Neutrophil–Lymphocyte Ratio in Fibromyalgia and Axial Spondyloarthritis: A Potential Biomarker for Diagnosis and Disease Activity
Abstract
1. Introduction
- (1)
- Patients with Fibromyalgia and Axial Spondyloarthritis have significantly higher NLR values than healthy controls, reflecting a heightened inflammatory or stress-related response.
- (2)
- Axial Spondyloarthritis patients exhibit the highest NLR values due to the well-established inflammatory nature of the disease, whereas Fibromyalgia patients will show intermediate NLR values between healthy controls and Axial Spondyloarthritis patients.
- (3)
- Higher NLR values are associated with greater disease severity in both Fibromyalgia and Axial Spondyloarthritis, as measured by disease-specific severity/activity scores.
- (4)
- Specific NLR cut-off values can be established to differentiate patients from controls and to classify disease severity/activity in both conditions.
2. Materials and Methods
2.1. Study Design
2.2. Participants
- (1)
- Fibromyalgia patients: Diagnosed based on clinical criteria and recruited from a specialized Fibromyalgia unit. These patients did not meet the criteria for any inflammatory joint disease.
- (2)
- Axial Spondyloarthritis patients: Diagnosed based on clinical and imaging criteria and recruited from a specialized unit. These patients did not meet the criteria for Fibromyalgia.
- (3)
- Healthy controls: Recruited from a complex low back pain unit. These individuals had no clinical diagnosis of Fibromyalgia or inflammatory joint disease and had undergone a recent blood test (within six months of study enrollment).
2.3. Measures
- -
- Common variables (all groups):
- Age, gender, and Body Mass Index (BMI);
- Cardiovascular risk factors: Arterial hypertension, dyslipidemia, diabetes mellitus, history of acute myocardial infarction, and history of stroke;
- Medication use: Regular Non-Steroidal Anti-Inflammatory Drug (NSAID) consumption (>3 times per week);
- C-Reactive Protein (CRP) levels (mg/dL). Measured at the tertiary hospital, using the same equipment and kit manufacturer.
- -
- Fibromyalgia and Axial Spondyloarthritis groups only:
- Disease duration: Time since symptom onset and time since diagnosis;
- Disease activity/severity scores;
- Fibromyalgia: Revised Fibromyalgia Impact Questionnaire (FIQR) [18].
- -
- Axial Spondyloarthritis group only:
- Current treatment with Disease-Modifying Antirheumatic Drugs (DMARDs): Conventional synthetic (csDMARDs), biological (bDMARDs), and targeted synthetic (tsDMARDs).
2.4. Statistical Analysis
- -
- Group comparisons: Differences between groups were calculated using the Kruskal–Wallis test for quantitative variables and the Chi-square test or Fisher’s exact test for categorical variables (depending on expected frequencies).
- -
- Correlation analysis: The correlation between the NLR and disease severity/activity scores (FIQR, ASDAS, and BASDAI) was calculated using Pearson’s correlation coefficients.
- -
- Cut-off determination: The NLR cut-off values for diagnosis and disease activity were calculated using Receiver Operating Characteristic (ROC) curves.
2.5. Ethical Considerations
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NLR | Neutrophil–Lymphocyte Ratio |
| BMI | Body Mass Index |
| NSAIDs | Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) |
| CRP | C-Reactive Protein |
| FIQR | Revised Fibromyalgia Impact Questionnaire |
| ASDAS | Axial Spondyloarthritis Disease Activity Score |
| BASDAI | Bath Ankylosing Spondylitis Disease Activity Index |
| csDMARD | Conventional synthetic Disease-Modifying Antirheumatic Drug |
| bDMARD | Biological Disease-Modifying Antirheumatic Drug |
| tsDMARD | Targeted synthetic Disease-Modifying Antirheumatic Drug |
| ROC | Receiver Operating Characteristic |
| AUC | Area Under the Curve |
| CI | Confidence Interval |
| ESR | Erythrocyte Sedimentation Rate |
References
- Friedman, G.D.; Klatsky, A.L.; Siegelaub, A.B. The leukocyte count as a predictor of myocardial infarction. N. Engl. J. Med. 1974, 290, 1275–1278. [Google Scholar] [CrossRef] [PubMed]
- Horne, B.D.; Anderson, J.L.; John, J.M.; Weaver, A.; Bair, T.L.; Jensen, K.R.; Renlund, D.G.; Muhlestein, J.B. Which white blood cell subtypes predict increased cardiovascularrisk? J. Am. Coll. Cardiol. 2005, 45, 1638–1643. [Google Scholar] [CrossRef] [PubMed]
- Adamstein, N.H.; MacFadyen, J.G.; Rose, L.M.; Glynn, R.J.; Dey, A.K.; Libby, P.; Tabas, I.A.; Mehta, N.N.; Ridker, P.M. The neutrophil-lymphocyte ratio and incident atherosclerotic events: Analyses from five contemporary randomized trials. Eur. Heart J. 2021, 42, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zeng, A.; Chen, B.; Chen, Y.; Zhou, R. Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in patients with systemic lupus erythematosus and their correlation with activity: A meta-analysis. Int. Immunopharmacol. 2019, 76, 105949. [Google Scholar] [CrossRef]
- Zinellu, A.; Mangoni, A.A. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratio and disease activity in rheumatoid arthritis: A systematic review and meta-analysis. Eur. J. Clin. Investig. 2023, 53, e13877. [Google Scholar] [CrossRef]
- Khorrampazhouh, N.; Omranzadeh, A.; Fazeli, B.; Zarifian, A.; Ghodsi, A.; Amirkhanlou, F.; Saberi, A.; Arekhi, S.; Tork, M.A.B.; Goudarzi, Z.; et al. A Systematic Review and Meta-analysis of Clinical Studies on Ankylosing Spondylitis and Neutrophil to Lymphocyte Ratio. Curr. Rheumatol. Rev. 2022, 18, 160–167. [Google Scholar] [CrossRef]
- Aktürk, S.; Büyükavcı, R. Evaluation of blood neutrophil-lymphocyte ratio and platelet distribution width as inflammatory markers in patients with fibromyalgia. Clin. Rheumatol. 2017, 36, 1885–1889. [Google Scholar] [CrossRef]
- El-Sawy, E.A.; Abdul Hakim, M.M.; El-Zohiery, A.; Salama, S.M. Significance of inflammatory markers in primary Fibromyalgia syndrome and their relation in assessing the disease severity. Egypt. J. Immunol. 2024, 31, 67–74. [Google Scholar]
- Al-Nimer, M.S.M.; Mohammad, T.A.M. Correlation of hematological indices and ratios derived from them with FIQR scores in fibromyalgia. Pak. J. Med. Sci. 2018, 34, 1219–1224. [Google Scholar] [CrossRef]
- Ege, F.; Isik, R. A Comparative Assessment of the Inflammatory Markers in Patients with Fibromyalgia under Duloxetine Treatment. Front. Biosci. (Landmark Ed.) 2023, 28, 161. [Google Scholar] [CrossRef]
- Rajaram Jayakrishnan, A.K.; Easwar, S.V.; Thattil, J.; Vignesh, M.; Rath, S.; Prithvi, A.; Marwaha, V.; Cb, M.; Surendran, S. Studying the Relation Between Fibromyalgia Severity and Neutrophil-to-Lymphocyte Ratio, Platelet-to-Lymphocyte Ratio, and Mean Platelet Volume. Cureus 2022, 14, e24847. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, R.; Paola, R.D.; Cuzzocrea, S.; Impellizzeri, D. Fibromyalgia: Pathogenesis, Mechanisms, Diagnosis and Treatment Options Update. Int. J. Mol. Sci. 2021, 22, 3891. [Google Scholar] [CrossRef]
- Goebel, A.; Krock, E.; Gentry, C.; Israel, M.R.; Jurczak, A.; Urbina, C.M.; Sandor, K.; Vastani, N.; Maurer, M.; Cuhadar, U.; et al. Passive transfer of fibromyalgia symptoms from patients to mice. J. Clin. Investig. 2021, 131, e144201. [Google Scholar] [CrossRef]
- Krock, E.; Morado-Urbina, C.E.; Menezes, J.; Hunt, M.A.; Sandström, A.; Kadetoff, D.; Tour, J.; Verma, V.; Kultima, K.; Haglund, L.; et al. Fibromyalgia patients with elevated levels of anti-satellite glia cell immunoglobulin G antibodies present with more severe symptoms. Pain 2023, 164, 1828–1840. [Google Scholar] [CrossRef]
- Fanton, S.; Menezes, J.; Krock, E.; Sandström, A.; Tour, J.; Sandor, K.; Jurczak, A.; Hunt, M.; Baharpoor, A.; Kadetoff, D.; et al. Anti-satellite glia cell IgG antibodies in fibromyalgia patients are related to symptom severity and to metabolite concentrations in thalamus and rostral anterior cingulate cortex. Brain Behav. Immun. 2023, 114, 371–382. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Tsilioni, I.; Bawazeer, M. Mast Cells, Neuroinflammation and Pain in Fibromyalgia Syndrome. Front. Cell Neurosci. 2019, 13, 353. [Google Scholar] [CrossRef]
- Staud, R. Cytokine and immune system abnormalities in fibromyalgia and other central sensitivity syndromes. Curr. Rheumatol. Rev. 2015, 11, 109–115. [Google Scholar] [CrossRef]
- Salgueiro, M.; García-Leiva, J.M.; Ballesteros, J.; Hidalgo, J.; Molina, R.; Calandre, E.P. Validation of a Spanish version of the Revised Fibromyalgia Impact Questionnaire (FIQR). Health Qual. Life Outcomes 2013, 11, 132. [Google Scholar] [CrossRef]
- Lukas, C.; Landewé, R.; Sieper, J.; Dougados, M.; Davis, J.; Braun, J.; van der Linden, S.; van der Heijde, D. Development of an ASAS-endorsed disease activity score (ASDAS) in patients with ankylosing spondylitis. Ann. Rheum. Dis. 2009, 68, 18–24. [Google Scholar] [CrossRef]
- Cardiel, M.H.; Londoño, J.D.; Gutiérrez, E.; Pacheco-Tena, C.; Vázquez-Mellado, J.; Burgos-Vargas, R. Translation, cross-cultural adaptation, and validation of the Bath Ankylosing Spondylitis Functional Index (BASFI), the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) and the Dougados Functional Index (DFI) in a Spanish speaking population with spondyloarthropathies. Clin. Exp. Rheumatol. 2003, 21, 451–458. [Google Scholar]
- Coşkun, B.N.; Öksüz, M.F.; Ermurat, S.; Tufan, A.N.; Oruçoğlu, N.; Doğan, A.; Dalkılıç, E.; Pehlivan, Y. Neutrophil lymphocyte ratio can be a valuable marker in defining disease activity in patients who have started anti-tumor necrosis factor (TNF) drugs for ankylosing spondylitis. Eur. J. Rheumatol. 2014, 1, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Kucuk, A.; Uslu, A.U.; Ugan, Y.; Bagcaci, S.; Karahan, A.Y.; Akarmut, A.; Sahin, A.; Kucuksen, S. Neutrophil-to-lymphocyte ratio is involved in the severity of ankylosing spondylitis. Bratisl. Lek. Listy 2015, 116, 722–725. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A.; Khurshid, S.; Bano, S.; Rasheed, U.; Zammurrad, S.; Khan, M.S.; Aziz, W.; Tahir, S. The Role of the Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio as Markers of Disease Activity in Ankylosing Spondylitis. Cureus 2019, 11, e6025. [Google Scholar] [CrossRef] [PubMed]
- Sen, R.; Kim, E.; Napier, R.J.; Cheng, E.; Fernandez, A.; Manning, E.S.; Anderson, E.R.; Maier, K.D.; Hashim, M.; Kerr, G.S.; et al. Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio as Biomarkers in Axial Spondyloarthritis: Observational Studies from the Program to Understand the Longterm Outcomes in Spondyloarthritis Registry. Arthritis Rheumatol. 2023, 75, 232–241. [Google Scholar] [CrossRef]
- Moon, D.H.; Kim, A.; Song, B.W.; Kim, Y.K.; Kim, G.T.; Ahn, E.Y.; So, M.W.; Lee, S.G. High Baseline Neutrophil-to-Lymphocyte Ratio Could Serve as a Biomarker for Tumor Necrosis Factor-Alpha Blockers and Their Discontinuation in Patients with Ankylosing Spondylitis. Pharmaceuticals 2023, 16, 379. [Google Scholar] [CrossRef]
- Merola, J.F.; McInnes, I.B.; Deodhar, A.A.; Dey, A.K.; Adamstein, N.H.; Quebe-Fehling, E.; Aassi, M.; Peine, M.; Mehta, N.N. Effect of Secukinumab on Traditional Cardiovascular Risk Factors and Inflammatory Biomarkers: Post Hoc Analyses of Pooled Data Across Three Indications. Rheumatol. Ther. 2022, 9, 935–955. [Google Scholar] [CrossRef]
- Bahadır, A.; Baltacı, D.; Türker, Y.; Türker, Y.; Iliev, D.; Öztürk, S.; Deler, M.H.; Sarıgüzel, Y.C. Is the neutrophil-to-lymphocyte ratio indicative of inflammatory state in patients with obesity and metabolic syndrome? Anatol. J. Cardiol. 2015, 15, 816–822. [Google Scholar] [CrossRef]
- Sarrafan-Chaharsoughi, Z.; Sinaii, N.; Demidowich, A.P.; Yanovski, J.A. The association of Neutrophil-to-Lymphocyte ratio with metabolic syndrome in U.S. Adults: Findings from the 1999–2018 National Health and Nutrition Examination survey. J. Clin. Transl. Endocrinol. 2024, 39, 100382. [Google Scholar] [CrossRef]
- Ion, R.M.; Sibianu, M.; Hutanu, A.; Beresescu, F.G.; Sala, D.T.; Flavius, M.; Rosca, A.; Constantin, C.; Scurtu, A.; Moriczi, R.; et al. A Comprehensive Summary of the Current Understanding of the Relationship between Severe Obesity, Metabolic Syndrome, and Inflammatory Status. J. Clin. Med. 2023, 12, 3818. [Google Scholar] [CrossRef]
- Tarp, S.; Bartels, E.M.; Bliddal, H.; Furst, D.E.; Boers, M.; Danneskiold-Samsøe, B.; Rasmussen, M.; Christensen, R. Effect of nonsteroidal antiinflammatory drugs on the C-reactive protein level in rheumatoid arthritis: A meta-analysis of randomized controlled trials. Arthritis Rheum. 2012, 64, 3511–3521. [Google Scholar] [CrossRef]
- Gökmen, F.; Akbal, A.; Reşorlu, H.; Gökmen, E.; Güven, M.; Aras, A.B.; Erbağ, G.; Kömürcü, E.; Akbal, E.; Coşar, M. Neutrophil-Lymphocyte Ratio Connected to Treatment Options and Inflammation Markers of Ankylosing Spondylitis. J. Clin. Lab. Anal. 2015, 29, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Bennett, R.M.; Bushmakin, A.G.; Cappelleri, J.C.; Zlateva, G.; Sadosky, A.B. Minimal clinically important difference in the fibromyalgia impact questionnaire. J. Rheumatol. 2009, 36, 1304–1311. [Google Scholar] [CrossRef]
- Bittar, M.; Deodhar, A. Axial Spondyloarthritis: A Review. JAMA 2025, 333, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Machado, P.M. Evolution of the axial spondyloarthritis disease activity score and uptake in clinical practice. Curr. Opin. Rheumatol. 2025. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Biswas, M.; Suvarna, R.; Krishnan, S.V.; Devasia, T.; Shenoy Belle, V.; Prabhu, K. The mechanistic role of neutrophil lymphocyte ratio perturbations in the leading non communicable lifestyle diseases. F1000Research 2022, 11, 960. [Google Scholar] [CrossRef]
- Singh, R.; Rai, J.; Pathak, A.; Rai, N.K. A study on the effect of fibromyalgia severity on sleep quality using inflammatory markers. Bioinformation 2024, 20, 1608–1612. [Google Scholar] [CrossRef]
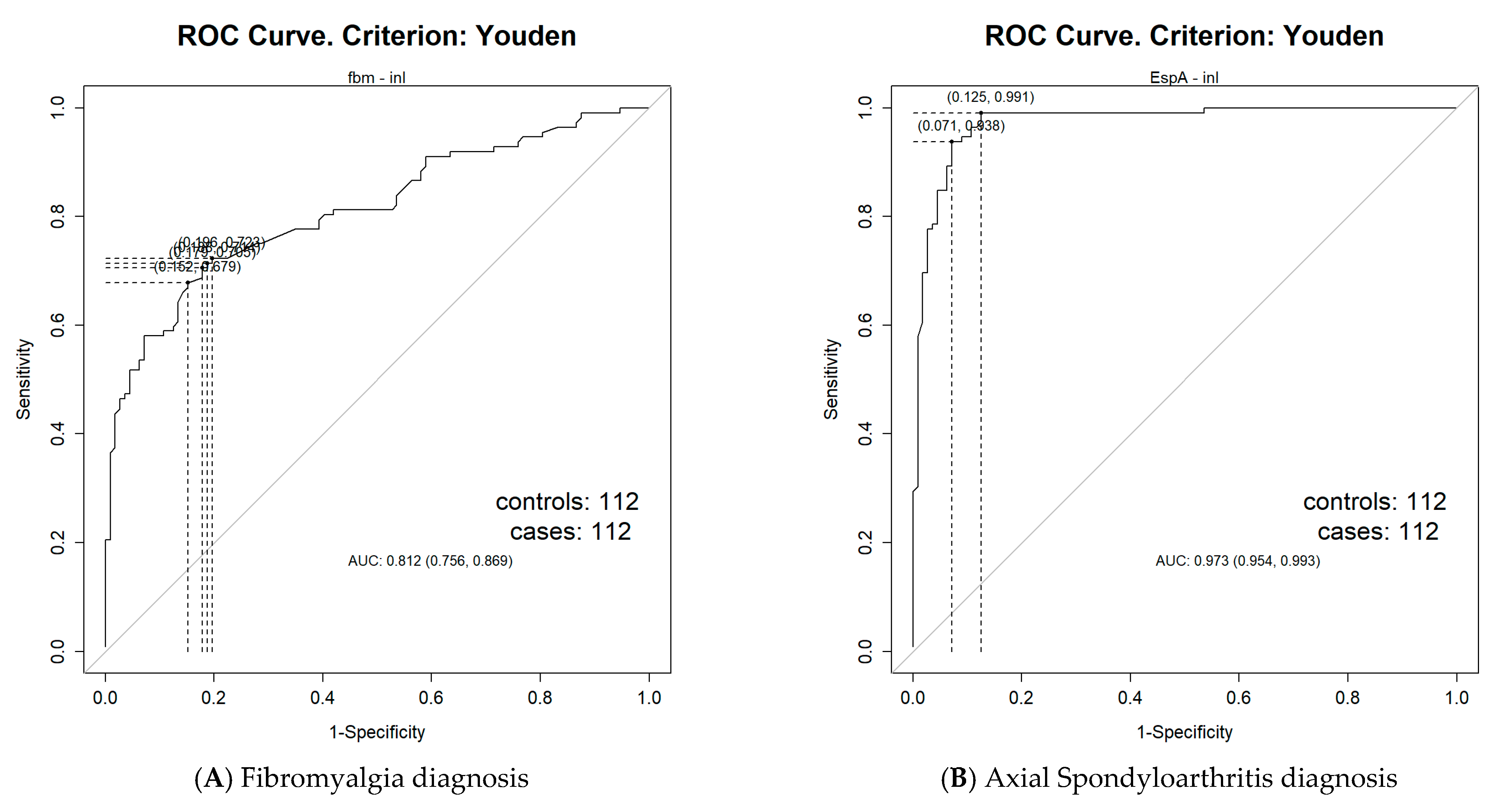
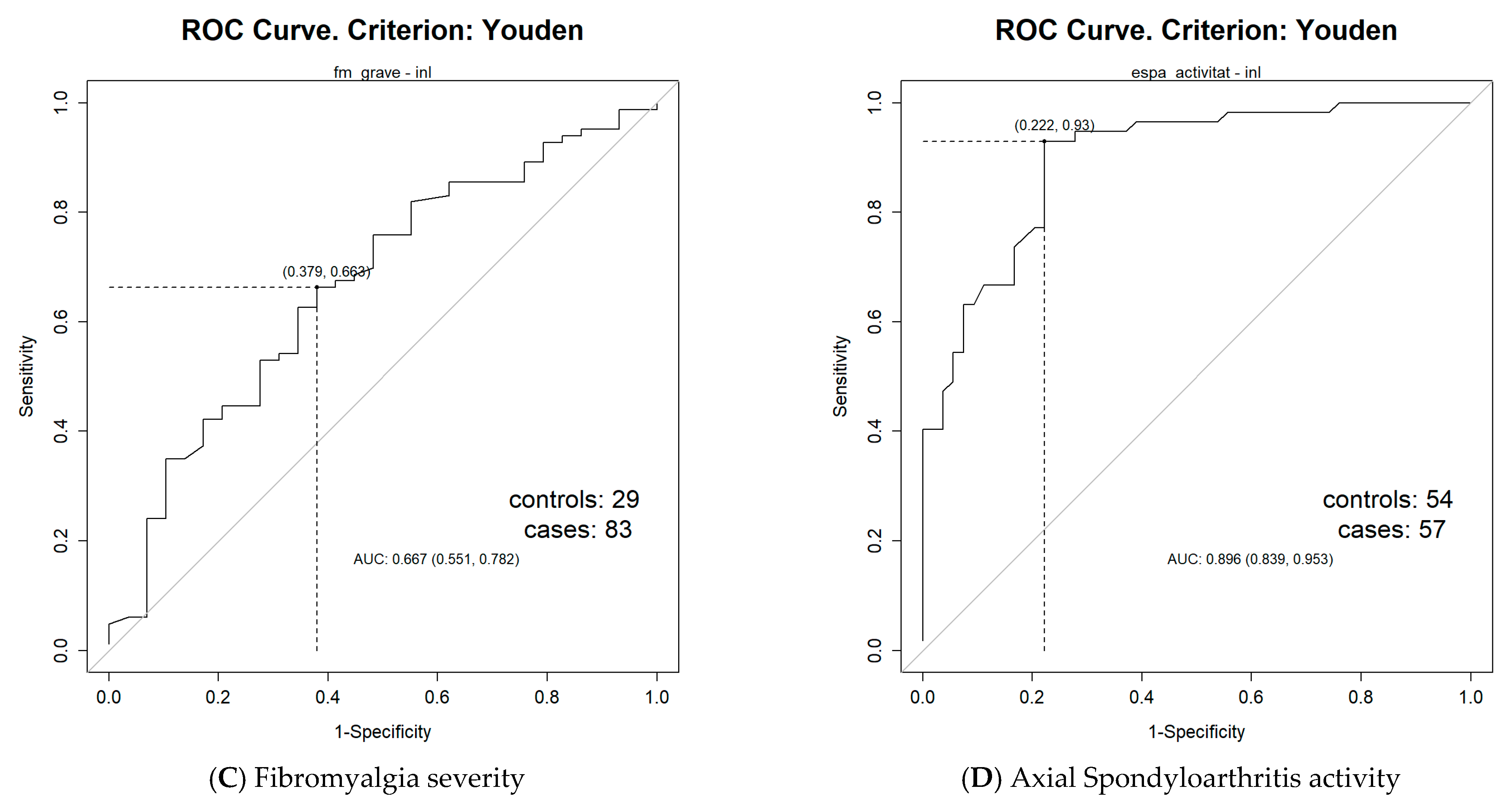
| Variables | FM (n = 112) | HC (n = 112) | axSpA (n = 112) | p Value FM-HC | p Value FM-AxSpA | p Value AxSpA-HC |
|---|---|---|---|---|---|---|
| Age, mean (SD) | 49.4 (±7.5) | 49.1 (±7.7) | 49.2 (±7.4) | 0.89 | 0.83 | 0.97 |
| Women, n (%) | 94 (83.9%) | 94 (83.9%) | 94 (83.9%) | 0.99 | 0.99 | 0.99 |
| NLR, mean (SD) | 1.8 (±0.5) | 1.4 (±0.2) | 2.1 (±0.3) | <0.001 | <0.001 | <0.001 |
| BMI, mean (SD) | 27.3 (±5.6) | 26.1 (±4.1) | 26.2 (±4.6) | 0.21 | 0.22 | 0.96 |
| AHT, n (%) | 20 (17.9%) | 21 (18.8%) | 20 (17.9%) | 0.99 | 0.98 | 0.98 |
| DLP, n (%) | 25 (22.3%) | 18 (16.1%) | 31 (27.7%) | 0.31 | 0.44 | 0.05 |
| DM, n (%) | 11 (9.8%) | 7 (6.2%) | 10 (8.9%) | 0.46 | 0.98 | 0.61 |
| AMI, n (%) | 1 (0.9%) | 1 (0.9%) | 2 (1.8%) | 0.99 | 0.98 | 0.98 |
| Stroke, n (%) | 1 (0.9%) | 0 (0.0%) | 2 (1.8%) | 0.98 | 0.98 | 0.50 |
| NSAIDs, n (%) | 29 (25.9%) | 28 (25%) | 58 (51.8%) | 0.98 | <0.001 | <0.001 |
| CRP (mg/dL), mean (SD) | 0.5 (±0.2) | 0.6 (±0.3) | 0.01 | |||
| Duration sso, (years), mean (SD) | 11.7 (±7.2) | 17.7 (±8.5) | ||||
| Duration sdg, (years), mean (SD) | 7.2 (±6.1) | 14.3 (±8.3) | ||||
| FIQR, mean (SD) | 71.8 (±14.3) | |||||
| FIQR ≥ 59, n (%) | 83 (74.1%) | |||||
| ASDAS, mean (SD) | 2.3 (±1.0) | |||||
| ASDAS ≥ 2.1, n (%) | 57 (50.9%) | |||||
| BASDAI, mean (SD) | 35.7 (±21.3) | |||||
| csDMARD, n (%) | 22 (19.6%) | |||||
| bDMARD, n (%) | 70 (62.5%) | |||||
| tsDMARD, n (%) | 14 (12.5%) |
| Variables | CC with NLR in FM | CC with NLR in AxSpA |
|---|---|---|
| CRP | 0.14 | 0.38 |
| FIQR | 0.15 | |
| ASDAS | 0.62 | |
| BASDAI | 0.57 |
| FIQR ≥ 59 (Severe FM) | FIQR < 59 (Moderate or Mild FM) | p Value | |
|---|---|---|---|
| NLR value | 1.9 (±0.5) | 1.7 (±0.4) | 0.008 |
| ASDAS ≥ 2.1 (High/Very High Disease Activity) | ASDAS < 2.1 (Inactive/Low Disease Activity) | p Value | |
|---|---|---|---|
| NLR value | 2.3 (±0.3) | 1.9 (±0.2) | <0.001 |
| FM Diagnosis | AxSpA Diagnosis | Severe FM | High/Very High Disease Activity in AxSpA | |
|---|---|---|---|---|
| NLR cut-off value | 1.54 | 1.61 | 1.64 | 1.95 |
| AUC = 0.81 (95% CI: 0.76–0.87) | AUC = 0.97 (95% CI: 0.95–0.99) | AUC = 0.66 (95% CI: 0.55–0.78) | AUC = 0.89 (95% CI: 0.84–0.95) | |
| SE = 70.54% | SE = 98.21% | SE = 66.27% | SE = 92.98% | |
| SP = 82.14% | SP = 91.96% | SP = 62.07% | SP = 77.78% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almirall, M.; Espartal, E.; Michelena, X.; Suso-Ribera, C.; Serrat, M.; Marsal, S.; Erra, A. Neutrophil–Lymphocyte Ratio in Fibromyalgia and Axial Spondyloarthritis: A Potential Biomarker for Diagnosis and Disease Activity. Biomedicines 2025, 13, 1497. https://doi.org/10.3390/biomedicines13061497
Almirall M, Espartal E, Michelena X, Suso-Ribera C, Serrat M, Marsal S, Erra A. Neutrophil–Lymphocyte Ratio in Fibromyalgia and Axial Spondyloarthritis: A Potential Biomarker for Diagnosis and Disease Activity. Biomedicines. 2025; 13(6):1497. https://doi.org/10.3390/biomedicines13061497
Chicago/Turabian StyleAlmirall, Miriam, Esther Espartal, Xabier Michelena, Carlos Suso-Ribera, Mayte Serrat, Sara Marsal, and Alba Erra. 2025. "Neutrophil–Lymphocyte Ratio in Fibromyalgia and Axial Spondyloarthritis: A Potential Biomarker for Diagnosis and Disease Activity" Biomedicines 13, no. 6: 1497. https://doi.org/10.3390/biomedicines13061497
APA StyleAlmirall, M., Espartal, E., Michelena, X., Suso-Ribera, C., Serrat, M., Marsal, S., & Erra, A. (2025). Neutrophil–Lymphocyte Ratio in Fibromyalgia and Axial Spondyloarthritis: A Potential Biomarker for Diagnosis and Disease Activity. Biomedicines, 13(6), 1497. https://doi.org/10.3390/biomedicines13061497






