Cytotoxic Molecules as Potential Biomarkers for Active and Inactive Systemic Lupus Erythematosus
Abstract
1. Introduction
2. Materials and Methods
2.1. Participant Recruitment
2.2. PBMC Collection
2.3. Serum Collection
2.4. Quantification of Molecules
2.5. CD8+ T-Cell Staining
2.6. Biomarker Association
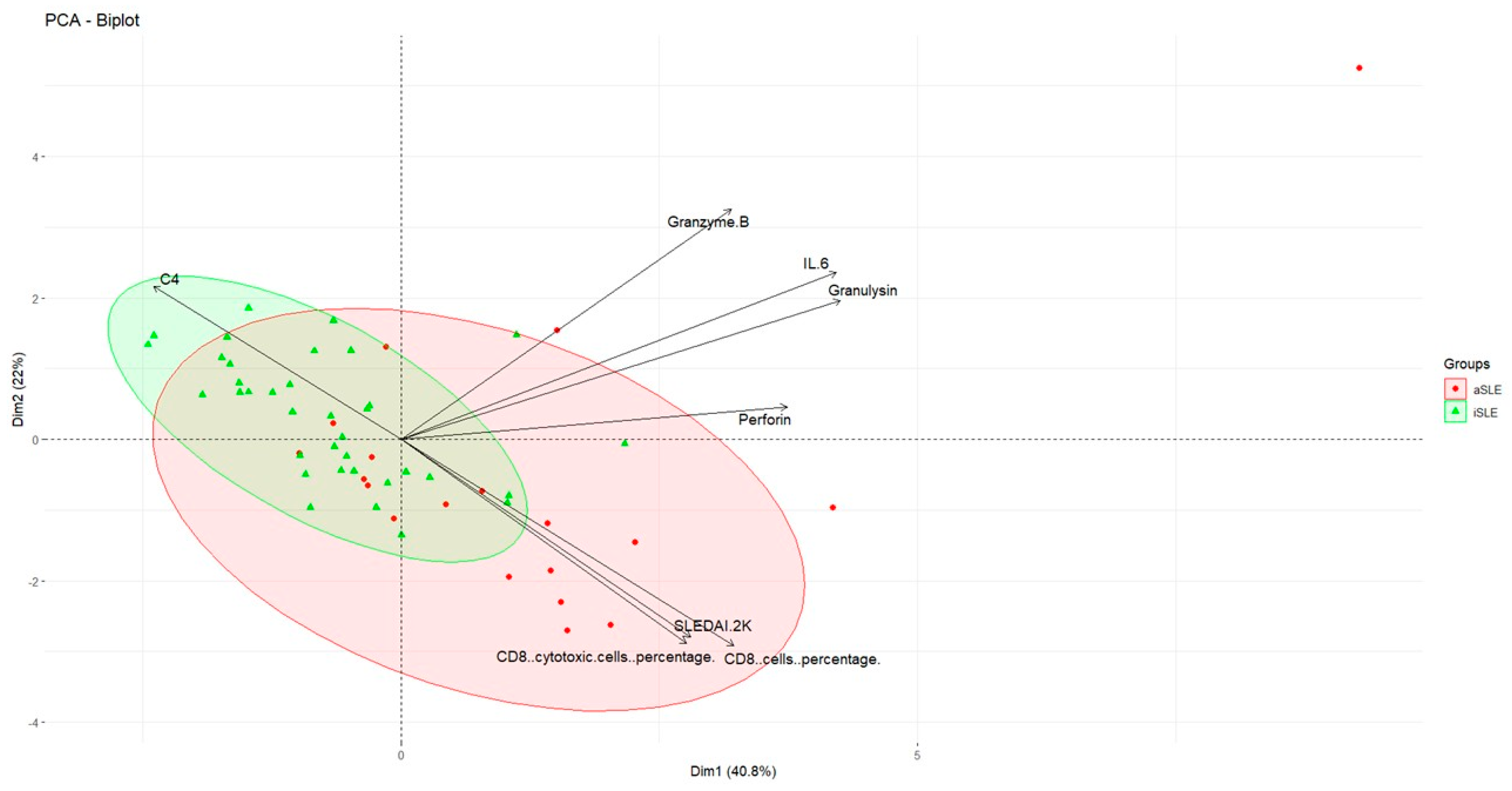
2.7. PCA-Based Clustering
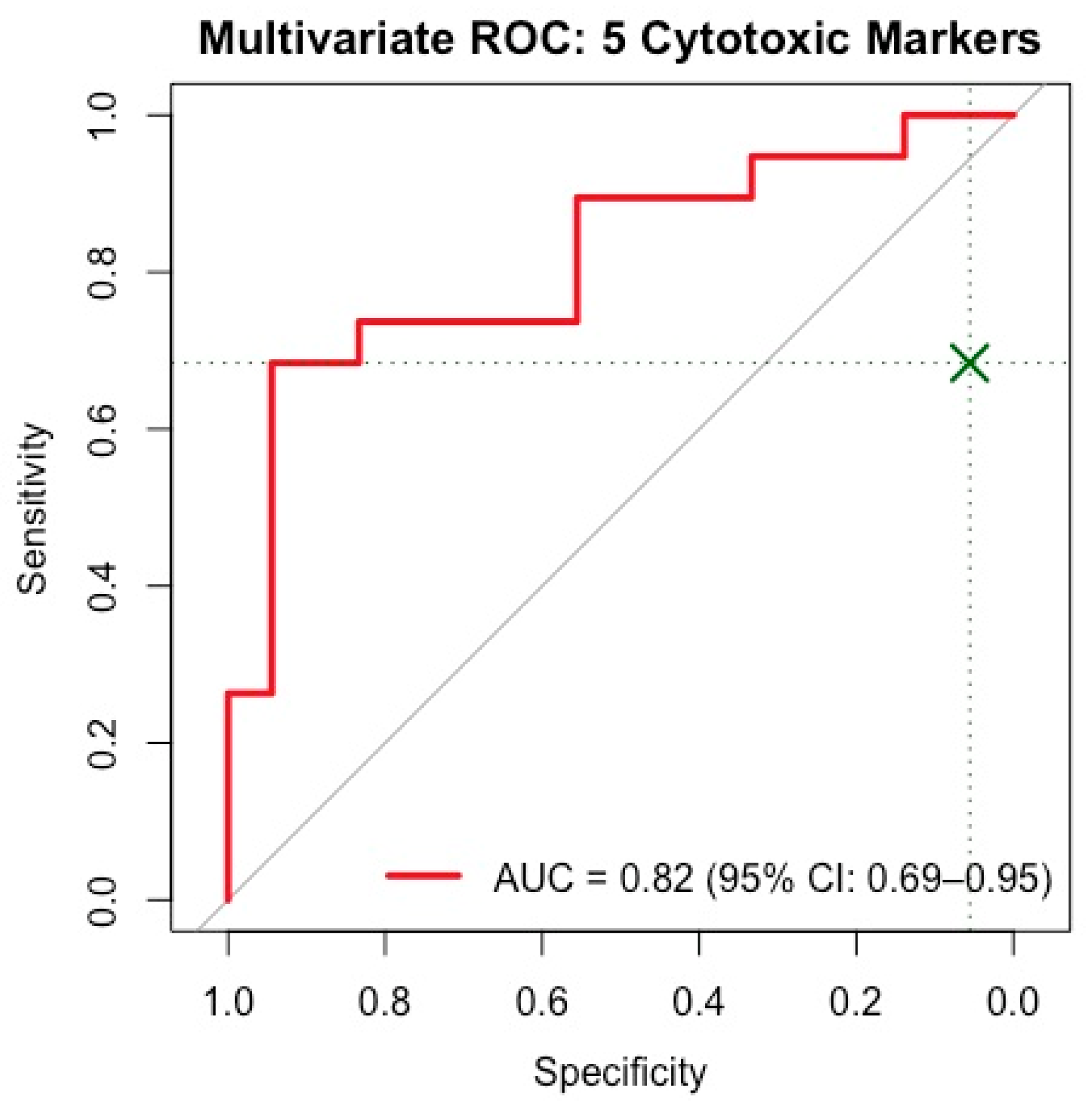
2.8. Multivariate ROC and Logistic Regression Analysis
2.9. Unsupervised Flow Cytometry Analysis
2.10. Statistical Analysis
3. Results
3.1. Characteristics of the Participants
3.2. Global Differences in Cytokine and Cytotoxic Profiles in SLE
3.3. Different Serum Biomarkers Are Associated with the Phases of SLE
3.4. Important Serum Biomarkers Differentiating SLE Activity Phases
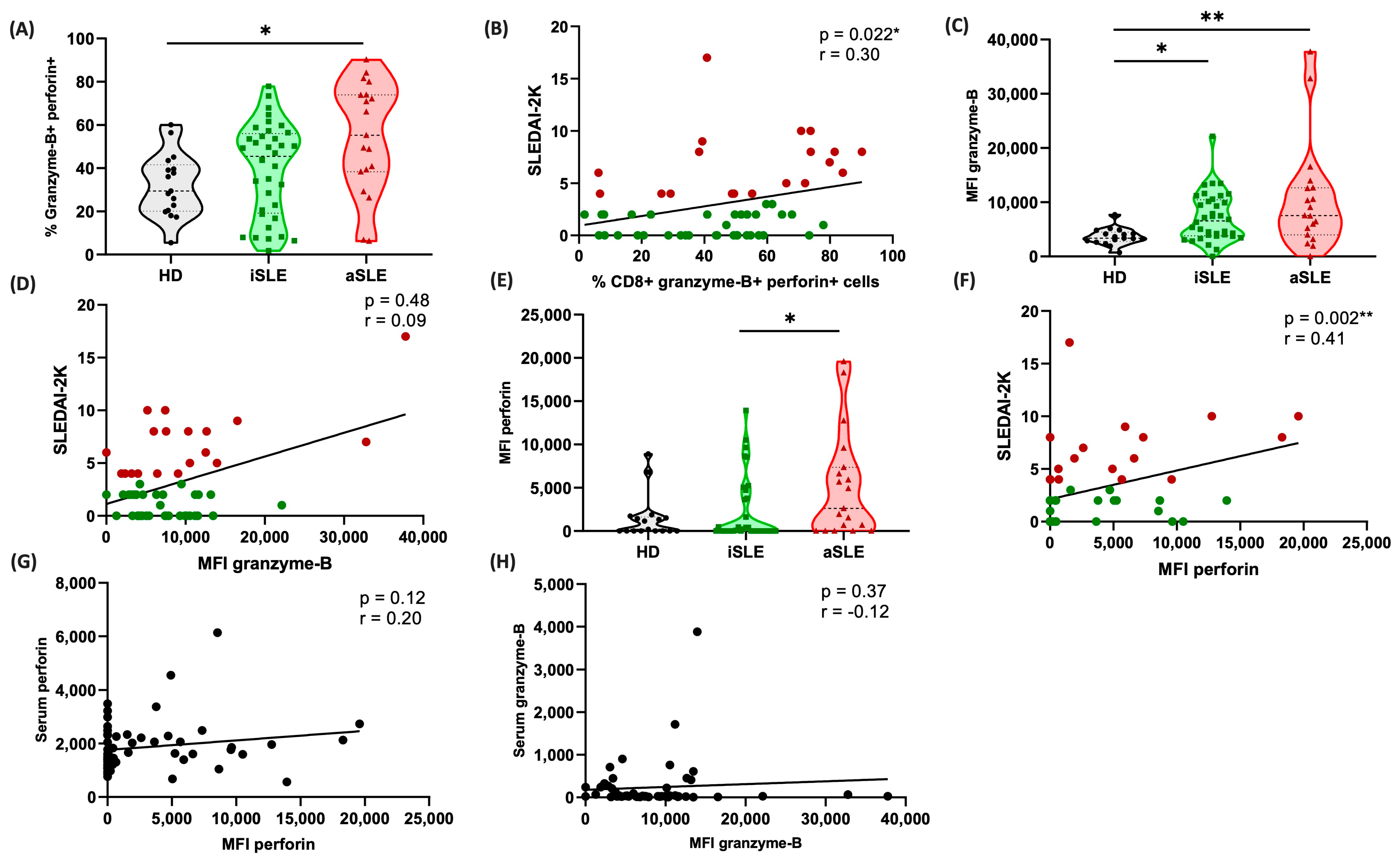
3.5. Cytotoxic Serum Markers Correlate with Disease Activity
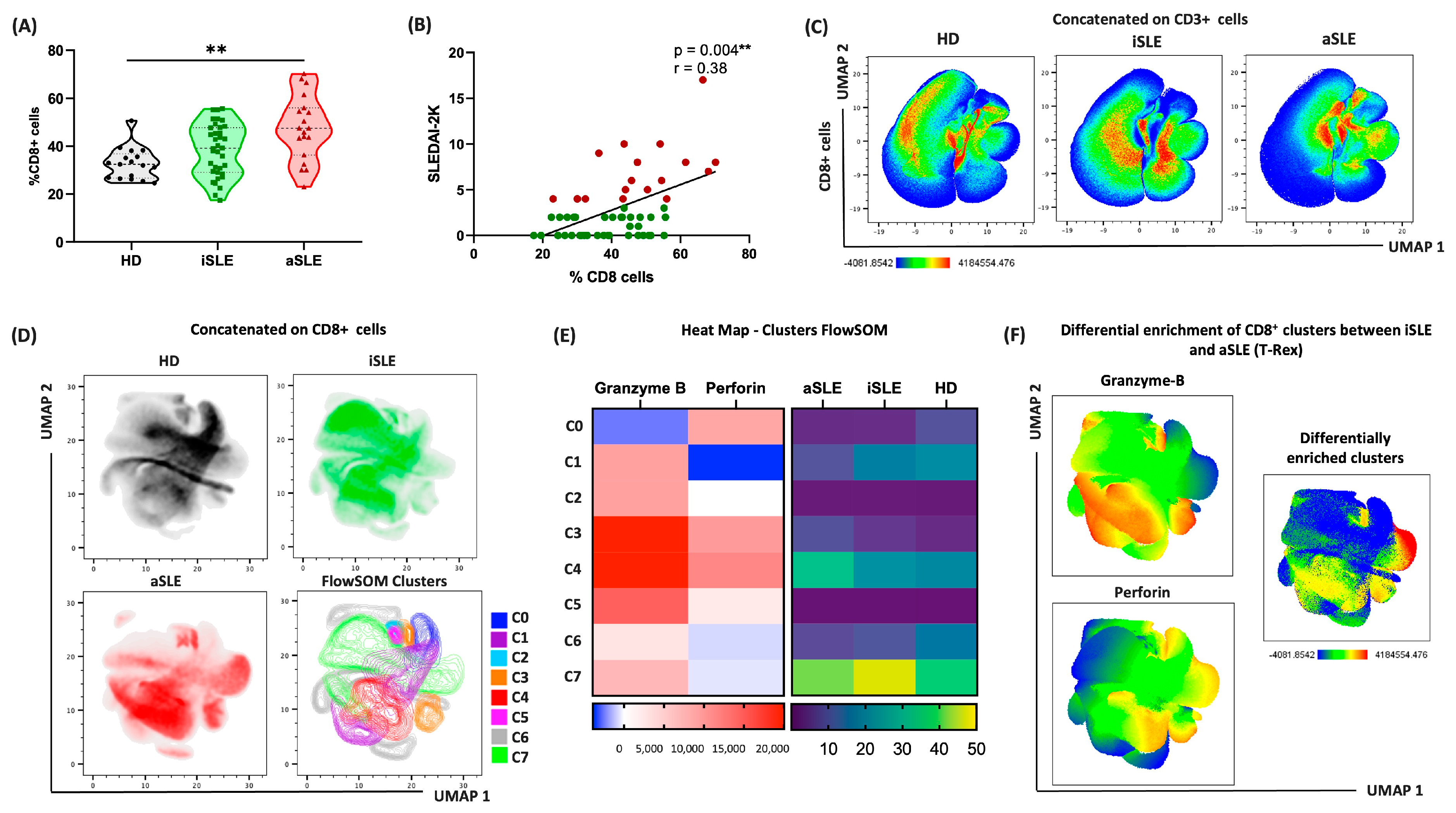
3.6. CD8+ Cells Drive Distinct Immune Patterns in Patients with Active and Inactive SLE
3.7. Cytotoxic CD8+ Cells Are Predominant in Patients with Active Disease
3.8. Cytotoxic Patterns of Cells and Proteins Differ Across the Phases of SLE Activity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SLE | Systemic Lupus Erythematosus |
| iSLE | Inactive Systemic Lus Erythematosus |
| aSLE | Active Systemic Lupus Erythematosus |
| SLEDAI-2K | Systemic Lupus Erythematosus Disease Activity Index 2000 |
| HD | Healthy Donors |
| PBMCs | Peripheral blood mononuclear cells |
| IL | Interleukin |
| TNF-α | Tumor necrosis factor-alpha |
| IFN-γ | Interferon gamma |
| PCA | Principal component analysis |
| IQR | Interquartile range |
References
- Kiriakidou, M.; Ching, C.L. Systemic Lupus Erythematosus. Ann. Intern. Med. 2020, 172, ITC81–ITC96. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Haas, M.; Glassock, R.; Zhao, M.H. Redefining lupus nephritis: Clinical implications of pathophysiologic subtypes. Nat. Rev. Nephrol. 2017, 13, 483–495. [Google Scholar] [CrossRef]
- Ameer, M.A.; Chaudhry, H.; Mushtaq, J.; Khan, O.S.; Babar, M.; Hashim, T.; Zeb, S.; Tariq, M.A.; Patlolla, S.R.; Ali, J.; et al. An Overview of Systemic Lupus Erythematosus (SLE) Pathogenesis, Classification, and Management. Cureus 2022, 14, e30330. [Google Scholar] [CrossRef] [PubMed]
- Richter, P.; Macovei, L.A.; Mihai, I.R.; Cardoneanu, A.; Burlui, M.A.; Rezus, E. Cytokines in Systemic Lupus Erythematosus-Focus on TNF-α and IL-17. Int. J. Mol. Sci. 2023, 24, 14413. [Google Scholar] [CrossRef] [PubMed]
- Pattanaik, S.S.; Das, B.K.; Tripathy, R.; Prusty, B.K.; Parida, M.K.; Tripathy, S.R.; Panda, A.K.; Ravindran, B.; Mukherjee, R. Machine learning identifies cytokine signatures of disease severity and autoantibody profiles in systemic lupus erythematosus—A pilot study. Sci Rep. 2024, 14, 28765. [Google Scholar] [CrossRef]
- Lourenco, E.V.; Cava, A.L. Cytokines in systemic lupus erythematosus. Curr. Mol. Med. 2009, 9, 242–254. [Google Scholar] [CrossRef]
- Ding, J.; Su, S.; You, T.; Xia, T.; Lin, X.; Chen, Z.; Zhang, L. Serum interleukin-6 level is correlated with the disease activity of systemic lupus erythematosus: A meta-analysis. Clinics 2020, 75, e1801. [Google Scholar] [CrossRef]
- Idborg, H.; Eketjäll, S.; Pettersson, S.; Gustafsson, J.T.; Zickert, A.; Kvarnström, M.; Oke, V.; Jakobsson, P.J.; Gunnarsson, I.; Svenungsson, E. TNF-α and plasma albumin as biomarkers of disease activity in systemic lupus erythematosus. Lupus Sci. Med. 2018, 5, e000260. [Google Scholar] [CrossRef]
- Arkatkar, T.; Du, S.W.; Jacobs, H.M.; Dam, E.M.; Hou, B.; Buckner, J.H.; Rawlings, D.J.; Jackson, S.W. B cell-derived IL-6 initiates spontaneous germinal center formation during systemic autoimmunity. J. Exp. Med. 2017, 214, 3207–3217. [Google Scholar] [CrossRef]
- Blanco, P.; Pitard, V.; Viallard, J.F.; Taupin, J.L.; Pellegrin, J.L.; Moreau, J.F. Increase in activated CD8+ T lymphocytes expressing perforin and granzyme B correlates with disease activity in patients with systemic lupus erythematosus. Arthritis Rheum. 2005, 52, 201–211. [Google Scholar] [CrossRef]
- Shah, D.; Kiran, R.; Wanchu, A.; Bhatnagar, A. Soluble granzyme B and cytotoxic T lymphocyte activity in the pathogenesis of systemic lupus erythematosus. Cell. Immunol. 2011, 269, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Lan-Lan, W.; Bei, C.; Jie, C.; Wei-Hua, F. Abnormal Fas/FasL and caspase-3-mediated apoptotic signaling pathways of T lymphocyte subset in patients with systemic lupus erythematosus. Cell. Immunol. 2006, 239, 121–128. [Google Scholar] [CrossRef]
- Abo-Elenein, A.; Shaaban, D.; Gheith, O. Flowcytometric study of expression of perforin and CD134 in patients with systemic lupus erythematosus. Egypt. J. Immunol. 2008, 15, 135–143. [Google Scholar] [PubMed]
- Vincent, F.B.; Kandane-Rathnayake, R.; Koelmeyer, R.; Harris, J.; Hoi, A.Y.; Mackay, F.; Morand, E.F. Associations of serum soluble Fas and Fas ligand (FasL) with outcomes in systemic lupus erythematosus. Lupus Sci. Med. 2020, 7, e000375. [Google Scholar] [CrossRef]
- Fava, A.; Petri, M. Systemic lupus erythematosus: Diagnosis and clinical management. J. Autoimmun. 2019, 96, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zucchi, D.; Cardelli, C.; Elefante, E.; Tani, C.; Mosca, M. Treat-to-Target in Systemic Lupus Erythematosus: Reality or Pipe Dream. J. Clin. Med. 2023, 12, 3348. [Google Scholar] [CrossRef] [PubMed]
- Parra Sánchez, A.R.; van Vollenhoven, R.F.; Morand, E.F.; Bruce, I.N.; Kandane-Rathnayake, R.; Weiss, G.; Tummala, R.; Al-Mossawi, H.; Sorrentino, A. Targeting DORIS Remission and LLDAS in SLE: A Review. Rheumatol. Ther. 2023, 10, 1459–1477. [Google Scholar] [CrossRef]
- Yee, C.S.; Farewell, V.T.; Isenberg, D.A.; Griffiths, B.; Teh, L.S.; Bruce, I.N.; Ahmad, Y.; Rahman, A.; Prabu, A.; Akil, M.; et al. The use of Systemic Lupus Erythematosus Disease Activity Index-2000 to define active disease and minimal clinically meaningful change based on data from a large cohort of systemic lupus erythematosus patients. Rheumatology 2011, 50, 982–988. [Google Scholar] [CrossRef]
- Suzuki, N.; Ichino, M.; Mihara, S.; Kaneko, S.; Sakane, T. Inhibition of Fas/Fas ligand-mediated apoptotic cell death of lymphocytes in vitro by circulating anti-Fas ligand autoantibodies in patients with systemic lupus erythematosus. Arthritis Rheum. 1998, 41, 344–353. [Google Scholar] [CrossRef]
- Narayanan, K.; Marwaha, V.; Shanmuganandan, K.; Shankar, S. Correlation between Systemic Lupus Erythematosus Disease Activity Index, C3, C4 and Anti-dsDNA Antibodies. Med. J. Armed Forces India 2010, 66, 102–107. [Google Scholar] [CrossRef]
- Peters, P.J.; Borst, J.; Oorschot, V.; Fukuda, M.; Krähenbühl, O.; Tschopp, J.; Slot, J.W.; Geuze, H.J. Cytotoxic T lymphocyte granules are secretory lysosomes, containing both perforin and granzymes. J. Exp. Med. 1991, 173, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Clayberger, C.; Krensky, A.M. Granulysin. Curr. Opin. Immunol. 2003, 15, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Raza, I.G.A.; Clarke, A.J. B Cell Metabolism and Autophagy in Autoimmunity. Front. Immunol. 2021, 12, 681105. [Google Scholar] [CrossRef]
- Orme, M.E.; Voreck, A.; Aksouh, R.; Ramsey-Goldman, R.; Schreurs, M.W.J. Systematic review of anti-dsDNA testing for systemic lupus erythematosus: A meta-analysis of the diagnostic test specificity of an anti-dsDNA fluorescence enzyme immunoassay. Autoimmun. Rev. 2021, 20, 102943. [Google Scholar] [CrossRef] [PubMed]
- Chiche, L.; Jourde-Chiche, N.; Whalen, E.; Presnell, S.; Gersuk, V.; Dang, K.; Anguiano, E.; Quinn, C.; Burtey, S.; Berland, Y.; et al. Modular transcriptional repertoire analyses of adults with systemic lupus erythematosus reveal distinct type I and type II interferon signatures. Arthritis Rheumatol. 2014, 66, 1583–1595. [Google Scholar] [CrossRef]
- Kok, H.M.; van den Hoogen, L.L.; van Roon, J.A.; Adriaansen, E.J.; Fritsch-Stork, R.D.; Nguyen, T.Q.; Goldschmeding, R.; Radstake, T.R.; Bovenschen, N. Systemic and local granzyme B levels are associated with disease activity, kidney damage and interferon signature in systemic lupus erythematosus. Rheumatology 2017, 56, 2129–2134. [Google Scholar] [CrossRef]
- Schwartz, N.; Rubinstein, T.; Burkly, L.C.; Collins, C.E.; Blanco, I.; Su, L.; Hojaili, B.; Mackay, M.; Aranow, C.; Stohl, W.; et al. Urinary TWEAK as a biomarker of lupus nephritis: A multicenter cohort study. Arthritis Res. Ther. 2009, 11, R143. [Google Scholar] [CrossRef]
- Chun, H.Y.; Chung, J.W.; Kim, H.A.; Yun, J.M.; Jeon, J.Y.; Ye, Y.M.; Kim, S.H.; Park, H.S.; Suh, C.H. Cytokine IL-6 and IL-10 as biomarkers in systemic lupus erythematosus. J. Clin. Immunol. 2007, 27, 461–466. [Google Scholar] [CrossRef]
- Liu, C.C.; Kao, A.H.; Manzi, S.; Ahearn, J.M. Biomarkers in systemic lupus erythematosus: Challenges and prospects for the future. Ther. Adv. Musculoskelet. Dis. 2013, 5, 210–233. [Google Scholar] [CrossRef]
- Asyraf, W.W.; Shahrir, M.M.; Asrul, W.; Norasyikin, A.W.; Hanita, O.; Kong, W.Y.; Azmi, M.T. The association between serum prolactin levels and interleukin-6 and systemic lupus erythematosus activity. Reumatismo 2018, 70, 241–250. [Google Scholar] [CrossRef]
- Moreno-Torres, V.; Castejón, R.; Martínez-Urbistondo, M.; Gutiérrez-Rojas, Á.; Vázquez-Comendador, J.; Tutor, P.; Durán-del Campo, P.; Mellor-Pita, S.; Rosado, S.; Vargas-Núñez, J.A. Serum cytokines to predict systemic lupus erythematosus clinical and serological activity. Clin. Transl. Sci. 2022, 15, 1676–1686. [Google Scholar] [CrossRef]
- Brattsand, R.; Linden, M. Cytokine modulation by glucocorticoids: Mechanisms and actions in cellular studies. Aliment Pharmacol Ther. 1996, 10 (Suppl. 2), 81–90, discussion 91–92. [Google Scholar] [CrossRef] [PubMed]
- Goodman, R.S.; Johnson, D.B.; Balko, J.M. Corticosteroids and Cancer Immunotherapy. Clin. Cancer Res. 2023, 29, 2580–2587. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.; Meng, C.; Ivashkiv, L.B. Inhibition of IL-2-induced Jak-STAT signaling by glucocorticoids. Proc. Natl. Acad. Sci. USA 2000, 97, 9573–9578. [Google Scholar] [CrossRef]
- Chen, X.; Oppenheim, J.J.; Winkler-Pickett, R.T.; Ortaldo, J.R.; Howard, O.M.Z. Glucocorticoid amplifies IL-2-dependent expansion of functional FoxP3+CD4+CD25+ T regulatory cells in vivo and enhances their capacity to suppress EAE. Eur. J. Immunol. 2006, 36, 2139–2149. [Google Scholar] [CrossRef] [PubMed]
- Konishi, A.; Suzuki, J.; Kuwahara, M.; Matsumoto, A.; Nomura, S.; Soga, T.; Yorozuya, T.; Yamashita, M. Glucocorticoid imprints a low glucose metabolism onto CD8 T cells and induces the persistent suppression of the immune response. Biochem. Biophys. Res. Commun. 2022, 588, 34–40. [Google Scholar] [CrossRef]
- Tokunaga, A.; Sugiyama, D.; Maeda, Y.; Warner, A.B.; Panageas, K.S.; Ito, S.; Togashi, Y.; Sakai, C.; Wolchok, J.D.; Nishikawa, H. Selective inhibition of low-affinity memory CD8+ T cells by corticosteroids. J. Exp. Med. 2019, 216, 2701–2713. [Google Scholar] [CrossRef]
- Duvall, M.G.; Barnig, C.; Cernadas, M.; Ricklefs, I.; Krishnamoorthy, N.; Grossman, N.L.; Bhakta, N.R.; Fahy, J.V.; Bleecker, E.R.; Castro, M.; et al. Natural killer cell-mediated inflammation resolution is disabled in severe asthma. Sci. Immunol. 2017, 2, eaam5446. [Google Scholar] [CrossRef]
- Allison, A.C. Mechanisms of action of mycophenolate mofetil. Lupus 2005, 14 (Suppl. 1), s2–s8. [Google Scholar] [CrossRef]
- Vögelin, M.; Biedermann, L.; Frei, P.; Vavricka, S.R.; Scharl, S.; Zeitz, J.; Sulz, M.C.; Fried, M.; Rogler, G.; Scharl, M. The Impact of Azathioprine-Associated Lymphopenia on the Onset of Opportunistic Infections in Patients with Inflammatory Bowel Disease. PLoS ONE 2016, 11, e0155218. [Google Scholar] [CrossRef]
- Zhang, L.; Du, F.; Jin, Q.; Sun, L.; Wang, B.; Tan, Z.; Meng, X.; Huang, B.; Zhan, Y.; Su, W.; et al. Identification and Characterization of CD8+ CD27+ CXCR3− T Cell Dysregulation and Progression-Associated Biomarkers in Systemic Lupus Erythematosus. Adv. Sci. 2023, 10, e2300123. [Google Scholar] [CrossRef] [PubMed]
- Fogagnolo, L.; Soares, T.C.B.; Senna, C.G.; Souza, E.M.; Blotta, M.H.S.L.; Cintra, M.L. Cytotoxic granules in distinct subsets of cutaneous lupus erythematosus. Clin. Exp. Dermatol. 2014, 39, 835–839. [Google Scholar] [CrossRef] [PubMed]
- Choy, J.C.; Kerjner, A.; Wong, B.W.; McManus, B.M.; Granville, D.J. Perforin mediates endothelial cell death and resultant transplant vascular disease in cardiac allografts. Am. J. Pathol. 2004, 165, 127–133. [Google Scholar] [CrossRef]
- Choy, J.C.; Cruz, R.P.; Kerjner, A.; Geisbrecht, J.; Sawchuk, T.; Fraser, S.A.; Hudig, D.; Bleackley, R.C.; Jirik, F.R.; McManus, B.M.; et al. Granzyme B induces endothelial cell apoptosis and contributes to the development of transplant vascular disease. Am. J. Transplant. 2005, 5, 494–499. [Google Scholar] [CrossRef]
- Voskoboinik, I.; Whisstock, J.C.; Trapani, J.A. Perforin and granzymes: Function, dysfunction and human pathology. Nat. Rev. Immunol. 2015, 15, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.M.; Jan Wu, Y.J.; Zheng, J.W.; Huang, L.Y.; Tan, K.P.; Chen, J.Y. T cell expressions of aberrant gene signatures and Co-inhibitory receptors (Co-IRs) as predictors of renal damage and lupus disease activity. J. Biomed. Sci. 2024, 31, 41. [Google Scholar] [CrossRef]
- Tilstra, J.S.; Avery, L.; Menk, A.V.; Gordon, R.A.; Smita, S.; Kane, L.P.; Chikina, M.; Delgoffe, G.M.; Shlomchik, M.J. Kidney-infiltrating T cells in murine lupus nephritis are metabolically and functionally exhausted. J. Clin. Investig. 2018, 128, 4884–4897. [Google Scholar] [CrossRef]
- McKinney, E.F.; Lee, J.C.; Jayne, D.R.W.; Lyons, P.A.; Smith, K.G.C. T-cell exhaustion, co-stimulation and clinical outcome in autoimmunity and infection. Nature 2015, 523, 612–616. [Google Scholar] [CrossRef]
- Lima, G.; Treviño-Tello, F.; Atisha-Fregoso, Y.; Llorente, L.; Fragoso-Loyo, H.; Jakez-Ocampo, J. Exhausted T cells in systemic lupus erythematosus patients in long-standing remission. Clin. Exp. Immunol. 2021, 204, 285–295. [Google Scholar] [CrossRef]
- Kosalka, J.; Jakiela, B.; Musial, J. Changes of memory B- and T-cell subsets in lupus nephritis patients. Folia Histochem. Cytobiol. 2016, 54, 32–41. [Google Scholar] [CrossRef]
- Jakiela, B.; Kosałka, J.; Plutecka, H.; Bazan-Socha, S.; Sanak, M.; Musiał, J. Facilitated expansion of Th17 cells in lupus nephritis patients. Clin. Exp. Immunol. 2018, 194, 283–294. [Google Scholar] [CrossRef] [PubMed]

| HD | SLE | iSLE | aSLE | |
|---|---|---|---|---|
| Total n | 17 | 55 | 36 | 19 |
| Female | 14 (82%) | 46 (83%) | 30 (83%) | 16 (84%) |
| Age, years | 26 (25–53) | 36 (28–46) | 36 (30–44) | 34 (28–51) |
| Body Mass Index | - | 25.3 (21.9–29.5) | 25.7 (22.0–30.4) | 24.6 (19.5–27.3) |
| Systolic Blood Pressure | - | 119 (109–129) | 120 (108–124) | 115 (109–131) |
| Diastolic Blood Pressure | - | 69 (61–76) | 65 (61–72) | 71 (62–77) |
| Comorbidities | ||||
| Diabetes | 0 | 4 (7.3%) | 2 (5.5%) | 2 (10.5%) |
| Hypertension | 0 | 16 (29.1%) | 10 (27%) | 6 (31.5%) |
| Hypothyroidism | 0 | 9 (16.4%) | 5 (13.8%) | 4 (21.0%) |
| Chronic Kidney Disease | 0 | 9 (16.4%) | 7 (19.4%) | 2 (10.5%) |
| Dyslipidemia | 0 | 1 (1.8%) | 1 (2.7%) | 0 (0%) |
| Myocardial infarction | 0 | 0 (0%) | 0 (0%) | 0 (0%) |
| Stroke | 0 | 2 (3.6%) | 1 (2.7%) | 1 (5.2%) |
| Lung disease | 0 | 3 (5.5%) | 1 (2.7%) | 2 (10.5%) |
| SLE duration, years | NA | 7 (3–18) | 6 (3–14.7) | 15 (5–24) |
| SLEDAI 2K | NA | 0 (0–2) | 6 (4–8) | |
| Neurological a | NA | 0 | 0 | 0 |
| Vascular | NA | 1 | 0 | 1 |
| Articular | NA | 1 | 0 | 1 |
| Muscular | NA | 0 | 0 | 0 |
| Renal b | NA | 19 | 0 | 19 |
| Cutaneous c | NA | 1 | 0 | 1 |
| Serositis | NA | 0 | 0 | 0 |
| Biochemical d | NA | 32 | 15 | 17 |
| Anti-dsDNA | NA | 30.2 (10.3, 124.4) | 29.3 (8.8, 123.8) | 36.5 (14.0, 321.7) |
| C3 | NA | 98.1 (73.0, 109.0) | 100.1 (79.5, 108.8) | 81.7 (70.4, 114.0) |
| C4 | NA | 14.0 (9.1, 20.0) | 15.0 (9.7, 20.6) | 10.5 (4.4, 15.5) |
| Hematological e | NA | 9 | 4 | 5 |
| Constitutional (Fever) | NA | 0 | 0 | 0 |
| Treatments | ||||
| Corticosteroids | NA | 21 (38.2%) | 9 (25.0%) | 12 (63.1%) |
| Antimalarials | NA | 36 (65.4%) | 24 (66.6%) | 12 (63.1%) |
| Immunosuppressants | ||||
| Mycophenolate | NA | 41 (74.5%) | 25 (69.4%) | 16 (84.2%) |
| Azathioprine | NA | 9 (16.4%) | 7 (19.44%) | 2 (10.5%) |
| Rituximab | NA | 1 (1.8%) | 1 (2.7%) | 0 (0%) |
| Other f | NA | 10 (18.8%) | 7 (19.4%) | 3 (15.7%) |
| Parameters | HD (Median; Q1–Q3) | SLE (Median; Q1–Q3) | p-Value |
|---|---|---|---|
| IL-2 | 47.87 (36.34–64.23) | 46.93 (36.34–89.13) | 0.75 |
| IL-4 | 9.36 (4.76–13.19) | 8.95 (5.92–21.59) | 0.45 |
| IL-6 | 17.57 (14.06–28.74) | 19.89 (14.48–30.67) | 0.37 |
| IL-10 | 5.68 (5.68–11.60) | 7.39 (5.68–15.77) | 0.29 |
| IFN-γ | 39.10 (24.47–97.56) | 52.07 (35.01–123.6) | 0.18 |
| IL-17A | 1.64 (1.37–3.09) | 2.14 (1.40–7.17) | 0.21 |
| TNF-α | 8.13 (5.59–12.98) | 14.04 (7.21–31.19) | 0.009 ** |
| Granzyme-A | 65.62 (41.95–114.7) | 91.16 (49.98–202.1) | 0.1 |
| Granzyme-B | 22.16 (17.49–40.19) | 35.03 (21.90–238.1) | 0.075 |
| sFas | 54.12 (28.38–75.57) | 199 (101.5–491.1) | <0.001 *** |
| sFas-L | 60.45 (51.31–67.78) | 44.19 (30.54–66.23) | 0.045 * |
| Perforin | 1757 (1538–2297) | 1628 (1214–2283) | 0.37 |
| Granulysin | 1044 (944.7–1398) | 1171 (672–1794) | 0.91 |
| Parameters | iSLE (Median; Q1–Q3) | aSLE (Median; Q1–Q3) | p-Value |
|---|---|---|---|
| IL-2 | 47.70 (36.34–85.72) | 42.68 (36.34–115.9) | 0.45 |
| IL-4 | 8.95 (5.92–21.59) | 10.01 (4.50–59.06) | 0.54 |
| IL-6 | 18.70 (14.06–26.48) | 24.26 (15.44–33.73) | 0.08 |
| IL-10 | 6.02 (5.68–13.02) | 9.75 (5.68–17.51) | 0.11 |
| IFN-γ | 51.61 (35.04–120.1) | 55.78 (35.01–150.7) | 0.74 |
| IL-17A | 2.5 (1.47–7.07) | 1.98 (1.3–7.17) | 0.46 |
| TNF-α | 15.13 (8.75–31.19) | 12.58 (5.44–73.87) | 0.35 |
| Granzyme-A | 84.02 (50.41–185.3) | 95.13 (46.67–416.8) | 0.32 |
| Granzyme-B | 35.47 (22.95–259.1) | 28.17 (13.08–88.15) | 0.38 |
| sFas | 202.9 (93.89–492.8) | 175.4 (104.5–491.1) | 0.66 |
| sFas-L | 37.15 (21.68–57.05) | 49.22 (36.48–74.09) | 0.03 * |
| Perforin | 1444 (1092–1849) | 2219 (1767–2491) | <0.001 *** |
| Granulysin | 963.6 (550.4–1445) | 1347 (1155–2576) | 0.005 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santana-Sánchez, P.; Ramírez-Pérez, A.A.; Alberti-Minutti, P.; Gajón, J.A.; Bonifaz, L.C.; Sánchez-Escobar, N.; Legorreta-Haquet, M.V.; Chávez-Sánchez, L.; Chávez-Rueda, A.K. Cytotoxic Molecules as Potential Biomarkers for Active and Inactive Systemic Lupus Erythematosus. Biomedicines 2025, 13, 1559. https://doi.org/10.3390/biomedicines13071559
Santana-Sánchez P, Ramírez-Pérez AA, Alberti-Minutti P, Gajón JA, Bonifaz LC, Sánchez-Escobar N, Legorreta-Haquet MV, Chávez-Sánchez L, Chávez-Rueda AK. Cytotoxic Molecules as Potential Biomarkers for Active and Inactive Systemic Lupus Erythematosus. Biomedicines. 2025; 13(7):1559. https://doi.org/10.3390/biomedicines13071559
Chicago/Turabian StyleSantana-Sánchez, Paola, Astrid Asminda Ramírez-Pérez, Paolo Alberti-Minutti, Julián A. Gajón, Laura C. Bonifaz, Norberto Sánchez-Escobar, María Victoria Legorreta-Haquet, Luis Chávez-Sánchez, and Adriana Karina Chávez-Rueda. 2025. "Cytotoxic Molecules as Potential Biomarkers for Active and Inactive Systemic Lupus Erythematosus" Biomedicines 13, no. 7: 1559. https://doi.org/10.3390/biomedicines13071559
APA StyleSantana-Sánchez, P., Ramírez-Pérez, A. A., Alberti-Minutti, P., Gajón, J. A., Bonifaz, L. C., Sánchez-Escobar, N., Legorreta-Haquet, M. V., Chávez-Sánchez, L., & Chávez-Rueda, A. K. (2025). Cytotoxic Molecules as Potential Biomarkers for Active and Inactive Systemic Lupus Erythematosus. Biomedicines, 13(7), 1559. https://doi.org/10.3390/biomedicines13071559









