging. Ageing Res. Rev. 2011, 10, 319–329. [Google Scholar] [CrossRef]
- Yuen, L. Gestational Diabetes Mellitus: Challenges for Different Ethnic Groups. World J. Diabetes 2015, 6, 1024. [Google Scholar] [CrossRef]
- Hoffmann, S.C.; Stanley, E.M.; Cox, E.D.; DiMercurio, B.S.; Koziol, D.E.; Harlan, D.M.; Kirk, A.D.; Blair, P.J. Ethnicity Greatly Influences Cytokine Gene Polymorphism Distribution. Am. J. Transplant. 2002, 2, 560–567. [Google Scholar] [CrossRef]
- Monod, C.; Kotzaeridi, G.; Linder, T.; Eppel, D.; Rosicky, I.; Filippi, V.; Tura, A.; Hösli, I.; Göbl, C.S. Prevalence of Gestational Diabetes Mellitus in Women with a Family History of Type 2 Diabetes in First- and Second-Degree Relatives. Acta Diabetol. 2022, 60, 345–351. [Google Scholar] [CrossRef]
- Pantham, P.; Aye, I.L.M.H.; Powell, T.L. Inflammation in Maternal Obesity and Gestational Diabetes Mellitus. Placenta 2015, 36, 709–715. [Google Scholar] [CrossRef]
- Song, S.; Zhang, Y.; Qiao, X.; Duo, Y.; Xu, J.; Peng, Z.; Zhang, J.; Chen, Y.; Nie, X.; Sun, Q.; et al. HOMA-IR as a Risk Factor of Gestational Diabetes Mellitus and a Novel Simple Surrogate Index in Early Pregnancy. Intl J. Gynecol. Obs. 2022, 157, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Quaresima, P.; Myers, S.H.; Pintaudi, B.; D’Anna, R.; Morelli, M.; Unfer, V. Gestational Diabetes Mellitus and Polycystic Ovary Syndrome, a Position Statement from EGOI-PCOS. Front. Endocrinol. 2025, 16, 1501110. [Google Scholar] [CrossRef]
- Hirano, T. Interleukin 6 in Autoimmune and Inflammatory Diseases: A Personal Memoir. Proc. Jpn. Acad. Ser. B 2010, 86, 717–730. [Google Scholar] [CrossRef] [PubMed]
- Spence, T.; Allsopp, P.J.; Yeates, A.J.; Mulhern, M.S.; Strain, J.J.; McSorley, E.M. Maternal Serum Cytokine Concentrations in Healthy Pregnancy and Preeclampsia. J. Pregnancy 2021, 2021, 1–33. [Google Scholar] [CrossRef]
- Kadivnik, M.; Plečko, D.; Kralik, K.; Arvaj, N.; Wagner, J. Role of IL-6, IL-10 and TNFα Gene Variants in Preterm Birth. J. Clin. Med. 2024, 13, 2429. [Google Scholar] [CrossRef]

| Study | Population Type | Population Size | Time | TNF-α (pg/mL) | IL-6 (ng/L) |
|---|---|---|---|---|---|
| Omazic et al., 2024 [36] | pregnant women |
| First trimester | decreased p = 0.64 | increased p = 0.37 |
| Muthuraman et al., 2024 [37] | Pregnant women | 27 Controls vs. 27 GDM | 24–28 weeks | decreased | N/A |
| Wang et al., 2020 [35] | Pregnant women | 68 Controls vs. 74 GDM | 24–28 weeks | increased p = 0.001 | N/A |
| Tenenbaum-Gavish et al., 2020 [38] | Pregnant women | 185 Controls vs. 20 GDM | First trimester | decreased p = 0.037 | increased p = 0.524 |
| Chiti et al., 2020 [39] | Pregnant women | 25 Controls vs. 25 GDM | Third trimester | decreased p = 0.900 | N/A |
| Siddiqui et al., 2019 [34] | Pregnant women | 50 Controls vs. 53 GDM | 12 to 26 weeks | N/A | increased p = 0.001 |
| Mohammed et al., 2018 [40] | Pregnant women | 100 Controls vs. 100 GDM | 24–28 weeks | increased p < 0.05 | N/A |
| Zhang et al., 2017 [41] | Pregnant women | 60 Controls vs. 60 GDM | 24–28 weeks | increased p = 0.32 | increased p = 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varthaliti, A.; Lygizos, V.; Fanaki, M.; Pergialiotis, V.; Papapanagiotou, A.; Pappa, K.; Theodora, M.; Daskalaki, M.A.; Antsaklis, P.; Daskalakis, G. The Role of IL-6 and TNF-α as Early Biomarkers in the Prediction and Diagnosis of Gestational Diabetes Mellitus. Biomedicines 2025, 13, 1627. https://doi.org/10.3390/biomedicines13071627
Varthaliti A, Lygizos V, Fanaki M, Pergialiotis V, Papapanagiotou A, Pappa K, Theodora M, Daskalaki MA, Antsaklis P, Daskalakis G. The Role of IL-6 and TNF-α as Early Biomarkers in the Prediction and Diagnosis of Gestational Diabetes Mellitus. Biomedicines. 2025; 13(7):1627. https://doi.org/10.3390/biomedicines13071627
Chicago/Turabian StyleVarthaliti, Antonia, Vasilios Lygizos, Maria Fanaki, Vasilios Pergialiotis, Angeliki Papapanagiotou, Kalliopi Pappa, Marianna Theodora, Maria Anastasia Daskalaki, Panos Antsaklis, and George Daskalakis. 2025. "The Role of IL-6 and TNF-α as Early Biomarkers in the Prediction and Diagnosis of Gestational Diabetes Mellitus" Biomedicines 13, no. 7: 1627. https://doi.org/10.3390/biomedicines13071627
APA StyleVarthaliti, A., Lygizos, V., Fanaki, M., Pergialiotis, V., Papapanagiotou, A., Pappa, K., Theodora, M., Daskalaki, M. A., Antsaklis, P., & Daskalakis, G. (2025). The Role of IL-6 and TNF-α as Early Biomarkers in the Prediction and Diagnosis of Gestational Diabetes Mellitus. Biomedicines, 13(7), 1627. https://doi.org/10.3390/biomedicines13071627










