Analysis of Vitamin D and VDR Expression in Women with Advanced Endometriosis: A Case–Control Study in Thailand
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Data Collection and Measurements
2.3. Statistical Analysis
3. Results
4. Discussion
4.1. Serum Vitamin D Levels in Women with Endometriosis Compared to Controls
4.2. Vitamin D Receptor (VDR) Expression
4.3. Serum Vitamin D Levels and VDR Expression
4.4. Serum Vitamin D Level and Endometriosis Severity
4.5. VDR Expression and Endometriosis Severity
4.6. Strengths and Limitations
4.7. Clinical Implications and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 25(OH)D | 25-Hydroxyvitamin D |
| AST | Aspartate aminotransferase |
| ALT | Alanine aminotransferase |
| BMI | Body mass index |
| COC | Combined oral contraception |
| Cu-IUD | Copper intrauterine device |
| C/S | Cesarean section |
| DMPA | Depot medroxyprogesterone acetate |
| GFR | Glomerular filtration rate |
| IHC | Immunohistochemical |
| TAH | Total abdominal hysterectomy |
| TR | Tubal resection |
| SPF | Sun protection factor |
| VAS | Visual analog scale |
| VDR | Vitamin D receptor |
References
- Sampson, J.A. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am. J. Obstet. Gynecol. 1927, 14, 422–469. [Google Scholar] [CrossRef]
- Zondervan, K.T.; Becker, C.M.; Missmer, S.A.; Longo, D.L. Endometriosis. N. Engl. J. Med. 2020, 382, 1244–1256. [Google Scholar] [CrossRef] [PubMed]
- Chapron, C.; Marcellin, L.; Borghese, B.; Santulli, P. Rethinking mechanisms, diagnosis and management of endometriosis. Nat. Rev. Endocrinol. 2019, 15, 666–682. [Google Scholar] [CrossRef]
- Farhangnia, P.; Noormohammadi, M.; Delbandi, A.-A. Vitamin D and reproductive disorders: A comprehensive review with a focus on endometriosis. Reprod. Health 2024, 21, 61. [Google Scholar] [CrossRef] [PubMed]
- Velarde, M.C.; Bucu, M.E.M.; E Habana, M.A. Endometriosis as a highly relevant yet neglected gynecologic condition in Asian women. Endocr. Connect. 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Liao, M.; Huang, Y.; Hang, F.; Ma, N.; Hu, Q.; Wang, J.; Jin, Y.; Qin, A.; Naem, A. Association between vitamin D and endometriosis among American women: National Health and Nutrition Examination Survey. PLoS ONE 2024, 19, e0296190. [Google Scholar] [CrossRef] [PubMed]
- Simoens, S.; Dunselman, G.; Dirksen, C.; Hummelshoj, L.; Bokor, A.; Brandes, I.; Brodszky, V.; Canis, M.; Colombo, G.L.; DeLeire, T.; et al. The burden of endometriosis: Costs and quality of life of women with endometriosis and treated in referral centres. Hum. Reprod. 2012, 27, 1292–1299. [Google Scholar] [CrossRef]
- E Bulun, S.; Yilmaz, B.D.; Sison, C.; Miyazaki, K.; Bernardi, L.; Liu, S.; Kohlmeier, A.; Yin, P.; Milad, M.; Wei, J. Endometriosis. Endocr. Rev. 2019, 40, 1048–1079. [Google Scholar] [CrossRef]
- Delbandi, A.-A.; Mahmoudi, M.; Shervin, A.; Akbari, E.; Jeddi-Tehrani, M.; Sankian, M.; Kazemnejad, S.; Zarnani, A.-H. Eutopic and ectopic stromal cells from patients with endometriosis exhibit differential invasive, adhesive, and proliferative behavior. Fertil. Steril. 2013, 100, 761–769. [Google Scholar] [CrossRef]
- Lips, P. Vitamin D physiology. Prog. Biophys. Mol. Biol. 2006, 92, 4–8. [Google Scholar] [CrossRef]
- Agic, A.; Xu, H.; Altgassen, C.; Noack, F.; Wolfler, M.M.; Diedrich, K.; Friedrich, M.; Taylor, R.N.; Hornung, D. Relative Expression of 1,25-Dihydroxyvitamin D3 Receptor, Vitamin D 1α-Hydroxylase, Vitamin D 24-Hydroxylase, and Vitamin D 25-Hydroxylase in Endometriosis and Gynecologic Cancers. Reprod. Sci. 2007, 14, 486–497. [Google Scholar] [CrossRef]
- Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D; Ross, A.C., Taylor, C.L., Yaktine, A.L., Del Valle, H.B., Eds.; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Giustina, A.; Bilezikian, J.P.; A Adler, R.; Banfi, G.; Bikle, D.D.; Binkley, N.C.; Bollerslev, J.; Bouillon, R.; Brandi, M.L.; Casanueva, F.F.; et al. Consensus Statement on Vitamin D Status Assessment and Supplementation: Whys, Whens, and Hows. Endocr. Rev. 2024, 45, 625–654. [Google Scholar] [CrossRef]
- Jiang, Z.; Pu, R.; Li, N.; Chen, C.; Li, J.; Dai, W.; Wang, Y.; Hu, J.; Zhu, D.; Yu, Q.; et al. High prevalence of vitamin D deficiency in Asia: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2021, 63, 3602–3611. [Google Scholar] [CrossRef]
- Jennings, B.S.; Hewison, M. Vitamin D and Endometriosis: Is There a Mechanistic Link? Cell Biochem. Funct. 2024, 43, e70037. [Google Scholar] [CrossRef] [PubMed]
- Yarmolinskaya, M.; Denisova, A.; Tkachenko, N.; Ivashenko, T.; Bespalova, O.; Tolibova, G.; Tral, T. Vitamin D significance in pathogenesis of endometriosis. Gynecol. Endocrinol. 2021, 37, 40–43. [Google Scholar] [CrossRef]
- Buggio, L.; Somigliana, E.; Pizzi, M.N.; Dridi, D.; Roncella, E.; Vercellini, P. 25-Hydroxyvitamin D Serum Levels and Endometriosis: Results of a Case–Control Study. Reprod. Sci. 2018, 26, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Somigliana, E.; Panina-Bordignon, P.; Murone, S.; Di Lucia, P.; Vercellini, P.; Vigano, P. Vitamin D reserve is higher in women with endometriosis. Hum. Reprod. 2007, 22, 2273–2278. [Google Scholar] [CrossRef] [PubMed]
- De Pascali, F.; Casarini, L.; Kuhn, C.; Simoni, M.; Mahner, S.; Jeschke, U.; von Schönfeldt, V. Nuclear expression of VDR and AHR is mutually exclusive in glandular cells in endometriosis. Histochem. 2021, 156, 391–399. [Google Scholar] [CrossRef]
- Jafari, M.; Khodaverdi, S.; Sadri, M.; Moradi, Z.; Mohammadi, T.; Heidari, S.; Sales, Z.A.; Delbandi, A.-A. Association Between Vitamin D Receptor (VDR) and Vitamin D Binding Protein (VDBP) Genes Polymorphisms to Endometriosis Susceptibility in Iranian Women. Reprod. Sci. 2021, 28, 3491–3497. [Google Scholar] [CrossRef]
- Cho, M.-C.; Kim, J.H.; Jung, M.H.; Cho, I.A.; Jo, H.C.; Shin, J.K.; Lee, S.A.; Choi, W.J.; Lee, J.H. Analysis of vitamin D-binding protein (VDBP) gene polymorphisms in Korean women with and without endometriosis. Clin. Exp. Reprod. Med. 2019, 46, 132–139. [Google Scholar] [CrossRef]
- Lee, H.-H.; Mun, M.-J.; Kim, T.-H.; Kim, Y.-S.; Jang, W.-C.; Hwang, J.-Y. Relationships between vitamin D receptor genetic polymorphisms and endometriosis in Korean women. Clin. Exp. Obstet. Gynecol. 2019, 46, 876–880. [Google Scholar] [CrossRef]
- Kahlon, B.K.; Simon-Collins, M.; Nylander, E.; Segars, J.; Singh, B. A systematic review of vitamin D and endometriosis: Role in pathophysiology, diagnosis, treatment, and prevention. F&S Rev. 2022, 4, 1–14. [Google Scholar] [CrossRef]
- Rajatanavin, N.; Kanokrungsee, S.; Aekplakorn, W. Vitamin D status in Thai dermatologists and working-age Thai population. J. Dermatol. 2018, 46, 206–212. [Google Scholar] [CrossRef]
- Jaruratanasirikul, S.; Boonrusmee, S.; Kasemsripitak, S.; Saengkaew, T.; Chimrung, K.; Sriplung, H. Vitamin D status in non-pregnant women of reproductive age: A study in Southern Thailand. Sci. Rep. 2023, 13, 15264. [Google Scholar] [CrossRef] [PubMed]
- Pludowski, P.; Takacs, I.; Boyanov, M.; Belaya, Z.; Diaconu, C.C.; Mokhort, T.; Zherdova, N.; Rasa, I.; Payer, J.; Pilz, S. Clinical Practice in the Prevention, Diagnosis and Treatment of Vitamin D Deficiency: A Central and Eastern European Expert Consensus Statement. Nutrients 2022, 14, 1483. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. Med. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Berisha, A.T.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef]
- Canis, M.; Donnez, J.G.; Guzick, D.S.; Halme, J.K.; Rock, J.A.; Schenken, R.S.; Vernon, M.W. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil. Steril. 1997, 67, 817–821. [Google Scholar] [CrossRef]
- Price, P.M.; Ganugapati, U.; Gatalica, Z.; Kakadekar, A.B.; Macpherson, J.; Quenneville, L.M.; Rees, H.M.; Slodkowska, E.; Suresh, J.; Yu, D.H.B.M.M.F.; et al. Reinventing Nuclear Histo-score Utilizing Inherent Morphologic Cutoffs: Blue-brown Color H-score (BBC-HS). Appl. Immunohistochem. Mol. Morphol. 2023, 31, 500–506. [Google Scholar] [CrossRef]
- Delbandi, A.-A.; Torab, M.; Abdollahi, E.; Khodaverdi, S.; Rokhgireh, S.; Moradi, Z.; Heidari, S.; Mohammadi, T. Vitamin D deficiency as a risk factor for endometriosis in Iranian women. J. Reprod. Immunol. 2021, 143, 103266. [Google Scholar] [CrossRef]
- Denisova, A.S.; Yarmolinskaya, M.I.; Tkachenko, N.N.; Игоревна, Я.М. Assessment of 25(OH)D status in patients with genital endometriosis and clinical efficacy of cholecalciferol in the treatment of the disease. J. Obstet. Women’s Dis. 2021, 70, 125–133. [Google Scholar] [CrossRef]
- Anastasi, E.; Fuggetta, E.; De Vito, C.; Migliara, G.; Viggiani, V.; Manganaro, L.; Granato, T.; Panici, P.B.; Angeloni, A.; Porpora, M.G. Low levels of 25-OH vitamin D in women with endometriosis and associated pelvic pain. cclm 2017, 55, e282–e284. [Google Scholar] [CrossRef] [PubMed]
- Harris, H.R.; Chavarro, J.E.; Malspeis, S.; Willett, W.C.; Missmer, S.A. Dairy-Food, Calcium, Magnesium, and Vitamin D Intake and Endometriosis: A Prospective Cohort Study. Am. J. Epidemiol. 2013, 177, 420–430. [Google Scholar] [CrossRef]
- Farland, L.V.; Degnan, W.J.; Harris, H.R.; Han, J.; Cho, E.; VoPham, T.; Kvaskoff, M.; A Missmer, S. Recreational and residential sun exposure and risk of endometriosis: A prospective cohort study. Hum. Reprod. 2020, 36, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Al-Lami, R.A.; Taha, S.A.; Jalloul, R.J.; Taylor, H.S. Women with endometriosis in the United States: National Survey of Family Growth, 2011–2019. J. Endometr. Uterine Disord. 2024, 8, 100081. [Google Scholar] [CrossRef]
- Siwamogsatham, O.; Ongphiphadhanakul, B.; Tangpricha, V. Vitamin D deficiency in Thailand. J. Clin. Transl. Endocrinol. 2015, 2, 48–49. [Google Scholar] [CrossRef][Green Version]
- Nagpal, S.; Na, S.; Rathnachalam, R. Noncalcemic Actions of Vitamin D Receptor Ligands. Endocr. Rev. 2005, 26, 662–687. [Google Scholar] [CrossRef]
- Hii, C.S.; Ferrante, A. The Non-Genomic Actions of Vitamin D. Nutrients 2016, 8, 135. [Google Scholar] [CrossRef]
- Czogalla, B.; Deuster, E.; Liao, Y.; Mayr, D.; Schmoeckel, E.; Sattler, C.; Kolben, T.; Hester, A.; Fürst, S.; Burges, A.; et al. Cytoplasmic VDR expression as an independent risk factor for ovarian cancer. Histochem. 2020, 154, 421–429. [Google Scholar] [CrossRef]
- Matasariu, D.R.; Mandici, C.E.; Ursache, A.; Bausic, A.I.G.; Bujor, I.E.; Cristofor, A.E.; Boiculese, L.V.; Grigore, M.; Bratila, E.; Lozneanu, L. Vitamin D and Mitosis Evaluation in Endometriosis: A Step toward Discovering the Connection? Biomedicines 2023, 11, 2102. [Google Scholar] [CrossRef]
- Deuster, E.; Jeschke, U.; Ye, Y.; Mahner, S.; Czogalla, B. Vitamin D and VDR in Gynecological Cancers—A Systematic Review. Int. J. Mol. Sci. 2017, 18, 2328. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, J.F.P.; Borges, M.V.d.O.; Soares, A.A.; dos Santos, J.C.; de Oliveira, A.B.B.; da Costa, C.H.B.; Cruz, M.S.; Bortolin, R.H.; de Freitas, R.C.C.; Dantas, P.M.S.; et al. The impact of vitamin D supplementation on VDR gene expression and body composition in monozygotic twins: Randomized controlled trial. Sci. Rep. 2020, 10, 11943. [Google Scholar] [CrossRef]
- Cermisoni, G.C.; Alteri, A.; Corti, L.; Rabellotti, E.; Papaleo, E.; Viganò, P.; Sanchez, A.M. Vitamin D and Endometrium: A Systematic Review of a Neglected Area of Research. Int. J. Mol. Sci. 2018, 19, 2320. [Google Scholar] [CrossRef]
- Gursoy, O.; Eren, C.Y.; Gürer, H.G. The relationship between ovarian endometrosis and vitamin D level. Sağlık Akad. Derg. 2022, 9, 265–271. [Google Scholar] [CrossRef]
- Qiu, Y.; Yuan, S.; Wang, H. Vitamin D status in endometriosis: A systematic review and meta-analysis. Arch. Gynecol. Obstet. 2020, 302, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sheng, S.; Pan, Z.; Zhao, L.; Yang, C.; Li, C.; Wang, F. Immune and endocrine regulation in endometriosis: What we know. J. Endometr. Uterine Disord. 2023, 4, 100049. [Google Scholar] [CrossRef]
- Zondervan, K.T.; Becker, C.M.; Koga, K.; Missmer, S.A.; Taylor, R.N.; Viganò, P. Endometriosis. Nat. Rev. Dis. Primers 2018, 4, 9. [Google Scholar] [CrossRef]
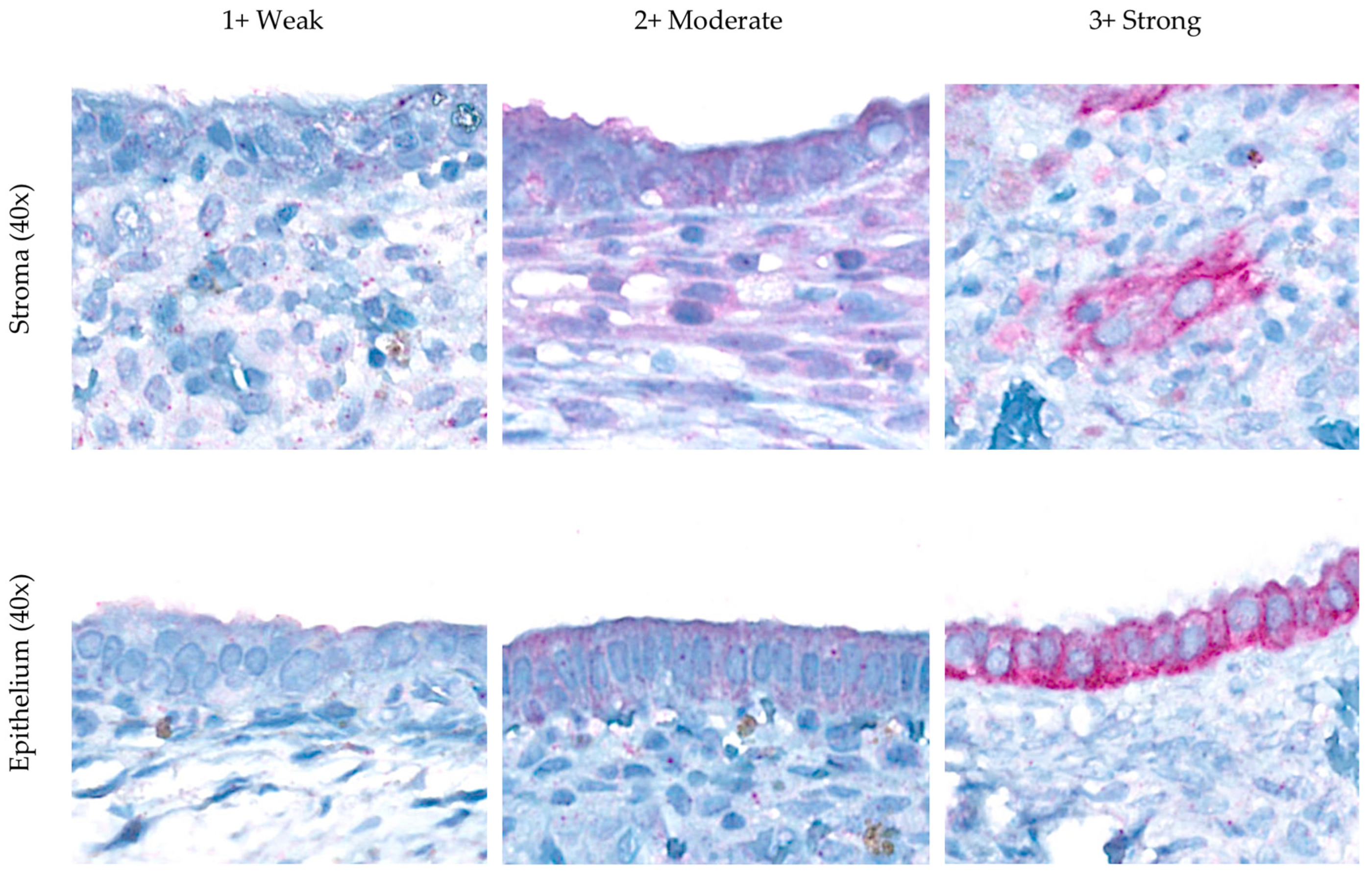
| Characteristic | Endometriosis | Control | p-Value * |
|---|---|---|---|
| Age (years) | 33.36 (5.41) | 33.35 (5.63) | 0.938 |
| BMI (kg/m2) | 21.76 [19.93, 23.19] | 21.50 [19.24, 23.87] | 0.927 |
| BMI | 0.841 | ||
| 3 (8.33) | 6 (8.33) | |
| 23 (63.89) | 46 (63.89) | |
| 3 (8.33) | 6 (8.33) | |
| 7 (19.44) | 14 (19.44) | |
| Living with a partner | 25 (69.44) | 47 (65.28) | 0.668 |
| 48 [29, 108] | 120 [68, 156] | 0.024 |
| Pregnancy history | |||
| 9 (25.00) | 34 (47.22) | 0.028 |
| 4 (11.11) | 13 (18.06) | 0.344 |
| Contraception | 3 (8.33) | 29 (40.28) | 0.002 |
| Education | 0.130 | ||
| 5 (13.89) | 19 (26.39) | |
| 31 (86.11) | 53 (73.61) | |
| Occupation environment | |||
| 0 | 0 | |
| 36 (100.00) | 72 (100.00) | |
| 115.00 [75.00, 150.00] | 75.00 [47.50, 142.50] | 0.304 |
| 74.30 [29.00, 109.00] | 45 [00.00, 83.80] | 0.133 |
| 3 (8.33) | 10 (13.89) | 0.469 |
| Facial SPF | 0.893 | ||
| 4 (11.11) | 10 (13.89) | |
| 2 (5.56) | 3 (4.17) | |
| 30 (83.33) | 59 (81.94) | |
| Body SPF | 0.310 | ||
| 8 (22.22) | 29 (40.27) | |
| 8 (22.22) | 8 (11.11) | |
| 3 (8.34) | 4 (5.56) | |
| 17 (47.22) | 31 (43.06) | |
| Outdoor exercise | 0.334 | ||
| 32 (88.89) | 62 (86.11) | |
| Consumption | |||
| 34 (94.44) | 72 (100.00) | >0.999 |
| 21 (58.33) | 48 (66.67) | 0.385 |
| Residence | 0.003 | ||
| 17 (47.22) | 57 (79.17) | |
| 19 (52.78) | 15 (20.83) |
| Vitamin D | Endometriosis | Control | Median Difference | (95% CI) |
|---|---|---|---|---|
| Unadjusted | 20.45 [16.10, 25.10] | 21.10 [18.20, 26.70] | –1.67 | –4.16, 0.83 |
| Adjusted model 1 * | 20.11 | 23.01 | −2.90 | −5.26, −0.54 |
| Adjusted model 2 † | 21.47 | 22.33 | −0.86 | −3.47, 1.75 |
| Adjusted model 3 ‡ | 20.70 | 22.71 | −2.01 | −4.50, 0.48 |
| Vitamin D | Endometriosis | Control | OR (95% CI) | |||
|---|---|---|---|---|---|---|
| Univariable | Multivariable | |||||
| Crude | AOR Model 1 * | AOR Model 2 † | AOR Model 3 ‡ | |||
| ≥20 ng/mL | 19 (52.78) | 40 (55.56) | 1 | 1 | 1 | 1 |
| <20 ng/mL | 17 (47.22) | 32 (44.44) | 1.12 (0.45, 2.77) | 1.99 (0.63, 6.35) | 0.72 (0.24, 2.13) | 1.28 (0.39, 4.16) |
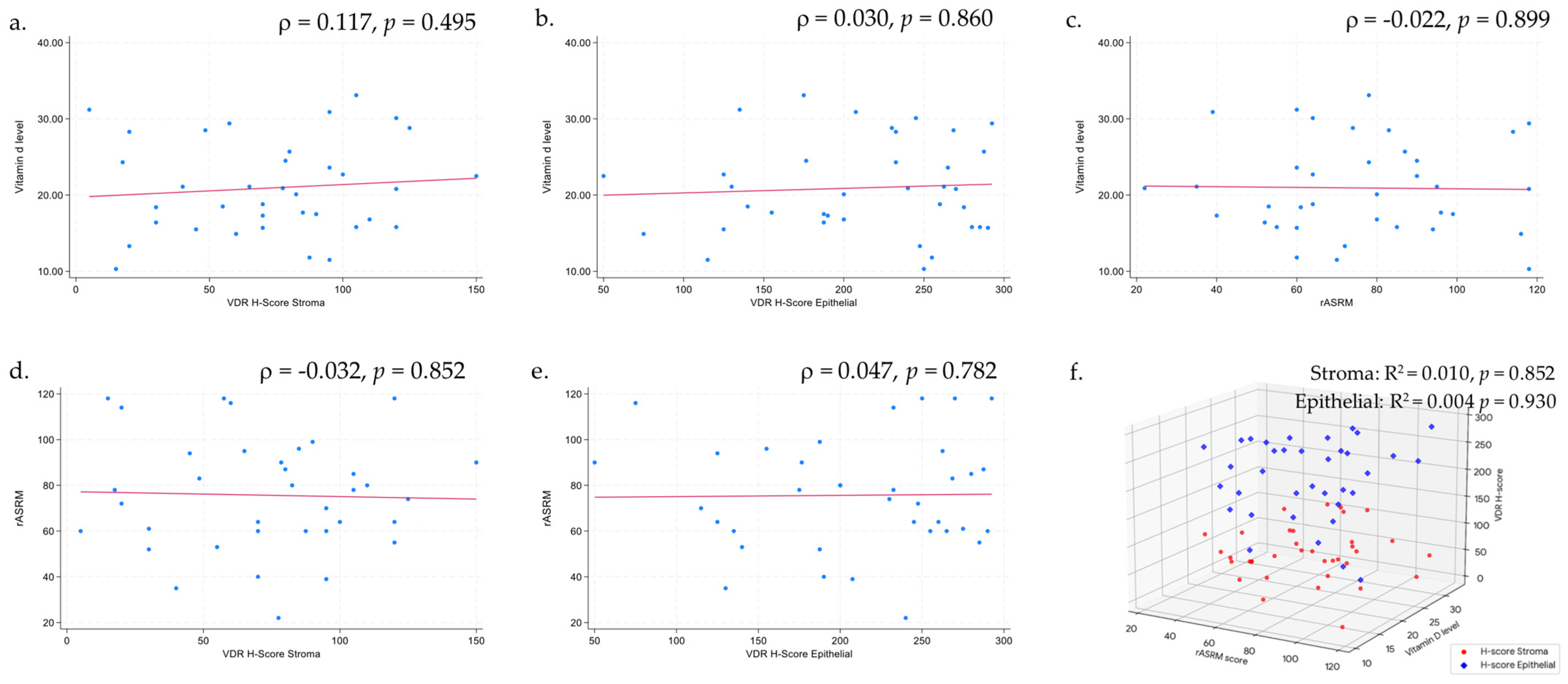
| Vitamin D | VDR H-Score Stroma | p-Value * | Difference (95% CI) † | VDR H-Score Epithelial | p-Value * | Difference (95% CI) † |
|---|---|---|---|---|---|---|
| Normal | 81.25 (51.86) | 0.876 | Reference | 190.63 (46.83) | 0.908 | Reference |
| Insufficient | 77.13 (37.67) | −4.12 (−46.82, 38.59) | 217.50 (69.45) | 26.88 (−50.69, 104.44) | ||
| Deficient | 68.57 (31.10) | −12.68 (−55.70, 30.35) | 206.96 (67.83) | 16.34 (−61.81, 94.49) | ||
| Severe deficiency | 65.83 (44.18) | −15.42 (−73.38, 42.54) | 206.67 (79.42) | 16.04 (−89.24, 121.32) |
| Vitamin D | Endometriosis Severity | p-Value * | OR (95% CI) † | |
|---|---|---|---|---|
| Stage 3 | Stage 4 | |||
| Vitamin D status | ||||
| 1 (2.78) | 3 (8.33) | 0.390 | 1 |
| 3 (8.34) | 29 (80.55) | 3.22 (0.05, 57.99) | |
| Hypovitaminosis D subgroup | ||||
| 2 (5.56) | 13 (36.11) | 2.17 (0.14, 33.80) | |
| 1 (2.78) | 13 (36.11) | 4.33 (0.24, 115.67) | |
| 0 | 3 (8.33) | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Layanun, V.; Somboonporn, W.; Aupongkaroon, P.; Kleebkaow, P.; Chaisuriya, N.; Pluthikarmpae, N. Analysis of Vitamin D and VDR Expression in Women with Advanced Endometriosis: A Case–Control Study in Thailand. Biomedicines 2025, 13, 1605. https://doi.org/10.3390/biomedicines13071605
Layanun V, Somboonporn W, Aupongkaroon P, Kleebkaow P, Chaisuriya N, Pluthikarmpae N. Analysis of Vitamin D and VDR Expression in Women with Advanced Endometriosis: A Case–Control Study in Thailand. Biomedicines. 2025; 13(7):1605. https://doi.org/10.3390/biomedicines13071605
Chicago/Turabian StyleLayanun, Vitet, Woraluk Somboonporn, Pinya Aupongkaroon, Pilaiwan Kleebkaow, Nipon Chaisuriya, and Naree Pluthikarmpae. 2025. "Analysis of Vitamin D and VDR Expression in Women with Advanced Endometriosis: A Case–Control Study in Thailand" Biomedicines 13, no. 7: 1605. https://doi.org/10.3390/biomedicines13071605
APA StyleLayanun, V., Somboonporn, W., Aupongkaroon, P., Kleebkaow, P., Chaisuriya, N., & Pluthikarmpae, N. (2025). Analysis of Vitamin D and VDR Expression in Women with Advanced Endometriosis: A Case–Control Study in Thailand. Biomedicines, 13(7), 1605. https://doi.org/10.3390/biomedicines13071605






