Betulinic Acid ω-Triphenylphosphonium Alkyl Esters: Antiproliferative Activities and In Silico Pharmacokinetic Profiles
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemistry
2.1.1. Isolation and Purification of Betulinic Acid (BA)
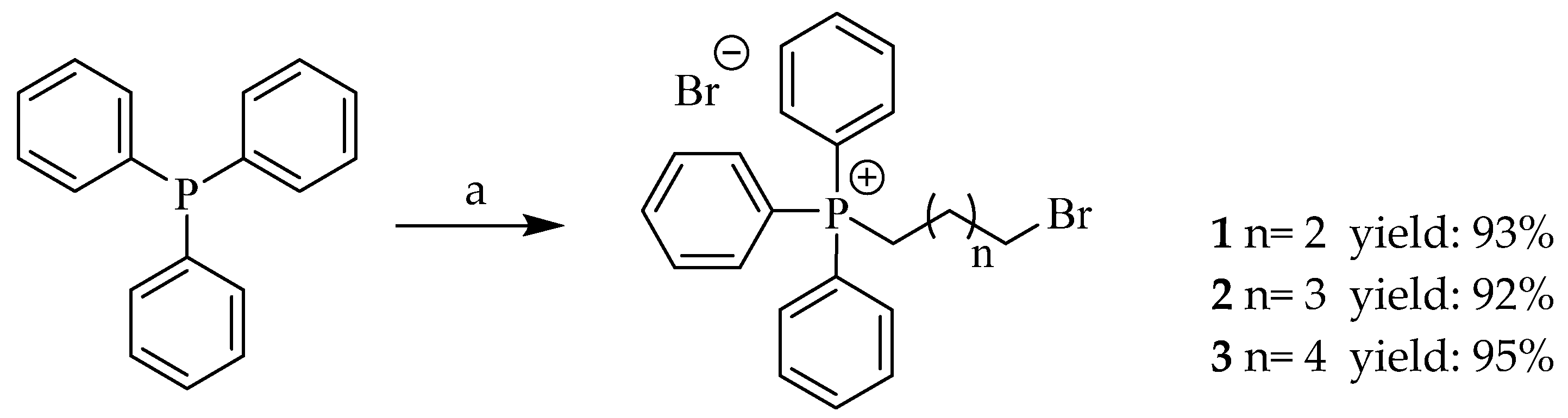
2.1.2. General Procedure for the Synthesis of Triphenylphosphonium Bromide Salts (1–3)
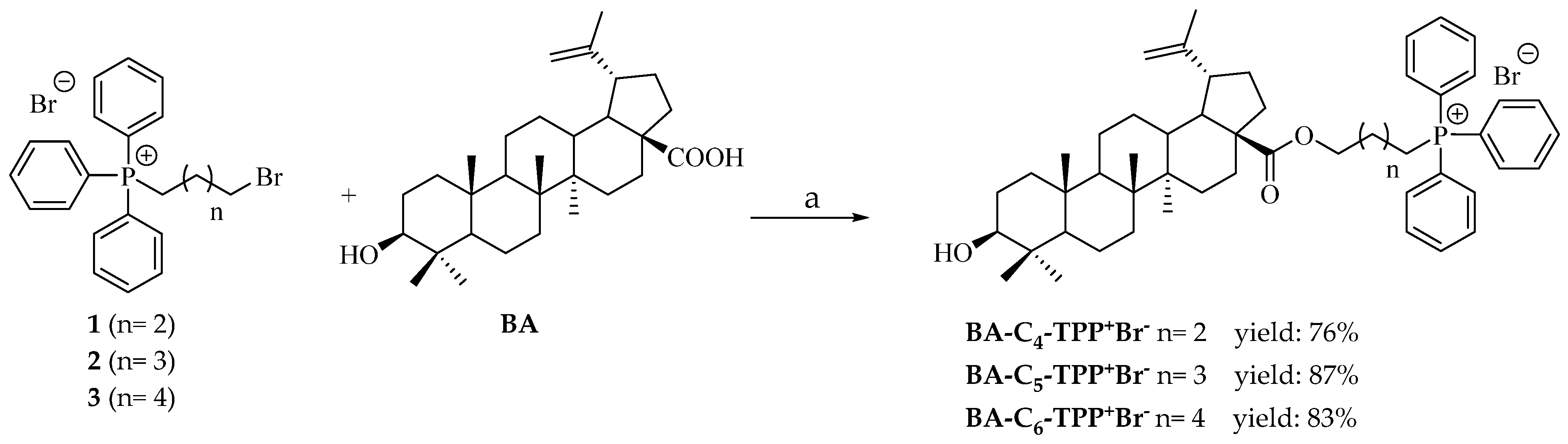
2.1.3. General Procedure for the Synthesis of Betulinic Acid Esters with Triphenylphosphonium Bromide Salts (BA-C4-TPP+Br−, BA-C5-TPP+Br− and BA-C6-TPP+Br−)
2.2. Biological Activity
2.2.1. Cell Culture
2.2.2. Cell Viability Assay
2.2.3. Oxygen Consumption Assay
2.2.4. LDH Release Assay
2.2.5. Annexin V/Propidium Iodide Double Staining Assay
2.2.6. Statistics
3. Results and Discussion
3.1. Chemistry
3.2. Biological Activity
3.2.1. In Vitro Cell Growth Inhibition
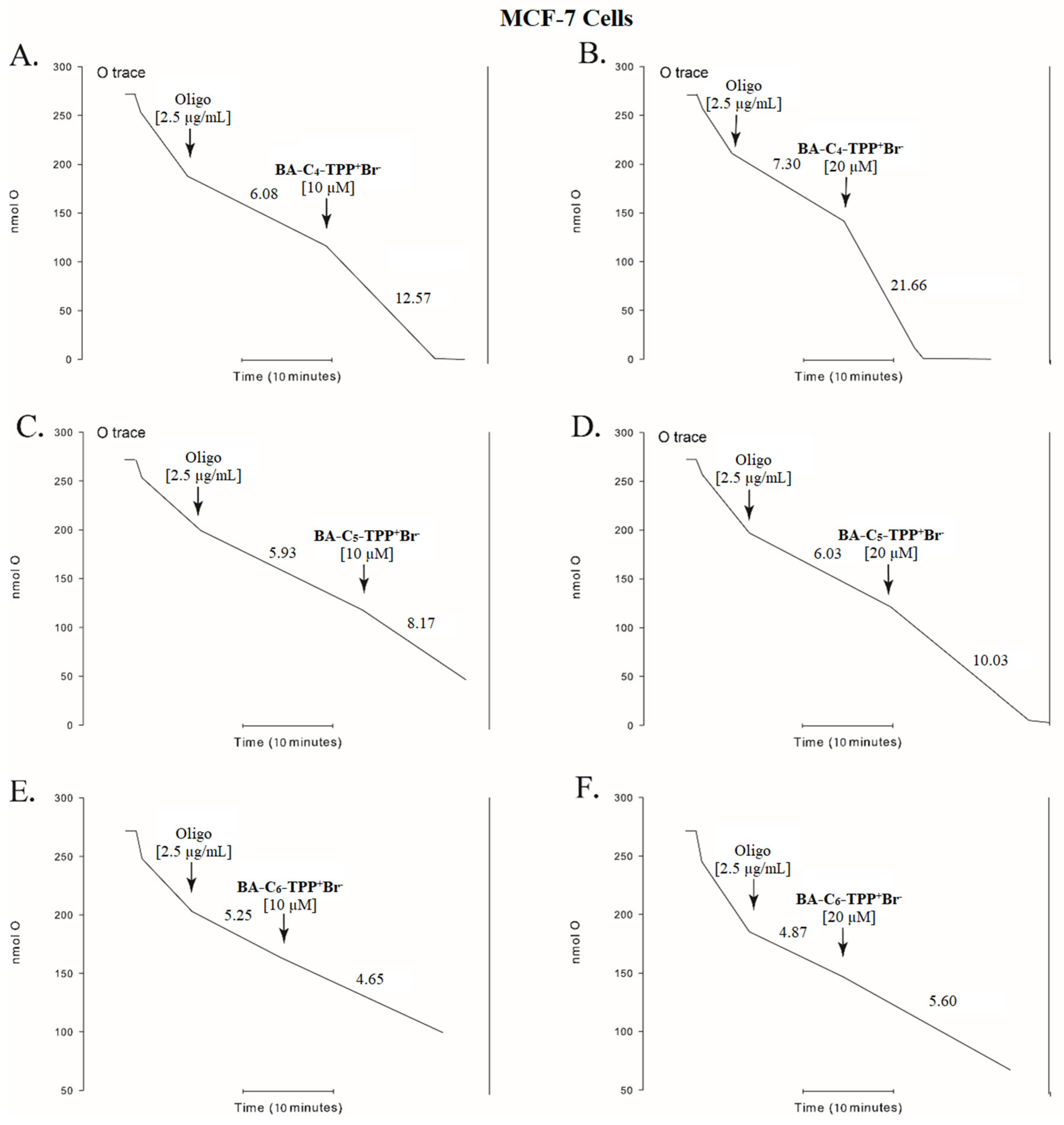
3.2.2. Oxygen Consumption
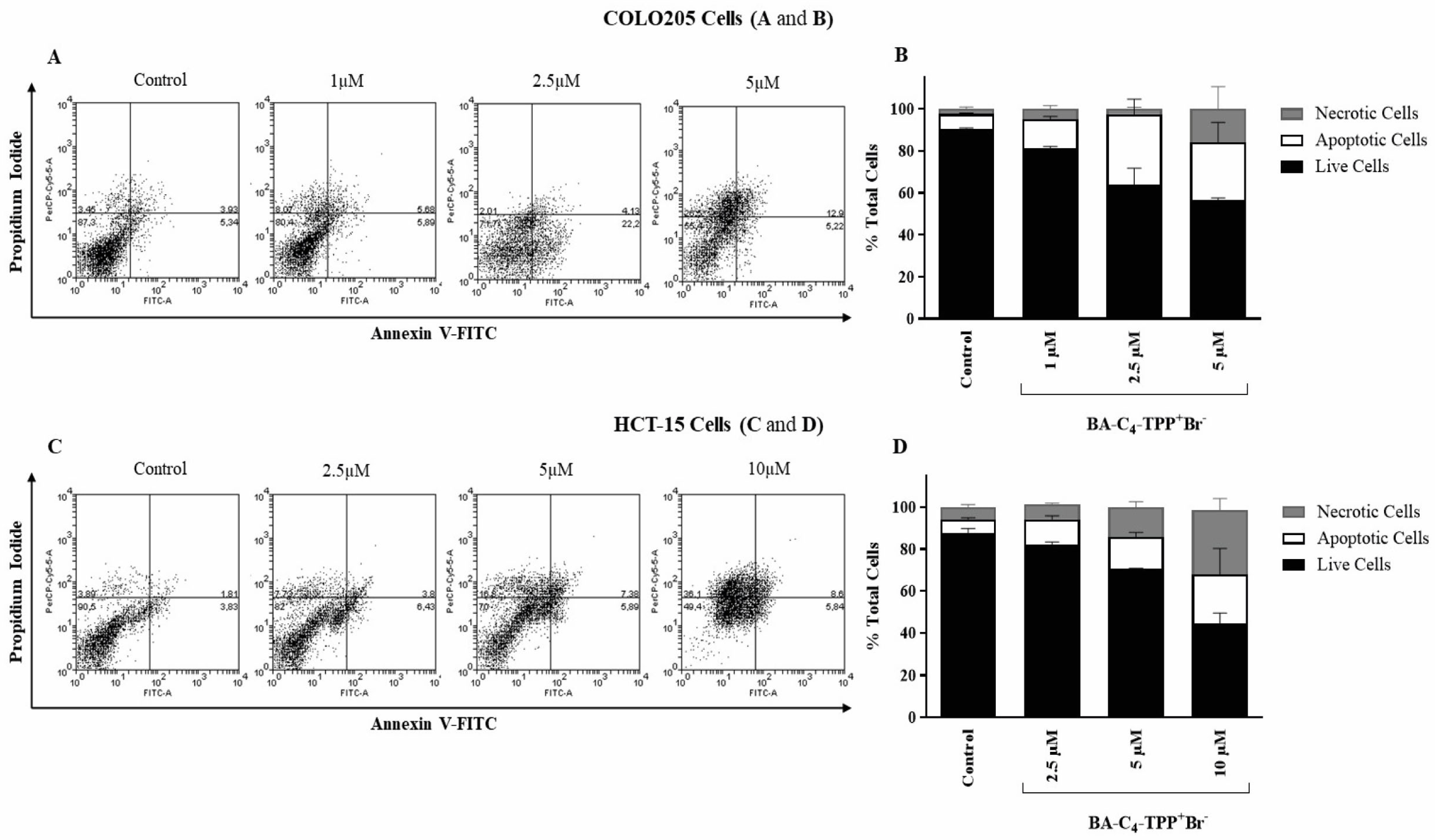
3.2.3. Effect of BA-C4-TPP+Br− on Membrane Permeability in Human Colorectal Carcinoma Cells
3.2.4. Analysis of the Induction of Apoptosis by BA-C4-TPP+Br− in Human Colorectal Carcinoma Cells
3.3. In Silico Pharmacokinetic Profile of BA and Related Compounds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, D.-M.; Xu, H.-G.; Wang, L.; Li, Y.-J.; Sun, P.-H.; Wu, X.-M.; Wang, G.-J.; Chen, W.-M.; Ye, W.-C. Betulinic Acid and Its Derivatives as Potential Antitumor Agents. Med. Res. Rev. 2015, 35, 1127–1155. [Google Scholar] [CrossRef] [PubMed]
- Sawai, S.; Saito, K. Triterpenoid Biosynthesis and Engineering in Plants. Front. Plant Sci. 2011, 2, 25. [Google Scholar] [CrossRef] [PubMed]
- Abe, I.; Rohmer, M.; Prestwich, G.D. Enzymatic Cyclization of Squalene and Oxidosqualene to Sterols and Triterpenes. Chem. Rev. 1993, 93, 2189–2206. [Google Scholar] [CrossRef]
- Fujioka, T.; Kashiwada, Y.; Kilkuskie, R.E.; Cosentino, L.M.; Ballas, L.M.; Jiang, J.B.; Janzen, W.P.; Chen, I.-S.; Lee, K.-H. Anti-AIDS Agents, 11. Betulinic Acid and Platanic Acid as Anti-HIV Principles from Syzigium Claviflorum, and the Anti-HIV Activity of Structurally Related Triterpenoids. J. Nat. Prod. 1994, 57, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Pisha, E.; Chai, H.; Lee, I.-S.; Chagwedera, T.E.; Farnsworth, N.R.; Cordell, G.A.; Beecher, C.W.W.; Fong, H.H.S.; Kinghorn, A.D.; Brown, D.M.; et al. Discovery of Betulinic Acid as a Selective Inhibitor of Human Melanoma That Functions by Induction of Apoptosis. Nat. Med. 1995, 1, 1046–1051. [Google Scholar] [CrossRef]
- Tan, Y.; Yu, R.; Pezzuto, J.M. Betulinic Acid-Induced Programmed Cell Death in Human Melanoma Cells Involves Mitogen-Activated Protein Kinase Activation. Clin. Cancer Res. 2003, 9, 2866–2875. [Google Scholar]
- Galgon, T.; Wohlrab, W.; Dräger, B. Betulinic Acid Induces Apoptosis in Skin Cancer Cells and Differentiation in Normal Human Keratinocytes. Exp. Dermatol. 2005, 14, 736–743. [Google Scholar] [CrossRef]
- Kessler, J.H.; Mullauer, F.B.; de Roo, G.M.; Medema, J.P. Broad in Vitro Efficacy of Plant-Derived Betulinic Acid against Cell Lines Derived from the Most Prevalent Human Cancer Types. Cancer Lett. 2007, 251, 132–145. [Google Scholar] [CrossRef]
- Chintharlapalli, S.; Papineni, S.; Ramaiah, S.K.; Safe, S. Betulinic Acid Inhibits Prostate Cancer Growth through Inhibition of Specificity Protein Transcription Factors. Cancer Res. 2007, 67, 2816–2823. [Google Scholar] [CrossRef]
- Eichenmüller, M.; von Schweinitz, D.; Kappler, R. Betulinic Acid Treatment Promotes Apoptosis in Hepatoblastoma Cells. Int. J. Oncol. 2009, 35, 873–879. [Google Scholar] [CrossRef]
- Eichenmüller, M.; Hemmerlein, B.; von Schweinitz, D.; Kappler, R. Betulinic Acid Induces Apoptosis and Inhibits Hedgehog Signalling in Rhabdomyosarcoma. Br. J. Cancer 2010, 103, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Chintharlapalli, S.; Papineni, S.; Lei, P.; Pathi, S.; Safe, S. Betulinic Acid Inhibits Colon Cancer Cell and Tumor Growth and Induces Proteasome-Dependent and -Independent Downregulation of Specificity Proteins (Sp) Transcription Factors. BMC Cancer 2011, 11, 371. [Google Scholar] [CrossRef] [PubMed]
- Domingues, R.M.A.; Sousa, G.D.A.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P. Eucalyptus Globulus Biomass Residues from Pulping Industry as a Source of High Value Triterpenic Compounds. Ind. Crops Prod. 2010, 31, 65–70. [Google Scholar] [CrossRef]
- Herz, W.; Santhanam, P.S.; Wahlberg, I. 3-Epi-Betulinic Acid, a New Triterpenoid from Picramnia Pentandra. Phytochemistry 1972, 11, 3061–3063. [Google Scholar] [CrossRef]
- Robinson, F.P.; Martel, H. Betulinic Acid from Arbutus Menziesii. Phytochemistry 1970, 9, 907–909. [Google Scholar] [CrossRef]
- Pinilla, J.M.; López-Padilla, A.; Vicente, G.; Fornari, T.; Quintela, J.C.; Reglero, G. Recovery of Betulinic Acid from Plane Tree (Platanus acerifolia L.). J. Supercrit. Fluids 2014, 95, 541–545. [Google Scholar] [CrossRef]
- Plánder, S.; Simon, B.; Béni, S.; Alberti, Á.; Kéry, Á.; Székely, E. Identification of Triterpenes and β-Sitosterol in the Bark of Plane Tree Extracts. Period. Polytech. Chem. Eng. 2019, 63, 340–347. [Google Scholar] [CrossRef]
- O’Connell, M.M.; Bentley, M.D.; Campbell, C.S.; Cole, B.J.W. Betulin and Lupeol in Bark from Four White-Barked Birches. Phytochemistry 1988, 27, 2175–2176. [Google Scholar] [CrossRef]
- Šiman, P.; Filipová, A.; Tichá, A.; Niang, M.; Bezrouk, A.; Havelek, R. Effective Method of Purification of Betulin from Birch Bark: The Importance of Its Purity for Scientific and Medicinal Use. PLoS ONE 2016, 11, e0154933. [Google Scholar] [CrossRef]
- Kim, D.S.H.L.; Chen, Z.; Nguyen, V.T.; Pezzuto, J.M.; Qiu, S.; Lu, Z.-Z. A Concise Semi-Synthetic Approach to Betulinic Acid from Betulin. Synth. Commun. 1997, 27, 1607–1612. [Google Scholar] [CrossRef]
- Flekhter, O.B.; Nigmatullina, L.R.; Baltina, L.A.; Karachurina, L.T.; Galin, F.Z.; Zarudii, F.S.; Tolstikov, G.A.; Boreko, E.I.; Pavlova, N.I.; Nikolaeva, S.N.; et al. Synthesis of Betulinic Acid from Betulin Extract and Study of the Antiviral and Antiulcer Activity of Some Related Terpenoids. Pharm. Chem. J. 2002, 36, 484–487. [Google Scholar] [CrossRef]
- Carlson, R.M.; Krasutsky, P.A.; Nesterenko, V.V. Method for Manufacturing Betulinic Acid, 2001.
- Carlson, R.M.; Krasutsky, P.A.; Nesterenko, V.V. Methods for Manufacturing Betulinic Acid, 2002.
- Barthel, A.; Stark, S.; Csuk, R. Oxidative Transformations of Betulinol. Tetrahedron 2008, 64, 9225–9229. [Google Scholar] [CrossRef]
- Csuk, R.; Schmuck, K.; Schäfer, R. A Practical Synthesis of Betulinic Acid. Tetrahedron Lett. 2006, 47, 8769–8770. [Google Scholar] [CrossRef]
- Wickholm, N.; Alakurtti, S.; Yli-Kauhaluoma, J.; Koskimies, S. Method for Preparation of Betulinic Acid, April 4, 2013.
- Suárez-Rozas, C.; Cassels, B.K. A Centum of Valuable Plant Bioactives. In Chapter 6—Betulinic Acid; Mushtaq, M., Anwar, F., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 117–142. [Google Scholar] [CrossRef]
- Dai, L.; Li, D.; Cheng, J.; Liu, J.; Deng, L.-H.; Wang, L.-Y.; Lei, J.-D.; He, J. Water Soluble Multiarm-Polyethylene Glycol–Betulinic Acid Prodrugs: Design, Synthesis, and in Vivo Effectiveness. Polym. Chem. 2014, 5, 5775–5783. [Google Scholar] [CrossRef]
- Spivak, A.Y.; Nedopekina, D.A.; Khalitova, R.R.; Gubaidullin, R.R.; Odinokov, V.N.; Bel’skii, Y.P.; Bel’skaya, N.V.; Khazanov, V.A. Triphenylphosphonium Cations of Betulinic Acid Derivatives: Synthesis and Antitumor Activity. Med. Chem. Res. 2017, 26, 518–531. [Google Scholar] [CrossRef]
- Ye, Y.; Zhang, T.; Yuan, H.; Li, D.; Lou, H.; Fan, P. Mitochondria-Targeted Lupane Triterpenoid Derivatives and Their Selective Apoptosis-Inducing Anticancer Mechanisms. J. Med. Chem. 2017, 60, 6353–6363. [Google Scholar] [CrossRef]
- He, H.; Li, D.-W.; Yang, L.-Y.; Fu, L.; Zhu, X.-J.; Wong, W.-K.; Jiang, F.-L.; Liu, Y. A Novel Bifunctional Mitochondria-Targeted Anticancer Agent with High Selectivity for Cancer Cells. Sci. Rep. 2015, 5, 13543. [Google Scholar] [CrossRef]
- Forrest, M.D. Why Cancer Cells Have a More Hyperpolarised Mitochondrial Membrane Potential and Emergent Prospects for Therapy. bioRxiv 2015, 25197. [Google Scholar] [CrossRef]
- Spivak, A.Y.; Nedopekina, D.A.; Gubaidullin, R.R.; Dubinin, M.V.; Belosludtsev, K.N. Conjugation of Natural Triterpenic Acids with Delocalized Lipophilic Cations: Selective Targeting Cancer Cell Mitochondria. J. Pers. Med. 2021, 11, 470. [Google Scholar] [CrossRef]
- Dickinson, B.C.; Lin, V.S.; Chang, C.J. Preparation and Use of MitoPY1 for Imaging Hydrogen Peroxide in Mitochondria of Live Cells. Nat. Protoc. 2013, 8, 1249–1259. [Google Scholar] [CrossRef]
- Chamberlain, G.R.; Tulumello, D.V.; Kelley, S.O. Targeted Delivery of Doxorubicin to Mitochondria. ACS Chem. Biol. 2013, 8, 1389–1395. [Google Scholar] [CrossRef]
- Lim, S.-Y.; Hong, K.-H.; Kim, D.I.; Kwon, H.; Kim, H.-J. Tunable Heptamethine–Azo Dye Conjugate as an NIR Fluorescent Probe for the Selective Detection of Mitochondrial Glutathione over Cysteine and Homocysteine. J. Am. Chem. Soc. 2014, 136, 7018–7025. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, X.; Hu, M.; Zhu, C.; Guo, Z. A Mitochondrion-Targeting Copper Complex Exhibits Potent Cytotoxicity against Cisplatin-Resistant Tumor Cells through Multiple Mechanisms of Action. Chem. Sci. 2014, 5, 2761–2770. [Google Scholar] [CrossRef]
- Lee, M.H.; Park, N.; Yi, C.; Han, J.H.; Hong, J.H.; Kim, K.P.; Kang, D.H.; Sessler, J.L.; Kang, C.; Kim, J.S. Mitochondria-Immobilized PH-Sensitive Off–On Fluorescent Probe. J. Am. Chem. Soc. 2014, 136, 14136–14142. [Google Scholar] [CrossRef]
- Sommerwerk, S.; Heller, L.; Kerzig, C.; Kramell, A.E.; Csuk, R. Rhodamine B Conjugates of Triterpenoic Acids Are Cytotoxic Mitocans Even at Nanomolar Concentrations. Eur. J. Med. Chem. 2017, 127, 1–9. [Google Scholar] [CrossRef]
- Spivak, A.Y.; Nedopekina, D.A.; Shakurova, E.R.; Khalitova, R.R.; Gubaidullin, R.R.; Odinokov, V.N.; Dzhemilev, U.M.; Bel’skii, Y.P.; Bel’skaya, N.V.; Stankevich, S.A.; et al. Synthesis of Lupane Triterpenoids with Triphenylphosphonium Substituents and Studies of Their Antitumor Activity. Russ. Chem. Bull. 2013, 62, 188–198. [Google Scholar] [CrossRef]
- Spivak, A.Y.; Keiser, J.; Vargas, M.; Gubaidullin, R.R.; Nedopekina, D.A.; Shakurova, E.R.; Khalitova, R.R.; Odinokov, V.N. Synthesis and Activity of New Triphenylphosphonium Derivatives of Betulin and Betulinic Acid against Schistosoma Mansoni in Vitro and in Vivo. Bioorg. Med. Chem. 2014, 22, 6297–6304. [Google Scholar] [CrossRef]
- Nedopekina, D.A.; Gubaidullin, R.R.; Odinokov, V.N.; Maximchik, P.V.; Zhivotovsky, B.; Bel’skii, Y.P.; Khazanov, V.A.; Manuylova, A.V.; Gogvadze, V.; Spivak, A.Y. Mitochondria-Targeted Betulinic and Ursolic Acid Derivatives: Synthesis and Anticancer Activity. MedChemComm 2017, 8, 1934–1945. [Google Scholar] [CrossRef]
- Tsepaeva, O.V.; Nemtarev, A.V.; Abdullin, T.I.; Grigor’eva, L.R.; Kuznetsova, E.V.; Akhmadishina, R.A.; Ziganshina, L.E.; Cong, H.H.; Mironov, V.F. Design, Synthesis, and Cancer Cell Growth Inhibitory Activity of Triphenylphosphonium Derivatives of the Triterpenoid Betulin. J. Nat. Prod. 2017, 80, 2232–2239. [Google Scholar] [CrossRef]
- Tsepaeva, O.V.; Nemtarev, A.V.; Salikhova, T.I.; Abdullin, T.I.; Grigor Eva, L.R.; Khozyainova, S.A.; Mironov, V.F. Synthesis, Anticancer, and Antibacterial Activity of Betulinic and Betulonic Acid C-28-Triphenylphosphonium Conjugates with Variable Alkyl Linker Length. Anticancer Agents Med. Chem. 2020, 20, 286–300. [Google Scholar] [CrossRef]
- Grymel, M.; Zawojak, M.; Adamek, J. Triphenylphosphonium Analogues of Betulin and Betulinic Acid with Biological Activity: A Comprehensive Review. J. Nat. Prod. 2019, 82, 1719–1730. [Google Scholar] [CrossRef]
- Jara, J.A.; Castro-Castillo, V.; Saavedra-Olavarría, J.; Peredo, L.; Pavanni, M.; Jaña, F.; Letelier, M.E.; Parra, E.; Becker, M.I.; Morello, A.; et al. Antiproliferative and Uncoupling Effects of Delocalized, Lipophilic, Cationic Gallic Acid Derivatives on Cancer Cell Lines. Validation in Vivo in Singenic Mice. J. Med. Chem. 2014, 57, 2440–2454. [Google Scholar] [CrossRef]
- Sandoval-Acuña, C.; Fuentes-Retamal, S.; Guzmán-Rivera, D.; Peredo-Silva, L.; Madrid-Rojas, M.; Rebolledo, S.; Castro-Castillo, V.; Pavani, M.; Catalán, M.; Maya, J.D.; et al. Destabilization of Mitochondrial Functions as a Target against Breast Cancer Progression: Role of TPP+-Linked-Polyhydroxybenzoates. Toxicol. Appl. Pharmacol. 2016, 309, 2–14. [Google Scholar] [CrossRef]
- Ahmad, V.U.; Rahman, A.-U. Handbook of Natural Products Data. Volume 2: Pentacyclic Triterpenoids; Elsevier Science: Amsterdam, The Netherlands, 1994. [Google Scholar]
- Garcez, F.R.; Garcez, W.S.; Miguel, D.L.S.; Serea, A.A.T.; Prado, F.C. Chemical Constituents from Terminalia Glabrescens. J. Braz. Chem. Soc. 2003, 14, 461–465. [Google Scholar] [CrossRef]
- Garcez, F.R.; Garcez, W.S.; Santana, A.L.B.D.; Alves, M.M.; Matos, M.d.F.C.; Scaliante, A.d.M. Bioactive Flavonoids and Triterpenes from Terminalia Fagifolia (Combretaceae). J. Braz. Chem. Soc. 2006, 17, 1223–1228. [Google Scholar] [CrossRef]
- Bisoli, E.; Garcez, W.S.; Hamerski, L.; Tieppo, C.; Garcez, F.R. Bioactive Pentacyclic Triterpenes from the Stems of Combretum Laxum. Molecules 2008, 13, 2717–2728. [Google Scholar] [CrossRef]
- Satiraphan, M.; Pamonsinlapatham, P.; Sotanaphun, U.; Sittisombut, C.; Raynaud, F.; Garbay, C.; Michel, S.; Cachet, X. Lupane Triterpenes from the Leaves of the Tropical Rain Forest Tree Hopea Odorata Roxb. and Their Cytotoxic Activities. Biochem. Syst. Ecol. 2012, 44, 407–412. [Google Scholar] [CrossRef]
- Saha, P.C.; Chatterjee, T.; Pattanayak, R.; Das, R.S.; Mukherjee, A.; Bhattacharyya, M.; Guha, S. Targeting and Imaging of Mitochondria Using Near-Infrared Cyanine Dye and Its Application to Multicolor Imaging. ACS Omega 2019, 4, 14579–14588. [Google Scholar] [CrossRef]
- Saha, P.C.; Das, R.S.; Das, S.; Sepay, N.; Chatterjee, T.; Mukherjee, A.; Bera, T.; Kar, S.; Bhattacharyya, M.; Sengupta, A.; et al. Live-Cell Mitochondrial Targeted NIR Fluorescent Covalent Labeling of Specific Proteins Using a Dual Localization Effect. Bioconjug. Chem. 2023, 34, 1407–1417. [Google Scholar] [CrossRef]
- Scherließ, R. The MTT Assay as Tool to Evaluate and Compare Excipient Toxicity in Vitro on Respiratory Epithelial Cells. Int. J. Pharm. 2011, 411, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Rozas, C.; Simpson, S.; Fuentes-Retamal, S.; Catalán, M.; Ferreira, J.; Theoduloz, C.; Mella, J.; Cabezas, D.; Cassels, B.K.; Yáñez, C.; et al. Antiproliferative and Proapoptotic Activities of Aza-Annulated Naphthoquinone Analogs. Toxicol. Vitr. 2019, 54, 375–390. [Google Scholar] [CrossRef] [PubMed]
- Kippo, T.; Hamaoka, K.; Ryu, I. Bromine Radical-Mediated Sequential Radical Rearrangement and Addition Reaction of Alkylidenecyclopropanes. J. Am. Chem. Soc. 2013, 135, 632–635. [Google Scholar] [CrossRef]
- Chen, X.; Khairallah, G.N.; O’Hair, R.A.J.; Williams, S.J. Fixed-Charge Labels for Simplified Reaction Analysis: 5-Hydroxy-1,2,3-Triazoles as Byproducts of a Copper(I)-Catalyzed Click Reaction. Tetrahedron Lett. 2011, 52, 2750–2753. [Google Scholar] [CrossRef]
- Hill, W.E.; Islam, M.Q.; Webb, T.R.; McAuliffe, C.A. Solution Studies of Gold(I) Complexes of n-Hexyldimethylphosphine, n-Butyldiphenylphosphine, 1-Dimethylphosphino-6-Diphenylphosphinohexane, 1,6-Bis(Dimethylphosphino)Hexane and 1,6-Bis(Diphenylphosphino)Hexane. Inorganica Chim. Acta 1989, 157, 215–222. [Google Scholar] [CrossRef]
- Riss, T.; Niles, A.; Moravec, R.; Karassina, N.; Vidugiriene, J. Cytotoxicity Assays: In Vitro Methods to Measure Dead Cells; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Indianapolis, IN, USA; Bethesda (MD): Promega Corporation: Madison, WI, USA, 2004. [Google Scholar]
- Vagia, E.; Mahalingam, D.; Cristofanilli, M. The Landscape of Targeted Therapies in TNBC. Cancers 2020, 12, 916. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yu, C.-W. Epigenetic Modulations in Triple-Negative Breast Cancer: Therapeutic Implications for Tumor Microenvironment. Pharmacol. Res. 2024, 204, 107205. [Google Scholar] [CrossRef] [PubMed]
- Dubinin, M.V.; Semenova, A.A.; Ilzorkina, A.I.; Penkov, N.V.; Nedopekina, D.A.; Sharapov, V.A.; Khoroshavina, E.I.; Davletshin, E.V.; Belosludtseva, N.V.; Spivak, A.Y.; et al. Mitochondria-Targeted Prooxidant Effects of Betulinic Acid Conjugated with Delocalized Lipophilic Cation F16. Free Radic. Biol. Med. 2021, 168, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Shin, A.E.; Giancotti, F.G.; Rustgi, A.K. Metastatic Colorectal Cancer: Mechanisms and Emerging Therapeutics. Trends Pharmacol. Sci. 2023, 44, 222–236. [Google Scholar] [CrossRef]
- Suárez-Rozas, C.; Jara, J.A.; Cortés, G.; Rojas, D.; Araya-Valdés, G.; Molina-Berrios, A.; González-Herrera, F.; Fuentes-Retamal, S.; Aránguiz-Urroz, P.; Campodónico, P.R.; et al. Antimigratory Effect of Lipophilic Cations Derived from Gallic and Gentisic Acid and Synergistic Effect with 5-Fluorouracil on Metastatic Colorectal Cancer Cells: A New Synthesis Route. Cancers 2024, 16, 2980. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. ILOGP: A Simple, Robust, and Efficient Description of n-Octanol/Water Partition Coefficient for Drug Design Using the GB/SA Approach. J. Chem. Inf. Model. 2014, 54, 3284–3301. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings1PII of Original Article: S0169-409X(96)00423-1. The Article Was Originally Published in Advanced Drug Delivery Reviews 23 (1997) 3. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Li, F.; Goila-Gaur, R.; Salzwedel, K.; Kilgore, N.R.; Reddick, M.; Matallana, C.; Castillo, A.; Zoumplis, D.; Martin, D.E.; Orenstein, J.M.; et al. PA-457: A Potent HIV Inhibitor That Disrupts Core Condensation by Targeting a Late Step in Gag Processing. Proc. Natl. Acad. Sci. USA 2003, 100, 13555–13560. [Google Scholar] [CrossRef]
- Wang, D.; Lu, W.; Li, F. Pharmacological Intervention of HIV-1 Maturation. Acta Pharm. Sin. B 2015, 5, 493–499. [Google Scholar] [CrossRef]
- Silva, F.S.G.; Oliveira, P.J.; Duarte, M.F. Oleanolic, Ursolic, and Betulinic Acids as Food Supplements or Pharmaceutical Agents for Type 2 Diabetes: Promise or Illusion? J. Agric. Food Chem. 2016, 64, 2991–3008. [Google Scholar] [CrossRef]
- Udeani, G.O.; Zhao, G.-M.; Geun Shin, Y.; Cooke, B.P.; Graham, J.; Beecher, C.W.W.; Kinghorn, A.D.; Pezzuto, J.M. Pharmacokinetics and Tissue Distribution of Betulinic Acid in CD-1 Mice1. Biopharm. Drug Dispos. 1999, 20, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.E.; Blum, R.; Wilton, J.; Doto, J.; Galbraith, H.; Burgess, G.L.; Smith, P.C.; Ballow, C. Safety and Pharmacokinetics of Bevirimat (PA-457), a Novel Inhibitor of Human Immunodeficiency Virus Maturation, in Healthy Volunteers. Antimicrob. Agents Chemother. 2007, 51, 3063–3066. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.F.; Ogundele, A.; Forrest, A.; Wilton, J.; Salzwedel, K.; Doto, J.; Allaway, G.P.; Martin, D.E. Phase I and II Study of the Safety, Virologic Effect, and Pharmacokinetics/Pharmacodynamics of Single-Dose 3-O-(3′,3′-Dimethylsuccinyl)Betulinic Acid (Bevirimat) against Human Immunodeficiency Virus Infection. Antimicrob. Agents Chemother. 2007, 51, 3574–3581. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Xiao, Y.; Fu, B.; Qin, Z. TPP-Based Mitocans: A Potent Strategy for Anticancer Drug Design. RSC Med. Chem. 2020, 11, 858–875. [Google Scholar] [CrossRef]
- Wang, J.Y.; Li, J.Q.; Xiao, Y.M.; Fu, B.; Qin, Z.H. Triphenylphosphonium (TPP)-Based Antioxidants: A New Perspective on Antioxidant Design. ChemMedChem 2020, 15, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Guengerich, F.P. Role of Cytochrome P450 Enzymes in Drug-Drug Interactions. Adv. Pharmacol. 1997, 43, 7–35. [Google Scholar] [CrossRef] [PubMed]
- Zanger, U.M.; Schwab, M. Cytochrome P450 Enzymes in Drug Metabolism: Regulation of Gene Expression, Enzyme Activities, and Impact of Genetic Variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar] [CrossRef] [PubMed]


| Compound | IC50 (μM) | ||||
|---|---|---|---|---|---|
| MRC-5 | AGS | SK-MES-1 | J82 | HL-60 | |
| BA-C4-TPP+ | 0.21 ± 0.01 | 0.35 ± 0.02 | 0.32 ± 0.08 | 0.84 ± 0.02 | 0.22 ± 0.04 |
| BA-C5-TPP+ | 0.83 ± 0.02 | 0.77 ± 0.08 | 0.47 ± 0.04 | 1.22 ± 0.01 | 0.63 ± 0.05 |
| BA-C6-TPP+ | 1.35 ± 0.1 | 1.27 ± 0.02 | 0.68 ± 0.01 | 1.71 ± 0.05 | 0.91 ± 0.01 |
| BA | 56.70 ± 4.3 | 14.12 ± 0.5 | 44.83 ± 2.7 | 32.81 ± 1.7 | 21.92 ± 1.5 |
| Etoposide | 3.96 ± 0.21 | 0.46 ± 0.04 | 2.61 ± 0.01 | 2.85 ± 0.02 | 0.82 ± 0.01 |
| Compound | IC50 (μM) | |||||
|---|---|---|---|---|---|---|
| HCT-15 | COLO205 | |||||
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | |
| BA-C4-TPP+Br− | 1.46 ± 0.17 | 0.61 ± 0.16 | 0.52 ± 0.12 | 0.88 ± 0.22 | 0.45 ± 0.09 | 0.13 ± 0.01 |
| BA-C5-TPP+Br− | 5.17 ± 1.32 | 1.93 ± 0.36 | 1.93 ± 0.32 | 4.32 ± 2.4 | 1.62 ± 1.15 | 1.61 ± 0.02 |
| BA-C6-TPP+Br− | 9.04 ± 2.8 | 2.89 ± 0.24 | 2.4 ± 0.13 | 4.87 ± 1.43 | 0.84 ± 0.39 | 0.97 ± 0.29 |
| BA | 43.83 ± 3.64 | 9.47 ± 3.1 | 3.89 ± 1.3 | 97.68 ± 1.02 | 74.6 ± 2.87 | 34.34 ± 2.58 |
| Compound | IC50 (μM) | |||||
|---|---|---|---|---|---|---|
| MCF-7 | MDA-MB-231 | AU565 | ||||
| 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | |
| BA-C4-TPP+Br− | 3.88 ± 0.45 | 0.95 ± 0.34 | 1.70 ± 0.44 | 1.01 ± 0.09 | 1.66 ± 0.12 | 0.60 ± 0.05 |
| BA-C5-TPP+Br− | 8.68 ± 1.27 | 1.77 ± 0.40 | 5.50 ± 0.60 | 2.53 ± 0.65 | 5.91 ± 0.08 | 1.02 ± 0.18 |
| BA-C6-TPP+Br− | 7.69 ± 1.32 | 4.62 ± 0.53 | 7.28 ± 0.53 | 2.66 ± 0.08 | 6.37 ± 0.31 | 1.33 ± 0.12 |
| BA | - | - | - | - | 33.09 ± 0.17 | 9.29 ± 0.98 |
| Physicochemical Properties | Lipinski’s Rule | Veber’s Rule | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Molecule | log P b | Log S c | MW d | MlogP e | ∑HBD f | ∑O + N g | TPSA a | Rotatable bonds | % ABS h |
| BA | 6.14 | −7.71 | 456.70 | 5.82 | 2 | 3 | 57.53 | 2 | 89.2 |
| 4 | 6.79 | −8.76 | 584.83 | 5.87 | 2 | 6 | 100.9 | 7 | 74.2 |
| BA-C4-TPP+Br− | 9.22 | −13.85 | 854.01 | 9.06 | 1 | 3 | 60.12 | 11 | 88.3 |
| BA-C5-TPP+Br− | 9.52 | −14.09 | 868.01 | 9.21 | 1 | 3 | 60.12 | 12 | 88.3 |
| BA-C6-TPP+Br− | 9.74 | −13.33 | 882.04 | 9.36 | 1 | 3 | 60.12 | 13 | 88.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suárez-Rozas, C.; Duarte-Salinas, C.; Gajardo-De la Fuente, J.; Salgado-Figueroa, P.; Salas-Norambuena, J.; Cassels, B.K.; Theoduloz, C.; Jara, J.A.; Fuentes-Retamal, S.; Campodónico, P.R.; et al. Betulinic Acid ω-Triphenylphosphonium Alkyl Esters: Antiproliferative Activities and In Silico Pharmacokinetic Profiles. Biomedicines 2025, 13, 1539. https://doi.org/10.3390/biomedicines13071539
Suárez-Rozas C, Duarte-Salinas C, Gajardo-De la Fuente J, Salgado-Figueroa P, Salas-Norambuena J, Cassels BK, Theoduloz C, Jara JA, Fuentes-Retamal S, Campodónico PR, et al. Betulinic Acid ω-Triphenylphosphonium Alkyl Esters: Antiproliferative Activities and In Silico Pharmacokinetic Profiles. Biomedicines. 2025; 13(7):1539. https://doi.org/10.3390/biomedicines13071539
Chicago/Turabian StyleSuárez-Rozas, Cristian, Claudia Duarte-Salinas, Javier Gajardo-De la Fuente, Paola Salgado-Figueroa, Julio Salas-Norambuena, Bruce K. Cassels, Cristina Theoduloz, José A. Jara, Sebastián Fuentes-Retamal, Paola R. Campodónico, and et al. 2025. "Betulinic Acid ω-Triphenylphosphonium Alkyl Esters: Antiproliferative Activities and In Silico Pharmacokinetic Profiles" Biomedicines 13, no. 7: 1539. https://doi.org/10.3390/biomedicines13071539
APA StyleSuárez-Rozas, C., Duarte-Salinas, C., Gajardo-De la Fuente, J., Salgado-Figueroa, P., Salas-Norambuena, J., Cassels, B. K., Theoduloz, C., Jara, J. A., Fuentes-Retamal, S., Campodónico, P. R., Soto-Delgado, J., & Catalán, M. (2025). Betulinic Acid ω-Triphenylphosphonium Alkyl Esters: Antiproliferative Activities and In Silico Pharmacokinetic Profiles. Biomedicines, 13(7), 1539. https://doi.org/10.3390/biomedicines13071539








