Evaluation of the Diagnosis and Antibiotic Therapy of Sepsis in the Emergency Department: A Retrospective Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
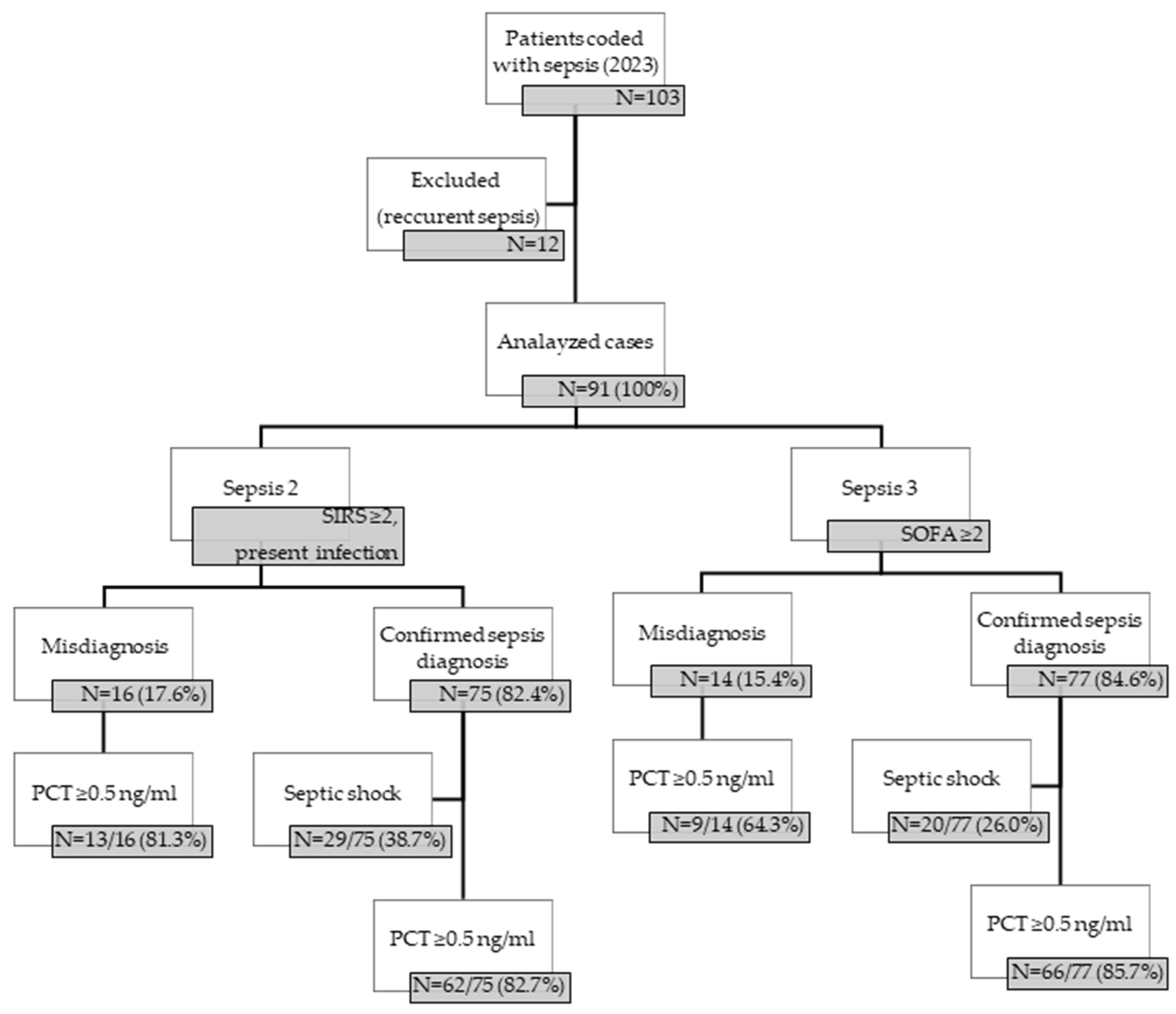
2.2. Inclusion and Exclusion Criteria
2.3. Data Collection
2.4. Main Outcome Measures
2.5. Statistical Analyses
3. Results
3.1. Risk Factors and Sources of Infection
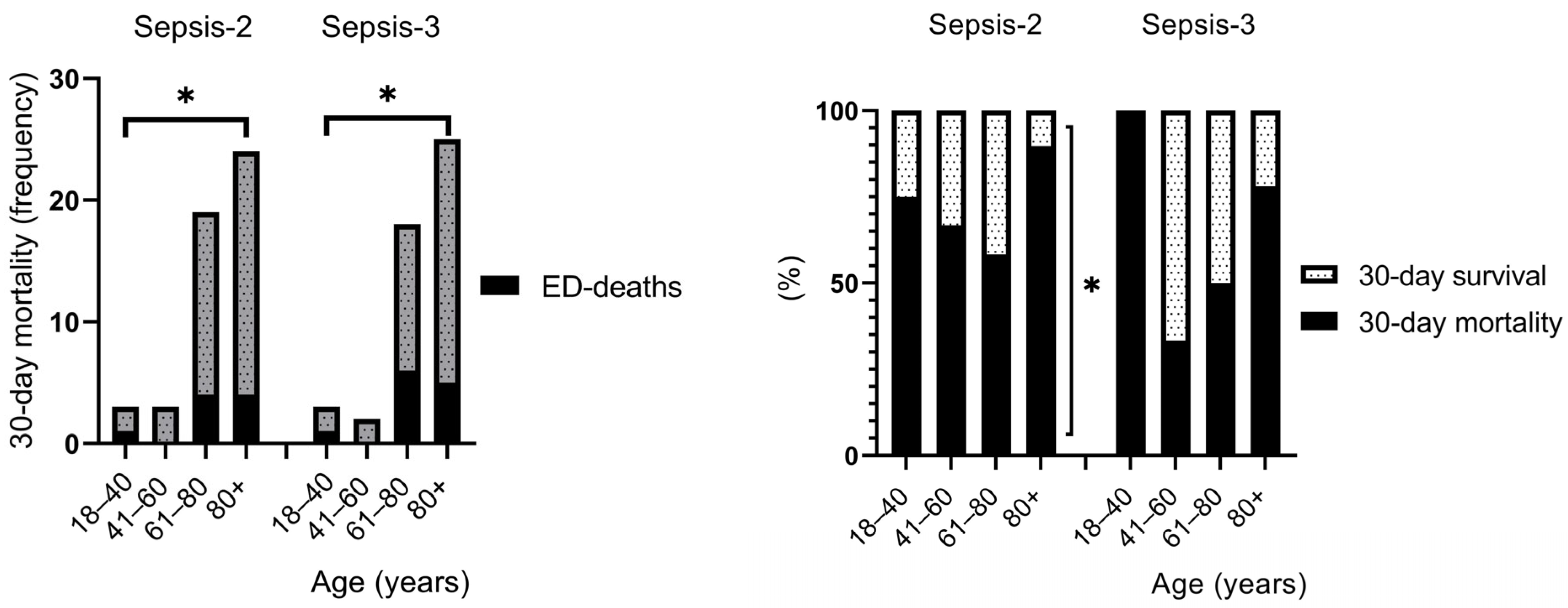
3.2. Risk Factors and Clinical Outcomes
3.3. Triage Flow (Triage Duration) and Clinical Outcomes
3.4. Empirical Antibiotic Administration and Clinical Outcomes
3.5. Investigated Laboratory Parameters in Sepsis
4. Discussion
4.1. Risk Factors and Clinical Outcomes
4.2. Triage Flow (Triage Duration) and Clinical Outcomes
4.3. Empirical Antibiotic Administration and Clinical Outcomes
4.4. Investigated Laboratory Parameters in Sepsis
4.5. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CCI | Charlson Comorbidity Index |
| ECDC | European Center for Disease Prevention and Control |
| ED | Emergency Department |
| HAI | Hospital-acquired Infection |
| HETS | Hungarian Emergency Triage System |
| ICU | Intensive Care Unit |
| LOS | Length of Stay |
| qSOFA | Quick Sequential Organ Failure Assessment |
| RTI | Respiratory Tract Infection |
| Sepsis-2 | International Sepsis Definitions Conference, 2001 |
| Sepsis-3 | Third International Consensus Conference 2016 |
| SIRS | Systemic Inflammatory Response Syndrome |
| SOFA | Sequential Organ Failure Assessment |
| SSTI | Skin and Soft Tissue Infection |
| UTI | Urinary Tract Infection |
References
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, Regional, and National Sepsis Incidence and Mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef]
- Fleischmann, C.; Scherag, A.; Adhikari, N.K.; Hartog, C.S.; Tsaganos, T.; Schlattmann, P.; Angus, D.C.; Reinhart, K. Assessment of Global Incidence and Mortality of Hospital-Treated Sepsis. Current Estimates and Limitations. Am. J. Respir. Crit. Care Med. 2016, 193, 259–272. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Report on the Epidemiology and Burden of Sepsis: Current Evidence, Identifying Gaps and Future Directions; World Health Organization: Geneva, Switzerland, 2020; p. 12.
- Surveillance Report. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/healthcare-associated-infections-acquired-intensive-care-units-2021.pdf (accessed on 28 March 2025).
- Yealy, D.M.; Mohr, N.M.; Shapiro, N.I.; Venkatesh, A.; Jones, A.E.; Self, W.H. Early Care of Adults with Suspected Sepsis in the Emergency Department and out-of-Hospital Environment: A Consensus-Based Task Force Report. Ann. Emerg. Med. 2021, 78, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Luka, S.; Golea, A.; Vesa, S.C.; Leahu, C.E.; Zaganescu, R.; Ionescu, D. Can We Improve Mortality Prediction in Patients with Sepsis in the Emergency Department? Medicina 2024, 60, 1333. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Spoto, S.; Nobile, E.; Carna, E.P.R.; Fogolari, M.; Caputo, D.; De Florio, L.; Valeriani, E.; Benvenuto, D.; Costantino, S.; Ciccozzi, M.; et al. Best Diagnostic Accuracy of Sepsis Combining Sirs Criteria or Qsofa Score with Procalcitonin and Mid-Regional Pro-Adrenomedullin Outside Icu. Sci. Rep. 2020, 10, 16605. [Google Scholar] [CrossRef]
- Seymour, C.W.; Liu, V.X.; Iwashyna, T.J.; Brunkhorst, F.M.; Rea, T.D.; Scherag, A.; Rubenfeld, G.; Kahn, J.M.; Shankar-Hari, M.; Singer, M.; et al. Assessment of Clinical Criteria for Sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 762–774. [Google Scholar] [CrossRef]
- Bidart, J.P.M.; Rosa, R.G.; Bessel, M.; Pedrotti, L.G.; Goldani, L.Z. Mortality Predictors in Patients with Suspected Sepsis in the Emergency Department of a Tertiary Care Hospital: A Retrospective Cohort Study. Int. J. Emerg. Med. 2024, 17, 74. [Google Scholar] [CrossRef]
- Gaddis, M.L.; Gaddis, G.M. Detecting Sepsis in an Emergency Department: Sirs Vs. Qsofa. Mo. Med. 2021, 118, 253–258. [Google Scholar]
- Tong-Minh, K.; Welten, I.; Endeman, H.; Hagenaars, T.; Ramakers, C.; Gommers, D.; van Gorp, E.; van der Does, Y. Predicting Mortality in Adult Patients with Sepsis in the Emergency Department by Using Combinations of Biomarkers and Clinical Scoring Systems: A Systematic Review. BMC Emerg. Med. 2021, 21, 70. [Google Scholar] [CrossRef]
- Arefian, H.; Heublein, S.; Scherag, A.; Brunkhorst, F.M.; Younis, M.Z.; Moerer, O.; Fischer, D.; Hartmann, M. Hospital-Related Cost of Sepsis: A Systematic Review. J. Infect. 2017, 74, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Pruinelli, L.; Westra, B.L.; Yadav, P.; Hoff, A.; Steinbach, M.; Kumar, V. Delay within the 3-Hour Surviving Sepsis Campaign Guideline on Mortality for Patients with Severe Sepsis and Septic Shock. Crit. Care Med. 2018, 46, 500–505. [Google Scholar] [CrossRef]
- Liu, V.X.; Fielding-Singh, V.; Greene, J.D.; Baker, J.M.; Iwashyna, T.J.; Bhattacharya, J.; Escobar, G.J. The Timing of Early Antibiotics and Hospital Mortality in Sepsis. Am. J. Respir. Crit. Care Med. 2017, 196, 856–863. [Google Scholar] [CrossRef]
- van Zanten, A.R.; Brinkman, S.; Arbous, M.S.; Abu-Hanna, A.; Levy, M.M.; de Keizer, N.F. Guideline Bundles Adherence and Mortality in Severe Sepsis and Septic Shock. Crit. Care Med. 2014, 42, 1890–1898. [Google Scholar] [CrossRef]
- Kumar, H.G.; Kanakaraju, K.; Manikandan, V.A.C.; Patel, V.; Pranay, C. The Relationship between Serum Albumin Levels and Sepsis in Patients Admitted to a Tertiary Care Center in India. Cureus 2024, 16, e59424. [Google Scholar] [CrossRef]
- Cao, Y.; Su, Y.; Guo, C.; He, L.; Ding, N. Albumin Level Is Associated with Short-Term and Long-Term Outcomes in Sepsis Patients Admitted in the Icu: A Large Public Database Retrospective Research. Clin. Epidemiol. 2023, 15, 263–273. [Google Scholar] [CrossRef]
- Ulldemolins, M.; Roberts, J.A.; Rello, J.; Paterson, D.L.; Lipman, J. The Effects of Hypoalbuminaemia on Optimizing Antibacterial Dosing in Critically Ill Patients. Clin. Pharmacokinet. 2011, 50, 99–110. [Google Scholar] [CrossRef]
- Neilson, H.K.; Fortier, J.H.; Finestone, P.J.; Ogilby, C.M.; Liu, R.; Bridges, E.J.; Garber, G.E. Diagnostic Delays in Sepsis: Lessons Learned from a Retrospective Study of Canadian Medico-Legal Claims. Crit. Care Explor. 2023, 5, e0841. [Google Scholar] [CrossRef]
- Gupta, S.; Jaswani, P.; Sharma, R.K.; Agrawal, S.; Prasad, N.; Sahu, C.; Gupta, A.; Prasad, K.N. Procalcitonin as a Diagnostic Biomarker of Sepsis: A Tertiary Care Centre Experience. J. Infect. Public Health 2019, 12, 323–329. [Google Scholar] [CrossRef]
- Hall, M.J.; Williams, S.J.; DeFrances, C.J.; Golosinskiy, A. Inpatient Care for Septicemia or Sepsis: A Challenge for Patients and Hospitals. Natl. Cent. Health Stat. 2011, 65, 1–8. [Google Scholar]
- Fathi, M.; Markazi-Moghaddam, N.; Ramezankhani, A. A Systematic Review on Risk Factors Associated with Sepsis in Patients Admitted to Intensive Care Units. Aust. Crit. Care 2019, 32, 155–164. [Google Scholar] [CrossRef]
- Reaven, M.S.; Rozario, N.L.; McCarter, M.S.J.; Heffner, A.C. Incidence and Risk Factors Associated with Early Death in Patients with Emergency Department Septic Shock. Acute Crit. Care 2022, 37, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Shibata, J.; Osawa, I.; Ito, H.; Soeno, S.; Hara, K.; Sonoo, T.; Nakamura, K.; Goto, T. Risk Factors of Sepsis among Patients with Qsofa<2 in the Emergency Department. Am. J. Emerg. Med. 2021, 50, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Baggs, J.; Jernigan, J.A.; Halpin, A.L.; Epstein, L.; Hatfield, K.M.; McDonald, L.C. Risk of Subsequent Sepsis within 90 Days after a Hospital Stay by Type of Antibiotic Exposure. Clin. Infect. Dis. 2018, 66, 1004–1012. [Google Scholar] [CrossRef] [PubMed]
- Hayden, G.E.; Tuuri, R.E.; Scott, R.; Losek, J.D.; Blackshaw, A.M.; Schoenling, A.J.; Nietert, P.J.; Hall, G.A. Triage Sepsis Alert and Sepsis Protocol Lower Times to Fluids and Antibiotics in the Ed. Am. J. Emerg. Med. 2016, 34, 1–9. [Google Scholar] [CrossRef]
- Rosenqvist, M.; Bengtsson-Toni, M.; Tham, J.; Lanbeck, P.; Melander, O.; Akesson, P. Improved Outcomes after Regional Implementation of Sepsis Alert: A Novel Triage Model. Crit. Care Med. 2020, 48, 484–490. [Google Scholar] [CrossRef]
- Grosman-Rimon, L.; Rivlin, L.; Spataro, R.; Zhu, Z.; Casey, J.; Tory, S.; Solanki, J.; Wegier, P. Trend of Mortality and Length of Stay in the Emergency Department Following Implementation of a Centralized Sepsis Alert System. Digit Health 2024, 10, 20552076241250255. [Google Scholar] [CrossRef]
- Mitzkewich, M. Sepsis Screening in Triage to Decrease Door-to-Antibiotic Time. J. Emerg. Nurs. 2019, 45, 254–256. [Google Scholar] [CrossRef]
- Molnar, G.; Gyarmathy, V.A.; Takacs, J.; Sandor, S.; Kiss, B.; Fazakas, J.; Kanizsai, P.L. Differentiating Sepsis from Similar Groups of Symptoms at Triage Level in Emergency Care. Physiol. Int. 2021, 108, 106–120. [Google Scholar] [CrossRef]
- D’Onofrio, V.; Meersman, A.; Magerman, K.; Waumans, L.; van Halem, K.; Cox, J.A.; van der Hilst, J.C.; Cartuyvels, R.; Messiaen, P.; Gyssens, I.C. Audit of Empirical Antibiotic Therapy for Sepsis and the Impact of Early Multidisciplinary Consultation on Patient Outcomes. Int. J. Antimicrob. Agents 2021, 58, 106379. [Google Scholar] [CrossRef]
- Joo, Y.M.; Chae, M.K.; Hwang, S.Y.; Jin, S.C.; Lee, T.R.; Cha, W.C.; Jo, I.J.; Sim, M.S.; Song, K.J.; Jeong, Y.K.; et al. Impact of Timely Antibiotic Administration on Outcomes in Patients with Severe Sepsis and Septic Shock in the Emergency Department. Clin. Exp. Emerg. Med. 2014, 1, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Siewers, K.; Abdullah, S.; Sorensen, R.H.; Nielsen, F.E. Time to Administration of Antibiotics and Mortality in Sepsis. J. Am. Coll. Emerg. Physicians Open 2021, 2, e12435. [Google Scholar] [CrossRef] [PubMed]
- Seok, H.; Song, J.; Jeon, J.H.; Choi, H.K.; Choi, W.S.; Moon, S.; Park, D.W. Timing of Antibiotics in Septic Patients: A Prospective Cohort Study. Clin. Microbiol. Infect. 2020, 26, 1495–1500. [Google Scholar] [CrossRef]
- Lindberg, O.; De Geer, L.; Chew, M.S. Nonadherence to Antibiotic Guidelines in Patients Admitted to Icu with Sepsis Is Associated with Increased Mortality: A Registry-Based, Retrospective Cohort Study. Eur. J. Anaesthesiol. 2020, 37, 113–120. [Google Scholar] [CrossRef]
- Lu, J.; Wei, Z.; Jiang, H.; Cheng, L.; Chen, Q.; Chen, M.; Yan, J.; Sun, Z. Lactate Dehydrogenase Is Associated with 28-Day Mortality in Patients with Sepsis: A Retrospective Observational Study. J. Surg. Res. 2018, 228, 314–321. [Google Scholar] [CrossRef]
- Qian, X.; Sheng, Y.; Jiang, Y.; Xu, Y. Association between Lactate Dehydrogenase and Ventilator-Associated Pneumonia Risk: An Analysis of the Mimic Database 2001–2019. BMC Pulm. Med. 2024, 24, 273. [Google Scholar] [CrossRef]
- Xiao, Y.; He, S.; Cheng, X.; Peng, L.; Tian, Y.; Li, T.; He, J.; Hao, P.; Chong, W.; Hai, Y.; et al. Elevated Lactate Dehydrogenase Predicts Pneumonia in Spontaneous Intracerebral Hemorrhage. Heliyon 2024, 10, e26109. [Google Scholar] [CrossRef]



| Source of Infection | Guideline-Adherent Agent Selection |
|---|---|
| Respiratory tract infections (RTIs) | beta lactam + macrolide ceftriaxone + clarithromycin/azithromycin |
| Urinary tract infections (UTIs) | beta lactam + aminoglycoside ceftriaxone ± amikacin |
| Skin and soft tissue infection (SSTIs) | beta lactams |
| Endocarditis | beta lactam + beta lactamase inhibitor amoxicillin + clavulanic acid |
| Abdominal infections | beta lactam + nitroimidazole ceftriaxone ± metronidazole |
| Parameters | Confirmed Sepsis Diagnosis | p-Values | |||
|---|---|---|---|---|---|
| Sepsis-2 | Sepsis-3 | ||||
| N = 75 | 100% | N = 77 | 100% | ||
| Gender (Male) | 40 | 53.3 | 37 | 48.1 | 0.780 |
| Age | |||||
| 18–40 years | 4 | 5.3 | 3 | 3.9 | 0.720 |
| 41–60 years | 6 | 8.3 | 6 | 7.8 | 1.000 |
| 61–80 years | 36 | 48.0 | 36 | 46.8 | 1.000 |
| 80+ years | 29 | 38.7 | 32 | 41.6 | 0.880 |
| Antibiotic allergy | 9 | 12.0 | 8 | 10.4 | 0.804 |
| CCI-Charlson Comorbidity Index | |||||
| 0 | 2 | 2.7 | 1 | 1.3 | 0.620 |
| 1 | 2 | 2.7 | 2 | 0.3 | 1.000 |
| 2 | 4 | 5.3 | 4 | 5.2 | 1.000 |
| 3 | 2 | 2.7 | 3 | 3.9 | 1.000 |
| 4 | 8 | 10.7 | 7 | 9.1 | 0.794 |
| >4 | 57 | 76.0 | 60 | 77.9 | 1.000 |
| Hospitalization in the last month | 27 | 36.0 | 26 | 33.8 | 0.874 |
| Source of infection | |||||
| Respiratory tract infections (RTIs) | 25 | 33.3 | 27 | 35.1 | 1.000 |
| Urinary tract infections (UTIs) | 28 | 37.3 | 26 | 33.8 | 0.874 |
| Mixed (RTI and UTI) | 13 | 17.3 | 13 | 16.9 | 1.000 |
| Skin and soft tissue infection (SSTIs) | 7 | 9.3 | 6 | 7.8 | 0.781 |
| Endocarditis | 1 | 1.3 | 1 | 1.3 | 1.000 |
| Abdominal infections | 1 | 1.3 | 2 | 2.6 | 1.000 |
| Not known | 0 | 0 | 2 | 2.6 | 0.497 |
| Parameters | Sepsis-2 | Sepsis-3 | p-Values | Sepsis-2 | Sepsis-3 | p-Values | ||
|---|---|---|---|---|---|---|---|---|
| N = 75 | 100% | N = 77 | 100% | CCI (Mean ± SD, Median) | ||||
| Discharged home | 6 | 8 | 5 | 6.5 | 0.766 | 6.50 ± 2.07 (6) | 6.80 ± 2.17 (6) | 0.820 |
| Moved to another hospital ward | 49 | 65.3 | 49 | 63.6 | 1 | 6.49 ± 3.24 (6) | 6.33 ± 3.02 (6) | 0.797 |
| Removed to ICU | 11 | 14.6 | 11 | 14.3 | 1 | 5.46 ± 3.05 (7) | 5.55 ± 3.05 (7) | 1.000 |
| Death in the ED | 9 | 12.0 | 12 | 15.6 | 0.646 | 7.44 ± 2.30 (7) | 7.08 ± 2.33 (7) | 0.721 |
| 18–40 years | 1/4 | 25.0 | 1/3 | 33.3 | 1 | 9.00 ± 0.00 (9) | 9.00 ± 0.00 (9) | 1.000 |
| 41–60 years | 0/6 | 0.0 | 0/6 | 0.0 | 1 | - | - | - |
| 61–80 years | 4/36 | 11.1 | 6/36 | 16.7 | 0.738 | 7.50 ± 3.51 (7.5) | 6.67 ± 3.08 (5.5) | 0.701 |
| 80+ years | 4/29 | 13.8 | 5/32 | 15.6 | 1 | 7.00 ± 0.82 (7) | 7.20 ± 0.84 (7) | 0.729 |
| 30-day mortality | 49 | 65.3 | 48 | 62.3 | 0.897 | 6.67 ± 2.92 (7) | 6.75 ± 2.88 (7) | 0.897 |
| 18–40 years | 3/4 | 75.0 | 3/3 | 100.0 | 1 | 3.33 ± 4.93 (1) | 3.33 ± 4.93 (1) | 1.000 |
| 41–60 years | 3/6 | 50.0 | 2/6 | 33.33 | 1 | 2.33 ± 1.16 (3) | 2.00 ± 1.41 (2) | 0.789 |
| 61–80 years | 19/36 | 52.8 | 18/36 | 50.0 | 1 | 7.21 ± 2.70 (7) | 7.17 ± 2.71 (7) | 0.961 |
| 80+ years | 24/29 | 82.8 | 25/32 | 78.1 | 1 | 7.21 ± 2.32 (7) | 7.24 ± 2.28 (7) | 0.962 |
| 90- and 180-day mortality | 58 | 77.3 | 57 | 74.0 | 0.902 | 6.81 ± 3.05 (7) | 6.75 ± 2.92 (7) | 0.932 |
| 18–40 years | 3/4 | 75.0 | 3/3 | 100 | 1 | 3.33 ± 4.93 (1) | 3.33 ± 4.93 (1) | 1.000 |
| 41–60 years | 4/6 | 66.7 | 3/6 | 50.0 | 1 | 2.75 ± 1.26 (3) | 2.00 ± 1.41 (2) | 0.667 |
| 61–80 years | 24/36 | 66.7 | 23/36 | 63.9 | 1 | 7.25 ± 2.86 (7) | 6.91 ± 2.59 (7) | 0.659 |
| 80+ years | 27/29 | 93.1 | 28/32 | 87.5 | 1 | 7.41 ± 2.56 (7) | 7.43 ± 2.52 (7) | 0.909 |
| LOS 30-day mortality (mean ± SD, median-days) | 4.31 ± 5.39 (1) | 3.60 ± 5.03 (1) | 0.509 | 6.67 ± 2.92 (7) | 6.75 ± 2.88 (7) | 0.897 | ||
| LOS 30-day survival group (mean ± SD, median-days) | 22.88 ± 20.36 (16) | 22.93 ± 21.61 (14) | 0.994 | 6.08 ± 3.25 (6) | 5.72 ± 2.75 (5) | 0.665 | ||
| 18–40 years | 6.00 ± 0.00 (6) | - | - | - | - | - | ||
| 41–60 years | 52.00 ± 41.22 (69) | 40.75 ± 40.48 (38) | 0.732 | 5.33 ± 4.16 (4) | 4.50 ± 3.79 (3) | 0.793 | ||
| 61–80 years | 22.53 ± 14.23 (17) | 23.67 ± 18.4 (17.5) | 0.840 | 6.06 ± 2.79 (6) | 5.56 ± 2.31 (5.5) | 0.564 | ||
| 80+ years | 10.00 ± 4.06 (8) | 10.86 ± 4.26 (10) | 0.734 | 7.80 ± 3.56 (7) | 6.86 ± 3.24 (6) | 0.643 | ||
| Parameters Sepsis 2 (N = 75) Sepsis 3 (N = 77) | Triage Category | ||||
|---|---|---|---|---|---|
| I. N = 3 (4.0%) N = 4 (5.2%) | II. N = 21 (28.0%) N = 23 (29.9%) | III. N = 43 (57.3%) N = 44 (57.1%) | IV. N = 8 (10.7%) N = 6 (7.8%) | p-Values 1.000 | |
| Triage duration (mean ± SD, median in minutes) Sepsis 2 Sepsis 3 | 19.67 ± 9.74 (16) 15.5 ± 11.10 (13) | 11.05 ± 14.23 (6) 10.43 ± 13.83 (6) | 13.47 ± 23.83 (8) 13.0 ± 23.64 (8) | 11.50 ± 5.32 (13.5) 9.33 ± 4.46 (9) | 0.522 |
| ED-LOS (mean ± SD, median in hours) Sepsis 2 Sepsis 3 | 7.25 ± 5.30 (7.4) 5.25 ± 5.21 (4) | 4.80 ± 3.38 (4.2) 4.65 ± 3.04 (5) | 6.34 ± 3.56 (5.2) 5.80 ± 3.53 (5) | 7.03 ± 6.48 (4.5) 4.0 ± 1.63 (4.5) | 0.393 |
| ED-mortality | |||||
| Sepsis 2 | 3/3 (100%) | 2/21 (9.5%) | 4/43 (9.3%) | 0/8 (0%) | 0.955 |
| Sepsis 3 | 4/4 (100%) | 4/23 (17.4%) | 4/44 (9.1%) | 0/6 (0%) | |
| Total LOS (mean ± SD, median in days) Sepsis 2 Sepsis 3 | 0.33 ± 0.47 (0) 0.25 ± 0.43 (0) | 6.33 ± 11.37 (1) 8.61 ± 16.42 (1) | 13.93 ± 17.76 (10) 13.32 ± 17.74 (9) | 9.13 ± 4.20 (8.5) 8.83 ± 4.22 (7.5) | 0.888 |
| 30-day mortality Sepsis 2 Sepsis 3 | 3/3 (100%) 4/4 (100%) | 16/21 (76.2%) 16/23 (69.6%) | 31/43 (72.1%) 27/44 (61.4%) | 4/8 (50.0%) 2/6 (33.3%) | 0.891 |
| 90- and 180-day mortality Sepsis 2 Sepsis 3 | 3/3 (100%) 4/4 (100%) | 18/21 (85.7%) 18/23 (82.6%) | 32/43 (74.4%) 32/44 (72.7%) | 5/8 (62.5%) 2/6 (33.3%) | 0.761 |
| Parameters | Frequency Sepsis 2: N = 75, 100% Sepsis 3: N = 77, 100% | 30-Day Mortality Sepsis 2 Sepsis 3 | p-Values | 90- and 180-Day Mortality Sepsis 2 Sepsis 3 | p-Values | LOS (Mean ± SD, Median-Days) Sepsis 2 Sepsis 3 | p-Values |
|---|---|---|---|---|---|---|---|
| Antibiotic therapy | |||||||
| no antibiotic therapy at the ED | 32 (42.7%) 31 (40.3%) | 22/32 (68.8%) 21/31 (67.7%) | 0.678 | 25/32 (78.1%) 24/31 (77.4%) | 0.434 | 7.44 ± 7.52 (6.5) 7.48 ± 8.54 (4) | 0.860 |
| antibiotic therapy at the ED | 43 (57.3%) 46 (59.7%) | 27/43 (62.8%) 27/46 (58.7%) | 33/43 (76.7%) 33/46 (71.7%) | 13.21 ± 18.83 (5) 13.17 ± 19.82 (5) | |||
| Administration time | Sepsis-2: N = 43 (100%) Sepsis-3: N = 46 (100%) | ||||||
| within 3 h | 4/43 (9.3%) 3/46 (6.5%) | 2/4 (50.0%) 2/3 (66.6%) | 0.800 | 3/4 (75.0%) 3/3 (100%) | 0.342 | 6.00 ± 7.66 (4) 2.67 ± 4.62 (0) | 0.382 |
| between 3 and 6 h | 20/43 (46.5%) 22/46 (47.8%) | 13/20 (65.0%) 12/22 (54.5%) | 17/20 (85.0%) 16/22 (72.7%) | 16.00 ± 23.55 (4.5) 16.77 ± 24.88 (6) | |||
| between 6 and 24 h | 19/43 (44.2%) 21/46 (45.7%) | 12/19 (63.2%) 13/21 (61.9%) | 13/19 (68.42%) 14/19 (73.7%) | 11.79 ± 15.15 (6) 10.90 ± 14.67 (5) | |||
| Parameters | No AB Therapy | Guideline Adherent AB Therapy | Guideline Non-Adherent AB Therapy | p-Values |
|---|---|---|---|---|
| Frequency | ||||
| Sepsis 2 | 32/75 (42.7%) | 18/43 (41.9%) | 25/43 (58.1%) | <0.001 ** |
| Sepsis 3 | 31/77 (40.6% | 19/46 (41.3%) | 27/46 (58.7%) | |
| Death at the ED | ||||
| Sepsis 2 | 5/32 (15.6%) | 2/18 (11.1%) | 2/25 (8.0%) | 0.136 |
| Sepsis 3 | 7/31 (22.6%) | 3/19 (15.8%) | 2/27 (7.4%) | |
| 30-day mortality | ||||
| Sepsis 2 | 22/31 (71.0%) | 9/19 (47.4%) | 18/26 (69.2%) | 0.058 |
| Sepsis 3 | 21/31 (67.7%) | 10/19 (52.6%) | 17/27 (63.0%) | |
| 90- and 180-day mortality | ||||
| Sepsis 2 | 25/31 (80.6%) | 11/19 (57.9%) | 22/26 (84.6%) | 0.041 * |
| Sepsis 3 | 24/31 (77.4%) | 12/19 (63.2%) | 21/27 (77.8%) | |
| LOS (mean ± SD, median-days) | ||||
| Sepsis 2 | 7.43 ± 7.54 (6.5) | 13.72 ± 18.03 (7) | 12.84 ± 19.71 (3) | 0.051 |
| Sepsis 3 | 7.48 ± 8.85 (4) | 11.26 ± 16.98 (6) | 14.52 ± 21.49 (4) | |
| Probability of survival | ||||
| Log-rank (Mantel–Cox) test | X2 = 3.342 | |||
| (median survival—days) | X2 = 4.131 | |||
| Sepsis 2 | 11 vs. 37 vs. 4 | 0.181 | ||
| Sepsis 3 | 9 vs. 37 vs. 4 | 0.042 * | ||
| Parameters (Mean ± SD, Median) | Confirmed Sepsis Diagnosis Sepsis 2 (N = 75) Sepsis 3 (N = 77) | ||
|---|---|---|---|
| 30-Day Mortality N = 49/75 N = 48/77 | 30-Day Survival N = 26/75 N = 29/77 | p-Values | |
| PCT (ng/mL) | 27.80 ± 83.35 (3.8) 33.33 ± 87.49 (4.9) | 76.95 ± 228.1 (3.8) 110.2 ± 255.9 (2.9) | 0.191 0.067 |
| WBC (G/L) | 18.98 ± 16.73 (17.1) 19.57 ± 17.51 (17.4) | 19.63 ± 18.41 (16.8) 18.19 ± 16.91 (15.2) | 0.989 0.735 |
| CRP (mg/dL) | 217.2 ± 129.5 (195.3) 231.2 ± 131.7 (205.6) | 169.0 ± 124.1 (141.3) 205.0 ± 134.5 (179.4) | 0.149 0.405 |
| LDH (mg/dL) | 393.00 ± 326.5 (267) 458.1 ± 367.0 (325) | 252.5 ± 82.50 (255) 236.6 ± 84.99 (227) | 0.071 0.004 * |
| Albumin (g/L) | 26.12 ± 6.62 (26) 26.21 ± 6.06 (26) | 29.81 ± 6.06 (30) 28.55 ± 7.41 (28) | 0.050 * 0.191 |
| lactate (mmol/L) | 5.11 ± 4.02 (3.5) 5.17 ± 3.94 (4) | 3.59 ± 2.99 (2.4) 3.34 ± 2.92 (2.4) | 0.170 0.067 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varga, E.; Somodi, S.; Molnár, M.; Ujvárosy, D.; Gaál, K.; Vaskó, A.; Szabó, Z.; Bácskay, I.; Lekli, I.; Fésüs, A. Evaluation of the Diagnosis and Antibiotic Therapy of Sepsis in the Emergency Department: A Retrospective Observational Study. Biomedicines 2025, 13, 1566. https://doi.org/10.3390/biomedicines13071566
Varga E, Somodi S, Molnár M, Ujvárosy D, Gaál K, Vaskó A, Szabó Z, Bácskay I, Lekli I, Fésüs A. Evaluation of the Diagnosis and Antibiotic Therapy of Sepsis in the Emergency Department: A Retrospective Observational Study. Biomedicines. 2025; 13(7):1566. https://doi.org/10.3390/biomedicines13071566
Chicago/Turabian StyleVarga, Eszter, Sándor Somodi, Máté Molnár, Dóra Ujvárosy, Krisztina Gaál, Attila Vaskó, Zoltán Szabó, Ildikó Bácskay, István Lekli, and Adina Fésüs. 2025. "Evaluation of the Diagnosis and Antibiotic Therapy of Sepsis in the Emergency Department: A Retrospective Observational Study" Biomedicines 13, no. 7: 1566. https://doi.org/10.3390/biomedicines13071566
APA StyleVarga, E., Somodi, S., Molnár, M., Ujvárosy, D., Gaál, K., Vaskó, A., Szabó, Z., Bácskay, I., Lekli, I., & Fésüs, A. (2025). Evaluation of the Diagnosis and Antibiotic Therapy of Sepsis in the Emergency Department: A Retrospective Observational Study. Biomedicines, 13(7), 1566. https://doi.org/10.3390/biomedicines13071566







