Plantar Fasciitis Pathophysiology and the Potential Role of Mesenchymal Stem Cell-Derived Extracellular Vesicles as Therapy
Abstract
1. Introduction
2. The Anatomy of the Plantar Fascia and the Pathophysiology of Plantar Fasciitis
3. Clinical Presentation and Diagnosis
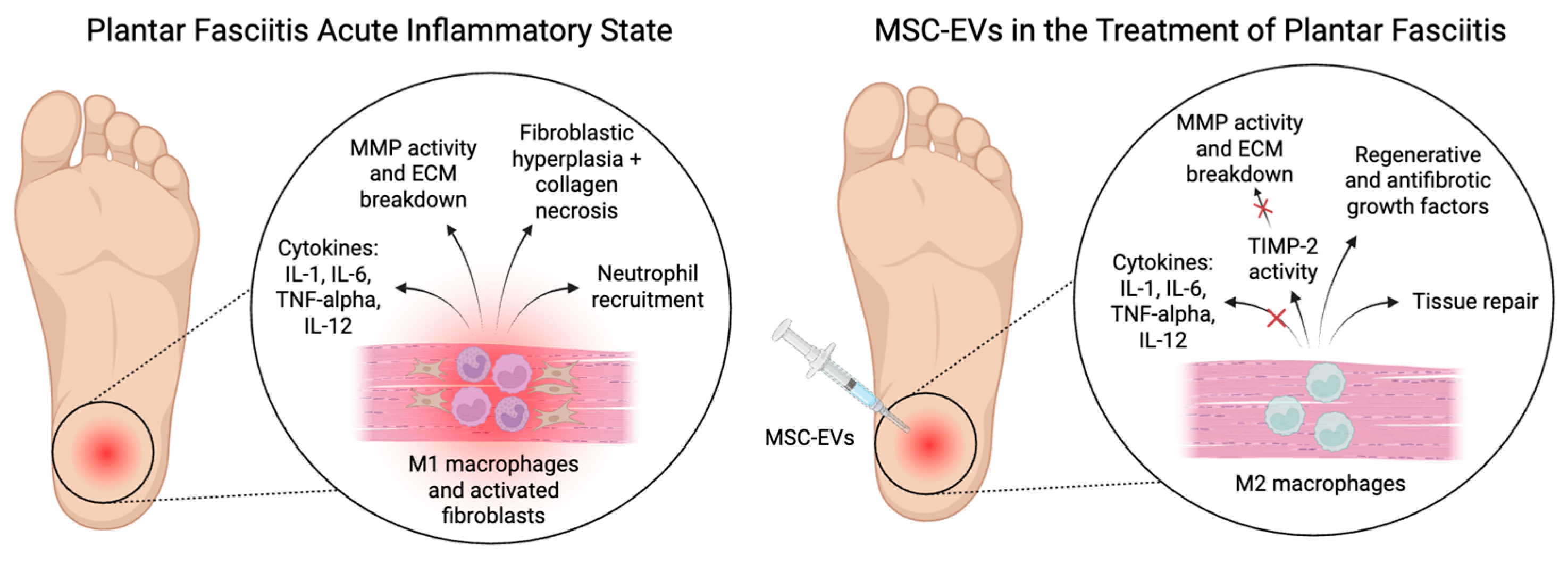
4. Cellular Response in Plantar Fasciitis
5. Current Treatment Strategies
5.1. Conservative Treatments
5.2. Advanced Therapies
6. Extracellular Vesicles for Therapy for Plantar Fasciitis
6.1. What Are Extracellular Vesicles?
6.2. Mechanisms of Action in Plantar Fasciitis
6.3. Preclinical Evidence of Foot Tendinopathy Treatment with MSC-EVs
| Author | Animal Model | Model Type | Treatment | Results |
|---|---|---|---|---|
| Wang et al. (2019) [59] | Rat | Enzyme-induced Achilles tendon injury | Tendon stem cell-derived EVs (TSC-EVs) | Reduced MMP-3 expression (p = 0.0052), induced tenomodulin expression, promoted collagen remodeling (p < 0.05), and improved biomechanical properties, indicating enhanced functional recovery. |
| Zhang et al. (2020) [76] | Rat | Achilles tendon injury | TSC-EVs | Promoted tenocyte proliferation (p < 0.0001) and migration (p < 0.0001); suppressed inflammation (p < 0.05) and apoptosis (p < 0.01) via the PI3K/AKT and MAPK/ERK1/2 signaling pathways, supporting the role of EVs in regulating tendon homeostasis. |
| Shen et al. (2023) [58] | Mouse | Achilles tendon injury | Interferon-γ-primed adipose-derived stem cell EVs (ASC-EVs) | Significantly reduced inflammation by suppressing NF-κB activity (p < 0.05) and downregulating pro-inflammatory genes Il1b and Ifng (p < 0.05); treated tendons exhibited reduced gap formation and rupture rates with enhanced collagen deposition (p < 0.05). |
| Li et al. (2020) [78] | Rat | Achilles tendon injury | Hydroxycamptothecin-primed EVs (HCPT-EVs) from human umbilical cord stem cells | Demonstrated superior anti-adhesion effects (p < 0.001) by inhibiting fibroblast proliferation (p < 0.001), viability (p < 0.001), and myofibroblast differentiation; activated the endoplasmic reticulum stress pathway to counteract fibrosis. |
| Hayashi et al. (2022) [79] | Rat | Achilles tendinopathy | Bone marrow MSC-derived EVs from early-passage cells | Exhibited glycan patterns associated with enhanced therapeutic efficacy, whereas late-passage cells showed altered glycosylation linked to reduced regenerative potential, emphasizing the importance of optimizing EV sources for clinical applications. |
| Chen et al. (2024) [80] | Mouse | Achilles tendon rupture | Dendritic cell-derived exosomes (DEXs) | Enhanced tendon healing by promoting collagen type I synthesis (p < 0.05), inhibiting collagen type III (p < 0.05), and facilitating tendon cell differentiation (p < 0.05); improved the inflammatory microenvironment by shifting M1 macrophages to M2 via the PI3K/AKT pathway and reducing key inflammatory cytokines (p < 0.05). |
| Parafioriti et al. 2011 [81] | Rat | Surgical Achilles tendon rupture | Single PRP injection (0.25 mL) | PRP improved tendon remodeling in the first week by enhancing tendon-like continuity, but after 2, 4, and 6 weeks, no difference was observed between PRP-treated and control groups in histology, immunostaining, or RT-PCR (p = 0.2). A single PRP injection was not effective for long-term healing. |
| Rajabi et al. (2015) [82] | Rat | Crush lesion on Achilles tendon | Aquatic activity and PRP injection | Significant increase in fibroblast number (p < 0.05), cellular density (p < 0.05), and collagen deposition (p < 0.05) in the combined treatment group, indicating effective tendon healing; no significant difference in tendon diameter among groups. |
| Chen et al. (2014) [83] | Rat | Collagenase-induced Achilles tendinopathy | Tendon-derived stem cells (TDSCs) and PRP | PRP treatment improved tendon healing, histology, and biomechanics (p < 0.01); PRP + TDSC combination further enhanced healing. PRP activated FAK/ERK1/2 pathways (p < 0.01) and tenocyte-related genes (p < 0.01). TDSC injection alone had little effect. |
| Yan et al. (2017) [84] | Rabbit | Collagenase-induced Achilles tendinopathy | Leukocyte-rich (Lr-PRP) and leukocyte-poor PRP (Lp-PRP) injections | MRI scans showed that Lp-PRP decreased signal intensity on T2 mapping compared to Lr-PRP and saline (p < 0.05), signifying less inflammatory edema in Lp-PRP. Lp-PRP decreased levels of IL-6 and increased levels of TIMP-1 at the lesion compared to Lr-PRP and saline (p < 0.05). Histology scoring showed significant improvement of the Lp-PRP group compared to Lr-PRP and saline (p < 0.05). Lp-PRP was found to have greater healing effects than Lr-PRP. |
| Lyras et al. (2010) [85] | Rabbit | Achilles tendon rupture model (transection) | Single PRP injection (1 mL) | Levels of TGF-β1 were compared between the PRP and injection groups. At weeks 1–2 post-injection, TGF-β1 levels were significantly higher in the PRP group (p < 0.0001). At weeks 3–4, TGF-β1 levels were significantly higher in the control group (p < 0.0001). The PRP group showed better healing overall with less inflammatory cells and vessels observed at 4 weeks. |
6.4. Limitations in Clinical Translation
7. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trojian, T.; Tucker, A.K. Plantar Fasciitis. Am. Fam. Physician 2019, 99, 744–750. [Google Scholar] [PubMed]
- Rhim, H.C.; Kwon, J.; Park, J.; Borg-Stein, J.; Tenforde, A.S. A Systematic Review of Systematic Reviews on the Epidemiology, Evaluation, and Treatment of Plantar Fasciitis. Life 2021, 11, 1287. [Google Scholar] [CrossRef] [PubMed]
- Tong, K.B.; Furia, J. Economic burden of plantar fasciitis treatment in the United States. Am. J. Orthop. 2010, 39, 227–231. [Google Scholar]
- Landorf, K.B.; Kaminski, M.R.; Munteanu, S.E.; Zammit, G.V.; Menz, H.B. Health-related quality of life is substantially worse in individuals with plantar heel pain. Sci. Rep. 2022, 12, 15652. [Google Scholar] [CrossRef] [PubMed]
- Lemont, H.; Ammirati, K.M.; Usen, N. Plantar fasciitis: A degenerative process (fasciosis) without inflammation. J. Am. Podiatr. Med. Assoc. 2003, 93, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Rabadi, D.; Seo, S.; Wong, B.; Chung, D.; Rai, V.; Agrawal, D.K. Immunopathogenesis, early Detection, current therapies and prevention of plantar Fasciitis: A concise review. Int. Immunopharmacol. 2022, 110, 109023. [Google Scholar] [CrossRef]
- Danilkowicz, R.; Murawski, C.; Pellegrini, M.; Walther, M.; Valderrabano, V.; Angthong, C.; Adams, S. Nonoperative and Operative Soft-Tissue and Cartilage Regeneration and Orthopaedic Biologics of the Foot and Ankle: An Orthoregeneration Network Foundation Review. Arthroscopy 2022, 38, 2350–2358. [Google Scholar] [CrossRef]
- Daher, M.; Covarrubias, O.; Herber, A.; Oh, I.; Gianakos, A.L. Platelet-Rich Plasma vs Extracorporeal Shock Wave Therapy in the Treatment of Plantar Fasciitis at 3-6 Months: A 579 Systematic Review and Meta-analysis of Randomized Controlled Trials. Foot Ankle Int. 2024, 45, 796–803. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- Phinney, D.G.; Pittenger, M.F. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells 2017, 35, 851–858. [Google Scholar] [CrossRef]
- Wearing, S.C.; Smeathers, J.E.; Urry, S.R.; Hennig, E.M.; Hills, A.P. The pathomechanics of plantar fasciitis. Sports Med. 2006, 36, 585–611. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, S.K.; Cerrato, R. Plantar fasciitis: Evaluation and treatment. J. Am. Acad. Orthop. Surg. 2008, 16, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.K.; Kitaoka, H.B.; An, K.N.; Chao, E.Y. Biomechanical evaluation of longitudinal arch stability. Foot Ankle 1993, 14, 353–357. [Google Scholar] [CrossRef]
- De Garceau, D.; Dean, D.; Requejo, S.M.; Thordarson, D.B. The association between diagnosis of plantar fasciitis and Windlass test results. Foot Ankle Int. 2003, 24, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.E.; Latt, D.L. Chronic Plantar Fasciitis is Mediated by Local Hemodynamics: Implications for Emerging Therapies. N. Am. J. Med. Sci. 2015, 7, 1–5. [Google Scholar] [CrossRef]
- Luffy, L.; Grosel, J.; Thomas, R.; So, E. Plantar fasciitis: A review of treatments. J. Am. Acad. Physician Assist. 2018, 31, 20–24. [Google Scholar] [CrossRef]
- Thompson, J.V.; Saini, S.S.; Reb, C.W.; Daniel, J.N. Diagnosis and management of plantar fasciitis. J. Am. Osteopath. Assoc. 2014, 114, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Draghi, F.; Gitto, S.; Bortolotto, C.; Draghi, A.G.; Ori Belometti, G. Imaging of plantar fascia disorders: Findings on plain radiography, ultrasound and magnetic resonance imaging. Insights Imaging 2017, 8, 69–78. [Google Scholar] [CrossRef]
- Mantovani, A.; Dinarello, C.A.; Molgora, M.; Garlanda, C. Interleukin-1 and Related Cytokines in the Regulation of Inflammation and Immunity. Immunity 2019, 50, 778–795. [Google Scholar] [CrossRef]
- Grebenciucova, E.; VanHaerents, S. Interleukin 6: At the interface of human health and disease. Front. Immunol. 2023, 14, 1255533. [Google Scholar] [CrossRef]
- Zelova, H.; Hosek, J. TNF-alpha signalling and inflammation: Interactions between old acquaintances. Inflamm. Res. 2013, 62, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Trinchieri, G. Proinflammatory and immunoregulatory functions of interleukin-12. Int. Rev. Immunol. 1998, 16, 365–396. [Google Scholar] [CrossRef]
- Younesi, F.S.; Miller, A.E.; Barker, T.H.; Rossi, F.M.V.; Hinz, B. Author Correction: Fibroblast and myofibroblast activation in normal tissue repair and fibrosis. Nat. Rev. Mol. Cell Biol. 2024, 25, 671. [Google Scholar] [CrossRef] [PubMed]
- Gerarduzzi, C.; Di Battista, J.A. Myofibroblast repair mechanisms post-inflammatory response: A fibrotic perspective. Inflamm. Res. 2017, 66, 451–465. [Google Scholar] [CrossRef]
- Siu, W.S.; Ma, H.; Ko, C.H.; Shiu, H.T.; Cheng, W.; Lee, Y.W.; Kot, C.H.; Leung, P.C.; Lui, P.P.Y. Rat Plantar Fascia Stem/Progenitor Cells Showed Lower Expression of Ligament Markers and Higher Pro-Inflammatory Cytokines after Intensive Mechanical Loading or Interleukin-1beta Treatment In Vitro. Cells 2023, 12, 2222. [Google Scholar] [CrossRef]
- Donley, B.G.; Moore, T.; Sferra, J.; Gozdanovic, J.; Smith, R. The efficacy of oral nonsteroidal anti-inflammatory medication (NSAID) in the treatment of plantar fasciitis: A randomized, prospective, placebo-controlled study. Foot Ankle Int. 2007, 28, 20–23. [Google Scholar] [CrossRef]
- Pfeffer, G.; Bacchetti, P.; Deland, J.; Lewis, A.; Anderson, R.; Davis, W.; Alvarez, R.; Brodsky, J.; Cooper, P.; Frey, C.; et al. Comparison of custom and prefabricated orthoses in the initial treatment of proximal plantar fasciitis. Foot Ankle Int. 1999, 20, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; McKeon, P.; Hertel, J. Does the use of orthoses improve self-reported pain and function measures in patients with plantar fasciitis? A meta-analysis. Phys. Ther. Sport. 2009, 10, 12–18. [Google Scholar] [CrossRef]
- Shetty, C.D.a.V. Healing Heels: A Meta-analysis of Platelet-rich Plasma vs Corticosteroid Injections in Plantar Fasciitis Treatment. J. Foot Ankle Surg. 2024, 11, 169–176. [Google Scholar] [CrossRef]
- Ling, Y.; Wang, S. Effects of platelet-rich plasma in the treatment of plantar fasciitis: A meta-analysis of randomized controlled trials. Medicine 2018, 97, e12110. [Google Scholar] [CrossRef]
- Middleton, K.K.; Barro, V.; Muller, B.; Terada, S.; Fu, F.H. Evaluation of the effects of platelet-rich plasma (PRP) therapy involved in the healing of sports-related soft tissue injuries. Iowa Orthop. J. 2012, 32, 150–163. [Google Scholar] [PubMed]
- Chou, A.C.; Ng, S.Y.; Koo, K.O. Endoscopic Plantar Fasciotomy Improves Early Postoperative Results: A Retrospective Comparison of Outcomes After Endoscopic Versus Open Plantar Fasciotomy. J. Foot Ankle Surg. 2016, 55, 9–15. [Google Scholar] [CrossRef]
- Mao, D.W.; Chandrakumara, D.; Zheng, Q.; Kam, C.; King, C.K.K. Endoscopic plantar fasciotomy for plantar fasciitis: A systematic review and network meta-analysis of the English literature. Foot 2019, 41, 63–73. [Google Scholar] [CrossRef]
- Johannsen, F.; Konradsen, L.; Hansen, P.; Brinch, S.; Nybing, J.U.; Krogsgaard, M.R. The Effect of Endoscopic Partial Plantar Fasciotomy on Morphologic and Functional Properties of the Foot. Foot Ankle Int. 2023, 44, 415–423. [Google Scholar] [CrossRef]
- Barrett, S.L.; Day, S.V.; Pignetti, T.T.; Robinson, L.B. Endoscopic plantar fasciotomy: A multi-surgeon prospective analysis of 652 cases. J. Foot Ankle Surg. 1995, 34, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Arshad, Z.; Aslam, A.; Razzaq, M.A.; Bhatia, M. Gastrocnemius Release in the Management of Chronic Plantar Fasciitis: A Systematic Review. Foot Ankle Int. 2022, 43, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.; Cavaliere, R.; Molchan, L. Biologics in the Treatment of Plantar Fasciitis. Clin. Podiatr. Med. Surg. 2021, 38, 245–259. [Google Scholar] [CrossRef]
- Werber, B. Amniotic Tissues for the Treatment of Chronic Plantar Fasciosis and Achilles Tendinosis. J. Sports Med. (Hindawi Publ. Corp.) 2015, 2015, 219896. [Google Scholar] [CrossRef]
- Gelberman, R.H.; Shen, H.; Kormpakis, I.; Rothrauff, B.; Yang, G.; Tuan, R.S.; Xia, Y.; Sakiyama-Elbert, S.; Silva, M.J.; Thomopoulos, S. Effect of adipose-derived stromal cells and BMP12 on intrasynovial tendon repair: A biomechanical, biochemical, and proteomics study. J. Orthop. Res. 2016, 34, 630–640. [Google Scholar] [CrossRef]
- Gelberman, R.H.; Linderman, S.W.; Jayaram, R.; Dikina, A.D.; Sakiyama-Elbert, S.; Alsberg, E.; Thomopoulos, S.; Shen, H. Combined Administration of ASCs and BMP-12 Promotes an M2 Macrophage Phenotype and Enhances Tendon Healing. Clin. Orthop. Relat. Res. 2017, 475, 2318–2331. [Google Scholar] [CrossRef]
- Shen, H.; Kormpakis, I.; Havlioglu, N.; Linderman, S.W.; Sakiyama-Elbert, S.E.; Erickson, I.E.; Zarembinski, T.; Silva, M.J.; Gelberman, R.H.; Thomopoulos, S. The effect of mesenchymal stromal cell sheets on the inflammatory stage of flexor tendon healing. Stem Cell Res. Ther. 2016, 7, 144. [Google Scholar] [CrossRef]
- Citeroni, M.R.; Ciardulli, M.C.; Russo, V.; Della Porta, G.; Mauro, A.; El Khatib, M.; Di Mattia, M.; Galesso, D.; Barbera, C.; Forsyth, N.R.; et al. In Vitro Innovation of Tendon Tissue Engineering Strategies. Int. J. Mol. Sci. 2020, 21, 6726. [Google Scholar] [CrossRef]
- Perucca Orfei, C.; Bowles, A.C.; Kouroupis, D.; Willman, M.A.; Ragni, E.; Kaplan, L.D.; Best, T.M.; Correa, D.; de Girolamo, L. Human Tendon Stem/Progenitor Cell Features and Functionality Are Highly Influenced by in vitro Culture Conditions. Front. Bioeng. Biotechnol. 2021, 9, 711964. [Google Scholar] [CrossRef] [PubMed]
- Lui, P.P. Identity of tendon stem cells--how much do we know? J. Cell Mol. Med. 2013, 17, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Lovati, A.B.; Corradetti, B.; Lange Consiglio, A.; Recordati, C.; Bonacina, E.; Bizzaro, D.; Cremonesi, F. Characterization and differentiation of equine tendon-derived progenitor cells. J. Biol. Regul. Homeost. Agents 2011, 25, S75–S84. [Google Scholar] [PubMed]
- Zhang, J.; Wang, J.H. Characterization of differential properties of rabbit tendon stem cells and tenocytes. BMC Musculoskelet. Disord. 2010, 11, 10. [Google Scholar] [CrossRef]
- Neri, S. Genetic Stability of Mesenchymal Stromal Cells for Regenerative Medicine Applications: A Fundamental Biosafety Aspect. Int. J. Mol. Sci. 2019, 20, 2406. [Google Scholar] [CrossRef]
- Cazzell, S.; Stewart, J.; Agnew, P.S.; Senatore, J.; Walters, J.; Murdoch, D.; Reyzelman, A.; Miller, S.D. Randomized Controlled Trial of Micronized Dehydrated Human Amnion/Chorion Membrane (dHACM) Injection Compared to Placebo for the Treatment of Plantar Fasciitis. Foot Ankle Int. 2018, 39, 1151–1161. [Google Scholar] [CrossRef]
- Jia, Y.; Yu, L.; Ma, T.; Xu, W.; Qian, H.; Sun, Y.; Shi, H. Small extracellular vesicles isolation and separation: Current techniques, pending questions and clinical applications. Theranostics 2022, 12, 6548–6575. [Google Scholar] [CrossRef]
- Liebmann, K.; Castillo, M.A.; Jergova, S.; Best, T.M.; Sagen, J.; Kouroupis, D. Modification of Mesenchymal Stem/Stromal Cell-Derived Small Extracellular Vesicles by Calcitonin Gene Related Peptide (CGRP) Antagonist: Potential Implications for Inflammation and Pain Reversal. Cells 2024, 13, 484. [Google Scholar] [CrossRef]
- Li, M.X.; Hu, S.; Lei, H.H.; Yuan, M.; Li, X.; Hou, W.K.; Huang, X.J.; Xiao, B.W.; Yu, T.X.; Zhang, X.H.; et al. Tumor-derived miR-9-5p-loaded EVs regulate cholesterol homeostasis to promote breast cancer liver metastasis in mice. Nat. Commun. 2024, 15, 10539. [Google Scholar] [CrossRef]
- Cao, M.; Isaac, R.; Yan, W.; Ruan, X.; Jiang, L.; Wan, Y.; Wang, J.; Wang, E.; Caron, C.; Neben, S.; et al. Cancer-cell-secreted extracellular vesicles suppress insulin secretion through miR-122 to impair systemic glucose homeostasis and contribute to tumour growth. Nat. Cell Biol. 2022, 24, 954–967. [Google Scholar] [CrossRef]
- Lindenbergh, M.F.S.; Koerhuis, D.G.J.; Borg, E.G.F.; van ‘t Veld, E.M.; Driedonks, T.A.P.; Wubbolts, R.; Stoorvogel, W.; Boes, M. Bystander T-Cells Support Clonal T-Cell Activation by Controlling the Release of Dendritic Cell-Derived Immune-Stimulatory Extracellular Vesicles. Front. Immunol. 2019, 10, 448. [Google Scholar] [CrossRef]
- Kouroupis, D.; Kaplan, L.D.; Huard, J.; Best, T.M. CD10-Bound Human Mesenchymal Stem/Stromal Cell-Derived Small Extracellular Vesicles Possess Immunomodulatory Cargo and Maintain Cartilage Homeostasis under Inflammatory Conditions. Cells 2023, 12, 1824. [Google Scholar] [CrossRef]
- D’Ippolito, E.; Plantamura, I.; Bongiovanni, L.; Casalini, P.; Baroni, S.; Piovan, C.; Orlandi, R.; Gualeni, A.V.; Gloghini, A.; Rossini, A.; et al. miR-9 and miR-200 Regulate PDGFRbeta-Mediated Endothelial Differentiation of Tumor Cells in Triple-Negative Breast Cancer. Cancer Res. 2016, 76, 5562–5572. [Google Scholar] [CrossRef]
- Cao, J.Y.; Wang, B.; Tang, T.T.; Wen, Y.; Li, Z.L.; Feng, S.T.; Wu, M.; Liu, D.; Yin, D.; Ma, K.L.; et al. Exosomal miR-125b-5p deriving from mesenchymal stem cells promotes tubular repair by suppression of p53 in ischemic acute kidney injury. Theranostics 2021, 11, 5248–5266. [Google Scholar] [CrossRef]
- Zhu, D.; Johnson, T.K.; Wang, Y.; Thomas, M.; Huynh, K.; Yang, Q.; Bond, V.C.; Chen, Y.E.; Liu, D. Macrophage M2 polarization induced by exosomes from adipose-derived stem cells contributes to the exosomal proangiogenic effect on mouse ischemic hindlimb. Stem Cell Res. Ther. 2020, 11, 162. [Google Scholar] [CrossRef]
- Shen, H.; Lane, R.A. Extracellular Vesicles From Primed Adipose-Derived Stem Cells Enhance Achilles Tendon Repair by Reducing Inflammation and Promoting Intrinsic Healing. Stem Cells 2023, 41, 617–627. [Google Scholar] [CrossRef]
- Wang, Y.; He, G.; Guo, Y.; Tang, H.; Shi, Y.; Bian, X.; Zhu, M.; Kang, X.; Zhou, M.; Lyu, J.; et al. Exosomes from tendon stem cells promote injury tendon healing through balancing synthesis and degradation of the tendon extracellular matrix. J. Cell Mol. Med. 2019, 23, 5475–5485. [Google Scholar] [CrossRef]
- Zhang, J.; Nie, D.; Rocha, J.L.; Hogan, M.V.; Wang, J.H. Characterization of the structure, cells, and cellular mechanobiological response of human plantar fascia. J. Tissue Eng. 2018, 9, 2041731418801103. [Google Scholar] [CrossRef]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuna, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef]
- Mittal, R.; Patel, A.P.; Debs, L.H.; Nguyen, D.; Patel, K.; Grati, M.; Mittal, J.; Yan, D.; Chapagain, P.; Liu, X.Z. Intricate Functions of Matrix Metalloproteinases in Physiological and Pathological Conditions. J. Cell Physiol. 2016, 231, 2599–2621. [Google Scholar] [CrossRef]
- Folorunso, O.S.; Sinha, N.R.; Singh, A.; Xi, L.; Pulimamidi, V.K.; Cho, W.J.; Mittal, S.K.; Chauhan, S.K. Tissue Inhibitor of Metalloproteinase-2 Promotes Wound Healing by Suppressing Matrix Metalloproteinases and Inflammatory Cytokines in Corneal Epithelial Cells. Am. J. Pathol. 2024, 195, 754–769. [Google Scholar] [CrossRef]
- Arpino, V.; Brock, M.; Gill, S.E. The role of TIMPs in regulation of extracellular matrix proteolysis. Matrix Biol. 2015, 44–46, 247–254. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Yang, H.; Li, J.; Cai, Q.; Shapiro, I.M.; Risbud, M.V. Tumor necrosis factor-alpha- and interleukin-1beta-dependent matrix metalloproteinase-3 expression in nucleus pulposus cells requires cooperative signaling via syndecan 4 and mitogen-activated protein kinase-NF-kappaB axis: Implications in inflammatory disc disease. Am. J. Pathol. 2014, 184, 2560–2572. [Google Scholar] [CrossRef]
- Uchida, K.; Takano, S.; Matsumoto, T.; Nagura, N.; Inoue, G.; Itakura, M.; Miyagi, M.; Aikawa, J.; Iwase, D.; Minatani, A.; et al. Transforming growth factor activating kinase 1 regulates extracellular matrix degrading enzymes and pain-related molecule expression following tumor necrosis factor-alpha stimulation of synovial cells: An in vitro study. BMC Musculoskelet. Disord. 2017, 18, 283. [Google Scholar] [CrossRef]
- Mukaro, V.R.; Quach, A.; Gahan, M.E.; Boog, B.; Huang, Z.H.; Gao, X.; Haddad, C.; Mahalingam, S.; Hii, C.S.; Ferrante, A. Small tumor necrosis factor receptor biologics inhibit the tumor necrosis factor-p38 signalling axis and inflammation. Nat. Commun. 2018, 9, 1365. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, B.; Li, Y.; Liu, X.; Guo, S.; Wang, C.; Li, S.; Wang, D. The Role of Vascular Endothelial Growth Factor in Tendon Healing. Front. Physiol. 2021, 12, 766080. [Google Scholar] [CrossRef]
- Roberts, J.H.; Halper, J. Growth Factor Roles in Soft Tissue Physiology and Pathophysiology. Adv. Exp. Med. Biol. 2021, 1348, 139–159. [Google Scholar] [CrossRef]
- Farooq, M.; Khan, A.W.; Kim, M.S.; Choi, S. The Role of Fibroblast Growth Factor (FGF) Signaling in Tissue Repair and Regeneration. Cells 2021, 10, 3242. [Google Scholar] [CrossRef]
- Maddaluno, L.; Urwyler, C.; Werner, S. Fibroblast growth factors: Key players in regeneration and tissue repair. Development 2017, 144, 4047–4060. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, H.; Shi, M.; Zhang, T.; Lu, W.; Yang, S.; Cui, Q.; Li, Z. Potential Mechanisms of the Impact of Hepatocyte Growth Factor Gene-Modified Tendon Stem Cells on Tendon Healing. Front. Cell Dev. Biol. 2021, 9, 659389. [Google Scholar] [CrossRef]
- Cui, Q.; Wang, Z.; Jiang, D.; Qu, L.; Guo, J.; Li, Z. HGF inhibits TGF-beta1-induced myofibroblast differentiation and ECM deposition via MMP-2 in Achilles tendon in rat. Eur. J. Appl. Physiol. 2011, 111, 1457–1463. [Google Scholar] [CrossRef]
- Gao, X.; Gao, L.F.; Kong, X.Q.; Zhang, Y.N.; Jia, S.; Meng, C.Y. Mesenchymal stem cell-derived extracellular vesicles carrying miR-99b-3p restrain microglial activation and neuropathic pain by stimulating autophagy. Int. Immunopharmacol. 2023, 115, 109695. [Google Scholar] [CrossRef]
- Quintero, D.; Perucca Orfei, C.; Kaplan, L.D.; de Girolamo, L.; Best, T.M.; Kouroupis, D. The roles and therapeutic potentialof mesenchymal stem/stromal cells and their extracellular vesicles in 692 tendinopathies. Front. Bioeng. Biotechnol. 2023, 11, 1040762. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, H.; Cui, Q.; Han, P.; Yang, S.; Shi, M.; Zhang, T.; Zhang, Z.; Li, Z. Tendon stem cell-derived exosomes regulate inflammation and promote the high-quality healing of injured tendon. Stem Cell Res. Ther. 2020, 11, 402. [Google Scholar] [CrossRef]
- Vergadi, E.; Ieronymaki, E.; Lyroni, K.; Vaporidi, K.; Tsatsanis, C. Akt Signaling Pathway in Macrophage Activation and M1/M2 Polarization. J. Immunol. 2017, 198, 1006–1014. [Google Scholar] [CrossRef]
- Li, J.; Yao, Z.; Xiong, H.; Cui, H.; Wang, X.; Zheng, W.; Qian, Y.; Fan, C. Extracellular vesicles from hydroxycamptothecin primed umbilical cord stem cells enhance anti-adhesion potential for treatment of tendon injury. Stem Cell Res. Ther. 2020, 11, 500. [Google Scholar] [CrossRef]
- Hayashi, Y.; Yimiti, D.; Sanada, Y.; Ding, C.; Omoto, T.; Ogura, T.; Nakasa, T.; Ishikawa, M.; Hiemori, K.; Tateno, H.; et al. The therapeutic capacity of bone marrow MSC-derived extracellular vesicles in Achilles tendon healing is passage-dependent and indicated by specific glycans. FEBS Lett. 2022, 596, 1047–1058. [Google Scholar] [CrossRef]
- Chen, R.; Ai, L.; Zhang, J.; Jiang, D. Dendritic Cell-Derived Exosomes Promote Tendon Healing and Regulate Macrophage Polarization in Preventing Tendinopathy. Int. J. Nanomed. 2024, 19, 11701–11718. [Google Scholar] [CrossRef] [PubMed]
- Parafioriti, A.; Armiraglio, E.; Del Bianco, S.; Tibalt, E.; Oliva, F.; Berardi, A.C. Single injection of platelet-rich plasma in a rat Achilles tendon tear model. Muscles Ligaments Tendons J. 2011, 1, 41–47. [Google Scholar] [PubMed]
- Rajabi, H.; Sheikhani Shahin, H.; Norouzian, M.; Mehrabani, D.; Dehghani Nazhvani, S. The Healing Effects of Aquatic Activities and Allogenic Injection of Platelet-Rich Plasma (PRP) on Injuries of Achilles Tendon in Experimental Rat. World J. Plast. Surg. 2015, 4, 66–73. [Google Scholar] [PubMed]
- Chen, L.; Liu, J.P.; Tang, K.L.; Wang, Q.; Wang, G.D.; Cai, X.H.; Liu, X.M. Tendon derived stem cells promote platelet-rich plasma healing in collagenase-induced rat achilles tendinopathy. Cell Physiol. Biochem. 2014, 34, 2153–2168. [Google Scholar] [CrossRef]
- Yan, R.; Gu, Y.; Ran, J.; Hu, Y.; Zheng, Z.; Zeng, M.; Heng, B.C.; Chen, X.; Yin, Z.; Chen, W.; et al. Intratendon Delivery of Leukocyte-Poor Platelet-Rich Plasma Improves Healing Compared With Leukocyte-Rich Platelet-Rich Plasma in a Rabbit Achilles Tendinopathy Model. Am. J. Sports Med. 2017, 45, 1909–1920. [Google Scholar] [CrossRef]
- Lyras, D.N.; Kazakos, K.; Tryfonidis, M.; Agrogiannis, G.; Botaitis, S.; Kokka, A.; Drosos, G.; Tilkeridis, K.; Verettas, D. Temporal and spatial expression of TGF-beta1 in an Achilles tendon section model after application of platelet-rich plasma. Foot Ankle Surg. 2010, 16, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrugger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef]
- Mizenko, R.R.; Feaver, M.; Bozkurt, B.T.; Lowe, N.; Nguyen, B.; Huang, K.W.; Wang, A.; Carney, R.P. A critical systematic review of extracellular vesicle clinical trials. J. Extracell. Vesicles 2024, 13, e12510. [Google Scholar] [CrossRef]
- Rezaie, J.; Feghhi, M.; Etemadi, T. A review on exosomes application in clinical trials: Perspective, questions, and challenges. Cell Commun. Signal 2022, 20, 145. [Google Scholar] [CrossRef]
- Witwer, K.W.; Thery, C. Extracellular vesicles or exosomes? On primacy, precision, and popularity influencing a choice of nomenclature. J. Extracell. Vesicles 2019, 8, 1648167. [Google Scholar] [CrossRef]
- Drohat, P.; Baron, M.; Kaplan, L.D.; Best, T.M.; Kouroupis, D. Long-Acting Extracellular Vesicle-Based Biologics in Osteoarthritis Immunotherapy. Bioengineering 2025, 12, 525. [Google Scholar] [CrossRef]
- Burnouf, T.; Agrahari, V.; Agrahari, V. Extracellular Vesicles As Nanomedicine: Hopes And Hurdles In Clinical Translation. Int. J. Nanomed. 2019, 14, 8847–8859. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Wang, H.; Li, Q.; Chen, Z. Extracellular Vesicles: A Review of Their Therapeutic Potentials, Sources, Biodistribution, and Administration Routes. Int. J. Nanomed. 2025, 20, 3175–3199. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.; Ughetto, S.; Mahjoum, S.; Nair, A.V.; Breakefield, X.O. Uptake, functionality, and re-release of extracellular vesicle-encapsulated cargo. Cell Rep. 2022, 39, 110651. [Google Scholar] [CrossRef] [PubMed]
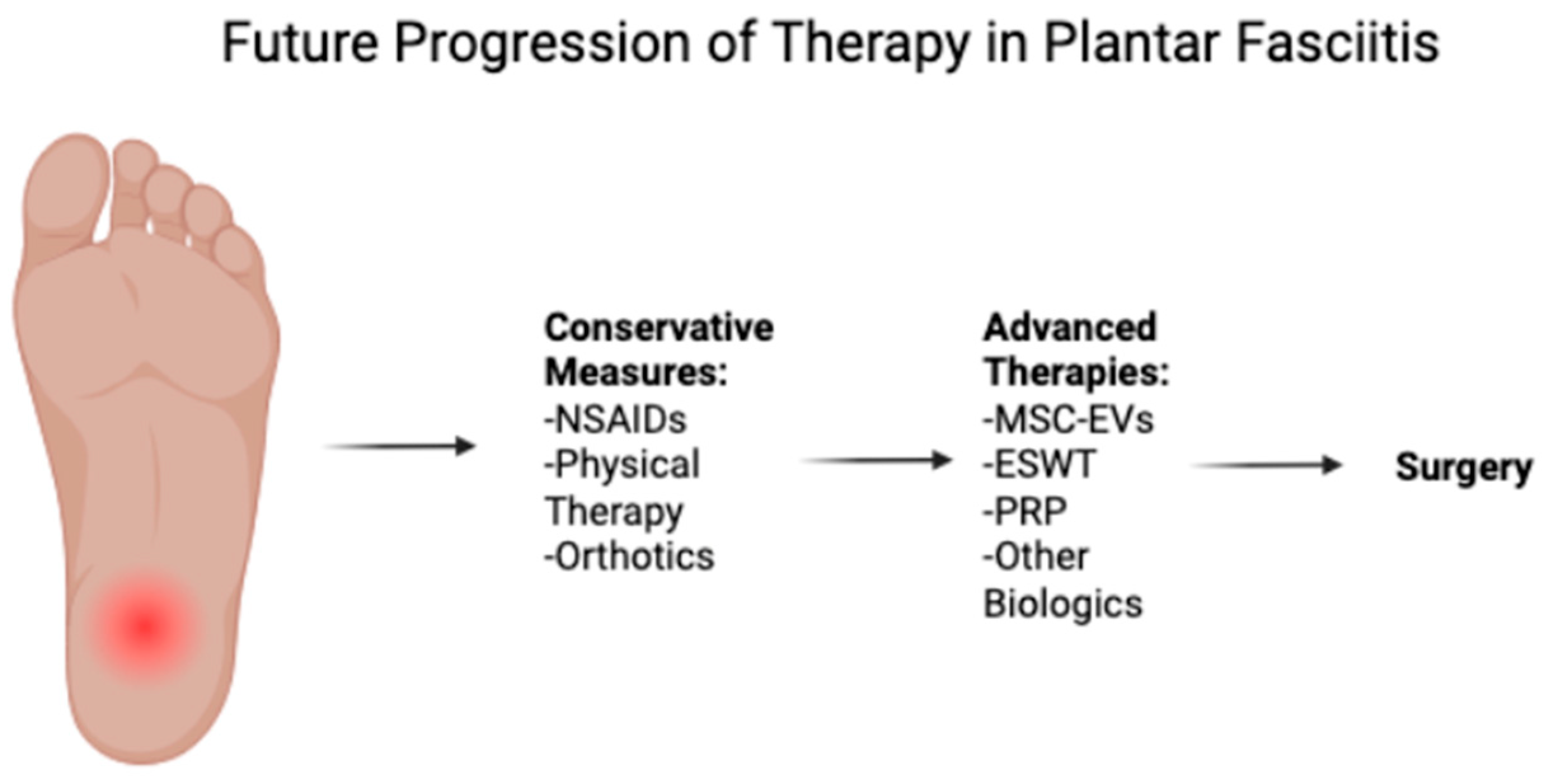

| Treatment Strategy | Advantages | Limitations and Drawbacks | |
|---|---|---|---|
| Conservative treatment | NSAIDs | Manages pain and inflammation in the acute setting. Inexpensive and accessible. | Can cause adverse gastrointestinal and kidney side effects with long-term use. Does not treat the root cause. |
| Stretching exercises | Addresses the root cause. Non-invasive. | Does not improve symptoms acutely. Normally it must be used in combination with another therapy. | |
| Orthotics | Improves foot biomechanics. It can be customized to the patient. Non-invasive. | Equivocal clinical data. | |
| Corticosteroid injections | Acutely reduces pain and inflammation. Functions locally. | Only offers short-term relief of symptoms. Equivocal clinical data. Carries several risks such as infection and plantar fascia rupture. Several injections are often required. | |
| Extracorporeal shock-wave therapy | Strong clinical efficacy on par with surgical intervention. Non-invasive. Quick recovery time. | Costly and generally not covered by insurance. Not universally available. | |
| Advanced therapies | PRP injections | Uses autologous blood. Platelets may assist healing response. | Costly and generally not covered by insurance. Equivocal clinical data. |
| Stem cell injections | Anti-inflammatory and regenerative properties. Minimally invasive compared to surgery. | Limited data. Potential for immune rejection. | |
| Surgery | Useful in recalcitrant cases. Different options to meet individual needs. Addresses the root problem. | Invasive with high risk profile. Long recovery period. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liebmann, K.; Kimbrough, D.W.; Best, T.M.; Kouroupis, D.; Rodriguez Materon, S. Plantar Fasciitis Pathophysiology and the Potential Role of Mesenchymal Stem Cell-Derived Extracellular Vesicles as Therapy. Biomedicines 2025, 13, 1528. https://doi.org/10.3390/biomedicines13071528
Liebmann K, Kimbrough DW, Best TM, Kouroupis D, Rodriguez Materon S. Plantar Fasciitis Pathophysiology and the Potential Role of Mesenchymal Stem Cell-Derived Extracellular Vesicles as Therapy. Biomedicines. 2025; 13(7):1528. https://doi.org/10.3390/biomedicines13071528
Chicago/Turabian StyleLiebmann, Kevin, D. Wood Kimbrough, Thomas M. Best, Dimitrios Kouroupis, and Solangel Rodriguez Materon. 2025. "Plantar Fasciitis Pathophysiology and the Potential Role of Mesenchymal Stem Cell-Derived Extracellular Vesicles as Therapy" Biomedicines 13, no. 7: 1528. https://doi.org/10.3390/biomedicines13071528
APA StyleLiebmann, K., Kimbrough, D. W., Best, T. M., Kouroupis, D., & Rodriguez Materon, S. (2025). Plantar Fasciitis Pathophysiology and the Potential Role of Mesenchymal Stem Cell-Derived Extracellular Vesicles as Therapy. Biomedicines, 13(7), 1528. https://doi.org/10.3390/biomedicines13071528







