Temporal Changes in Mitochondria-Centric Excitotoxic Responses Following Severe Penetrating Traumatic Brain Injury
Abstract
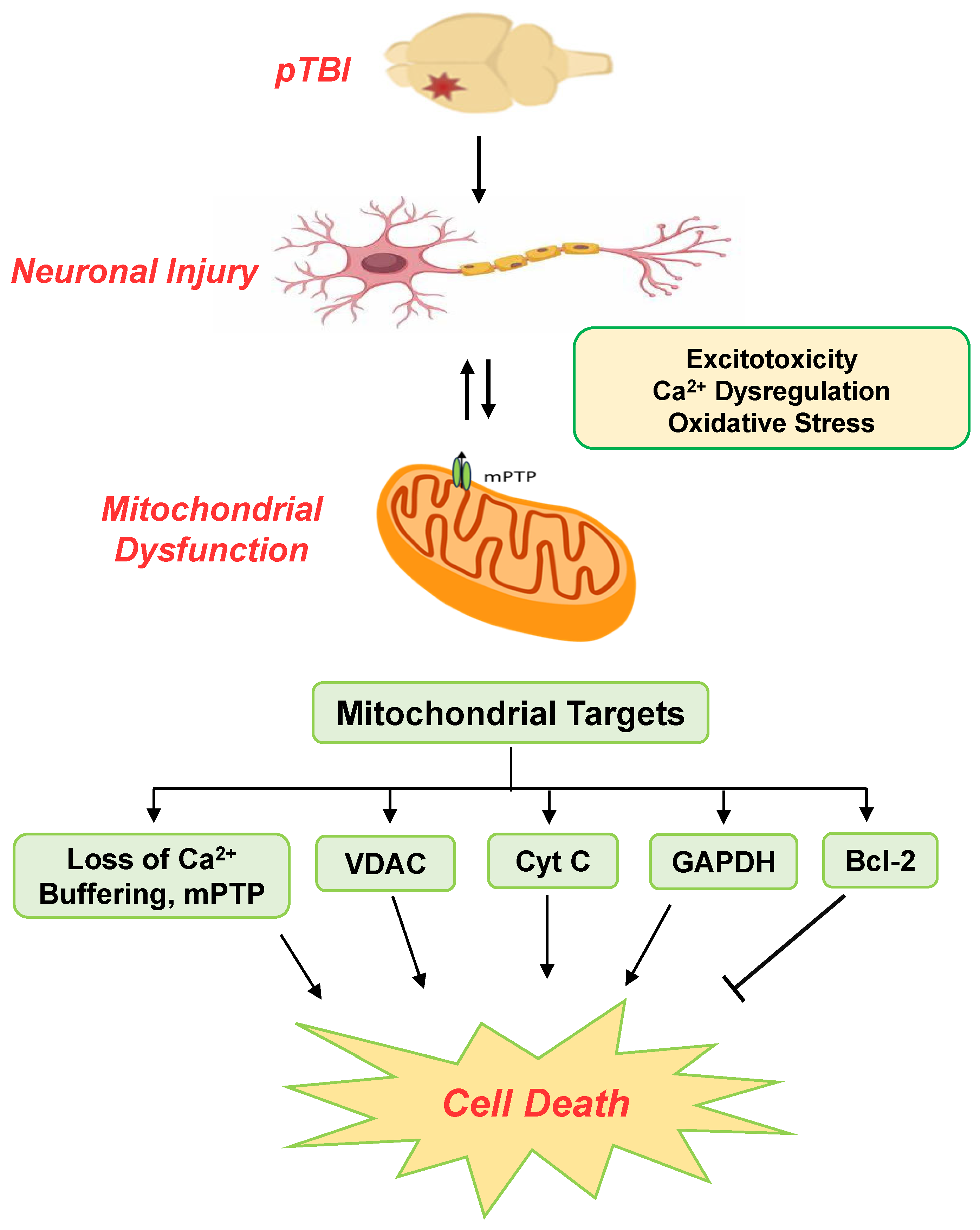
1. Introduction
2. Material and Methods
2.1. Reagents
2.2. Animals
2.3. Penetrating Traumatic Brain Injury (pTBI)
2.4. Mitochondrial Isolation
2.5. Mitochondrial Calcium Buffering Capacity
2.6. Western Blots
2.7. Statistical Analysis
3. Results
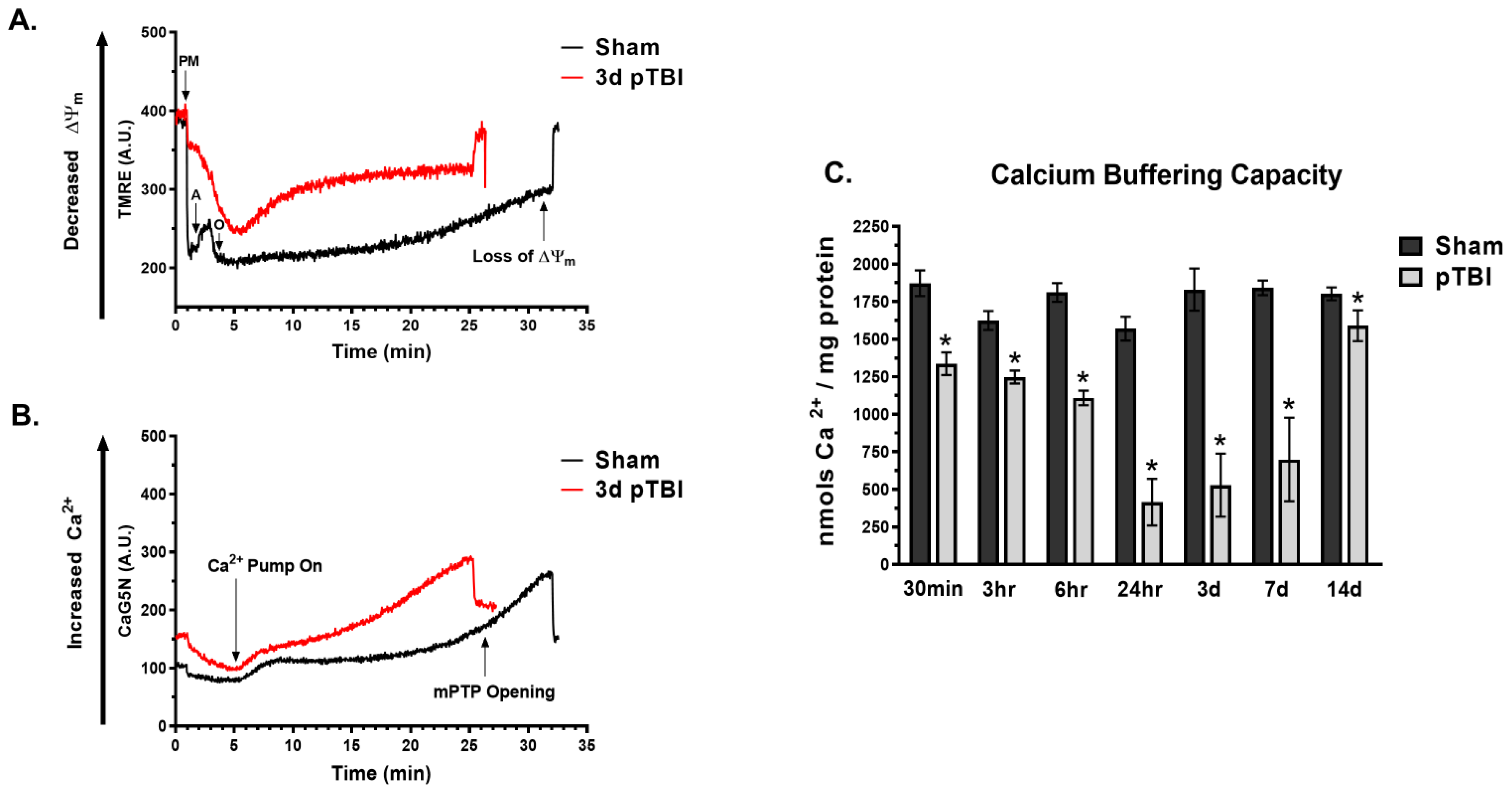
3.1. Temporal Loss of Calcium Buffering Capacity Post-pTBI
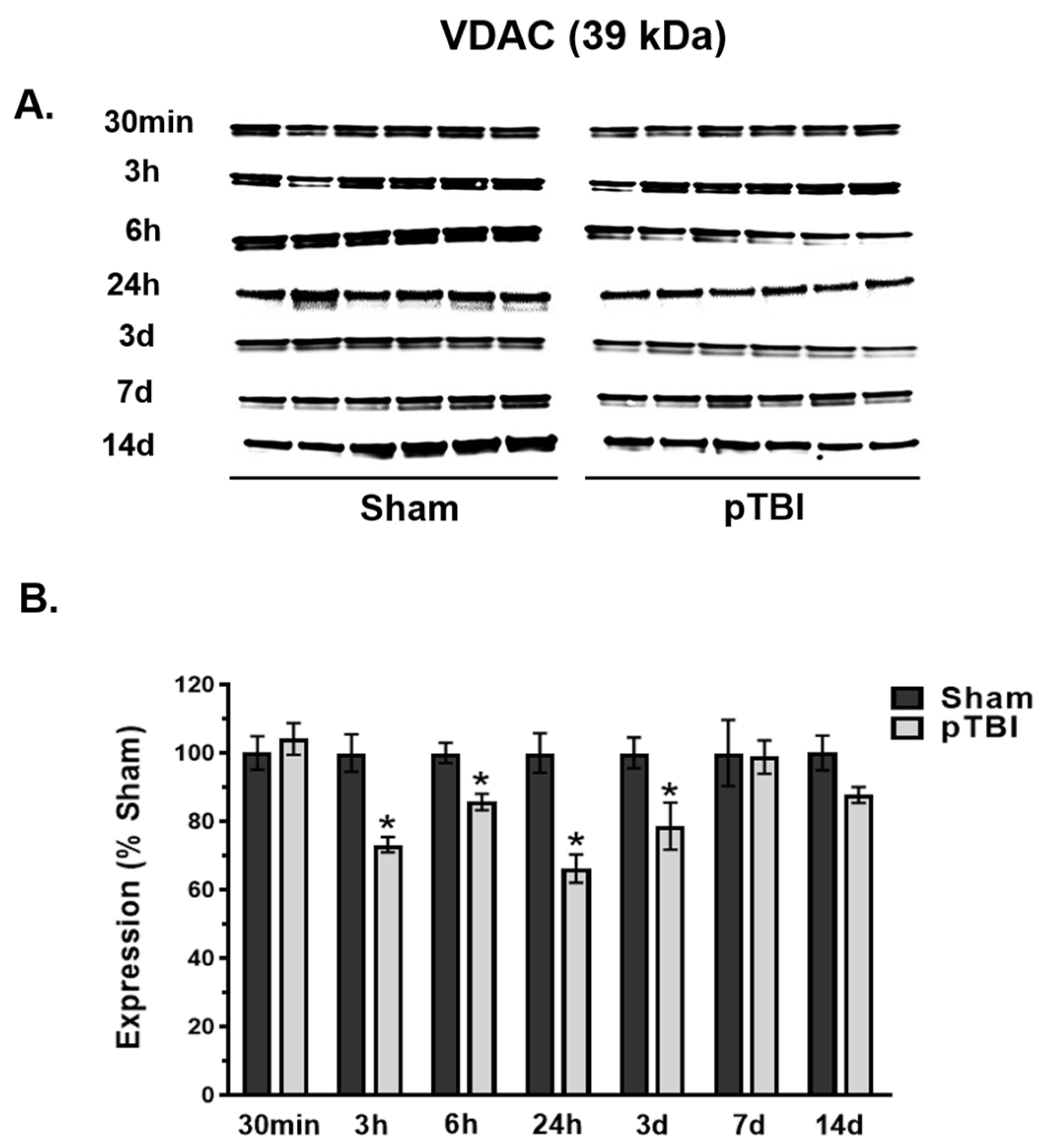
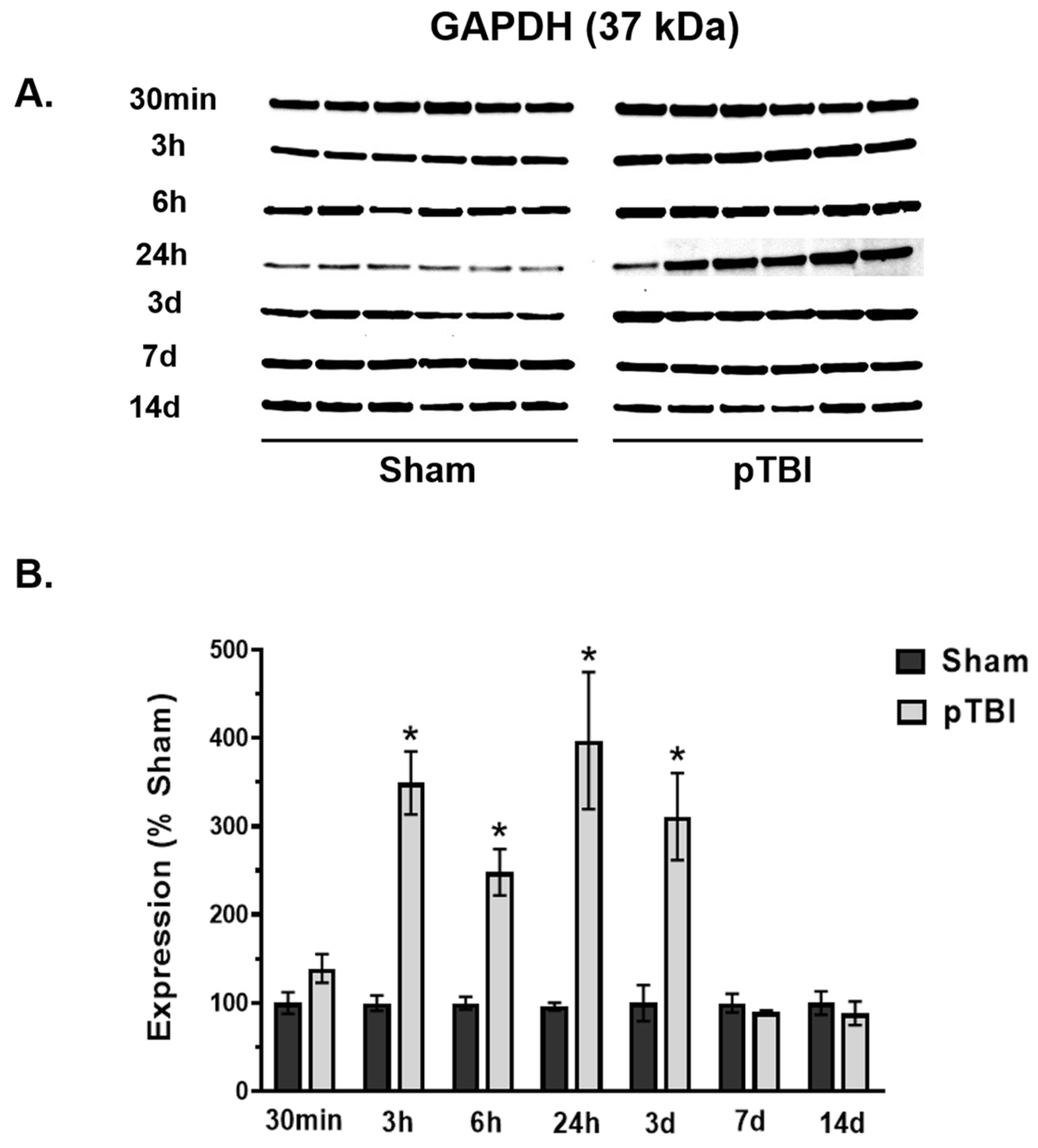
3.2. Time Course of Decreased Mitochondrial Membrane Integrity Markers Post-pTBI
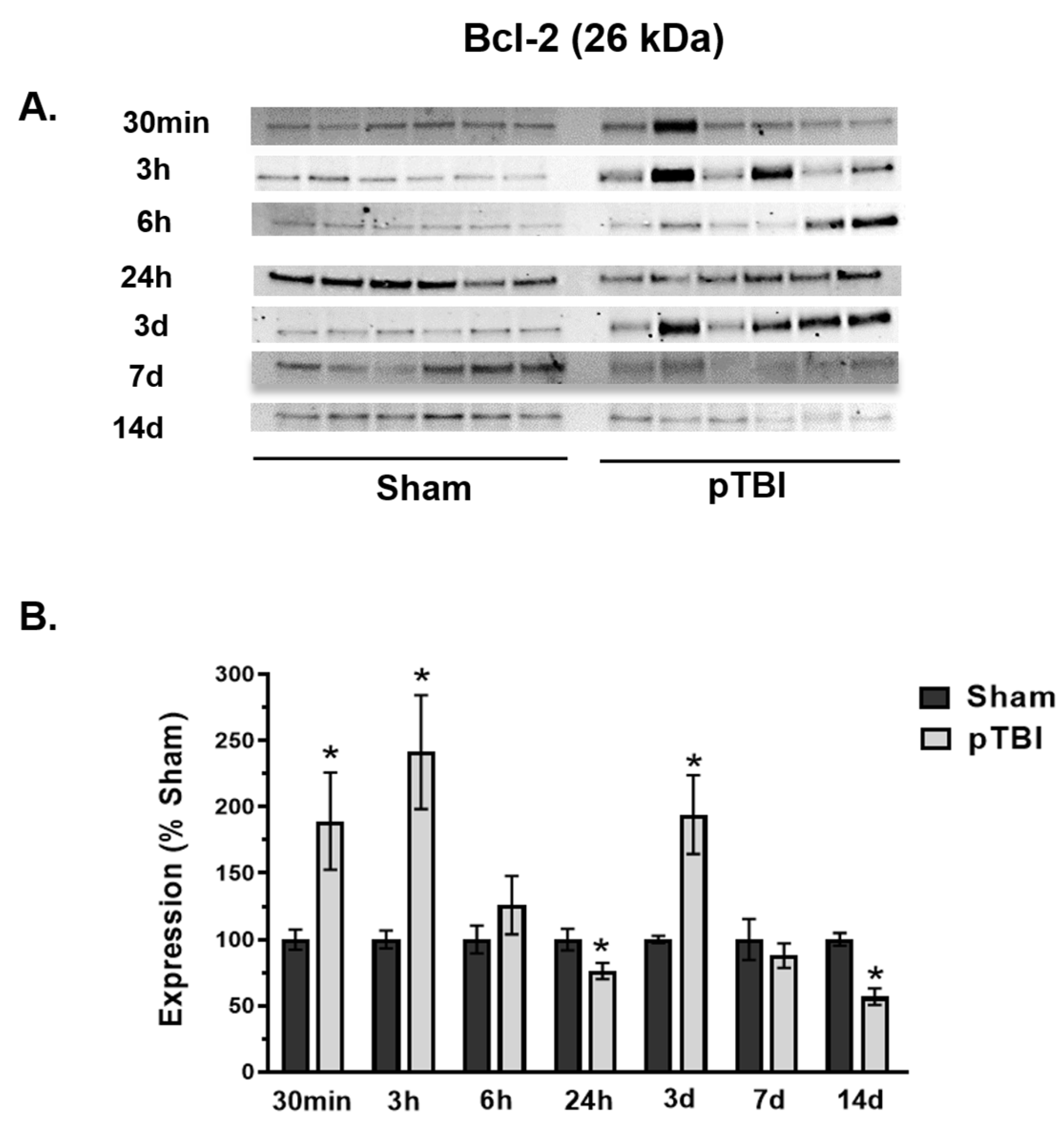
3.3. Temporal Changes in Mitochondrial Cell Death Markers Post-pTBI
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Dengler, B.A.; Agimi, Y.; Stout, K.; Caudle, K.L.; Curley, K.C.; Sanjakdar, S.; Rone, M.; Dacanay, B.; Fruendt, J.C.; Phillips, J.B.; et al. Epidemiology, patterns of care and outcomes of traumatic brain injury in deployed military settings: Implications for future military operations. J. Trauma Acute Care Surg. 2022, 93, 220–228. [Google Scholar] [CrossRef] [PubMed]
- McKee, A.C.; Robinson, M.E. Military-related traumatic brain injury and neurodegeneration. Alzheimer’s Dement. 2014, 10 (Suppl. S3), S242–S253. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, W.B.; Joseph, B.; Spry, M.; Vekaria, H.J.; Saatman, K.E.; Sullivan, P.G. Acute Mitochondrial Impairment Underlies Prolonged Cellular Dysfunction after Repeated Mild Traumatic Brain Injuries. J. Neurotrauma 2019, 36, 1252–1263. [Google Scholar] [CrossRef] [PubMed]
- Kilbaugh, T.J.; Karlsson, M.; Byro, M.; Bebee, A.; Ralston, J.; Sullivan, S.; Duhaime, A.-C.; Hansson, M.J.; Elmer, E.; Margulies, S.S. Mitochondrial bioenergetic alterations after focal traumatic brain injury in the immature brain. Exp. Neurol. 2015, 271, 136–144. [Google Scholar] [CrossRef]
- Pandya, J.D.; Pauly, J.R.; Nukala, V.N.; Sebastian, A.H.; Day, K.M.; Korde, A.S.; Maragos, W.F.; Hall, E.D.; Sullivan, P.G. Post-injury administration of mitochondrial uncouplers increases tissue sparing and improves behavioral outcome following traumatic brain injury in rodents. J. Neurotrauma 2007, 24, 798–811. [Google Scholar] [CrossRef]
- Sullivan, P.G.; Rabchevsky, A.G.; Keller, J.N.; Lovell, M.; Sodhi, A.; Hart, R.P.; Scheff, S.W. Intrinsic differences in brain and spinal cord mitochondria: Implication for therapeutic interventions. J. Comp. Neurol. 2004, 474, 524–534. [Google Scholar] [CrossRef]
- Prins, M.; Greco, T.; Alexander, D.; Giza, C.C. The pathophysiology of traumatic brain injury at a glance. Dis. Models Mech. 2013, 6, 1307–1315. [Google Scholar] [CrossRef]
- Bodnar, C.N.; Roberts, K.N.; Higgins, E.K.; Bachstetter, A.D. A systematic review of closed head injury models of mild traumatic brain injury in mice and rats. J. Neurotrauma 2019, 36, 1683–1706. [Google Scholar] [CrossRef]
- Xiong, Y.; Mahmood, A.; Chopp, M. Animal models of traumatic brain injury. Nat. Rev. Neurosci. 2013, 14, 128–142. [Google Scholar] [CrossRef]
- Marklund, N. Rodent models of traumatic brain injury: Methods and challenges. In Injury Models of the Central Nervous System: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2016; pp. 29–46. [Google Scholar]
- Plantman, S. Novel rodent models of penetrating traumatic brain injury. Neural Regen. Res. 2015, 10, 1047–1049. [Google Scholar] [CrossRef]
- Cheng, G.; Kong, R.H.; Zhang, L.M.; Zhang, J.N. Mitochondria in traumatic brain injury and mitochondrial-targeted multipotential therapeutic strategies. Br. J. Pharmacol. 2012, 167, 699–719. [Google Scholar] [CrossRef] [PubMed]
- Modi, H.R.; Musyaju, S.; Ratcliffe, M.; Shear, D.A.; Scultetus, A.H.; Pandya, J.D. Mitochondria-Targeted Antioxidant Therapeutics for Traumatic Brain Injury. Antioxidants 2024, 13, 303. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Youngblood, H.; Wu, C.; Zhang, Q. Mitochondria as a target for neuroprotection: Role of methylene blue and photobiomodulation. Transl. Neurodegener. 2020, 9, 19. [Google Scholar] [CrossRef]
- Faden, A.I.; Demediuk, P.; Panter, S.S.; Vink, R. The role of excitatory amino acids and NMDA receptors in traumatic brain injury. Science 1989, 244, 798–800. [Google Scholar] [CrossRef]
- Rego, A.C.; Ward, M.W.; Nicholls, D.G. Mitochondria control ampa/kainate receptor-induced cytoplasmic calcium deregulation in rat cerebellar granule cells. J. Neurosci. 2001, 21, 1893–1901. [Google Scholar] [CrossRef]
- Stavsky, A.; Stoler, O.; Kostic, M.; Katoshevsky, T.; Assali, E.A.; Savic, I.; Amitai, Y.; Prokisch, H.; Leiz, S.; Daumer-Haas, C.; et al. Aberrant activity of mitochondrial NCLX is linked to impaired synaptic transmission and is associated with mental retardation. Commun. Biol. 2021, 4, 666. [Google Scholar] [CrossRef]
- Abdul-Muneer, P.M.; Chandra, N.; Haorah, J. Interactions of oxidative stress and neurovascular inflammation in the pathogenesis of traumatic brain injury. Mol. Neurobiol. 2015, 51, 966–979. [Google Scholar] [CrossRef]
- Brookes, P.S.; Yoon, Y.; Robotham, J.L.; Anders, M.W.; Sheu, S.S. Calcium, ATP, and ROS: A mitochondrial love-hate triangle. Am. J. Physiol.-Cell Physiol. 2004, 287, C817–C833. [Google Scholar] [CrossRef]
- Görlach, A.; Bertram, K.; Hudecova, S.; Krizanova, O. Calcium and ROS: A mutual interplay. Redox Biol. 2015, 6, 260–271. [Google Scholar] [CrossRef]
- Mazur, H.; Merlavsky, V.; Manko, B.; Manko, V. mPTP opening differently affects electron transport chain and oxidative phosphorylation at succinate and NAD-dependent substrates oxidation in permeabilized rat hepatocytes. Ukr. Biochem. J. 2020, 92, 14–23. [Google Scholar] [CrossRef]
- Feno, S.; Butera, G.; Vecellio Reane, D.; Rizzuto, R.; Raffaello, A. Crosstalk between Calcium and ROS in Pathophysiological Conditions. Oxid. Med. Cell Longev. 2019, 2019, 9324018. [Google Scholar] [CrossRef] [PubMed]
- Pandya, J.D.; Leung, L.Y.; Flerlage, W.J.; Gilsdorf, J.S.; Bryant, Y.D.; Shear, D. Comprehensive profile of acute mitochondrial dysfunction in a preclinical model of severe penetrating TBI. Front. Neurol. 2019, 10, 605. [Google Scholar] [CrossRef] [PubMed]
- Pandya, J.D.; Leung, L.Y.; Hwang, H.M.; Yang, X.; Deng-Bryant, Y.; Shear, D.A. Time-Course Evaluation of Brain Regional Mitochondrial Bioenergetics in a Pre-Clinical Model of Severe Penetrating Traumatic Brain Injury. J. Neurotrauma 2021, 38, 2323–2334. [Google Scholar] [CrossRef] [PubMed]
- Pandya, J.D.; Musyaju, S.; Modi, H.R.; Cao, Y.; Flerlage, W.J.; Huynh, L.; Kociuba, B.; Visavadiya, N.P.; Kobeissy, F.; Wang, K.; et al. Comprehensive evaluation of mitochondrial redox profile, calcium dynamics, membrane integrity and apoptosis markers in a preclinical model of severe penetrating traumatic brain injury. Free Radic. Biol. Med. 2023, 198, 44–58. [Google Scholar] [CrossRef]
- Musyaju, S.; Modi, H.R.; Shear, D.A.; Scultetus, A.H.; Pandya, J.D. Time Course of Mitochondrial Antioxidant Markers in a Preclinical Model of Severe Penetrating Traumatic Brain Injury. Int. J. Mol. Sci. 2025, 26, 906. [Google Scholar] [CrossRef]
- Williams, A.J.; Hartings, J.A.; Lu, X.C.; Rolli, M.L.; Tortella, F.C. Penetrating ballistic-like brain injury in the rat: Differential time courses of hemorrhage, cell death, inflammation, and remote degeneration. J. Neurotrauma 2006, 23, 1828–1846. [Google Scholar] [CrossRef]
- Brown, M.R.; Sullivan, P.G.; Geddes, J.W. Synaptic mitochondria are more susceptible to Ca2+ overload than nonsynaptic mitochondria. J. Biol. Chem. 2006, 281, 11658–11668. [Google Scholar] [CrossRef]
- Chalmers, S.; Nicholls, D.G. The relationship between free and total calcium concentrations in the matrix of liver and brain mitochondria. J. Biol. Chem. 2003, 278, 19062–19070. [Google Scholar] [CrossRef]
- Avery, M.A.; Rooney, T.M.; Pandya, J.D.; Wishart, T.M.; Gillingwater, T.H.; Geddes, J.W.; Sullivan, P.G.; Freeman, M.R. WldS prevents axon degeneration through increased mitochondrial flux and enhanced mitochondrial Ca2+ buffering. Curr. Biol. 2012, 22, 596–600. [Google Scholar] [CrossRef]
- Pandya, J.D.; Pauly, J.R.; Sullivan, P.G. The optimal dosage and window of opportunity to maintain mitochondrial homeostasis following traumatic brain injury using the uncoupler FCCP. Exp. Neurol. 2009, 218, 381–389. [Google Scholar] [CrossRef]
- Musyaju, S.; Modi, H.R.; Flerlage, W.J.; Scultetus, A.H.; Shear, D.A.; Pandya, J.D. Revert total protein normalization method offers a reliable loading control for mitochondrial samples following TBI. Anal. Biochem. 2023, 680, 115301. [Google Scholar] [CrossRef] [PubMed]
- Deng-Bryant, Y.; Leung, L.Y.; Pandya, J.; Yang, W.; Gilsdorf, J.; Shear, D. Global metabolomics analysis in rats following penetrating ballistic-like brain injury. J. Neurotrauma 2016, 33, A54. [Google Scholar]
- Ladak, A.A.; Enam, S.A.; Ibrahim, M.T. A Review of the Molecular Mechanisms of Traumatic Brain Injury. World Neurosurg. 2019, 131, 126–132. [Google Scholar] [CrossRef]
- Kunz, A.; Dirnagl, U.; Mergenthaler, P. Acute pathophysiological processes after ischaemic and traumatic brain injury. Best Pr. Res. Clin. Anaesthesiol. 2010, 24, 495–509. [Google Scholar] [CrossRef]
- Furuta, T.; Nakagawa, I.; Yokoyama, S.; Morisaki, Y.; Saito, Y.; Nakase, H. Melatonin-Induced Postconditioning Suppresses NMDA Receptor through Opening of the Mitochondrial Permeability Transition Pore via Melatonin Receptor in Mouse Neurons. Int. J. Mol. Sci. 2022, 23, 3822. [Google Scholar] [CrossRef]
- Peng, T.I.; Jou, M.J.; Sheu, S.S.; Greenamyre, J.T. Visualization of NMDA receptor-induced mitochondrial calcium accumulation in striatal neurons. Exp. Neurol. 1998, 149, 1–12. [Google Scholar] [CrossRef]
- Garbincius, J.F.; Elrod, J.W. Mitochondrial calcium exchange in physiology and disease. Physiol. Rev. 2022, 102, 893–992. [Google Scholar] [CrossRef]
- Rizzuto, R.; Marchi, S.; Bonora, M.; Aguiari, P.; Bononi, A.; De Stefani, D.; Giorgi, C.; Leo, S.; Rimessi, A.; Siviero, R.; et al. Ca2+ transfer from the ER to mitochondria: When, how and why. Biochim. Biophys. Acta (BBA) Bioenerg. 2009, 1787, 1342–1351. [Google Scholar] [CrossRef]
- Thapak, P.; Gomez-Pinilla, F. The bioenergetics of traumatic brain injury and its long-term impact for brain plasticity and function. Pharmacol. Res. 2024, 208, 107389. [Google Scholar] [CrossRef]
- Wan, B.; LaNoue, K.F.; Cheung, J.Y.; Scaduto, R.C., Jr. Regulation of citric acid cycle by calcium. J. Biol. Chem. 1989, 264, 13430–13439. [Google Scholar] [CrossRef]
- Veech, R.L.; Valeri, C.R.; VanItallie, T.B. The mitochondrial permeability transition pore provides a key to the diagnosis and treatment of traumatic brain injury. IUBMB Life 2012, 64, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.T. Altered calcium signaling following traumatic brain injury. Front. Pharmacol. 2012, 3, 60. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Pizzo, P.; Filadi, R. Calcium, mitochondria and cell metabolism: A functional triangle in bioenergetics. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2019, 1866, 1068–1078. [Google Scholar] [CrossRef] [PubMed]
- Contreras, L.; Drago, I.; Zampese, E.; Pozzan, T. Mitochondria: The calcium connection. Biochim. Biophys. Acta (BBA) Bioenerg. 2010, 1797, 607–618. [Google Scholar] [CrossRef]
- Morciano, G.; Bonora, M.; Campo, G.; Aquila, G.; Rizzo, P.; Giorgi, C.; Wieckowski, M.R.; Pinton, P. Mechanistic Role of mPTP in Ischemia-Reperfusion Injury. In Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2017; Volume 982, pp. 169–189. [Google Scholar] [CrossRef]
- Panel, M.; Ruiz, I.; Brillet, R.; Lafdil, F.; Teixeira-Clerc, F.; Nguyen, C.T.; Calderaro, J.; Gelin, M.; Allemand, F.; Guichou, J.F.; et al. Small-Molecule Inhibitors of Cyclophilins Block Opening of the Mitochondrial Permeability Transition Pore and Protect Mice from Hepatic Ischemia/Reperfusion Injury. Gastroenterology 2019, 157, 1368–1382. [Google Scholar] [CrossRef]
- Hånell, A.; Greer, J.E.; McGinn, M.J.; Povlishock, J.T. Traumatic brain injury-induced axonal phenotypes react differently to treatment. Acta Neuropathol. 2015, 129, 317–332. [Google Scholar] [CrossRef]
- Readnower, R.D.; Pandya, J.D.; McEwen, M.L.; Pauly, J.R.; Springer, J.E.; Sullivan, P.G. Post-injury administration of the mitochondrial permeability transition pore inhibitor, NIM811, is neuroprotective and improves cognition after traumatic brain injury in rats. J. Neurotrauma 2011, 28, 1845–1853. [Google Scholar] [CrossRef]
- Zhou, B.; Kreuzer, J.; Kumsta, C.; Wu, L.; Kamer, K.J.; Cedillo, L.; Zhang, Y.; Li, S.; Kacergis, M.C.; Webster, C.M.; et al. Mitochondrial Permeability Uncouples Elevated Autophagy and Lifespan Extension. Cell 2019, 177, 299–314. [Google Scholar] [CrossRef]
- Ludtmann, M.H.R.; Angelova, P.R.; Horrocks, M.H.; Choi, M.L.; Rodrigues, M.; Baev, A.Y.; Berezhnov, A.V.; Yao, Z.; Little, D.; Banushi, B.; et al. α-synuclein oligomers interact with ATP synthase and open the permeability transition pore in Parkinson’s disease. Nat. Commun. 2018, 9, 2293. [Google Scholar] [CrossRef]
- Peng, T.I.; Jou, M.J. Oxidative stress caused by mitochondrial calcium overload. Ann. N. Y. Acad. Sci. 2010, 1201, 183–188. [Google Scholar] [CrossRef]
- Garrido, C.; Galluzzi, L.; Brunet, M.; Puig, P.E.; Didelot, C.; Kroemer, G. Mechanisms of cytochrome c release from mitochondria. Cell Death Differ. 2006, 13, 1423–1433. [Google Scholar] [CrossRef] [PubMed]
- Lewén, A.; Fujimura, M.; Sugawara, T.; Matz, P.; Copin, J.C.; Chan, P.H. Oxidative stress-dependent release of mitochondrial cytochrome c after traumatic brain injury. J. Cereb. Blood Flow Metab. 2001, 21, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, P.G.; Keller, J.N.; Bussen, W.L.; Scheff, S.W. Cytochrome c release and caspase activation after traumatic brain injury. Brain Res. 2002, 949, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, A.; Scorrano, L. Mitochondria: From cell death executioners to regulators of cell differentiation. Trends Cell Biol. 2014, 24, 761–770. [Google Scholar] [CrossRef]
- Hiebert, J.B.; Shen, Q.; Thimmesch, A.R.; Pierce, J.D. Traumatic brain injury and mitochondrial dysfunction. Am. J. Med. Sci. 2015, 350, 132–138. [Google Scholar] [CrossRef]
- Darwish, R.S.; Amiridze, N.S. Detectable levels of cytochrome C and activated caspase-9 in cerebrospinal fluid after human traumatic brain injury. Neurocrit. Care 2010, 12, 337–341. [Google Scholar] [CrossRef]
- Mattson, M.P. Excitotoxicity. In Neurodegeneration; Wiley: Hoboken, NJ, USA, 2017; pp. 37–45. [Google Scholar]
- Luetjens, C.M.; Bui, N.T.; Sengpiel, B.; Münstermann, G.; Poppe, M.; Krohn, A.J.; Bauerbach, E.; Krieglstein, J.; Prehn, J.H. Delayed mitochondrial dysfunction in excitotoxic neuron death: Cytochrome c release and a secondary increase in superoxide production. J. Neurosci. 2000, 20, 5715–5723. [Google Scholar] [CrossRef]
- Li, Z.; Jo, J.; Jia, J.M.; Lo, S.C.; Whitcomb, D.J.; Jiao, S.; Cho, K.; Sheng, M. Caspase-3 activation via mitochondria is required for long-term depression and AMPA receptor internalization. Cell 2010, 141, 859–871. [Google Scholar] [CrossRef]
- Opii, W.O.; Nukala, V.N.; Sultana, R.; Pandya, J.D.; Day, K.M.; Merchant, M.L.; Klein, J.B.; Sullivan, P.G.; Butterfield, D.A. Proteomic identification of oxidized mitochondrial proteins following experimental traumatic brain injury. J. Neurotrauma 2007, 24, 772–789. [Google Scholar] [CrossRef]
- Tristan, C.; Shahani, N.; Sedlak, T.W.; Sawa, A. The diverse functions of GAPDH: Views from different subcellular compartments. Cell. Signal. 2011, 23, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Tarze, A.; Deniaud, A.; Le Bras, M.; Maillier, E.; Molle, D.; Larochette, N.; Zamzami, N.; Jan, G.; Kroemer, G.; Brenner, C. GAPDH, a novel regulator of the pro-apoptotic mitochondrial membrane permeabilization. Oncogene 2007, 26, 2606–2620. [Google Scholar] [CrossRef] [PubMed]
- Saunders, P.A.; Chalecka-Franaszek, E.; Chuang, D.M. Subcellular distribution of glyceraldehyde-3-phosphate dehydrogenase in cerebellar granule cells undergoing cytosine arabinoside-induced apoptosis. J. Neurochem. 1997, 69, 1820–1828. [Google Scholar] [CrossRef] [PubMed]
- Colell, A.; Ricci, J.E.; Tait, S.; Milasta, S.; Maurer, U.; Bouchier-Hayes, L.; Fitzgerald, P.; Guio-Carrion, A.; Waterhouse, N.J.; Li, C.W.; et al. GAPDH and autophagy preserve survival after apoptotic cytochrome c release in the absence of caspase activation. Cell 2007, 129, 983–997. [Google Scholar] [CrossRef] [PubMed]
- Ameri, K.; Rajah, A.M.; Nguyen, V.; Sanders, T.A.; Jahangiri, A.; Delay, M.; Donne, M.; Choi, H.J.; Tormos, K.V.; Yeghiazarians, Y.; et al. Nuclear localization of the mitochondrial factor HIGD1A during metabolic stress. PLoS ONE 2013, 8, e62758. [Google Scholar] [CrossRef]
- Mansur, N.R.; Meyer-Siegler, K.; Wurzer, J.C.; Sirover, M.A. Cell cycle regulation of the glyceraldehyde-3-phosphate dehydrogenase/uracil DNA glycosylase gene in normal human cells. Nucleic Acids Res. 1993, 21, 993–998. [Google Scholar] [CrossRef]
- Schuppe-Koistinen, I.; Moldéus, P.; Bergman, T.; Cotgreave, I.A. S-thiolation of human endothelial cell glyceraldehyde-3-phosphate dehydrogenase after hydrogen peroxide treatment. Eur. J. Biochem. 1994, 221, 1033–1037. [Google Scholar] [CrossRef]
- Grant, C.M.; Quinn, K.A.; Dawes, I.W. Differential protein S-thiolation of glyceraldehyde-3-phosphate dehydrogenase isoenzymes influences sensitivity to oxidative stress. Mol. Cell. Biol. 1999, 19, 2650–2656. [Google Scholar] [CrossRef]
- Itakura, M.; Nakajima, H.; Kubo, T.; Semi, Y.; Kume, S.; Higashida, S.; Kaneshige, A.; Kuwamura, M.; Harada, N.; Kita, A.; et al. Glyceraldehyde-3-phosphate Dehydrogenase Aggregates Accelerate Amyloid-β Amyloidogenesis in Alzheimer Disease. J. Biol. Chem. 2015, 290, 26072–26087. [Google Scholar] [CrossRef]
- Dutysheva, E.A.; Mikhaylova, E.R.; Trestsova, M.A.; Andreev, A.I.; Apushkin, D.Y.; Utepova, I.A.; Serebrennikova, P.O.; Akhremenko, E.A.; Aksenov, N.D.; Bon, E.I.; et al. Combination of a Chaperone Synthesis Inducer and an Inhibitor of GAPDH Aggregation for Rehabilitation after Traumatic Brain Injury: A Pilot Study. Pharmaceutics 2022, 15, 7. [Google Scholar] [CrossRef]
- Lazarev, V.F.; Dutysheva, E.A.; Komarova, E.Y.; Mikhaylova, E.R.; Guzhova, I.V.; Margulis, B.A. GAPDH-targeted therapy–A new approach for secondary damage after traumatic brain injury on rats. Biochem. Biophys. Res. Commun. 2018, 501, 1003–1008. [Google Scholar] [CrossRef]
- Nakajima, H.; Itakura, M.; Kubo, T.; Kaneshige, A.; Harada, N.; Izawa, T.; Azuma, Y.T.; Kuwamura, M.; Yamaji, R.; Takeuchi, T. Glyceraldehyde-3-phosphate Dehydrogenase (GAPDH) Aggregation Causes Mitochondrial Dysfunction during Oxidative Stress-induced Cell Death. J. Biol. Chem. 2017, 292, 4727–4742. [Google Scholar] [CrossRef] [PubMed]
- Vervliet, T.; Parys, J.B.; Bultynck, G. Bcl-2 proteins and calcium signaling: Complexity beneath the surface. Oncogene 2016, 35, 5079–5092. [Google Scholar] [CrossRef] [PubMed]
- Pinton, P.; Ferrari, D.; Magalhães, P.; Schulze-Osthoff, K.; Di Virgilio, F.; Pozzan, T.; Rizzuto, R. Reduced loading of intracellular Ca2+ stores and downregulation of capacitative Ca2+ influx in Bcl-2-overexpressing cells. J. Cell Biol. 2000, 148, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.; Dubyak, G.; Chen, L.; Nuñez, G.; Miesfeld, R.L.; Distelhorst, C.W. Evidence that BCL-2 represses apoptosis by regulating endoplasmic reticulum-associated Ca2+ fluxes. Proc. Natl. Acad. Sci. USA 1994, 91, 6569–6573. [Google Scholar] [CrossRef]
- Tsujimoto, Y.; Shimizu, S. VDAC regulation by the Bcl-2 family of proteins. Cell Death Differ. 2000, 7, 1174–1181. [Google Scholar] [CrossRef]
- Rossé, T.; Olivier, R.; Monney, L.; Rager, M.; Conus, S.; Fellay, I.; Jansen, B.; Borner, C. Bcl-2 prolongs cell survival after Bax-induced release of cytochrome c. Nature 1998, 391, 496–499. [Google Scholar] [CrossRef]
- Deng, H.; Yue, J.K.; Zusman, B.E.; Nwachuku, E.L.; Abou-Al-Shaar, H.; Upadhyayula, P.S.; Okonkwo, D.O.; Puccio, A.M. B-Cell Lymphoma 2 (Bcl-2) and Regulation of Apoptosis after Traumatic Brain Injury: A Clinical Perspective. Medicina 2020, 56, 300. [Google Scholar] [CrossRef]
- Camello-Almaraz, C.; Gomez-Pinilla, P.J.; Pozo, M.J.; Camello, P.J. Mitochondrial reactive oxygen species and Ca2+ signaling. Am. J. Physiol.-Cell Physiol. 2006, 291, C1082–C1088. [Google Scholar] [CrossRef]
- Dykens, J.A. Isolated cerebral and cerebellar mitochondria produce free radicals when exposed to elevated Ca2+ and Na+: Implications for neurodegeneration. J. Neurochem. 1994, 63, 584–591. [Google Scholar] [CrossRef]
- Budd, S.L.; Nicholls, D.G. A reevaluation of the role of mitochondria in neuronal Ca2+ homeostasis. J. Neurochem. 1996, 66, 403–411. [Google Scholar] [CrossRef]
- Ichas, F.; Jouaville, L.S.; Mazat, J.P. Mitochondria are excitable organelles capable of generating and conveying electrical and calcium signals. Cell 1997, 89, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Ichas, F.; Mazat, J.P. From calcium signaling to cell death: Two conformations for the mitochondrial permeability transition pore. Switching from low- to high-conductance state. Biochim. Biophys. Acta (BBA) Bioenerg. 1998, 1366, 33–50. [Google Scholar] [CrossRef]
- Rizzuto, R.; Bernardi, P.; Pozzan, T. Mitochondria as all-round players of the calcium game. J. Physiol. 2000, 529 Pt 1, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Starkov, A.A.; Fiskum, G. Regulation of brain mitochondrial H2O2 production by membrane potential and NAD(P)H redox state. J. Neurochem. 2003, 86, 1101–1107. [Google Scholar] [CrossRef]
- La Barbera, L.; Nobili, A.; Cauzzi, E.; Paoletti, I.; Federici, M.; Saba, L.; Giacomet, C.; Marino, R.; Krashia, P.; Melone, M.; et al. Upregulation of Ca2+-binding proteins contributes to VTA dopamine neuron survival in the early phases of Alzheimer’s disease in Tg2576 mice. Mol. Neurodegener. 2022, 17, 76. [Google Scholar] [CrossRef]
- Ryan, K.C.; Laboy, J.T.; Norman, K.R. Deregulation of Mitochondrial Calcium Handling Due to Presenilin Loss Disrupts Redox Homeostasis and Promotes Neuronal Dysfunction. Antioxidants 2022, 11, 1642. [Google Scholar] [CrossRef]
- Cooper, G.; Kang, S.; Perez-Rosello, T.; Guzman, J.N.; Galtieri, D.; Xie, Z.; Kondapalli, J.; Mordell, J.; Silverman, R.B.; Surmeier, D.J. A Single Amino Acid Determines the Selectivity and Efficacy of Selective Negative Allosteric Modulators of CaV1.3 L-Type Calcium Channels. ACS Chem. Biol. 2020, 15, 2539–2550. [Google Scholar] [CrossRef]
- Kagan, V.E.; Bayir, A.; Bayir, H.; Stoyanovsky, D.; Borisenko, G.G.; Tyurina, Y.Y.; Wipf, P.; Atkinson, J.; Greenberger, J.S.; Chapkin, R.S.; et al. Mitochondria-targeted disruptors and inhibitors of cytochrome c/cardiolipin peroxidase complexes: A new strategy in anti-apoptotic drug discovery. Mol. Nutr. Food Res. 2009, 53, 104–114. [Google Scholar] [CrossRef]
- Cernak, I.; Stoica, B.; Byrnes, K.R.; Di Giovanni, S.; Faden, A.I. Role of the cell cycle in the pathobiology of central nervous system trauma. Cell Cycle 2005, 4, 1286–1293. [Google Scholar] [CrossRef]
- Luan, Y.; Jiang, L.; Luan, Y.; Xie, Y.; Yang, Y.; Ren, K.D. Mitophagy and Traumatic Brain Injury: Regulatory Mechanisms and Therapeutic Potentials. Oxid. Med. Cell Longev. 2023, 2023, 1649842. [Google Scholar] [CrossRef]
- Zotey, V.; Andhale, A.; Shegekar, T.; Juganavar, A. Adaptive Neuroplasticity in Brain Injury Recovery: Strategies and Insights. Cureus 2023, 15, e45873. [Google Scholar] [CrossRef]
- Bylicky, M.A.; Mueller, G.P.; Day, R.M. Mechanisms of Endogenous Neuroprotective Effects of Astrocytes in Brain Injury. Oxid. Med. Cell Longev. 2018, 2018, 6501031. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Modi, H.R.; Musyaju, S.; Scultetus, A.H.; Pandya, J.D. Temporal Changes in Mitochondria-Centric Excitotoxic Responses Following Severe Penetrating Traumatic Brain Injury. Biomedicines 2025, 13, 1520. https://doi.org/10.3390/biomedicines13071520
Modi HR, Musyaju S, Scultetus AH, Pandya JD. Temporal Changes in Mitochondria-Centric Excitotoxic Responses Following Severe Penetrating Traumatic Brain Injury. Biomedicines. 2025; 13(7):1520. https://doi.org/10.3390/biomedicines13071520
Chicago/Turabian StyleModi, Hiren R., Sudeep Musyaju, Anke H. Scultetus, and Jignesh D. Pandya. 2025. "Temporal Changes in Mitochondria-Centric Excitotoxic Responses Following Severe Penetrating Traumatic Brain Injury" Biomedicines 13, no. 7: 1520. https://doi.org/10.3390/biomedicines13071520
APA StyleModi, H. R., Musyaju, S., Scultetus, A. H., & Pandya, J. D. (2025). Temporal Changes in Mitochondria-Centric Excitotoxic Responses Following Severe Penetrating Traumatic Brain Injury. Biomedicines, 13(7), 1520. https://doi.org/10.3390/biomedicines13071520





