Management of Advanced Ovarian Cancer: Current Clinical Practice and Future Perspectives
Abstract
1. Introduction
2. First-Line Management of Advanced Ovarian Cancer
2.1. Chemotherapy
2.2. Angiogenesis and Bevacizumab
2.3. Poly-ADP Ribose (PARP) Inhibitors in First-Line Therapy
3. Management of Recurrent Disease
3.1. Platinum-Sensitive Recurrent Ovarian Cancer
3.2. Platinum-Resistant Recurrent Ovarian Cancer
4. Emerging and Future Therapeutic Directions
4.1. Immunotherapy Approaches
4.1.1. Immune Checkpoint Inhibitors
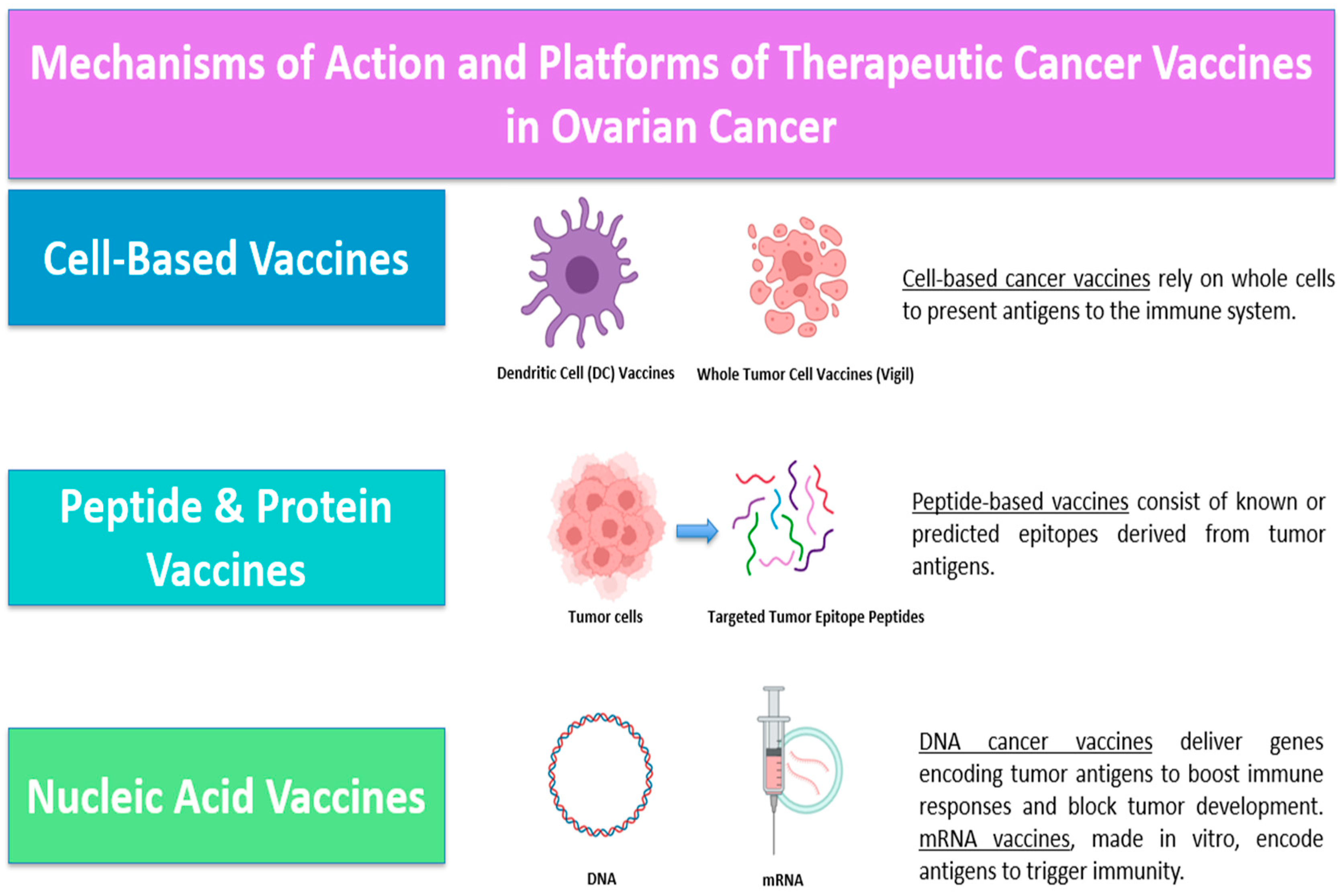
4.1.2. Cancer Vaccines
4.1.3. Adoptive Cell Therapy (ACT)
4.1.4. Oncolytic Virus
4.1.5. Cytokine Therapy
4.2. Antibody-Drug Conjugates (ADCs)
4.2.1. Targeting Folate Receptor Alpha
4.2.2. Targeting Trophoblast Cell Surface Antigen 2 (TROP2)
4.2.3. Targeting Cadherin 6 (CDH6)
4.2.4. Targeting Claudin 6 (CLDN6)
4.2.5. Targeting Mesothelin (MSLN)
4.2.6. Targeting MUC16
4.2.7. Targeting NaPi2b
4.2.8. Targeting Tissue Factor (TF)
4.2.9. Targeting B7-H4
4.2.10. Targeting HER-2
4.3. The Role of the Glucocorticoid Receptor
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CRS | Cytoreductive Surgery |
| CC | Completeness of Cytoreduction |
| PARP | Poly-ADP Ribose Polymerase |
| NACT | Neoadjuvant Chemotherapy |
| VEGF | Vascular Endothelial Growth Factor |
| PFS | Progression Free Survival |
| OS | Overall Survival |
| EMA | European Medicines Agency |
| FDA | Food and Drug Administration |
| HR | Homologous Recombination |
| SSBs | Single-Strand Breaks |
| DSBs | Double-Strand Breaks |
| HRD | Homologous Recombination Deficiency |
| HGSOG | High-Grade Serous Ovarian Cancer |
| PFI | Progression Free Survival |
| PLD | Pegylated Liposomal Doxorubicin |
| FRa | Folate Receptor a |
| ADC | Antibody-Drug Complex |
| DOR | Duration Of Response |
| ORR | Objective Response Rate |
| DC | Dendritic Cell |
| ACT | Adoptive Cell Therapy |
| NK | Natural Killer |
| ADCs | Antibody-Drug Conjugates |
| TF | Tissue Factor |
References
- Huo, X.; Tian, T.; Zhang, X.; Zhou, N. Comparative effectiveness and safety of treatment regimens for recurrent advanced ovarian cancer: A systematic review and network meta-analysis. World J. Surg. Oncol. 2025, 23, 134. [Google Scholar] [CrossRef] [PubMed]
- Doubeni, C.A.; Doubeni, A.R.; Myers, A.E. Diagnosis and Management of Ovarian Cancer. Am. Fam. Physician 2016, 93, 937–944. [Google Scholar] [PubMed]
- Armstrong, D.K.; Alvarez, R.D.; Backes, F.J.; Bakkum-Gamez, J.N.; Barroilhet, L.; Behbakht, K.; Berchuck, A.; Chen, L.M.; Chitiyo, V.C.; Cristea, M.; et al. NCCN Guidelines® Insights: Ovarian Cancer, Version 3.2022: Featured Updates to the NCCN Guidelines. J. Natl. Compr. Cancer Netw. 2022, 20, 972–980. [Google Scholar] [CrossRef]
- Neesham, D.; Richards, A.; McGauran, M. Advances in epithelial ovarian cancer. Aust. J. Gen. Pract. 2020, 49, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Deraco, M.; Baratti, D.; Kusamura, S.; Laterza, B.; Balestra, M.R. Surgical technique of parietal and visceral peritonectomy for peritoneal surface malignancies. J. Surg. Oncol. 2009, 100, 321–328. [Google Scholar] [CrossRef]
- Sugarbaker, P. Cytoreductive surgery using peritonectomy and visceral resections for peritoneal surface malignancy. Transl. Gastrointest. Cancer 2013, 2, 54–74. [Google Scholar] [CrossRef]
- Mehta, S.S.; Bhatt, A.; Glehen, O. Cytoreductive Surgery and Peritonectomy Procedures. Indian J. Surg. Oncol. 2016, 7, 139–151. [Google Scholar] [CrossRef]
- Xiang, L.; Ye, S.; Yang, H. Total pelvic peritonectomy for ovarian cancer with extensive peritoneal carcinomatosis in pelvic cavity. Gynecol. Oncol. 2019, 154, 651–652. [Google Scholar] [CrossRef]
- Kyriazanos, I.; Papageorgiou, D.; Zoulamoglou, M.; Marougkas, M.; Stamos, N.; Ivros, N.; Kalles, V. Total extraperitoneal access for parietal peritonectomy for peritoneal surface malignancy: The ‘cocoon’ technique. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 251, 258–262. [Google Scholar] [CrossRef]
- Smith, J.A.; Medina, P.; Miao, M.; Moore, K.N. A review of mirvetuximab soravtansine-gynx in folate receptor alpha-expressing platinum-resistant ovarian cancer. Am. J. Health Syst. Pharm. 2025, 82, 522–536. [Google Scholar] [CrossRef]
- Arora, T.; Mullangi, S.; Vadakekut, E.S.; Lekkala, M.R. Epithelial Ovarian Cancer. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Marth, C.; Reimer, D.; Zeimet, A.G. Front-Line Therapy of Advanced Epithelial Ovarian Cancer: Standard Treatment. Ann. Oncol. 2017, 28, viii36–viii39. [Google Scholar] [CrossRef] [PubMed]
- Ledermann, J.A. First-Line Treatment of Ovarian Cancer: Questions and Controversies to Address. Ther. Adv. Med. Oncol. 2018, 10, 1758835918768232. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.K.; Brady, M.F.; Penson, R.T.; Huang, H.; Birrer, M.J.; Walker, J.L.; DiSilvestro, P.A.; Rubin, S.C.; Martin, L.P.; Davidson, S.A.; et al. Weekly vs. Every-3-Week Paclitaxel and Carboplatin for Ovarian Cancer. N. Engl. J. Med. 2016, 374, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Ngoi, N.Y.; Syn, N.L.; Goh, R.M.; Goh, B.C.; Huang, R.Y.-J.; Soon, Y.Y.; James, E.; Cook, A.; Clamp, A.; Tan, D.S. Weekly versus Tri-Weekly Paclitaxel with Carboplatin for First-Line Treatment in Women with Epithelial Ovarian Cancer. Cochrane Database Syst. Rev. 2022, 2, CD012007. [Google Scholar] [CrossRef]
- Clamp, A.R.; James, E.C.; McNeish, I.A.; Dean, A.; Kim, J.-W.; O’Donnell, D.M.; Gallardo-Rincon, D.; Blagden, S.; Brenton, J.; Perren, T.J.; et al. Weekly Dose-Dense Chemotherapy in First-Line Epithelial Ovarian, Fallopian Tube, or Primary Peritoneal Cancer Treatment (ICON8): Overall Survival Results from an Open-Label, Randomised, Controlled, Phase 3 Trial. Lancet Oncol. 2022, 23, 919–930. [Google Scholar] [CrossRef]
- American Society of Clinical Oncology. ASCO-SEP®: Medical Oncology Self-Evaluation Program; American Society of Clinical Oncology: Alexandria, VA, USA, 2024; p. 750. ISBN 979-8-9888294-2-3. [Google Scholar]
- Lukanović, D.; Kobal, B.; Černe, K. Ovarian Cancer: Treatment and Resistance to Pharmacotherapy. Reprod. Med. 2022, 3, 127–140. [Google Scholar] [CrossRef]
- Gaillard, S.; Lacchetti, C.; Armstrong, D.K.; Cliby, W.A.; Edelson, M.I.; Garcia, A.A.; Ghebre, R.G.; Gressel, G.M.; Lesnock, J.L.; Meyer, L.A.; et al. Neoadjuvant Chemotherapy for Newly Diagnosed, Advanced Ovarian Cancer: ASCO Guideline Update. JCO 2025, 43, 868–891. [Google Scholar] [CrossRef]
- Berek, J.S.; Renz, M.; Kehoe, S.; Kumar, L.; Friedlander, M. Cancer of the Ovary, Fallopian Tube, and Peritoneum: 2021 Update. Intl. J. Gynecol. Obs. 2021, 155, 61–85. [Google Scholar] [CrossRef] [PubMed]
- Vergote, I.; Tropé, C.G.; Amant, F.; Kristensen, G.B.; Ehlen, T.; Johnson, N.; Verheijen, R.H.M.; Van Der Burg, M.E.L.; Lacave, A.J.; Panici, P.B.; et al. Neoadjuvant Chemotherapy or Primary Surgery in Stage IIIC or IV Ovarian Cancer. N. Engl. J. Med. 2010, 363, 943–953. [Google Scholar] [CrossRef]
- Kehoe, S.; Hook, J.; Nankivell, M.; Jayson, G.C.; Kitchener, H.; Lopes, T.; Luesley, D.; Perren, T.; Bannoo, S.; Mascarenhas, M.; et al. Primary Chemotherapy versus Primary Surgery for Newly Diagnosed Advanced Ovarian Cancer (CHORUS): An Open-Label, Randomised, Controlled, Non-Inferiority Trial. Lancet 2015, 386, 249–257. [Google Scholar] [CrossRef]
- Fagotti, A.; Ferrandina, M.G.; Vizzielli, G.; Pasciuto, T.; Fanfani, F.; Gallotta, V.; Margariti, P.A.; Chiantera, V.; Costantini, B.; Gueli Alletti, S.; et al. Randomized Trial of Primary Debulking Surgery versus Neoadjuvant Chemotherapy for Advanced Epithelial Ovarian Cancer (SCORPION-NCT01461850). Int. J. Gynecol. Cancer 2020, 30, 1657–1664. [Google Scholar] [CrossRef]
- Garcia, J.; Hurwitz, H.I.; Sandler, A.B.; Miles, D.; Coleman, R.L.; Deurloo, R.; Chinot, O.L. Bevacizumab (Avastin®) in Cancer Treatment: A Review of 15 Years of Clinical Experience and Future Outlook. Cancer Treat. Rev. 2020, 86, 102017. [Google Scholar] [CrossRef]
- Garcia, A.; Singh, H. Bevacizumab and Ovarian Cancer. Ther. Adv. Med. Oncol. 2013, 5, 133–141. [Google Scholar] [CrossRef]
- Polajžer, S.; Černe, K. Precision Medicine in High-Grade Serous Ovarian Cancer: Targeted Therapies and the Challenge of Chemoresistance. Int. J. Mol. Sci. 2025, 26, 2545. [Google Scholar] [CrossRef]
- Burger, R.A.; Brady, M.F.; Bookman, M.A.; Fleming, G.F.; Monk, B.J.; Huang, H.; Mannel, R.S.; Homesley, H.D.; Fowler, J.; Greer, B.E.; et al. Incorporation of Bevacizumab in the Primary Treatment of Ovarian Cancer. N. Engl. J. Med. 2011, 365, 2473–2483. [Google Scholar] [CrossRef]
- Tewari, K.S.; Burger, R.A.; Enserro, D.; Norquist, B.M.; Swisher, E.M.; Brady, M.F.; Bookman, M.A.; Fleming, G.F.; Huang, H.; Homesley, H.D.; et al. Final Overall Survival of a Randomized Trial of Bevacizumab for Primary Treatment of Ovarian Cancer. JCO 2019, 37, 2317–2328. [Google Scholar] [CrossRef]
- Perren, T.J.; Swart, A.M.; Pfisterer, J.; Ledermann, J.A.; Pujade-Lauraine, E.; Kristensen, G.; Carey, M.S.; Beale, P.; Cervantes, A.; Kurzeder, C.; et al. A Phase 3 Trial of Bevacizumab in Ovarian Cancer. N. Engl. J. Med. 2011, 365, 2484–2496. [Google Scholar] [CrossRef]
- Oza, A.M.; Cook, A.D.; Pfisterer, J.; Embleton, A.; Ledermann, J.A.; Pujade-Lauraine, E.; Kristensen, G.; Carey, M.S.; Beale, P.; Cervantes, A.; et al. Standard Chemotherapy with or without Bevacizumab for Women with Newly Diagnosed Ovarian Cancer (ICON7): Overall Survival Results of a Phase 3 Randomised Trial. Lancet Oncol. 2015, 16, 928–936. [Google Scholar] [CrossRef]
- Marchetti, C.; Muzii, L.; Romito, A.; Benedetti Panici, P. First-Line Treatment of Women with Advanced Ovarian Cancer: Focus on Bevacizumab. OTT 2019, 12, 1095–1103. [Google Scholar] [CrossRef]
- Pfisterer, J.; Joly, F.; Kristensen, G.; Rau, J.; Mahner, S.; Pautier, P.; El-Balat, A.; Kurtz, J.E.; Canzler, U.; Sehouli, J.; et al. Optimal Treatment Duration of Bevacizumab (BEV) Combined with Carboplatin and Paclitaxel in Patients (Pts) with Primary Epithelial Ovarian (EOC), Fallopian Tube (FTC) or Peritoneal Cancer (PPC): A Multicenter Open-Label Randomized 2-Arm Phase 3 ENGOT/GCIG Trial of the AGO Study Group, GINECO, and NSGO (AGO-OVAR 17/BOOST, GINECO OV118, ENGOT Ov-15, NCT01462890). JCO 2021, 39, 5501. [Google Scholar] [CrossRef]
- Dewani, D.; Jaiswal, A.; Karwade, P. Poly(Adenosine Diphosphate Ribose) Polymerase (PARP) Inhibitors in the Treatment of Advanced Ovarian Cancer: A Narrative Review. Cureus 2024, 16, e68463. [Google Scholar] [CrossRef] [PubMed]
- Masvidal Hernandez, M.; Cros Costa, S.; Salvador Coloma, C.; Quilez Cutillas, A.; Barretina-Ginesta, M.-P.; Cotes Sanchís, A. First-Line PARP Inhibitor Maintenance Treatment in Ovarian Carcinoma for Older Adult Women: A Review of the Current Literature. Clin. Transl. Oncol. 2024, 27, 417–424. [Google Scholar] [CrossRef]
- Garg, V.; Oza, A.M. Treatment of Ovarian Cancer Beyond PARP Inhibition: Current and Future Options. Drugs 2023, 83, 1365–1385. [Google Scholar] [CrossRef]
- da Cunha Colombo Bonadio, R.R.; Fogace, R.N.; Miranda, V.C.; Diz, M.D.P.E. Homologous Recombination Deficiency in Ovarian Cancer: A Review of Its Epidemiology and Management. Clinics 2018, 73, e450s. [Google Scholar] [CrossRef]
- Moore, K.; Colombo, N.; Scambia, G.; Kim, B.-G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2018, 379, 2495–2505. [Google Scholar] [CrossRef] [PubMed]
- DiSilvestro, P.; Banerjee, S.; Colombo, N.; Scambia, G.; Kim, B.-G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; et al. Overall Survival with Maintenance Olaparib at a 7-Year Follow-Up in Patients with Newly Diagnosed Advanced Ovarian Cancer and a BRCA Mutation: The SOLO1/GOG 3004 Trial. JCO 2023, 41, 609–617. [Google Scholar] [CrossRef]
- Banerjee, S.; Moore, K.N.; Colombo, N.; Scambia, G.; Kim, B.-G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; et al. Maintenance Olaparib for Patients with Newly Diagnosed Advanced Ovarian Cancer and a BRCA Mutation (SOLO1/GOG 3004): 5-Year Follow-up of a Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Oncol. 2021, 22, 1721–1731. [Google Scholar] [CrossRef] [PubMed]
- González-Martín, A.; Pothuri, B.; Vergote, I.; DePont Christensen, R.; Graybill, W.; Mirza, M.R.; McCormick, C.; Lorusso, D.; Hoskins, P.; Freyer, G.; et al. Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2391–2402. [Google Scholar] [CrossRef]
- Monk, B.J.; Barretina-Ginesta, M.P.; Pothuri, B.; Vergote, I.; Graybill, W.; Mirza, M.R.; McCormick, C.C.; Lorusso, D.; Moore, R.G.; Freyer, G.; et al. Niraparib First-Line Maintenance Therapy in Patients with Newly Diagnosed Advanced Ovarian Cancer: Final Overall Survival Results from the PRIMA/ENGOT-OV26/GOG-3012 Trial. Ann. Oncol. 2024, 35, 981–992. [Google Scholar] [CrossRef]
- Monk, B.J.; Parkinson, C.; Lim, M.C.; O’Malley, D.M.; Oaknin, A.; Wilson, M.K.; Coleman, R.L.; Lorusso, D.; Bessette, P.; Ghamande, S.; et al. A Randomized, Phase III Trial to Evaluate Rucaparib Monotherapy as Maintenance Treatment in Patients with Newly Diagnosed Ovarian Cancer (ATHENA–MONO/GOG-3020/ENGOT-Ov45). JCO 2022, 40, 3952–3964. [Google Scholar] [CrossRef]
- O’Malley, D.M.; Monk, B.J.; Lim, M.C.; Fuentes Pradera, J.; Buscema, J.; Wilson, M.K.; De Vivo, R.; Herzog, T.J.; Zagouri, F.; Oza, A.M.; et al. Final Safety Results from ATHENA–MONO (GOG-3020/ENGOT-Ov45), a Randomized, Placebo-Controlled, Double-Blind, Phase 3 Trial Evaluating Rucaparib Monotherapy as Maintenance Treatment in Patients with Newly Diagnosed Ovarian Cancer. JCO 2024, 42, 5554. [Google Scholar] [CrossRef]
- Coleman, R.L.; Fleming, G.F.; Brady, M.F.; Swisher, E.M.; Steffensen, K.D.; Friedlander, M.; Okamoto, A.; Moore, K.N.; Efrat Ben-Baruch, N.; Werner, T.L.; et al. Veliparib with First-Line Chemotherapy and as Maintenance Therapy in Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2403–2415. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Pautier, P.; Pignata, S.; Pérol, D.; González-Martín, A.; Berger, R.; Fujiwara, K.; Vergote, I.; Colombo, N.; Mäenpää, J.; et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2416–2428. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Leary, A.; Pignata, S.; Cropet, C.; González-Martín, A.; Marth, C.; Nagao, S.; Vergote, I.; Colombo, N.; Mäenpää, J.; et al. Olaparib plus Bevacizumab First-Line Maintenance in Ovarian Cancer: Final Overall Survival Results from the PAOLA-1/ENGOT-Ov25 Trial. Ann. Oncol. 2023, 34, 681–692. [Google Scholar] [CrossRef]
- Tavares, V.; Marques, I.S.; Melo, I.G.D.; Assis, J.; Pereira, D.; Medeiros, R. Paradigm Shift: A Comprehensive Review of Ovarian Cancer Management in an Era of Advancements. IJMS 2024, 25, 1845. [Google Scholar] [CrossRef] [PubMed]
- Foley, O.W.; Rauh-Hain, J.A.; del Carmen, M.G. Recurrent Epithelial Ovarian Cancer: An Update on Treatment. Oncology 2013, 27, 288–294, 298. [Google Scholar] [PubMed]
- Cortez, A.J.; Tudrej, P.; Kujawa, K.A.; Lisowska, K.M. Advances in Ovarian Cancer Therapy. Cancer Chemother. Pharmacol. 2018, 81, 17–38. [Google Scholar] [CrossRef] [PubMed]
- González-Martín, A.; Harter, P.; Leary, A.; Lorusso, D.; Miller, R.E.; Pothuri, B.; Ray-Coquard, I.; Tan, D.S.P.; Bellet, E.; Oaknin, A.; et al. Newly Diagnosed and Relapsed Epithelial Ovarian Cancer: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2023, 34, 833–848. [Google Scholar] [CrossRef]
- Aghajanian, C.; Blank, S.V.; Goff, B.A.; Judson, P.L.; Teneriello, M.G.; Husain, A.; Sovak, M.A.; Yi, J.; Nycum, L.R. OCEANS: A Randomized, Double-Blind, Placebo-Controlled Phase III Trial of Chemotherapy with or without Bevacizumab in Patients with Platinum-Sensitive Recurrent Epithelial Ovarian, Primary Peritoneal, or Fallopian Tube Cancer. J. Clin. Oncol. 2012, 30, 2039–2045. [Google Scholar] [CrossRef]
- Aghajanian, C.; Goff, B.; Nycum, L.R.; Wang, Y.V.; Husain, A.; Blank, S.V. Final Overall Survival and Safety Analysis of OCEANS, a Phase 3 Trial of Chemotherapy with or without Bevacizumab in Patients with Platinum-Sensitive Recurrent Ovarian Cancer. Gynecol. Oncol. 2015, 139, 10–16. [Google Scholar] [CrossRef]
- Coleman, R.L.; Brady, M.F.; Herzog, T.J.; Sabbatini, P.; Armstrong, D.K.; Walker, J.L.; Kim, B.-G.; Fujiwara, K.; Tewari, K.S.; O’Malley, D.M.; et al. Bevacizumab and Paclitaxel–Carboplatin Chemotherapy and Secondary Cytoreduction in Recurrent, Platinum-Sensitive Ovarian Cancer (NRG Oncology/Gynecologic Oncology Group Study GOG-0213): A Multicentre, Open-Label, Randomised, Phase 3 Trial. Lancet Oncol. 2017, 18, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Pujade-Lauraine, E.; Ledermann, J.A.; Selle, F.; Gebski, V.; Penson, R.T.; Oza, A.M.; Korach, J.; Huzarski, T.; Poveda, A.; Pignata, S.; et al. Olaparib Tablets as Maintenance Therapy in Patients with Platinum-Sensitive, Relapsed Ovarian Cancer and a BRCA1/2 Mutation (SOLO2/ENGOT-Ov21): A Double-Blind, Randomised, Placebo-Controlled, Phase 3 Trial. Lancet Oncol. 2017, 18, 1274–1284. [Google Scholar] [CrossRef]
- Poveda, A.; Floquet, A.; Ledermann, J.A.; Asher, R.; Penson, R.T.; Oza, A.M.; Korach, J.; Huzarski, T.; Pignata, S.; Friedlander, M.; et al. Olaparib Tablets as Maintenance Therapy in Patients with Platinum-Sensitive Relapsed Ovarian Cancer and a BRCA1/2 Mutation (SOLO2/ENGOT-Ov21): A Final Analysis of a Double-Blind, Randomised, Placebo-Controlled, Phase 3 Trial. Lancet Oncol. 2021, 22, 620–631. [Google Scholar] [CrossRef] [PubMed]
- Mirza, M.R.; Monk, B.J.; Herrstedt, J.; Oza, A.M.; Mahner, S.; Redondo, A.; Fabbro, M.; Ledermann, J.A.; Lorusso, D.; Vergote, I.; et al. Niraparib Maintenance Therapy in Platinum-Sensitive, Recurrent Ovarian Cancer. N. Engl. J. Med. 2016, 375, 2154–2164. [Google Scholar] [CrossRef] [PubMed]
- Matulonis, U.; Herrstedt, J.; Oza, A.; Mahner, S.; Redondo, A.; Berton, D.; Berek, J.; Haslund, C.; Marmé, F.; González-Martín, A.; et al. Final Overall Survival and Long-Term Safety in the ENGOT-OV16/NOVA Phase III Trial of Niraparib in Patients with Recurrent Ovarian Cancer (LBA 6). Gynecol. Oncol. 2023, 176, S31–S32. [Google Scholar] [CrossRef]
- Coleman, R.L.; Oza, A.M.; Lorusso, D.; Aghajanian, C.; Oaknin, A.; Dean, A.; Colombo, N.; Weberpals, J.I.; Clamp, A.; Scambia, G.; et al. Rucaparib Maintenance Treatment for Recurrent Ovarian Carcinoma after Response to Platinum Therapy (ARIEL3): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet 2017, 390, 1949–1961. [Google Scholar] [CrossRef]
- Coleman, R.L.; Oza, A.; Lorusso, D.; Aghajanian, C.; Oaknin, A.; Dean, A.; Colombo, N.; Weberpals, J.; Clamp, A.; Scambia, G.; et al. O003/#557 Overall Survival Results from ARIEL3: A Phase 3 Randomized, Double-Blind Study of Rucaparib vs Placebo Following Response to Platinum-Based Chemotherapy for Recurrent Ovarian Carcinoma. In Proceedings of the Oral Abstracts (Regular and Late-Breaking Submission); BMJ Publishing Group Ltd.: London, UK, 2022; pp. A3.2–A4. [Google Scholar]
- Coleman, R.L.; Spirtos, N.M.; Enserro, D.; Herzog, T.J.; Sabbatini, P.; Armstrong, D.K.; Kim, J.-W.; Park, S.-Y.; Kim, B.-G.; Nam, J.-H.; et al. Secondary Surgical Cytoreduction for Recurrent Ovarian Cancer. N. Engl. J. Med. 2019, 381, 1929–1939. [Google Scholar] [CrossRef]
- Harter, P.; Sehouli, J.; Vergote, I.; Ferron, G.; Reuss, A.; Meier, W.; Greggi, S.; Mosgaard, B.J.; Selle, F.; Guyon, F.; et al. Randomized Trial of Cytoreductive Surgery for Relapsed Ovarian Cancer. N. Engl. J. Med. 2021, 385, 2123–2131. [Google Scholar] [CrossRef]
- Shi, T.; Zhu, J.; Feng, Y.; Tu, D.; Zhang, Y.; Zhang, P.; Jia, H.; Huang, X.; Cai, Y.; Yin, S.; et al. Secondary Cytoreduction Followed by Chemotherapy versus Chemotherapy Alone in Platinum-Sensitive Relapsed Ovarian Cancer (SOC-1): A Multicentre, Open-Label, Randomised, Phase 3 Trial. Lancet Oncol. 2021, 22, 439–449. [Google Scholar] [CrossRef]
- Jiang, R.; Feng, Y.; Chen, Y.; Cheng, X.; Shi, T.; Gao, W.; Jia, H.; Jiang, S.; Guo, Y.; Huang, X.; et al. Surgery versus No Surgery in Platinum-Sensitive Relapsed Ovarian Cancer: Final Overall Survival Analysis of the SOC-1 Randomized Phase 3 Trial. Nat. Med. 2024, 30, 2181–2188. [Google Scholar] [CrossRef]
- Pujade-Lauraine, E.; Hilpert, F.; Weber, B.; Reuss, A.; Poveda, A.; Kristensen, G.; Sorio, R.; Vergote, I.; Witteveen, P.; Bamias, A.; et al. Bevacizumab Combined with Chemotherapy for Platinum-Resistant Recurrent Ovarian Cancer: The AURELIA Open-Label Randomized Phase III Trial. JCO 2014, 32, 1302–1308. [Google Scholar] [CrossRef] [PubMed]
- Kokori, E.; Olatunji, G.; Komolafe, R.; Abraham, I.C.; Ukoaka, B.; Samuel, O.; Ayodeji, A.; Ogunbowale, I.; Ezenwoba, C.; Aderinto, N. Mirvetuximab Soravtansine: A Breakthrough in Targeted Therapy for Platinum-Resistant Ovarian Cancer. Medicine 2024, 103, e38132. [Google Scholar] [CrossRef] [PubMed]
- Matulonis, U.A.; Lorusso, D.; Oaknin, A.; Pignata, S.; Dean, A.; Denys, H.; Colombo, N.; Van Gorp, T.; Konner, J.A.; Marin, M.R.; et al. Efficacy and Safety of Mirvetuximab Soravtansine in Patients with Platinum-Resistant Ovarian Cancer with High Folate Receptor Alpha Expression: Results from the SORAYA Study. JCO 2023, 41, 2436–2445. [Google Scholar] [CrossRef] [PubMed]
- Coffman, L.G.; You, B.; Hamilton, E.P.; Garcia, Y.G.; Moore, K.N.; Sonnenburg, D.; Scambia, G.; Derio, S.; Pepin, J.T.; Klasa-Mazurkiewicz, D.; et al. 746P Phase III MIRASOL Trial: Updated Overall Survival Results of Mirvetuximab Soravtansine (MIRV) vs. Investigator’s Choice Chemotherapy (ICC) in Patients (Pts) with Platinum-Resistant Ovarian Cancer (PROC) and High Folate Receptor-Alpha (FRα) Expression. Ann. Oncol. 2024, 35, S566. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Makker, V.; Oaknin, A.; Oh, D.-Y.; Banerjee, S.; González-Martín, A.; Jung, K.H.; Ługowska, I.; Manso, L.; Manzano, A.; et al. Efficacy and Safety of Trastuzumab Deruxtecan in Patients with HER2-Expressing Solid Tumors: Primary Results From the DESTINY-PanTumor02 Phase II Trial. J. Clin. Oncol. 2024, 42, 47–58. [Google Scholar] [CrossRef]
- Marcus, L.; Fashoyin-Aje, L.A.; Donoghue, M.; Yuan, M.; Rodriguez, L.; Gallagher, P.S.; Philip, R.; Ghosh, S.; Theoret, M.R.; Beaver, J.A.; et al. FDA Approval Summary: Pembrolizumab for the Treatment of Tumor Mutational Burden-High Solid Tumors. Clin. Cancer Res. 2021, 27, 4685–4689. [Google Scholar] [CrossRef]
- Monk, B.J.; Colombo, N.; Oza, A.M.; Fujiwara, K.; Birrer, M.J.; Randall, L.; Poddubskaya, E.V.; Scambia, G.; Shparyk, Y.V.; Lim, M.C.; et al. Chemotherapy with or without Avelumab Followed by Avelumab Maintenance versus Chemotherapy Alone in Patients with Previously Untreated Epithelial Ovarian Cancer (JAVELIN Ovarian 100): An Open-Label, Randomised, Phase 3 Trial. Lancet Oncol. 2021, 22, 1275–1289. [Google Scholar] [CrossRef]
- Pujade-Lauraine, E.; Fujiwara, K.; Ledermann, J.A.; Oza, A.M.; Kristeleit, R.; Ray-Coquard, I.-L.; Richardson, G.E.; Sessa, C.; Yonemori, K.; Banerjee, S.; et al. Avelumab Alone or in Combination with Chemotherapy versus Chemotherapy Alone in Platinum-Resistant or Platinum-Refractory Ovarian Cancer (JAVELIN Ovarian 200): An Open-Label, Three-Arm, Randomised, Phase 3 Study. Lancet Oncol. 2021, 22, 1034–1046. [Google Scholar] [CrossRef]
- Moore, K.N.; Bookman, M.; Sehouli, J.; Miller, A.; Anderson, C.; Scambia, G.; Myers, T.; Taskiran, C.; Robison, K.; Mäenpää, J.; et al. Atezolizumab, Bevacizumab, and Chemotherapy for Newly Diagnosed Stage III or IV Ovarian Cancer: Placebo-Controlled Randomized Phase III Trial (IMagyn050/GOG 3015/ENGOT-OV39). JCO 2021, 39, 1842–1855. [Google Scholar] [CrossRef]
- Kurtz, J.-E.; Pujade-Lauraine, E.; Oaknin, A.; Belin, L.; Leitner, K.; Cibula, D.; Denys, H.; Rosengarten, O.; Rodrigues, M.; De Gregorio, N.; et al. Atezolizumab Combined with Bevacizumab and Platinum-Based Therapy for Platinum-Sensitive Ovarian Cancer: Placebo-Controlled Randomized Phase III ATALANTE/ENGOT-Ov29 Trial. JCO 2023, 41, 4768–4778. [Google Scholar] [CrossRef]
- O’Cearbhaill, R.; Zamarin, D.; Sill, M.; Duong, H.; Waggoner, S.; Grisham, R.; Backes, F.; Mannel, R.; Tanyi, J.; Powell, M.; et al. LB005/#1605 Randomized, Phase II/III Study of Pegylated Liposomal Doxorubicin, Bevacizumab, and Atezolizumab in Platinum-Resistant Ovarian Cancer (NRG-GY009): Clinical Outcomes by PD-L1 and T Cell Infiltration Status. Int. J. Gynecol. Cancer 2024, 34, A10. [Google Scholar]
- Marmé, F.; Harter, P.; Redondo, A.; Reuss, A.; Ray-Coquard, I.L.; Lindemann, K.; Kurzeder, C.; Van Nieuwenhuysen, E.; Bellier, C.; Pietzner, K.; et al. Atezolizumab versus Placebo in Combination with Bevacizumab and Non-Platinum-Based Chemotherapy in Recurrent Ovarian Cancer: Final Overall and Progression-Free Survival Results from the AGO-OVAR 2.29/ENGOT-Ov34 Study. JCO 2024, 42, LBA5501. [Google Scholar] [CrossRef]
- González-Martín, A.; Rubio, M.J.; Heitz, F.; Depont Christensen, R.; Colombo, N.; Van Gorp, T.; Romeo, M.; Ray-Coquard, I.; Gaba, L.; Leary, A.; et al. Atezolizumab Combined With Platinum and Maintenance Niraparib for Recurrent Ovarian Cancer with a Platinum-Free Interval >6 Months: ENGOT-OV41/GEICO 69-O/ANITA Phase III Trial. JCO 2024, 42, 4294–4304. [Google Scholar] [CrossRef]
- Trillsch, F.; Okamoto, A.; Kim, J.-W.; Reuss, A.; Pérez, M.J.R.; Vardar, M.A.; Salutari, V.; Frenel, J.-S.; Kärkkäinen, H.; Colombo, N.; et al. 43O Durvalumab (D) + Carboplatin/Paclitaxel (CP) + Bevacizumab (B) Followed by D, B + Olaparib (O) Maintenance (Mtx) for Newly Diagnosed Advanced Ovarian Cancer (AOC) without a Tumour BRCA1/BRCA2 Mutation (Non-tBRCAm): Updated Results from DUO-O. ESMO Open 2024, 9, 103550. [Google Scholar] [CrossRef]
- Monk, B.J.; Oaknin, A.; O’Malley, D.M.; Wilson, M.; Lorusso, D.; Westin, S.; Oza, A.M.; Zagouri, F.; Herzog, T.J.; Mikheeva, O.; et al. LBA30 ATHENA-COMBO, a Phase III, Randomized Trial Comparing Rucaparib (RUCA) + Nivolumab (NIVO) Combination Therapy vs RUCA Monotherapy as Maintenance Treatment in Patients (Pts) with Newly Diagnosed Ovarian Cancer (OC). Ann. Oncol. 2024, 35, S1223–S1224. [Google Scholar] [CrossRef]
- Hardy-Bessard, A.-C.; Moore, K.N.; Mirza, M.R.; Asselain, B.; Redondo, A.; Pfisterer, J.; Pignata, S.; Provencher, D.M.; Cibula, D.; Reyners, A.K.L.; et al. ENGOT-OV44/FIRST Study: A Randomized, Double-Blind, Adaptive, Phase III Study of Standard of Care (SOC) Platinum-Based Therapy ± Dostarlimab Followed by Niraparib ± Dostarlimab Maintenance as First-Line (1L) Treatment of Stage 3 or 4 Ovarian Cancer (OC). JCO 2020, 38, TPS6101. [Google Scholar] [CrossRef]
- Powell, M.A. Combination Therapy for Newly Diagnosed BRCA-Nonmutated Advanced Epithelial Ovarian Cancer. Available online: https://ascopost.com/issues/april-25-2025/combination-therapy-for-newly-diagnosed-brca-nonmutated-advanced-epithelial-ovarian-cancer/ (accessed on 5 May 2025).
- Vergote, I.; Sehouli, J.; Salutari, V.; Zola, P.; Madry, R.; Wenham, R.M.; Korach, J.; Pautier, P.; Cibula, D.; Lheureux, S.; et al. ENGOT-OV43/KEYLYNK-001: A Phase III, Randomized, Double-Blind, Active- and Placebo-Controlled Study of Pembrolizumab plus Chemotherapy with Olaparib Maintenance for First-Line Treatment of BRCA-Nonmutated Advanced Epithelial Ovarian Cancer. JCO 2019, 37, TPS5603. [Google Scholar] [CrossRef]
- Colombo, N.; Coleman, R.L.; Wu, X.; Köse, F.; Wenham, R.M.; Sebastianelli, A.; Hasegawa, K.; Zsiros, E.; De La Motte Rouge, T.; Bidziński, M.; et al. ENGOT-Ov65/KEYNOTE-B96: Phase 3, Randomized, Double-Blind Study of Pembrolizumab versus Placebo plus Paclitaxel with Optional Bevacizumab for Platinum-Resistant Recurrent Ovarian Cancer. JCO 2022, 40, TPS5617. [Google Scholar] [CrossRef]
- Kefas, J.; Flynn, M. Unlocking the Potential of Immunotherapy in Platinum-Resistant Ovarian Cancer: Rationale, Challenges, and Novel Strategies. Cancer Drug Resist. 2024, 7, 39. [Google Scholar] [CrossRef]
- Haines, N.A.; Fowler, M.G.; Zeh, B.G.; Kriete, C.B.; Bai, Q.; Wakefield, M.R.; Fang, Y. Unlocking the ‘Ova’-Coming Power: Immunotherapy’s Role in Shaping the Future of Ovarian Cancer Treatment. Med. Oncol. 2024, 41, 67. [Google Scholar] [CrossRef]
- Chen, K.; Wang, J.; Yang, M.; Deng, S.; Sun, L. Immunotherapy in Recurrent Ovarian Cancer. Biomedicines 2025, 13, 168. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Fang, C.; Liu, X.; Liao, Q.; Wu, N.; Wang, J. Immunotherapy and the Ovarian Cancer Microenvironment: Exploring Potential Strategies for Enhanced Treatment Efficacy. Immunology 2024, 173, 14–32. [Google Scholar] [CrossRef] [PubMed]
- Barve, M.; Aaron, P.; Manning, L.; Bognar, E.; Wallraven, G.; Horvath, S.; Stanbery, L.; Nemunaitis, J. Pilot Study of Combination Gemogenovatucel-T (Vigil) and Durvalumab in Women With Relapsed BRCA-Wt Triple-Negative Breast or Ovarian Cancer. Clin. Med. Insights Oncol. 2022, 16, 11795549221110501. [Google Scholar] [CrossRef] [PubMed]
- Leary, A.; Gladieff, L.; Sabatier, R.; Paoletti, X.; Marmé, F.; Van Gorp, T.; Alexandre, J.; Grellety, T.; Angelergues, A.; Simmet, V.; et al. TEDOVA/GINECO-OV244b/ENGOT-Ov58 Trial: Neo-Epitope Based Vaccine OSE2101 Alone or in Combination with Pembrolizumab vs Best Supportive Care (BSC) as Maintenance in Platinum-Sensitive Recurrent Ovarian Cancer with Disease Control after Platinum. JCO 2023, 41, TPS5618. [Google Scholar] [CrossRef]
- Mirza, M.R.; Pietzner, K.; Van Nieuwenhuysen, E.; Marth, C.; Boere, I.; Lindemann, K.; Fernebro, J.; Hietanen, S.; Brasiuniene, B.; Pors, K.; et al. A Randomized Trial of Olaparib, Durvalumab, and Cancer Vaccine, UV1, as Maintenance Therapy in Patients with BRCAwt with Recurrent Ovarian Cancer: ENGOT-OV56/NSGO-CTU-DOVACC Trial. JCO 2023, 41, TPS5615. [Google Scholar] [CrossRef]
- Sarivalasis, A.; Morotti, M.; Mulvey, A.; Imbimbo, M.; Coukos, G. Cell Therapies in Ovarian Cancer. Ther. Adv. Med. Oncol. 2021, 13, 17588359211008399. [Google Scholar] [CrossRef] [PubMed]
- Verdegaal, E.M.E.; Santegoets, S.J.; Welters, M.J.P.; de Bruin, L.; Visser, M.; van der Minne, C.E.; de Kok, P.M.; Loof, N.M.; Boekestijn, S.; Roozen, I.; et al. Timed Adoptive T Cell Transfer during Chemotherapy in Patients with Recurrent Platinum-Sensitive Epithelial Ovarian Cancer. J. Immunother. Cancer 2023, 11, e007697. [Google Scholar] [CrossRef]
- Pedersen, M.; Westergaard, M.C.W.; Milne, K.; Nielsen, M.; Borch, T.H.; Poulsen, L.G.; Hendel, H.W.; Kennedy, M.; Briggs, G.; Ledoux, S.; et al. Adoptive Cell Therapy with Tumor-Infiltrating Lymphocytes in Patients with Metastatic Ovarian Cancer: A Pilot Study. Oncoimmunology 2018, 7, e1502905. [Google Scholar] [CrossRef]
- Zhang, T.; Jou, T.H.-T.; Hsin, J.; Wang, Z.; Huang, K.; Ye, J.; Yin, H.; Xing, Y. Talimogene Laherparepvec (T-VEC): A Review of the Recent Advances in Cancer Therapy. J. Clin. Med. 2023, 12, 1098. [Google Scholar] [CrossRef]
- Borella, F.; Carosso, M.; Chiparo, M.P.; Ferraioli, D.; Bertero, L.; Gallio, N.; Preti, M.; Cusato, J.; Valabrega, G.; Revelli, A.; et al. Oncolytic Viruses in Ovarian Cancer: Where Do We Stand? A Narrative Review. Pathogens 2025, 14, 140. [Google Scholar] [CrossRef]
- Van der Meer, J.M.R.; Maas, R.J.A.; Guldevall, K.; Klarenaar, K.; de Jonge, P.K.J.D.; Evert, J.S.H.; van der Waart, A.B.; Cany, J.; Safrit, J.T.; Lee, J.H.; et al. IL-15 Superagonist N-803 Improves IFNγ Production and Killing of Leukemia and Ovarian Cancer Cells by CD34+ Progenitor-Derived NK Cells. Cancer Immunol. Immunother. 2021, 70, 1305–1321. [Google Scholar] [CrossRef]
- Cohen, C.A.; Shea, A.A.; Heffron, C.L.; Schmelz, E.M.; Roberts, P.C. Interleukin-12 Immunomodulation Delays the Onset of Lethal Peritoneal Disease of Ovarian Cancer. J. Interferon Cytokine Res. 2016, 36, 62–73. [Google Scholar] [CrossRef]
- Mollaoglu, G.; Tepper, A.; Falcomatà, C.; Potak, H.T.; Pia, L.; Amabile, A.; Mateus-Tique, J.; Rabinovich, N.; Park, M.D.; LaMarche, N.M.; et al. Ovarian Cancer-Derived IL-4 Promotes Immunotherapy Resistance. Cell 2024, 187, 7492.e22–7510.e22. [Google Scholar] [CrossRef] [PubMed]
- Kaser, E.C.; Lequio, M.; Zhu, Z.; Hunzeker, Z.E.; Heslin, A.J.; D’mello, K.P.; Xiao, H.; Bai, Q.; Wakefield, M.R.; Fang, Y. Ovarian Cancer Immunotherapy En Route: IL9 Inhibits Growth of Ovarian Cancer and Upregulates Its Expression of Ox40L and 4-1BBL. EJGO 2022, 43, 163. [Google Scholar] [CrossRef]
- Wang, S.; Guo, J.; Tang, Y.; Zheng, R.; Song, M.; Sun, W. Effects of recombinant human interleukin-24 alone and in combination with cisplatin on the growth of ovarian cancer cells in vitro. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2014, 30, 33–36. [Google Scholar] [PubMed]
- Feroz, B.; Marth, C.; Zeimet, A.G. Antibody–Drug Conjugates in Ovarian Cancer. memo 2024, 17, 130–134. [Google Scholar] [CrossRef]
- Sato, S.; Shoji, T.; Jo, A.; Otsuka, H.; Abe, M.; Tatsuki, S.; Chiba, Y.; Takatori, E.; Kaido, Y.; Nagasawa, T.; et al. Antibody-Drug Conjugates: The New Treatment Approaches for Ovarian Cancer. Cancers 2024, 16, 2545. [Google Scholar] [CrossRef]
- O’Malley, D.M.; Moore, K.N.; Vergote, I.; Martin, L.P.; Gilbert, L.; Gonzalez Martin, A.; Nepert, D.L.; Ruiz-Soto, R.; Birrer, M.J.; Matulonis, U.A. Safety Findings from FORWARD II: A Phase 1b Study Evaluating the Folate Receptor Alpha (FRα)-Targeting Antibody-Drug Conjugate (ADC) Mirvetuximab Soravtansine (IMGN853) in Combination with Bevacizumab, Carboplatin, Pegylated Liposomal Doxorubicin (PLD), or Pembrolizumab in Patients (Pts) with Ovarian Cancer. JCO 2017, 35, 5553. [Google Scholar] [CrossRef]
- Gilbert, L.; Oaknin, A.; Matulonis, U.A.; Mantia-Smaldone, G.M.; Lim, P.C.; Castro, C.M.; Provencher, D.; Memarzadeh, S.; Method, M.; Wang, J.; et al. Safety and Efficacy of Mirvetuximab Soravtansine, a Folate Receptor Alpha (FRα)-Targeting Antibody-Drug Conjugate (ADC), in Combination with Bevacizumab in Patients with Platinum-Resistant Ovarian Cancer. Gynecol. Oncol. 2023, 170, 241–247. [Google Scholar] [CrossRef]
- Vergote, I.; Armstrong, D.; Scambia, G.; Teneriello, M.; Sehouli, J.; Schweizer, C.; Weil, S.C.; Bamias, A.; Fujiwara, K.; Ochiai, K.; et al. A Randomized, Double-Blind, Placebo-Controlled, Phase III Study to Assess Efficacy and Safety of Weekly Farletuzumab in Combination with Carboplatin and Taxane in Patients with Ovarian Cancer in First Platinum-Sensitive Relapse. JCO 2016, 34, 2271–2278. [Google Scholar] [CrossRef]
- Herzog, T.J.; Pignata, S.; Ghamande, S.A.; Rubio, M.-J.; Fujiwara, K.; Vulsteke, C.; Armstrong, D.K.; Sehouli, J.; Coleman, R.L.; Gabra, H.; et al. Randomized Phase II Trial of Farletuzumab plus Chemotherapy versus Placebo plus Chemotherapy in Low CA-125 Platinum-Sensitive Ovarian Cancer. Gynecol. Oncol. 2023, 170, 300–308. [Google Scholar] [CrossRef]
- Oaknin, A.; Fariñas-Madrid, L.; García-Duran, C.; Martin, L.P.; O’Malley, D.M.; Schilder, R.J.; Uyar, D.; Moroney, J.W.; Diaz, J.P.; Spira, A.I.; et al. Luveltamab Tazevibulin (STRO-002), an Anti-Folate Receptor Alpha (FolRα) Antibody Drug Conjugate (ADC), Safety and Efficacy in a Broad Distribution of FolRα Expression in Patients with Recurrent Epithelial Ovarian Cancer (OC): Update of STRO-002-GM1 Phase 1 Dose Expansion Cohort. JCO 2023, 41, 5508. [Google Scholar] [CrossRef]
- Oaknin, A.; Lee, J.-Y.; Cibula, D.; Lee, Y.C.; Schilder, R.J.; Auranen, A.; Gao, B.; Tan, D.S.P.; Oza, A.M.; Miller, R.; et al. Efficacy and Safety of Luveltamab Tazevibulin vs Investigator’s Choice of Chemotherapy in Patients with Recurrent Platinum-Resistant Ovarian Cancer (PROC) Expressing Folate Receptor Alpha (FRα): The REFRaME-01 (GOG-3086, ENGOT-79ov, and APGOT-OV9) Phase 2/3 Study. JCO 2024, 42, TPS5637. [Google Scholar] [CrossRef]
- Martin, L.P.; Uyar, D.; Madariaga, A.; Spira, A.I.; Oaknin, A.; Schilder, R.; Alia, E.M.G.; Hamilton, E.P.; Naumann, R.W.; Lorusso, D.; et al. 749P Luveltamab Tazevibulin, an Antifolate Receptor Alpha (FRα) Antibody-Drug Conjugate (ADC), in Combination with Bevacizumab (Bev) in Patients with Recurrent High-Grade Epithelial Ovarian Cancer (EOC): STRO-002-GM2 Phase I Study. Ann. Oncol. 2024, 35, S568–S569. [Google Scholar] [CrossRef]
- Call, J.A.; Orr, D.W.; Anderson, I.C.; Richardson, D.L.; Chen, Z.; Ma, S.; Hunder, N.N.H.; Hamilton, E.P. Phase 1/2 Study of PRO1184, a Novel Folate Receptor Alpha-Directed Antibody-Drug Conjugate, in Patients with Locally Advanced and/or Metastatic Solid Tumors. JCO 2023, 41, TPS3157. [Google Scholar] [CrossRef]
- Lee, E.K.; Yeku, O.; Winer, I.; Hamilton, E.P.; Richardson, D.L.; Zhang, J.; Konecny, G.E.; Anderson, I.C.; Wu, X.; Orr, D.; et al. 719MO A Phase I/II Study of Rinatabart Sesutecan (Rina-S) in Patients with Advanced Ovarian or Endometrial Cancer. Ann. Oncol. 2024, 35, S550. [Google Scholar] [CrossRef]
- Genmab. A Phase 3 Randomized, Open-Label Study of Rinatabart Sesutecan (Rina-S) Versus Treatment of Investigator’s Choice (IC) in Patients with Platinum Resistant Ovarian Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT06619236 (accessed on 2 February 2025).
- Oaknin, A.; Ang, J.E.; Rha, S.Y.; Yonemori, K.; Kristeleit, R.; Lin, C.-C.; Satoh, T.; Garcia, P.E.; Sendur, M.A.N.; Rodríguez, L.M.; et al. 714MO Datopotamab Deruxtecan (Dato-DXd) in Patients with Endometrial (EC) or Ovarian Cancer (OC): Results from the Phase II TROPION-PanTumor03 Study. Ann. Oncol. 2024, 35, S547–S548. [Google Scholar] [CrossRef]
- Wang, D.; Wang, K.; An, R.; Yu, G.; Zhang, K.; Wang, D.; Jiang, K.; Gao, Y.; Cheng, Y.; Liu, Y.; et al. 715MO Safety and Efficacy of Sacituzumab Tirumotecan (Sac-TMT) in Patients (Pts) with Previously Treated Advanced Endometrial Carcinoma (EC) and Ovarian Cancer (OC) from a Phase II Study. Ann. Oncol. 2024, 35, S548. [Google Scholar] [CrossRef]
- Song, Z.; Chen, L.; Dang, Q.; Tang, D.; Liu, T.; Wang, L.; Wang, J.; Liu, C.; Qu, X.; Li, X.; et al. 717MO SHR-A1921 in Platinum-Resistant Ovarian Cancer (PROC): Data from a First-in-Human (FIH) Phase I Study. Ann. Oncol. 2024, 35, S549. [Google Scholar] [CrossRef]
- Daiich-Sankyo. A Study of DS-6000a in Subjects With Advanced Renal Cell Carcinoma and Ovarian Tumors. 2024. Available online: https://clinicaltrials.gov/ct2/show/NCT04707248 (accessed on 2 February 2025).
- Moore, K.; Philipovskiy, A.; Harano, K.; Rini, B.; Sudo, K.; Kitano, S.; Spigel, D.; Lin, J.; Kundu, M.; Hagihara, K.; et al. Raludotatug Deruxtecan Monotherapy among Patients with Previously Treated Ovarian Cancer: Subgroup Analysis of a First-in-Human Phase I Study. Gynecol. Oncol. 2024, 174, 79–80. [Google Scholar] [CrossRef]
- Daiichi-Sankyo. A Study of Raludotatug Deruxtecan (R-DXd) in Subjects with Platinum-Resistant, High-Grade Ovarian, Primary Peritoneal, or Fallopian Tube Cancer. 2025. Available online: https://clinicaltrials.gov/ct2/show/NCT06161025 (accessed on 2 February 2025).
- Konecny, G.E.; Hendrickson, A.E.W.; Winterhoff, B.; Adjei, A.A.; Kung, A.; Miller, L.-L.; Press, M.F.; Qazi, I.; Scholler, N.; Dokainish, H.; et al. 721MO Phase I, Two-Part, Multicenter First-in-Human (FIH) Study of TORL-1-23: A Novel Claudin 6 (CLDN6) Targeting Antibody Drug Conjugate (ADC) in Patient with Advanced Solid Tumors. Ann. Oncol. 2024, 35, S551. [Google Scholar] [CrossRef]
- Santin, A.D.; Vergote, I.; González-Martín, A.; Moore, K.; Oaknin, A.; Romero, I.; Diab, S.; Copeland, L.J.; Monk, B.J.; Coleman, R.L.; et al. Safety and Activity of Anti-Mesothelin Antibody-Drug Conjugate Anetumab Ravtansine in Combination with Pegylated-Liposomal Doxorubicin in Platinum-Resistant Ovarian Cancer: Multicenter, Phase Ib Dose Escalation and Expansion Study. Int. J. Gynecol. Cancer 2023, 33, 562–570. [Google Scholar] [CrossRef]
- Lheureux, S.; Alqaisi, H.; Cohn, D.E.; Chern, J.-Y.; Duska, L.R.; Jewell, A.; Corr, B.; Winer, I.S.; Girda, E.; Crispens, M.A.; et al. A Randomized Phase II Study of Bevacizumab and Weekly Anetumab Ravtansine or Weekly Paclitaxel in Platinum-Resistant or Refractory Ovarian Cancer NCI Trial#10150. JCO 2022, 40, 5514. [Google Scholar] [CrossRef]
- Liu, J.; Burris, H.; Wang, J.S.; Barroilhet, L.; Gutierrez, M.; Wang, Y.; Vaze, A.; Commerford, R.; Royer-Joo, S.; Choeurng, V.; et al. An Open-Label Phase I Dose-Escalation Study of the Safety and Pharmacokinetics of DMUC4064A in Patients with Platinum-Resistant Ovarian Cancer. Gynecol. Oncol. 2021, 163, 473–480. [Google Scholar] [CrossRef]
- Liu, J.; Moore, K.; Birrer, M.; Berlin, S.; Matulonis, U.; Infante, J.; Wolpin, B.; Poon, K.; Firestein, R.; Xu, J.; et al. Phase I Study of Safety and Pharmacokinetics of the Anti-MUC16 Antibody-Drug Conjugate DMUC5754A in Patients with Platinum-Resistant Ovarian Cancer or Unresectable Pancreatic Cancer. Ann. Oncol. 2016, 27, 2124–2130. [Google Scholar] [CrossRef]
- Richardson, D.; Concin, N.; Hays, J.; Fidalgo, J.A.P.; Pothuri, B.; Banerjee, S.; Ghamande, S.; Coquard, I.R.; Germanova, A.; Rimel, B.; et al. UPLIFT (ENGOT-OV67/GOG-3048): Results from the Phase II Trial of Upifitamab Rilsodotin (UpRi; XMT-1536), a NaPi2b-Directed Dolaflexin Antibody-Drug Conjugate in Platinum-Resistant Ovarian Cancer. Gynecol. Oncol. 2024, 190, S56. [Google Scholar] [CrossRef]
- Blank, S.; Mahdi, H.; Tehrani, O.; Ghamande, S.; Jain, S.; Nicacio, L.; Soumaoro, I.; O’Malley, D.M. 882TiP InnovaTV 208: New Weekly Dosing Cohort in the Phase II Study of Tisotumab Vedotin in Platinum-Resistant Ovarian Cancer. Ann. Oncol. 2020, 31, S646. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Naito, Y.; Gaillard, S.; Shimoi, T.; Chung, V.; Davis, A.A.; Proia, T.; Ayyoub, A.; Kulkarni, M.; Upadhyay, S.; et al. 606O Initial Results from a First-in-Human Study of the B7-H4-Directed Antibody-Drug Conjugate (ADC) AZD8205 (Puxitatug Samrotecan) in Patients with Advanced/Metastatic Solid Tumors. Ann. Oncol. 2024, 35, S485–S486. [Google Scholar] [CrossRef]
- Patnaik, A.; Lakhani, N.J.; Xu, P.; Nazarenko, N.N.; Call, J.A. Phase 1 Study of SGN-B7H4V, a Novel, Investigational Vedotin Antibody–Drug Conjugate Directed to B7-H4, in Patients with Advanced Solid Tumors (SGNB7H4V-001, Trial in Progress). JCO 2022, 40, TPS3155. [Google Scholar] [CrossRef]
- Hamilton, E.P.; Chaudhry, A.; Spira, A.I.; Adams, S.; Abuhadra, N.; Giordano, A.; Parajuli, R.; Han, H.S.; Weise, A.M.; Marchesani, A.; et al. XMT-1660: A Phase 1b Trial of a B7-H4 Targeted Antibody Drug Conjugate (ADC) in Breast, Endometrial, and Ovarian Cancers. JCO 2023, 41, TPS3154. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Oh, D.-Y.; Naito, Y.; Shimizu, T.; Chung, V.; Park, H.; Gaillard, S.; Wang, F.; Cooper, Z.A.; Kinneer, K.; et al. First-in-Human Study of the B7-H4 Antibody-Drug Conjugate (ADC) AZD8205 in Patients with Advanced/Metastatic Solid Tumors. JCO 2022, 40, TPS3153. [Google Scholar] [CrossRef]
- Perez, C.A.; Henry, J.T.; Lakhani, N.; Call, J.A.; Hamilton, E.P.; Colon-Otero, G.; Diamond, J.R.; O’Neil, B.; Kalyan, A.; Sonpavde, G.P.; et al. 660MO First-in-Human Study of SGN-B7H4V, a B7-H4-Directed Vedotin ADC, in Patients with Advanced Solid Tumors: Preliminary Results of a Phase I Study (SGNB7H4V-001). Ann. Oncol. 2023, 34, S464–S465. [Google Scholar] [CrossRef]
- Wang, D.; Li, H.; Xing, Y.; Wu, M.; Lin, Z.; Li, J. 378MO Efficacy and Safety of Disitamab Vedotin in Treatment of HER2 Expressed Advanced or Recurrent Gynecological Cancers. Ann. Oncol. 2024, 35, S1546. [Google Scholar] [CrossRef]
- ClinicalTrials.gov; Seagen Inc. (a wholly owned subsidiary of Pfizer). A Study of Disitamab Vedotin in Previously Treated Solid Tumors That Express HER2; U.S. National Library of Medicine: Bethesda, MD, USA, 2025. [Google Scholar]
- Buonaiuto, R.; Neola, G.; Cecere, S.C.; Caltavituro, A.; Cefaliello, A.; Pietroluongo, E.; De Placido, P.; Giuliano, M.; Arpino, G.; De Angelis, C. Glucocorticoid Receptor and Ovarian Cancer: From Biology to Therapeutic Intervention. Biomolecules 2023, 13, 653. [Google Scholar] [CrossRef]
- Olawaiye, A.B.; Kim, J.-W.; Bagameri, A.; Bishop, E.; Chudecka-Głaz, A.; Devaux, A.; Gladieff, L.; Gordinier, M.E.; Korach, J.; McCollum, M.E.; et al. Clinical Trial Protocol for ROSELLA: A Phase 3 Study of Relacorilant in Combination with Nab-Paclitaxel versus Nab-Paclitaxel Monotherapy in Advanced Platinum-Resistant Ovarian Cancer. J. Gynecol. Oncol. 2024, 35, e111. [Google Scholar] [CrossRef] [PubMed]
- Giudice, E.; Salutari, V.; Sassu, C.M.; Ghizzoni, V.; Carbone, M.V.; Vertechy, L.; Fagotti, A.; Scambia, G.; Marchetti, C. Relacorilant in Recurrent Ovarian Cancer: Clinical Evidence and Future Perspectives. Expert Rev. Anticancer Ther. 2024, 24, 649–655. [Google Scholar] [CrossRef]
- Colombo, N.; Van Gorp, T.; Matulonis, U.A.; Oaknin, A.; Grisham, R.N.; Fleming, G.F.; Olawaiye, A.B.; Nguyen, D.D.; Greenstein, A.E.; Custodio, J.M.; et al. Relacorilant + Nab-Paclitaxel in Patients with Recurrent, Platinum-Resistant Ovarian Cancer: A Three-Arm, Randomized, Controlled, Open-Label Phase II Study. JCO 2023, 41, 4779–4789. [Google Scholar] [CrossRef]
| Study | Treatment Strategy | Setting | No. of Patients | Primary Endpoint | Outcome | Adverse Events | Clinical Trial ID |
|---|---|---|---|---|---|---|---|
| GOG-0218 [27,28] | CTx (carboplatin AUC6 + paclitaxel 175 mg/m2, 6 cycles) • Control: + placebo (C2–22) • Bev-initiation: + bevacizumab 15 mg/kg (C2–6) → placebo (C7–22) • Bev-throughout: + bevacizumab 15 mg/kg (C2–22) | Newly diagnosed stage III (incompletely resectable) or stage IV EOC, FTC, or PPC | 1873 | PFS | PFS: 10.3 mo (control) vs. 11.2 mo (bev-initiation, HR = 0.91) vs. 14.1 mo (bev-throughout, HR = 0.72, p < 0.001) OS: No significant difference | HTN: 7.2% (control) vs. 16.5% (bev-initiation) vs. 22.9% (bev-throughout) GI perf: 1.2% vs. 2.8% vs 2.6% | NCT00262847 |
| ICON7 [29,30] | CTx (carboplatin AUC5–6 + paclitaxel 175 mg/m2, 6 cycles) • Control: CTx only • Bev arm: + bevacizumab 7.5 mg/kg (C1–5/6) → maintenance bev (up to C18 or until PD) | First-line (incl. high-risk early & advanced disease) | 1528 | PFS | • PFS: 22.4 (control) → 24.1 mo (bev arm) (HR = 0.87, p = 0.04) • OS overall: No difference (44.6 vs 45.5 mo, p = 0.85) | ↑ HTN (18% vs 2%), rare GI events with bev | NCT00483782 |
| AGO-OVAR 17/BOOST [32] | Bev 15 mo vs. 30 mo + CTx (carboplatin AUC5 + paclitaxel 175 mg/m2 × 6) | First-line, FIGO stage IIb–IV EOC, FTC, or PPC | 927 | PFS | PFS & OS: No significant benefit with Bev 30 vs. Bev15 | AEs ≥ G3 (Bev15 vs. Bev30): HTN 2.7% vs. 4.5%, thrombosis 2.2% vs 3.2%, fistula 3.1% vs. 1.1%, GI perf 0.2% vs. 0.9%, proteinuria 0.7% vs. 1.4%, hemorrhage 0.2% vs. 0.9%, MI 0% vs. 1.1%. | NCT01462890 |
| Study | Treatment Strategy | Setting | No. of Patients | Primary Endpoint | Outcome | Adverse Events | Clinical Trial ID |
|---|---|---|---|---|---|---|---|
| SOLO1/GOG-3004 [37,38,39] | Maintenance olaparib 300 mg BID, up to 2 yrs vs. placebo after response to 1L platinum-based CTx | Newly diagnosed FIGO stage III–IV high-grade serous or endometrioid EOC/FTC/PPC with BRCA1/2 mutation | 391 | PFS | Median PFS: 56 mo (olaparib) vs. 13.8 mo (placebo); HR = 0.33 7-yr OS: 67.0% (olaparib) vs. 46.5% (placebo); HR = 0.55 | Most common grade ≥ 3: anemia (21.9%) MDS/AML: low incidence | NCT01844986 |
| PRIMA/ENGOT-OV26/GOG-3012 [40,41] | Maintenance niraparib QD up to 3 yrs vs. placebo after response to 1L platinum-based CTx | Newly diagnosed FIGO stage III–IV high-grade serous or endometrioid EOC/FTC/PPC | 733 | PFS (HRD & overall population) | HRD: median PFS 21.9 mo (niraparib) vs. 10.4 mo (placebo); HR = 0.43 Overall: median PFS 13.8 mo (niraparib) vs. 8.2 mo (placebo); HR = 0.62 Final OS: NS; (HR = 1.01) | Most common grade ≥ 3: anemia (31%), thrombocytopenia (28.7%), neutropenia (12.8%) MDS/AML: <2.5% | NCT02655016 |
| ATHENA-MONO/GOG-3020/ENGOT-ov45 [42,43] | Rucaparib BID up to 2 yrs vs. placebo after response to 1 L platinum-based CTx | Newly diagnosed FIGO III–IV HGSOC | 538 | PFS (HRD, ITT) | HRD: PFS 28.7 mo (rucaparib)vs. 11.3 mo (placebo); HR = 0.47 ITT: PFS 20.2 mo (rucaparib) vs. 9.2 mo (placebo); HR = 0.52 | Most common grade ≥ 3: anemia (28.7%), neutropenia (14.6%), ↑ALT/AST (10.6%), thrombocytopenia (7.3%) | NCT03522246 |
| VELIA/GOG-3005 [44] | Control: Carboplatin + Paclitaxel + Placebo (6 cycles) → Placebo maintenance Veliparib combination only: Carboplatin + Paclitaxel + Veliparib (6 cycles) → Placebo maintenance Veliparib throughout: Carboplatin + Paclitaxel + Veliparib (6 cycles) → Veliparib maintenance (400 mg BID, up to 30 cycles) | Newly diagnosed FIGO stage III–IV HGSOC | 1140 | PFS (BRCA, HRD, ITT) | BRCA-mut: PFS 34.7 mo (veliparib-throughout) vs. 22.0 mo (control); HR = 0.44 HRD: PFS 31.9 mo (veliparib-throughout) vs. 20.5 mo (control); HR = 0.57 ITT: PFS 23.5 mo (veliparib-throughout) vs. 17.3 mo (control); HR = 0.68 | Anemia, thrombocytopenia more frequent with veliparib + chemo | NCT02470585 |
| PAOLA-1/ENGOT-ov25 [45,46] | Control: Bev (15 mg/kg q3w for up to 15 mo) + Placebo maintenance (24 mo) Experimental: Bev (15 mg/kg q3w for up to 15 mo) + Olaparib (300 mg BID, up to 24 mo) | Newly diagnosed FIGO stage III–IV HGSOC in response after platinum-based CTx + bevacizumab | 806 | PFS | HRD+: PFS 37.2 mo (olaparib + bev) vs. 17.7 mo (control); HR = 0.33 HRD+/BRCAwt: PFS 28.1 mo vs. 16.6 mo; HR = 0.43 ITT: PFS 22.1 mo vs. 16.6 mo; HR = 0.59 OS (ITT): 56.5 vs. 51.6 mo; HR = 0.92; NS HRD+: OS 5-yr OS 65.5% vs. 48.4%; HR = 0.62 | AE(s) profile consistent with olaparib + bev | NCT02477644 |
| Study | Treatment Strategy | Setting | No. of Patients | PD-L1 Selection | Primary Endpoint | Outcome | Clinical Trial ID |
|---|---|---|---|---|---|---|---|
| JAVELIN-100 [70] | Avelumab + CTx followed by avelumab maintenance vs CTx followed by avelumab maintenance vs. CTx alone | First-line | 998 | No | PFS | Negative (futility analysis) | NCT02718417 |
| JAVELIN-200 [71] | Avelumab monotherapy or avelumab + PLD vs. PLD alone | Platinum-resistant recurrent | 566 | No | PFS, OS | Negative | NCT02580058 |
| IMagyn050 [72] | Atezolizumab + CTx (carboplatin-paclitaxel) + bevacizumab vs. placebo | First-line | 1301 | Yes (stratified) | PFS, OS | Negative | NCT03038100 |
| ATALANTE/ENGOT-ov29 [73] | Atezolizumab + CTx + bevacizumab vs. placebo | Platinum-sensitive recurrent | 614 | Yes (38% PD-L1+) | PFS | Negative | NCT02891824 |
| NRG-GY009 [74] | Atezolizumab + PLD + bevacizumab vs PLD + bevacizumab vs. PLD + atezolizumab | Platinum-resistant recurrent | 444 | Exploratory (PD-L1, TILs) | PFS, OS | Negative | NCT02839707 |
| AGO-OVAR 2.29 [75] | Atezolizumab + non-platinum CTx (weekly paclitaxel or PLD) + bevacizumab vs. placebo | Platinum-resistant recurrent | 574 | Yes (mandatory PD-L1) | PFS, OS | Negative | NCT03353831 |
| ANITA (ENGOT-OV41) [76] | Atezolizumab + platinum CTx followed by niraparib maintenance vs. placebo | Platinum-sensitive recurrent | 417 | Yes (36% PD-L1+) | PFS | Negative | NCT03598270 |
| DUO-O [77] | Durvalumab + platinum-based CTx + bevacizumab, followed by durvalumab + bevacizumab + olaparib maintenance vs. platinum-based CTx + bevacizumab followed by bevacizumab | First-line (non-BRCA) | 1130 | No | PFS | Positive (PFS) | NCT03737643 |
| ATHENA Combo [78] | Nivolumab + rucaparib vs. rucaparib monotherapy | First-line maintenance | 863 | No | PFS | Negative | NCT03522246 |
| FIRST (ENGOT-OV44) [79] | CTx (carboplatin-paclitaxel) ± bevacizumab followed by placebo maintenance vs. CTx (carboplatin-paclitaxel) ± bevacizumab followed by niraparib maintenance vs. Dostarlimab + CTx (carboplatin-paclitaxel) ± bevacizumab followed by niraparib + dostarlimab maintenance | First-line | ~1000 | Yes (PD-L1 tested) | PFS | Ongoing | NCT03602859 |
| KEYLYNK-001 [80,81] | Pembrolizumab + CTx (carboplatin-paclitaxel) ± bevacizumab → pembrolizumab + olaparib maintenance vs. CTx alone vs pembrolizumab alone maintenance | First-line (BRCA-wt) | 1367 | Yes (CPS ≥ 10) | PFS | Positive pembrolizumab/olaparib vs CTx; pembrolizumab alone negative in CPS ≥ 10; intriguing benefit without bevacizumab in exploratory analyses | NCT03740165 |
| KEYNOTE-B96 [82] | Pembrolizumab + weekly paclitaxel ± bevacizumab vs. placebo + weekly paclitaxel ± bevacizumab | Platinum-resistant recurrent | ~616 | Yes (CPS score) | PFS | Ongoing | NCT05116189 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papageorgiou, D.; Liouta, G.; Pliakou, E.; Zachariou, E.; Sapantzoglou, I.; Prokopakis, I.; Kontomanolis, E.N. Management of Advanced Ovarian Cancer: Current Clinical Practice and Future Perspectives. Biomedicines 2025, 13, 1525. https://doi.org/10.3390/biomedicines13071525
Papageorgiou D, Liouta G, Pliakou E, Zachariou E, Sapantzoglou I, Prokopakis I, Kontomanolis EN. Management of Advanced Ovarian Cancer: Current Clinical Practice and Future Perspectives. Biomedicines. 2025; 13(7):1525. https://doi.org/10.3390/biomedicines13071525
Chicago/Turabian StylePapageorgiou, Dimitrios, Galateia Liouta, Evangelia Pliakou, Eleftherios Zachariou, Ioakeim Sapantzoglou, Ioannis Prokopakis, and Emmanuel N. Kontomanolis. 2025. "Management of Advanced Ovarian Cancer: Current Clinical Practice and Future Perspectives" Biomedicines 13, no. 7: 1525. https://doi.org/10.3390/biomedicines13071525
APA StylePapageorgiou, D., Liouta, G., Pliakou, E., Zachariou, E., Sapantzoglou, I., Prokopakis, I., & Kontomanolis, E. N. (2025). Management of Advanced Ovarian Cancer: Current Clinical Practice and Future Perspectives. Biomedicines, 13(7), 1525. https://doi.org/10.3390/biomedicines13071525







