Longan Flower Ethanol Extract, Dimocarpus longan Lour, Mitigates Oxidative Damage and Inflammatory Responses While Promoting Sleep-Related Enzymes in Cell Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Longan Flower Extract (LFE)
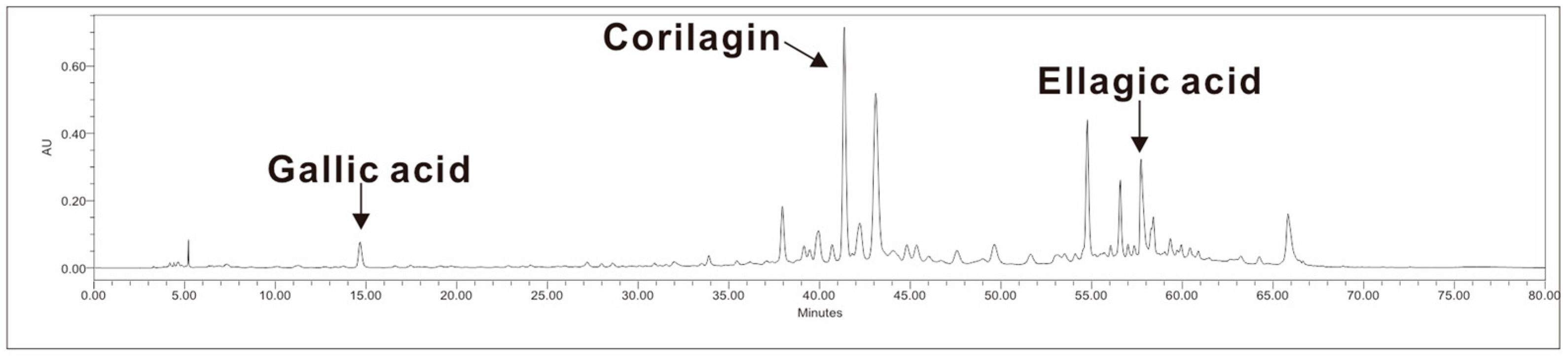
2.2. Analysis of Bioactive Compounds
2.3. Cell Lines
2.4. Cell Viability Assay
2.5. Measurement of TNF-α and IL-6
2.6. NO Assay
2.7. Measurement of ROS
2.8. GPx Activity Assay
2.9. SOD Activity Assay
2.10. GSH Assay
2.11. Total RNA Extraction and qPCR
2.12. Serotonin Measurement
2.13. Statistical Analysis
3. Results
3.1. Bioactive Compounds in LFE
3.2. Reduction in Inflammatory Markers
3.3. Reduction in Nitric Oxide (NO) Expression
3.4. Reduction in Reactive Oxygen Species (ROS) and Enhancement of Antioxidant Enzymes
3.5. Enhancement of Melatonin-Synthesis-Related Enzymes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LFE | longan flower extract |
| OPCs | Oligomeric proanthocyanidins |
| TPH1 | tryptophan hydroxylase 1 |
| DDC | DOPA decarboxylase |
| AANAT | aralkylamine N-acetyltransferase |
| ASMT | acetylserotonin O-methyltransferase |
| NO | Nitric oxide |
| ROS | reactive oxygen species |
| iNOS | inducible nitric oxide synthase |
| GPx | glutathione peroxidase |
| SOD | superoxide dismutase |
| LPS | Lipopolysaccharide |
References
- Garbarino, S.; Lanteri, P.; Bragazzi, N.L.; Magnavita, N.; Scoditti, E. Role of sleep deprivation in immune-related disease risk and outcomes. Commun. Biol. 2021, 4, 1304. [Google Scholar] [CrossRef] [PubMed]
- Uccella, S.; Cordani, R.; Salfi, F.; Gorgoni, M.; Scarpelli, S.; Gemignani, A.; Geoffroy, P.A.; De Gennaro, L.; Palagini, L.; Ferrara, M.; et al. Sleep Deprivation and Insomnia in Adolescence: Implications for Mental Health. Brain Sci. 2023, 13, 569. [Google Scholar] [CrossRef]
- Fietze, I.; Laharnar, N.; Koellner, V.; Penzel, T. The Different Faces of Insomnia. Front. Psychiatry 2021, 12, 683943. [Google Scholar] [CrossRef] [PubMed]
- Krystal, A.D.; Prather, A.A.; Ashbrook, L.H. The assessment and management of insomnia: An update. World Psychiatry 2019, 18, 337–352. [Google Scholar] [CrossRef] [PubMed]
- Bravo, R.; Matito, S.; Cubero, J.; Paredes, S.D.; Franco, L.; Rivero, M.; Rodríguez, A.B.; Barriga, C. Tryptophan-enriched cereal intake improves nocturnal sleep, melatonin, serotonin, and total antioxidant capacity levels and mood in elderly humans. Age 2013, 35, 1277–1285. [Google Scholar] [CrossRef]
- Morin, C.M.; Jarrin, D.C. Epidemiology of Insomnia: Prevalence, Course, Risk Factors, and Public Health Burden. Sleep Med. Clin. 2022, 17, 173–191. [Google Scholar] [CrossRef]
- Yuan, K.; Zheng, Y.B.; Wang, Y.J.; Sun, Y.K.; Gong, Y.M.; Huang, Y.T.; Chen, X.; Liu, X.X.; Zhong, Y.; Su, S.Z.; et al. A systematic review and meta-analysis on prevalence of and risk factors associated with depression, anxiety and insomnia in infectious diseases, including COVID-19: A call to action. Mol. Psychiatry 2022, 27, 3214–3222. [Google Scholar] [CrossRef]
- Höglund, P.; Hakelind, C.; Nordin, M.; Nordin, S. Risk factors for insomnia and burnout: A longitudinal population-based cohort study. Stress Health 2023, 39, 798–812. [Google Scholar] [CrossRef]
- Peng, C.; Wang, K.; Wang, J.; Wassing, R.; Eickhoff, S.B.; Tahmasian, M.; Chen, J. Neural correlates of insomnia with depression and anxiety from a neuroimaging perspective: A systematic review. Sleep Med. Rev. 2025, 81, 102093. [Google Scholar] [CrossRef]
- Riemann, D.; Dressle, R.J.; Benz, F.; Spiegelhalder, K.; Johann, A.F.; Nissen, C.; Hertenstein, E.; Baglioni, C.; Palagini, L.; Krone, L.; et al. Chronic insomnia, REM sleep instability and emotional dysregulation: A pathway to anxiety and depression? J. Sleep Res. 2025, 34, e14252. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Melatonin ameliorates anxiety-like behaviors induced by sleep deprivation in mice: Role of oxidative stress, neuroinflammation, autophagy and apoptosis. Brain Res. Bull. 2021, 174, 161–172. [Google Scholar] [CrossRef]
- Akinnusi, M.; Martinson, A.; El-Solh, A.A. Treatment of insomnia associated with alcohol and opioid use: A narrative review. Sleep Biol. Rhythm. 2024, 22, 429–445. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Wang, W.-H.; Chen, S.-H.; Chang, Y.-W.; Hung, L.-C.; Chen, C.-Y.; Chen, Y.-H. Lipopolysaccharide-Induced Nitric Oxide, Prostaglandin E2, and Cytokine Production of Mouse and Human Macrophages Are Suppressed by Pheophytin-b. Int. J. Mol. Sci. 2017, 18, 2637. [Google Scholar] [CrossRef]
- Harasym, J.; Oledzki, R. Effect of fruit and vegetable antioxidants on total antioxidant capacity of blood plasma. Nutrition 2014, 30, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Núñez, S.; Moliner, C.; Valero, M.S.; Mustafa, A.M.; Maggi, F.; Gómez-Rincón, C.; López, V. Antidiabetic and anti-obesity properties of a polyphenol-rich flower extract from Tagetes erecta L. and its effects on Caenorhabditis elegans fat storages. J. Physiol. Biochem. 2023, 79, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Durmaz, A.; Gun Kakasci, C. Pregnant women’s attitudes towards complementary and alternative medicine and the use of phytotherapy during the COVID-19 pandemic: A cross-sectional study. PLoS ONE 2024, 19, e0296435. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, S.; Ho, C.-T.; Bai, N. Phytochemical constituents and biological activities of longan (Dimocarpus longan Lour.) fruit: A review. Food Sci. Hum. Wellness 2020, 9, 95–102. [Google Scholar] [CrossRef]
- Yi, Y.; Liao, S.T.; Zhang, M.W.; Shi, J.; Zhang, R.F.; Deng, Y.Y.; Wei, Z.C. Physicochemical characteristics and immunomodulatory activities of three polysaccharide-protein complexes of longan pulp. Molecules 2011, 16, 6148–6164. [Google Scholar] [CrossRef]
- Wang, S.; Chen, X.; Li, Q.; Zhang, Y.; Rong, Y.; Feng, Y.; Liu, H.; Xu, J.; Yang, R.; Li, W. Comparative Study on the Mechanism of Macrophage Activation Induced by Polysaccharides from Fresh and Dried Longan. Nutrients 2024, 16, 1654. [Google Scholar] [CrossRef]
- Meng, F.-Y.; Ning, Y.-L.; Qi, J.; He, Z.; Jie, J.; Lin, J.-J.; Huang, Y.-J.; Li, F.-S.; Li, X.-H. Structure and Antitumor and Immunomodulatory Activities of a Water-Soluble Polysaccharide from Dimocarpus longan Pulp. Int. J. Mol. Sci. 2014, 15, 5140–5162. [Google Scholar] [CrossRef]
- Hsieh, M.-C.; Shen, Y.-J.; Kuo, Y.-H.; Hwang, L.S. Antioxidative Activity and Active Components of Longan (Dimocarpus longan Lour.) Flower Extracts. J. Agric. Food Chem. 2008, 56, 7010–7016. [Google Scholar] [CrossRef]
- Lin, C.C.; Chung, Y.C.; Hsu, C.P. Potential roles of longan flower and seed extracts for anti-cancer. World J. Exp. Med. 2012, 2, 78–85. [Google Scholar] [CrossRef]
- Hsu, C.P.; Lin, Y.H.; Zhou, S.P.; Chung, Y.C.; Lin, C.C.; Wang, S.C. Longan flower extract inhibits the growth of colorectal carcinoma. Nutr. Cancer 2010, 62, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.J.; Chang, Y.Y.; Hsu, C.L.; Liu, C.W.; Lin, Y.L.; Lin, Y.H.; Liu, K.C.; Chen, Y.C. Antiobesity and hypolipidemic effects of polyphenol-rich longan (Dimocarpus longans Lour.) flower water extract in hypercaloric-dietary rats. J. Agric. Food Chem. 2010, 58, 2020–2027. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F.; Ashraf, M. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules 2009, 14, 2167–2180. [Google Scholar] [CrossRef]
- Hong-In, P.; Neimkhum, W.; Punyoyai, C.; Sriyab, S.; Chaiyana, W. Enhancement of phenolics content and biological activities of longan (Dimocarpus longan Lour.) treated with thermal and ageing process. Sci. Rep. 2021, 11, 15977. [Google Scholar] [CrossRef] [PubMed]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Dinh, M.N.; Hitomi, M.; Al-Turaihi, Z.A.; Scott, J.G. Alamar Blue assay optimization to minimize drug interference and inter assay viability. MethodsX 2024, 13, 103024. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Zhu, Z.; Chen, X.; Chen, S.; Hu, C.; Guo, R.; Wu, Y.; Liu, Z.; Shu, X.; Jiang, M. Examination of the mechanism of Piezo ion channel in 5-HT synthesis in the enterochromaffin cell and its association with gut motility. Front. Endocrinol. 2023, 14, 1193556. [Google Scholar] [CrossRef]
- Doihara, H.; Nozawa, K.; Kojima, R.; Kawabata-Shoda, E.; Yokoyama, T.; Ito, H. QGP-1 cells release 5-HT via TRPA1 activation; a model of human enterochromaffin cells. Mol. Cell Biochem. 2009, 331, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.C.; Yang, M.D.; Li, P.C.; Fang, H.Y.; Huang, H.Y.; Chan, Y.C.; Bau, D.T. Effect of Oligomeric Proanthocyanidin on the Antioxidant Status and Lung Function of Patients with Chronic Obstructive Pulmonary Disease. Vivo 2018, 32, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.Y.; Cai, Y.Z.; Zhang, Y. Natural phenolic compounds from medicinal herbs and dietary plants: Potential use for cancer prevention. Nutr. Cancer 2010, 62, 1–20. [Google Scholar] [CrossRef]
- Wang, W.; Ige, O.O.; Ding, Y.; He, M.; Long, P.; Wang, S.; Zhang, Y.; Wen, X. Insights into the potential benefits of triphala polyphenols toward the promotion of resilience against stress-induced depression and cognitive impairment. Curr. Res. Food Sci. 2023, 6, 100527. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.; Cheng, D.; Tao, J.Y.; Zhang, S.L.; Pang, R.; Guo, Y.J.; Ye, P.; Dong, J.H.; Zhao, L. Anti-inflammatory and anti-oxidative effects of corilagin in a rat model of acute cholestasis. BMC Gastroenterol. 2013, 13, 79. [Google Scholar] [CrossRef]
- Liu, F.-C.; Liao, C.-C.; Lee, H.-C.; Chou, A.-H.; Yu, H.-P. Effects of Corilagin on Lipopolysaccharide-Induced Acute Lung Injury via Regulation of NADPH Oxidase 2 and ERK/NF-κB Signaling Pathways in a Mouse Model. Biology 2022, 11, 1058. [Google Scholar] [CrossRef]
- Zamora, R.; Vodovotz, Y.; Billiar, T.R. Inducible Nitric Oxide Synthase and Inflammatory Diseases. Mol. Med. 2000, 6, 347–373. [Google Scholar] [CrossRef]
- Xue, Q.; Yan, Y.; Zhang, R.; Xiong, H. Regulation of iNOS on Immune Cells and Its Role in Diseases. Int. J. Mol. Sci. 2018, 19, 3805. [Google Scholar] [CrossRef]
- Batty, M.; Bennett, M.R.; Yu, E. The Role of Oxidative Stress in Atherosclerosis. Cells 2022, 11, 3843. [Google Scholar] [CrossRef]
- Michaeloudes, C.; Abubakar-Waziri, H.; Lakhdar, R.; Raby, K.; Dixey, P.; Adcock, I.M.; Mumby, S.; Bhavsar, P.K.; Chung, K.F. Molecular mechanisms of oxidative stress in asthma. Mol. Asp. Med. 2022, 85, 101026. [Google Scholar] [CrossRef]
- Chen, X.; Guo, C.; Kong, J. Oxidative stress in neurodegenerative diseases. Neural Regen. Res. 2012, 7, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef] [PubMed]
- Rath, M.F.; Coon, S.L.; Amaral, F.G.; Weller, J.L.; Møller, M.; Klein, D.C. Melatonin Synthesis: Acetylserotonin O-Methyltransferase (ASMT) Is Strongly Expressed in a Subpopulation of Pinealocytes in the Male Rat Pineal Gland. Endocrinology 2016, 157, 2028–2040. [Google Scholar] [CrossRef] [PubMed]




| Gene | Primer Forward Sequence | Primer Reverse Sequence |
|---|---|---|
| GAPDH | CTGGGCTACACTGAGCACC | AAGTGGTCGTTGAGGGCAATG |
| TPH1 | ACGTCGAAAGTATTTTGCGGA | ACGGTTCCCCAGGTCTTAATC |
| DDC | ACCACAACATGCTGCTCCTTT | ATCAACGTGCAGCCATATGTCT |
| AANAT | TGTCAGCGCCTTTGAGATCG | CTCTGGACATAGGGTCAGGAA |
| ASMT | GGGCAGACGGAAAGTGCTC | CTGCACAAGCATGTTCAGAGA |
| Test Items (Unit) | Value |
|---|---|
| Total phenolic content (mg/g) | 171.4 ± 5.9 |
| Total flavonoid (mg/g) | 233.3 ± 16.7 |
| Oligomeric proanthocyanidins (OPCs) (mg/g) | 15.9 ± 2.1 |
| Corilagin (mg/g) | 37.7 ± 4.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, C.-C.; Hsieh, M.-C.; Chiang, W.-L.; Chen, Y.-W.; Huang, P.-C.; Yu, C.-H.; Lee, S.-Y.; Chung, T.-C.; Lu, H.-C.; Chang, Y.-W. Longan Flower Ethanol Extract, Dimocarpus longan Lour, Mitigates Oxidative Damage and Inflammatory Responses While Promoting Sleep-Related Enzymes in Cell Models. Biomedicines 2025, 13, 1588. https://doi.org/10.3390/biomedicines13071588
Ma C-C, Hsieh M-C, Chiang W-L, Chen Y-W, Huang P-C, Yu C-H, Lee S-Y, Chung T-C, Lu H-C, Chang Y-W. Longan Flower Ethanol Extract, Dimocarpus longan Lour, Mitigates Oxidative Damage and Inflammatory Responses While Promoting Sleep-Related Enzymes in Cell Models. Biomedicines. 2025; 13(7):1588. https://doi.org/10.3390/biomedicines13071588
Chicago/Turabian StyleMa, Chao-Chun, Ming-Chang Hsieh, Wei-Lun Chiang, Yi-Wen Chen, Pin-Chao Huang, Chin-Hsiu Yu, Shao-Yu Lee, Tin-Ching Chung, Hsi-Chi Lu, and Yu-Wei Chang. 2025. "Longan Flower Ethanol Extract, Dimocarpus longan Lour, Mitigates Oxidative Damage and Inflammatory Responses While Promoting Sleep-Related Enzymes in Cell Models" Biomedicines 13, no. 7: 1588. https://doi.org/10.3390/biomedicines13071588
APA StyleMa, C.-C., Hsieh, M.-C., Chiang, W.-L., Chen, Y.-W., Huang, P.-C., Yu, C.-H., Lee, S.-Y., Chung, T.-C., Lu, H.-C., & Chang, Y.-W. (2025). Longan Flower Ethanol Extract, Dimocarpus longan Lour, Mitigates Oxidative Damage and Inflammatory Responses While Promoting Sleep-Related Enzymes in Cell Models. Biomedicines, 13(7), 1588. https://doi.org/10.3390/biomedicines13071588







